Abstract
Consumer demand for natural pathogen-control agents for substitution of synthetic food preservatives and traditional antibiotics is increasing. This study aimed to reveal the distribution of lactic acid bacteria (LAB) in raw camel milk and to characterize their antimicrobial traits. The genetic identification by 16S rRNA sequencing of 58 LAB isolates showed the predominance of Enterococcus (24.2%), Lactococcus (22.4%) and Pediococcus (20.7%) genera in raw camel milk. These genera exhibited inhibitory activity against a broad spectrum of Gram-positive and Gram-negative bacteria including multidrug-resistant Salmonella. Among these LAB, two isolates—identified as Pediococcus pentosaceus CM16 and Lactobacillus brevis CM22—were selected for their strong bacteriocinogenic anti-listerial activity estimated at 1600 and 800 AU/mL, respectively. The bacteriocins produced were partially purified by ammonium sulphate precipitation and gel filtration and then biochemically characterized. The proteinaceous nature of bacteriocins was confirmed by the susceptibility to enzymes. These bacteriocins showed significant technological characteristics such as heat-resistance, and stability over a wide range of pH (2.0–10.0). In conclusion, these results indicated that Pediococcus pentosaceus CM16 and Lactobacillus brevis CM22 could be useful as potential probiotics. Moreover, their partially purified bacteriocins may play an important role as food preservatives and feed additives. To our knowledge, this is the first report describing the distribution of LAB population in raw camel milk and the characterization of their bacteriocins from the Arabian Peninsula of western Asia.
Keywords: Anti-listerial, bacteriocins, camel milk, food preservation, lactic acid bacteria
Introduction
Camel milk is a highly nutritious medium permissive for the growth of many diverse bacterial species that have important technological characteristics, health-promoting effects and the ability to produce many antimicrobials that might be used as food preservatives [1]. Although the microbiological characterization of this milk is worth investigating, few studies have been conducted on the microbiota of camel milk including lactic acid bacteria (LAB) [2]. LAB are the dominant population in raw milk, playing a key role in food fermentation processes and food preservation through the production of a variety of antimicrobials such as organic acids, hydrogen peroxide, antifungal peptides and bacteriocins [1]. Seven genera of LAB were identified in camel milk from different countries, including Enterococcus, Lactobacillus, Lactococcus, Streptococcus, Leuconostoc, Pediococcus and Weissella. LAB isolates were dominated by the genus Enterococcus in Kazakhstan and Iran [3], [4]. Whereas, in Sudan and Morocco, the genera Streptococcus and Lactobacillus were identified as the major groups, respectively [5], [6], [7]. Furthermore, the genus Lactococcus was one of the most represented genera in Morocco, Sudan, Kazakhstan and the United Arab Emirates [2], [3], [7], [8]. Besides being one of the major genera in Morocco and Kazakhstan, Leuconostoc was the most abundant in Kenya [3], [7], [9]. Pediococcus was the less represented genus in Morocco and Iran, whereas, the genus Weissella was detected only in Iran [4], [7].
Generally, LAB used as probiotics and their partially purified bacteriocins are isolated from food matrices in which those microorganisms are used. Therefore, the isolation of LAB from camels as potential probiotics and the characterization of their bacteriocins are necessary to control food-borne pathogens in the dairy industry, particularly in camel milk and its by-products. The beneficial effect of LAB and their bacteriocins is not limited to food preservation, but they are also considered as an alternative to traditional antibiotics—specifically in controlling the major global problem of antimicrobial resistance [10]. Despite advances in the treatment of food-borne diseases, pathogenic multidrug-resistant microorganisms are an important threat to both human and animal health worldwide.
Bacteriocins and bacteriocin-producing LAB have been isolated from raw camel milk and have demonstrated antimicrobial activity against a broad-spectrum of Gram-positive and Gram-negative bacterial strains. A recent study reported an inhibitory activity of the LAB strain Enterococcus faecium LCW44 isolated from raw camel milk against Listeria sp., Staphylococcus aureus and other LAB [11]. A Lactobacillus casei TN-2 strain isolated from fermented camel milk showed antimicrobial activity against Escherichia coli and Staphylococcus aureus. The purified bacteriocin produced by this strain, caseicin TN-2, exhibited a broad antimicrobial spectrum against food-borne pathogens including some antibiotic-resistant strains [12]. In addition, the Lactobacillus acidophilus AA105 strain isolated from raw camel milk strongly inhibited Staphylococcus sp., Bacillus sp., Salmonella paratyphi, Shigella sp. and Escherichia coli [13]. Benmechernene et al. [14] demonstrated the antimicrobial activity of a bacteriocin-producing Leuconostoc mesenteroides strain against other LAB, such as Lactobacillus sp., Lactococcus sp., and against several pathogenic bacteria, such as Escherichia coli, Staphylococcus aureus and Listeria sp.
This work aimed to study the distribution of the LAB population in raw camel milk and to identify food-control agents. We report LAB strains displaying antimicrobial activity against a broad spectrum of food-borne pathogens and aetiological agents of animal diseases including multidrug-resistant Salmonella. These strains and their bacteriocins could be promising in optimizing animal-feed additives and substituting synthetic preservatives towards the preservation of animal and human health.
Materials and methods
Raw camel milk sampling and isolation of LAB
Twenty raw camel milk samples were collected in sterile bottles from the two main habitats of camels in Kuwait, Al-Wafra (southernmost area of Kuwait) and Kabad (northwest region of Kuwait). All samples were transported in ice-boxes to the laboratory and analysed immediately upon arrival. LAB were isolated using the spread-plate method on de Man, Rogosa and Sharpe (MRS) agar (Thermo Fisher Scientific, Waltham, MA, USA). The plates were incubated at 37°C for 48 h under anaerobic conditions. After incubation, the colonies were counted, and representative colonies were selected (about 10% of the observed count) from each sample. Isolates possessing typical LAB characteristics (Gram-positive, catalase-negative, oxidase-negative) were inoculated into MRS broth and streaked to obtain pure cultures. Pure cultures were stored in glycerol (50%) at –80°C.
Genetic identification of LAB isolates
All LAB isolates were identified at the molecular level by 16S rRNA sequencing. Genomic DNA extraction from an overnight culture of the LAB was carried out using a GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer's instructions. PCR-mediated amplification of the 16S rDNA was carried out using a HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA, USA) under the following conditions: 94°C for 3 minutes, followed by 28 cycles of 94°C for 30 seconds; 53°C for 40 seconds and 72°C for 1 minute; after which a final elongation step at 72°C for 5 minutes was performed. Following PCR, all amplicon products from different samples were mixed in equal concentrations and purified using Ampure PB beads (Pacific Biosciences, Menlo Park, CA, USA). Purified PCR products were sequenced using PacBio Sequel chemistry following the manufacturer's protocols. The library for each sample was prepared using an SMRTbell Template Prep Kit (Pacific Biosciences) following the manufacturer's user guide. After completion of initial DNA sequencing, each library underwent a secondary analysis, Circular Consensus Sequencing, using PacBio's CCS2 algorithm.
Antimicrobial activity spectrum of LAB isolates
The antimicrobial activities of the identified isolates were determined according to the spot-on-the-lawn method as described by Hoover and Harlander [15]. LAB isolates were cultured in MRS broth at 37°C for 24 h, after which 1-μL aliquots were spotted on the surface of MRS agar and incubated at 37°C for 24 h under anaerobic conditions, then, the appropriate culture medium containing 0.8% (weight/volume) agar was inoculated with each indicator strain at 106 CFU/mL and overlaid on the LAB-spotted plates and incubated at the conditions required by each indicator strain. Results of triplicates were determined by measuring the diameter of the inhibition halos (clear zone) in millimetres. All indicator strains were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) including Salmonella enterica ATCC 13076, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Listeria monocytogenes ATCC 7644, Shigella flexneri ATCC 12022 and Pseudomonas aeruginosa ATCC 27853. The antimicrobial activity of the isolates was also tested against multidrug-resistant Salmonella isolated from local chicken caecum in a previous study [16].
Characterization of bacteriocins produced by LAB isolates
Evaluation of the anti-listerial potential of bacteriocins All LAB strains were tested for their ability to produce bacteriocins against Listeria monocytogenes ATCC 7644 by the well-diffusion assay as described previously [17]. The results of three assays were determined by measuring the diameter of the clear zone (in mm) around the wells. Zones of inhibition >5 mm were regarded as positive.
Bacteriocinogenic activity was measured by the well-diffusion assay following a two-fold serial dilution of the cell-free supernatant. The titre, in arbitrary units (AU) per millilitre, was defined as the reciprocal of the highest two-fold dilution still providing a distinct inhibition zone [18].
Partial purification of bacteriocins Cell-free supernatants of the bacteriocin-producing LAB strains were subjected to ammonium sulphate precipitation (40%, 60%, 80% and 100% saturation) according to Kumari et al. [19] and tested for anti-listerial activity using the well-diffusion assay as described previously [17]. Then, the partially purified bacteriocins were subjected to further purification by gel filtration chromatography (Superdex 75 10/300 GL; GE Healthcare Life Sciences, Chalfont St Giles, UK). Twenty-eight fractions of 0.5 mL each were collected on the chromatogram between 7 and 20 mL. The antimicrobial activity of the partially purified bacteriocins (1 μg) against Listeria monocytogenes ATCC 7644 was assayed by the agar well-diffusion method [17].
Biochemical characterization of bacteriocins The effect of enzymes, pH and temperature on bacteriocin activity was assessed on the partially purified bacteriocins. The bacteriocins were tested for their susceptibility to various enzymes (Sigma): trypsin, α-chymotrypsin, proteinase K, papain and protease (final concentration 1 mg/mL). Following incubation at 37°C for 2 h, reactions were heated at 80°C for 10 min to denature the enzymes and were then assayed for activity. As a control, a sample was treated with the enzyme buffer. To determine thermal stability, the bacteriocins were first heated for 3 h at 37°C, 60 min at 60°C and 80°C, 30 min at 100°C and 15 min at 121°C, and then cooled and assayed for activity. A non-heated control sample was kept at 4°C. The effect of pH on the bacteriocins was tested by adjusting the pH level between 2 and 10 (at increments of 2 pH unit) with sterile 1 m NaOH or 1 m HCl. Following incubation at 37°C for 1 h, the samples were re-adjusted to pH 6.5 and tested for anti-listerial activity. Untreated samples served as control.
Results and discussion
Genetic identification of LAB isolates
Fifty-eight bacterial colonies were characterized as possessing typical LAB characteristics. They were identified molecularly by direct sequencing of PCR-amplified 16S rDNA. The obtained sequences were compared with 16S rRNA sequences deposited in the RDPII (http://rdp.cme.msu.edu) and NCBI (https://www.ncbi.nlm.nih.gov) (Table 1). The isolates CM19, CM20, CM46, CM55 and CM61, which were classified to genus level (similarity index >98.7%), could be new species. The identity of these isolates will be further determined by whole genome sequencing. The distribution of the identified LAB isolates is summarized in Table 2. At the genus level, the dominant genus is Enterococcus (24.2%) followed by Lactococcus (22.4%), Pediococcus (20.7%), Lactobacillus (12%), Weissella (10.3%), Leuconostoc (6.9%) and Streptococcus (3.5%). These genera, which are typical dairy bacteria representing the most common LAB present in milk, have been identified in raw camel milk in several countries (Table 2). As Enterococcus can survive adverse conditions, including high-temperature and high-salinity environments [20], camel milk is typically dominated by this genus because of the high salt content in camel milk compared with other livestock animals. The predominance of this genus in raw camel milk was also reported in Morocco, Kazakhstan and Iran [3], [4], [21]. Lactococcus, which is a dominant genus in raw cow milk, was also detected in raw camel milk along with Pediococcus, Lactobacillus, Weissella, Leuconostoc and Streptococcus [2], [3], [4], [7], [21], [22].
Table 1.
Identified LAB isolates by 16S rRNA sequencing
| Isolate | Species | Identity % |
|---|---|---|
| CM1 | Lactobacillus salivarius | 99.9 |
| CM2 | Lactococcus garvieae | 99.9 |
| CM3 | Enterococcus faecium | 99.9 |
| CM4 | Lactobacillus reuteri | 99.9 |
| CM5 | Pediococcus acidilactici | 99.7 |
| CM6 | Lactococcus lactis | 99.9 |
| CM7 | Lactobacillus reuteri | 99.9 |
| CM8 | Pediococcus acidilactici | 99.7 |
| CM9 | Pediococcus acidilactici | 99.7 |
| CM10 | Lactobacillus fermentum | 99.9 |
| CM11 | Weissella sp. t4r2c13 | 99.9 |
| CM12 | Pediococcus acidilactici | 99.7 |
| CM13 | Pediococcus acidilactici | 99.7 |
| CM14 | Leuconostoc pseudomesenteroides | 100.0 |
| CM15 | Pediococcus pentosaceus | 100.0 |
| CM16 | Pediococcus pentosaceus | 100.0 |
| CM17 | Pediococcus pentosaceus | 99.0 |
| CM18 | Pediococcus pentosaceus | 99.0 |
| CM19 | Lactobacillus sp.a | 98.0 |
| CM20 | Pediococcus sp.a | 98.0 |
| CM21 | Enterococcus durans | 99.0 |
| CM22 | Lactobacillus brevis | 99.9 |
| CM23 | Pediococcus pentosaceus | 100.0 |
| CM26 | Enterococcus faecium | 99.9 |
| CM27 | Weissella confusa | 99.9 |
| CM28 | Weissella confusa | 99.9 |
| CM29 | Enterococcus gallinarum | 99.5 |
| CM30 | Pediococcus acidilactici | 99.7 |
| CM31 | Lactococcus lactis | 99.9 |
| CM32 | Lactobacillus reuteri | 99.9 |
| CM33 | Leuconostoc pseudomesenteroides | 100.0 |
| CM34 | Weissella sp. t4r2c13 | 99.9 |
| CM35 | Lactococcus lactis | 99.9 |
| CM36 | Lactococcus lactis | 99.9 |
| CM37 | Weissella confusa | 99.9 |
| CM38 | Lactococcus lactis | 99.9 |
| CM39 | Enterococcus faecium | 99.9 |
| CM40 | Lactococcus lactis | 99.9 |
| CM41 | Streptococcus infantarius subsp. infantarius | 99.9 |
| CM42 | Lactobacillus plantarum | 99.8 |
| CM43 | Lactococcus lactis | 99.9 |
| CM44 | Lactococcus lactis | 99.9 |
| CM45 | Weissella confusa | 99.9 |
| CM46 | Lactococcus sp.a | 97.8 |
| CM47 | Streptococcus subsp. infantarius | 99.9 |
| CM48 | Lactococcus lactis | 99.9 |
| CM49 | Leuconostoc pseudomesenteroides | 100.0 |
| CM50 | Lactococcus lactis | 98.9 |
| CM51 | Leuconostoc pseudomesenteroides | 100.0 |
| CM53 | Enterococcus faecium | 99.9 |
| CM54 | Enterococcus faecium | 99.9 |
| CM55 | Enterococcus sp.a | 98.3 |
| CM56 | Enterococcus faecium | 99.9 |
| CM57 | Enterococcus faecium | 99.9 |
| CM58 | Enterococcus faecium | 99.9 |
| CM59 | Enterococcus faecium | 99.9 |
| CM60 | Enterococcus faecium | 99.9 |
| CM61 | Enterococcus sp.a | 97.9 |
These isolates were classified to the genus level (similarity value <98.7%).
Table 2.
Distribution of LAB populations detected in raw camel milk
| Genus | Geographical area | Frequency | Reference |
|---|---|---|---|
| Enterococcus | Kuwait | 24.2 | This study |
| Morocco | 58.8 | Benkerroum et al.[21] | |
| Morocco | 10.8 | Khedid et al.[7] | |
| Iran | 51.0 | Davati et al.[4] | |
| Kazakhstan | 51.3 | Akhmetsadykova et al.[3] | |
| Lactococcus | Kuwait | 22.4 | This study |
| Morocco | 8.0 | Benkerroum et al.[21] | |
| Morocco | 25.8 | Khedid et al.[7] | |
| Kazakhstan | 10.9 | Akhmetsadykova et al.[3] | |
| Pediococcus | Kuwait | 20.7 | This study |
| Morocco | 28.2 | Benkerroum et al.[21] | |
| Morocco | 5.0 | Khedid et al.[7] | |
| Iran | 2.0 | Davati et al.[4] | |
| Lactobacillus | Kuwait | 12.0 | This study |
| Morocco | 37.5 | Khedid et al.[7] | |
| Iran | 11.0 | Davati et al.[4] | |
| Kazakhstan | 29.8 | Akhmetsadykova et al.[3] | |
| Weissella | Kuwait | 10.3 | This study |
| Iran | 2.0 | Davati et al.[4] | |
| Leuconostoc | Kuwait | 6.9 | This study |
| Morocco | 1.0 | Benkerroum et al.[21] | |
| Morocco | 11.7 | Khedid et al.[7] | |
| Iran | 5.0 | Davati et al.[4] | |
| Kazakhstan | 8.0 | Akhmetsadykova et al.[3] | |
| Streptococcus | Kuwait | 3.5 | This study |
| Morocco | 4.0 | Benkerroum et al.[21] | |
| Morocco | 9.2 | Khedid et al.[7] | |
| Somalia | 53.7 | Abera et al.[22] |
The most frequent species isolated were Enterococcus faecium (20.7%), Lactococcus lactis (17.2%), Pediococcus pentosaceus (9.8%), Pediococcus acidilactici (10.3%), Weissella confusa (6.9%), Leuconostoc pseudomesenteroides (6.9%) and Lactobacillus reuteri (5.2%). These species display important technological characteristics in the food industry: Enterococcus faecium plays a fundamental role in the manufacturing and ripening of a traditional European cheese originating from Mediterranean countries by adding a unique taste and flavour. This is possibly due to its proteolytic activity and its ability to hydrolyse milk fat. Apart from its role in the manufacturing of cheese, this genus acts as a preservative against various food-borne pathogens through producing antimicrobial peptides [23], [24]. Lactococcus lactis, Pediococcus pentosaceus, Pediococcus acidilactici, Weissella confusa, Leuconostoc pseudomesenteroides and Lactobacillus reuteri are used in the dairy industry as starter or adjunct cultures. In addition, they are currently available in the market as probiotics [1], [25], [26], [27], [28], [29].
Although many LAB are described as “generally recognized as safe”, some pathogenic LAB are responsible for human diseases [30]. In this study, Streptococcus infantarius subsp. infantarius represented 3.3% of the identified LAB isolates. It belongs to the Streptococcus bovis/Streptococcus equinus complex, some members of which are associated with endocarditis, bacteraemia and cancer of the colon [31]. This species was previously reported in fermented camel milk product from Sudan and Kenya [5], [32].
Antimicrobial activity of LAB isolates
From the LAB collection, representative isolates of each identified genus were tested for their antimicrobial activity against eight food-borne pathogens and aetiological agents causing animal diseases. Antimicrobial activities of these isolates ranged from 6- to 35-mm inhibition zones. Numerous isolates displayed strong antimicrobial activities against all the tested pathogens (Table 3). The results demonstrate that the antimicrobial activity of LAB against pathogens is species- and strain-dependent. This observation is in agreement with previous reports [2], [33]. The antimicrobial activity of these LAB was mainly due to the production of one or more active metabolites during their growth such as organic acids, hydrogen peroxide and bacteriocins.
Table 3.
Antimicrobial activity of representative LAB isolates against seven pathogens
| Bacteria | Antimicrobial activity (IZD)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| S. entericab | E. colic | St. aureusd | St. epidermidise | L. monocytogenesf | MDR-S. entericag | Sh. flexnerih | P. aeruginosai | ||
| CM1 | Lactobacillus salivarius | 35.0 ± 0.0 | 28.7 ± 1.5 | 28.0 ± 0.0 | 21.0 ± 1.0 | 28.0 ± 0.0 | 32.7 ± 0.6 | 16.0 ± 0.0 | — |
| CM2 | Lactococcus garvieae | 30.0 ± 0.0 | 12.0 ± 0.0 | 8.0 ± 0.0 | 6.0 ± 0.0 | 12.0 ± 0.0 | 19.0 ± 1.0 | 6.0 ± 0.0 | — |
| CM3 | Enterococcus faecium | 35.0 ± 1.0 | 35.0 ± 0.0 | 34.7 ± 0.6 | 20.0 ± 0.0 | 33.6 ± 1.5 | 27.0 ± 0.0 | 16.0 ± 0.0 | — |
| CM4 | Lactobacillus reuteri | 33.0 ± 1.7 | 30.0 ± 0.0 | 46.0 ± 0.0 | 14.3 ± 0.6 | 33.0 ± 1.0 | 33.7 ± 2.5 | 20.0 ± 0.0 | — |
| CM5 | Pediococcus acidilactici | 28.3 ± 1.5 | 34.7 ± 2.0 | 35.0 ± 0.0 | 27.0 ± 1.0 | 34.7 ± 2.0 | 27.0 ± 0.0 | 15.3 ± 1.5 | — |
| CM6 | Lactococcus lactis | 31.0 ± 1.0 | 25.0 ± 0.0 | 15.0 ± 0.0 | 17.3 ± 1.5 | 27.6 ± 1.5 | 25.0 ± 1.0 | 12.3 ± 2.5 | — |
| CM7 | Lactobacillus reuteri | 34.0 ± 1.0 | 21.7 ± 3.0 | 13.0 ± 1.0 | 14.0 ± 1.0 | 18.0 ± 0.0 | 18.0 ± 2.6 | 9.3 ± 1.5 | — |
| CM8 | Pediococcus acidilactici | 34.7 ± 2.5 | 25.0 ± 1.0 | 20.0 ± 0.0 | 19.7 ± 0.6 | 23.0 ± 1.0 | 28.0 ± 0.0 | 13.0 ± 2.0 | — |
| CM9 | Pediococcus acidilactici | 35.0 ± 1.0 | 34.7 ± 2.0 | 8.0 ± 0.0 | 35.0 ± 0.0 | 35.0 ± 1.0 | 34.0 ± 1.7 | 15.0 ± 0.0 | — |
| CM10 | Lactobacillus fermentum | 35.0 ± 1.0 | 33.0 ± 1.7 | 33.3 ± 2.5 | 28.0 ± 0.0 | 28.0 ± 0.0 | — | 16.0 ± 0.0 | — |
| CM11 | Weissella sp. T4R2C13 | 35.0 ± 2.6 | 35.0 ± 1.0 | 33.0 ± 2.0 | 23.0 ± 2.0 | 34.0 ± 1.0 | 34.3 ± 1.5 | 20.0 ± 0.0 | — |
| CM12 | Pediococcus acidilactici | 35.0 ± 2.0 | 22.0 ± 1.0 | 20.0 ± 0.0 | 18.0 ± 0.0 | 22.0 ± 0.0 | 29.3 ± 2.5 | 15.0 ± 0.0 | — |
| CM13 | Pediococcus acidilactici | 18.0 ± 1.0 | 35.0 ± 2.0 | 34.3 ± 2.0 | 23.7 ± 2.5 | 34.0 ± 0.0 | 16.3 ± 2.5 | 8.0 ± 0.0 | — |
| CM14 | Leuconostoc pseudomesenteroides | 30.0 ± 0.0 | 32.0 ± 2.0 | 15.0 ± 1.0 | 14.0 ± 2.0 | 25.0 ± 1.0 | 20.0 ± 0.0 | — | — |
| CM15 | Pediococcus pentosaceus | 26.0 ± 0.0 | 22.0 ± 0.0 | 23.0 ± 0.0 | 15.0 ± 0.0 | 22.0 ± 0.0 | 27.0 ± 2.0 | 16.0 ± 0.0 | — |
| CM16 | Pediococcus pentosaceus | 27.0 ± 2.0 | 23.0 ± 2.0 | 22.0 ± 0.0 | 14.0 ± 0.0 | 24.3 ± 1.5 | 28.0 ± 0.0 | 19.0 ± 2.0 | — |
| CM17 | Pediococcus pentosaceus | 34.7 ± 2.5 | 22.0 ± 0.0 | 29.0 ± 1.0 | 15.0 ± 1.0 | 22.0 ± 0.0 | 28.0 ± 0.0 | 15.0 ± 0.0 | — |
| CM18 | Pediococcus pentosaceus | 31.0 ± 1.0 | 25.0 ± 0.0 | 22.3 ± 2.5 | 16.0 ± 1.0 | 25.7 ± 3.0 | 28.0 ± 0.0 | 18.0 ± 2.0 | — |
| CM19 | Lactobacillus sp. | 14.0 ± 0.0 | 25.0 ± 0.0 | 28.0 ± 1.0 | 28.7 ± 3.0 | 29.0 ± 2.0 | 30.0 ± 2.0 | 16.0 ± 1.0 | — |
| CM20 | Pediococcus sp. | 31.0 ± 1.0 | — | 35.0 ± 0.0 | 34.7 ± 0.6 | 35.0 ± 3.0 | 30.0 ± 0.0 | — | — |
| CM21 | Enterococcus durans | 12.0 ± 0.0 | 29.0 ± 2.0 | 27.0 ± 2.0 | 17.0 ± 1.0 | 35.0 ± 1.0 | — | 15.0 ± 0.0 | — |
| CM22 | Lactobacillus brevis | 30.0 ± 0.0 | 17.0 ± 2.6 | 18.0 ± 0.0 | 8.0 ± 0.0 | 22.0 ± 0.0 | 23.0 ± 2.0 | 24.0 ± 0.0 | — |
| CM23 | Pediococcus pentosaceus | 35.0 ± 0.0 | 22.0 ± 1.0 | 25.0 ± 0 | 13.0 ± 1.0 | 28.0 ± 0.0 | 34.0 ± 2.0 | 27.0 ± 1.0 | — |
| CM27 | Weissella confusa | 26.0 ± 0.0 | 18.0 ± 0.0 | 25.0 ± 2.0 | 9.0 ± 1.0 | 19.7 ± 1.5 | 24.0 ± 1.0 | 23.0 ± 1.0 | 32.0 ± 0.0 |
| CM41 | Streptococcus infantarius | 17.0 ± 0.0 | 11.0 ± 0.0 | 16.5 ± 0.5 | 9.0 ± 1.0 | 14.0 ± 2.0 | 18.0 ± 1.0 | 14.0 ± 0.0 | 16.0 ± 1.0 |
| CM42 | Lactobacillus plantarum | 29.0 ± 1.0 | 24.0 ± 1.0 | 20.0 ± 0.0 | 17.0 ± 2.0 | 21.0 ± 1.0 | 27.0 ± 1.0 | 25.0 ± 0.0 | 32.0 ± 0.0 |
| CM47 | Streptococcus infantarius | 19.3 ± 0.6 | 13.0 ± 0.0 | 14.0 ± 0.0 | 8.0 ± 1.0 | 15.0 ± 1.0 | 19.0 ± 1.0 | 15.0 ± 1.0 | 31.0 ± 1.0 |
| CM57 | Weissella confusa | 25.0 ± 1.0 | 15.0 ± 0.0 | 17.0 ± 1.0 | 14.0 ± 1.0 | 17.0 ± 0.0 | 24.0 ± 2.0 | 20.0 ± 0.0 | 23.0 ± 1.0 |
ATCC, American Type Culture Collection; IZD, inhibition zone diameter; LAB, lactic acid bacteria; MDR, multidrug-resistant; — indicates no inhibition.
IZD, means of inhibition zone diameter of triplicate (mm) ± Standard Deviation.
IZD against Salmonella enterica ATCC 13076.
IZD against Escherichia coli ATCC 25922.
IZD against Staphylococcus aureus ATCC 25923.
IZD against Staphylococcus epidermidis ATCC 12228.
IZD against Listeria monocytogenes ATCC 7644.
IZD against multi-drug resistant Salmonella enterica.
IZD against Shigella flexneri ATCC 12022.
IZD against Pseudomonas aeruginosa ATCC 27853.
The antimicrobial activities of the isolates were also evaluated against a multidrug-resistant Salmonella strain isolated from local chicken's caecum in a previous study [16] and identified by 16S rRNA gene sequencing as a strain of Salmonella enterica subsp. enterica. This strain demonstrated its resistance to different groups of antibiotics whose modes of action involved the inhibition of either cell wall or protein synthesis. It displayed resistance to penicillin G (10 μg), ampicillin (10 μg), erythromycin (15 μg), clindamycin (10 μg), tetracycline (30 μg), vancomycin (30 μg) and bacitracin (10 μg). Interestingly, most of the tested isolates showed strong antimicrobial activity against this strain (Table 3).
Anti-listerial activity of the bacteriocins
Listeria monocytogenes is a ubiquitous pathogen responsible for listeriosis, which is potentially lethal in immunocompromised individuals [34]. It has the ability to grow at a wide range of temperatures (from 0°C to 50°C) and pH levels (as low as 4.5), and has been reported to be present in raw milk and cheese. As several listeriosis outbreaks have occurred following consumption of contaminated dairy products [23], effective antimicrobial agents against this pathogen are required. In this context, all LAB strains were tested for their ability to produce bacteriocins against Listeria monocytogenes ATCC 7644 by the well-diffusion assay as described previously [17]. Among these isolates, CM16 and CM22, which were identified as Pediococcus pentosaceus (NCBI accession number MH023512) and Lactobacillus brevis (NCBI accession number MH023515), respectively, showed anti-listerial activity estimated at 1600 and 800 AU/mL after neutralization of their cell-free supernatant at pH 6.5. The neutralized cell-free supernatant of these strains did not show a significant activity against the indicator strains listed in Table 3. Further tests will be conducted to evaluate their activity on other pathogenic bacteria. Some strains of Pediococcus pentosaceus are known for their production of the bacteriocins named pediocins and have been the focus of much research with regard to food preservation [26]. Regarding Lactobacillus brevis, a recent PCR-based study revealed the presence of genes encoding for the bacteriocin Brevicin 174A in five Lactobacillus brevis isolates using specific primers for this bacteriocin [35]. Few bacteriocins produced by this species isolated from various sources have been partially purified and characterized [36].
Partial purification of the bacteriocins
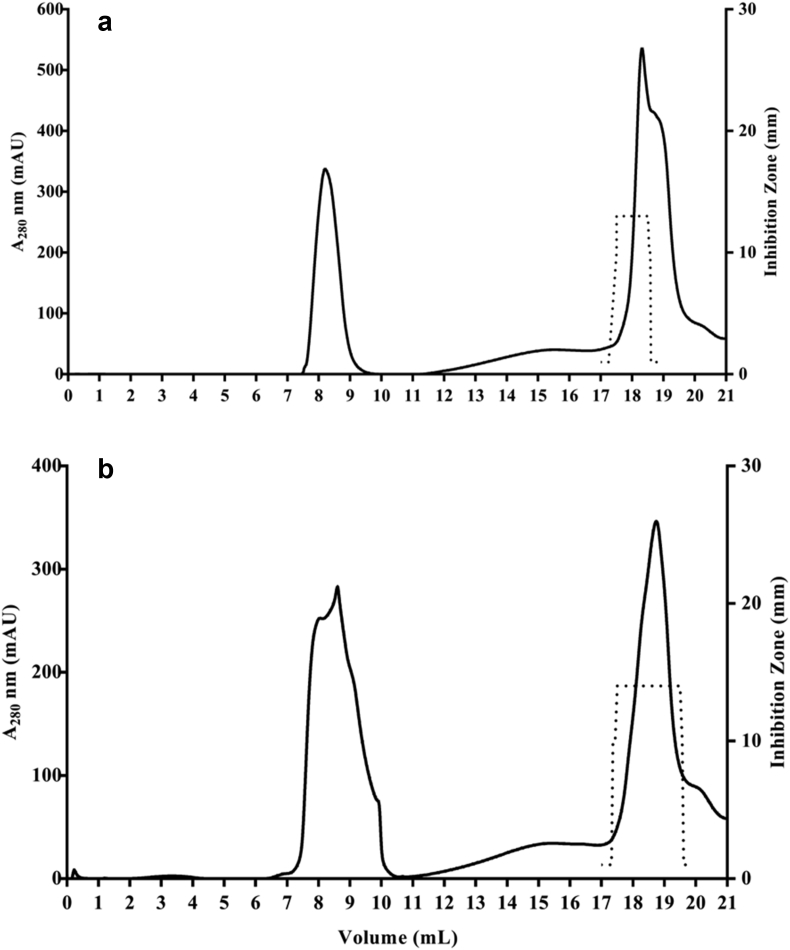
To prevent the growth of spoilage and pathogenic bacteria in food, bacteriocins are used as food preservatives, either by the addition of bacteriocin-producing strains or by direct addition of the semi-purified extracts. The optimal conditions for production of bacteriocins from CM16 and CM22 was determined as follows: overnight cultures of the isolate CM16 and CM22 were used to inoculate (1% v/v) 500 mL of MRS broth, (pH 6, incubation at 30°C, 120 rpm for 32 h for CM16 and pH 4, incubation at 37°C, 120 rpm for 36 h for CM22). The bacteriocins produced by CM16 and CM22 were partially purified from the culture supernatants with 40% and 60% ammonium sulphate, respectively, followed by further purification by gel filtration chromatography (Superdex 75 10/300 GL; GE Healthcare Life Sciences). Fig. 1 shows the chromatography profile of the bacteriocins from CM16 (Fig. 1a) and CM22 (Fig. 1b). Collected fractions were assayed for anti-listerial activity by the agar well-diffusion method. Active fractions were detected as a peak on the chromatogram between 17.5 and 18.5 mL for CM16 and between 17.5 and 18.5 mL for CM22. This elution volume corresponds to a molecular weight between 1.3 and 6.5 kDa as per the calibration.
Fig. 1.
Purification of bacteriocins after ammonium sulphate precipitation from Pediococcus pentosaceus CM16 (a) and Lactobacillus brevis CM22 isolates (b) by gel filtration chromatography using Superdex 75 10/300 GL. The peaks with antimicrobial activity were observed at 17.5–18.5 mL for CM16 and 17.5–18.5 mL for CM22. The dot plots represent the inhibition zone diameter of fractions (mm).
Characterization of the bacteriocins
To determine the biochemical properties of the antimicrobial compounds produced, the partially purified bacteriocins were tested for sensitivity to different enzymes, temperatures and pH levels (Table 4). Enzyme sensitivity assays demonstrated a complete elimination of the inhibitory activity of the bacteriocins produced by CM16 and CM22 after treatment with α-chymotrypsin, proteinase K, papain, trypsin and protease (Table 4). These results confirmed the proteinaceous nature of these bacteriocins. Moreover, the bacteriocins retained their anti-listerial activity after heat treatment up to 121°C for 15 min compared with those of the control sample kept at 4°C. The heat stability of these bacteriocins may be attributed to the ecological and environmental adaptation of the strains producing them—CM16 and CM22—which were isolated from camels living in a hot arid environment [37]. In addition, the bacteriocins retained their activity over a pH range of 2.0–10.0. These data indicate that the bacteriocins produced by CM16 and CM22 have the potential for use in the dairy industry as natural preservatives in pasteurized foods and fermented milk products in general and camel-milk-derived products in particular.
Table 4.
Effect of enzymes, pH and heat on anti-listerial activity of bacteriocins from Pediococcus pentosaceus CM16 and Lactobacillus brevis CM22 isolates
| Treatment | Antimicrobial activity of bacteriocins from CM16 isolate | Antimicrobial activity of bacteriocins from CM22 isolate |
|---|---|---|
| Enzymes | ||
| Control | ++ | ++ |
| α-chymotrypsin | — | — |
| Proteinase K | — | — |
| Papain | — | — |
| Trypsin | — | — |
| Protease | — | — |
| pH | ||
| Control | ++ | ++ |
| 2 | ++ | ++ |
| 6 | ++ | ++ |
| 8 | ++ | ++ |
| 10 | ++ | ++ |
| Heat/time | ||
| Control | ++ | ++ |
| 37°C/180 min | ++ | ++ |
| 60°C/60 min | ++ | ++ |
| 80°C/60 min | ++ | ++ |
| 100°C/30 min | + | ++ |
| 121°C/15 min | + | ++ |
Results of three assays were determined by measuring the diameter of the clear zone in mm around the wells. Interpretation of diameter of inhibition zone: —, no inhibition; +, 10–12 mm; ++, 12–14 mm.
Conclusions
There is an increasing interest in functional camel-milk-derived products. Therefore, the isolation and characterization of resident microbes and their functional traits are essential for their use as preservatives in these products. This study reported the genetic identification of diverse LAB isolated from raw camel milk with antimicrobial activity against a broad spectrum of pathogens. These isolates could be potentially used as a starter culture in the manufacture of fermented camel milk products. Moreover, two isolates, Pediococcus pentosaceus CM16 and Lactobacillus brevis CM22, were able to produce bacteriocins that were stable over a wide range of pH and temperature and having anti-listerial activity. These properties make them interesting candidates for application in food preservation and as feed additives. Further studies are needed to investigate the safety and probiotic properties of these isolated LAB strains.
Conflicts of interest
None declared.
Acknowledgements
The authors thank the Kuwait-MIT Centre for Natural Resources and the Environment (CNRE), Cambridge, MA, USA, Kuwait Foundation for the Advancement of Sciences (KFAS), Kuwait, and Kuwait Institute for Scientific Research, Kuwait, for their financial support. The technical assistance of Riaz Al-Dawi is gratefully acknowledged.
References
- 1.Quigley L., O’Sullivan O., Stanton C., Beresford T.P., Ross R.P., Fitzgerald G.F. The complex microbiota of raw milk. FEMS Microbiol Rev. 2013;37:664–698. doi: 10.1111/1574-6976.12030. [DOI] [PubMed] [Google Scholar]; Quigley L, O’Sullivan O, Stanton C, Beresford TP, Ross RP, Fitzgerald GF, et al. The complex microbiota of raw milk. FEMS Microbiol Rev 2013;37:664-698. [DOI] [PubMed]
- 2.Abushelaibi A., Al-Mahadin S., El-Tarabily K., Shah N.P., Ayyash M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT—Food Sci Technol. 2017;79:316–325. [Google Scholar]; Abushelaibi A, Al-Mahadin S, El-Tarabily K, Shah NP, Ayyash M. Characterization of potential probiotic lactic acid bacteria isolated from camel milk. LWT-Food Sci Technol 2017;79:316-325.
- 3.Akhmetsadykova S., Baubekova A., Konuspayeva G., Akhmetsadykov N., Loiseau G. Microflora identification of fresh and fermented camel milk from Kazakhstan. Emirates J Food Agric. 2014;26:327. [Google Scholar]; Akhmetsadykova S, Baubekova A, Konuspayeva G, Akhmetsadykov N, Loiseau G. Microflora identification of fresh and fermented camel milk from Kazakhstan. Emirates J Food Agric 2014;26:327.
- 4.Davati N., Yazdi F.T., Zibaee S., Shahidi F., Edalatian M.R. Study of lactic acid bacteria community from raw milk of Iranian one humped camel and evaluation of their probiotic properties. Jundishapur J Microbiol. 2015;8:1–6. doi: 10.5812/jjm.8(5)2015.16750. [DOI] [PMC free article] [PubMed] [Google Scholar]; Davati N, Yazdi FT, Zibaee S, Shahidi F, Edalatian MR. Study of lactic acid bacteria community from raw milk of Iranian one humped camel and evaluation of their probiotic properties. Jundishapur J Microbiol 2015;8:1-6. [DOI] [PMC free article] [PubMed]
- 5.Abdelgadir W., Nielsen D.S., Hamad S., Jakobsen M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int J Food Microbiol. 2008;127:215–219. doi: 10.1016/j.ijfoodmicro.2008.07.008. [DOI] [PubMed] [Google Scholar]; Abdelgadir W, Nielsen DS, Hamad S, Jakobsen M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int J Food Microbiol 2008;127:215-219. [DOI] [PubMed]
- 6.Hassan R.A., EL-Zubeir I.M.E., Babiker S.A. Chemical and microbial measurements of fermented camel milk “Garris” from transhumance and nomadic herds in Sudan. Aust J Basic Appl Sci. 2008;2:800–804. [Google Scholar]; Hassan RA, EL-Zubeir IME, Babiker SA. Chemical and microbial measurements of fermented camel milk “Garris” from transhumance and nomadic herds in Sudan. Aust J Basic Appl Sci 2008;2:800-804.
- 7.Khedid K., Faid M., Mokhtari A., Soulaymani A., Zinedine A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol Res. 2009;164:81–91. doi: 10.1016/j.micres.2006.10.008. [DOI] [PubMed] [Google Scholar]; Khedid K, Faid M, Mokhtari A, Soulaymani A, Zinedine A. Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol Res 2009;164:81-91. [DOI] [PubMed]
- 8.Ashmaig A., Hasan A., EL-Gaali E. Identification of lactic acid bacteria isolated from traditional Sudanese fermented camel’s milk (Gariss) Afr J Microbiol Res. 2009;3:451–457. [Google Scholar]; Ashmaig A, Hasan A, EL-Gaali E. Identification of lactic acid bacteria isolated from traditional Sudanese fermented camel’s milk (Gariss). Afr J Microbiol Res 2009;3:451-457.
- 9.Lore T.A., Mbugua S.K., Wangoh J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT—Food Sci Technol. 2005;38:125–130. [Google Scholar]; Lore TA, Mbugua SK, Wangoh J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT-Food Sci Technol 2005;38:125-130.
- 10.Cotter P.D., Ross R.P., Hill C. Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105. doi: 10.1038/nrmicro2937. [DOI] [PubMed] [Google Scholar]; Cotter PD, Ross RP, Hill C. Bacteriocins-a viable alternative to antibiotics? Nat Rev Microbiol 2013;11:95-105. [DOI] [PubMed]
- 11.Vimont A., Fernandez B., Hammami R., Ababsa A., Daba H., Fliss I. Bacteriocin-producing Enterococcus faecium LCW 44: a high potential probiotic candidate from raw camel milk. Front Microbiol. 2017;8:865. doi: 10.3389/fmicb.2017.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vimont A, Fernandez B, Hammami R, Ababsa A, Daba H, Fliss I. Bacteriocin-producing Enterococcus faecium LCW 44: a high potential probiotic candidate from raw camel milk. Front Microbiol 2017;8:865. [DOI] [PMC free article] [PubMed]
- 12.Lü X., Hu P., Dang Y., Liu B. Purification and partial characterization of a novel bacteriocin produced by Lactobacillus casei TN-2 isolated from fermented camel milk (Shubat) of Xinjiang Uygur Autonomous region, China. Food Control. 2014;43:276–283. [Google Scholar]; Lu X, Hu P, Dang Y, Liu B. Purification and partial characterization of a novel bacteriocin produced by Lactobacillus casei TN-2 isolated from fermented camel milk (Shubat) of Xinjiang Uygur Autonomous region, China. Food Control 2014;43:276-283.
- 13.Abo-Amer A.E. Inhibition of foodborne pathogens by a bacteriocin-like substance produced by a novel strain of Lactobacillus acidophilus isolated from camel milk. Appl Biochem Microbiol. 2013;49:270–279. [Google Scholar]; Abo-Amer AE. Inhibition of foodborne pathogens by a bacteriocin-like substance produced by a novel strain of Lactobacillus acidophilus isolated from camel milk. Appl Biochem Microbiol 2013;49:270-279.
- 14.Benmechernene Z., Chentouf H.F., Yahia B., Fatima G., Quintela-Baluja M., Calo-Mata P. Technological aptitude and applications of Leuconostoc mesenteroides bioactive strains isolated from Algerian raw camel milk. BioMed Res Int. 2013:1–14. doi: 10.1155/2013/418132. [DOI] [PMC free article] [PubMed] [Google Scholar]; Benmechernene Z, Chentouf, HF, Yahia B, Fatima G, Quintela-Baluja M, Calo-Mata P, et al. Technological aptitude and applications of Leuconostoc mesenteroides bioactive strains isolated from Algerian raw camel milk. BioMed Res Int 2013:1-14. [DOI] [PMC free article] [PubMed]
- 15.Hoover D.G., Harlander S.K. Screening methods for detecting bacteriocin activity. In: Hoover D.G., Steenson L.R., editors. Bacteriocins of lactic acid bacteria. Academic Press; San Diego, CA: 1993. pp. 23–39. [Google Scholar]; Hoover DG, Harlander SK. Screening methods for detecting bacteriocin activity. In: Hoover DG, Steenson LR, editors. Bacteriocins of lactic acid bacteria. San Diego, CA: Academic Press; 1993, p.23-39.
- 16.Al-Zenki S., Al-Nasser A., Al-Safar A., Alomirah H., Al-Haddad A., Hendriksen R.S. Prevalence and antibiotic resistance of Salmonella isolated from a poultry farm and processing plant environment in the State of Kuwait. Foodborne Pathog Dis. 2007;4:367–373. doi: 10.1089/fpd.2007.0017. [DOI] [PubMed] [Google Scholar]; Al-Zenki S, Al-Nasser A, Al-Safar A, Alomirah H, Al-Haddad A, Hendriksen RS, et al. Prevalence and antibiotic resistance of Salmonella isolated from a poultry farm and processing plant environment in the State of Kuwait. Foodborne Pathog Dis 2007;4:367-373. [DOI] [PubMed]
- 17.Tagg J.R., McGiven A.R. Assay system for bacteriocins. Appl Microbiol. 1971;21:943. doi: 10.1128/am.21.5.943-943.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tagg JR, McGiven AR. Assay system for bacteriocins. Appl Microbiol 1971;21:943. [DOI] [PMC free article] [PubMed]
- 18.Hernández D., Cardell E., Zárate V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J Appl Microbiol. 2005;99:77–84. doi: 10.1111/j.1365-2672.2005.02576.x. [DOI] [PubMed] [Google Scholar]; Hernandez D, Cardell E, Zarate V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J Appl Microbiol 2005;99:77-84. [DOI] [PubMed]
- 19.Kumari T., Borah D. Process optimization, partial purification and characterization of protease enzyme from Bacilus altitudinis. Int J Pharm Pharm Sci. 2012;4:483–489. [Google Scholar]; Kumari T, Borah D. Process optimization, partial purification and characterization of protease enzyme from Bacilus altitudinis. Int J Pharm Pharm Sci 2012;4:483-489.
- 20.McAuley C.M., Gobius K.S., Britz M.L., Craven H.M. Heat resistance of thermoduric enterococci isolated from milk. Int J Food Microbiol. 2012;154:162–168. doi: 10.1016/j.ijfoodmicro.2011.12.033. [DOI] [PubMed] [Google Scholar]; McAuley CM, Gobius KS, Britz ML, Craven HM. Heat resistance of thermoduric enterococci isolated from milk. Int J Food Microbiol 2012;154:162-168. [DOI] [PubMed]
- 21.Benkerroum N., Boughdadi A., Bennani N., Hidane K. Microbiological quality assessment of Moroccan camel’s milk and identification of predominating lactic acid bacteria. World J Microbiol Biotechnol. 2003;19:645–648. [Google Scholar]; Benkerroum N, Boughdadi A, Bennani N, Hidane K. Microbiological quality assessment of Moroccan camel’s milk and identification of predominating lactic acid bacteria. World J Microbiol Biotechnol 2003;19:645-648.
- 22.Abera T., Legesse Y., Mummed B., Urga B. Bacteriological quality of raw camel milk along the market value chain in Fafen zone, Ethiopian Somali regional state. BMC Res Notes. 2016;9:285. doi: 10.1186/s13104-016-2088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Abera T, Legesse Y, Mummed B, Urga B. Bacteriological quality of raw camel milk along the market value chain in Fafen zone, Ethiopian Somali regional state. BMC Res Notes 2016;9:285. [DOI] [PMC free article] [PubMed]
- 23.Khan H., Flint S., Yu P.-L. Enterocins in food preservation. Int J Food Microbiol. 2010;141:1–10. doi: 10.1016/j.ijfoodmicro.2010.03.005. [DOI] [PubMed] [Google Scholar]; Khan H, Flint S, Yu P-L. Enterocins in food preservation. Int J Food Microbiol 2010;141:1-10. [DOI] [PubMed]
- 24.Rahmeh R., Akbar A., Kishk M., Al Onaizi T., Al-Shatti A., Shajan A. Characterization of semipurified enterocins produced by Enterococcus faecium strains isolated from raw camel milk. J Dairy Sci. 2018;101:4944–4952. doi: 10.3168/jds.2017-13996. [DOI] [PubMed] [Google Scholar]; Rahmeh R, Akbar A, Kishk M, Al Onaizi T, Al-Shatti A, Shajan A, et al. Characterization of semipurified enterocins produced by Enterococcus faecium strains isolated from raw camel milk. J Dairy Sci 2018;101:4944-4952. [DOI] [PubMed]
- 25.Smit G., Smit B.A., Engels W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev. 2005;29:591–610. doi: 10.1016/j.femsre.2005.04.002. [DOI] [PubMed] [Google Scholar]; Smit G, Smit BA, Engels WJM. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev 2005;29:591-610. [DOI] [PubMed]
- 26.Papagianni M., Anastasiadou S. Pediocins: the bacteriocins of pediococci. Sources, production, properties and applications. Microb Cell Fact. 2009;8:3. doi: 10.1186/1475-2859-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Papagianni M, Anastasiadou S. Pediocins: the bacteriocins of pediococci. Sources, production, properties and applications. Microb Cell Fact 2009;8:3. [DOI] [PMC free article] [PubMed]
- 27.Goh H.F., Philip K. Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140434. [DOI] [PMC free article] [PubMed] [Google Scholar]; Goh HF, Philip K. Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS One 2015;10:e0140434. [DOI] [PMC free article] [PubMed]
- 28.Jans C., Bugnard J., Njage P.M.K., Lacroix C., Meile L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT—Food Sci Technol. 2012;47:371–379. [Google Scholar]; Jans C, Bugnard J, Njage PMK, Lacroix C, Meile L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT-Food Sci Technol 2012;47:371-379.
- 29.Hemme D., Foucaud-Scheunemann C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J. 2004;14:467–494. [Google Scholar]; Hemme D, Foucaud-Scheunemann C. Leuconostoc, characteristics, use in dairy technology and prospects in functional foods. Int Dairy J 2004;14:467-494.
- 30.Carr F.J., Chill D., Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]; Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol 2002;28:281-370. [DOI] [PubMed]
- 31.Corredoira J.C., Alonso M.P., García J.F., Casariego E., Coira A., Rodriguez A. Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: a prospective 16-year study. Eur J Clin Microbiol Infect Dis. 2005;24:250–255. doi: 10.1007/s10096-005-1314-x. [DOI] [PubMed] [Google Scholar]; Corredoira JC, Alonso MP, Garcia JF, Casariego E, Coira A, Rodriguez A, et al. Clinical characteristics and significance of Streptococcus salivarius bacteremia and Streptococcus bovis bacteremia: a prospective 16-year study. Eur J Clin Microbiol Infect Dis 2005;24:250-255. [DOI] [PubMed]
- 32.Jans C., Follador R., Hochstrasser M., Lacroix C., Meile L., Stevens M.J.A. Comparative genome analysis of Streptococcus infantarius subsp. infantarius CJ18, an African fermented camel milk isolate with adaptations to dairy environment. BMC Genom. 2013;14:200. doi: 10.1186/1471-2164-14-200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jans C, Follador R, Hochstrasser M, Lacroix C, Meile L, Stevens MJA. Comparative genome analysis of Streptococcus infantarius subsp. infantarius CJ18, an African fermented camel milk isolate with adaptations to dairy environment. BMC Genomics 2013;14:200. [DOI] [PMC free article] [PubMed]
- 33.Angmo K., Kumari A., Savitri, Bhalla T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT—Food Sci Technol. 2016;66:428–435. [Google Scholar]; Angmo K, Kumari A, Savitri, Bhalla TC. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci Technol 2016;66:428-435.
- 34.Radoshevich L., Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2017;16:32–46. doi: 10.1038/nrmicro.2017.126. [DOI] [PubMed] [Google Scholar]; Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol 2017;16:32-46. [DOI] [PubMed]
- 35.Azizi F., Habibi Najafi M.B., Edalatian Dovom M.R. The biodiversity of Lactobacillus spp. from Iranian raw milk Motal cheese and antibacterial evaluation based on bacteriocin-encoding genes. AMB Expr. 2017;7:176. doi: 10.1186/s13568-017-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Azizi F, Habibi Najafi MB, Edalatian Dovom MR. The biodiversity of Lactobacillus spp. from Iranian raw milk Motal cheese and antibacterial evaluation based on bacteriocin-encoding genes. AMB Express 2017;7:176. [DOI] [PMC free article] [PubMed]
- 36.Gautam N., Sharma N., Ahlawat O.P. Purification and characterization of bacteriocin produced by Lactobacillus brevis UN isolated from dhulliachar: a traditional food product of north east India. Indian J Microbiol. 2014;54:185–189. doi: 10.1007/s12088-013-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gautam N, Sharma N, Ahlawat OP. Purification and characterization of bacteriocin produced by Lactobacillus brevis UN isolated from dhulliachar: a traditional food product of north east India. Indian J Microbiol 2014;54:185-189. [DOI] [PMC free article] [PubMed]
- 37.Heredia-Castro P.Y., Mendez-Romero J.I., Hernandez-Mendoza A., Acedo-Felix E., Gonzalez-Cordova A.F., Vallejo-Cordoba B. Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese. J Dairy Sci. 2015;98:8285–8293. doi: 10.3168/jds.2015-10104. [DOI] [PubMed] [Google Scholar]; Heredia-Castro PY, Mendez-Romero JI, Hernandez-Mendoza A, Acedo-Felix E, Gonzalez-Cordova AF, Vallejo-Cordoba B. Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances produced by Lactobacillus spp. isolated from artisanal Mexican cheese. J Dairy Sci 2015;98:8285-8293. [DOI] [PubMed]