Abstract
BACKGROUND
Preoperative radiochemotherapy is widely used in locally advanced rectal cancer. It can improve local control of rectal cancer. However, some researchers believe it increases the incidence of surgical complications. They doubt its safety. Patients with locally advanced rectal cancer receive three different treatments in our hospital, including long-course radiochemotherapy, short-course radiotherapy, and surgery directly. We can compare their differences in postoperative complications.
AIM
To investigate surgical complications caused by different preoperative radiotherapy regimens.
METHODS
We retrospectively analyzed 1197 patients admitted between 2008 and 2010 with locally advanced rectal cancer. Three hundred and forty-six patients were treated with preoperative long-course radiochemotherapy (25 × 2 Gy) followed by total mesorectal excision (TME) 6–8 wk later, and 259 patients received short-course radiotherapy (10 × 3 Gy) and subsequently TME 7–10 d later. The remaining 592 patients underwent TME alone without neoadjuvant therapy. According to Clavien–Dindo classification, surgical complications were evaluated for up to 30 d after discharge from hospital.
RESULTS
There were no deaths in 30 d in all groups after treatment. The major complications were anastomotic leakage and perineal wound complications. The results suggested that both long-course [odds ratio (OR) = 3.624, 95% confidence interval (CI): 1.689–7.775, P = 0.001] and short-course (OR = 5.150, 95%CI: 1.828–14.515, P = 0.002) radiotherapy were associated with anastomotic leakage. Temporary ileostomy was a protective factor for anastomotic leakage (OR = 6.211, 95%CI: 2.525–15.385, P < 0.001). The severity of anastomotic leakage did not increase in patients following preoperative radiotherapy (P = 0.411). Compared with TME alone, short-course radiotherapy was associated with an increase in perineal wound complications (OR = 5.565, 95%CI: 2.203–14.057, P < 0.001), but long-course radiotherapy seemed safe regarding this complication (OR = 1.692, 95%CI: 0.651–4.394, P = 0.280). Although the severity of perineal wound complications increased in patients following short-course radiotherapy (P < 0.001), additional intervention was not necessary.
CONCLUSION
Radiotherapy increased the incidence but not severity of anastomotic leakage. Short-course radiotherapy was also accompanied with perineal wound complications, but intervention appeared unnecessary to ameliorate the complications.
Keywords: Rectal cancer, Radiotherapy, Surgical complications, Total mesorectal excision, Anastomotic leakage
Core tip: Preoperative radiotherapy is a promising treatment for rectal cancer. Our aim is to investigate surgical complications caused by radiotherapy. Both long-course and short-course radiotherapy increased the incidence of anastomotic leakage but did not affect the severity. Additional ileostomy was an effective method to reduce the risk of anastomotic leakage. Short-course radiotherapy was accompanied with increased incidence of perineal wound complications, but intervention appeared unnecessary to ameliorate the complications.
INTRODUCTION
Patients with locally advanced rectal cancer were recommended to receive neoadju-vant radiochemotherapy, especially for those with positive circumferential resection margin or extensive nodal involvement. It can improve local control for these patients. The rate of local recurrence has decreased significantly as a result of neoadjuvant radiochemotherapy. Some researchers believe that preoperative radiotherapy can improve survival of patients with resectable rectal cancer. It is suggested that patients who are sensitive to radiotherapy can achieve better prognosis. Approximately 15% of patients can achieve complete response after long-course neoadjuvant radioche-motherapy[1]. Different protocols for short-course radiotherapy that consists of 30 Gy in 10 fractions are recommended by the Chinese Anti-Cancer Association[2]. The biological equivalent dose of the short-course radiotherapy is similar to the commonly used regimen (5 × 5 Gy). Although the tumor regression is not as good as with long-course radiochemotherapy, with < 5% complete response rate, the advantages are no surgical delay, reduced toxicity from capecitabine, and avoidance of overtreatment of non-responders. However, some surgeons believe that preoperative radioche-motherapy increases surgical complications. Anastomotic leakage is thought to be associated with malnutrition resulting from radiotherapy[3]. Perineal wound complications after abdominoperineal resection (APR) are also considered to be associated with tissue edema and infection caused by radiotherapy. In addition, toxicity of radiochemotherapy may decrease patients’ tolerance to surgery.
The aim of this study was to evaluate surgical complications of patients with locally advanced rectal cancer following different neoadjuvant therapy and radical surgery. We compared the incidence and severity of surgical complications at 30 d after sur-gery in different groups and the contribution of neoadjuvant therapy to surgical complications.
MATERIALS AND METHODS
Patients
We performed a retrospective consecutive study of 1197 patients with mid-to-low rectal cancer (≤ 10 cm from anal verge) who received low anterior resection and APR at the Peking University Cancer Hospital between 2008 and 2010. Among them, 346 patients were treated with long-course chemoradiotherapy, and 259 received short-course radiotherapy. Radical resection was performed in all patients. The remaining 592 patients received total mesorectal excision (TME) immediately after rectal cancer was diagnosed. Surgical complications were evaluated for up to 30 d after discharge from hospital according to Clavien–Dindo classification. The median duration of admission for patients who underwent resection was 19 (range 5–81) d. Among them, 197 patients (16.3%) were hospitalized for > 30 d.
Radiotherapy
Two different neoadjuvant radiotherapy regimens were applied. Three hundred and forty-six patients received long-course preoperative radiochemotherapy that consisted of 50 Gy in 25 fractions with capecitabine (825 mg/m2, twice daily) as radiosensitizer. The other 259 patients were treated with short-course radiotherapy that consisted of 30 Gy in 10 fractions. Its biological equivalent dose was 36 Gy, which was close to the dose of 5 × 5 Gy radiation (37.5 Gy).
Surgery
TME was the standard approach for surgical treatment of rectal cancer. All patients underwent laparotomy at Beijing Cancer Hospital at 6–8 wk after long-course radiochemotherapy or 7–10 d after short-course radiotherapy. Low anterior resection (LAR) was performed in 894 patients. Temporary ileostomy was performed based on the pathological conditions during the operation. APR was performed in 303 patients.
Surgical complications
Surgical complications were evaluated using predetermined conditions of common complications (Table 1). The main complications are anastomotic leakage and perineal wound complications. The definition of anastomotic leakage was different from those in the literature. It was confirmed by detection of fluid collection through the drainage tubes. Digital rectal examinations were used to evaluate the size of the leakage. Computed tomography was not routinely performed unless puncture drainage or surgical reintervention was needed. The severity of these complications was evaluated by Clavien–Dindo classification (Table 2).
Table 1.
Definition of postoperative surgical complications (during admission and 30 d thereafter)
| Definition | |
| Anastomotic leakage | Any gas or feces collection around the anastomosis after low anterior resection in the drainage tubes; clinical suspicion confirmed by surgery |
| Perineal wound complications | Perineal wound dehiscence and wound necrosis after abdominoperineal resection resulting from infection |
| Ileus | Absence of bowel sounds or defecation after 5 d following surgery |
| Bleeding | Gastrointestinal or abdominal hemorrhage, decrease in hemoglobin level directly after surgery treated conservatively with blood transfusion or by reintervention |
| Intra-abdominal abscess | Any intra-abdominal fluid collection unrelated to the anastomosis or perineal wound |
| Abdominal wound complications | Fascial dehiscence, superficial wound infection |
| Urological complications | Ureter leakage, urinary incontinence, ureter stenosis |
| Intestinal necrosis | Caused by bowel ischemia |
| Gastrointestinal perforation | Intestinal contents discharge from abdominal cavity; clinical suspicion confirmed by surgery |
| Intravenous line infection | Fever, chills and increase in leukocyte count, excluding other infections; the symptoms disappear after removing the intravenous line |
| Stoma complications | Stoma necrosis, stoma infection, parastomal hernia |
| General complications | Cardiovascular, pulmonary, neurological events |
Table 2.
Clavien–Dindo classification of surgical complications
| Grade | Definition |
| I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiologic interventions. Allowed therapeutic regimens are drugs including antiemetics, antipyretics, analgesics and diuretics, and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside. |
| II | Requiring pharmacological treatment with drugs other than those allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included. |
| III | Requiring surgical, endoscopic or radiological intervention |
| IIIa | Intervention not under general anesthesia |
| IIIb | Intervention under general anesthesia |
| IV | Life-threatening complication (including CNS complications)1 requiring IC/ICU management |
| IVa | Single organ dysfunction (including dialysis) |
| IVb | Multiple organ dysfunction |
| V | Death as a result of complications |
Brain hemorrhage, ischemic stroke, or subarachnoid bleeding, but excluding transient ischemic attacks. Adapted from Clavien–Dindo classification. CNS: Central nervous system; IC: Intermediate care; ICU: Intensive care unit.
Statistical analysis
The association between neoadjuvant radiotherapy and surgical complications was analyzed using two-sided χ2 or Fisher’s exact test. The two key complications, anastomotic leakage and perineal wound complications, were also evaluated. The clinical variables included general information about the patients and tumor characteristics, as well treatment-related variables such as diverting ileostomy. Logistic regression was performed to investigate the independent factors associated with anastomotic leakage and perineal would complications. P < 0.05 was considered as statistically significant.
RESULTS
Groups and patient characteristics
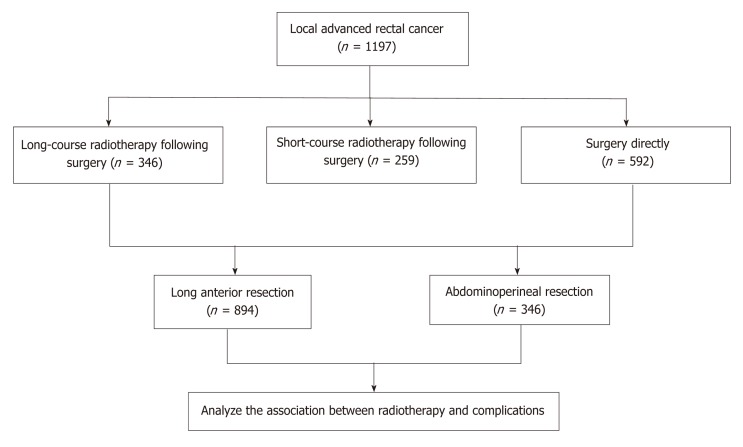
A total of 1197 patients with locally advanced rectal cancer who received LAR and APR were analyzed. They all underwent laparotomy. The patients were divided into three groups according to different preoperative therapy (Figure 1). Group 1: 346 patients treated with preoperative long-course chemoradiotherapy followed by TME 6–8 wk after. Group 2: 259 patients were treated with short-course radiotherapy (10 × 3 Gy) followed by TME 7–10 d after. Group 3: 592 patients received radical surgery only. Patient and tumor characteristics are summarized in Table 3. The median duration of admission for patients who underwent resection was 19 (range 5–81) d. One hundred and ninety-seven patients (16.3%) stayed in the hospital for > 30 d.
Figure 1.
Flow diagram of treatment.
Table 3.
Patient and treatment characteristics
| Characteristics | No. of patients |
| Age in yr | 59 (21–88) |
| Sex ratio, male: female | 721: 485 |
| Zubrod-ECOG-WHO | |
| 0 | 1060 |
| 1 | 106 |
| 2 | 40 |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| Preoperative treatment | |
| 25 × 2 Gy with capecitabine | 346 |
| 10 × 3 Gy | 259 |
| None | 592 |
| Distance from anal verge | |
| ≤ 5 cm | 817 |
| 5–10 cm | 389 |
| Surgery | |
| LAR | 894 |
| APR | 303 |
| Hartmann procedure | 27 |
| No resection | 7 |
| Diverting stoma after LAR | |
| Yes | 313 |
| No | 581 |
| Pathological TNM classification | |
| pT0 | 70 |
| pT1 | 59 |
| pT2 | 326 |
| pT3 | 661 |
| pT4 | 81 |
| pN0 | 687 |
| pN1 | 284 |
| pN2 | 226 |
ECOG-WHO: Eastern Cooperative Oncology Group-World Health Organization; LAR: Low anterior resection; APR: Abdominoperineal resection.
Treatment-related postoperative complications
Eight hundred and ninety-four patients underwent LAR, and 303 patients received APR. There were no deaths within 30 d after surgery. Forty-three patients required surgical reintervention. We analyzed 12 different complications, including anastomotic leakage, perineal wound complications, ileus, bleeding, intra-abdominal abscess, abdominal wound complications, urological complications, intestinal necrosis, gastrointestinal perforation, intravenous line infection, stoma complications, and general complications. Anastomotic leakage and perineal wound complications were the two major complications after resection. The severity of postoperative complications is summarized in Table 4. There were no significant differences in the grade of treatment-related complications except for perineal wound complications. Higher grade of perineal wound complication was observed in patients following short-course radiotherapy.
Table 4.
Postoperative complications (events during admission and 30 d thereafter)
| Complications | Treatment group | No. of patients | Total | Grade 1 | Grade 2 | Grade 3a | Grade 3b | Grade 4a | Grade 4b | Grade 5 | P value |
| Anastomotic leakage (LAR) | 1 | 236 | 16 | 10 | 6 | 0.411 | |||||
| 2 | 151 | 9 | 3 | 6 | |||||||
| 3 | 507 | 23 | 16 | 7 | |||||||
| Perineal wound complications (APR) | 1 | 110 | 13 | 9 | 3 | 1 | < 0.001 | ||||
| 2 | 108 | 29 | 14 | 13 | 2 | ||||||
| 3 | 85 | 8 | 4 | 3 | 1 | ||||||
| Ileus | 1 | 346 | 11 | 2 | 5 | 4 | 0.069 | ||||
| 2 | 259 | 14 | 13 | 1 | |||||||
| 3 | 592 | 14 | 2 | 12 | |||||||
| Bleeding | 1 | 346 | 10 | 1 | 8 | 1 | 0.485 | ||||
| 2 | 259 | 10 | 2 | 7 | |||||||
| 3 | 592 | 14 | 2 | 10 | 2 | ||||||
| Intra-abdominal abscess | 1 | 346 | 12 | 2 | 5 | 4 | 1 | 0.932 | |||
| 2 | 259 | 10 | 5 | 2 | 3 | ||||||
| 3 | 592 | 20 | 3 | 8 | 6 | 3 | |||||
| Abdominal wound complications | 1 | 346 | 10 | 7 | 2 | 1 | 0.474 | ||||
| 2 | 259 | 10 | 6 | 4 | |||||||
| 3 | 592 | 14 | 11 | 2 | 1 | ||||||
| Urological complications | 1 | 346 | 17 | 10 | 4 | 3 | 0.154 | ||||
| 2 | 259 | 16 | 5 | 8 | 3 | ||||||
| 3 | 592 | 20 | 12 | 6 | 2 | ||||||
| Intestinal necrosis | 1 | 346 | 2 | 2 | 0.689 | ||||||
| 2 | 259 | 3 | 2 | 1 | |||||||
| 3 | 592 | 4 | 4 | ||||||||
| Gastrointestinal perforation | 1 | 346 | 0 | 1.000 | |||||||
| 2 | 259 | 0 | |||||||||
| 3 | 592 | 0 | |||||||||
| Intravenous line infection | 1 | 346 | 8 | 8 | 0.641 | ||||||
| 2 | 259 | 8 | 8 | ||||||||
| 3 | 592 | 12 | 12 | ||||||||
| Stoma complications | 1 | 269 | 11 | 4 | 7 | 0.702 | |||||
| 2 | 228 | 14 | 3 | 11 | |||||||
| 3 | 119 | 5 | 2 | 3 | |||||||
| General complications | 1 | 346 | 36 | 21 | 15 | 0.520 | |||||
| 2 | 259 | 34 | 17 | 17 | |||||||
| 3 | 592 | 54 | 28 | 25 | 1 |
Group 1: Patients received 25 × 2 Gy radiation with capecitabine; Group 2: Patients received 10 × 3 Gy radiation; Group 3: Patients did not receive radiation; LAR: Low anterior resection; APR: Abdominoperineal resection.
In 894 patients who received LAR, anastomotic leakage was the most obvious complication. Anastomotic leakage developed in 48 (5.4%) patients. Nineteen (2.1%) patients who required surgical reintervention were classified as Grade 3b according to the Clavien–Dindo classification. Our data suggested that preoperative radiotherapy (P = 0.001) and diverting ileostomy (P < 0.001) were significant independent factors (Table 5). Both long-course [odds ratio (OR) = 3.624, 95% confidence interval (CI): 1.689–7.775, P = 0.001] and short-course (OR = 5.150, 95%CI: 1.828–14.515, P = 0.002) neoadjuvant radiotherapy increased the incidence of anastomotic leakage (6.78%, 5.96%, and 4.54% in Groups 1, 2, and 3, respectively), but neither was associated with the severity of the complication (P = 0.411) (Table 4). Temporary diverting ileostomy was a protective factor to reduce the incidence of anastomotic leakage (OR = 6.211, 95%CI: 2.525–15.385, P < 0.001). The majority of patients with neoadjuvant radiotherapy underwent additional surgery of temporary ileostomy, especially in those with short-course radiotherapy (69.4%, 83.1%, and 7.4% in Groups 1, 2, and 3, respectively, P < 0.001).
Table 5.
Logistic regression analysis of anastomotic leakage
| Characteristics |
Anastomotic leakage |
|||
| χ2 | OR | 95%CI of Exp(B) | P value | |
| Age | 1.748 | 1.542 | 0.812–2.928 | 0.186 |
| Sex | 1.081 | 1.410 | 0.738–2.695 | 0.299 |
| Distance from anal verge | 0.508 | 1.276 | 0.653–2.496 | 0.476 |
| Pathological T stage | 13.089 | 2.620 | 1.555–4.415 | < 0.001 |
| Pathological N stage | 1.402 | 0.768 | 0.496–1.189 | 0.236 |
| Preoperative radiotherapy | 14.029 | 0.001 | ||
| Long course CRT/surgery directly | 10.931 | 3.624 | 1.689–7.775 | 0.001 |
| short course RT/surgery directly | 9.614 | 5.150 | 1.828–14.515 | 0.002 |
| diverting stoma | 15.804 | 6.211 | 2.525–15.385 | < 0.001 |
OR: Odds ratio; CRT: Chemoradiotherapy; RT: Radiotherapy.
Three hundred and three patients received APR. More than 16.5% of patients suffered from perineal wound complications. The incidence of perineal wound complications in the three groups was 11.8%, 26.8%, and 9.4%, respectively. Short-course chemoradiotherapy was closely associated with perineal wound complications (OR = 5.565, 95%CI: 2.203–14.057, P < 0.001). In contrast, long-course radio-chemotherapy did not significantly influence development of perineal wound complications (OR = 1.692, 95%CI: 0.651–4.394, P = 0.280) (Table 6). The grade of these complications differed significantly among the three groups (P < 0.001) (Table 4). Patients receiving short-course radiotherapy had higher-grade perineal wound complications. However, there were no Grade 3b perineal wound complications, and none of these patients required surgical reintervention.
Table 6.
Logistic regression analysis of perineal wound complications
| Characteristics |
Perineal wound complications |
|||
| χ2 | OR | 95%CI of Exp(B) | P value | |
| Age | 1.576 | 1.508 | 0.794–2.865 | 0.209 |
| Sex | 1.542 | 1.513 | 0.787–2.907 | 0.214 |
| Distance from anal verge | 1.045 | 1.779 | 0.590–5.376 | 0.307 |
| Pathological T stage | 0.391 | 1.121 | 0.784–1.602 | 0.532 |
| Pathological N stage | 0.791 | 1.227 | 0.782–1.927 | 0.374 |
| Preoperative radiotherapy | 16.757 | <0.001 | ||
| long course CRT/surgery directly | 1.166 | 1.692 | 0.651–4.394 | 0.280 |
| short course RT/surgery directly | 13.184 | 5.565 | 2.203–14.057 | <0.001 |
OR: Odds ratio; CRT: Chemoradiotherapy; RT: Radiotherapy.
Reintervention
Among all the studied patients, only 43 with Grade 3b complications required reintervention. The reasons included anastomotic leakage, ileus, bleeding, intra-abdominal abscess, abdominal wound complications, urological complications, and intestinal necrosis. Some patients with anastomotic leakage required reintervention. The reintervention rate for anastomotic leakage repair in in all three groups did not differ significantly (37.5%, 66.7%, and 30.4% in Groups 1, 2, and 3, respectively, P = 0.411), indicating that neither long-course nor short-course radiotherapy increased the need for reintervention. The increase in perineal wound complications caused by short-course radiotherapy was mild. None of the patients with perineal wound complications required reintervention.
DISCUSSION
Neoadjuvant radiochemotherapy has become important to reduce local recurrence of locally advanced rectal cancer. In China, an increasing number of patients have been recommended to receive radiotherapy before surgery, but an increase in postoperative complications if patients receive preoperative radiation has been a major concern[4]. The present study compared the major postoperative complications associated with long-course and short-course radiotherapy followed by TME in a large series of patients with locally advanced rectal cancer. Compared to those without radiotherapy, the increase in surgical complications caused by two different preoperative radiotherapy regimens was acceptable, and postoperative mortality did not increase. No patients died within 30 d after surgery, although preoperative radiotherapy might have been associated with anastomotic leakage and perineal wound complications. Temporary ileostomy prevented the occurrence and severity of anastomotic leakage. The grade of surgical complications did not differ significantly, except for perineal wounds, which did not always require surgical reintervention.
Several studies have investigated whether preoperative radiotherapy increases surgical complications[5]. The conclusions were not in agreement. Most of these studies have suggested that preoperative radiotherapy does not increase postoperative morbidity[6]. However, the complications in patients with neoadjuvant radiotherapy seem to be more severe, as demonstrated by the need for more surgical reintervention to treat the complications[7]. Our study indicated that long-course and short-course chemoradiotherapy were not associated with increased incidence or grade of complications. Conservative measures do not have any benefit after radiotherapy[8]. Reintervention is more often used for patients who receive radiotherapy if anastomotic leakage cannot be healed. This was one of the major setbacks of radiotherapy.
Anastomotic leakage is the most serious surgical complication after LAR for rectal cancer. It occurs in 3.5%–25.0% of patients after surgery[9,10]. The rates reported varied according to different definitions being used. In some studies, anastomotic leakage was diagnosed by computed tomography, magnetic resonance imaging, or radio-graphy. The incidence rates were usually higher by imaging diagnosis than clinical observation. In this study, we defined leakage by the presence of gas or feces around the anastomosis in the drainage tubes. Radiology was used only when surgical reintervention was required. There were many risk factors believed to be associated with anastomotic leakage, such as male gender, lower location of the tumor, and preoperative radiotherapy[11,12]. Neoadjuvant radiotherapy has been implicated as a causative factor for the increased rate of anastomotic leakage[13]. It might be due to the local effect of radiation, and subsequently increased technical difficulty during the operation. Radiotherapy may also decrease the oxygen supply to the local tissue around the anastomosis. It can slow down the healing process and cause leakage. In our study, the rate for all these patients was 5.4%. It was a low incidence and within the acceptable range compared with other complications.
It is agreed that preoperative radiotherapy is associated with an increase in anastomotic leakage. Patients with radiotherapy may need a longer time for healing of leakage. As a result, a temporary defunctioning ileostomy was constructed during rectal surgery for patients who underwent preoperative radiotherapy, and it was reversed after 6 mo[14]. It is believed that ileostomy can reduce anastomotic leakage[15]. In the present study, more patients with radiotherapy had a defunctioning ileostomy than those who underwent surgery alone. Defunctioning ileostomy also decreases the grade of leakage as the feces are diverted[16]. This may be an effective approach to avoid surgical reintervention caused by anastomotic leakage.
Perineal wound complications are commonly seen after APR. Previously reported rates varied between 5.9% and 31.0%[17,18]. Most perineal wound complications, such as wound gaping, are mild and do not require a prolonged stay in hospital or surgical reintervention. However, severe wound complications can impair quality of life[19]. For example, patients with open wounds are usually accompanied with pain and movement limitation. They might also delay subsequent adjuvant chemotherapy, which may result in worse prognosis. Previous studies have shown that the rate of perineal wound complications increases in patients who receive preoperative radiotherapy[4,20]. Our study also showed a significant increase in perineal wound complications after short-course radiochemotherapy compared with patients who received long-course raidochemotherapy and those who did not receive neoadjuvant treatment. In addition, the grade of the complications was also higher in the short-course radiotherapy group. The grade of these complications was always 3a. However, no reintervention was required to manage these complications.
Although our study had a large patient cohort, it was limited by its retrospective nature. It is difficult to fully evaluate late complications for > 30 d after hospital discharge, and the incidence might have been underestimated.
The use of preoperative radiochemotherapy for rectal cancer has been debated for decades, including indications, methods, TRG, and so on. Preoperative chemora-diotherapy may be affected by several factors, such as carcinoembryonic antigen and histological regression score. Acellular mucin pools are also thought to be a useful predictor for complete response in several studies[21,22], but it is controversial. The association between postoperative complications and mucin pools is worth study. From 2008-2010, which is the recruitment period, we had not begun to detect routinely regression rate and mucin pool in our hospital. Therefore, these data were not collected for this study. It is one of the limitations of our study.
Since the sample includes more 1000 patients, we can compare the local control, survival, and quality of life among different groups. We can compare differences of clinical effect between different preoperative therapies, and additional studies are necessary in the future.
In conclusion, preoperative radiotherapy was associated with two major surgical complications: anastomotic leakage and perineal wound complications. There were no significant differences in other complications. Both long-course and short-course radiochemotherapy increased the incidence of anastomotic leakage, but the grade remained close to that in patients treated with surgery alone. A temporary defunctioning ileostomy seemed to be an effective method to reduce the risk of anastomotic leakage for patients who received radiotherapy. Short-course radiochemotherapy increased the incidence and grade of perineal wound complications. Reintervention may not be necessary to ameliorate the perineal wound complications as the damage is usually low grade.
ARTICLE HIGHLIGHTS
Research background
Preoperative radiochemotherapy can improve local control of rectal cancer. However, some researchers believe it increases the incidence of surgical complications. Patients with locally advanced rectal cancer receive three different treatments in our hospital, including long-course radiochemotherapy, short-course radiotherapy, and surgery directly. We can compare diffe-rences in their postoperative complications.
Research motivation
Some surgeons suspect that preoperative radiochemotherapy increases surgical complications, such as anastomotic leakage. As a result, surgeons are more likely to do additional diverting ileostomy for these patients. Our motivation is to determine if radiochemotherapy increases the incidence of complications or only increases the severity of complications. These findings can guide our treatment strategies.
Research objectives
To investigate surgical complications caused by three different preoperative radiotherapy regimens. It includes the incidence and severity of complications.
Research methods
This is a retrospective study. We analyzed 1197 patients with locally advanced rectal cancer between 2008 and 2010. Three hundred and forty-six patients were treated with preoperative long-course radiochemotherapy, and 259 patients received short-course radiotherapy (10 × 3 Gy) before surgery. The remaining 592 patients underwent total mesorectal excision (TME) alone without neoadjuvant therapy. The incidence of surgical complications was evaluated for up to 30 d after discharge from hospital. Severity was also studied according to Clavien–Dindo classi-fication.
Research results
The major complications were anastomotic leakage and perineal wound complications. Both long-course and short-course radiotherapy were associated with incidence of anastomotic leakage, but the severity of anastomotic leakage did not increase in patients following preoperative radiotherapy. Temporary ileostomy can reduce incidence of anastomotic leakage. Compared with TME alone, short-course radiotherapy was associated with an increase in incidence and severity of perineal wound complications. Long-course radiotherapy seemed safe regarding this complication.
Research conclusions
Radiotherapy increased incidence but not severity of anastomotic leakage. Short-course radiotherapy was also accompanied with perineal wound complications. However, intervention appeared unnecessary to ameliorate the complications. The increase of complications seems to be acceptable. Our surgeons are more likely to use diverting ileostomy for patients with preo-perative radiotherapy.
Research perspectives
We determined the advantages and disadvantages of preoperative radiotherapy, and this knowledge will inform our selection of different preoperative treatments. Our study is a retrospective study with a large sample size. In our opinion, a prospective randomized controlled study needs to be designed and performed.
Footnotes
Informed consent statement: This study is a retrospective study. As confirmed by the Ethics Committee of Beijing Cancer Hospital, no additional informed consent statement is required.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: December 27, 2019
First decision: January 11, 2019
Article in press: March 26, 2019
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arias F, Gurzu S S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
Contributor Information
Tian-Cheng Zhan, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Colorectal Surgery, Peking University Cancer Hospital and Institute, Beijing Cancer Hospital, Beijing 100142, China.
Da-Kui Zhang, Department of General Surgery, China–Japan Friendship Hospital, Beijing 100029, China.
Jin Gu, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Colorectal Surgery, Peking University Cancer Hospital and Institute, Beijing Cancer Hospital, Beijing 100142, China.
Ming Li, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Colorectal Surgery, Peking University Cancer Hospital and Institute, Beijing Cancer Hospital, Beijing 100142, China. limingmd@126.com.
References
- 1.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 2.Zhan T, Gu J, Li M, Du C. Intermediate-fraction neoadjuvant radiotherapy for rectal cancer. Dis Colon Rectum. 2013;56:422–432. doi: 10.1097/DCR.0b013e31828576c6. [DOI] [PubMed] [Google Scholar]
- 3.Yamano T, Yoshimura M, Kobayashi M, Beppu N, Hamanaka M, Babaya A, Tsukamoto K, Noda M, Matsubara N, Tomita N. Malnutrition in rectal cancer patients receiving preoperative chemoradiotherapy is common and associated with treatment tolerability and anastomotic leakage. Int J Colorectal Dis. 2016;31:877–884. doi: 10.1007/s00384-016-2507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, Heriot A, Joseph D, McLachlan SA, McClure B, Ngan SY. Acute Adverse Events and Postoperative Complications in a Randomized Trial of Preoperative Short-course Radiotherapy Versus Long-course Chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04) Ann Surg. 2017;265:882–888. doi: 10.1097/SLA.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 5.Swellengrebel HA, Marijnen CA, Verwaal VJ, Vincent A, Heuff G, Gerhards MF, van Geloven AA, van Tets WF, Verheij M, Cats A. Toxicity and complications of preoperative chemoradiotherapy for locally advanced rectal cancer. Br J Surg. 2011;98:418–426. doi: 10.1002/bjs.7315. [DOI] [PubMed] [Google Scholar]
- 6.Milgrom SA, Goodman KA, Nash GM, Paty PB, Guillem JG, Temple LK, Weiser MR, Garcia-Aguilar J. Neoadjuvant radiation therapy prior to total mesorectal excision for rectal cancer is not associated with postoperative complications using current techniques. Ann Surg Oncol. 2014;21:2295–2302. doi: 10.1245/s10434-014-3624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonker FH, Tanis PJ, Coene PP, van der Harst E Dutch Surgical Colorectal Audit Group. Impact of Neoadjuvant Radiotherapy on Complications After Hartmann Procedure for Rectal Cancer. Dis Colon Rectum. 2015;58:931–937. doi: 10.1097/DCR.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 8.Johnston MJ, Robertson GM, Frizelle FA. Management of late complications of pelvic radiation in the rectum and anus: a review. Dis Colon Rectum. 2003;46:247–259. doi: 10.1007/s10350-004-6530-4. [DOI] [PubMed] [Google Scholar]
- 9.Akiyoshi T, Ueno M, Fukunaga Y, Nagayama S, Fujimoto Y, Konishi T, Kuroyanagi H, Yamaguchi T. Incidence of and risk factors for anastomotic leakage after laparoscopic anterior resection with intracorporeal rectal transection and double-stapling technique anastomosis for rectal cancer. Am J Surg. 2011;202:259–264. doi: 10.1016/j.amjsurg.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Vermeer TA, Orsini RG, Daams F, Nieuwenhuijzen GA, Rutten HJ. Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: Incidence, risk factors and treatment. Eur J Surg Oncol. 2014;40:1502–1509. doi: 10.1016/j.ejso.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240:260–268. doi: 10.1097/01.sla.0000133185.23514.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Choi GS, Kim SH, Kim HR, Kim NK, Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, Bae BN, Son GM, Lee SI, Kang H. Multicenter analysis of risk factors for anastomotic leakage after laparoscopic rectal cancer excision: the Korean laparoscopic colorectal surgery study group. Ann Surg. 2013;257:665–671. doi: 10.1097/SLA.0b013e31827b8ed9. [DOI] [PubMed] [Google Scholar]
- 13.Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, Wang L, Wang J. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum. 2016;59:934–942. doi: 10.1097/DCR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 14.Hain E, Maggiori L, Manceau G, Zappa M, Prost à la Denise J, Panis Y. Persistent Asymptomatic Anastomotic Leakage After Laparoscopic Sphincter-Saving Surgery for Rectal Cancer: Can Diverting Stoma Be Reversed Safely at 6 Months? Dis Colon Rectum. 2016;59:369–376. doi: 10.1097/DCR.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 15.Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 16.Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207–214. doi: 10.1097/SLA.0b013e3180603024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han JG, Wang ZJ, Qian Q, Dai Y, Zhang ZQ, Yang JS, Li F, Li XB. A prospective multicenter clinical study of extralevator abdominoperineal resection for locally advanced low rectal cancer. Dis Colon Rectum. 2014;57:1333–1340. doi: 10.1097/DCR.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 18.Musters GD, Sloothaak DA, Roodbeen S, van Geloven AA, Bemelman WA, Tanis PJ. Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis. 2014;29:1151–1157. doi: 10.1007/s00384-014-1967-y. [DOI] [PubMed] [Google Scholar]
- 19.Colov EP, Klein M, Gögenur I. Wound Complications and Perineal Pain After Extralevator Versus Standard Abdominoperineal Excision: A Nationwide Study. Dis Colon Rectum. 2016;59:813–821. doi: 10.1097/DCR.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 20.De Nardi P, Summo V, Vignali A, Capretti G. Standard versus extralevator abdominoperineal low rectal cancer excision outcomes: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:2997–3006. doi: 10.1245/s10434-015-4368-8. [DOI] [PubMed] [Google Scholar]
- 21.Gurzu S, Bara T, Bara T, Jr, Kadar Z, Molnar C, Kovecsi A, Jung I. Clinical significance of carcinoembryonic antigen expression of acellular mucin pools after preoperative chemoradiotherapy of rectal carcinoma. Cancer Biother Radiopharm. 2014;29:295–297. doi: 10.1089/cbr.2014.1640. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds IS, McNamara DA, Kay EW, O'Neill B, Deasy J, Burke JP. The significance of mucin pools following neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Surg Oncol. 2018;118:1129–1134. doi: 10.1002/jso.25247. [DOI] [PubMed] [Google Scholar]