Abstract
LncRNA SOX21 antisense RNA 1 (SOX21-AS1) dysregulated in many types of human cancer, and functioned as tumor suppressor or promoter depending on tumor types. However, there was no report about the role of SOX21-AS1 in nephroblastoma. In the present study, we first found that SOX21-AS1 expression was elevated in nephroblastoma tissues and cell lines compared with adjacent normal tissues and normal human embryonic kidney cell line, respectively. Moreover, we observed nephroblastoma patients with large tumor size, advanced National Wilms Tumor Study (NWTS) stage or unfavorable histopathological type, and patients that had higher SOX21-AS1 expression levels than nephroblastoma patients with small tumor size, early NWTS stage or favorable histopathological type. The in vitro studies suggested that knockdown of SOX21-AS1 suppressed nephroblastoma cell proliferation and colony formation, and induced cell-cycle arrest through up-regulating p57 expression. In conclusion, our study suggests that SOX21-AS1 functions as oncogenic lncRNA in nephroblastoma, which may provide a novel insight for nephroblastoma carcinogenesis.
Keywords: biomarker, lncRNA, nephroblastoma, SOX21-AS1, Wilms tumor
Introduction
Nephroblastoma, also known as Wilms’ tumor, is the most common pediatric kidney cancer accounting for approximately 8% of all malignant tumors in children [1]. The incidence rate of nephroblastoma is approximately 2/100 million [2]. Nephroblastoma is generally discovered under the age of 5 years, and the average age of morbidity is approximately 3 years [3]. Recent decades, the overall survival rate of nephroblast cases has risen to exceeding 90% due to the advance of systemic treatment including surgery, radiotherapy and chemotherapy [4]. In China, the rapid population growth is resulting in a large number of new nephroblastoma cases, which cause serious influence on children’s health [5,6]. Thus, a better understanding of nephroblastoma progression would be a substantial advancement for identifying sensitive molecular biomarkers to predict high risk patients and guide treatment.
Long non-coding RNA (lncRNA) is class of non-coding RNAs with >200 nucleotides in length and no protein coding capacity [7,8]. Recently, lncRNA H19 and LINC00473 have been confirmed to be involved in the development and progression of nephroblastoma [9–13].
LncRNA SOX21 antisense RNA 1 (SOX21-AS1) is located at chromosome 13q32.1 and transcribed into a 2986 nt transcript [14]. In Alzheimer’s disease, knocking down SOX21-AS1 alleviated neuronal oxidative stress and suppressed neuronal apoptosis through up-regulating FZD3/5 and activating Wnt signaling pathway [15]. Moreover, increasing studies showed that SOX21-AS1 functioned as cancer-associated lncRNA in several kinks of human tumor, such as oral cancer [16], lung cancer [17], colorectal cancer [18,19], hepatocellular carcinoma [20] and glioblastoma [21]. However, there was no report about the role of SOX21-AS1 in nephroblastoma. In our early study, five potential associated lncRNAs were screened and measured in nephroblastoma tissues and adjacent normal tissues, and SOX21-AS1 expression level was the highest in nephroblastoma tissues among five lncRNAs. Therefore, the aim of the present study was to explore the clinical value and biological function of SOX21-AS1 in nephroblastoma.
Materials and methods
Ethical statement
The present study was approved by the Ethical Review Committee of Linyi Central Hospital and Maternal and Child Health Care Family Planning Service Center of Yishui County. Written informed consent was obtained from all the patients. The present study was carried out in accordance with the World Medical Association Declaration of Helsinki
Sample collection
Forty pairs of nephroblastoma tissues and corresponding adjacent normal tissues were collected from Linyi Central Hospital or Maternal and Child Health Care Family Planning Service Center of Yishui County. All specimens were confirmed by at least two pathologists, and stored at −80°C refrigerator. All cases have not received preoperative chemotherapy or radiotherapy.
Cell lines
The nephroblastoma cell lines (WiT49 and WT-CLS1) and normal human embryonic kidney cell line (HEK293) were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, MA, U.S.A.) supplemented with 10% FBS (Gibco, Rockville, MD, U.S.A.) at a humidified incubator with 5% CO2 and 37°C.
RNA extraction and quantitative real-time PCR
The total RNAs were extracted from nephroblastoma tissues or cells with TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instruction. Then, total RNAs were used for template to reverse-transcribed into cDNAs using PrimeScript RT Master Mix (Takara Biomedical Technology, Beijing, China). The quantitative real-time PCR (qRT-PCR) was conducted with One Step TB Green PrimeScript RT-PCR Kit (Takara Biomedical Technology, Beijing, China) at ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, U.S.A.) following the manufacturer’s protocols. Gene expression was normalized to GAPDH.
RNA interference
The SOX21-AS1-specific small interfering RNA (siRNA-SOX21-AS1) and negative control siRNA (siRNA-NC) were purchased from GenePharma Co., Ltd (Shanghai, China). The siRNAs were transfected into nephroblastoma cells through Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instruction.
Cell counting kit-8 (CCK-8) assay
The Cell counting kit-8 (CCK-8) assay was utilized to estimate the effect of SOX21-AS1 on nephroblastoma cell proliferation according to the manufacturer’s instructions. The nephroblastoma cells were transfected with siRNA-SOX21-AS1 for 1, 2, 3 or 4 days, and 10 μl of CCK-8 was added to the culture medium in each well of 96-well plates. After 1 h of incubation at 37°C, the optical density at 450 nm was detected at a microplate monitor.
Colony formation assay
The transfected nephroblastoma cells (500 cells/well) were plated in six-well plates, and replacing the culture medium every 4 days. After 10 days culture, the colonies were fixed with methanol, and stained with 0.1% Crystal Violet (Beyotime, Shanghai, China). Finally, the specific number of colonies (>50 cells) was manually counted at light microscope.
Cell cycle assay
After 48-h transfection, the nephroblastoma cells were fixed in ethanol for 1 h and then incubated with RNase A. Afterward, nephroblastoma cells were stained with propidium iodide (Sigma-Aldrich, Louis, MO, U.S.A.) for 30 min at room temperature without light, and were analyzed by using flow cytometer (BD Biosciences, U.S.A.). The percentages of cells in the G0-G1, S, and G2-M phases were counted and compared.
Western blot
The proteins were extracted from nephroblastoma cells by using RIPA Lysis Buffer (Beyotime, Shanghai, China), and quantified through BCA protein assay kit (Beyotime, Shanghai, China). Equal quantities of protein were isolated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride. Subsequently, membranes were blocked in 5% non-fat milk for 1 h at room temperature, and incubated with anti-p57 or anti-β-actin (Cell Signaling Technology, Danvers, MA, U.S.A.) overnight at 4°C. Next, membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, and visualized using an enhanced chemiluminescence detection system (Beyotime, Shanghai, China). Finally, the bands were quantified by densitometry using Quantity One software.
Statistical analysis
All data were presented from at least three independent experiments and analyzed by SPSS 19.0 software (SPSS Inc., Chicago, IL, U.S.A.). The two-tailed Student’s t-test was used to estimate the statistical significance between two groups. P value <0.05 was considered statistically significant.
Results
The expression of SOX21-AS1 is up-regulated in nephroblastoma
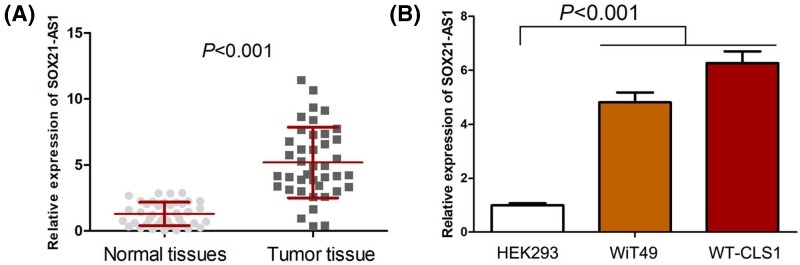
To identify SOX21-AS1 expression status in nephroblastoma, we measured SOX21-AS1 expression in nephroblastoma tissue samples and cell lines through using qRT-PCR. As shown in the Figure 1A, SOX21-AS1 expression was remarkably increased in nephroblastoma tissues compared with corresponding adjacent normal tissues. We also found nephroblastoma cell lines indicated higher SOX21-AS1 expression than normal human embryonic kidney cell line (Figure 1B).
Figure 1. The expression of SOX21-AS1 is up-regulated in nephroblastoma.
(A) SOX21-AS1 expression was remarkably increased in nephroblastoma tissues (n=40) compared with corresponding adjacent normal tissues (n=40). (B) SOX21-AS1 expression in nephroblastoma cell lines was higher than normal human embryonic kidney cell line.
The association between SOX21-AS1 expression and clinicopathological features in nephroblastoma patients
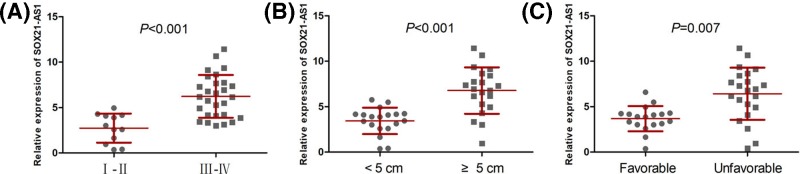
In order to know the clinical value of SOX21-AS1 in patients with nephroblastoma, all nephroblastoma patients were classified into different groups based on clinicopathological features including tumor size (<5 cm vs. ≥5 cm), NWTS (National Wilms Tumor Study) stage (I-II vs. III-IV) and histopathological type (favorable vs. unfavorable). As shown in the Figure 2A–C, nephroblastoma patients with ≥5 cm, III-IV or unfavorable had profoundly higher SOX21-AS1 expression levels than nephroblastoma patients with <5 cm, I-II or favorable, respectively.
Figure 2. The association between SOX21-AS1 expression and clinicopathological features in nephroblastoma patients.
(A) Nephroblastoma patients with III-IV stage (n=28) had higher SOX21-AS1 expression levels than nephroblastoma patients with I-II stage (n=12). (B) Nephroblastoma patients with ≥5 cm (n=21) had higher SOX21-AS1 expression levels than nephroblastoma patients with <5 cm (n=19). (C) Nephroblastoma patients with unfavorable histopathological type (n=22) had higher SOX21-AS1 expression levels than nephroblastoma patients with favorable histopathological type (n=18).
Knockdown of SOX21-AS1 suppresses nephroblastoma cell proliferation and colony formation, and induces cell-cycle arrest
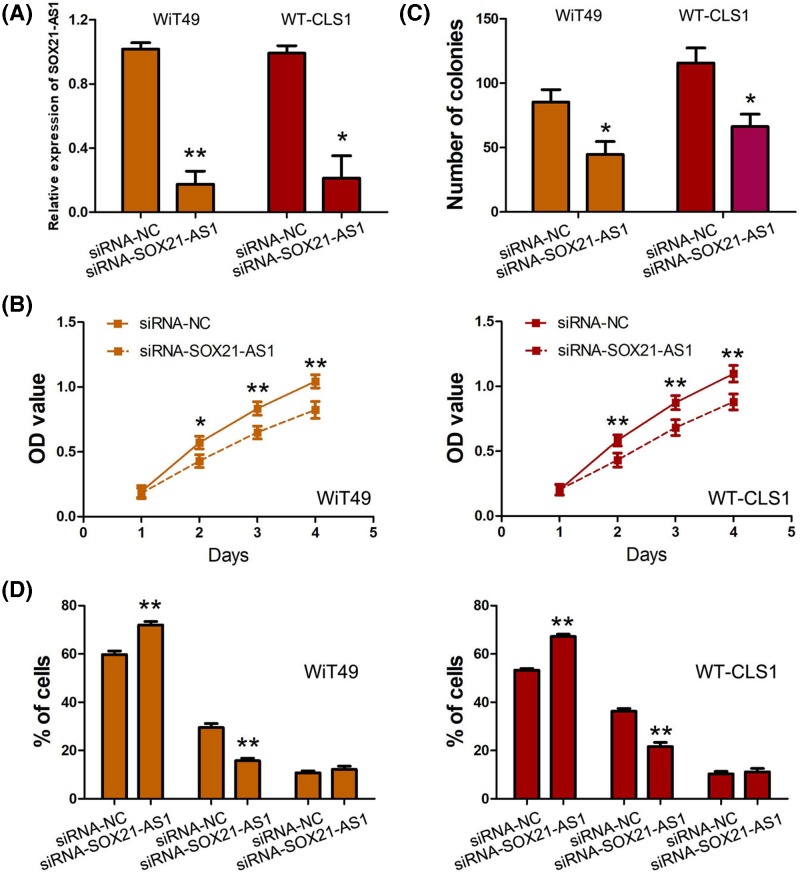
As shown in the Figure 1B, SOX21-AS1 expression levels were markedly low in nephroblastoma cell lines. Thus, we performed loss-of-function study through siRNA-SOX21-AS1 transfection in nephroblastoma cells. The efficient silencing of SOX21-AS1 in nephroblastoma cells was confirmed by using qRT-PCR (Figure 3A). We conducted CCK-8 assay to assess the effect of SOX21-AS1 expression on nephroblastoma cell proliferation, and found silencing SOX21-AS1 expression obviously impaired cell proliferation ability of nephroblastoma cells (Figure 3B). Moreover, we conducted the colony formation assay to further confirm the role of SOX21-AS1 on nephroblastoma cell growth. Similarly, we observed that silencing SOX21-AS1 expression inhibited the number of colony formation in nephroblastoma cells (Figure 3C). In addition, cycle analysis by flow cytometry suggested that silencing SOX21-AS1 expression induced the cycle arrest at G1/G0 phase in nephroblastoma cells (Figure 3D).
Figure 3. The biological function of SOX21-AS1 in nephroblastoma cells.
(A) The efficient silencing of SOX21-AS1 in nephroblastoma cells was confirmed by using qRT-PCR. (B) Knockdown of SOX21-AS1 suppressed nephroblastoma cell proliferation. (C) Knockdown of SOX21-AS1 expression inhibited the number of colony formation in nephroblastoma cells. (D) Knockdown of SOX21-AS1 expression induced the cycle arrest at G1/G0 phase in nephroblastoma cells. (*, P<0.01; **, P<0.001).
Knockdown of SOX21-AS1 promotes p57 expression in nephroblastoma cells
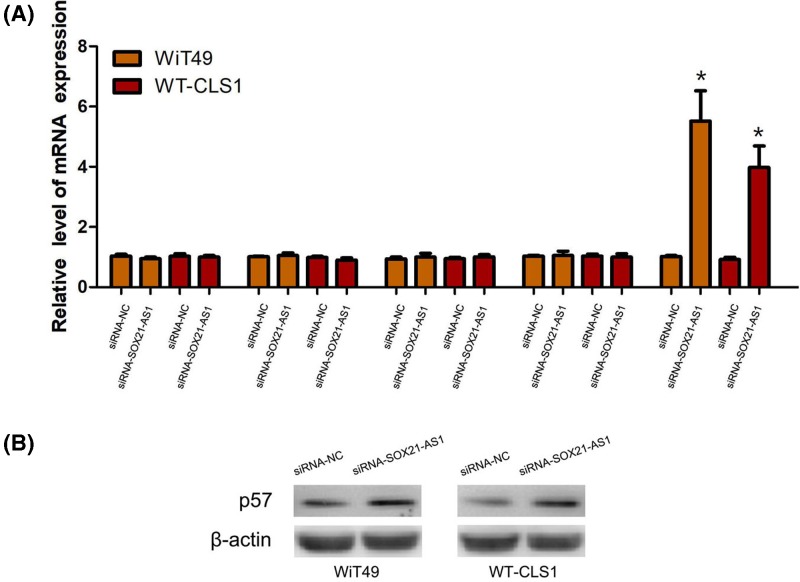
To investigate the mechanism of SOX21-AS1 on nephroblastoma cell proliferation, the mRNA levels of p15, p16, p21, p27 and p57 were determined using qRT-PCR following silencing SOX21-AS1 expression in nephroblastoma cells. The results of qRT-PCR showed that silencing SOX21-AS1 expression definitely elevated mRNA level of p57, but had no effect on mRNA levels of p15, p16, p21 and p27 (Figure 4A). Furthermore, we performed Western blot to confirm the influence of SOX21-AS1 on the p57 protein expression, and found that silencing SOX21-AS1 expression remarkably increased p57 protein expression in nephroblastoma cells (Figure 4B).
Figure 4. The molecular mechanism of SOX21-AS1 in nephroblastoma cells.
(A) The mRNA levels of p15, p16, p21, p27 and p57 were determined using qRT-PCR following silencing SOX21-AS1 expression in nephroblastoma cells. (B) Knockdown of SOX21-AS1 expression increased p57 protein expression in nephroblastoma cells. (*, P<0.01).
Discussion
SOX21-AS1 has been showed to be dysregulated in many types of human cancer. Originally, Yang et al. [16] comprehensively analyzed transcriptome profiles from two pairs of oral squamous cell carcinoma and corresponding adjacent normal tissues, and identified SOX21-AS1 for the following study due to a CpG-rich region upstream of SOX21-AS1. Then, SOX21-AS1 expression levels were found to be down-regulated in oral squamous cell carcinoma tissue samples compared with matching normal tissue samples [16]. Furthermore, the clinical correlation analysis showed low SOX21-AS1 expression was correlated with advanced clinical stage and large tumor size, and univariate and multivariate Cox’s regression analysis suggested low SOX21-AS1 expression acted as an independent unfavorable prognostic factor for disease-specific survival in patients with oral squamous cell carcinoma [16]. Afterward, Paul et al. [21] also performed lncRNA transcript profiling from 19 glioblastoma samples and 9 control brain samples, but found there was no significant difference of SOX21-AS1 expression between glioblastoma samples and control brain samples. Furthermore, they performed survival analysis from the TCGA glioblastoma RNA-Seq data, and identified SOX21-AS1 as a good prognostic predictor for overall survival in glioblastoma patients [21]. On the contrary, recent more evidence suggested SOX21-AS1 expression was increased in human cancers. Lu et al. [17] performed and analyzed TCGA lung cancer RNA-Seq data, and found that SOX21-AS1 expression was increased approximately 5-fold in lung cancer tissues compared with normal lung tissues. Then, they confirmed high SOX21-AS1 expression status in lung cancer tissues and cell lines through qRT-PCR, and observed patients with high SOX21-AS1 expression tended to have large tumor size or advanced TNM stage [17]. Besides, Kaplan–Meier survival analysis suggested higher SOX21-AS1 expression correlated with worse overall survival, and univariate and multivariate analyses indicated that high SOX21-AS1 expression was an independent biomarker for unfavorable overall survival in lung cancer patients [17]. Similarly, Wei et al. [20] found SOX21-AS1 expression was increased in hepatocellular carcinoma tissues and cell lines respectively compared with adjacent normal liver tissues and normal liver epithelial cell line, and SOX21-AS1 overexpression was associated with large tumor size, high Edmonson grade, vascular invasion and cirrhosis in patients with hepatocellular carcinoma. The survival analyses revealed that cases with high SOX21-AS1 expression had short survival time compared with cases with low SOX21-AS1 expression, and high SOX21-AS1 expression served as an independent unfavorable predictor for overall survival in patients with hepatocellular carcinoma [20]. In colon cancer, Wang et al. [18] comprehensively analyzed TCGA colon cancer RNA-Seq data, and found that SOX21-AS1 expression was obviously up-regulated in colon cancer tissues. In addition, Wei et al. [19] performed qRT-PCR to confirm high levels of lncRNA SOX21-AS1 in colorectal cancer tissue samples and cell lines. Meanwhile, they observed aberrant high SOX21-AS1 expression predicted unfavorable clinical outcome in colorectal cancer patients [19]. The expression pattern and clinical significance of SOX21-AS1 were still unknown in nephroblastoma. In our study, we first found that SOX21-AS1 expression was elevated in nephroblastoma tissues and cell lines compared with adjacent normal tissues and normal human embryonic kidney cell line, respectively. Moreover, we observed nephroblastoma patients with large tumor size, advanced NWTS stage or unfavorable histopathological type had higher SOX21-AS1 expression levels than nephroblastoma patients with small tumor size, early NWTS stage or favorable histopathological type, respectively.
SOX21-AS1 functioned as tumor suppressor or promoter depending on tumor types. In oral cancer cells, Yang et al. [16] indicated that SOX21-AS1 overexpression markedly inhibited cell proliferation and invasion. However, Wei et al. [20] found that down-regulation of SOX21-AS1 expression inhibited proliferation and invasion, arrested cell cycle process, and accelerated cell apoptosis through increasing p21 expression in hepatocellular carcinoma. In colorectal cancer cells, Wei et al. [19] that showed SOX21-AS1 sponged miR-145 to enhance tumor cell proliferation and invasion in vivo, and tumor growth in vitro through up-regulating myosin VI. Moreover, Lu et al. [17] found that inhibition of SOX21-AS1 expression repressed lung cancer cell proliferation and cell cycle progression through up-regulating p57 expression. Similarly, we also found knockdown of SOX21-AS1 suppressed nephroblastoma cell proliferation and colony formation, and induced cell-cycle arrest through elevating p57 expression.
In conclusion, SOX21-AS1 expression is increased in nephroblastoma tissues and cells, and has relationships with tumor size, NWTS stage and histopathological type in nephroblastoma cases. Down-regulation of SOX21-AS1 expression mediates p57 to inhibit nephroblastoma cell proliferation, colony formation and cell-cycle progression.
Abbreviations
- CCK-8
cell counting kit-8
- lncRNA
long non-coding RNA
- NWTS
National Wilms Tumor Study
- qRT-PCR
quantitative real-time PCR
- SOX21-AS1
SOX21 antisense RNA 1
Author Contribution
Xiangguo Sun: studied concept and design; analyzed and interpreted the data; revised the manuscript. Jingxiu Zhang, Tianzhao Hou, Xueliang Qi, Jihong Wang: executed experiment and statistical analysis.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
References
- 1.Martinez C.H., Dave S. and Izawa J. (2010) Wilms’ tumor. Adv. Exp. Med. Biol. 685, 196–209 10.1007/978-1-4419-6448-9_18 [DOI] [PubMed] [Google Scholar]
- 2.Al-Hussain T., Ali A. and Akhtar M. (2014) Wilms tumor: an update. Adv. Anat. Pathol. 21, 166–173 10.1097/PAP.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 3.Dome J.S., Graf N., Geller J.I., Fernandez C.V., Mullen E.A., Spreafico F.. et al. (2015) Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 33, 2999–3007 10.1200/JCO.2015.62.1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szychot E., Apps J. and Pritchard-Jones K. (2014) Wilms’ tumor: biology, diagnosis and treatment. Transl. Pediatrics 3, 12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Han L., He Z., Li X., Yang S., Yang J.. et al. (2018) Advances in limb salvage treatment of osteosarcoma. J. Bone Oncol. 10, 36–40 10.1016/j.jbo.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Lei C., Xiang B., Li F., Wang C., Wang Q.. et al. (2017) Extrarenal teratoma with nephroblastoma in the retroperitoneum: case report and literature review. Medicine 96, e8670 10.1097/MD.0000000000008670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarroux J., Morillon A. and Pinskaya M. (2017) History, discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 1008, 1–46 10.1007/978-981-10-5203-3_1 [DOI] [PubMed] [Google Scholar]
- 8.Cao M., Zhao J. and Hu G. (2019) Genome-wide methods for investigating long noncoding RNAs. Biomed. Pharmacother. 111, 395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto K., Morison I.M., Taniguchi T. and Reeve A.E. (1997) Epigenetic changes at the insulin-like growth factor II/H19 locus in developing kidney is an early event in Wilms tumorigenesis. PNAS 94, 5367–5371 10.1073/pnas.94.10.5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frevel M.A., Sowerby S.J., Petersen G.B. and Reeve A.E. (1999) Methylation sequencing analysis refines the region of H19 epimutation in Wilms tumor. J. Biol. Chem. 274, 29331–29340 10.1074/jbc.274.41.29331 [DOI] [PubMed] [Google Scholar]
- 11.Cerrato F., Sparago A., Verde G., De Crescenzo A., Citro V., Cubellis M.V.. et al. (2008) Different mechanisms cause imprinting defects at the IGF2/H19 locus in Beckwith-Wiedemann syndrome and Wilms’ tumour. Hum. Mol. Genet. 17, 1427–1435 10.1093/hmg/ddn031 [DOI] [PubMed] [Google Scholar]
- 12.Hubertus J., Lacher M., Rottenkolber M., Muller-Hocker J., Berger M., Stehr M.. et al. (2011) Altered expression of imprinted genes in Wilms tumors. Oncol. Rep. 25, 817–823 10.3892/or.2010.1113 [DOI] [PubMed] [Google Scholar]
- 13.Zhu S., Fu W., Zhang L., Fu K., Hu J., Jia W.. et al. (2018) LINC00473 antagonizes the tumour suppressor miR-195 to mediate the pathogenesis of Wilms tumour via IKKalpha. Cell Prolif. 51, e12416 10.1111/cpr.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo X., Qiu Y., Jiang Y., Chen F., Jiang L., Zhou Y.. et al. (2018) Long non-coding RNA implicated in the invasion and metastasis of head and neck cancer: possible function and mechanisms. Mol. Cancer 17, 14 10.1186/s12943-018-0763-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi P., Lin W.R., Zhang M., Huang D., Ni S.J., Zhu X.L.. et al. (2018) E2F1 induces LSINCT5 transcriptional activity and promotes gastric cancer progression by affecting the epithelial-mesenchymal transition. Cancer Manag. Res. 10, 2563–2571 10.2147/CMAR.S171652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang C.M., Wang T.H., Chen H.C., Li S.C., Lee M.C., Liou H.H.. et al. (2016) Aberrant DNA hypermethylation-silenced SOX21-AS1 gene expression and its clinical importance in oral cancer. Clin. Epigenetics 8, 129 10.1186/s13148-016-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu X., Huang C., He X., Liu X., Ji J., Zhang E.. et al. (2017) A novel long non-coding RNA, SOX21-AS1, indicates a poor prognosis and promotes lung adenocarcinoma proliferation. Cell. Physiol. Biochem.: Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 42, 1857–1869 10.1159/000479543 [DOI] [PubMed] [Google Scholar]
- 18.Wang W.J., Li H.T., Yu J.P., Han X.P., Xu Z.P., Li Y.M.. et al. (2019) A Competing Endogenous RNA Network Reveals Novel Potential lncRNA, miRNA, and mRNA Biomarkers in the Prognosis of Human Colon Adenocarcinoma. J. Surg. Res. 235, 22–33 10.1016/j.jss.2018.09.053 [DOI] [PubMed] [Google Scholar]
- 19.Wei A.W. and Li L.F. (2017) Long non-coding RNA SOX21-AS1 sponges miR-145 to promote the tumorigenesis of colorectal cancer by targeting MYO6. Biomed. Pharmacother. 96, 953–959 [DOI] [PubMed] [Google Scholar]
- 20.Wei C., Wang H., Xu F., Liu Z. and Jiang R. (2018) LncRNA SOX21-AS1 is associated with progression of hepatocellular carcinoma and predicts prognosis through epigenetically silencing p21. Biomed. Pharmacother. 104, 137–144 [DOI] [PubMed] [Google Scholar]
- 21.Paul Y., Thomas S., Patil V., Kumar N., Mondal B., Hegde A.S.. et al. (2018) Genetic landscape of long noncoding RNA (lncRNAs) in glioblastoma: identification of complex lncRNA regulatory networks and clinically relevant lncRNAs in glioblastoma. Oncotarget 9, 29548–29564 10.18632/oncotarget.25434 [DOI] [PMC free article] [PubMed] [Google Scholar]