Abstract
Ecto-5′-nucleotidase (NT5E) is a glycosylphosphatidylinositol anchored cell surface protein, and has been suggested to be dysregulated in most types of human cancer including gastric cancer. The aim of the present study was to present more evidence about the clinical and prognostic value of Ecto-5′-nucleotidase in gastric cancer patients, and preliminarily explore the biological function of Ecto-5′-nucleotidase in gastric cancer cells. In our study, high Ecto-5′-nucleotidase expression was observed in gastric cancer tissues and cell lines, respectively, compared with normal gastric mucosa tissues cells. Meanwhile, TCGA database also indicated that Ecto-5′-nucleotidase expression levels were notably elevated in gastric cancer tissues compared with normal gastric mucosa tissues. Furthermore, high-expression of Ecto-5′-nucleotidase was obviously associated with advanced clinical stage, deep tumor invasion, lymph node metastasis and distant metastasis in gastric cancer patients. The survival analyses of TCGA database and our study consistent suggested high Ecto-5′-nucleotidase expression was negatively correlated with overall survival time in gastric cancer patients. The univariate and multivariate Cox proportional hazards regression model showed high Ecto-5′-nucleotidase expression was an independent poor prognostic factor for gastric cancer patients. Moreover, silencing of Ecto-5′-nucleotidase expression suppressed cell proliferation, migration and invasion in vitro in gastric cancer. In conclusion, Ecto-5′-nucleotidase is a credible prognostic biomarker, and serves as a potential therapeutic target in gastric cancer.
Keywords: biomarkers, gastric cancer, NT5E, prognosis
Introduction
Gastric cancer is the sixth most common cancer worldwide accounting for 1,033,701 newly diagnosed patients in 2018 [1]. Although the incidence and mortality of gastric cancer have been declining in the past decades, gastric cancer remains the second leading cause of cancer death in the world with an estimated 782,685 deaths in 2018 [1]. With the performance of clinical trials in gastric cancer, molecular targeted therapy was gradually considered as an important part of the clinical therapy such as trastuzumab [2] and apatinib [3]. Unfortunately, the prognosis of gastric cancer remains unfavorable with a 5-year overall survival of <20% [4,5]. Therefore, it is necessary to identify more novel biomarkers for guiding system management or developing effective therapeutic target in gastric cancer patients.
Ecto-5′-nucleotidase (also known as CD73) is glycosylphosphatidylinositol anchored cell surface protein, and has been suggested to be dysregulated in most types of human cancer [6,7]. In gastric cancer, Ecto-5′-nucleotidase was originally found to be methylated in primary gastric cancer tissues [8]. Subsequently, Ecto-5′-nucleotidase gene mutation was observed in gastric cancer metastasis, and predicted prognosis in gastric cancer [9,10]. Moreover, Ecto-5′-nucleotidase overexpression was found in gastric cancer tissues and serums, and associated with clinical progression in gastric cancer patients [11,12]. Due to limited sample size in previous study about the clinical significance of Ecto-5′-nucleotidase protein in gastric cancer, we present more evidence about the correlations between Ecto-5′-nucleotidase protein expression and clinicopathological characteristics including prognosis in gastric cancer patients. In addition, we tried to preliminarily explore the biological function of Ecto-5′-nucleotidase in gastric cancer cells.
Materials and methods
Tissue samples
The present study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Medical University (No. 20150132), The Second Affiliated Hospital of Shaanxi University of Chinese Medicine (No. 2015-CL013) and Sheyang County People’s Hospital (No. 15043-H), and complied with the Declaration of Helsinki. All patients were aware of the present study and signed an informed consent agreement. All patients provided a written informed consent prior to the study. A total of 131 paraffin-embedded gastric cancer tissues and 45 paraffin-embedded normal gastric mucosa tissues were collected through surgery or biopsy from gastric cancer patients who were not subjected to neoadjuvant radiotherapy and chemotherapy at The First Affiliated Hospital of Xi’an Medical University, The Second Affiliated Hospital of Shaanxi University of Chinese Medicine or Sheyang County People’s Hospital. The clinicopathological characteristics such as age, gender, histological type, clinical stage, tumor depth, lymph node metastasis and distant metastasis were included in the standard questionnaire, which was used to record each gastric cancer patient in the present study.
TCGA database
Analysis of TCGA database was performed at the GEPIA (Gene Expression Profiling Interactive Analysis, http://gepia.cancer-pku.cn/) platform. The Ecto-5′-nucleotidase expression profiles in gastric cancer samples (n=408) and normal gastric mucosa samples (n=211) were showed from TCGA database. The prognostic value of Ecto-5′-nucleotidase was assessed in gastric cancer cohort (n=384) from TCGA database through Kaplan–Meier method and log-rank test.
Cell lines
Four human gastric cancer cell lines (AGS, SGC-7901, BGC-823 and NCI-N87) and human gastric mucosal epithelial cell line (GES-1) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), and cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Rockville, MD, U.S.A.) supplemented with 10% fetal bovine serum (FBS, Gibco, Rockville, MD, U.S.A.) in a humidified incubator at 37°C with 5% CO2.
Immunohistochemistry
Immunohistochemical analysis was conducted to detect Ecto-5′-nucleotidase protein expression in gastric cancer tissues and normal gastric mucosa tissues. Paraffin-embedded tissue samples were sectioned at 4-μm thick. Then, sections were deparaffinized in xylene twice for 10 min, rehydrated through graded ethanol to distilled water. After conducting antigen retrieval using a microwave for 5 min at 95°C, 3% hydrogen peroxide and 5% non-fat dried milk were respectively used to block endogenous peroxidase activity and non-specific binding activity. Subsequently, the sections were incubated with the primary Ecto-5′-nucleotidase antibody (1:100 dilution; Abcam, MA, U.S.A.) overnight at 4°C, and horseradish peroxidase-labeled anti-goat IgG secondary antibody for 30 min at room temperature. The target protein was stained with 3,3′-diaminobenzidine (DAB; Zhongshan biotech, Beijing, China) in the sections. Finally, all the sections were dehydrated and sealed after hematoxylin counterstain was completed.
The Ecto-5′-nucleotidase staining was estimated semi-quantitatively according to the combined scores of the staining intensity and percentage of positive-staining tumor cells by at least two pathologists who were blinded to the clinical data. The staining strength was graded as follows: 0 score, no coloring; 1 score, slightly yellow; 2 score, brown yellow; 3 score, tan. The percentage of positive cells was graded as follows: 0 score, positive cells <1%; 1 score, the number of positive cells ranged from 2 to 25%; 2 score, the number of positive cells ranged from 26 to 50%; 3 score, the number of positive cells ranged from 51 to 75%; 4 score, the number of positive cells >75%. Final score ≥6 and <6 were respectively defined as high-expression of Ecto-5′-nucleotidase and low-expression of Ecto-5′-nucleotidase.
Western blot
Gastric cancer cells were lysed in RIPA buffer (Beyotime, Shanghai, China), and protein concentrations were detected by using BCA Protein Assay Kit (Beyotime, Shanghai, China). After the protein concentration of each specimen was adjusted, equal protein of each specimen was separated at sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA, U.S.A.). Then, the membrane was blocked by 5% non-fat dried milk for 1 h, and incubated with the primary Ecto-5′-nucleotidase antibody (1:1000 dilution; Abcam, MA, U.S.A.) overnight at 4°C. In next day, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. The protein bands were visualized by using the enhanced chemiluminescence Detection System (Thermo Fisher Scientific, Waltham, MA, U.S.A.).
siRNA transfection
Small interfering RNA targeting Ecto-5′-nucleotidase (si-Ecto-5′-nucleotidase) and a scrambled negative control (si-NC) were purchased from Thermo Fisher Scientific. Gastric cancer cells were cultured 24 h, and transiently transfected with either si-Ecto-5′-nucleotidase or si-NC by using Lipofectamine® RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. Knockdown efficiency was estimated by Western blot.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was evaluated using Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Kumamoto, Japan). Transfected gastric cancer cells (3 × 103) were seeded into 96-well plates (Corning, NY, U.S.A.). About 10 μl of CCK-8 solution was then added to each well containing 100 μl of serum-free medium at 24, 48, 72 and 96 h. Then, the cells were incubated for 2 h at 37°C. Cell viability was determined in absorbance at 450 nm by a microplate reader. All assays were repeated three times.
Transwell migration and invasion assays
Cell migration and invasion abilities were estimated by Transwell invasion and migration assays. For invasion assay, transwell chambers (8 μm pores, BD Bioscience, San Jose, CA, U.S.A.) were pre-coated with Matrigel (BD Bioscience, San Jose, CA, U.S.A.) in the upper chamber. For migration assay, there was no Matrigel in the upper chamber. Briefly, 5 × 104 cells were first starved in serum-free medium, and placed in the upper chamber. Meanwhile, medium with 10% FBS added to the lower chamber. After incubating 18 h for migration and 24 h for invasion, the cells at the bottom surface of the filter membrane were stained with Giemsa solution and photographed in five random fields per insert under a light microscopy. Experiments were repeated at least three times.
Statistical analysis
Statistical analysis was conducted by SPSS version 17.0 (Chicago, IL, U.S.A.). Comparisons between two groups were evaluated using Student’s t-test. The two-tailed Chi-square test or Fisher’s exact test was used to determine the significance of correlation between Ecto-5′-nucleotidase expression and clinicopathological features of gastric cancer. The overall survival curve was drawn by Kaplan–Meier method, and the significant differences of survival curves were evaluated by the log-rank test. Univariate and multivariate Cox proportional hazards regression model were used to estimate the independent predictor for overall survival of gastric cancer patients. Differences were considered to be significant at P<0.05.
Results
The expression of Ecto-5′-nucleotidase in gastric cancer
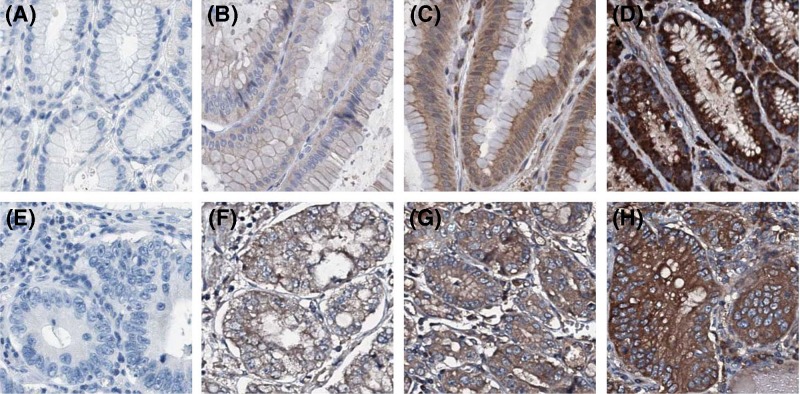
To investigate Ecto-5′-nucleotidase expression in human gastric cancer, we first observed the difference of Ecto-5′-nucleotidase expression between gastric cancer tissues and normal gastric mucosa tissues at TCGA database. In comparison with normal gastric mucosa tissues, Ecto-5′-nucleotidase expression levels were notably elevated in gastric cancer tissues (P<0.001, Figure 1A). To further confirm the expression of Ecto-5′-nucleotidase in gastric cancer, we performed immunohistochemical staining to detect Ecto-5′-nucleotidase protein expression in 131 gastric cancer tissues (Figure 2A–D) and 45 normal gastric mucosa tissues (Figure 2E-H). High-expression of Ecto-5′-nucleotidase was found in 72 of 131 (55.0%) gastric cancer tissues and 9 of 45 (20.0%) normal gastric mucosa tissues. Then, the statistical analysis showed Ecto-5′-nucleotidase expression definitely up-regulated in gastric cancer tissues compared with normal gastric mucosa tissues (P<0.001, Table 1).
Figure 1. The expression of Ecto-5′-nucleotidase in gastric cancer.
(A) Ecto-5′-nucleotidase expression levels are notably elevated in gastric cancer tissues compared with normal gastric mucosa tissues. (B) Gastric cancer cell lines display higher level of Ecto-5′-nucleotidase expression than human gastric mucosal epithelial cell line.
Figure 2. Immunohistochemical staining of Ecto-5′-nucleotidase.
(A) Negative expression of Ecto-5′-nucleotidase in normal gastric mucosa tissues. (B) Weak expression of Ecto-5′-nucleotidase in normal gastric mucosa tissues. (C) Moderate expression of Ecto-5′-nucleotidase in normal gastric mucosa tissues. (D) Strong expression of Ecto-5′-nucleotidase in normal gastric mucosa tissues. (E) Negative expression of Ecto-5′-nucleotidase in gastric cancer tissues. (F) Weak expression of Ecto-5′-nucleotidase in gastric cancer tissues. (G) Moderate expression of Ecto-5′-nucleotidase in gastric cancer tissues. (H) Strong expression of Ecto-5′-nucleotidase in gastric cancer tissues.
Table 1. Ecto-5′-nucleotidase expression in between gastric cancer tissues and normal gastric mucosa tissues.
| Group | n | Ecto-5′-nucleotidase expression | P | |
|---|---|---|---|---|
| High (%) | Low (%) | |||
| Normal | 45 | 9(20.0) | 36(80.0) | <0.001 |
| Tumor | 131 | 72(55.0) | 59(45.0) | |
The expression of Ecto-5′-nucleotidase expression was also measured in human gastric cancer cell lines (AGS, SGC-7901, BGC-823 and NCI-N87) and human gastric mucosal epithelial cell line (GES-1) through Western blot. The result of Western blot indicated that gastric cancer cell lines displayed higher level of Ecto-5′-nucleotidase expression than human gastric mucosal epithelial cell line (Figure 1B).
Associations between Ecto-5′-nucleotidase expression and clinicopathological parameters in gastric cancer
The clinical relationship between Ecto-5′-nucleotidase expression and clinicopathological parameters of gastric cancer was further explored Chi-square test or Fisher’s exact test. The results showed that the high-expression of Ecto-5′-nucleotidase was obviously associated with advanced clinical stage (P<0.001, Table 2), deep tumor invasion (P=0.004, Table 2), lymph node metastasis (P=0.002, Table 2) and distant metastasis (P=0.007, Table 2). However, the expression of Ecto-5′-nucleotidase had no statistical association with gender, age, histological grade and HP infection.
Table 2. Correlations between Ecto-5′-nucleotidase expression and clinicopathological parameters in gastric cancer.
| Parameters | n | High expression (%) | Low expression (%) | P |
|---|---|---|---|---|
| Gender | ||||
| Female | 48 | 29(60.4) | 19(39.6) | 0.340 |
| Male | 83 | 43(51.8) | 40(48.2) | |
| Age (years) | ||||
| <50 | 55 | 29(52.7) | 26(47.3) | 0.662 |
| ≥50 | 76 | 43(56.6) | 33(43.4) | |
| Histological type | ||||
| Differentiated | 76 | 39(51.3) | 37(48.7) | 0.324 |
| Undifferentiated | 55 | 33(60.0) | 22(40.0) | |
| Clinical stage | ||||
| I-II | 59 | 22(37.3) | 37(62.7) | <0.001 |
| III-IV | 72 | 50(69.4) | 22(30.6) | |
| Tumor depth | ||||
| T1-T2 | 66 | 28(42.4) | 38(57.6) | 0.004 |
| T3-T4 | 65 | 44(67.7) | 21(32.3) | |
| Lymph node metastasis | ||||
| N0-N1 | 67 | 28(41.8) | 39(58.2) | 0.002 |
| N2-N3 | 64 | 44(68.8) | 20(31.3) | |
| Distant metastasis | ||||
| M0 | 119 | 61(51.3) | 58(48.7) | 0.007 |
| M1 | 12 | 11(91.7) | 1(8.3) | |
| HP infection | ||||
| Absent | 90 | 48(53.3) | 42(46.7) | 0.579 |
| Present | 41 | 24(58.5) | 17(41.5) | |
Relationship between Ecto-5′-nucleotidase expression and overall survival in gastric cancer
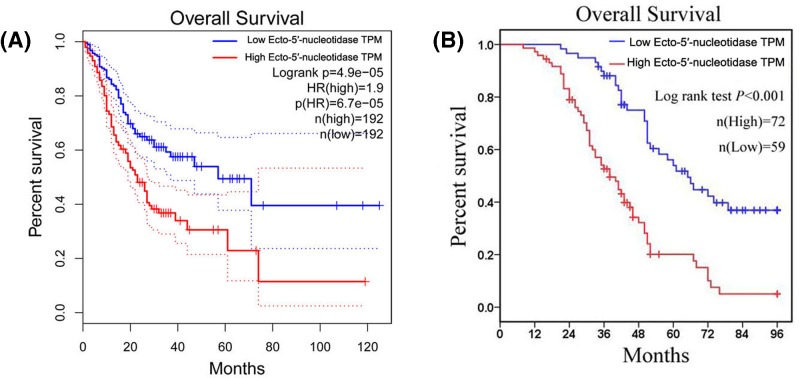
The relationship between Ecto-5′-nucleotidase expression and overall survival was primarily evaluated at gastric cancer cohort from TCGA database. We observed that high Ecto-5′-nucleotidase expression was negatively correlated with overall survival time in gastric cancer patients (P<0.001, Figure 3A). Then, the prognostic value of Ecto-5′-nucleotidase expression was further explored in gastric cancer patients from our study through Kaplan–Meier method and the log-rank test. The result revealed that gastric cancer patients with high-expression Ecto-5′-nucleotidase had unfavorable overall survival compared with those with low-expression of Ecto-5′-nucleotidase (P<0.001, Figure 3B). Furthermore, we executed univariate and multivariate Cox regression analysis for identifying the independent predictor for overall survival in gastric cancer patients. As shown in Table 3, we found clinical stage, tumor depth (P<0.001), lymph node metastasis (P<0.001), distant metastasis (P<0.001) and Ecto-5′-nucleotidase expression (P<0.001) were prognostic factors for overall survival in gastric cancer patients. Then, the result of multivariate Cox regression analysis showed high Ecto-5′-nucleotidase expression was an independent poor prognostic factor for gastric cancer patients (P=0.007, HR and 95%CI: 1.974 and 1.209–3.224, Table 3).
Figure 3. The prognostic value of Ecto-5′-nucleotidase expression in gastric cancer patients.
The relationship between Ecto-5′-nucleotidase expression and overall survival is evaluated at gastric cancer cohort from TCGA database (A) and our study (B) (TPM: Transcripts Per Million).
Table 3. Univariate and multivariate Cox regression analyses of overall survival in gastric cancer.
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Gender | ||||||
| (Female vs. Male) | 0.873 | 0.569–1.339 | 0.535 | |||
| Age | ||||||
| (<50 vs. ≥50) | 1.018 | 0.668–1.551 | 0.934 | |||
| Histological grade | ||||||
| (Differentiated vs. Undifferentiated) | 0.996 | 0.650–1.528 | 0.987 | |||
| Clinical stage | ||||||
| (I-II vs. III-IV) | 3.946 | 2.408–6.465 | <0.001 | 2.494 | 0.837–7.432 | 0.101 |
| Tumor depth | ||||||
| (T1-T2 vs. T3-T4) | 2.423 | 1.570–3.742 | <0.001 | 1.863 | 1.167–2.975 | 0.009 |
| Lymph node metastasis | ||||||
| (N0-N1 vs. N2-N3) | 3.535 | 2.193–5.698 | <0.001 | 1.081 | 0.381–3.067 | 0.884 |
| Distant metastasis | ||||||
| (M0 vs. M1) | 11.757 | 5.849–23.631 | <0.001 | 5.743 | 2.708–12.183 | <0.001 |
| HP infection | ||||||
| (Absent vs. Present) | 1.099 | 0.704–1.714 | 0.679 | |||
| Ecto-5′-nucleotidase | ||||||
| (Low expression vs. High expression) | 3.082 | 1.967–4.828 | <0.001 | 1.974 | 1.209–3.224 | 0.007 |
Abbreviations: 95%CI: 95% confidence interval; HR, hazard ratio.
The biological function of Ecto-5′-nucleotidase in gastric cancer
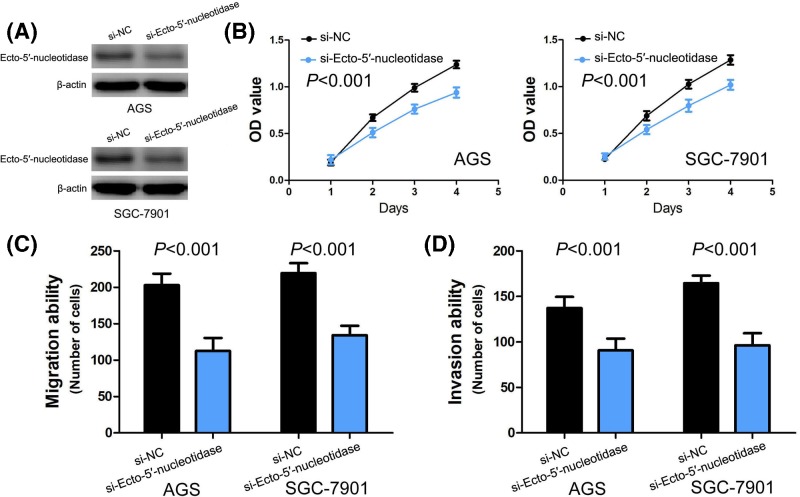
To identify the effect of Ecto-5′-nucleotidase on gastric cancer cell proliferation, migration and invasion in vitro, we conducted loss-of-function in gastric cancer cells. Based on the expression of Ecto-5′-nucleotidase in four human gastric cancer cell lines (AGS, SGC-7901, BGC-823 and NCI-N87), AGS and SGC-7901 cells were chosen for following loss-of-function studies, and the knockdown efficiency of si-Ecto-5′-nucleotidase in AGS and SGC-7901 cells was estimated by Western blot (Figure 4A). We performed CCK-8 assay to assess the effect of Ecto-5′-nucleotidase on gastric cancer cell proliferation, and found that silencing of Ecto-5′-nucleotidase expression dramatically inhibited cell proliferation in AGS and SGC-7901 cells (P<0.001, Figure 4B). Then, the effect of Ecto-5′-nucleotidase on gastric cancer cell migration and invasion was estimated by transwell migration and invasion assays. We found silencing of Ecto-5′-nucleotidase expression also profoundly suppressed cell migration and invasion in AGS and SGC-7901 cells (P<0.001, Figure 4C–D).
Figure 4. The biological function of Ecto-5′-nucleotidase in gastric cancer.
(A) The knockdown efficiency of si-Ecto-5′-nucleotidase in AGS and SGC-7901 cells is estimated by Western blot. (B) The effect of Ecto-5′-nucleotidase on cell proliferation is evaluated in AGS and SGC-7901 cells by CCK-8 assay (P<0.001 vs. si-NC). (C) The impact of Ecto-5′-nucleotidase on cell migration is assessed in AGS and SGC-7901 cells by transwell migration assay (P<0.001 vs. si-NC). (D) The effect of Ecto-5′-nucleotidase on cell invasion is estimated in AGS and SGC-7901 cells by transwell invasion assay (P<0.001 vs. si-NC).
Discussion
Ecto-5′-nucleotidase is a glycosyl-phosphatidylinositol anchored membrane glycoprotein [13]. Originally, Ecto-5′-nucleotidase had been found to strengthen endothelial barrier function and depress leukokinesis, and be markedly affected by hypoxia and inflammatory mediators [14,15]. Subsequently, more and more human cancers have been suggested to display high Ecto-5′-nucleotidase expression, such as lung cancer [16,17], breast cancer [18], colorectal cancer [19,20], pancreatic cancer [21], gallbladder cancer [22], prostate cancer [23,24], thyroid cancer [25], and head and neck cancer [26]. However, there was discrepancy in endometrial carcinoma. Aliagas et al. [27] reported that high levels of Ecto-5′-nucleotidaseexpression were observed endometrial carcinoma samples compared with their non-pathological endometrial counterparts. On the contrary, Bowser et al. [28] showed Ecto-5′-nucleotidase expression levels were remarkably low and poorly differentiated in endometrial carcinoma samples compared with normal endometrium samples. In gastric cancer, high-expression of Ecto-5′-nucleotidase was observed in gastric cancer tissues and serums compared with normal gastricmucosal tissues and serum of healthy individuals, respectively [11,12]. In our research, we present more evidence about Ecto-5′-nucleotidase expression in gastric cancer. On the one hand, the result of TCGA database indicated that Ecto-5′-nucleotidase expression levels were notably elevated in gastric cancer tissues compared with normal gastric mucosa tissues. On the other hand, high Ecto-5′-nucleotidase expression was observed in gastric cancer tissues and cell lines through immunohistochemistry and Western blot. Furthermore, we explored the clinical relationship between Ecto-5′-nucleotidase expression and clinicopathological parameters of gastric cancer, and found high expression of Ecto-5′-nucleotidase was obviously associated with advanced clinical stage, deep tumor invasion, lymph node metastasis and distant metastasis in gastric cancer patients. Similarly, Lu et al. [12] also suggested high Ecto-5′-nucleotidase was associated with unfavorable differentiation, deep tumor invasion, more metastatic lymph nodes, distant metastasis and advanced clinical stage in gastric cancer patients. Moreover, Inoue et al. [16] showed Ecto-5′-nucleotidase overexpression was correlated with gender, smoking and histological classification in non-small cell lung cancer patients. In breast cancer patients, Yu et al. [18] showed Ecto-5′-nucleotidase overexpression was correlated with high tumor grades and present lymph node metastasis. Meanwhile, Turcotte et al. [29] found that Ecto-5′-nucleotidase overexpression predicted trastuzumab resistance in breast cancer patients. Similarly, Cushman et al. [30] also found Gene expression of Ecto-5′-nucleotidase was identified as potential biomarker for predicting cetuximab sensitivity in colorectal cancer patients. In prostate cancer patients, Yang et al. [23] suggested patients with lymph node metastasis had higher levels of Ecto-5′-nucleotidase expression than patients without lymph node metastasis. Besides, Bertonia et al. [25] showed Ecto-5′-nucleotidase overexpression was associated with large tumor size, more metastatic lymph nodes and high risk of recurrence in patients with papillary thyroid carcinoma.
The prognostic value of Ecto-5′-nucleotidase expression was estimated in many kinds of human tumor including gastric cancer [11,12], lung cancer [16,17], gallbladder cancer [22], prostate cancer [23], head and neck cancer [26,31,32,33], breast cancer [34,35], colorectal cancer [36], melanoma [37], ovarian cancer [38] and so on. Interestingly, Wang Rong et al. [39] and Jiang Tao et al. [40] respectively performed meta-analysis to estimate the prognostic value of Ecto-5′-nucleotidase expression in human cancers, and consistently found high Ecto-5′-nucleotidase expression was an efficient biomarker predicting unfavorable prognosis in human cancers. In acute lymphoblastic leukemia patients, high expression of Ecto-5′-nucleotidase was associated with unfavorable prognosis in earlier small studies [41,42]. However, high level of Ecto-5′-nucleotidase expression was found no effect on the prognosis of patients with acute lymphoblastic leukemia in larger and prospective study [43]. Due to limited sample size in previous study, we further present evidence about the association between Ecto-5′-nucleotidase expression and overall survival in gastric cancer. In our study, we found high Ecto-5′-nucleotidase expression was negatively correlated with overall survival time in gastric cancer patients both from TCGA database and our study. Meanwhile, high Ecto-5′-nucleotidase expression was identified as an independent poor prognostic factor for gastric cancer patients through univariate and multivariate Cox proportional hazards regression model. Therefore, high Ecto-5′-nucleotidase expression is credible biomarker for predicting poor clinical outcome in gastric cancer patients.
The biological function of Ecto-5′-nucleotidase in gastric cancer has not been reported. In our study, we preliminarily explored the effect of Ecto-5′-nucleotidase on gastric cancer cell proliferation, migration and invasion. We found silencing of Ecto-5′-nucleotidase expression profoundly suppressed cell proliferation, migration and invasion in gastric cancer. Similarly, Wu Ruimin et al. showed Ecto-5′-nucleotidase enhanced cell proliferation in vitro and tumor growth in vivo in colorectal cancer [20]. Moreover, Yu Jiangang suggested up-regulation of Ecto-5′-nucleotidase expression accelerated breast cancer cell proliferation [18]. Besides, Ren Zhen-Hu et al. found down-regulation of Ecto-5′-nucleotidaseexpression inhibited cell invasion, migration and epithelial-mesenchymal transition process in head and neck cancer [26].
The limit of our research was lack of the molecular mechanism of Ecto-5′-nucleotidase in gastric cancer. However, Ecto-5′-nucleotidase has been found to activate multiple oncogenic signaling pathway. In breast cancer, Ecto-5′-nucleotidase overexpression enhanced AKT/GSK-3β/β-catenin/cyclinD1 signaling pathway [18]. Moreover, inhibition of Ecto-5′-nucleotidase could depress epithelial-mesenchymal transition process and AKT/GSK-3β signal pathway in renal cell cancer [44]. Besides, Zhu Jianjie et al reported Ecto-5′-nucleotidase is a target of miR-30a-5p, and positively regulated EGFR signaling in non-small cell lung cancer [17]. Similarly, Ren Zhen-Hu et al also found Ecto-5′-nucleotidase stimulated adenosine A3R, and activate of EGF/EGFR signaling in head and neck squamous cell carcinoma [26]. In future research, we will try to investigate the molecular mechanism of Ecto-5′-nucleotidase in gastric cancer.
In conclusion, Ecto-5′-nucleotidase expression is increased in gastric cancer tissues and cells, and associated with advanced clinical stage, deep tumor invasion, lymph node metastasis and distant metastasis in gastric cancer patients. Silencing of Ecto-5′-nucleotidase expression suppresses cell proliferation, migration and invasion in gastric cancer.
Abbreviations
- CCK-8
Cell Counting Kit-8
Author Contribution
Sifeng Hu and Xiankun Yin designed the experiment, interpreted the data, and prepared the manuscript. Sifeng Hu, Fanmei Meng, Xiankun Yin, Changling Cao and Guangyong Zhang conducted the experiment and collected the data, and reviewed the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A. and Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A.. et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 3.Brower V. (2016) Apatinib in treatment of refractory gastric cancer. Lancet Oncol. 17, e137 10.1016/S1470-2045(16)00138-8 [DOI] [PubMed] [Google Scholar]
- 4.Karimi P., Islami F., Anandasabapathy S., Freedman N.D. and Kamangar F. (2014) Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomarkers Prev. 23, 700–713 10.1158/1055-9965.EPI-13-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guggenheim D.E. and Shah M.A. (2013) Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 107, 230–236 10.1002/jso.23262 [DOI] [PubMed] [Google Scholar]
- 6.Kordass T., Osen W. and Eichmuller S.B. (2018) Controlling the immune suppressor: transcription factors and microRNAs regulating CD73/NT5E. Front. Immunol. 9, 813 10.3389/fimmu.2018.00813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Liao X., Yu J. and Zhou P. (2018) Role of CD73 in Disease: promising prognostic indicator and therapeutic target. Curr. Med. Chem. 25, 2260–2271 10.2174/0929867325666180117101114 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita S., Tsujino Y., Moriguchi K., Tatematsu M. and Ushijima T. (2006) Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2′-deoxycytidine treatment and oligonucleotide microarray. Cancer Sci. 97, 64–71 10.1111/j.1349-7006.2006.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H., Li F., Zhu Y., Li T., Huang H., Lin T.. et al. (2016) Whole-exome sequencing to identify somatic mutations in peritoneal metastatic gastric adenocarcinoma: a preliminary study. Oncotarget 7, 43894–43906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunakawa Y., Cao S., Volz N.B., Berger M.D., Yang D., Parekh A.. et al. (2017) Genetic variations in immunomodulatory pathways to predict survival in patients with locoregional gastric cancer. Pharmacogenomics J. 17, 528–534 10.1038/tpj.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Wang J., Li J., Zhang H., Guo S., Yan M.. et al. (2016) Identification of serum biomarkers for gastric cancer diagnosis using a human proteome microarray. Mol. Cell. Proteomics 15, 614–623 10.1074/mcp.M115.051250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X.X., Chen Y.T., Feng B., Mao X.B., Yu B. and Chu X.Y. (2013) Expression and clinical significance of CD73 and hypoxia-inducible factor-1alpha in gastric carcinoma. World J. Gastroenterol. 19, 1912–1918 10.3748/wjg.v19.i12.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allard D., Allard B., Gaudreau P.O., Chrobak P. and Stagg J. (2016) CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy 8, 145–163 10.2217/imt.15.106 [DOI] [PubMed] [Google Scholar]
- 14.Allard B., Longhi M.S., Robson S.C. and Stagg J. (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 10.1111/imr.12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viviani L.G., Piccirillo E., Cheffer A., De Rezende L., Ulrich H., Carmona-Ribeiro A.M.. et al. (2018) Be aware of aggregators in the search for potential human Ecto-5′-nucleotidase inhibitors. Molecules 23, pii:E1876 10.3390/molecules23081876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue Y., Yoshimura K., Kurabe N., Kahyo T., Kawase A., Tanahashi M.. et al. (2017) Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 8, 8738–8751 10.18632/oncotarget.14434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J., Zeng Y., Li W., Qin H., Lei Z., Shen D.. et al. (2017) CD73/NT5E is a target of miR-30a-5p and plays an important role in the pathogenesis of non-small cell lung cancer. Mol. Cancer 16, 34 10.1186/s12943-017-0591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J., Wang X., Lu Q., Wang J., Li L., Liao X.. et al. (2018) Extracellular 5′-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3beta/beta-catenin/cyclinD1 signaling pathway. Int. J. Cancer 142, 959–967 10.1002/ijc.31112 [DOI] [PubMed] [Google Scholar]
- 19.Xie M., Qin H., Luo Q., Huang Q., He X., Yang Z.. et al. (2017) MicroRNA-30a regulates cell proliferation and tumor growth of colorectal cancer by targeting CD73. BMC Cancer 17, 305 10.1186/s12885-017-3291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu R., Chen Y., Li F., Li W., Zhou H., Yang Y.. et al. (2016) Effects of CD73 on human colorectal cancer cell growth in vivo and in vitro. Oncol. Rep. 35, 1750–1756 10.3892/or.2015.4512 [DOI] [PubMed] [Google Scholar]
- 21.Haun R.S., Quick C.M., Siegel E.R., Raju I., Mackintosh S.G. and Tackett A.J. (2015) Bioorthogonal labeling cell-surface proteins expressed in pancreatic cancer cells to identify potential diagnostic/therapeutic biomarkers. Cancer Biol. Ther. 16, 1557–1565 10.1080/15384047.2015.1071740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong L., Wen Y., Miao X. and Yang Z. (2014) NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 355, 365–374 10.1007/s00441-013-1752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q., Du J. and Zu L. (2013) Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol. Oncol. Res. 19, 811–814 10.1007/s12253-013-9648-7 [DOI] [PubMed] [Google Scholar]
- 24.Song Y., Song C. and Yang S. (2018) Tumor-suppressive function of miR-30d-5p in prostate cancer cell proliferation and migration by targeting NT5E. Cancer Biother. Radiopharm. 33, 203–211 10.1089/cbr.2018.2457 [DOI] [PubMed] [Google Scholar]
- 25.Bertoni A.P.S., Bracco P.A., De Campos R.P., Lutz B.S., Assis-Brasil B.M., Meyer E.L.S.. et al. (2019) Activity of Ecto-5′-nucleotidase (NT5E/CD73) is increased in papillary thyroid carcinoma and its expression is associated with metastatic lymph nodes. Mol. Cell. Endocrinol. 479, 54–60 10.1016/j.mce.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 26.Ren Z.H., Lin C.Z., Cao W., Yang R., Lu W., Liu Z.Q.. et al. (2016) CD73 is associated with poor prognosis in HNSCC. Oncotarget 7, 61690–61702 10.18632/oncotarget.11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aliagas E., Vidal A., Texido L., Ponce J., Condom E. and Martin-Satue M. (2014) High expression of ecto-nucleotidases CD39 and CD73 in human endometrial tumors. Mediators Inflamm. 2014, 509027 10.1155/2014/509027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowser J.L., Blackburn M.R., Shipley G.L., Molina J.G., Dunner K. Jr and Broaddus R.R. (2016) Loss of CD73-mediated actin polymerization promotes endometrial tumor progression. J. Clin. Invest. 126, 220–238 10.1172/JCI79380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turcotte M., Allard D., Mittal D., Bareche Y., Buisseret L., Jose V.. et al. (2017) CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 77, 5652–5663 10.1158/0008-5472.CAN-17-0707 [DOI] [PubMed] [Google Scholar]
- 30.Cushman S.M., Jiang C., Hatch A.J., Shterev I., Sibley A.B., Niedzwiecki D.. et al. (2015) Gene expression markers of efficacy and resistance to cetuximab treatment in metastatic colorectal cancer: results from CALGB 80203 (Alliance). Clin. Cancer Res. 21, 1078–1086 10.1158/1078-0432.CCR-14-2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnin N., Armandy E., Carras J., Ferrandon S., Battiston-Montagne P., Aubry M.. et al. (2016) MiR-422a promotes loco-regional recurrence by targeting NT5E/CD73 in head and neck squamous cell carcinoma. Oncotarget 7, 44023–44038 10.18632/oncotarget.9829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt T.J., Gevensleben H., Dietrich J., Kristiansen G., Bootz F., Landsberg J.. et al. (2018) Detailed analysis of adenosine A2a receptor (ADORA2A) and CD73 (5′-nucleotidase, ecto, NT5E) methylation and gene expression in head and neck squamous cell carcinoma patients. Oncoimmunology 7, e1452579 10.1080/2162402X.2018.1452579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandapathil M., Boduc M., Netzer C., Guldner C., Roessler M., Wallicek-Dworschak U.. et al. (2018) CD73 expression in lymph node metastases in patients with head and neck cancer. Acta Otolaryngol. 138, 180–184 10.1080/00016489.2017.1378436 [DOI] [PubMed] [Google Scholar]
- 34.Loi S., Pommey S., Haibe-Kains B., Beavis P.A., Darcy P.K., Smyth M.J.. et al. (2013) CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 11091–11096 10.1073/pnas.1222251110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Supernat A., Markiewicz A., Welnicka-Jaskiewicz M., Seroczynska B., Skokowski J., Sejda A.. et al. (2012) CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl. Immunohistochem. Mol. Morphol. 20, 103–107 10.1097/PAI.0b013e3182311d82 [DOI] [PubMed] [Google Scholar]
- 36.Wu X.R., He X.S., Chen Y.F., Yuan R.X., Zeng Y., Lian L.. et al. (2012) High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J. Surg. Oncol. 106, 130–137 10.1002/jso.23056 [DOI] [PubMed] [Google Scholar]
- 37.Morello S., Capone M., Sorrentino C., Giannarelli D., Madonna G., Mallardo D.. et al. (2017) Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J. Transl. Med. 15, 244 10.1186/s12967-017-1348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turcotte M., Spring K., Pommey S., Chouinard G., Cousineau I., George J.. et al. (2015) CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 75, 4494–4503 10.1158/0008-5472.CAN-14-3569 [DOI] [PubMed] [Google Scholar]
- 39.Wang R., Zhang Y., Lin X., Gao Y. and Zhu Y. (2017) Prognositic value of CD73-adenosinergic pathway in solid tumor: A meta-analysis and systematic review. Oncotarget 8, 57327–57336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang T., Xu X., Qiao M., Li X., Zhao C., Zhou F.. et al. (2018) Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer 18, 267 10.1186/s12885-018-4073-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pieters R., Huismans D.R., Loonen A.H., Peters G.J., Hahlen K., Van Der Does-Van Den Berg A.. et al. (1992) Relation of 5′-nucleotidase and phosphatase activities with immunophenotype, drug resistance and clinical prognosis in childhood leukemia. Leuk. Res. 16, 873–880 10.1016/0145-2126(92)90033-4 [DOI] [PubMed] [Google Scholar]
- 42.Veerman A.J., Hogeman P.H., Van Zantwijk C.H. and Bezemer P.D. (1985) Prognostic value of 5′nucleotidase in acute lymphoblastic leukemia with the common-ALL phenotype. Leuk. Res. 9, 1227–1229 10.1016/0145-2126(85)90149-3 [DOI] [PubMed] [Google Scholar]
- 43.Wieten E., Van Der Linden-Schrever B.E., Sonneveld E., Veerman A.J. and Pieters R. (2011) CD73 (5′-nucleotidase) expression has no prognostic value in children with acute lymphoblastic leukemia. Leukemia 25, 1374–1376 10.1038/leu.2011.174 [DOI] [PubMed] [Google Scholar]
- 44.Peng D., Hu Z., Wei X., Ke X., Shen Y. and Zeng X. (2019) NT5E inhibition suppresses the growth of sunitinib-resistant cells and EMT course and AKT/GSK-3beta signaling pathway in renal cell cancer. IUBMB Life 71, 113–124 10.1002/iub.1942 [DOI] [PubMed] [Google Scholar]