Abstract
In recent years, because of improved cancer screening, detection and treatment modalities, a rapid increase in the population of colorectal and other cancer survivors has been observed. The increasing population has justified the requirement of preventive strategies such as lifestyle modifications with regard to obesity, physical activity, diet and smoking. Physical activity may prevent approximately 15% of the colon cancers. Furthermore, several observational studies have demonstrated the efficacy and dose-dependent and anti-cancer effects of exercise on decreasing the mortality and risk of recurrence before and after the colorectal cancer (CRC) diagnosis. However, the required exercise dose, type and intensity are yet unclear. The results of randomised prospective studies are expected to determine the optimal amount, type and intensity of exercise and formulate the most appropriate exercise plan and guidelines, according to the requirements and comorbidities of the patients. In addition, recent studies have focused on the molecular and genetic mechanisms underlying the effect of physical activity on disease outcomes and recurrence rates. This review aimed to investigate the effects of physical activity and the biological basis of these effects in preventing the risk and recurrence of CRC and decreasing the hazards of cancer and cancer treatment.
Keywords: Colorectal cancer, Exercise, Physical activity
Core tip: This review aimed to investigate the effects of physical activity and the biological basis of these effects in preventing the risk and recurrence of colorectal cancer (CRC) and decreasing the hazards of cancer and cancer treatment. Several observational studies have demonstrated the efficacy and dose-dependent and anti-cancer effects of exercise on decreasing the mortality and risk of recurrence before and after the CRC diagnosis. However, the required exercise dose, type and intensity are yet unclear. The results of randomised prospective studies are expected.
INTRODUCTION
Colorectal cancer (CRC) is the third most common type of cancer and the fourth most common cause of cancer-related death worldwide[1,2]. A significant improvement has been observed in the 5-year survival rates with early screening programs, new treatment modalities and individualised treatments. The 5-year survival rates of patients with stage 1-3 CRC, accounting for approximately 75% of patients with CRC, has approached 65%[3]. Although long-term survival is poor in metastatic disease, patients have been living for more than 2 years because of new developments in treatment strategies[4].
The aetiology of CRC is multifactorial. In addition to genetic factors, lifestyle and environmental risk factors have substantial effects on CRC development. Several risk factors have been identified for CRC (low-fibre and high-fat diet, sedentary lifestyle, diabetes, obesity, smoking, alcohol, advanced age and inflammatory bowel disease). In recent years, the increase in CRC incidence is attributed to the increase in the elderly population, changes in dietary habits and increased risk factors such as smoking, low physical activity and obesity. Despite the advances in treatment strategies, new therapies have limited impact on cure rates and survival. Therefore, a tendency towards selecting adjuvant treatment strategies such as physical activity and exercise has been observed[4-6].
Epidemiological studies have shown that lifestyle factors and obesity affect the development of various types of cancer, particularly CRC[7]. A synergistic association is observed between physical inactivity and obesity. The International Agency for Research on Cancer (IARC) has reported that 25% of all the cancer cases worldwide are caused by obesity and sedentary lifestyle[8].
Obesity and decreased physical activity are associated with colon cancers that have P53 overexpression and KRAS mutation[9,10]. Physical activity may prevent approximately 15% of the colon cancers[8]. An umbrella review, including 19 reviews, 26 meta-analyses and 541 original studies, evaluating physical activity and cancer risk, has shown that regular physical activity is beneficial in preventing 7 types of cancers (colon, breast, endometrium, lung, oesophagus, pancreas and meningioma)[11]. The effect of physical activity on cancer risk is much stronger in breast and colon cancer than in the other types of cancers[12]. In recent studies, a dose-dependent effect of exercise has been reported[13,14].
More than 40% of the patients diagnosed with CRCs have comorbid diseases (diabetes, obesity, chronic obstructive pulmonary disease and heart failure)[15]. Physical activity decreases the risk of developing comorbid diseases in patients with CRC and improves the disease outcomes in patients with comorbid diseases. Several studies have been conducted regarding the effects of exercise on cancer prevention and the outcome in patients with cancer. Most studies focus on CRC and breast cancer. Furthermore, observational studies regarding cancer prevention and exercise are predominant and have often tested aerobic exercise programs. In these studies, physical activities were generally measured as metabolic equivalents hour/week (MET-hour/week). Because these studies include heterogeneous physical activity applications and are survey-based and subjective, significant errors in physical activity measurement have been reported. The studies showed the benefit of increased physical activity after the known predictors (stage, tumour differentiation and treatment status) were adjusted in the analysis. Furthermore, most studies suggested that the beneficial effect of physical activity is independent of the body mass index (BMI) and physical fitness. In recent years, exercise in patients with CRC is used as both primary and secondary prevention and CRC treatment.
CRC AND PRIMARY PREVENTION
Recently, several studies regarding cancer prevention and treatment strategies are directed towards novel approaches alternative to pharmacological treatments. Physical activity has been known to decrease the incidence of age-related chronic diseases such as cardiovascular disease, diabetes, hypertension and metabolic syndrome. Recent studies have additionally revealed that exercise aids the prevention of CRC.
The protective role of physical activity can be correlated with the incidence of precancerous colorectal polyps[16]. In an epidemiological study, it was observed that those who exercised for ≥ 1 h per week had a lower prevalence of colon polyps and adenoma than those who exercised for < 1 h[17]. In this study, exercise decreased the risk of polyp development throughout the entire colon, regardless of a specific area of the colon. In another study, exercise was reported to decrease the total number of intestinal polyps by 50% and the number of large polyps by 67%[18].
A meta-analysis showed that physical activity resulted in a 24% decrease in colon cancer risk [risk ratio (RR): 0.76, 95% confidence interval (CI): 0.72–0.81][19]. In a population of more than 150000 people, 940 colon and 390 rectal cancer cases were detected in a 6-year study period of an epidemiological study, evaluating the association between the risk of colon cancer and physical activity.In this study, 21% of men and 16.5% of women were physically active (> 7 h of regular physical activity), and it was reported that the risk of colon cancer decreased by 40% in the people exercising 7 h a week[20,21].
In the studies evaluating the association between CRC and physical activity, the intensity, type and area-specific effect of physical activity have been evaluated. A meta-analysis showed that increasing doses of physical activity considerably decreased the risk of colon cancer[20].
In a study by Mahmood et al[21], no statistically significant decrease in the risk of CRC was detected with occupational, transport and household activities. However, recreational activity significantly decreased the risk of CRC. Although physical activity decreases the risk of colon cancer, the association between physical activity and colon cancer are yet unclear. Furthermore, the duration and intensity of physical activity required to optimally decrease the risk of CRC are unknown.
In recent studies, the epidemiological differences in proximal and distal colon cancers and different genetic and environmental risk factors are reported to show different molecular features. Accordingly, modified treatment approaches have been adopted for proximal and distal colon cancers[22]. In a study, it was reported that physical activity had more effect on the risk of distal colon cancer than that of proximal colon cancers[23].
In a meta-analysis study involving 21 studies, the association between physical activity and colon cancer did not differ with the anatomical location[14]. Moreover, data regarding rectal cancer are insufficient[24]. Some studies describe a similar decrease in the risk of colon cancer, whereas other studies have not demonstrated the benefit of physical activity in rectal cancer[19,25,26]. In a meta-analysis, no significant decrease in risk was found with regard to the incidence of rectal cancer among physically active subjects (RR: 1.15, 95%CI: 0.83–1.64)[27]. In a recent meta-analysis, higher physical activity was found to be associated with a decreased risk of colon by 16% and rectal cancer by 13%[28].
According to some studies, the anti-cancer effect of exercise depends on the carcinogenic exposure and the duration of physical activity. In a preclinical study, exercising during and before chemical exposure in a chemically induced intestinal tumour rat model resulted in a significant decrease in the number of tumours. However, exercise following chemical exposure did not have any effect[29].
In early physical activity and cancer studies, physical activity has been reported to decrease the risk of colon cancer, particularly in men; however, it does not decrease the risk in women[30]. Mechanisms associated with gender differences are yet unknown. Sex hormones are considered to have a protective effect against colon cancer; thus, the difference observed in the studies may be because of a decreased protective effect caused by decreased estrogen levels in women who exercise more. However, in the meta-analysis of 20 studies in 2011, no difference was observed between the genders in terms of physical activity and the risk of CRC[31]. Moreover, in a study involving postmenopausal women, women undergoing hormone replacement therapy were shown to have a lower risk of colon cancer, and physical activity did not provide any additional benefit. However, it has been shown that physical activity has a protective effect against colon cancer in postmenopausal women who are not undergoing hormone replacement therapy[32]. In a meta-analysis evaluating 19 cohort studies, the risk of CRC in physically active women and men was reported to be decreased by 29%and 22%, respectively[33].
CRC AND SECONDARY PREVENTION
Because of early diagnosis and advances in the treatment of cancers, > 25 million people have been diagnosed with cancer worldwide, and this number is increasing each day. This population has been estimated to increase to > 75 million in the next 3 decades[34]. Despite positive developments, patients experience several long-term health issues and physical and psychological issues following cancer treatment. Compared with the general population, studies have shown that secondary malignancy, cardiovascular disease, diabetes, osteoporosis, sleep disorders, anxiety, depression and decreased functional capacity exhibit a higher risk, in addition to the risk of recurrence, in patients with cancer. These comorbid conditions can be caused by cancer treatment, in addition to genetic predisposing and lifestyle factors[35,36]. In recent years, there has been an increasing awareness of lifestyle modifications because of the increased population of cancer survivors.
With regard to primary prevention, the role of physical activity during and after CRC treatment is yet unclear. Compared with other cancer survivors, CRC survivors have been observed to have a higher rate of physical inactivity[37]. In one study, 68% of CRC survivors were physically inactive after a curative treatment[38]. Despite the benefits of physical activity, only 23.5% of CRC survivors followed the exercise guidelines[39]. In another study, 21%–42% of CRC survivors could exercise according to the guidelines recommended by the American College of Sports Medicine (ACSM)/American Cancer Society (ACS)/National Comprehensive Cancer Network, 6 months following the curative treatment[40]. Adherence to exercise and dietary guidelines in patients with cancer is associated with a lower incidence ofcancer and lower cancer-specific and all-cause mortality[41].
Despite advances in the diagnosis and treatment, the rate of recurrence for locally advanced colon cancer is 40%[42]. In addition to the risk of recurrence, CRC survivors experience the late and long-term effects of cancer treatment[43,44]. Lifestyle interventions such as an improved diet and exercise are recommended to improve the side effects of cancer and cancer treatment. Physical activity improves clinical conditions, such as weakness, quality of life, muscle strength, lymphedema, depression, functional status, and decreases the risk of recurrence of cancer before and after the diagnosis of CRC and cancer-specific and overall mortality[38,45-47]. Because exercise decreases the risk of developing chronic diseases such as cardiovascular disease and diabetes, there is a decrease in the all-cause mortality[48].
In a meta-analysis evaluating 16 breast and 7 CRC studies comparing low and high levels of post-diagnostic physical activity, a 42% (RR: 0.58, 95%CI: 0.48–0.70) decreasein the risk of all-cause mortality and 39% decrease in CRC-specific mortality (RR 0.61, 95%CI: 0.40–0.92) were detected[49]. In CRC survivors who adhered to the new guidelines, CRC-specific mortality has been shown to be 10%–40% less and all-cause mortality to be 20%–50% less[50-53]. Clearly, post-diagnostic physical activity is associated with an improvement in disease outcomes in patients with CRC; however, whether the pre-diagnostic physical activity affects the CRC survival remains unclear[54]. A meta-analysis evaluating prospective cohort studies has shown a 25% decrease (HR: 0.75, 95%CI: 0.65–0.87) in CRC-specific mortality in those who participated in any physical activity at any level before diagnosis compared with those who did not participate at all. Furthermore, the pre-diagnostic physical activity decreased the all-cause mortality[55]. The clinical benefit rate increased when the level of physical activity was increased. Whether the effect of the post-diagnostic physical activity on CRC survival is influenced by the pre-diagnostic physical activity remains unknown. A new meta-analysis included both pre-diagnostic (CRC-specific mortality; HR: 0.79, 95%CI: 0.71–0.89, total mortality HR: 0.81, 95%CI: 0.72–0.91) and post-diagnostic physical activity (CRC-specific mortality; HR: 0.77, 95%CI: 0.63–0.94, all mortality HR: 0.71, 95%CI: 0.63–0.81)and confirmed its association with improved disease outcomes[56]. Furthermore, a recent study demonstrated the benefit of both pre- and post-diagnostic activity in postmenopausal women with CRC[57].
CRC is associated with multiple gene mutations (such as APC, KRAS, PIK3CA and TP53). Recent studies to identify patients with CRC who may benefit from exercise evaluated the association between physical activity and cancer outcomes using molecular [KRAS, PIK3CA, BRAF and microsatellite instability (MSI)] or genetic markers [P27 (CDKN1B)-positive, B-catenin (CTNNB1)-negative, PTGS2 (prostaglandin-endoperoxide synthase 2/COX-2)-positive or the insulin receptor substrate (IRS1)-low/negative protein expressions][58-62]. The study results showed that P27, B-catenin, COX-2 and IRS1 expression significantly modified the association between the post-diagnostic physical activity and CRC-specific survival. Physical activity significantly has been shown to improve CRC-specific survival after diagnosis in patients with tumours with increased P27 and COX-2 expression and decreased B-catenin and IRS1 expression[58-62]. Hardikar et al[63] showed that the beneficial effect of physical activity was not specific to the molecular phenotype of CRC (BRAF mutation, KRAS mutation and MSI status).
To date, few studies have evaluated the feasibility and reliability of increased physical activity compared with the standard levels. In a study by Brown et al[5], stage 1–3 colon cancer survivors were included in anaerobic exercise program for 150 min/wk (14 patients, low doses) or 300 min/wk (12 patients, high doses) for 6 months, and 13 patients were randomised into the control group. In this study, changes in the prognostic markers such as soluble intercellular adhesion molecule (sICAM-1) and vascular adhesion molecule 1 (sVCAM-1), which are associated with early death and disease recurrence, were evaluated among colon cancer survivors[64,65]. Increased exercise and physical activity (300 min/wk) were associated with decreased mortality and risk of recurrence in CRC survivors. Furthermore, sICAM-1 reduction was achieved in both exercise arms; however, sVCAM-1 did not decrease. Moreover, sICAM-1 may be associated with the anti-cancer effect of exercise; however, this finding requires further confirmation[66].
Although observational studies have shown the beneficial association between physical activity and survival after CRC treatment, no randomised controlled trials have been conducted. The first prospective phase 3 randomised clinical trial, Challenge Trial (the Colon Health and Lifelong Exercise Trials), continues to evaluate the effect of 3-year exercise on survival in stage 2 and 3 CRC survivors[67]. Furthermore, there are many on-going studies focusing on CRC and exercise (Table 1).
Table 1.
Ongoing trials on colorectal cancer and exercise
| Study | Conditions | Interventions | Title | Status |
| NCT01325909 | Rectal cancer | Exercise programme | Exercise training in colorectal cancer patients | Completed |
| NCT03515356 | Colorectal cancer | Motivational interviewing-walk intervention/physical activity education pamphlet | Exercise to reduce chemotherapy-ınduced peripheral neuropathy | Recruiting |
| NCT01924897 | Colorectal cancer | Exercise training | Preop cardiopulmonary exercise testing and exercise training in colorectal patients | Unknown |
| NCT00985400 | Colorectal cancer | Exercise programme/telephone-based intervention | Doctor-recommended home-based exercise program or relaxation training in improving physical function and controlling symptoms in patients with stage IV or recurrent colon cancer that cannot be removed by surgery | Active,not recruiting |
| NCT00230646 | Colon cancer/rectal cancer | Exercise counseling | Promoting physical activity after colorectal cancer | Completed |
| NCT01133132 | Colon cancer | Survivorship CHESS (mobile comprehensive health enhancement support system) | Interactive cancer communication system directed physical activity enhancement for colon cancer survivors | Completed |
| NCT02597075 | Metastatic colorectal cancer | Standard therapy+physical activity program/standard therapy | Physical activity in patients with metastatic colorectal cancer who receive palliative first line chemotherapy | Recruiting |
| NCT02191969 | Colorectal Cancer/fatigue | Walk with ease | Physical activity ıntervention for older patients during chemotherapy for colorectal cancer | Recruiting |
| NCT02966054 | Colon cancer/rectal cancer | Digital health physical activity ıntervention group | Self-monitoring and reminder texts to ıncrease physical activity after cancer:a pilot randomized controlled trial | Completed |
| NCT00373022 | Colorectal Cancer/depression/anxiety disorder | Exercise programme | Moderate physical activity in helping patients recover physically and emotionally from stage II or stage III colorectal cancer | Completed |
| NCT01708824 | Colorectal cancer | Physical activity/dietary | Diet and physical activity intervention in CRC survivors | Unknown |
| NCT03232814 | Colorectal cancer | Group-based walking | Walk on:a community-based approach to ıncrease physical activity among men treated for colorectal cancer | Withdrawn |
| NCT01991847 | Colorectal cancer | Physical activity | Tertiary prevention by exercise in colorectal cancer therapy | Completed |
| NCT02056691 | Colorectal cancer | Exercise programme/muscle biopsy | Exercise induced changes in colorectal cancer tissues | Completed |
| NCT00819208 | Colorectal cancer/anxiety/depression/fatigue/sleep disorders | Exercise intervention | Health education materials with or without a physical activity program for patients who have undergone treatment for high risk stage II or stage III colon cancer | Recruiting |
| NCT02347852 | Colorectal neoplasms | Regorafenib | Assessment of physical activity during therapy with regorafenib for metastatic colorectal cancer | Completed |
| NCT02780284 | Colorectal cancer | Physical activity intervention | Microbiome, exercise tracking study | Unknown |
| NCT02250053 | Colon cancer (stage 2 and 3) | Exercise | Exercise and colon cancer | Unknown |
| NCT03049124 | Rectal cancer | Exercise | Exercise for adults diagnosed with rectal cancer | Recruiting |
| NCT03082495 | Rectal cancer | Exercise | Exercise during and after neoadjuvant rectal cancer treatment | Recruiting |
| NCT03111823 | Stage IV colorectal cancer | Exercise intervention | Exercise program during chemotherapy in metastatic colorectal cancer | Terminated |
| NCT00977613 | Colorectal cancer | Exercise counseling | Adherence to a recommended exercise regimen in colorectal cancer patients | Completed |
| NCT03291951 | Colon cancer | Resistance training | Focus on reducing dose-limiting toxicities in colon cancer with resistance exercise study | Enrolling |
| NCT02264496 | Colorectal cancer | Exercise | Prospective randomised trial of exercise and/pr antioxidants in colorectal cancer patients undergoing surgery | Completed |
| NCT03120104 | Rectal cancer | Pelvic floor muscle exercise | Physical exercise for colorectal cancer patients after transanal total mesorectal excision | Recruiting |
| NCT03186638 | Colorectal cancer (stage 1-3) | Exercise intervention/ibuprofen | Exercise and low-dose ibuprofen for cognitive ımpairment in colorectal cancer patients receiving chemotheapy | Recruiting |
| NCT00668161 | Colon cancer prevention | Exercise | Effect of exercise on biomarkers of colon cancer risk | Completed |
| NCT02538913 | Rectal neoplasms | Exercise training | Exercise training for rectal cancer patients | Recruiting |
| NCT02724306 | Colon polyps/adenomas | Active lifestyle programme | Physical activity intervention with people at ıncreased risk of developing colon cancer | Completed |
| NCT01859442 | Locally advanced rectal cancer | Structured responsive interval exercise training programme | The effects of cancer therapies and exercise on mitochondrial energetics and fitness | Completed |
| NCT01914068 | Rectal cancer | Supervised exercise in hospital | The effects of a 9 wk exercise programme on fitness and quality of life in rectal cancer patients after chemoradiotherapy and before surgery | Completed |
| NCT02057991 | Colorectal cancer/anxiety/depression/fatigue | Educational intervention/CAM exercise therapy (mindfulness exercise video) | Mindfulness-based exercise video in educating Hispanic/Latino patients with colorectal cancer and their caregivers | Terminated |
| NCT02403024 | Colorectal cancer | Interval walking | Feasibility and Efficacy of Interval Walking in patients with colorectal cancer | Completed |
| NCT02188342 | Colorectal cancer | High intensity interval training | Assessing the effectiveness of a preoperative high intensity interval training programme in older colorectal cancer patients | Completed |
| NCT03336229 | Colorectal cancer | Exercise Intervention | Enhancing fitness with preoperative exercise in colorectal cancer surgery | Not yet recruiting |
| NCT02586701 | Colorectal cancer | Supervised /non-supervised exercise | Supervised versus non-supervised exercise on adherence and functional outcomes in colorectal patients | Completed |
| NCT01210313 | Colorectal cancer | Physical activity | Physical activity for reduction of recurrence rate after adjuvant chemotherapy for localised colorectal carcinoma | Completed |
| NCT02299596 | Colorectal cancer | Physical activity | Physical activity in relation to surgical procedures | Recruiting |
| NCT03361150 | Colorectal cancer | Physical activity | High-intensity interval vs moderate continuous training in surgical prehabilitation | Completed |
| NCT02889276 | Colorectal cancer | Unsupervised activity/Functional resistance training | Effects of functional exercise on fitness and QoL in cancer survivors | Recruiting |
| NCT02895464 | Colorectal cancer | Exercise | Feasibility of home-based preoperative exercise in older people | Completed |
| NCT02499939 | Colorectal cancer | Exercise/ultrasound therapy | Ultrasound therapy and therapeutic exercise for chemotherapy ınduced peripheral neuropathy | Completed |
| NCT02522520 | Colorectal cancer | Pedometer intervention | Pedometer Intervention and health effects for sedentary colorectal cancer patients during adjuvant chemotherapy | Recruiting |
| NCT02442583 | Colorectal cancer | Brochure regarding sedentary behavior | Reducing sedentary behaviors among colorectal cancer survivors | Completed |
EXERCISE IN PATIENTS WITH CRC UNDERGOING TREATMENT
Reportedly, exercise improves the surgical tolerance of CRC and decreases the hospital stay after surgery[68]. Exercise before CRC surgery may improve postoperative results. However, one review concluded that whether exercise before CRC surgery reflected improvement in peri- and post-operative outcomes was unclear[69]. A phase 3 randomised prospective study, PHYSSURG-C study, evaluating the effect of pre-and post-operative physical activity on the post-operative morbidity and mortality after CRC surgery is on-going (NCT 02299596)[70]. Exercise has been shown to improve the quality of life and decrease few side effects in several patient groups receiving adjuvant therapy[44]. In addition, exercise increases the completion rate of chemotherapy in patients with CRC[71].
Neoadjuvant chemotherapy and radiotherapy can cause severe acute toxicity in locally advanced rectal cancer. Few studies have shown that exercise is feasible and safe during neoadjuvant therapy in rectal cancer[72]. The EXERT study, which is evaluating the effect of exercise on the clinical outcomes and side effects of exercise performed during and after neoadjuvant treatment in locally advanced rectal cancer, is on-going (NCT03082495)[73].
In patients with cancer, the disease itself and each treatment modality applied (surgery, chemotherapy and radiotherapy) can create specific side effects and complications that affect their daily life. Side effects such as fatigue, pain, muscle weakness, peripheral neuropathy, cardiovascular and pulmonary complications, endocrine changes, anaemia, immune dysfunction, sleep disorders, depression, anxiety, gastrointestinal disturbance and skin changes can develop during the treatment. The most common side effects during the treatment in patients with CRC are generalised and muscle weakness. In these patients, physical exercise programs improve the symptoms and side effects of chemotherapy, along with the quality of life[5,74,75]. In the Cochrane study, which included 56 randomised trials involving patients with cancer-associated fatigue, exercise was found to decrease cancer-associated fatigue and improve depression and sleep disorders[76]. The 6- and 12-wk home-based exercise programs, which are easier to apply, have been shown to significantly improve physical fitness in CRC survivors and to be effective and applicable in increasing the level of physical activity[77,78]. The CASUS (Cancer Survivor Study), a prospective observational study investigating the effect of physical activity and nutrition on the quality of life and disease recurrence and survival in CRC survivors, is on-going[79].
Patients with metastatic disease have very low participation rates in exercise programs. Prospective studies investigating the effect of exercise on clinical outcomes have shown that physical activity improves prognosis in patients with CRC even in advanced stage patients[52]. In a study regarding patients with stage 4 CRC, an improvement in fatigue, functional capacity, sleep quality and the quality of life was reported in patients after 8 weeks of home-based exercise programs[80]. Moreover, low intensity physical activities in patients with stage 4 metastatic cancer are recommended. The studies have shown no clear association between the physical activity and overall or CRC-specific survival in patients with metastatic CRC[81,82].
The CALGB89803/ALLIANCE study, a prospective observational study, investigated the effect of physical activity on survival in patients with colon cancer before and after recurrence 6 months post-completion of adjuvant therapy. After adjusting the potential factors that can affect the survival, a statistically significant 29% improvement in mortality was observed in physically active patients with recurrent colon cancer[83]. The physical activity prior to recurrence appears to be a factor that affects prognosis in patients with recurrent colon cancer.
OBSTACLES, CONTRAINDICATIONS AND COMPLICATIONS FOR EXERCISE
Although it is known that exercise has beneficial effects in patients with CRC and survivors, there are some obstacles to participation of patients in exercise regimens. Fatigue is the most frequently reported obstacle to exercise in patients with CRC[84]. More than 60% of patients with cancer complain of weakness during and after treatment. Fatigue decreases the quality of life and physical activity. The cause of fatigue in patients with cancer is unknown, and may develop because of the disease itself or its treatment. Furthermore, it may occur because of other clinical issues such as depression, physical inactivity and sleep disorders. Although complaints such as nausea and pain can be treated effectively during cancer treatment, effective treatment for fatigue is yet unavailable. After treatment, fatigue decreases over time; however, 30% of the patients may continue to complain for years[85]. In addition, treatment-related side effects and comorbid conditions (cardiopulmonary disease and diabetes) may be an obstacle to exercise. CRC survivors with peripheral neuropathy have been shown to participate in lesser physical activity than those without neuropathy[86].
In addition to the effect of exercise on survival, several studies have evaluated the efficacy and reliability of physical activity applications (home-based, supervised and telephone-based counselling and interval walking) in CRC survivors. These studies have shown that aerobic and resistance exercises were safe and did not increase the risk of side effects throughout the chemotherapy[80,87-89].
Understanding the factors that impede exercise practices is important for directing patients with cancer to the appropriate exercise programs. However, data regarding the appropriate exercise dose and type to safely correct the results is insufficient. Guidelines should be developed according to each patient’s disease, age and comorbid condition. Clinicians can influence patients by encouraging them to exercise, thereby increasing patient participation in physical activity. However, in a study investigating the effect of oncologists’ exercise advice on patients, oncologists’ exercise re-commendations alone were shown to be insufficient to increase participation in exercise. Reportedly, exercise package programs may be ideal for increasing the exercise participation[90].
Contraindications for exercise are heart failure, acute infectious disease, metabolic disease (thyrotoxicosis and myxoedema) and mental and physical disorders[91]. Non-scheduled exercises can cause complications. Furthermore, intense physical activity, particularly in the early period, can result in deterioration of wound healing and parastomal hernias. Skeleton stability should be investigated before exercise, if patients are suspected of bone metastasis. Blood counts should be monitored in patients who are scheduled to participate in exercise during chemotherapy. For intensive and light exercises, thrombocytes should be at least 50000/µL and 20000–50000/µL, respectively. If haemoglobin is ≤ 8 mg/dL, exercise may cause cardiac ischemic complications because of increased O2 requirement. Neutropenia is not a contraindicated condition for exercise; however, caution should be exercised in terms of infection. Patients should avoid severe exercises because of the toxicity (nausea, vomiting, nephrotoxicity, cardiotoxicity and diarrhea) caused within 24 h of chemotherapy[24]. Severe peripheral neuropathies constitute the contraindications for exercises. Physical activity studies have reported no side effects because of exercise during and after cancer treatment[91].
EXERCISE AND MECHANISMS OF ACTION
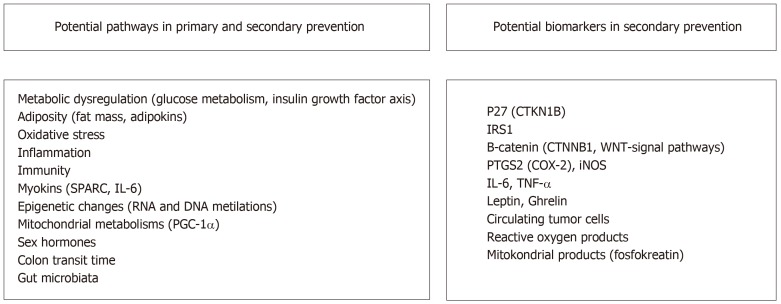
Although the association between exercise and prevention of CRC is definite, the molecular mechanism underlying the protective effect of exercise is yet unknown. The association between exercise and cancer is explained through several mechanisms. These mechanisms include metabolic dysregulation [involving insulin, glucose and insulin-like growth factor (IGF)], sex hormones, adiposity [changes in adipokines (leptin andadiponectin)], oxidative stress and inflammation and impaired immune function[42,92] (Figure 1).
Figure 1.
Biological potential pathways as explanatory mechanisms of the association between physical activity and primary and secondary prevention of colorectal cancer/potential biomarkers in secondary prevention. SPARC: Secretory protein acidic and rich in cysteine; PGC-1 α: Peroxisome proliferator-activated receptor gamma co-activator 1α; CTKN1B: Cyclin-dependent kinase inhibitor 1B; IRS1: İnsulin receptor substrate 1; CTNNB1: Catenin beta 1; PTGS2: Prostaglandin-endoperoxide synthase 2; COX-2: Cyclooxygenase-2; TNF- α: Tumor necrosıs factor alpha; iNOS: İnducible nitric oxide synthase.
Insulin pathway
Insulin influences DNA synthesis, cell survival, proliferation and differentiation using various cellular signalling pathways via insulin growth factor receptor IGF1R[93]. Elevated systemic IGF1 levels are associated with CRC risk[94]. In preclinical studies, exposure to insulin has been shown to induce colonic tumour cell development[95-97]. Moreover, observational studies have supported pre-clinical studies[98,99]. Insulin resistance is associated with higher CRC incidence and mortality[100]. In the studies, the degree of IGF1R over-expression has been shown to be correlated with the tumour stage in CRCs[101].
Physical activity decreases insulin resistance and the insulin levels affecting the IGF pathway and indirectly decreases the risk of CRC, recurrence and mortality. With regard to the IGF pathway, heterogeneity is observed in response to exercise. In some studies, an increase in IGF-1 and IGFBP-3 levels was observed with exercise, whereas in some cases, a decrease was observed. This clinical condition may be because of several factors affecting the association between the IGF pathway and exercise[102]. Despite heterogeneous results, decreased IGF-1 levels and increased IGFBP-3 levels may be a reasonable mechanism underlying the inverse correlation between CRC and physical activity[103]. The association between exercise and CRC cannot be explained using a single mechanism because exercise and interrelated factors exert varying effects.
Inflammation
Inflammation is known to be a risk factor for various chronic diseases (obesity and metabolic syndrome) including cancer. Inflammation plays an important role in cancer development and progression[104]. Although the underlying mechanisms are yet unclear, the inflammatory process appears to be an important pathway associated with the risk of CRC. Physical activity can decrease systemic inflammation and improve immune function[105]. Proinflammatory cytokines such as IL-6, C-reactive protein and tumour necrosis factor (TNF)-α are associated with an increased risk of cancer. Various studies have demonstrated the effects of physical activity on IL-6 in the colon cancer model. In a study conducted by Mehl et al[106], a decrease in plasma IL-6 was observed in APCmin/+ male mice after treadmill running. This result has been shown to be associated with fewer polyps.
New preclinical studies have shown that inflammation is associated with polyp formation and progression and that the cyclooxygenase isoenzymes (COX-1 and -2) particularly play an important role in intestinal tumour formation[103]. Administration of nonsteroidal anti-inflammatory drugs that inhibit the COX enzyme is known to be associated with a decreased risk of colon cancer (RR: 0.60, 95%CI: 0.40–0.89)[107,108]. Physical activity results in a local anti-inflammatory effect by decreasing COX-2 and iNOS (inducible nitric oxide synthase) expression in the colon mucosa. Adipocyte, energy balance, insulin, adipokines, estrogen and other factors known to play a role in carcinogenesis have been shown to affect the inflammatory response. Therefore, these factors may be involved in the indirect effect of physical activity on inflammatory processes in cancer[108,109].
Myokines
Myokine secretion from the skeletal muscles may be involved in the protective effect of exercise. Studies have shown that exercise-induced myokines include IL-6, IL-8, IL-15, brain neurotrophic factor and leukaemia inhibitory factor released from the muscle fibres. Exercise enhances the insulin sensitivity through these cytokines and decreases the production of proinflammatory cytokines[110-112].
Recent studies have shown that the secretory protein acidic and rich in cysteine (SPARC protein), a cellular matrix protein known as a myokine, is released from the muscle tissue after exercise and is involved in intercellular interaction and cell differentiation. The SPARC protein forms a biological association between colon tumorigenesis and physical activity. The SPARC protein prevents CRC development by increasing apoptosis[113]. In a study conducted by Aoi et al[114], the antiproliferative and proapoptotic effects of the SPARC protein in colon cancer cells has been shown.
Immunity
The mechanisms underlying the protective effect of exercise on the risk of colon cancer are complex. The role of exercise in the immune system in cancer prevention is yet unclear. Recently, macrophages and T cells have been the important factors in colon cancer pathogenesis. Accumulation of intra-tumoral macrophages is associated with poor prognosis in colon cancer[115,116]. Exercise-induced changes in the immune system are a possible mechanism. In a preclinical study, exercise was shown to affect the immune cell parameters in the mucosal tissue of APCmin/+ mice. Exercise decreased the expression of macrophage and regulatory T-cell markers and increased the number of cytotoxic T-cells[117]. Other preclinical studies confirmed that exercise was particularly influenced immune cells such as T lymphocytes and macrophages[118,119]. Furthermore, exercise may have a positive effect on immune aging[120]. Exercise has been shown to increase natural killer cell cytotoxicity, monocyte and macrophage number and function and the CD8 T-cell ratio. Furthermore, it has been shown to decrease the increased antigen presentation, inflammation and number of proinflammatory monocytes and prevent the accumulation of aged T-cells[121-123]. These mechanisms demonstrate the complexity of interaction between the risk of cancer and physical activity. Further studies are required to fully understand the associations among immunity, exercise and cancer.
Other mechanisms
In CRC, there are other suggested mechanisms (micro RNA, global DNA methylation, intestinal microbiota, colon transit time and mitochondrial dysfunction) underlying the effects of exercise on tumorigenesis. In recent years, it has been suggested that intestinal microbiota is associated with CRC incidence and progression and may predict the response to immunotherapy. Diet and lifestyle changes alter the intestinal microbiota[124,125]. Several studies have shown that some gut microbes such as anaerobic bacteria significantly increased in patients with CRC; however, further investigation is required to assess the importance of these bacteria and their metabolites in CRC pathogenesis. Moreover, the effect of lifestyle on the anticancer immune response is yet unclear[126].
Another mechanism that explains the association between exercise and CRC is that the exercise decreases the colon transit time. Thus, the interaction of intra-colonic chemicals with colonic mucosa is limited[127]. Moreover, CRC development and risk factors such as obesity and aging are associated with mitochondrial dysfunction. In a recent study, it was shown that the peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α), the major regulator of mitochondrial functions, may be a biomarker involved in the protective effect of physical activity in patients with CRC[128].
BIOMARKERS AND THE EFFECT OF EXERCISE ON PATIENTS WITH CRC
Physical activity before and after the diagnosis in CRC is associated with improved disease outcomes and decreased risk of recurrence; however, the underlying molecular mechanisms are unknown. Various observational studies have focused on whether the different molecular properties of CRC affect the association between physical activity and survival. As observed in the standard oncology treatments, every treatment does not have the same effect on every patient. Similarly, physical activity should not be expected to benefit all patients. The molecular basis of the association between CRC and physical activity must be highlighted, and tumour biomarkers or patient characteristics that can predict the response to exercise must be identified. Using molecular markers and protein expression, a patient subgroup that can benefit the most from physical activity can be determined (Figure 1).
P27
P27 loss is common in CRC. P27 is a cyclin-dependent kinase inhibitor that is additionally associated with the insulin pathway. High insulin and IGF-1 levels result in P27 downregulation. In response to energy restriction and physical activity, P27 expression increases[59]. High P27 (CTKN1B) levels are associated with cell cycle termination[129]. The benefit of physical activity may be affected by tumour P27 status. In a preclinical study, patients with colorectal tumours that expressed P27 experienced greater benefit from physical activity after diagnosis than those with a P27 loss. People with ≥ 18 met h/week of physical activity after diagnosis showed a 67% decrease in colon cancer-specific mortality compared with less active subjects. No statistically significant association was observed between the patients with tumour and loss of P27[59].
B-catenin
The B-catenin (WNT) signalling pathway plays an important role in CRC development, energy metabolism, adipogenesis, obesity and metabolic diseases. The activation of the WNT signalling pathway because of a loss of APC and its major mediator CTNNB1 (B-catenin) results in cell growth independent of the energy balance[130]. Physical activity alters the WNT-CTNNB1 signal in the mouse colonic mucosa and the WNT-CTNNB1 signalling pathway affects the cellular sensitivity to physical activity[131].
In one study, patients with early-stage CRC with CTNNB1-negative tumours, who had ≥ 18 met hour/week of physical activity after diagnosis, showed a 67% decrease in the risk of CRC-specific mortality compared with that in inactive patients. However, no correlation was observed in CTNNB1-positive patients[60]. In a study evaluating whether B-catenin predicted the benefit of exercise in patients with metastatic colon cancer receiving chemotherapy, it was found that exercise did not affect the survival (HR: 0.98, 95%CI: 0.32–2.97). However, patients with weak staining for B-catenin in the exercise program had a lower mortality rate (HR: 0.39, 95%CI: 0.025–6.1)[132]. In clinical practice, CTNNB1 status can be used as a predictive biomarker in response to exercise applications[133].
PTGS2 (COX-2) and TNF-α
Physical activity can affect inflammation-induced cell growth. In preclinical studies, exercise has been shown to alter chemically induced COX-2 (cyclooxygenase-2) expression and cell proliferation in the colon. CRC-specific survival may vary based on the PTGS2 (prostaglandin-endoperoxide synthase 2/COX-2) expression status[134]. Among 382 patients with PTGS2 (COX-2)-positive CRC, those with the highest physical activity had an 82% decreased CRC-specific mortality compared with the least active patients. However, the protective effect was not observed in 223 patients with PTGS2-negative CRC[61]. High TNF-α expression in colon tumour tissue is associated with positive lymph node stage and colon cancer recurrence[135]. In patients with colon cancer, the circulating TNF-alpha levels were observed to be decreased by exercise[88].
Insulin, IGF-1 and IRS1
Diabetic patients and patients with metabolic syndrome have an increased risk of CRC recurrence[136]. In addition, increased insulin and IGF-1 levels in patients with CRC are associated with a poor prognosis[137]. In one study, postoperative physical activity in CRC survivors decreased insulin levels and insulin resistance and increased IGF-1 (17.8%, P: 0.007) and IGFBP-3 (30.3%, P: 00013) levels[88]. However, the decrease in insulin and IGF levels is known to decrease the risk of CRC and improve survival outcomes[104].
İnsulin receptor substrate 1 (IRS1), insulin and IGF are mediators in the insulin signalling pathway, and downregulation of IRS1 is associated with insulin resistance[138]. In a study evaluating 371 patients with stage 1–3 CRC, post-diagnostic physical activity significantly improved the CRC-specific survival in patients with low IRS1-expressing tumours,with a hazard ratio (HR) of 0.15 (95%CI: 0.02–1.38) in the IRS1-negative group, 0.45 (95%CI:0.19–1.03) in IRS1-low group and 1.32 (95%CI: 0.50–3.53) in IRS1-high group.If confirmed by other studies, it may be used as a predictive marker to identify the patient groups that will benefit the most from exercise[62].
Leptin and Ghrelin
Preclinical studies have shown that exposure of colon cancer cells to adipocytes and pre-adipocytes increases cell proliferation[139]. Exercise decreases visceral obesity. Physical activity and exercise decrease inflammatory adipocytes and increase anti-inflammatory adipokines[140]. Leptin and ghrelin are important regulating hormones in the intake and consumption of energy and weight control. Decreased body fat mass and percentage is associated with an increase in ghrelin levels[141]. Ghrelin was found to be associated with increased proliferation and invasion in CRC by endogenous and autocrine effects[142]. The endocrine and autocrine effects of ghrelin in CRC vary. Aerobic exercise causes a decrease in leptin and an increase in ghrelin[143,144]. In one study, because of the increase in adipose tissue, a decrease in ghrelin levels and an increase in the risk of colon cancer were determined[145]. In a study evaluating the effect of 8 weeks of exercise on plasma leptin and ghrelin levels in patients with CRC, a significant increase in ghrelin levels was observed in the exercise group after 8 wk. Plasma leptin levels and insulin resistance didnot differ significantly. Plasma ghrelin levels were negatively correlated with the body fat percentage. If confirmed by other studies, ghrelin hormones can be used as biomarkers to demonstrate the benefit of exercise.
Other biomarkers
Genetic and epigenetic changes induced by reactive oxygen products may contribute to CRC progression. Chronic exercise may decrease the risk of recurrence by decreasing the systemic oxidative stress[146,147].
Circulating tumour cells predict stage 1–3 colon cancer recurrence and mortality[148]. In a study, patients with stage 1–3 colon cancer were randomised into two groups and were subjected to aerobic exercise for 150 min/wk and aerobic exercise for 300 min/wk for 6 mo. After 6 mo, a significant decrease was observed in tumour cells circulating in both low- and high-dose exercise arms. However, no exercise dose-response association was observed[149]. The mechanism underlying the effect of exercise against the circulating tumour cells is yet unknown.
In a recent study, it was observed that the central carbon metabolism was affected, and a significant decrease in phosphocreatine levels was observed in the tumour models that responded to exercise. This finding indicates a change in tumour energy metabolism after exercise in CRC. Furthermore, this study showed that the underlying mechanism of exercise benefit may be the modifications in tumour cell mitochondrial metabolism[150]. Aerobic exercise has been shown to be effective through various intra-tumoral and systemic mechanisms in cancer onset, progression and metastasis. Furthermore, exercise can activate different biological mechanisms at different intensities[151].
EXERCISE AND GUIDELINES
The ACS and ACSM have developed physical activity and dietary guidelines for patients with cancer. To recommend specific exercise programs, the patient’s comorbid conditions and exercise contraindications should be carefully evaluated through medical screening. In addition, the patient’s age, gender, type of cancer treatment and physical performance should be considered. Echocardiography should be performed in patients who have a history of cardiac disease or a cardiotoxic chemotherapy regimen. Patients with a history of severe smoking and suspected pulmonary dysfunction should undergo a respiratory function test[24]. If no contraindications (presence of widespread lytic bone metastases, severe thrombocytopenia, anaemia, fever or presence of active infection or safety issues) are present, it is advisable to offer moderate personalised aerobic exercise programs for most patients. Exercise programs vary according to the type, intensity and frequency of exercise[152]. For colon cancer survivors, supervised exercise is an appropriate program[153]. In patients with advanced cancer, the applicability of exercise programs is limited.
Initial intensity and duration of the exercise should be determined according to the functional capacity and comorbid status of the patient. Patients should be initially subjected to exercise with less intensity and duration; furthermore, it should be gradually increased based on patients’ medical conditions. For adult patients with cancer who have fatigue, the American Society of Clinical Oncology manual recommends 150 min/wk of moderate aerobic exercise (fast walking, cycling or stretching) and two or three power-boosting exercises (weight lifting)[154].
Recommendation of the 2012 ACS guideline: It is recommended to avoid inactivity and to return to normal daily activity immediately after diagnosis, aiming for at least 150 min/wk of moderate or 75 min/wk of vigorous aerobic exercise, including exercises that require strength at least 2 d/wk[36]. However, it is recommended that this amount must be increased to 225 min (45 min/d, 5 d/wk) in appropriate patients. All patients with cancer should be encouraged to participate in regular physical activity during their lifetime.
CONCLUSION
In recent years, various studies have been conducted regarding the effect of exercise on cancer prevention and clinical outcomes. Increasing observational and experimental evidence suggests that exercise can modify the biology of CRC. Based on the results of the study, lifestyle interventions that provide an improvement in diet and exercise are recommended as an effective method to prevent CRC and improve the negative effects of cancer and its treatment. However, the required exercise dose, type and intensity are yet unclear. The results of randomised prospective studies are expected to determine the optimal amount of exercise, type and intensity and develop the most appropriate exercise plan according to the requirements and comorbidities of the patients and eventually formulate more useful guidelines. In this review, we outlined the beneficial effects of exercise in the prevention and treatment of CRC and the potential biological mechanisms underlying these beneficial effects.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: February 26, 2019
First decision: March 15, 2019
Article in press: May 3, 2019
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Fogli L, Zhu Q S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
Contributor Information
Zeynep Oruç, Department of Medical Oncology, Mersin City Hospital, Mersin 33000, Turkey.
Muhammed Ali Kaplan, Department of Medical Oncology, Faculty of Medicine, Dicle University, Diyarbakır 21280, Turkey. drmalikaplan@hotmail.com.
References
- 1.World Cancer Research Fund International. Colorectal cancer statistics. Available from: http://wcrf.org/int/cancer-facts-figures/data-specific-cancers/colorectal-cancer-statistics.
- 2.World Cancer Research Fund. Colorectal Cancer 2011 Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011. Available from: https://free-ebooks.com/ebooks/colorectal-cancer-2011-report-food-nutrition-physical-activity-and-the-prevention-of-colorectal-cancer/
- 3.American Cancer Society. Cancer Statistics Center. Available from: https://cancerstatisticscenter.cancer.org/#!/
- 4.Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr Physiol. 2012;2:2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11 Suppl 2:S94–100. [PubMed] [Google Scholar]
- 9.Zhang ZF, Zeng ZS, Sarkis AS, Klimstra DS, Charytonowicz E, Pollack D, Vena J, Guillem J, Marshall JR, Cordon-Cardo C. Family history of cancer, body weight, and p53 nuclear overexpression in Duke's C colorectal cancer. Br J Cancer. 1995;71:888–893. doi: 10.1038/bjc.1995.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slattery ML, Anderson K, Curtin K, Ma K, Schaffer D, Edwards S, Samowitz W. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res. 2001;483:73–81. doi: 10.1016/s0027-5107(01)00228-7. [DOI] [PubMed] [Google Scholar]
- 11.Rezende LFM, Sá TH, Markozannes G, Rey-López JP, Lee IM, Tsilidis KK, Ioannidis JPA, Eluf-Neto J. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826–833. doi: 10.1136/bjsports-2017-098391. [DOI] [PubMed] [Google Scholar]
- 12.Kruk J, Czerniak U. Physical activity and its relation to cancer risk: updating the evidence. Asian Pac J Cancer Prev. 2013;14:3993–4003. doi: 10.7314/apjcp.2013.14.7.3993. [DOI] [PubMed] [Google Scholar]
- 13.Behrens G, Leitzmann MF. The association between physical activity and renal cancer: systematic review and meta-analysis. Br J Cancer. 2013;108:798–811. doi: 10.1038/bjc.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- 15.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez NF, Stierman B, Saab S, Mahajan D, Yeung H, Francois F. Physical activity reduces risk for colon polyps in a multiethnic colorectal cancer screening population. BMC Res Notes. 2012;5:312. doi: 10.1186/1756-0500-5-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baltgalvis KA, Berger FG, Peña MM, Davis JM, Carson JA. The interaction of a high-fat diet and regular moderate intensity exercise on intestinal polyp development in Apc Min/+ mice. Cancer Prev Res (Phila) 2009;2:641–649. doi: 10.1158/1940-6207.CAPR-09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao A, Connell CJ, Jacobs EJ, McCullough ML, Patel AV, Calle EE, Cokkinides VE, Thun MJ. Amount, type, and timing of recreational physical activity in relation to colon and rectal cancer in older adults: the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:2187–2195. [PubMed] [Google Scholar]
- 20.Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33:S530–550; discussion S609-610. doi: 10.1097/00005768-200106001-00025. [DOI] [PubMed] [Google Scholar]
- 21.Mahmood S, English DR, MacInnis RJ, Karahalios A, Owen N, Milne RL, Giles GG, Lynch BM. Domain-specific physical activity and the risk of colorectal cancer: results from the Melbourne Collaborative Cohort Study. BMC Cancer. 2018;18:1063. doi: 10.1186/s12885-018-4961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 23.Boyle T, Heyworth J, Bull F, McKerracher S, Platell C, Fritschi L. Timing and intensity of recreational physical activity and the risk of subsite-specific colorectal cancer. Cancer Causes Control. 2011;22:1647–1658. doi: 10.1007/s10552-011-9841-5. [DOI] [PubMed] [Google Scholar]
- 24.Halle M, Schoenberg MH. Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch Arztebl Int. 2009;106:722–727. doi: 10.3238/arztebl.2009.0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slattery ML, Edwards S, Curtin K, Ma K, Edwards R, Holubkov R, Schaffer D. Physical activity and colorectal cancer. Am J Epidemiol. 2003;158:214–224. doi: 10.1093/aje/kwg134. [DOI] [PubMed] [Google Scholar]
- 26.Colbert LH, Hartman TJ, Malila N, Limburg PJ, Pietinen P, Virtamo J, Taylor PR, Albanes D. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–268. [PubMed] [Google Scholar]
- 27.Harriss DJ, Atkinson G, Batterham A, George K, Cable NT, Reilly T, Haboubi N, Renehan AG Colorectal Cancer, Lifestyle, Exercise And Research Group. Lifestyle factors and colorectal cancer risk (2): a systematic review and meta-analysis of associations with leisure-time physical activity. Colorectal Dis. 2009;11:689–701. doi: 10.1111/j.1463-1318.2009.01767.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly SA, Zhao L, Jung KC, Hua K, Threadgill DW, Kim Y, de Villena FP, Pomp D. Prevention of tumorigenesis in mice by exercise is dependent on strain background and timing relative to carcinogen exposure. Sci Rep. 2017;7:43086. doi: 10.1038/srep43086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calton BA, Lacey JV, Jr, Schatzkin A, Schairer C, Colbert LH, Albanes D, Leitzmann MF. Physical activity and the risk of colon cancer among women: a prospective cohort study (United States) Int J Cancer. 2006;119:385–391. doi: 10.1002/ijc.21840. [DOI] [PubMed] [Google Scholar]
- 31.Wolin KY, Yan Y, Colditz GA. Physical activity and risk of colon adenoma: a meta-analysis. Br J Cancer. 2011;104:882–885. doi: 10.1038/sj.bjc.6606045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mai PL, Sullivan-Halley J, Ursin G, Stram DO, Deapen D, Villaluna D, Horn-Ross PL, Clarke CA, Reynolds P, Ross RK, West DW, Anton-Culver H, Ziogas A, Bernstein L. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2007;16:517–525. doi: 10.1158/1055-9965.EPI-06-0747. [DOI] [PubMed] [Google Scholar]
- 33.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 35.Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 2011;4:502–511. doi: 10.1158/1940-6207.CAPR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Rogers LQ, Alfano CM, Thomson CA, Courneya KS, Meyerhardt JA, Stout NL, Kvale E, Ganzer H, Ligibel JA. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65:167–189. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- 37.McGowan EL, Speed-Andrews AE, Rhodes RE, Blanchard CM, Culos-Reed SN, Friedenreich CM, Courneya KS. Sport participation in colorectal cancer survivors: an unexplored approach to promoting physical activity. Support Care Cancer. 2013;21:139–147. doi: 10.1007/s00520-012-1501-0. [DOI] [PubMed] [Google Scholar]
- 38.Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. J Clin Oncol. 2008;26:4480–4487. doi: 10.1200/JCO.2007.15.7917. [DOI] [PubMed] [Google Scholar]
- 39.Chung JY, Lee DH, Park JH, Lee MK, Kang DW, Min J, Kim DI, Jeong DH, Kim NK, Meyerhardt JA, Jones LW, Jeon JY. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer. 2013;21:1605–1612. doi: 10.1007/s00520-012-1703-5. [DOI] [PubMed] [Google Scholar]
- 40.Brown JC, Schmitz KH. The prescription or proscription of exercise in colorectal cancer care. Med Sci Sports Exerc. 2014;46:2202–2209. doi: 10.1249/MSS.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol Biomarkers Prev. 2016;25:1018–1028. doi: 10.1158/1055-9965.EPI-16-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedenreich CM, Shaw E, Neilson HK, Brenner DR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl) 2017;95:1029–1041. doi: 10.1007/s00109-017-1558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL American College of Sports Medicine. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 44.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, Miller P, Mitchell DC, Demark-Wahnefried W. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301:1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buffart LM, Thong MS, Schep G, Chinapaw MJ, Brug J, van de Poll-Franse LV. Self-reported physical activity: its correlates and relationship with health-related quality of life in a large cohort of colorectal cancer survivors. PLoS One. 2012;7:e36164. doi: 10.1371/journal.pone.0036164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeTroye A, Christner M, Eganhouse D, Manning B, Sunkin E, Gregory T. The effects of physical activity on survival in patients with colorectal cancer. JAAPA. 2018;31:21–25. doi: 10.1097/01.JAA.0000529767.60402.00. [DOI] [PubMed] [Google Scholar]
- 49.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 50.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 51.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 52.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 53.Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, Fuchs CS. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169:2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, Lane DS, Wactawski-Wende J, Hou L, Jackson RD, Kampman E, Newcomb PA. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer Causes Control. 2012;23:1939–1948. doi: 10.1007/s10552-012-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133:1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 56.Wu W, Guo F, Ye J, Li Y, Shi D, Fang D, Guo J, Li L. Pre- and post-diagnosis physical activity is associated with survival benefits of colorectal cancer patients: a systematic review and meta-analysis. Oncotarget. 2016;7:52095–52103. doi: 10.18632/oncotarget.10603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton MK. Higher levels of physical activity significantly increase survival in women with colorectal cancer. CA Cancer J Clin. 2013;63:83–84. doi: 10.3322/caac.21175. [DOI] [PubMed] [Google Scholar]
- 58.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res. 2016;22:4766–4775. doi: 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 59.Meyerhardt JA, Ogino S, Kirkner GJ, Chan AT, Wolpin B, Ng K, Nosho K, Shima K, Giovannucci EL, Loda M, Fuchs CS. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15:5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morikawa T, Kuchiba A, Yamauchi M, Meyerhardt JA, Shima K, Nosho K, Chan AT, Giovannucci E, Fuchs CS, Ogino S. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305:1685–1694. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamauchi M, Lochhead P, Imamura Y, Kuchiba A, Liao X, Qian ZR, Nishihara R, Morikawa T, Shima K, Wu K, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT, Ogino S. Physical activity, tumor PTGS2 expression, and survival in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1142–1152. doi: 10.1158/1055-9965.EPI-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanyuda A, Kim SA, Martinez-Fernandez A, Qian ZR, Yamauchi M, Nishihara R, Morikawa T, Liao X, Inamura K, Mima K, Cao Y, Zhang X, Wu K, Chan AT, Giovannucci EL, Meyerhardt JA, Fuchs CS, Shivdasani RA, Ogino S. Survival Benefit of Exercise Differs by Tumor IRS1 Expression Status in Colorectal Cancer. Ann Surg Oncol. 2016;23:908–917. doi: 10.1245/s10434-015-4967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardikar S, Newcomb PA, Campbell PT, Win AK, Lindor NM, Buchanan DD, Makar KW, Jenkins MA, Potter JD, Phipps AI. Prediagnostic Physical Activity and Colorectal Cancer Survival: Overall and Stratified by Tumor Characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24:1130–1137. doi: 10.1158/1055-9965.EPI-15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okugawa Y, Miki C, Toiyama Y, Koike Y, Inoue Y, Kusunoki M. Serum level of soluble vascular cell adhesion molecule 1 is a valuable prognostic marker in colorectal carcinoma. Dis Colon Rectum. 2009;52:1330–1336. doi: 10.1007/DCR.0b013e3181a0d144. [DOI] [PubMed] [Google Scholar]
- 65.Toiyama Y, Miki C, Inoue Y, Okugawa Y, Koike Y, Yokoe T, Tanaka K, Kusunoki M. Soluble intercellular adhesion molecule-1 as a prognostic marker for stage II colorectal cancer patients. Ann Surg Oncol. 2008;15:1617–1624. doi: 10.1245/s10434-008-9874-5. [DOI] [PubMed] [Google Scholar]
- 66.Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH. Dose-response Effects of Aerobic Exercise Among Colon Cancer Survivors: A Randomized Phase II Trial. Clin Colorectal Cancer. 2018;17:32–40. doi: 10.1016/j.clcc.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Courneya KS, Vardy JL, O'Callaghan CJ, Friedenreich CM, Campbell KL, Prapavessis H, Crawford JJ, O'Brien P, Dhillon HM, Jonker DJ, Chua NS, Lupichuk S, Sanatani MS, Gill S, Meyer RM, Begbie S, Bonaventura T, Burge ME, Turner J, Tu D, Booth CM. Effects of a Structured Exercise Program on Physical Activity and Fitness in Colon Cancer Survivors: One Year Feasibility Results from the CHALLENGE Trial. Cancer Epidemiol Biomarkers Prev. 2016;25:969–977. doi: 10.1158/1055-9965.EPI-15-1267. [DOI] [PubMed] [Google Scholar]
- 68.Ahn KY, Hur H, Kim DH, Min J, Jeong DH, Chu SH, Lee JW, Ligibel JA, Meyerhardt JA, Jones LW, Jeon JY, Kim NK. The effects of inpatient exercise therapy on the length of hospital stay in stages I-III colon cancer patients: randomized controlled trial. Int J Colorectal Dis. 2013;28:643–651. doi: 10.1007/s00384-013-1665-1. [DOI] [PubMed] [Google Scholar]
- 69.Boereboom C, Doleman B, Lund JN, Williams JP. Systematic review of pre-operative exercise in colorectal cancer patients. Tech Coloproctol. 2016;20:81–89. doi: 10.1007/s10151-015-1407-1. [DOI] [PubMed] [Google Scholar]
- 70.Onerup A, Angenete E, Bock D, Börjesson M, Fagevik Olsén M, Grybäck Gillheimer E, Skullman S, Thörn SE, Haglind E, Nilsson H. The effect of pre- and post-operative physical activity on recovery after colorectal cancer surgery (PHYSSURG-C): study protocol for a randomised controlled trial. Trials. 2017;18:212. doi: 10.1186/s13063-017-1949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, Travier N, Van Den Buijs BJ, Backx FJ, Los M, Erdkamp FL, Bloemendal HJ, Koopman M, De Roos MA, Verhaar MJ, Ten Bokkel-Huinink D, Van Der Wall E, Peeters PH, May AM. Effects of an Exercise Program in Colon Cancer Patients undergoing Chemotherapy. Med Sci Sports Exerc. 2016;48:767–775. doi: 10.1249/MSS.0000000000000855. [DOI] [PubMed] [Google Scholar]
- 72.Morielli AR, Usmani N, Boulé NG, Tankel K, Severin D, Nijjar T, Joseph K, Courneya KS. A Phase I Study Examining the Feasibility and Safety of an Aerobic Exercise Intervention in Patients With Rectal Cancer During and After Neoadjuvant Chemoradiotherapy. Oncol Nurs Forum. 2016;43:352–362. doi: 10.1188/16.ONF.352-362. [DOI] [PubMed] [Google Scholar]
- 73.Morielli AR, Usmani N, Boulé NG, Severin D, Tankel K, Nijjar T, Joseph K, Fairchild A, Courneya KS. Exercise during and after neoadjuvant rectal cancer treatment (the EXERT trial): study protocol for a randomized controlled trial. Trials. 2018;19:35. doi: 10.1186/s13063-017-2398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Otto SJ, Korfage IJ, Polinder S, van der Heide A, de Vries E, Rietjens JA, Soerjomataram I. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2015;23:1237–1250. doi: 10.1007/s00520-014-2480-0. [DOI] [PubMed] [Google Scholar]
- 75.Andersen C, Adamsen L, Moeller T, Midtgaard J, Quist M, Tveteraas A, Rorth M. The effect of a multidimensional exercise programme on symptoms and side-effects in cancer patients undergoing chemotherapy--the use of semi-structured diaries. Eur J Oncol Nurs. 2006;10:247–262. doi: 10.1016/j.ejon.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee MK, Kim NK, Jeon JY. Effect of the 6-week home-based exercise program on physical activity level and physical fitness in colorectal cancer survivors: A randomized controlled pilot study. PLoS One. 2018;13:e0196220. doi: 10.1371/journal.pone.0196220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 79.Soares-Miranda L, Abreu S, Silva M, Peixoto A, Ramalho R, da Silva PC, Costa C, Teixeira JP, Gonçalves C, Moreira P, Mota J, Macedo G. Cancer Survivor Study (CASUS) on colorectal patients: longitudinal study on physical activity, fitness, nutrition, and its influences on quality of life, disease recurrence, and survival. Rationale and design. Int J Colorectal Dis. 2017;32:75–81. doi: 10.1007/s00384-016-2671-x. [DOI] [PubMed] [Google Scholar]
- 80.Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, Basford JR. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811–821. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walter V, Jansen L, Knebel P, Chang-Claude J, Hoffmeister M, Brenner H. Physical activity and survival of colorectal cancer patients: Population-based study from Germany. Int J Cancer. 2017;140:1985–1997. doi: 10.1002/ijc.30619. [DOI] [PubMed] [Google Scholar]
- 82.Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109:814–822. doi: 10.1038/bjc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeon J, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Wigler DS, Atienza D, Messino M, Kindler H, Venook A, Fuchs CS, Meyerhardt JA. Impact of physical activity after cancer diagnosis on survival in patients with recurrent colon cancer: Findings from CALGB 89803/Alliance. Clin Colorectal Cancer. 2013;12:233–238. doi: 10.1016/j.clcc.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang DW, Chung JY, Lee MK, Lee J, Park JH, Kim DI, Jones LW, Ahn JB, Kim NK, Jeon JY. Exercise barriers in Korean colorectal cancer patients. Asian Pac J Cancer Prev. 2014;15:7539–7545. doi: 10.7314/apjcp.2014.15.18.7539. [DOI] [PubMed] [Google Scholar]
- 85.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91:822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Packel LB, Prehn AW, Anderson CL, Fisher PL. Factors influencing physical activity behaviors in colorectal cancer survivors. Am J Health Promot. 2015;30:85–92. doi: 10.4278/ajhp.140103-QUAN-7. [DOI] [PubMed] [Google Scholar]
- 87.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22:54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 88.Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, Lee JW, Chu SH, Meyerhardt JA, Ligibel J, Jones LW, Kim NK, Jeon JY. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21:2537–2545. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- 89.Courneya KS, Friedenreich CM, Quinney HA, Fields AL, Jones LW, Fairey AS. A randomized trial of exercise and quality of life in colorectal cancer survivors. Eur J Cancer Care (Engl) 2003;12:347–357. doi: 10.1046/j.1365-2354.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 90.Park JH, Lee J, Oh M, Park H, Chae J, Kim DI, Lee MK, Yoon YJ, Lee CW, Park S, Jones LW, Kim NK, Kim SI, Jeon JY. The effect of oncologists' exercise recommendations on the level of exercise and quality of life in survivors of breast and colorectal cancer: A randomized controlled trial. Cancer. 2015;121:2740–2748. doi: 10.1002/cncr.29400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rajarajeswaran P, Vishnupriya R. Exercise in cancer. Indian J Med Paediatr Oncol. 2009;30:61–70. doi: 10.4103/0971-5851.60050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Na HK, Oliynyk S. Effects of physical activity on cancer prevention. Ann N Y Acad Sci. 2011;1229:176–183. doi: 10.1111/j.1749-6632.2011.06105.x. [DOI] [PubMed] [Google Scholar]
- 93.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 94.Chi F, Wu R, Zeng YC, Xing R, Liu Y. Circulation insulin-like growth factor peptides and colorectal cancer risk: an updated systematic review and meta-analysis. Mol Biol Rep. 2013;40:3583–3590. doi: 10.1007/s11033-012-2432-z. [DOI] [PubMed] [Google Scholar]
- 95.Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev. 1996;5:1013–1015. [PubMed] [Google Scholar]
- 96.MacDonald RS, Thornton WH, Jr, Bean TL. Insulin and IGE-1 receptors in a human intestinal adenocarcinoma cell line (CACO-2): regulation of Na+ glucose transport across the brush border. J Recept Res. 1993;13:1093–1113. doi: 10.3109/10799899309063266. [DOI] [PubMed] [Google Scholar]
- 97.Corpet DE, Jacquinet C, Peiffer G, Taché S. Insulin injections promote the growth of aberrant crypt foci in the colon of rats. Nutr Cancer. 1997;27:316–320. doi: 10.1080/01635589709514543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Limburg PJ, Stolzenberg-Solomon RZ, Vierkant RA, Roberts K, Sellers TA, Taylor PR, Virtamo J, Cerhan JR, Albanes D. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin Gastroenterol Hepatol. 2006;4:1514–1521. doi: 10.1016/j.cgh.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–1154. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 100.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 101.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 102.Devin JL, Bolam KA, Jenkins DG, Skinner TL. The Influence of Exercise on the Insulin-like Growth Factor Axis in Oncology: Physiological Basis, Current, and Future Perspectives. Cancer Epidemiol Biomarkers Prev. 2016;25:239–249. doi: 10.1158/1055-9965.EPI-15-0406. [DOI] [PubMed] [Google Scholar]
- 103.Sax AT, Jenkins DG, Devin JL, Hughes GI, Bolam KA, Skinner TL. The insulin-like growth factor axis: A biological mechanism linking physical activity to colorectal cancer survival. Cancer Epidemiol. 2014;38:455–459. doi: 10.1016/j.canep.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 104.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol (1985) 2005;98:1534–1540. doi: 10.1152/japplphysiol.00566.2004. [DOI] [PubMed] [Google Scholar]
- 106.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol (1985) 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 107.Oshima M, Taketo MM. COX selectivity and animal models for colon cancer. Curr Pharm Des. 2002;8:1021–1034. doi: 10.2174/1381612023394953. [DOI] [PubMed] [Google Scholar]
- 108.Guffey CR, Fan D, Singh UP, Murphy EA. Linking obesity to colorectal cancer: recent insights into plausible biological mechanisms. Curr Opin Clin Nutr Metab Care. 2013;16:595–600. doi: 10.1097/MCO.0b013e328362d10b. [DOI] [PubMed] [Google Scholar]
- 109.Murphy EA, Enos RT, Velázquez KT. Influence of Exercise on Inflammation in Cancer: Direct Effect or Innocent Bystander? Exerc Sport Sci Rev. 2015;43:134–142. doi: 10.1249/JES.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 110.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 111.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 112.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30 Suppl:S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–816. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, Yoshikawa T. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 115.Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology. 2010;215:242–249. doi: 10.1016/j.imbio.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Kang JC, Chen JS, Lee CH, Chang JJ, Shieh YS. Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. J Surg Oncol. 2010;102:242–248. doi: 10.1002/jso.21617. [DOI] [PubMed] [Google Scholar]
- 117.McClellan JL, Steiner JL, Day SD, Enos RT, Davis MJ, Singh UP, Murphy EA. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int J Oncol. 2014;45:861–868. doi: 10.3892/ijo.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawanishi N, Yano H, Mizokami T, Takahashi M, Oyanagi E, Suzuki K. Exercise training attenuates hepatic inflammation, fibrosis and macrophage infiltration during diet induced-obesity in mice. Brain Behav Immun. 2012;26:931–941. doi: 10.1016/j.bbi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 119.Rogers CJ, Zaharoff DA, Hance KW, Perkins SN, Hursting SD, Schlom J, Greiner JW. Exercise enhances vaccine-induced antigen-specific T cell responses. Vaccine. 2008;26:5407–5415. doi: 10.1016/j.vaccine.2008.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11:404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 121.Woods JA, Davis JM. Exercise, monocyte/macrophage function, and cancer. Med Sci Sports Exerc. 1994;26:147–156. doi: 10.1249/00005768-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Shimizu K, Suzuki N, Imai T, Aizawa K, Nanba H, Hanaoka Y, Kuno S, Mesaki N, Kono I, Akama T. Monocyte and T-cell responses to exercise training in elderly subjects. J Strength Cond Res. 2011;25:2565–2572. doi: 10.1519/JSC.0b013e3181fc5e67. [DOI] [PubMed] [Google Scholar]
- 123.Goh J, Ladiges WC. Exercise enhances wound healing and prevents cancer progression during aging by targeting macrophage polarity. Mech Ageing Dev. 2014;139:41–48. doi: 10.1016/j.mad.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 124.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382–396. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 125.Song M, Chan AT. The Potential Role of Exercise and Nutrition in Harnessing the Immune System to Improve Colorectal Cancer Survival. Gastroenterology. 2018;155:596–600. doi: 10.1053/j.gastro.2018.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jahani-Sherafat S, Alebouyeh M, Moghim S, Ahmadi Amoli H, Ghasemian-Safaei H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol Hepatol Bed Bench. 2018;11:101–109. [PMC free article] [PubMed] [Google Scholar]
- 127.Oettlé GJ. Effect of moderate exercise on bowel habit. Gut. 1991;32:941–944. doi: 10.1136/gut.32.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Souza-Teixeira F, Alonso-Molero J, Ayán C, Vilorio-Marques L, Molina AJ, González-Donquiles C, Dávila-Batista V, Fernández-Villa T, de Paz JA, Martín V. PGC-1α as a Biomarker of Physical Activity-Protective Effect on Colorectal Cancer. Cancer Prev Res (Phila) 2018;11:523–534. doi: 10.1158/1940-6207.CAPR-17-0329. [DOI] [PubMed] [Google Scholar]
- 129.Zhu Z, Jiang W, Thompson HJ. Effect of corticosterone administration on mammary gland development and p27 expression and their relationship to the effects of energy restriction on mammary carcinogenesis. Carcinogenesis. 1998;19:2101–2106. doi: 10.1093/carcin/19.12.2101. [DOI] [PubMed] [Google Scholar]
- 130.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 131.Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, Yang CS. Voluntary exercise inhibits intestinal tumorigenesis in Apc(Min/+) mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer. 2008;8:316. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiarotto JA, Akbarali R, Bellotti L, Dranitsaris G. A structured group exercise program for patients with metastatic cancer receiving chemotherapy and CTNNB1 (β-catenin) as a biomarker of exercise efficacy. Cancer Manag Res. 2017;9:495–501. doi: 10.2147/CMAR.S147054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morikawa T, Kuchiba A, Lochhead P, Nishihara R, Yamauchi M, Imamura Y, Liao X, Qian ZR, Ng K, Chan AT, Meyerhardt JA, Giovannucci E, Fuchs CS, Ogino S. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with β-catenin (CTNNB1) status. Cancer Res. 2013;73:1600–1610. doi: 10.1158/0008-5472.CAN-12-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Demarzo MM, Martins LV, Fernandes CR, Herrero FA, Perez SE, Turatti A, Garcia SB. Exercise reduces inflammation and cell proliferation in rat colon carcinogenesis. Med Sci Sports Exerc. 2008;40:618–621. doi: 10.1249/MSS.0b013e318163274d. [DOI] [PubMed] [Google Scholar]
- 135.Grimm M, Lazariotou M, Kircher S, Höfelmayr A, Germer CT, von Rahden BH, Waaga-Gasser AM, Gasser M. Tumor necrosis factor-α is associated with positive lymph node status in patients with recurrence of colorectal cancer-indications for anti-TNF-α agents in cancer treatment. Cell Oncol (Dordr) 2011;34:315–326. doi: 10.1007/s13402-011-0027-7. [DOI] [PubMed] [Google Scholar]
- 136.Jeon JY, Jeong DH, Park MG, Lee JW, Chu SH, Park JH, Lee MK, Sato K, Ligibel JA, Meyerhardt JA, Kim NK. Impact of diabetes on oncologic outcome of colorectal cancer patients: colon vs. rectal cancer. PLoS One. 2013;8:e55196. doi: 10.1371/journal.pone.0055196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wolpin BM, Meyerhardt JA, Chan AT, Ng K, Chan JA, Wu K, Pollak MN, Giovannucci EL, Fuchs CS. Insulin, the insulin-like growth factor axis, and mortality in patients with nonmetastatic colorectal cancer. J Clin Oncol. 2009;27:176–185. doi: 10.1200/JCO.2008.17.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Durai R, Yang W, Gupta S, Seifalian AM, Winslet MC. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20:203–220. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- 139.Amemori S, Ootani A, Aoki S, Fujise T, Shimoda R, Kakimoto T, Shiraishi R, Sakata Y, Tsunada S, Iwakiri R, Fujimoto K. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol. 2007;292:G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- 140.Kim ES, Im JA, Kim KC, Park JH, Suh SH, Kang ES, Kim SH, Jekal Y, Lee CW, Yoon YJ, Lee HC, Jeon JY. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity (Silver Spring) 2007;15:3023–3030. doi: 10.1038/oby.2007.360. [DOI] [PubMed] [Google Scholar]
- 141.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 142.Waseem T, Javaid-Ur-Rehman, Ahmad F, Azam M, Qureshi MA. Role of ghrelin axis in colorectal cancer: a novel association. Peptides. 2008;29:1369–1376. doi: 10.1016/j.peptides.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 143.Foster-Schubert KE, McTiernan A, Frayo RS, Schwartz RS, Rajan KB, Yasui Y, Tworoger SS, Cummings DE. Human plasma ghrelin levels increase during a one-year exercise program. J Clin Endocrinol Metab. 2005;90:820–825. doi: 10.1210/jc.2004-2081. [DOI] [PubMed] [Google Scholar]
- 144.Pérusse L, Collier G, Gagnon J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Nadeau A, Zimmet PZ, Bouchard C. Acute and chronic effects of exercise on leptin levels in humans. J Appl Physiol (1985) 1997;83:5–10. doi: 10.1152/jappl.1997.83.1.5. [DOI] [PubMed] [Google Scholar]
- 145.Sobhani I, Zeitoun JD, Chanteloup E, Charachon A, Levy M, Delchier JC. Obesity and Cancer: yin/yan effects of nutrition. Open Obes J. 2010;2:38–42. [Google Scholar]
- 146.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–173. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 147.Slattery ML, Herrick JS, Mullany LE, Stevens JR, Wolff RK. Diet and lifestyle factors associated with miRNA expression in colorectal tissue. Pharmgenomics Pers Med. 2016;10:1–16. doi: 10.2147/PGPM.S117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bork U, Rahbari NN, Schölch S, Reissfelder C, Kahlert C, Büchler MW, Weitz J, Koch M. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer. 2015;112:1306–1313. doi: 10.1038/bjc.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brown JC, Rhim AD, Manning SL, Brennan L, Mansour AI, Rustgi AK, Damjanov N, Troxel AB, Rickels MR, Ky B, Zemel BS, Courneya KS, Schmitz KH. Effects of exercise on circulating tumor cells among patients with resected stage I-III colon cancer. PLoS One. 2018;13:e0204875. doi: 10.1371/journal.pone.0204875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lu M, Sanderson SM, Zessin A, Ashcraft KA, Jones LW, Dewhirst MW, Locasale JW, Hsu DS. Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab. 2018;6:14. doi: 10.1186/s40170-018-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016;76:4032–4050. doi: 10.1158/0008-5472.CAN-16-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thompson WR, Gordon NF, Pescatello LS. 2010. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed. Lippincott Williams and Wilkins; p. 380. [Google Scholar]
- 153.Sellar CM, Bell GJ, Haennel RG, Au HJ, Chua N, Courneya KS. Feasibility and efficacy of a 12-week supervised exercise intervention for colorectal cancer survivors. Appl Physiol Nutr Metab. 2014;39:715–723. doi: 10.1139/apnm-2013-0367. [DOI] [PubMed] [Google Scholar]
- 154.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB American Society of Clinical Oncology. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]