Abstract
Cardiac fibrosis secondary to long-term hypertension is known to promote cardiac dysfunction; however, few therapeutic agents are available for the treatment of this condition in clinical practice. The heptapeptide alamandine (Ala) has recently been identified as a component of the renin-angiotensin system (RAS), which exerts a protective effect against cardiac hypertrophy; however, it is unknown whether Ala may also be useful for the treatment of cardiac fibrosis. In the present study, the potential therapeutic effects of Ala on long-term hypertension-induced cardiac fibrosis were investigated in an aged, spontaneous hypertensive rat model. Weekly blood pressure (BP) measurements revealed that daily Ala treatment significantly decreased the systolic, diastolic and mean arterial BP compared with the control. Of note, the observed reduction in BP in Ala-treated animals markedly differed to that observed in rats treated with hydralazine (Hyd). Echocardiography further demonstrated that Ala treatment decreased the ratio of left ventricle mass to body weight, and alleviated structural and functional parameters associated with cardiac fibrosis, including left ventricular volume, ejection fraction and fractional shortening compared with the control and Hyd-treated groups. Furthermore, Ala deceased the density of cardiac fibrosis, as assessed by Masson and Sirius red staining; reduced expression of fibrotic proteins, including connective tissue growth factor, collagen I (COL1A1) and matrix metalloproteinase 9, was also observed. In addition, Ala treatment further decreased the expression of angiotensin II-induced fibrotic markers at the mRNA and protein levels in cultured cardiac fibroblasts; Ala-mediated inhibition of COL1A1 expression and Akt phosphorylation was inhibited via the Mas-related G protein receptor antagonist, PD123319. Collectively, the findings of the present study suggest that Ala is an effective anti-hypertensive peptide that can attenuate cardiac dysfunction and fibrosis induced by chronic hypertension, independent of BP.
Keywords: hypertension, vasoactive peptide, cardiac fibrosis, renin-angiotensin system
Introduction
Cardiac fibrosis is a common feature of cardiac remodeling that occurs in numerous types of cardiovascular diseases, including hypertension, myocardial infarction and cardiomyopathy (1). This condition is characterized by the inappropriate interstitial deposition of collagen and extracellular matrix (ECM) proteins, which impair normal organ structure and function (2,3). Cardiac fibroblasts (CFs) are the predominant cell type responsible for physiological ECM homeostasis and tissue healing following cardiac injury (4). During the process of fibrosis, CFs transdifferentiate into myofibroblasts, which are characterized by the expression of α-smooth muscle actin (α-SMA), a contractile protein and marker of profibrogenic cardiac fibroblast activation (5).
The renin-angiotensin system (RAS) is involved in various aspects of pathological cardiac remodeling, including cardiac hypertrophy and fibrosis (6,7). Angiotensin II (Ang II), the key member of RAS, has been demonstrated to promote myocardial remodeling and fibrosis via interactions with the Ang II type 1 (AT1) receptor (8,9). This process is distinguished by fibroblast cell proliferation and fibrogenic gene expression (10,11). Inhibition of the RAS with angiotensin-converting enzyme (ACE) inhibitors or AT1 receptor blockers promotes anti-hypertrophic and anti-fibrotic effects beyond reductions in blood pressure (BP) (12). Importantly, knowledge of the RAS and its inhibitors has substantially increased in recent decades; however, numerous biological effects of these drugs cannot be explained by the well-established mechanisms of action. Thus, current understanding of the RAS is far from complete.
Alamandine (Ala) is a recently identified component of the RAS. This heptapeptide differs from Ang (1–7) in that it contains an N-terminal alanine, rather than an aspartate residue (13). Functional studies have revealed that Ala can induce endothelial-dependent vasorelaxation, similar to the effect of Ang (1–7) (10,11). On the contrary, Ala can counteract the vasoconstriction induced by its precursor, angiotensin A (Ang A), without affecting Ang II-induced vasoconstriction (14). Furthermore, it has been reported that the vasodilatory effects of Ala are context dependent. Specifically, in the thoracic aorta and iliac artery, Ala promotes acetylcholine-mediated vasodilation; however, in the carotid artery, Ala does not affect the levels of acetylcholine or vascular tone (14). In the renal artery, it suppresses acetylcholine-induced vasodilation (14). In a isoproterenol-treated rat model of cardiac hypertrophy, the Mas-related G protein-coupled receptor D (MrgD) has been reported to function as a major target for Ala (13). Additionally, the role of Ala in cardiac fibrosis induced by hypertension and RAS overactivation remains unknown. In the present study, the potential therapeutic effects of Ala on fibrosis in a spontaneous hypertensive rat (SHR) model were investigated. We reported that Ala significantly attenuated cardiac fibrosis in aged SHR rats and that this effect may be independent of reductions in BP.
Materials and methods
Animals and grouping
Male rats (n=24; 50-weeks-old, mean weight, 380 g; Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China), including six normotensive Wistar-Kyoto (WKY) and 18 SHRs, were housed in a temperature-controlled room (23°C; 40–60% humidity; 21% oxygen) under a 12 h light/dark cycle, with free access to standard chow and tap water. Three groups of SHRs (n=6 per group) were subjected to 6-weeks of intraperitoneal administration of 50 µg/kg/day Ala (Phoenix Pharmaceuticals Inc., Burlingame, CA, USA), 10 mg/kg/day hydralazine (Hyd) or the equivalent volume of saline. Following the completion of treatment, all rats were examined via two-dimensional echocardiography, weighed and sacrificed. Isolated hearts were dried and weighed, and a portion of the left heart tissue was sectioned for staining. The remaining tissue was used for western blotting and reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
Measurement of tail artery BP
Systolic BP, diastolic BP and the mean arterial pressure were measured every week for 6 weeks in the tails of conscious rats, using a noninvasive computerized tail-cuff system (Kent Scientific Corporation, Torrington, CT, USA). To minimize stress-induced fluctuations, the rats were pre-trained by measuring BP daily for at least 1 week prior to the start of the experiments, and the total training duration was 2 weeks. The rats were maintained under 28°C for 10–20 min prior to analysis to allow the detection of tail artery pulsations and to achieve a steady pulse. The reported tail artery BP data were obtained by averaging 10 measurements.
Echocardiography
After the 6-week course of drug or saline administration, rats were anesthetized with continuous administration of 2% isoflurane in 2 l/min of 100% oxygen via a facemask. Then, transthoracic echocardiography was performed in all rats using an ultrasound system (Vevo 2100, VisualSonics, Inc., Toronto, Canada), with a 21-MHz probe. LV diameter were assessed by the measurement of the left ventricle (LV) mass and LV volumes in diastole and systole, and the LV mass to body weight ratio. LV ejection fraction (EF) and fractional shortening (FS) were calculated to evaluate LV function. Measurements over three consecutive cardiac cycles were averaged.
Masson and Sirius red staining
To assess the degree of fibrosis of cardiomyocytes, 5-µm cardiac sections were examined by Masson kit (cat. no. GP1016) and Sirius red kit (cat. no. GP1017; Service Biological Technology Co., Ltd, Wuhan, China, http://www.servicebio.cn/), according to the manufacturer's protocols. Briefly, the tissues were fixed with 4% paraformaldehyde at 4°C overnight, paraffin embedded and cut into sections (5 µm thick). For trichrome Masson staining, sections were de-paraffinized, rehydrated and rinsed with potassium dichromate mordant solution overnight. Next, sections were sequentially stained with iron hematoxylin for 3 min, acid ponceau fuchsin for 3–5 min, phosphomolybdic acid for 1–3 min, aniline blue for 3–6 min and differentiated in 1% glacial acetic acid solution. The slides were mounted with resin mounting medium. In addition, slides were stained with Sirius red solution for 8–10 mins and subsequently sealed with glycerol jelly. Three to five random fields (~30–50 cells per field) were selected from the three sections obtained from each animal for observation under a light microscope (Zeiss GmbH, Jena, Germany). Images were analyzed using Image-Pro Plus software version 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Culture of cardiac fibroblasts isolated from adult rats
Adult cardiac fibroblasts (CFs) were obtained from male WKY rats. Ventricular tissue was dissected, washed, minced and subjected to an initial digestion step in bacterial proteinase solution (4 U/ml) at room temperature. for 15 min; seven rounds of digestion were then performed at 37°C for 20 min in a solution containing a mixture of 1 mg/ml collagenase A and 0.5 mg/ml hyaluronidase. Following each cycle of digestion, the tissue was mechanically dissociated using a wide-mouth pipet; the supernatant containing dissociated cells was collected. Cells were resuspended in Iscove's Modified Dulbecco's medium (IMDM; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). Cells from all digestions were pooled and resuspended in IMDM, supplemented with 20% fetal calf serum (ScienCell Research Laboratories, Inc., San Diego, CA, USA), penicillin (100 U/ml), streptomycin (100 µg/ml), nonessential amino acids (1%), and 2-mercaptoethanol (0.1 mM). Cells (105) were first plated and incubated for 2 h at 37°C to allow the preferential attachment of fibroblasts. To confirm that the cells were CFs, and exclude endothelial cells and cardiomyocytes from the mixture, pure CF identification was performed by immunostaining with anti-vimentin (1:1,000; cat. no. ab92547), anti-CD31 (1:500; cat. no. ab28364; both Abcam, Cambridge, MA, USA) and anti-α-actin (1:1,000; cat. no. A5228; Sigma-Aldrich; Merck KGaA) antibodies at 4°C overnight. Next, cells were incubated with an Alexa-488-conjugated (1:2,000; cat. no. 111-545-003), or Cy™3-conjugated (1:2,000; cat. no. 111-165-003; both Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) secondary antibodies, at room temperature for 1 h. CFs from passages 3–8 were used for all experiments.
CFs were divided into groups and treated with PBS, PBS + Ang II, Ala, or Ala + Ang II. Pre-treatment with Ala (1 µM) or PBS control was performed for 30 min at 37°C, after which the PBS + Ang II and Ala + Ang II groups were treated at 37°C with 10−6 M Ang II (Sigma-Aldrich; Merck KGaA) for 24 h. For inhibition experiments, CFs were divided into four groups and treated with Ala (control), Ala + Ang II, Ala + PD123319 (10 µM; cat. no. s7098; Selleck Chemicals, Houston, TX, USA), or Ala + Ang II + PD123319. CF cell lysates were used for western blotting and RT-qPCR.
Western blotting
Heart tissues or cultured cells were homogenized in radioimmunoprecipitation assay lysis buffer (Thermo Fisher Scientific, Inc.) and sonicated. Cell debris was removed, and the supernatant was obtained by centrifugation at 12,000 × g for 10 min at 4°C. Protein concentration was determined by a bicinchoninic acid protein assay, and a total of 30–50 µg protein was separated on 10–15% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Following blocking with 5% fat-free milk solution, the proteins of interest were detected by using primary antibodies (4°C overnight) and a corresponding horseradish peroxidase-linked secondary antibody at room temperature for 1 h. The targets of the primary antibodies used were as follows: Connective tissue growth factor (CTGF, 1:500; cat. no. ab6992; Abcam); collagen I (COL-1; 1:500; Wanleibio Co., Ltd., Shanghai, China), matrix metalloproteinase 9 (MMP9; 1:1,000; cat. no. 13667), protein kinase B (Akt; 1:1,000; cat. no. 469) and phosphorylated (p)-Akt [(Ser 473) 1:1,000; all cat. no. 40601]; an antibody against GAPDH (1:1,000; cat. no. 5174; all Cell Signaling Technology, Inc., Danvers, MA, USA) was used as an internal control. The blots were developed with enhanced chemiluminescence reagent (Thermo Fisher Scientific, Inc.) and exposed on a ChemiDoc MP imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Experiments were performed in triplicate for each indicated protein. Images were analyzed using Image-Pro Plus software version 6.0.
RT-qPCR
Total RNA was isolated from CFs using TRIzol (Invitrogen; thermos Fisher Scientific, Inc., Waltham, MA, USA) and 1 µg of the RNA was then converted to cDNA using Transcriptor First Strand cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's protocols. qPCR reactions were performed on an ABI Prism 7900 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using TaqMan™ probe qPCR kit (Roche Diagnostics). The thermocycling conditions were as follows: 95°C for 10 min, followed by 45 cycles of 95°C for 10 sec and 60°C for 1 min. Taqman probes to detect COL1A1, collagen type III α 1 chain (COL3A1), α-SMA and CTGF were purchased from Roche Diagnostics. Each sample was analyzed in triplicate and target genes were normalized to the reference housekeeping gene GAPDH. Fold differences were then calculated for each treatment group using normalized Cq values for the control, using the 2−∆∆Cq method (15). Probes (5′-3′) used in the present study were: a-SMA forward, CCCAGCACCATGAAGATCA and reverse, CGCCGATCCAGACAGAAT; CTGF forward, GCTGACCTAGAGGAAAACATTAAGA and reverse, CCGGTAGGTCTTCACACTGG; Col1a1 forward, TCCTGGCAAGAACGGAGAT and reverse, CAGGAGGTCCACGCTCAC; Col3a1 forward, TCCCCTGGAATCTGTGAATC and reverse, TGAGTCGAATTGGGGAGAAT. GAPDH forward, GGCACAGTCAAGGCTGAGAATG and reverse, ATGGTGGTGAAGACGCCAGTA.
Statistical analyses
All data are presented as mean ± standard error of the mean of at least three independent experiments. Statistical significance among multiple groups was evaluated by one-way analysis of variance followed by a Bonferroni's post-hoc test, using GraphPad Prism 5.0 (GraphPad Software Inc., CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
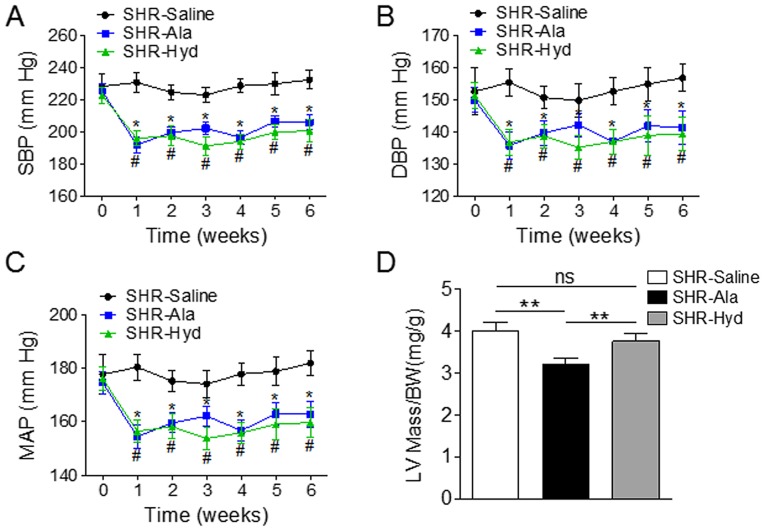
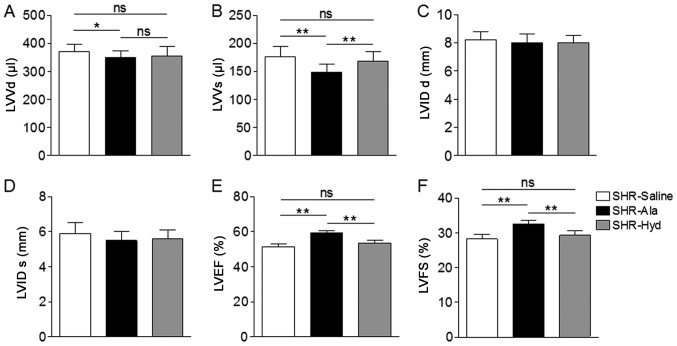
Aged SHRs were treated daily with Ala, Hyd, or saline as a control, for six weeks and BP was measured weekly in all animals. The systolic, diastolic and mean arterial BPs of Ala- and Hyd-treated animals were significantly lower than those of the saline-treated group (Fig. 1A-C). Ala administration also resulted in a significantly decreased ratio of LV mass to body weight compared with the control and Hyd-treated SHRs (Fig. 1D). Assessment of LV diameter and function by transthoracic echocardiography further revealed a significantly decreased LV volume in the systolic and diastolic phase compared with the control (Fig. 2A and B). On the contrary, Ala and Hyd treatment did not affect LV diameter along the long axis view (Fig. 2C and D). An improved EF and FS in Ala-treated SHRs were observed compared with the control and Hyd-treated SHRs (Fig. 2E and F).
Figure 1.
Ala treatment decreases BP and LV mass in a SHR model. SHRs were treated daily with Ala, Hyd or saline for 6 weeks, and tail arterial BP was measured weekly. (A-C) Measurements of SBP, DBP and MAP; n=6 per group. *P<0.05 vs. SHR-Ala, #P<0.05 vs. SHR-Hyd. (D) LV mass was measured by echocardiography; the ratio of LV mass to BW was compared among the three groups. **P<0.01. Data are presented as the mean ± standard error of the mean. Ala, alamandine; BW, body weight; DBP, diastolic blood pressure; Hyd, hydralazine; LV, left ventricle; MAP, mean arterial blood pressure; ns, no significance; SHR, spontaneous hypertensive rat; SBP, systolic blood pressure.
Figure 2.
Ala treatment attenuates cardiac dysfunction in SHRs. (A and B) LV volume at diastolic phase and systolic phase were measured by echocardiography. (C and D) LV septal diameters at diastolic phase and systolic phase were measured by echocardiography. (E and F) LVEF and LVFS were measured by echocardiography; n=6 per group. Data are presented as the mean ± standard error of the mean. *P<0.05, **P<0.01. Ala, alamandine; Hyd, hydralazine; LV, left ventricle; ns, no significance; LVVd, LV volume in diastole; LVVs, LV volume in systole; LVIDd, LV internal diameter in diastole; LVIDs, LV internal diameter in systole; LVEF, LV ejection fraction; LVFS, LV fractional shortening; SHR, spontaneous hypertensive rat.
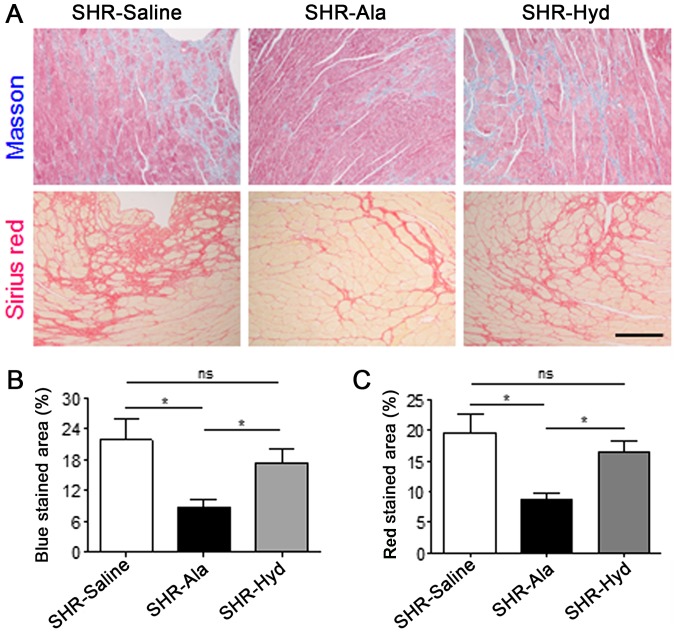
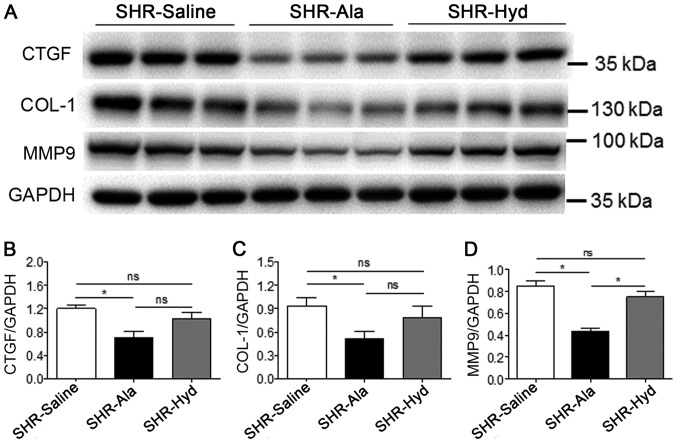
Subsequently, whether Ala affects the remodeling of cardiac tissues in SHRs was investigated in the present study. Masson and Sirius red staining of LV sections indicated that the degree of fibrosis in Ala-treated animals was significantly lower than in the control group; a marked reduction in fibrosis was observed in response to Hyd treatment (Fig. 3A-C). Furthermore, analysis of the expression of fibrosis-associated and remodeling marker proteins, including CTGF, COL-1 and MMP-9 in LV tissue, by western blotting revealed that, compared with the control group, Ala treatment significantly decreased expression levels of all three proteins. The expression levels of these markers were markedly lower in the Ala-treated group than the Hyd-treated group; however, the expression of MMP-9 exhibited a significant difference. Conversely, we reported that the protein expression levels of these markers were notably different between the control and Hyd-treated groups (Fig. 4A-D).
Figure 3.
Ala treatment attenuates cardiac fibrosis in SHRs. (A) Density and area of fibrosis in left ventricular tissue from Ala-, Hyd- and saline control-treated SHRs were assessed by Masson and Sirius red staining. Scale bar, 100 µm. (B and C) Quantification of Masson- and Sirius red-stained fibrotic area; n=6 per group. Data are presented as the mean ± standard error of the mean. *P<0.05. ns, no significance; Ala, alamandine; Hyd, hydralazine; SHR, spontaneous hypertensive rat.
Figure 4.
Ala treatment decreases fibrotic marker protein expression. (A) Protein expression levels of CTGF, COL-1 and MMP9 were assessed in LV tissue from Ala-, Hyd- and saline control-treated SHRs by western blotting with the indicated antibodies. (B-D) Quantification of relative CTGF, COL-1 and MMP9 expression levels, which were normalized to that of GAPDH; n=6 per group. Data are presented as mean ± standard error of the mean. *P<0.05. Ala, alamandine; COL-1, collagen I; CTGF, connective tissue growth factor; Hyd, hydralazine; MMP9, matrix metalloproteinase 9; ns, no significance; SHR, spontaneous hypertensive rat.
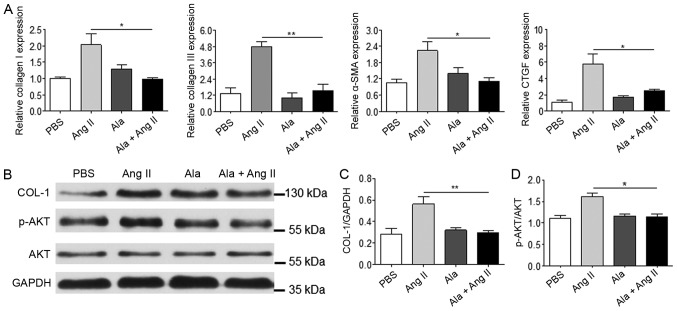
To further investigate the mechanism underlying the antifibrotic effects of Ala in vitro, primary CFs isolated from WKY rats were employed and treated with Ang II to mimic the cardiac fibrosis observed in SHRs. We observed that, compared with Ang II treatment alone, Ala pre-treatment significantly decreased the mRNA levels of several genes associated with fibrosis, including COL1A1, COL3A1, α-SMA and CTGF (Fig. 5A), which were induced in response to Ang II. In addition, Ala pre-treatment significantly decreased Ang II-induced protein expression of COL-1 and Akt phosphorylation, which are associated with fibrosis (Fig. 5B-D).
Figure 5.
Ala treatment decreases Ang II-induced fibrotic marker protein expression in cardiac fibroblasts. (A) mRNA levels of COL1A1, COL3A1, α-SMA and CTGF were assessed by reverse transcription-quantitative polymerase chain reaction. (B) Protein levels of COL-1 and p-Akt were assessed by western blotting. (C) Quantification of relative COL-I and (D) p-Akt levels, normalized to GAPDH and Akt, respectively. Data are presented as the mean ± standard error of the mean of triplicates and are representative of one experiment out of three performed. *P<0.05, **P<0.01. α-SMA, α-smooth muscle actin; Ala, alamandine; Akt, protein kinase B; Ang II, angiotensin II; COL1A1/COL-1, collagen I; COL3A1, collagen type III α 1 chain; CTGF, connective tissue growth factor; p, phosphorylated.
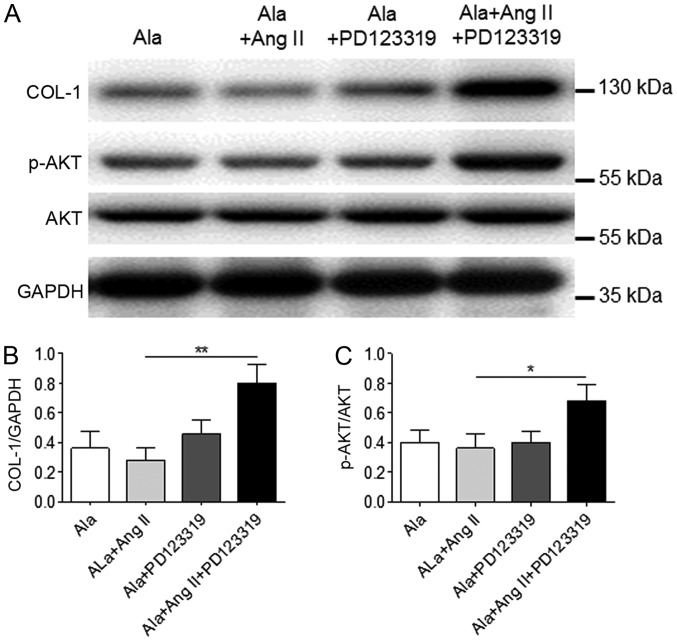
To determine whether the effects of Ala were mediated by the MrgD receptor in CFs, CFs were treated with a non-specific angiotensin AT2 receptor antagonist, PD123319, which also blocks the MrgD receptor (16). The results demonstrated that treatment with PD123319 significantly inhibited the effects of Ala on Ang II-induced COL-1 protein expression and Akt phosphorylation in CFs (Fig. 6).
Figure 6.
A Mas-related G protein-coupled receptor D antagonist inhibits the effects of Ala in cardiac fibroblasts. (A) Cells were treated with Ala, Ala + Ang II, Ala + PD123319 or Ala + Ang II + PD123319, and the protein expression levels of COL-1 and p-Akt were assessed by western blotting. (B and C) Quantification of relative COL-1 and p-Akt expression levels, which were normalized to GAPDH, respectively. Data are presented as the mean ± standard error of the mean of triplicates and are representative of one experiment out of three performed. *P<0.05, **P<0.01. Ala, alamandine; Akt, protein kinase B; Ang II, angiotensin II; COL-1, collagen I; p, phosphorylated.
Discussion
The RAS comprises numerous peptides and enzymes, including Ang I, Ang II, ACE1, ACE2, Ang A, Ang (1–7) and Ala (13,17,18). ACE1 cleaves the C-terminal of dipeptide of Ang I to form Ang II, and Ang A is thought to be generated from Ang II via the decarboxylation of aspartate (17,19). Ang II and Ang A promote vasoconstriction by binding to the AT1 receptor (20). Ala is the only one of these factors that may be derived from Ang A and Ang (1–7) (21), and has been reported to exhibit anti-hypertensive effects in SHRs and to reduces the effects of fibrosis in isoproterenol-treated rats (13); however, whether Ala can protect against the development of fibrosis in SHRs remains unknown.
The pathological mechanisms underlying cardiac fibrosis are complicated and not completely understood. Compared with cardiac hypertrophy, fibrosis can occur in pressure over-loaded ventricles, as well as in normotensive dilated ventricles and atria (3). Therefore, nonhemodynamic factors, including cardiac fibroblast cell proliferation and increased collage synthesis, have been proposed to serve a role in the development of fibrosis (22). In the present study, compared with the frequently used, short-term model of cardiac fibrosis, the therapeutic effects of Ala in a chronic, long-term model of cardiac fibrosis were investigated using 50 week-old SHRs. This model is notably similar to clinical patients with cardiac fibrosis than short-term models, including Ang II or isoproterenol-induced models. In addition, the present study aimed to identify the therapeutic, rather than protective, effects of Ala on cardiac fibrosis, which have been assessed previously, as well as other RAS signaling inhibitors (12,21,23,24). The results of the present study indicated that Ala exhibited better therapeutic effects against cardiac fibrosis than Hyd, a vasodilator used for treatment of hypertension; these treatments revealed a similar reduction in BP. Ala significantly decreased the expression levels of fibrotic markers compared with in the control group; however, the differences in the expression of Col-1 and CTGF did not exhibit significant differences compared with the Hyd group. Based on our in vitro data, it was proposed that the anti-fibrotic effects of Ala mainly occur in CFs. The results of the present study indicated that reductions in elevated BP may not be sufficient for the control of chronic cardiac fibrosis in SHRs. Hyd treatment resulted in a similar reduction of BP, but less improvement of cardiac fibrosis compared with Ala treatment. This suggested that Ala performed additional alleviation of cardiac fibrosis independent of BP reduction, which further suggests that this novel component of the RAS system may serve an important role in the reduction of chronic cardiac fibrosis in our in vivo model.
A role for Ang II in fibrosis has been suggested by several studies [reviewed in (22)]. Two types of RAS system inhibitors, including ACE inhibitors and antagonists of the Ang II receptor type 1 (AT1) receptor, have been reported to inhibit the effects of Ang II on cardiomyocytes and cardiac fibroblast cells (8), and to prevent cardiac hypertrophy and fibrosis (9,25). However, Ang II reactivation or aldosterone escape mediated by the potent profibrotic factor, aldosterone, during long-term RAS inhibition therapy can attenuate the clinical benefits of a RAS blockade, particularly on cardiac fibrosis (26). The results of the present study revealed that Ala exerts a significant antifibrotic effect on cardiac tissue in the chronic hypertension rat model, suggesting that it may have potential as a supplementary treatment for classical RAS inhibition by ACE inhibitors or AT1 receptor blockers. Furthermore, the results suggest that Ala may be able to reverse chronic hypertension-induced cardiac fibrosis in those with a genetic predisposition this disease; thus, Ala may be considered as a potential novel therapeutic target in clinical practice.
To further investigate the mechanism by which Ala prevents cardiac fibrosis, the effects of Ala on collagen synthesis and Akt activation in primary CFs from normal rats was investigated. Akt signaling is involved in cardiac fibrosis, and activated Akt is required for Ang II- and uremia-induced cardiac fibrosis (27,28). Ang II acts as a profibrotic factor for cardiac fibrosis via the AT1 receptor; the Ang II-mediated anti-apoptotic effects on cardiac fibroblast cells are also Akt dependent (29). In the present study, Ang II treatment was observed to promote a significant increase in the phosphorylation of Akt in CFs, whereas this was inhibited by Ala. In addition, the known AT2 receptor antagonist, PD123319 which also binds to MrgD receptor (16), suppresses the effects of Ala on collagen synthesis and Akt activation in primary CFs. Ala has no affinity for the AT2 receptor (13); the in vitro data of the present study demonstrated that PD123319 can suppress the inhibition of Akt phosphorylation induced by Ala treatment, which suggests that the AT2 receptor may not be involved in Ang II-mediated Akt activation.
A previous study reported that PD123319 is not a specific antagonist for the AT2 receptor, but can bind to MrgD receptors (13). AT2 is highly expressed in the developing fetus, but its expression is low in the adult cardiovascular system; however, increased AT2 expression is observed in conditions of inflammation, hypertension and atherosclerosis (30). This protein has vasoactive properties in human radial arteries (31). MrgD has been reported as the main receptor for Ala, which exhibits no affinity to MAS or AT2 (6); thus, it was proposed that Ala inhibits Ang II-induced Akt activation mainly via interactions with MrgD in the present study. MrgD is expressed predominantly in dorsal root ganglion neurons, which are key for pain perception (32,33). This protein is also stably expressed in an oncogenic fibroblast cell line, and in this context, overexpression of MrgD promotes cell growth (34). The known ligand of MrgD, β-alanine, has also been demonstrated to enhance spheroid formation induced by MrgD and tumorigenesis (18,34). The potential role of MrgD during cardiac remodeling is not fully understood; however, in the present study, the MrgD ligand Ala exhibited antiproliferative activity, unlike the effects of β-alanine on MrgD in fibroblasts (34). Therefore, the findings of the present study suggest that MrgD may induce a variety of signal transduction pathways upon interaction with different ligands, and this protein may represent a therapeutic target for cardiac remodeling.
Acknowledgements
We thank Dr Wei Sun and Ms. Ming Qiu of Nanjing Medical University (Nanjing, China) for the technical help and writing assistance.
Funding
The present study was supported by the Kangda College of Nanjing Medical University (grant no. 2015012) and the National Natural Science Foundation of China (grant no. 81400315).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LW and PL designed the study and wrote the manuscript. CL and XC performed and analyzed the experiments. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University and were conducted in accordance with National Institutes of Health guide for the Care and Use of Laboratory Animals (35) and comply with the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines) and the AVMA euthanasia guidelines 2013 (36).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol. 2014;70:64–73. doi: 10.1016/j.yjmcc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.CIR.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 3.Young MJ, Funder JW. The renin-angiotensin-aldosterone system in experimental mineralocorticoid-salt-induced cardiac fibrosis. Am J Physiol. 1996;271:E883–E888. doi: 10.1152/ajpendo.1996.271.5.E883. [DOI] [PubMed] [Google Scholar]
- 4.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/S0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 5.Davis J, Molkentin JD. Myofibroblasts: Trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: New insights from experimental animal models in hypertension research. J Mol Med (Berl) 2001;79:76–102. doi: 10.1007/s001090100210. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Ortega M, Lorenzo O, Rupérez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: Expanding the field. Hypertension. 2001;38:1382–1387. doi: 10.1161/hy1201.100589. [DOI] [PubMed] [Google Scholar]
- 8.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin II receptors on rat cardiac fibroblasts. Circulation. 1993;88:2849–2861. doi: 10.1161/01.CIR.88.6.2849. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Ohta K, Hamaguchi A, Yukimura T, Miura K, Iwao H. Angiotensin II induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension. 1995;25:1252–1259. doi: 10.1161/01.HYP.25.6.1252. [DOI] [PubMed] [Google Scholar]
- 10.Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation. 2000;101:1130–1137. doi: 10.1161/01.CIR.101.10.1130. [DOI] [PubMed] [Google Scholar]
- 11.González A, López B, Querejeta R, Diez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol. 2002;34:1585–1593. doi: 10.1006/jmcc.2002.2081. [DOI] [PubMed] [Google Scholar]
- 12.Simko F, Pechanova O, Pelouch V, Krajcirovicova K, Mullerova M, Bednarova K, Adamcova M, Paulis L. Effect of melatonin, captopril, spironolactone and simvastatin on blood pressure and left ventricular remodelling in spontaneously hypertensive rats. J Hypertens. 2009;(Suppl 27):S5–S10. doi: 10.1097/01.hjh.0000358830.95439.e8. [DOI] [PubMed] [Google Scholar]
- 13.Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, et al. Discovery and characterization of alamandine: A novel component of the renin-angiotensin system. Circ Res. 2013;112:1104–1111. doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 14.Habiyakare B, Alsaadon H, Mathai ML, Hayes A, Zulli A. Reduction of angiotensin A and alamandine vasoactivity in the rabbit model of atherogenesis: Differential effects of alamandine and Ang(1–7) Int J Exp Pathol. 2014;95:290–295. doi: 10.1111/iep.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Soares ER, Barbosa CM, Campagnole-Santos MJ, Santos RAS, Alzamora AC. Hypotensive effect induced by microinjection of Alamandine, a derivative of angiotensin-(1–7), into caudal ventrolateral medulla of 2K1C hypertensive rats. Peptides. 2017;96:67–75. doi: 10.1016/j.peptides.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Jankowski V, Vanholder R, van der Giet M, Tölle M, Karadogan S, Gobom J, Furkert J, Oksche A, Krause E, Tran TN, et al. Mass-spectrometric identification of a novel angiotensin peptide in human plasma. Arterioscler Thromb Vasc Biol. 2007;27:297–302. doi: 10.1161/01.ATV.0000253889.09765.5f. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, et al. Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem. 2004;279:23559–23564. doi: 10.1074/jbc.M314240200. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Smolders I, Vanderheyden P, Demaegdt H, Van Eeckhaut A, Vauquelin G, Lukaszuk A, Tourwé D, Chai SY, Albiston AL, et al. Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent. Hypertension. 2011;57:956–964. doi: 10.1161/HYPERTENSIONAHA.110.161836. [DOI] [PubMed] [Google Scholar]
- 20.Coutinho DC, Foureaux G, Rodrigues KD, Salles RL, Moraes PL, Murça TM, De Maria ML, Gomes ER, Santos RA, Guatimosim S, Ferreira AJ. Cardiovascular effects of angiotensin A: A novel peptide of the renin-angiotensin system. J Renin Angiotensin Aldosterone Syst. 2014;15:480–486. doi: 10.1177/1470320312474856. [DOI] [PubMed] [Google Scholar]
- 21.Qaradakhi T, Apostolopoulos V, Zulli A. Angiotensin (1–7) and Alamandine: Similarities and differences. Pharmacol Res. 2016;111:820–826. doi: 10.1016/j.phrs.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Ramires FJ, Zhou G, Ganjam VK, Weber KT. Fibrous tissue and angiotensin II. J Mol Cell Cardiol. 1997;29:2001–2012. doi: 10.1006/jmcc.1997.0451. [DOI] [PubMed] [Google Scholar]
- 23.Varo N, Etayo JC, Zalba G, Beaumont J, Iraburu MJ, Montiel C, Gil MJ, Monreal I, Díez J. Losartan inhibits the post-transcriptional synthesis of collagen type I and reverses left ventricular fibrosis in spontaneously hypertensive rats. J Hypertens. 1999;17:107–114. doi: 10.1097/00004872-199917010-00016. [DOI] [PubMed] [Google Scholar]
- 24.Brown L, Duce B, Miric G, Sernia C. Reversal of cardiac fibrosis in deoxycorticosterone acetate-salt hypertensive rats by inhibition of the renin-angiotensin system. J Am Soc Nephrol. 1999;10(Suppl 11):S143–S148. [PubMed] [Google Scholar]
- 25.Crawford DC, Chobanian AV, Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ Res. 1994;74:727–739. doi: 10.1161/01.RES.74.4.727. [DOI] [PubMed] [Google Scholar]
- 26.Athyros VG, Mikhailidis DP, Kakafika AI, Tziomalos K, Karagiannis A. Angiotensin II reactivation and aldosterone escape phenomena in renin-angiotensin-aldosterone system blockade: Is oral renin inhibition the solution? Expert Opin Pharmacother. 2007;8:529–535. doi: 10.1517/14656566.8.5.529. [DOI] [PubMed] [Google Scholar]
- 27.Zhao QD, Viswanadhapalli S, Williams P, Shi Q, Tan C, Yi X, Bhandari B, Abboud HE. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFkB signaling pathways. Circulation. 2015;131:643–655. doi: 10.1161/CIRCULATIONAHA.114.011079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CY, Hsu YJ, Hsu SC, Chen Y, Lee HS, Lin SH, Huang SM, Tsai CS, Shih CC. CB1 cannabinoid receptor antagonist attenuates left ventricular hypertrophy and Akt-mediated cardiac fibrosis in experimental uremia. J Mol Cell Cardiol. 2015;85:249–261. doi: 10.1016/j.yjmcc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Tian B, Liu J, Bitterman P, Bache RJ. Angiotensin II modulates nitric oxide-induced cardiac fibroblast apoptosis by activation of AKT/PKB. Am J Physiol Heart Circ Physiol. 2003;285:H1105–1112. doi: 10.1152/ajpheart.01139.2002. [DOI] [PubMed] [Google Scholar]
- 30.Arumugam S, Sreedhar R, Thandavarayan RA, Karuppagounder V, Krishnamurthy P, Suzuki K, Nakamura M, Watanabe K. Angiotensin receptor blockers: Focus on cardiac and renal injury. Trends Cardiovasc Med. 2016;26:221–228. doi: 10.1016/j.tcm.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Zulli A, Hare DL, Buxton BF, Widdop RE. Vasoactive role for angiotensin II type 2 receptors in human radial artery. Int J Immunopathol Pharmacol. 2014;27:79–85. doi: 10.1177/039463201402700110. [DOI] [PubMed] [Google Scholar]
- 32.Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/S0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 33.Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, et al. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura S, Uno M, Kaneta Y, Fukuchi K, Nishigohri H, Hasegawa J, Komori H, Takeda S, Enomoto K, Nara F, Agatsuma T. MRGD, a MAS-related G-protein coupled receptor, promotes tumorigenisis and is highly expressed in lung cancer. PLoS One. 2012;7:e38618. doi: 10.1371/journal.pone.0038618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Council NR. The National Academies Press; Washington, DC: 1996. Guide for the care and use of laboratory animals. [PubMed] [Google Scholar]
- 36.Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, et al. AVMA guidelines for the euthanasia of animals: 2013 Edition. American Veterinary Medical Association. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.