Abstract
Background
It remains unclear whether improving iron status increases malaria risk, and few studies have looked at the effect of host iron status on subsequent malaria infection. We therefore aimed to determine whether a child’s iron status influences their subsequent risk of malaria infection in sub-Saharan Africa.
Methods
We assayed iron and inflammatory biomarkers from community-based cohorts of 1309 Kenyan and 1374 Ugandan children aged 0–7 years and conducted prospective surveillance for episodes of malaria. Poisson regression models were fitted to determine the effect of iron status on the incidence rate ratio (IRR) of malaria using longitudinal data covering a period of 6 months. Models were adjusted for age, sex, parasitemia, inflammation, and study site.
Results
At baseline, the prevalence of iron deficiency (ID) was 36.9% and 34.6% in Kenyan and Ugandan children, respectively. ID anemia (IDA) affected 23.6% of Kenyan and 17.6% of Ugandan children. Malaria risk was lower in children with ID (IRR, 0.7; 95% confidence interval [CI], 0.6, 0.8; P < .001) and IDA (IRR, 0.7; 95% CI, 0.6, 0.9; P = .006). Low transferrin saturation (<10%) was similarly associated with lower malaria risk (IRR, 0.8; 95% CI, 0.6, 0.9; P = .016). However, variation in hepcidin, soluble transferrin receptors (sTfR), and hemoglobin/anemia was not associated with altered malaria risk.
Conclusions
ID appears to protect against malaria infection in African children when defined using ferritin and transferrin saturation, but not when defined by hepcidin, sTfR, or hemoglobin. Additional research is required to determine causality.
Clinical Trials Registration
ISRCTN32849447
Keywords: iron status, iron deficiency, malaria risk, African children
Decreased ferritin and transferrin saturation are associated with protection against malaria in African children. Hepcidin, soluble transferrin receptor, and hemoglobin concentrations are not associated with malaria protection. These findings may reflect differences in parasite iron acquisition.
Iron deficiency (ID) and malaria remain important public health problems in African children [1, 2]. ID, the most common nutrient deficiency in preschool African children [3], is associated with poor brain development and long-term behavioral and cognitive impairments [4]. Similarly, malaria has devastating health effects in African children. In 2015, malaria caused an estimated 292000 deaths in African children aged <5 years [2] and remains a persistent and widespread problem in Africa, infecting 24% of the population at any one time [5].
The safety of iron supplementation has been a long-standing concern among policy makers and clinicians in malaria-endemic areas [6, 7]. In these areas, the World Health Organization (WHO) recommends iron supplementation in conjunction with effective malaria prevention and treatment strategies [8]. However, randomized, controlled trials of iron supplementation have reported conflicting findings [9, 10]. Furthermore, it is unclear whether iron supplementation might be unsafe because it improves iron status itself, thus resulting in a long-term increase in the risk of malaria.
Few observational studies have investigated the effect of iron status on malaria risk. These studies indicate that ID is associated with a reduced risk of both mild and severe Plasmodium falciparum malaria in African children [11–15] but have largely used ferritin-based definitions of ID. Little is known about whether other indicators of iron status (including hepcidin, hemoglobin, soluble transferrin receptors [sTfR], and transferrin saturation [TSAT]) influence malaria risk in humans. In mouse models, hepcidin has been shown to play a role in preventing superinfection by depriving the Plasmodium liver stage of iron [16], but studies in children have reported mixed findings [17, 18]. Two previous studies have reported that hemoglobin concentrations do not influence malaria risk [19, 20], while in vitro culture indicates otherwise [21]. There are no specific reports of the influence of sTfR and TSAT on malaria in humans.
In this study, our aim was to determine whether iron status influences the subsequent risk of malaria infection in 2683 Kenyan and Ugandan children, thus making this the largest observational study on iron status and risk of malaria to date with the most comprehensive range of iron markers.
METHODS
Ethical Approval
Ethical approval was provided by the Scientific Ethics Review Unit of the Kenya Medical Research Institute for the Kenyan cohort and the Uganda Virus Research Institute and the London School of Hygiene and Tropical Medicine for the Ugandan cohort.
Study Population
This study used data from 2 African community-based cohorts of children in Kilifi, Kenya, and Entebbe, Uganda.
Kenya Cohort
The Kenya cohort included 3 community cohorts: Junju, Ngerenya, and malaria RTS,S vaccine. Junju and Ngerenya are ongoing rolling cohorts evaluating malaria immunity as described elsewhere [22]. The malaria RTS,S vaccine cohort was the RTS,S/AS01E vaccine trial against malaria that was conducted between 2007 and 2008 with continued active malaria surveillance for 8 years [23]. Within these cohorts, children are followed to a maximum age of 13 years with annual cross-sectional bleeds. The follow-ups involved weekly visits to assess for fever; if the temperature was above 37.5°C, a malaria blood film was taken. Iron biomarkers were measured from a single cross-sectional bleed based on the availability of plasma samples archived at −80°C.
Uganda Cohort
The Entebbe Mother and Baby Study is a prospective birth cohort study that was originally designed as a randomized, double-blind, placebo-controlled trial (ISRCTN32849447) to determine whether anti-helminthic treatment during pregnancy and early childhood was associated with differential responses to vaccination or incidence of infections such as pneumonia, diarrhea, or malaria [24]. Blood samples were collected at birth and at subsequent birthdays up to age 5 years. Markers of iron status were assayed from a single birthday based on the availability of stored samples. The study included longitudinal active surveillance of malaria and other infections during fortnightly home visits and quarterly clinic visits.
Longitudinal parasitemia data were obtained from active surveillance during the 6 months following measurement of iron biomarkers. For the study, 94% of Kenyan and 90% of Ugandan children were followed for 6 months, while the length of follow-up for the remainder ranged from 1 to 5 months. We chose a follow-up period of not more than 6 months since iron status may change over a longer follow-up period. Secondary analyses included a 1-year follow-up period. Clinical malaria data included microscopy-confirmed density of asexual P. falciparum parasitemia and temperature. Genotyping of hemoglobin types was conducted using polymerase chain reaction assay [25] with DNA extracted by Qiagen DNA Blood Mini Kit (Qiagen, West Sussex, United Kingdom).
Measurement of Iron and Inflammatory Biomarkers
The assayed biomarkers of iron status included plasma ferritin (chemiluminescent microparticle immunoassay [CMI], Abbott Architect), hepcidin (DRG hepcidin 25 [bioactive] high sensitive enzyme-linked immunosorbent assay [ELISA] kit, DRG Diagnostics), sTfR (Human sTfR ELISA, BioVendor, CZ), iron (MULTIGENT iron calorimetric assay, Abbott Architect), transferrin (CMI, Abbott Architect), and hemoglobin (Medonic CA 530 hemoglobinometer). Since biomarkers of iron are influenced by inflammation, C-reactive protein (CRP) (MULTIGENT CRP Vario assay, Abbott Architect) was assayed to adjust for inflammation [26].
Definitions
The following 2 definitions of ID were used: based on low ferritin defined as plasma ferritin <12 µg/L or <30 µg/L in the presence of inflammation (CRP >5 mg/L) in children aged <5 years or <15 µg/L in children aged ≥5 years [26], and TSAT <10% (calculated as iron in µmol/L/(transferrin in g/L × 25.1) × 100) [27]. TSAT was calculated in Kenya only because Ugandan plasma samples were stored in EDTA, which chelates iron. We did not define ID by hepcidin or sTfR since there are no internationally established cutoffs. Anemia was defined as hemoglobin <11 g/dL in children aged 0 to 4 years or hemoglobin <11.5 g/dL in children aged >4 years while ID anemia (IDA) was defined as low ferritin and anemia [28]. A malaria episode was defined as parasitemia and temperature >37.5°C. All malaria episodes that occurred during the follow-up period were included except those that occurred within 14 days of an initial presentation, which were regarded as recrudescence.
Statistical Analyses
All analyses were conducted using STATA 13.0 (StataCorp, College Station, TX). Iron biomarkers (except hemoglobin) were loge-transformed to normalize their distributions. Geometric means of iron biomarkers and proportions of ID and anemia were computed. Two-tailed Student t tests were used to test for difference in means between groups. Poisson regression models of counts of malaria episodes were fitted as predicted by iron status (ID/anemia/individual iron biomarkers) and were adjusted for age, sex, parasitemia, inflammation, and study site. Difference in individual length of follow-up was accounted for in the model by including the length of follow-up as “exposure” in the model. We accounted for multiple episodes using robust cluster variance estimation, which takes into account correlations between multiple events. Secondary analyses involved excluding children with parasitemia or inflammation at baseline to mitigate the effects of concurrent infection on iron status [29]. We used Cox proportional hazards analyses to evaluate the temporal effect of iron status on malaria risk. A P value of <.05 was considered significant.
We searched the PubMed and Google Scholar databases with search terms that included “ID or ferritin or hepcidin or sTfR or TSAT or hemoglobin or anemia and malaria children.” We found 5 longitudinal studies that investigated the effect of ID on malaria risk. A metaanalysis of the current study and 4 previous longitudinal studies that reported risk ratios of the relationship between ID and malaria risk was performed using the “metan” command in STATA.
RESULTS
Baseline Characteristics of the Study Population
A total of 1309 Kenyan and 1374 Ugandan children aged 0–7 years and 1–5 years, respectively, were included in the analyses. Table 1 shows the characteristics of the study participants. At baseline, the prevalence of ID and IDA were 36.9% and 23.6% in Kenyan and 34.6% and 17.6% in Ugandan children, respectively. The prevalence of ID based on TSAT (measured in Kenya only) was 52.4%. The prevalence of malaria parasitemia was higher in Kenyan (20.1%) compared to Ugandan children (6.7%). During the 6-month follow-up, 31.1% of Kenyan and 14.3% of Ugandan children experienced at least 1 episode of malaria infection. Malaria incidence rate per child-year of follow-up was 0.6 in Kenya and 0.3 in Uganda.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Kenya (n = 1309) | Uganda (n = 1374) |
|---|---|---|
| Mean age in years (range) | 2.3 (0.0, 7.1) | 2.3 (1.0, 5.1) |
| Males, n/total (%) | 668/1309 (51.0) | 696/1374 (51.7) |
| Malaria parasitemia, n/total (%) | 261/1296 (20.1) | 92/1371 (6.7) |
| Inflammation,a n/total (%) | 334/1264 (26.4) | 316/1337 (23.6) |
| Iron deficiency,b n/total (%) | ||
| Low ferritin | 457/1237 (36.9) | 438/1267 (34.6) |
| TSAT < 10% | 637/1215 (52.4) | n/a |
| Anemia,c n/total (%) | 526/765 (68.8) | 533/1312 (40.6) |
| Iron deficiency anemia,d n/total (%) | 172/729 (23.6) | 213/1209 (17.6) |
| Sickle cell trait, n (%) | 157/1057 (14.9) | 224/1355 (16.5) |
| Ferritin, n (geometric mean ± SD) in µg/L | 1237 (20.8 ± 3.0) | 1267 (20.8 ± 2.9) |
| Hepcidin, n (geometric mean ± SD) in µg/L | 1202 (5.6 ± 3.6) | 1333 (6.8 ± 3.3) |
| Soluble transferrin receptors, n (geometric mean ±SD) in mg/L | 1296 (17.8 ± 1.5) | 1343 (6.7 ± 2.0) |
| Hemoglobin, n (geometric mean ±SD) in g/dL | 765 (10.1 ± 1.2) | 1312 (11.0 ± 1.1) |
| TSAT, n (geometric mean ±SD) in % | 1215 (9.3 ± 2.2) | n/a |
Malaria incidence rate per child-year of follow-up was 0.6 in Kenya and 0.3 in Uganda.
Abbreviations: n/a, not available; SD, standard deviation; TSAT, transferrin saturation.
aInflammation was defined as C-reactive protein >5 mg/L.
bIron deficiency was defined using 2 definitions: low ferritin defined as plasma ferritin <12 µg/L or <30 µg/L in the presence of inflammation in children aged <5 years or <15 µg/L in children aged ≥5 years, and transferrin saturation <10% (available in 1215 Kenyan children only and not available (n/a) in Uganda).
cAnemia was defined as hemoglobin <11 g/dL in children aged 0 to 4 years or hemoglobin <11.5 g/dL in children aged >4 years. The range of hemoglobin was 5.1–14.7 in Kenya and 5.4–18.5 in Uganda. The interquartile range was 9.4–11.3 in Kenya and 10.3–12.1 in Uganda. Only 33 (1.6%) had severe anemia (Hb <7 g/dL in children aged <5 years or <8 g/dL in children aged >5 years).
dIron deficiency anemia was defined as low ferritin and anemia.
Higher Ferritin Concentrations and TSAT Are Positively Associated With Malaria Infection
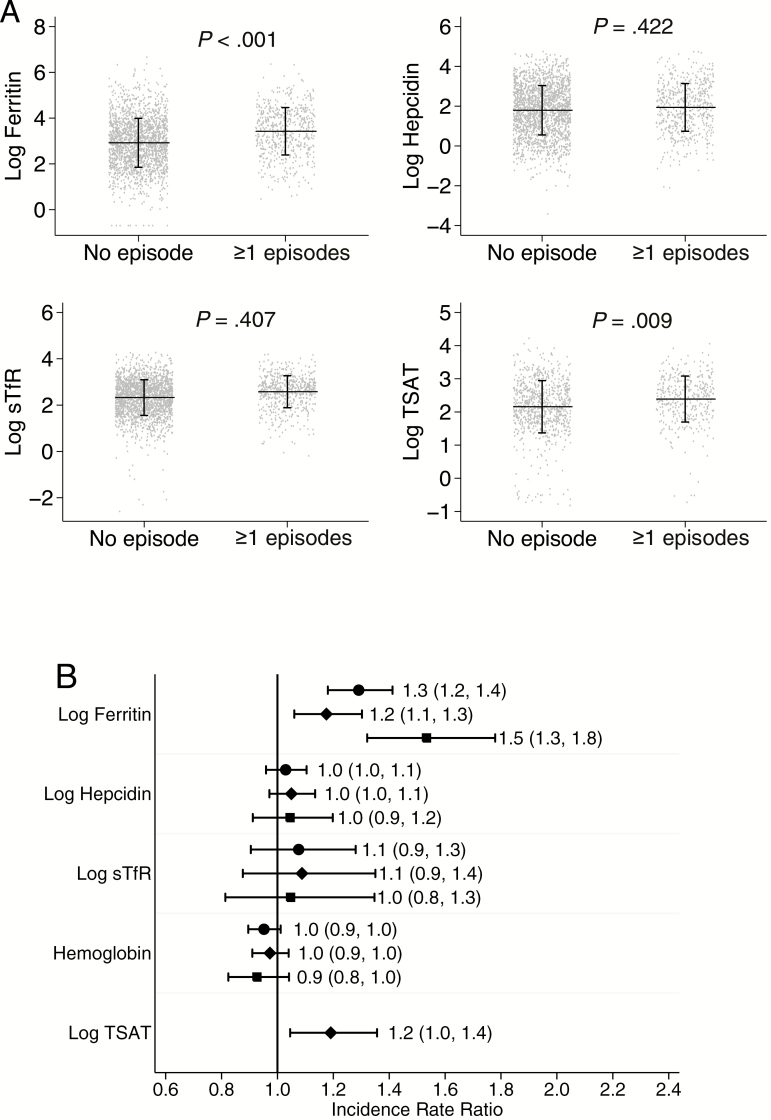
Concentrations of ferritin and TSAT, but not other iron markers, were higher in children who subsequently developed a malaria episode (Figure 1A). Similarly, a unit increase in log ferritin was associated with an increased incidence rate ratio (IRR) for malaria overall (IRR, 1.3; 95% confidence interval [CI], 1.2, 1.4; P < .001) and in each cohort individually (Figure 1B). A unit increase in log TSAT was also associated with a 20% increased risk of malaria in Kenyan children (IRR, 1.2; 95% CI, 1.05, 1.4; P = .009). However, hepcidin, sTfR, and hemoglobin concentrations were not associated with subsequent risk of malaria (Figure 1B).
Figure 1.
The effect of iron status on subsequent malaria. A, Scatter plots of iron biomarkers stratified by no subsequent malaria or one or more subsequent malaria episodes. Horizontal line indicates mean while vertical line indicates standard deviation. P value was derived from Poisson regression model. B, Adjusted incidence rate ratios for the effect of iron biomarkers on subsequent malaria episodes. Circle marker indicates overall, diamond Kenya and square Uganda. Labels indicate incidence rate ratio and 95% confidence intervals. Poisson regression models were adjusted for age, sex, parasitemia, inflammation, length of follow-up and study site. Maximum length of follow-up was 6 months. Abbreviations: sTfR, soluble transferrin receptor; TSAT, transferrin saturation.
ID Defined Using Ferritin or TSAT Protects Against Malaria Risk
ID defined by low ferritin concentrations and IDA were associated with a 30% reduction in the incidence of malaria infection (IRR, 0.7; 95% CI, 0.6, 0.8; P < .001 and IRR, 0.7; 95% CI, 0.6, 0.9; P = .006, respectively). These findings were consistent for the individual cohorts (Table 2). Likewise, ID defined by low TSAT reduced the risk of malaria in Kenyan children (IRR, 0.8; 95% CI, 0.6, 0.9; P = .016). However, anemia itself was not significantly associated with variation in malaria risk (Table 2).
Table 2.
Incidence of Malaria by Iron Status and Anemia
| Kilifi, Kenya | Entebbe, Uganda | Overall | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | No. | No. of Episodes | Incidence | IRR (95% CI) |
P Value | No. | No. of Episodes | Incidence | IRR (95% CI) |
P Value | No. | No. of Episodes | Incidence | IRR (95% CI) |
P Value |
| No ID (iron replete) | 780 | 286 | 0.8 | 1 | … | 829 | 140 | 0.3 | 1 | … | 1609 | 426 | 0.6 | 1 | … |
| ID (low ferritin)a | 457 | 93 | 0.4 | 0.8 (0.6, 0.9) | .018 | 438 | 45 | 0.2 | 0.5 (0.4, 0.7) | <.001 | 895 | 138 | 0.3 | 0.7 (0.6, 0.8) | <.001 |
| No ID (TSAT ≥10%) | 578 | 224 | 0.8 | 1 | … | n/a | n/a | n/a | n/a | n/a | 578 | 224 | 0.8 | 1 | … |
| ID (TSAT <10%)b | 637 | 159 | 0.5 | 0.8 (0.6, 0.9) | .016 | n/a | n/a | n/a | n/a | n/a | 637 | 159 | 0.5 | 0.8 (0.6, 0.9) | .016 |
| No anemia | 239 | 97 | 0.8 | 1 | … | 779 | 93 | 0.2 | 1 | … | 1018 | 190 | 0.4 | 1 | … |
| Anemiac | 526 | 219 | 0.9 | 1.0 (0.8, 1.2) | .77 | 533 | 93 | 0.4 | 1.2 (0.9, 1.6) | .16 | 1059 | 312 | 0.6 | 1.1 (0.9, 1.3) | .17 |
| No IDA | 557 | 257 | 1.0 | 1 | … | 996 | 154 | 0.3 | 1 | … | 1553 | 411 | 0.5 | 1 | … |
| IDAd | 172 | 47 | 0.6 | 0.8 (0.6, 1.1) | .27 | 213 | 23 | 0.2 | 0.5 (0.3, 0.7) | <.001 | 385 | 70 | 0.4 | 0.7 (0.6, 0.9) | .006 |
Poisson regression models were adjusted for age, sex, parasitemia, inflammation, length of follow-up, and study site. Maximum length of follow-up was 6 months. Incidence defined as number of malaria episodes per child-year of follow-up. The number of episodes ranged from 0–5 in Kenya and 0–6 in Uganda; 101 Kenyan and 45 Ugandan children had multiple episodes.
Abbreviations: CI, confidence interval; ID, iron deficiency; IDA, iron deficiency anemia; IRR, incidence rate ratio; n/a, not available; TSAT, transferrin saturation.
aIron deficiency (low ferritin) was defined as plasma ferritin <12 µg/L or <30 µg/L in the presence of inflammation (C-reactive protein >5 mg/L) in children aged <5 years or <15 µg/L in children aged ≥5 years otherwise iron replete.
bTransferrin saturation data were available in 1215 Kenyan children. Not available (n/a) for Uganda.
cAnemia was defined as hemoglobin <11 g/dL in children aged 0 to 4 years or hemoglobin <11.5 g/dL in children aged >4 years.
dIron deficiency anemia was defined as low ferritin and anemia.
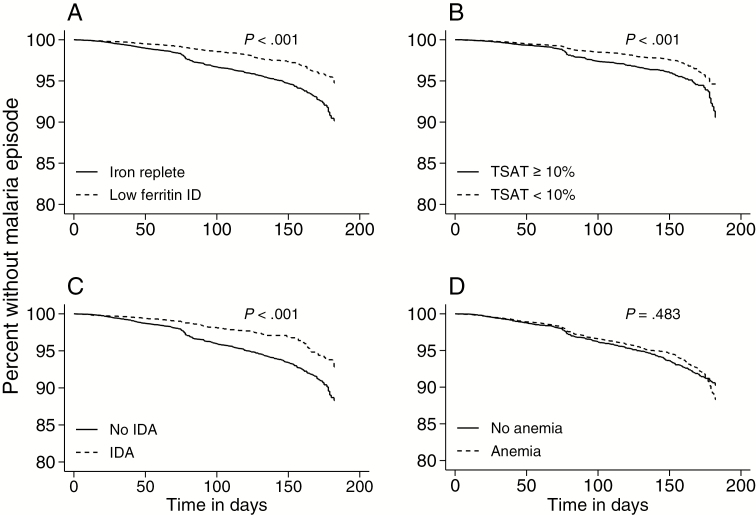
In Cox proportional hazards models, ID defined by low ferritin, ID defined by low TSAT, and IDA were associated with 40%, 20%, and 30% reduced risk of malaria, respectively (Figure 2A–C), for the 6 months of follow-up compared to iron-replete children. However, anemia was not associated with malaria risk (Figure 2D and Supplementary Table 1). We observed similar results regardless of whether the follow-up period was extended to 1 year, the children had malaria parasitemia or inflammation at baseline, age, or following adjustment for sickle cell trait (Supplementary Figures 1–5).
Figure 2.
Kaplan-Meier curves of time to first malaria episode according to (A) iron deficiency (ID) defined by low ferritin, (B) ID defined by transferrin saturation (<10%), (C ) ID anemia, and (D) anemia. P values were derived from log-rank tests for equality of survivor functions. Abbreviations: ID, iron deficiency; IDA, iron deficiency anemia; TSAT, transferrin saturation.
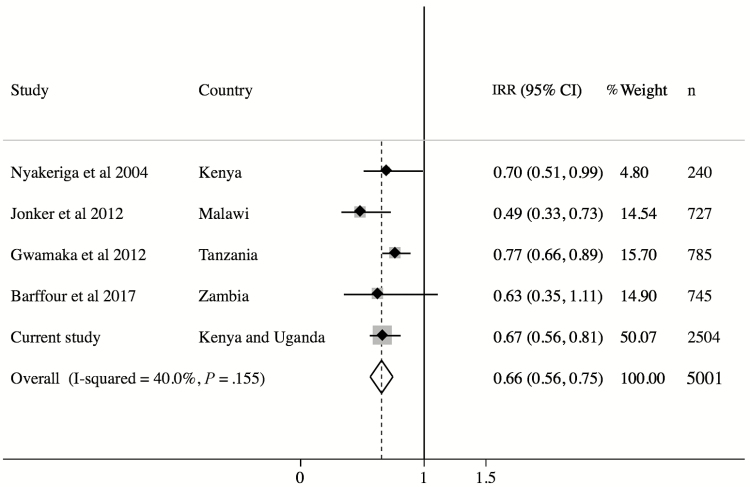
A metaanalysis of observational studies examining the influence of ID on malaria risk is shown in Figure 3. All the studies report that ID, using a ferritin-based definition, protects against malaria infection, despite differences in study site, length of follow-up, and definition of ID (Supplementary Table 2). The overall estimate indicates that ID is associated with a 34% lower risk of malaria infection. We report the largest study to date.
Figure 3.
Metaanalysis of observational studies examining the relationship between iron deficiency (ID) and malaria risk. Study-specific estimates and their relative contribution (percentage weight and sample size) to overall estimates are shown. Definitions of ID varied by study: Nyakeriga et al 2004 [11], ferritin <12 µg/L plus transferrin saturation <10%; Jonker et al 2012 [12], ferritin <30 µg/L; Gwamaka et al 2012 [13], ferritin <30 µg/L if C-reactive protein (CRP) <8.2 mg/L or ferritin <70 µg/L if CRP >8.2 mg/L; Barffour et al 2017 [14], ferritin <12 µg/L in children aged <5 years or <15 µg/L in children aged ≥5 years; and current study, ferritin <12 µg/L or <30 µg/L if CRP >5 mg/L in children aged <5 years or <15 µg/L in children aged ≥5 years. Abbreviations: CI, confidence interval; IRR, incidence rate ratio.
DISCUSSION
In this study, we report an observational analysis of the influence of iron status on subsequent malaria risk in 2683 Kenyan and Ugandan children. We found that ID, defined using either ferritin or TSAT, and IDA were associated with a lower risk of subsequent malaria infection. However, anemia (or hemoglobin concentrations), hepcidin, and sTfR were not significantly associated with variation in malaria risk.
Consistent with our findings, previous observational studies have reported that ID based on low ferritin concentrations confers protection against malaria infection in African children [11–15]. Nyakeriga et al reported a 30% reduction in clinical malaria during a 6-month follow-up of Kenyan children aged 8 months to 8 years [11]. Similarly, studies in Malawi (aged 6–60 months with 1 year of follow-up) [12] and Tanzania (birth to 3 years follow-up) [13] reported reduced malaria risk of 45% and 23%, respectively. Recently, a study in Zambian children aged 4–8 years followed for 6 months reported an increased risk of malaria in children with high ferritin concentrations [14]. These estimates are similar to our finding of a 30% reduction in malaria risk in iron-deficient children (0–7 years). The metaanalysis of these studies indicated that ID is associated with a 34% lower risk of malaria infection.
Also, we found that ID defined by TSAT was associated with a 20% reduction in the subsequent risk of malaria and that TSAT was positively associated with malaria incidence. Using a combination of low TSAT and low ferritin, Nyakeriga et al reported a 30% reduction in clinical malaria among iron-deficient children [11]. In support of our findings, P. falciparum has been demonstrated to obtain iron from transferrin using in vitro parasite culture [30]. Furthermore, Clark et al demonstrated that parasitized red blood cells utilize serum iron [31]. These studies indicate that increasing bioavailable transferrin-bound iron may predispose an individual to increased risk of malaria. Indeed, we show that higher TSAT may increase malaria risk in children.
So, how might ID protect against malaria infection? In in vitro parasite cultures, iron-deficient human erythrocytes are poorly infected by P. falciparum compared to those that are iron replete, and this protective effect is reversed by iron supplementation [32]. In mouse models, Matsuzaki-Moriya et al showed that during ID, macrophages cleared parasitized erythrocytes more efficiently, suggesting that either erythrocytes produced under iron-deficient conditions are easily phagocytized by macrophages or that there is an enhancement of macrophage function during ID [33]. Furthermore, ID has also been shown to upregulate nitric oxide, which has antiparasitic properties against Plasmodium [34]. Another possible explanation is that during ID, zinc is incorporated in place of iron during heme synthesis, leading to formation of zinc protoporphyrin that, in turn, is thought to inhibit formation of hemozoin (the parasite survival pigment) in a manner similar to quinolines [35].
We also report that anemia (or hemoglobin concentration) does not influence subsequent malaria risk. Similar observations from 2 previous studies have been reported. A recent longitudinal study in Papua New Guinean infants aged 3 months and followed for 1 year reported a nonsignificant association between lower hemoglobin concentrations and subsequent malaria infection [19]. Ghosh et al made a similar observation in Indian children [20]. Indeed, in both studies and in ours, it was found that anemic children have a nonsignificant increase in malaria risk rather than protection from malaria. In contrast, Goheen et al reported that anemia was associated with decreased in vitro growth rate of P. falciparum [21]. However, it is possible that in vitro parasite growth rate might not mimic direct malaria susceptibility for children. Moreover, hemoglobin has low sensitivity and specificity in determining body iron status due to the overlap of values in iron-deficient and iron-replete individuals [36] and the multiple overlapping causes of low hemoglobin concentrations in African children [37]. It remains unclear whether the malaria parasite utilizes heme iron in hemoglobin or has other sources and mechanisms of iron acquisition. There are suggestions that the parasite may utilize storage iron since bioavailable iron content increases in parasitized red blood cells as the parasite develops from ring stage to schizont [31].
We hypothesized that increased hepcidin concentrations may reduce malaria risk through sequestration of iron within macrophages and enterocytes [38], thereby starving liver-stage Plasmodium [16]. Since the parasite requires iron for growth, it has been suggested that withholding iron from hepatocytes inhibits the development of malaria [16]. Furthermore, high cord blood hepcidin has been associated with decreased risk of clinical malaria, although not parasitemia or severe malaria, in Tanzanian infants [18]. However, in agreement with our study in an independent cohort of Kenyan children [17], we found no association between hepcidin concentrations and clinical malaria episodes. Differences in age or environmental factors may account for the different findings. Moreover, the high prevalence of ID, which is normally associated with decreased hepcidin and reduced the risk of malaria in our participants, may counter a possible protective role of hepcidin.
Our data indicate that a child’s erythropoietic drive (as measured by sTfR) does not influence their subsequent risk of malaria infection. The expression of sTfR increases with both ID and expanded erythropoiesis (with the latter being more influential) [39], factors that might have opposing effects on malaria risk. For example, increased erythropoiesis may increase the risk of malaria since Plasmodium parasites preferentially infect young red blood cells [40], whereas ID may be protective. These opposing effects could explain why sTfR concentrations were not associated with malaria risk in our study. It is also known that malaria itself causes increased sTfR concentrations [39, 41].
A major challenge in this study is that iron biomarkers are influenced by infections and inflammatory processes that may confound the effect of iron status on malaria infection [29]. To mitigate the potential confounding effects of infection on iron biomarkers, we excluded children with inflammation or malaria parasitemia at the time of iron measurement in secondary analyses and observed similar results (Supplementary Figure 2). Additionally, other potential confounders such as age, sex, length of follow-up, and study site were adjusted for in regression models. Limitations of the study were lack of TSAT concentrations in Ugandan children and that only febrile malaria was included for the malaria episodes. Strengths of our study included its large size (n = 2683 children) across 2 study sites and that we used multiple iron biomarkers in order to determine their individual effects on malaria risk, making it the largest and most definitive observational study to address the question of iron status and risk of malaria infection.
Our findings, which are in agreement with other studies, suggest that ID protects children against malaria infection and thus that improving iron status may predispose African children to infection. Interestingly, of the iron biomarkers, only higher concentrations of ferritin and TSAT were predictive of increased rates of subsequent malaria, perhaps reflecting differences in their relationship to parasite mechanisms of iron acquisition. Although WHO recommends iron supplementation coupled with malaria treatment and prevention strategies in malaria-endemic areas [8], these strategies remain difficult to implement. Thus, it is important to establish whether improved iron status increases malaria risk since this would necessitate long-term malaria prevention and treatment programs. However, our findings and those from other studies do not necessarily imply causality since observational studies may be subject to confounding and reverse causation, for example, prior malaria exposure might lead to both ID (due to raised hepcidin concentrations blocking iron absorption) and the acquisition of protective immunity against malaria, while malaria itself increases ferritin levels. Since ID prevents children from reaching their developmental potential, it is important to establish causality in the iron–malaria relationship. Thus, these data warrant additional large-scale studies, including studies that utilize genetic variants associated with iron status to infer causality (Mendelian randomization) and prospective interventional trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank our colleagues Barnes Kitsao, Jennifer Musyoki, and Jedidah Mwacharo at the Kenya Medical Research Institute (KEMRI)-Wellcome Trust Research Programme for their support in retrieving archived samples. We also thank the team at the Entebbe Mother and Baby Study under the Medical Research Council (MRC)/Uganda Virus Research Institute and London School of Hygiene and Tropical Medicine Uganda Research Unit. This study is published with permission from the Director of KEMRI.
Financial support. This work was funded by the Wellcome Trust (grant 110255/Z/15/Z to S. H. A., grant 202800/Z/16/Z to T. N. W., and grant 106289/Z/14/Z to A. J. M.) and the DELTAS Africa Initiative (DEL-15-003). The DELTAS Africa Initiative is an independent funding scheme of the Alliance for Accelerating Excellence in Science in Africa under the African Academy of Sciences and is supported by the New Partnership for Africa’s Development Planning and Coordinating Agency with funding from the Wellcome Trust (107769/Z/10/Z) and the UK government. The Ugandan cohort was supported by Wellcome Trust grants (064693, 079110, and 95778 to A. M. E.) with additional support from the Medical Research Council and Department for International Development, UK Government under the MRC/DfID concordat.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kyu HH, Pinho C, Wagner JA, et al. . Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013. JAMA Pediatr 2016; 170:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. World Malaria Report 2015. WHO: Geneva, Switzerland, 2015. [Google Scholar]
- 3. Petry N, Olofin I, Hurrell R, et al. . The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 2016; 8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr 2011; 141:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snow RW, Sartorius B, Kyalo D, et al. . The prevalence of Plasmodium falciparum in sub-Saharan Africa since 1900. Nature 2017; 550:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suchdev PS, Leeds IL, McFarland DA, Flores R. Is it time to change guidelines for iron supplementation in malarial areas?J Nutr 2010; 140:875–6. [DOI] [PubMed] [Google Scholar]
- 7. Brittenham GM. Safety of iron fortification and supplementation in malaria-endemic areas. Nestle Nutr Inst Workshop Ser 2012; 70:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. Guideline: daily iron supplementation in infants and children. WHO: Geneva, Switzerland, 2016. [PubMed] [Google Scholar]
- 9. Sazawal S, Black RE, Ramsan M, et al. . Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet 2006; 367:133–43. [DOI] [PubMed] [Google Scholar]
- 10. Zlotkin S, Newton S, Aimone AM, et al. . Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA 2013; 310:938–47. [DOI] [PubMed] [Google Scholar]
- 11. Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. . Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis 2004; 190:439–47. [DOI] [PubMed] [Google Scholar]
- 12. Jonker FAM, Calis JCJ, van Hensbroek MB, et al. . Iron status predicts malaria risk in Malawian preschool children. PLoS One 2012; 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gwamaka M, Kurtis JD, Sorensen BE, et al. . Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012; 54:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barffour MA, Schulze KJ, Coles CL, et al. . High iron stores in the low malaria season increase malaria risk in the high transmission season in a prospective cohort of rural Zambian children. J Nutr 2017; 147:1531–6. [DOI] [PubMed] [Google Scholar]
- 15.Moya-Alvarez V, Cottrell G, Ouedraogo S, Accrombessi M, Massougbodgi A, Cot M. High iron levels are associated with increased malaria risk in infants during the first year of life in Benin. Am J Trop Med Hyg 2017; 97:497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Portugal S, Carret C, Recker M, et al. . Host-mediated regulation of superinfection in malaria. Nat Med 2011; 17:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkinson SH, Uyoga SM, Armitage AE, et al. . Malaria and age variably but critically control hepcidin throughout childhood in Kenya. EBioMedicine 2015; 2:1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brickley EB, Spottiswoode N, Kabyemela E, et al. . Cord blood hepcidin: cross-sectional correlates and associations with anemia, malaria, and mortality in a Tanzanian birth cohort study. Am J Trop Med Hyg 2016; 95:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lombardo P, Vaucher P, Rarau P, Mueller I, Favrat B, Senn N. Hemoglobin levels and the risk of malaria in Papua New Guinean infants: a nested cohort study. Am J Trop Med Hyg 2017; 97:1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh SK, Yadav RS, Das BS, Sharma VP. Influence of nutritional and haemoglobin status on malaria infection in children. Indian J Pediatr 1995; 62:321–6. [DOI] [PubMed] [Google Scholar]
- 21. Goheen MM, Wegmüller R, Bah A, et al. . Anemia offers stronger protection than sickle cell trait against the erythrocytic stage of falciparum malaria and this protection is reversed by iron supplementation. EBioMedicine 2016; 14:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis 2005; 191:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bejon P, Lusingu J, Olotu A, et al. . Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med 2008; 359:2521–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elliott AM, Kizza M, Quigley MA, et al. . The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447]. Clin Trials 2007; 4:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waterfall CM, Cobb BD. Single tube genotyping of sickle cell anaemia using PCR-based SNP analysis. Nucleic Acids Res 2001; 29:E119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Vitamin and mineral nutrition information system. WHO: Geneva, Switzerland, 2011. [Google Scholar]
- 27. Yamanishi H, Iyama S, Yamaguchi Y, Kanakura Y, Iwatani Y. Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin Chem 2003; 49:175–8. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. WHO: Geneva, Switzerland, 2001. [Google Scholar]
- 29. Aguilar R, Moraleda C, Quintó L, et al. . Challenges in the diagnosis of iron deficiency in children exposed to high prevalence of infections. PLoS One 2012; 7:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollack S, Fleming J. Plasmodium falciparum takes up iron from transferrin. Br J Haematol 1984; 58:289–93. [DOI] [PubMed] [Google Scholar]
- 31. Clark M, Fisher NC, Kasthuri R, Cerami Hand C. Parasite maturation and host serum iron influence the labile iron pool of erythrocyte stage Plasmodium falciparum. Br J Haematol 2013; 161:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark MA, Goheen MM, Fulford A, et al. . Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat Commun 2014; 5:4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuzaki-Moriya C, Tu L, Ishida H, et al. . A critical role for phagocytosis in resistance to malaria in iron-deficient mice. Eur J Immunol 2011; 41:1365–75. [DOI] [PubMed] [Google Scholar]
- 34. Fritsche G, Larcher C, Schennach H, Weiss G. Regulatory interactions between iron and nitric oxide metabolism for immune defense against Plasmodium falciparum infection. J Infect Dis 2001; 183:1388–94. [DOI] [PubMed] [Google Scholar]
- 35. Iyer JK, Shi L, Shankar AH, Sullivan DJ Jr. Zinc protoporphyrin IX binds heme crystals to inhibit the process of crystallization in Plasmodium falciparum. Mol Med 2003; 9:175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cook D, Smith J. Evaluation of the iron status of a population. Blood 1976; 48:449–55. [PubMed] [Google Scholar]
- 37. Foote EM, Sullivan KM, Ruth LJ, et al. . Determinants of anemia among preschool children in rural, western Kenya. Am J Trop Med Hyg 2013; 88:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nemeth E, Tuttle MS, Powelson J, et al. . Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004; 306:2090–3. [DOI] [PubMed] [Google Scholar]
- 39. Beguin Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin Chim Acta 2003; 329:9–22. [DOI] [PubMed] [Google Scholar]
- 40. Pasvol G, Weatherall DJ, Wilson RJ. The increased susceptibility of young red cells to invasion by the malarial parasite Plasmodium falciparum. Br J Haematol 1980; 45:285–95. [DOI] [PubMed] [Google Scholar]
- 41. Verhoef H, West CE, Ndeto P, Burema J, Beguin Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. Am J Clin Nutr 2001; 74:767–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.