Abstract
Enteroviruses are RNA viruses within the Picornaviridae family. Enteroviruses derive their name from the way they are typically transmitted via the intestinal tract. They commonly infect millions of people every year and often do not cause severe disease in immunocompetent patients with few exceptions. Aseptic meningitis is a classic manifestation and is usually self-limited, however, can lead to severe neurological complications in an immunocompromised individual. It has been well-described that patients with hypogammaglobulinemia are predisposed to developing chronic enteroviral meningoencephalitis [1]. This is the first reported case of enteroviral meningoencephalitis in a patient being treated for psoriatic arthritis with rituximab. Here we describe a 46-year-old female who presented with altered mental status, fever, and myalgia. Polymerase chain reaction (PCR) of her cerebrospinal fluid (CSF) confirmed the presence of enterovirus. In the immunocompromised patient with encephalopathy, it is important to consider an enteroviral infection. This case adds to the present body of knowledge about enteroviral infections in immunocompromised hosts.
Keywords: Rituximab, Enterovirus, Enteroviral meningoencephalitis, Viral encephalopathy, Psoriatic arthritis
Introduction
Rituximab is a chimeric anti-CD20 monoclonal antibody that induces a long-lasting B-cell depletion. It is presently FDA-approved for the treatment of non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangitis, microscopic polyangitis, and most recently pemphigus vulgaris [2]. It has also been successfully used as a second-line agent in the treatment of psoriatic arthritis [3]. Typically, the drug has a respectable safety profile. There is a Black Box Warning for rituximab for a potentially fatal first-time infusion reaction, severe mucocutaneous reaction, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Enteroviral meningoencephalitis is a lesser known but clinically significant complication of rituximab that has been recognized the recent years.
Enteroviruses remain the most common cause of viral meningitis in the United States with encephalitis recognized as a potential complication. Among immunocompetent individuals, children are affected more frequently than adults. Typical meningitis presentation in adults can include fever, headache, stiff neck, vesicular lesions, and lymphocytic pleocytosis on CSF. Rapid viral detection is imperative in establishing a proper diagnosis that can ultimately improve patient outcome and avoid unnecessary antibacterial usage and testing.
There have been several cases of enteroviral meningoencephalitis described in patients on rituximab since the first reported case in 2003 [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]] (Table 1). All of these patients were receiving rituximab in the setting of hematologic diseases. We report a novel case of enteroviral meningoencephalitis in a patient on prolonged rituximab therapy for psoriatic arthritis.
Table 1.
Review of rituximab-associated enteroviral meningoencephalitis cases in the literature.
| Age & Sex | Indication for Rituximab | Time since last Rituximab | Symptoms | CSF Findings | Treatment & Outcome | |
|---|---|---|---|---|---|---|
| Quartier et al. [4] | 10M | AITP | 18 months post first cycle, 11 months post second cycle | -Altered cognitive function -Aphasia -Sensorimotor deafness -Mild fever -Extrapyramidal syndrome |
-Lymphocytosis -Increased protein -EV RNA |
-High dose IVIG & Pleconaril -Resolution with residual learning disabilities & behavioral problems |
| Quartier et al. [4] | 55M | FL | 6 months | -Fever -Headaches -Diffuse paresthesia -Trouble concentrating -Sensorimotor deafness -Diplopia -Pyramidal syndrome -Ataxia |
-Lymphocytosis -Increased protein -EV RNA |
-High dose IVIG & Pleconaril -Resolution with residual deafness & mild pyramidal syndrome |
| Garzo-Caldas et al. [5] | 66M | WM | 1 month | -Drowsiness -Fever -Encephalopathy |
-Lymphocytosis -Increased protein -Normal glucose -PCR negative (Enterovirus detection on right temporal lobe biopsy) |
-IVIG -Transient improvement, died 7 months later from pneumonia |
| Ganjoo et al. [6] | 42M | DLBCL | 16 months (NR) |
-Progressive neurologic debilitating dysfunction -Dysarthria -Nystagmus -Psychomotor retardation |
-Lymphocytosis -Increased protein -Normal glucose -EV RNA |
-Died before planned administration of IVIG |
| Shaheen et al. [7] | 5M | BL | During treatment | -Fever -Seizures -Impaired consciousness -Globally increased tone -Brisk reflexes -Positive Babinski reflex |
-Lymphocytosis -Increased protein -EV RNA |
-IVIG -Resolution with residual impaired cognition, aphasia, and movement disorders |
| Grisarui et al. [8] | 6 patients described: 65 33 34 67 62 62 |
FL FL DLBCL MCL FL FL |
Various | -Fever -Weight loss -Cognitive impairment -Hypoalbuminemia -Headache -Cough -Reduced DLCO -Muscle weakness -Rash -Tinnitus -Axonal polyneuropathy -Hearing loss -Gait disturbances |
-Lymphocytosis -Increased protein -EV RNA -Decreased glucose |
-IVIG, death -IVIG, Resolution -No specific treatment, Resolution -IVIG, Resolution with residual hearing loss -IVIG, death -IVIG, Resolution |
| Kassab et al. [9] | 66F | FL | 2 months, on maintenance therapy | -Fever -Asthenia -Psychomotor retardation -Aphasia -Facial paralysis -Spastic movements -Impaired consciousness |
-Lymphocytosis -Increased protein -Normal to mildly increased glucose on 3 CSF samples -EV RNA |
-No specific treatment -Died 32 days after admission |
| Schilthuizen et al. [10] | 64F | MZL | During maintenance therapy | -Fever -Nausea/Vomiting -Weight loss/Fatigue |
-Lymphocytosis -Increased protein -EV RNA |
-IVIG -Resolution |
| Padate et al. [11] | 75M | DLBCL | 7 months | -Gastroenteritis -Septicemia -Low GCS -Fever -Confusion -Agitation, disinhibition |
-Lymphocytosis -Increased protein -CSF initially negative to enterovirus, transformed to positive for EV RNA |
-Broad-spectrum antibiotics, acyclovir, ganciclovir, IVIG -Clinical improvement followed by neurological deterioration and death due to intercurrent infection |
| Servais et al. [12] | 61F | DLBCL | 4 months | -Confusion -Ataxia -Cerebellar syndrome |
-Lymphocytosis -Increased protein -Normal glucose -Initially negative, positive for EV RNA 2 months later |
-IVIG -Resolution with neurological improvement |
| Kiani-Alikhan et al. [13] | 53M | DLBCL | During treatment | -Fever -Ataxia -Difficulty concentrating -Dysarthria -Cerebellar signs -Positive Babinski reflex |
-Lymphocytosis -Initially negative, positive for EV RNA 1 month later |
-IVIG Neurological deterioration and death |
| Palacios et al. [14] | 28F | Evans Syndrome & ITP | 12 years | -Sensorineural hearing loss -Fever -Neurocognitive decline |
-Lymphocytosis -Increased protein -Initially persistently negative, EV RNA found on brain biopsy and CSF per CDC |
-IVIG -Died following left MCA ischemic stroke while in hospice |
| Our Patient | 46F | Psoriatic arthritis | 4 months | -Fever -Myalgias -Cough -Obtundation -Generalized stiffness -Decreased visual acuity |
-Lymphocytosis -Increased protein -Normal glucose -EV RNA |
-IVIG -Residual deficits including cortical blindness, hallucinations, discoordination, intermittent altered mentation |
AITP, autoimmune thrombocytopenia; FL, follicular lymphoma; WM, Waldenstrom’s macroglobulinemia; DLBCL, diffuse large B cell lymphoma; BL, Burkitt lymphoma; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; GCS, Glasgow Coma Scale; EV, enteroviral; ITP, idiopathic thrombocytopenic purpura; CDC, Centers for Disease Control and Prevention.
Case presentation
A 46-year-old African American female presented to an outside hospital in late November 2017 due to altered mentation and flu-like symptoms. Her past medical history was significant for bipolar disorder, 1 seizure episode at age 17, and psoriatic arthritis treated with rituximab infusions since 2014. Her last known rituximab infusion was July 2017. Prior to seeking medical care, the patient tried multiple doses of Theraflu (acetaminophen, dextromethorphan, and phenylephrine) because she attributed her symptomatology to influenza.
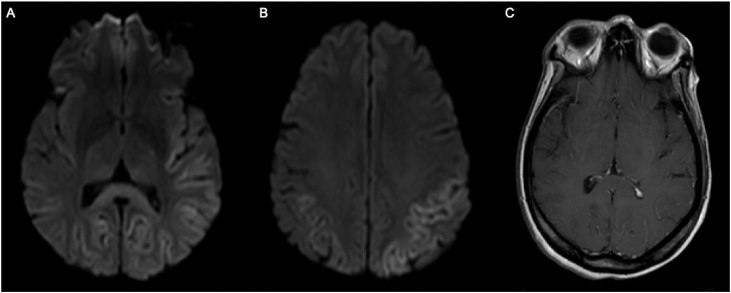
She complained of a subjective tactile fever, myalgias, and cough. Initial physical exam revealed the patient to be normotensive and afebrile, but she did have nuchal rigidity, was obtunded and showed generalized stiffness with her arms flexed and legs extended. Initial emergency department workup included a CT scan of the brain without contrast that showed no mass effect or hemorrhages and normal gray-white differentiation. Complete blood count (CBC) on admission showed a leukocytosis of 18,000/uL with an absolute neutrophil count of 15,600/uL. C-reactive protein (CRP) was elevated at 156.8 mg/L. A lumbar puncture showed a high WBC count of 161/uL with 70% lymphocytes, 25% PMNs, 98 mg/dL protein, and 55 mg/dL glucose. The patient was placed on isolation precautions and empirically started on ceftriaxone, vancomycin, and acyclovir. A CSF BioFire Meningitis/Encephalitis Panel revealed the presence of enterovirus. As a result, empiric antimicrobials were discontinued. On subsequent hospital days, the patient continued to have generalized stiffness, was unable to follow commands, and remained nonverbal. She experienced recurrent febrile episodes as well as multiple witnessed generalized tonic-clonic seizures. An EEG showed abnormal bihemispheric slowing suggestive of nonspecific diffuse encephalopathy. Attempted brain MRI on hospital day 5 was not successful. The patient did not improve, had recurrent febrile episodes with leukocytosis, and frequent seizures. A brain MRI under sedation revealed restricted diffusion in the bilateral cortical and subcortical regions of the parietal and posterior temporal lobes, splenium of the corpus callosum, and bilaterally in the posterior thalamus (Fig. 1). These findings raised concern for posterior reversible encephalopathy syndrome (PRES) at the outside facility.
Fig. 1.
MRI Brain: Fig. 1A Axial DWI. Restricted diffusion in posterior temporal, parietal, and bilateral occipital lobe cortex. Restricted diffusion in splenium of corpus callosum and bilateral posterior thalami. Fig. 1B Axial DWI. Restricted diffusion in parietal lobe cortex. Fig. 1C Axial T1. Leptomeningeal enhancement.
The patient was transferred to our tertiary care facility. On arrival, the patient remained altered with disorientation to person, place, and time. She showed left gaze preference and was unable to follow simple one-step commands. Her reflexes, muscle tone, and sensorium were normal. CRP was elevated at 64.2 mg/L. Her liver function studies and pancreatic studies were unremarkable. Intracranial and extracranial CT angiography showed no abnormalities. Brain MRI showed restricted diffusion in the splenium of the corpus callosum. Left greater than right restricted diffusion in the temporoparietal cortex was present. Subtle leptomeningeal enhancement on the left was also observed. Folate, vitamin B12, vitamin B1, and TSH levels were all within the normal reference ranges.
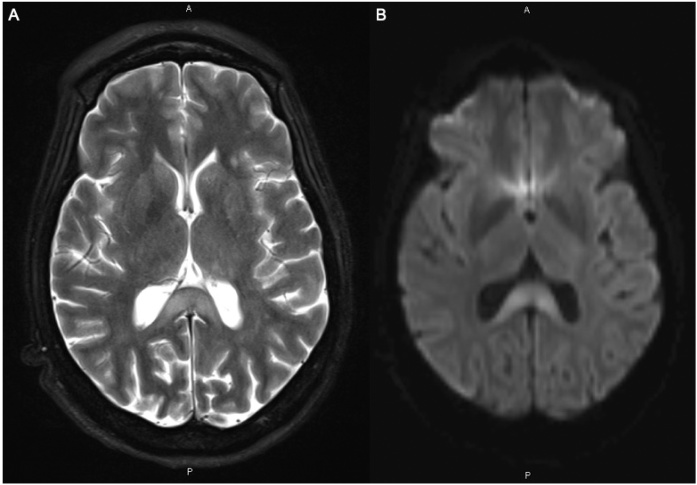
The patient improved clinically and was able to follow one-step commands. However, she had intermittent episodes of agitation requiring four-point restraints. She was placed on olanzapine as needed with haloperidol for breakthrough agitation. Follow-up brain MRI two weeks later showed stable FLAIR and T2 signal abnormalities within the splenium of the corpus callosum and thalami with no residual hypointensity on ADC map images, indicating no persisting restricted diffusion in these locations. Previous temporoparietal diffusion hyperintensities also had returned to normal and no new areas of signal abnormalities were identified (Fig. 2). At this point, the patient was awaiting placement in a skilled nursing facility. Her mentation and orientation continued to improve. Her episodes of aggression and agitation lessened.
Fig. 2.
MRI Brain: Fig. 2A Axial T2. T2 hyperintensity persists in splenium of corpus callosum. Fig. 2B Axial DWI. Residual hyperintense signal in splenium of corpus callosum is present, in the absence of ADC map hypointensity. This combination of findings indicates “T2 shine through” effect, which follows the acute phase of cytotoxic edema after approximately 2 weeks. The distinction is important as this pattern reflects chronic injury as opposed to new or ongoing injury. Diffusion weighted hyperintensity associated with the prior restricted diffusion in the cortex has subsided, suggesting “pseudonormalization” after ischemic/infectious insult.
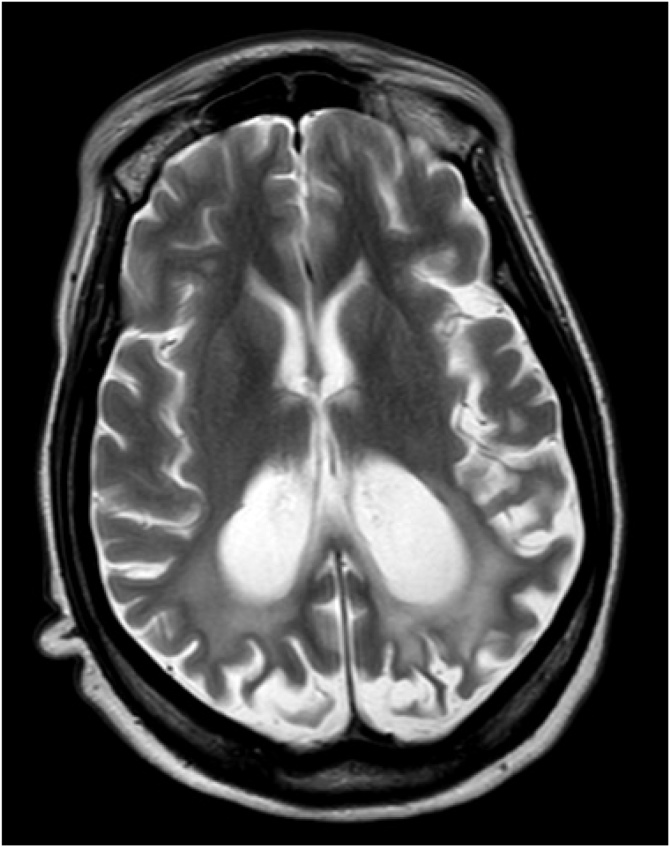
A month after admission, the patient complained of decreased visual acuity. She was found to have decreased bilateral blink-to-threat reflexes with left-gaze preference. However, she was able to distinguish colors and track around the room. As a result, a repeat brain MRI was done on January 10, 2018. New cortical-restricted diffusion was present in the bilateral parietal and occipital lobes. Stable abnormal diffusion abnormality within the splenium of the corpus callosum and thalami remained (Fig. 3). These findings were concerning for Creutzfeldt-Jakob Disease but a 14-3-3 protein analysis was found to be within the normal reference range and real-time quaking-induced conversion failed to detect abnormal prion proteins.
Fig. 3.
MRI Brain Axial FLAIR: Hyperintense signal in cortex of bilateral parietal lobes indicates cortical injury, and corresponds to the original location of signal abnormality in cortex.
Repeat CSF PCR for enterovirus was negative as well as a swab of the oropharynx. Cerebrospinal fluid testing for California (La Crosse) encephalitis, John Cunningham virus, Mycobacterium tuberculosis, Eastern equine encephalitis virus, Cryptococcus neoformans, VZV, HSV-1, HSV-2, CMV, VDRL for neurosyphilis, and angiotensin converting enzyme for neurosarcoidosis all proved to be negative. An India ink stain was performed and proved to be negative. Serum and plasma testing for hepatitis B virus, hepatitis C virus, HIV-1, HIV-2, John Cunningham virus, Borrelia burgdorferi, Borrelia mayonii, Borrelia Garinii, and Borrelia afzelii were all negative. The prior hospital had tested a CSF sample for West Nile virus and Streptococcus pneumoniae antigen, both of which were negative. Blood and CSF cultures showed no growth for bacteria or fungi. The patient did have a positive QuantiFERON-TB Gold test. However, a follow-up chest X-ray was negative, AFB sputum culture ultimately showed no growth for 42 days, and sputum PCR for Mycobaterium tuberculosis was negative.
A serum Epilepsy Autoimmune Evaluation (Mayo Clinic Laboratories) failed to reveal any of the 19 autoantibodies tested. A serum NeoCerebellar Degeneration Paraneoplastic Profile with Recombx (Quest Diagnostics) was unremarkable for any autoantibodies indicative of cerebellar degeneration. CSF and serum paraneoplastic autoantibody evaluations were negative. The patient’s IgM, IgG, and IgA levels were all within the normal reference ranges. She ultimately received a five-day course of 400 mg/kg/day IVIG. Although the patient was not at baseline, she was discharged in an improved clinical condition with decreased visual acuity. She was instructed to follow-up as an outpatient with neurology, psychiatry, and infectious disease.
Discussion
The presence of enterovirus in the patient’s CSF was established with the use of a CSF BioFire Meningitis/Encephalitis Panel. This panel is a molecular test used to assess the presence of 14 bacterial, viral, and fungal pathogens. It has an overall sensitivity of 94.2% and specificity of 99.9% [15]. The patient later had a repeat CSF PCR analysis that was negative for the presence of enterovirus. However, there are documented cases in the literature in which a CSF PCR for enterovirus was negative yet positive confirmation was obtained through a brain biopsy [5,14]. Our patient did not undergo a brain biopsy.
The cortical and subcortical parietal and posterior temporal lobe diffusion abnormalities observed on brain MRI (Fig. 1) prior to transfer to our facility raised concern for posterior reversible encephalopathy syndrome (PRES). PRES typically has an acute presentation and commonly manifests as seizures, visual disturbances, headache, and altered mental status [17]. Hypertension is seen in over 70% of patients, and renal injury is frequently present [18]. There are cases in the literature of PRES occurring within hours to days after the infusion of rituximab with resolution of symptoms in 1–2 days and no lasting residual deficits [19]. Our patient persistently had a blood urea nitrogen level under 12 mg/dL and a creatinine under 0.90 mg/dL. Her last rituximab infusion was 4 months prior to presentation which is inconsistent with prior cases of PRES after rituximab. In addition, she continues to have neurological deficits including decreased visual acuity. Given the aforementioned, PRES is an unlikely etiology.
Determining the etiology of encephalitis can be diagnostically challenging and even careful examination of a brain biopsy specimen can fall short [16]. Prior reported cases of enteroviral meningoencephalitis in association with rituximab have demonstrated thalamus and basal ganglia involvement on MRI, which is similar to what we have observed in our patient [6,7,9]. Autoimmune encephalitis was considered highly unlikely as all neurologic antibody studies were negative. Although this does not completely exclude autoimmune encephalitis as a possible etiology, it is interesting to note that rituximab is recommended as part of the treatment for autoimmune encephalitis, even in the absence of an identified antibody [20].
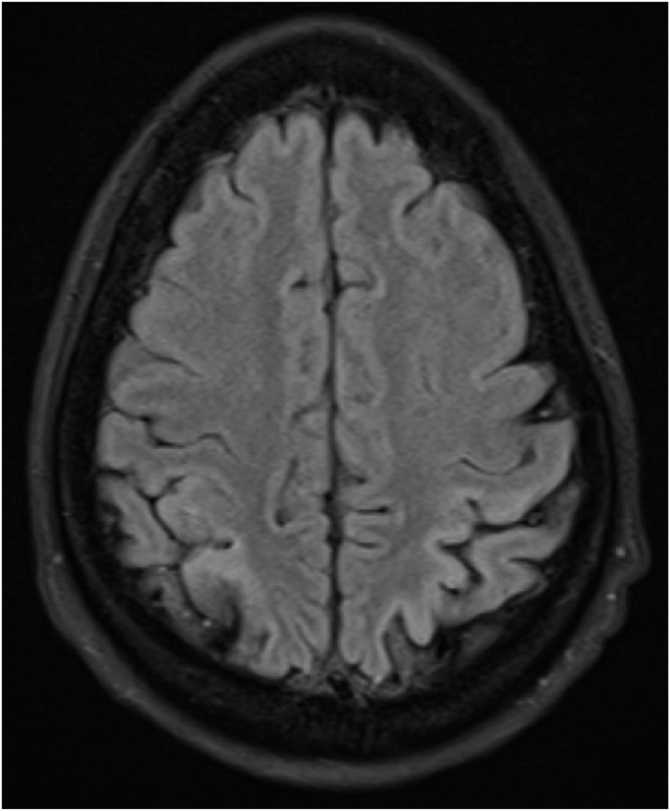
Previously, the administration of high-dose IVIG and pleconaril, an antipicornaviral agent, were the mainstay of treatment for enteroviral meningoencephalitis [1,4,10]. However, there is very little evidence in the present body of medical literature to support this. In addition, pleconaril is no longer available in the United States. Previous reports of patients with rituximab related enteroviral meningoencephalitis describe lasting neurologic residual deficits and behavioral problems [4,7,8]. This is consistent with our patient’s current state. She has cortical blindness, episodes of altered mental status with agitation and visual hallucinations, aphasia requiring speech therapy, altered balance and discoordination requiring physical therapy. Her most recent MRI done 10 months after discharge shows ex vacuo dilatation of the occipital horns of the lateral ventricles and bilateral occipital lobe encephalomalacia (Fig. 4). Chronic meningoencephalitis is a rare and potentially fatal complication of a typically nonthreatening pathogen. It should be an important clinical consideration in an immunocompromised patient on prolonged rituximab therapy.
Fig. 4.
MRI Brain Axial T2: regional bilateral enlargement of the posterior bodies and trigones of lateral ventricles indicates deep white matter volume loss. Cortical loss and thinning correspond to locations where prior studies had shown FLAIR and T2 hyperintense signal, restricted diffusion on DWI, and leptomeningeal enhancement.
Conflict of interests
On behalf of all authors, there are no conflicts of interests to declare.
CRediT authorship contribution statement
Roberto Tellez: Writing - original draft, Writing - review & editing. Allison M. Lastinger: Conceptualization, Supervision, Writing - review & editing. Jeffery P. Hogg: Writing - review & editing.
Acknowledgements
None.
Contributor Information
Roberto Tellez, Email: rt0004@mix.wvu.edu.
Allison M. Lastinger, Email: alastinger@hsc.wvu.edu.
Jeffery P. Hogg, Email: jhogg@hsc.wvu.edu.
References
- 1.Radanović I., Rkman D., Zekan P., Kutleša M., Baršić B. Chronic meningoencephalitis caused by echo virus 6 in a patient with common variable immunodeficiency. Wien Klin Wochenschr. 2017;130(1-2):70–72. doi: 10.1007/s00508-017-1289-5. [DOI] [PubMed] [Google Scholar]
- 2.Joly P., Maho-Vaillant M., Prost-Squarcioni C. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389(10083):2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 3.Cantini F., Niccoli L., Nannini C. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47(2):183–192. doi: 10.1016/j.semarthrit.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Quartier P., Tournilhac O., Archimbaud C. Enteroviral meningoencephalitis after anti‐CD20 (Rituximab) treatment. Clin Infect Dis. 2003;36(3):e47–e49. doi: 10.1086/345746. [DOI] [PubMed] [Google Scholar]
- 5.Garzo-Caldas N., Ruiz-Sainz E., Vila-Bedmar S. Enteroviral T-cell encephalitis related to immunosuppressive therapy including rituximab. Neurology. 2017;89(4):408–409. doi: 10.1212/WNL.0000000000004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganjoo K., Raman R., Sobel R., Pinto H. Opportunistic enteroviral meningoencephalitis: an unusual treatable complication of rituximab therapy. Leuk Lymphoma. 2009;50(4):673–675. doi: 10.1080/10428190902782210. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen N., Mussai F. Enteroviral encephalitis in a child with CNS relapse of Burkitt leukemia treated with rituximab. J Pediatr Hematol Oncol. 2019;41(1):e27–e29. doi: 10.1097/MPH.0000000000001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grisariu S., Vaxman I., Gatt M. Enteroviral infection in patients treated with rituximab for non-Hodgkin lymphoma: a case series and review of the literature. Hematol Oncol. 2016;35(4):591–598. doi: 10.1002/hon.2365. [DOI] [PubMed] [Google Scholar]
- 9.Kassab S., Saghi T., Boyer A. Fatal case of enterovirus 71 infection and rituximab therapy, France, 2012. Emerg Infect Dis. 2013;19(8) doi: 10.3201/eid1908.130202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilthuizen C., Berenschot H.W., Levin M.D. Enteroviral encephalitis in a patient with a marginal zone lymphoma treated with rituximab. Neth J Med. 2010;68:221–223. [PubMed] [Google Scholar]
- 11.Padate B., Keidan J. Enteroviral meningoencephalitis in a patient with non-Hodgkin’s lymphoma treated previously with rituximab. Clin Lab Haematol. 2006;28(1):69–71. doi: 10.1111/j.1365-2257.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Servais S., Caers J., Warling O. Enteroviral meningoencephalitis as complication of rituximab therapy in a patient treated for diffuse large B-cell lymphoma. Br J Haematol. 2010;150(3):379–381. doi: 10.1111/j.1365-2141.2010.08202.x. [DOI] [PubMed] [Google Scholar]
- 13.Kiani-Alikhan S., Skoulidis F., Barroso A. Enterovirus infection of neuronal cells post-rituximab. Br J Haematol. 2009;146(3):333–335. doi: 10.1111/j.1365-2141.2009.07748.x. [DOI] [PubMed] [Google Scholar]
- 14.Palacios T., Bartelt L., Scheld W. Fatal coxsackie meningoencephalitis in a patient with B-cell lymphopenia and hypogammaglobulinemia following rituximab therapy. Ann Allergy Asthma Immunol. 2015;115(2):148–150. doi: 10.1016/j.anai.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2016. FilmArray Meningitis/Encephalitis Panel- BioFire diagnostics FimArray Meningitis/Encephalitis Panel- BioFire Diagnostics.http://www.biofiredx.com/research/ Available from. [Google Scholar]
- 16.Gelfand J., Genrich G., Green A., Tihan T., Cree B. Encephalitis of unclear origin diagnosed by brain biopsy. JAMA Neurol. 2015;72(1):66. doi: 10.1001/jamaneurol.2014.2376. [DOI] [PubMed] [Google Scholar]
- 17.Fugate J., Claassen D., Cloft H., Kallmes D., Kozak O., Rabinstein A. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427–432. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hobson E., Craven I., Blank S. Posterior reversible encephalopathy syndrome: a truly treatable neurologic illness. Perit Dial Int. 2012;32(6):590–594. doi: 10.3747/pdi.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqi A. Rituximab as a possible cause of posterior reversible encephalopathy syndrome. Aust Med J. 2011;4(9):513–515. doi: 10.4066/AMJ.2011.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prüss H. Postviral autoimmune encephalitis. Curr Opin Neurol. 2017;30(3):327–333. doi: 10.1097/WCO.0000000000000445. [DOI] [PubMed] [Google Scholar]