Abstract
Steroid treatment has become recognized as an important risk factor for avascular osteonecrosis of the femoral head. However, not all patients who receive long-term, high-dose steroids develop osteonecrosis, indicating that there are individual differences in occurrence.
We explored the relationship between polymorphisms and steroid-induced osteonecrosis of the femoral head (SONFH) incidence with variables. We used a multilevel mixed-effects logistic regression model, which is an expansion of logistic regression, for each type of steroid, primary disease, drug dose, applied duration, and single-nucleotide polymorphism (SNP). We also conducted a dose-response meta-analysis to analyze the cumulative dosage and SONFH risk in mutation carriers. There were significant correlations between the ABCB1 rs1045642 mutant and SONFH in the prednisone-use and methylprednisolone/prednisone-use populations. The ABCB1 rs2032582 mutant homozygote had a protective effect in the methylprednisolone/prednisolone renal transplant population. For ApoB rs693, mutation increased the incidence of SONFH in prednisone-use and methylprednisolone/prednisolone-use populations and renal transplant patients. For ApoB rs1042031, mutation increased the risk of SONFH in the prednisone-use population. The PAI-1 rs1799768 mutation had a protective effect on the SONFH risk prednisone-use and renal transplant populations. ABCB1 rs1045642 mutations have a protective effect against SONFH, and ApoB rs693 and rs1042031 increase the SONFH risk. Cumulative dosage and treatment duration had little effect on the results. In addition, there was a dose-effect correlation in ABCB1 rs1045642 and rs2032582 mutation carriers.
Keywords: meta analysis, single nucleotide polymorphisms, steroids
Introduction
Steroid-induced osteonecrosis of the femoral head (SONFH), which leads to collapse of the femoral head and articular dysfunction, has an incidence of 9–40% amongst patients receiving steroid treatment [1]. The exact pathology of SONFH is still unclear and might be related to lipid metabolism disorders, abnormal microcirculation, insufficient blood supply, inflammation, and bone marrow mesenchymal stem cell osteogenesis differentiation dysfunction. The abnormal blood supply leads to the apoptosis of osteocytes and osteoblasts, followed by bone loss and reduced bone mineral density [2]. Lipid metabolism dysfunction is also an important pathology, and steroid application may lead to an increase in blood lipid levels and then to the development of intravascular lipid embolism in the microvasculature [3]. Embolism accumulation might affect microcirculation and increase the pressure of the intramedullary cavity, eventually leading to the death of bone cells. Long-term or mass steroid application is the critical pathogenesis of SONFH [4]. However, not all patients who receive long-term, high-dose steroids will develop SONFH, indicating that there are individual differences in the occurrence of SONFH.
To date, several meta-analyses have been published and shown that PAI-1 4G/5G (rs1799768) [5], ABCB1 C3435T (rs1045642) [5–7] and CYP3A activity [8] are associated with SONFH incidence. However, the results of the ABCB1 G2677T/A polymorphism are still disputed [5–7]. Our previous research reported only the single-nucleotide polymorphisms (SNPs) that appeared in more than three studies and indicated that ABCB1 rs1045642 has a protective effect on SONFH in an allelic model and that the ApoB rs693 and rs1042031 mutations promote the pathogenesis of SONFH. ABCB1 rs2032582, MTHFR rs1801133, and PAI-1 rs1799768 were not correlated with SONFH incidence. However, heterogeneity still exists in the previous results, and further analysis of the characteristics of the included studies is needed. Therefore, this research further analyzes the effects of primary disease, type of steroids, cumulative steroid dosage, and treatment duration on the results.
Methods
We used the meta-analysis of observational studies in epidemiology guidelines in the present study [9].
Data source and search strategy
Two authors independently performed a literature search of the PubMed, Embase, Cochrane Library, and Chinese public databases, including the China National Knowledge Infrastructure, the China Biology Medicine Database, the China Science Periodical Database (Wanfang Database), and the VIP Journal Integration Platform. The search included studies published through 29 July 2018. The following terms were used in the search strategy: hormone, glucocorticoid, steroid, corticosteroid, osteonecrosis, femoral, femur, femoris, whirlbone, polymorphism, SNP, genetic, mutation, genotype, allele, allelic, and variation. The search strategy is shown in Supplementary Table S1. Divergence in the search results was resolved by discussion.
Inclusion and exclusion criteria
The studies were included in our meta-analysis if they met the following criteria: (1) case-control or cohort studies comparing a population that suffered SONFH with a population that did not suffer after steroid treatment, (2) studies assessing the associations between genetic polymorphisms and SONFH, and (3) studies reporting the frequencies of specific alleles or the effect sizes of individual genotypes between cases and controls. Studies were excluded from the analysis for the following reasons: (1) noncase-control or noncohort studies, (2) the case group included SONFH patients with other etiologies or SONFH patients who were not reported separately, (3) the control group included an ONFH population without steroid application or a healthy population, (4) non-SNP-related studies, (5) studies about family heredity, and (6) studies that did not report data pertaining to allelic frequencies or effect size. In addition, conference reports, editor comments, reviews, case reports, and academic dissertations were excluded from the analysis.
Data extraction and quality assessment
Two authors independently extracted the following data from each eligible study: first author’s name, publication year, research location, sample size, average subject age, primary disease, steroid type, cumulative steroid dose, treatment duration, and genes of interest. If the average or median value of the cumulative steroid dosage in each group was not reported, it was estimated. The cumulative dose was generally calculated by multiplying the daily average dose and the treatment duration. For studies with a fixed treatment strategy, the cumulative dosage was calculated by multiplying the dosage of each treatment cycle by the total number of cycles. In the present study, we defined the average body surface area as 1.6 m2 and the average body weight as 60 kg. For closed-interval categories, the value assigned to each dosage or duration, such as the average or median, was the midpoint. For open-interval categories, the value was estimated as the limit value multiplied by 1.2 [10]. Because of the different types of steroids used in the included studies, it was necessary to convert the doses of different types of steroids to the equivalent dose of prednisone. The conversion standard is 5 mg prednisone = 5 mg prednisolone = 4 mg methylprednisolone = 0.75 mg dexamethasone [11]. The Newcastle-Ottawa Scale (NOS), a validated tool for evaluating the quality of observation studies, was used to evaluate the methodological quality of the included studies; the scale includes the following three subscales: selection, comparability, and exposure [12].
Statistical analysis
Association analysis was performed using five genetic models: allelic (W vs M), dominant (WW+WM vs MM), recessive (WW vs WM+MM), heterozygous (WM vs MM), and homozygous (WW vs MM) models [13]. W is the wild allele and M is the mutation allele. The odds ratios (ORs) and their 95% CIs were used to assess the strength of the associations between polymorphisms and SONFH incidence. The I2 statistic was used to estimate the degree of heterogeneity amongst the studies. Cochran’s Q statistics were also taken as measures of between-study heterogeneity. Generally, a significance level of P<0.1 suggests the existence of significant heterogeneity to expand a test’s sensitivity [13]. If the I2 ≥ 50% (Q test, P<0.1), the random-effect model was used; otherwise, the fixed-effect model was used. Then, we attempted to use a multilevel mixed-effects logistic regression model, which is an expansion of logistic regression, for each type of steroid, primary disease, drug dose, applied duration, and SNP. The factors of different genotypes and other variables were included as fixed effects, and different studies were considered random effects.
Finally, we conducted a dose-response meta-analysis across the cumulative steroid dosage [14]. To derive the dose-response curve, we modeled the dose using restricted cubic splines with three knots at fixed percentiles of 10, 50, and 90% of the distribution [15]. All tests were two-tailed, and a p-value less than 0.05 was considered statistically significant except in Cochran’s Q test.
Results
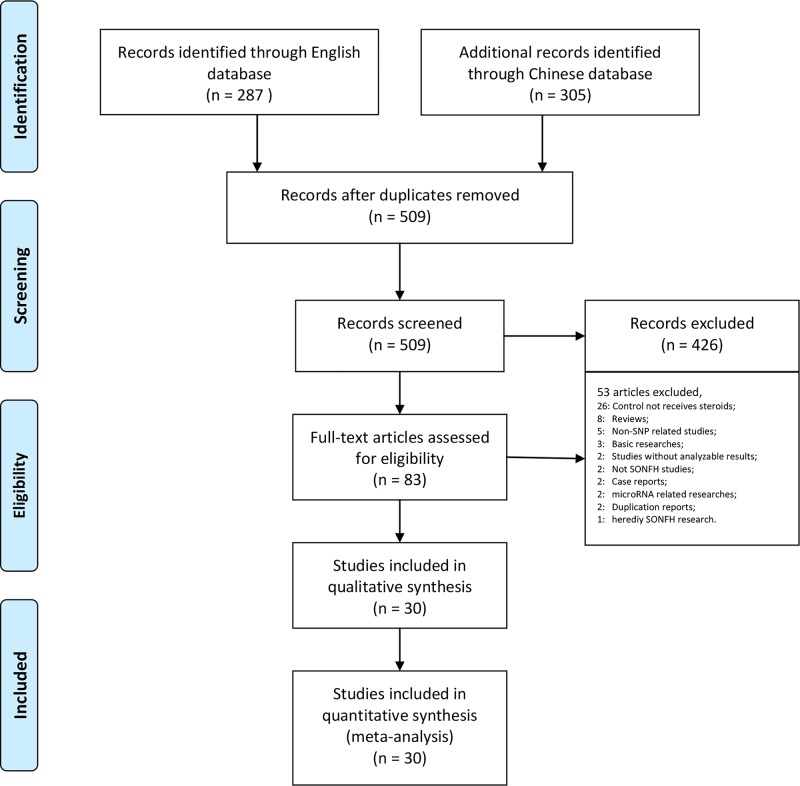
Our research returned 287 English articles and 305 Chinese articles after removing duplicates. After screening the titles and abstracts, 509 of these articles were excluded. The full texts of 83 articles were assessed, amongst which studies were excluded for the following reasons: the control group did not receive steroid therapy (26); reviews (8); non-SNP-related studies (5); basic studies (3); studies without frequencies or effect size results (2); non-steroid-related ONFH studies (2); case reports (2); microRNA-related studies (2); duplicated reports (2); and heredity SONFH study (1). Ultimately, we included 30 studies that assessed a total of 7553 patients in our meta-analysis [16–45] (Figure 1, Table 1).
Figure 1. Preferred reporting items for systematic review and meta-analysis flow chart illustrating the process by which the studies included in our analysis were selected.
Table 1. Characters of included studies.
| Author | Year | Local | Sample size | Average age# | Type of steroids | Primary disease | Cumulative dose in Exp group | Cumulative dose in Control group | Treatment duration | Genes | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al. [16] | 2017 | China | 193 (78/115) | 40 (18–48) | Prednisone | Various | 1400 mg | 2400 mg | 1Y | GRG | 9 |
| Plesa et al. [17] | 2017 | Caucasian | 304 (32/272) | NA | Prednisone Dexamethasone | ALL | 50,400 mg | 54,149 mg | 120W | BCL2L11 | 9 |
| Karol et al.[18] | 2015 | Multinational | 2955 (400/2555) | NA | Prednisone Dexamethasone | ALL | 24,275 mg | 24,275 mg | 1500D | GWAS | 7 |
| X Wei [19] | 2015 | China | 75 (45/30) | 39 ± 10 | Prednisone | Various | 4800 mg | 4800 mg | 6M | ApoA1;ApoB; ApoE | 8 |
| Zhang et al. [20] | 2014 | China | 200 (94/106) | 44.5 (18–82) | Prednisolone | Various | 7300 mg | 7300 mg | 1Y | ABCB1 | 7 |
| Y Xue [21] | 2014 | China | 322 (105/217) | 39 (18–48) | Prednisone | Various | 1388 mg | 2400 mg | 1Y | ABCB1 | 9 |
| Y Cui [22] | 2014 | China | 424 (223/201) | 42.27 ± 15.71 | Prednisolone | Various | 7300 mg | 7300 mg | 1Y | ApoA5 | 7 |
| P Zeng [23] | 2014 | China | 206 (108/98) | 40 ± 10 | Prednisone | Various | 2400 mg | 2400 mg | 3M | ApoB | 8 |
| Zhang et al. [24] | 2013 | China | 200 (94/106) | 44.5 (18–82) | Prednisolone | Various | 7300 mg | 7300 mg | 1Y | PAI–1 | 7 |
| Wang et al.[25] | 2013 | China | 200 (94/106) | 44.5 (18–82) | Prednisolone | Various | 7300 mg | 7300 mg | 1Y | PON-1 | 6 |
| Y Li [26] | 2012 | China | 123 (70/53) | 29 (18–73) | Prednisone | Various | 480 mg | 480 mg | 6M | ABCB1 | 6 |
| W Fang [27] | 2011 | China | 134 (63/71) | 35.17 ± 11.73 | Prednisone | Various | 6991 mg | 5014 mg | 6M | ApoB; CYP1A2 | 8 |
| W Fang [28] | 2011 | China | 134 (63/71) | 35.17 ± 11.73 | Prednisone | Various | 6991 mg | 5014 mg | 6M | Factor V;GR;CBP; ApoB;CYP1A2 | 8 |
| J Bond [29] | 2011 | U.K. | 110 (43/67) | NA | Dexamethasone | ALL | 68,333 mg | 68,333 mg | 143W | PAI-1 | 8 |
| W He [30] | 2009 | China | 48 (31/17) | 32 (12–59) | Prednisone | Hemoglobinopathies | 480 mg | 480 mg | 2M | CYP3A4/ ABCB1 | 7 |
| W He [31] | 2009 | China | 48 (31/17) | 18–60 | Prednisone | Various | 480 mg | 480 mg | 6M | CYP3A4 | 7 |
| Kuribayashi et al. [32] | 2008 | Japan | 157 (34/123) | 35 (9–64) | Methylprednisolone Prednisolone | RT | 3227 mg | 3207 mg | 6M | ABCB1; ApoB; CBP | 9 |
| D French [33] | 2008 | U.S.A. | 361 (51/310) | NA (10–20) | Prednisone Dexamethasone | ALL | 15,139 mg | 15,139 mg | NA | ABCB1;PAI–1 et al. 11 Genes## | 7 |
| Wang et al.[34] | 2008 | China | 53 (16/37) | 35 (16–78) | Methylprednisolone | SARS | 5672 mg | 4187 mg | NA | TNF-a | 8 |
| Tamura et al. [35] | 2007 | Japan | 157 (34/123) | 35 (9–64) | Methylprednisolone Prednisolone | RT | 3251 mg | 3215 mg | 6M | GR;CYP3A4; CBP;NCoA2 | 9 |
| XY Yang [36] | 2007 | China | 127 (21/106) | 34 (11–67) | Methylprednisolone Prednisone | SLE | 89,173 mg | 89,173 mg | 5Y | ABCB1 | 9 |
| Hirata et al.[37] | 2007 | Japan | 112 (20/92) | NA | Methylprednisolone Prednisone | RT | 3223 mg | 3223 mg | 6M | ApoA | 7 |
| Hirata et al.[38] | 2007 | Japan | 158 (34/124) | 36.1 (9–64) | Methylprednisolone Prednisolone | RT | 3223 mg | 3223 mg | 6M | ApoB | 7 |
| Ekmekci et al. [39] | 2006 | Turkey | 57 (19/38) | 34.2 ± 9.3 | NA | RT | NA | NA | 20.6M | Factor V, Prothrombin | 7 |
| Celik et al.[40] | 2006 | Turkey | 50 (11/39) | 41 ± 11.79 | Prednisolone | RT | 8835 mg | 6322 mg | 18M | Factor V, Prothrombin, MTHFR | 8 |
| Relling et al. [41] | 2004 | U.S.A. | 64 (25/39) | 8.6 (2.7–18.8) | Prednisone | ALL | 17,028 mg | 17,867 mg | 1.23Y | MDR1(ABCB1) et al. 13 Genes### | 8 |
| Asano et al. [42] | 2004 | Japan | 137 (31/106) | 36 (9–63) | Methylprednisolone Prednisolone | RT | 3228 mg | 3174 mg | NA | PAI-1; MTHFR | 8 |
| Asano et al. [43] | 2003 | Japan | 80 (26/54) | NA | NA | RT | NA | NA | 2Y | CYP3A4; CYP2D6; CYP2C19 | 7 |
| Asano et al. [44] | 2003 | Japan | 136 (30/106) | 35.5 (9–63) | Methylprednisolone Prednisolone | RT | 3227 mg | 3207 mg | 6M | ABCB1 | 8 |
| Ferrari et al. [45] | 2002 | Switzerland | 228 (26/202) | 50 ± 12 | Prednisone | RT | 9600 mg | 9600 mg | 8Y | PAI-1 | 8 |
ALL, acute lymphoblastic leukemia; GWAS, genome-wide association study; NA, not available; RT, renal transplant; SARS, severe acute respiratory syndrome.
Mean ± standardization; mean/median (minimum–maximum).
TYMS; VDR; BGLAP; ESR1; LRP5; MTHFR; PAI-1; ABCB1(MDR1); PTH; PTHR; ACP5.
CYP3A4; CYP3A5; TPMT; UGT1A1; TYMS; GSTT1; GSTM1; RFC; MTHFR; GRG(NR3C1); MDR1(ABCB1); VDR; GSTP1.
All studies were case-control designs, except one study, which was a cohort design [18]. The primary diseases included organ transplantation, hematological disease, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), nephrotic syndrome, and ophthalmic disease. In addition, two studies by Wei He [30,31], two studies by Weibao Fang [27,28], and studies by Masaaki and Kyoko [32,35] included the same patient groups but did not assess the same SNPs. The NOS scores ranged from six to nine points, and the overall quality of the observational studies was ideal (Table 1). The details of NOS scores for each study are shown in Supplementary Table S2.
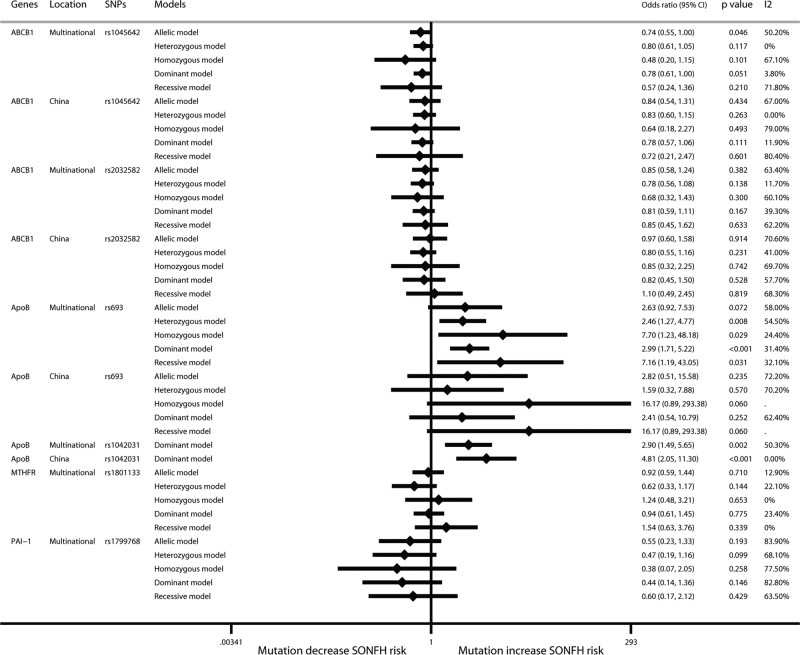
Amongst the preliminary results of SNPs that were included in more than three studies, an allelic model showed that the ABCB1 rs1045642 mutation had a protective effect against SONFH (OR: 0.74; 95% CI: 0.55–1.00; P=0.046). ApoB rs693 (heterozygous model: OR: 2.46; 95% CI: 1.27–4.77; P=0.008; homozygous model: OR: 7.70; 95% CI: 1.23–48.16; P=0.029; dominant model: OR: 2.99; 95% CI: 1.71–5.21; P<0.001; recessive model: OR: 7.16; 95% CI: 1.19–43.02; P=0.031) and rs1042031 (dominant model: OR: 2.90; 95% CI: 1.49–5.66; P=0.002) mutations increased the risk of SONFH. ABCB1 rs2032582, MTHFR rs1801133, and PAI-1 rs1799768 had no significant relationships with SONFH (Figure 2). Other SNPs were not included in the analysis because they did not appear in more than three studies. A further analysis was performed in the present study using a multilevel mixed-effects logistic regression model. The details of the included studies for each group analysis are listed in Supplementary Table S3.
Figure 2. Forest plot of SONFH risk of SNP carriers that are included in more than three studies by traditional meta-analysis.
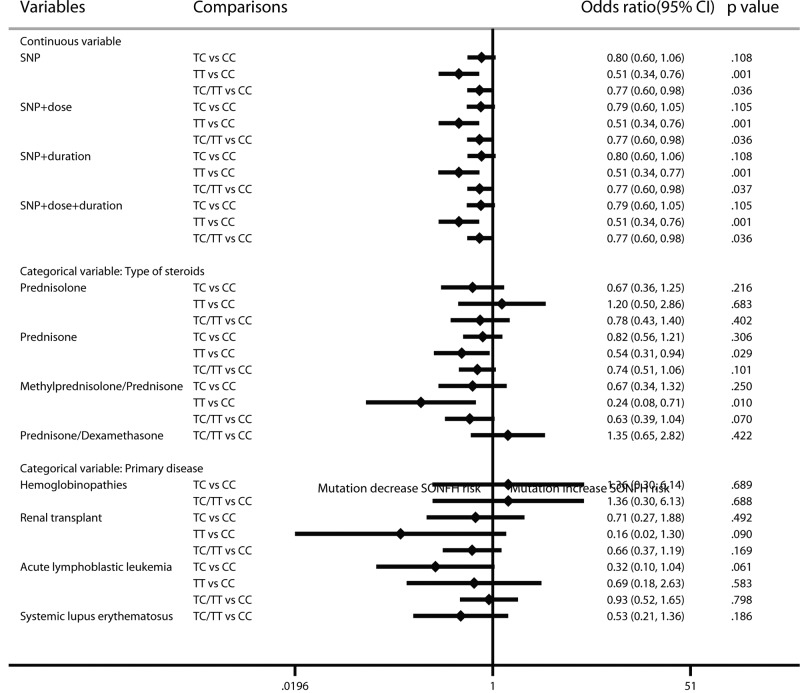
In the results of the ABCB1 rs1045642 multilevel mixed-effects regression model, the mutant homozygote and the mutant carrier could significantly protect against SONFH after steroid application (TT vs CC genotype: OR: 0.51; 95% CI: 0.34–0.76; P=0.001; TC/TT vs CC: OR: 0.77; 95% CI: 0.60–0.98; P=0.036). The results showed no significant changes in the cumulative dosage and treatment duration. According to the analyses of categorical variables of steroid type, in the prednisone-use population, mutant homozygotes had a significant protective effect compared with the wild-type population (TT vs CC: OR: 0.54; 95% CI: 0.31–0.94; P=0.029), and the results were similar in the methylprednisolone/prednisone-use population (TT vs CC: OR: 0.24; 95% CI: 0.08–0.71; P=0.010). The analysis of primary disease showed no significant results. It should be noted that the studies that did not report primary disease type or that included several types of primary disease were not included in the categorical analysis of primary disease (Figure 3).
Figure 3. Forest plot of ABCB1 rs1045642 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
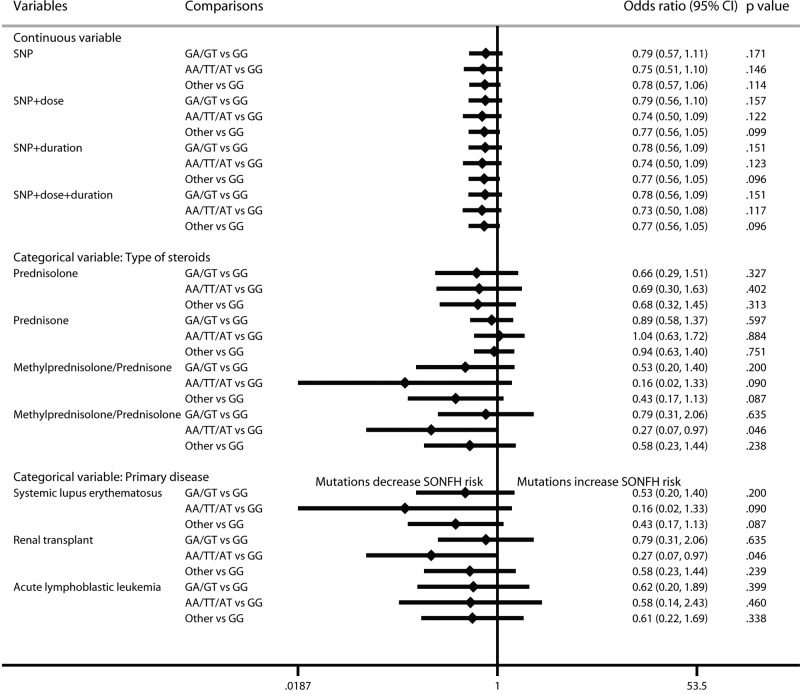
For ABCB1 rs2032582, the negative results did not change after considering cumulative dose and treatment duration. In the categorical analysis, the protective effect of the mutant was only found in the methylprednisolone/prednisolone-use renal transplant population (OR: 0.27; 95% CI: 0.07–0.97; P=0.046). However, this result was based on a single study and had a large standard error (SE = 0.66); thus, this result needs to be confirmed (Figure 4).
Figure 4. Forest plot of ABCB1 rs2032582 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
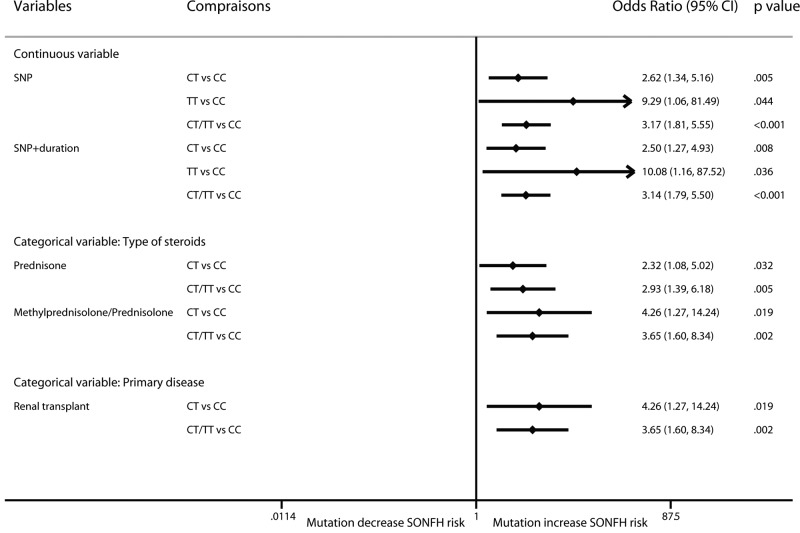
For ApoB rs693, mutation increased the incidence of SONFH (CT vs TT: OR: 2.62; 95% CI: 1.34–5.16; P=0.005; TT vs CC: OR: 9.29; 95% CI: 1.06–81.49; P=0.044; CT/TT vs CC: OR: 3.16; 95% CI: 1.81–5.55; P<0.001) (Figure 5). Due to a large number of zero events, the regression results did not converge after considering the cumulative dose. The results were not changed after considering treatment duration. In addition, in prednisone-use and methylprednisolone/prednisolone-use populations and renal transplant patients, mutation increased the risk of SONFH.
Figure 5. Forest plot of ApoB rs693 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
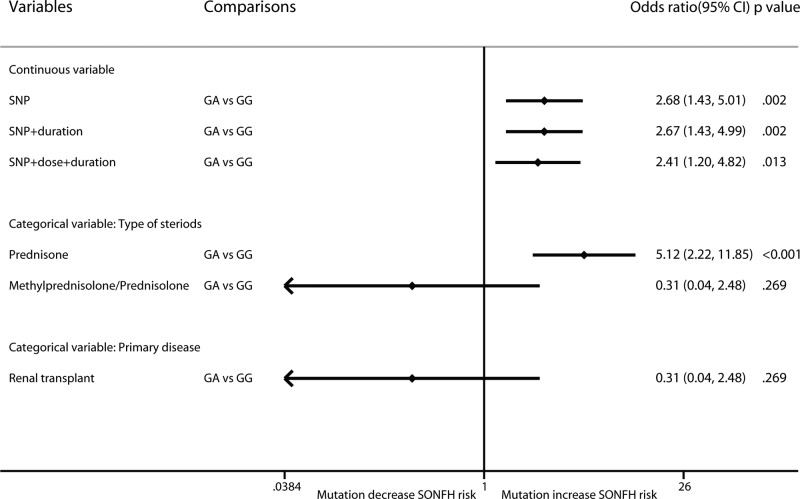
For ApoB rs1042031, mutation increased the risk of SONFH (GA vs GG: OR: 2.68; 95% CI: 1.43–5.01; P=0.002) (Figure 6). The results were not obviously changed after considering cumulative dose and treatment duration. In the categorical analysis, mutation increased the risk of SONFH in the prednisone-use population (OR: 5.12; 95% CI: 2.22–11.85; P<0.001), but not in the methylprednisolone/prednisolone-use renal transplant population (OR: 0.31; 95% CI: 0.04–2.48; P=0.269).
Figure 6. Forest plot of ApoB rs1042031 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
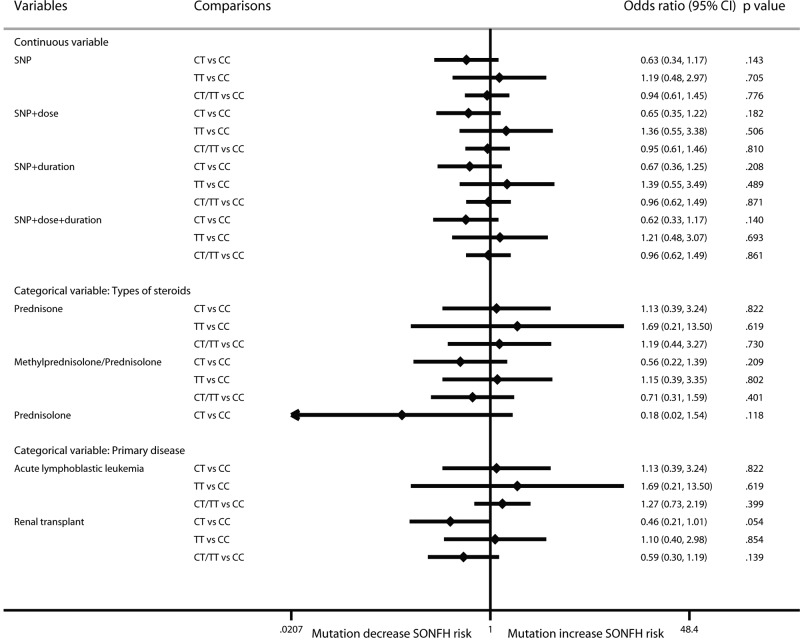
For MTHFR rs1801133, there was no significant correlation between mutation and SONFH risk (CT vs CC: OR: 0.63; 95% CI: 0.34–1.17; P=0.143; TT vs CC: OR: 1.19; 95% CI: 0.48–2.97; P=0.705; CT/TT vs CC: OR: 0.94; 95% CI: 0.61–1.45; P=0.776). The results did not change after considering the continuous and categorical variables (Figure 7).
Figure 7. Forest plot of MTHFR rs1801133 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
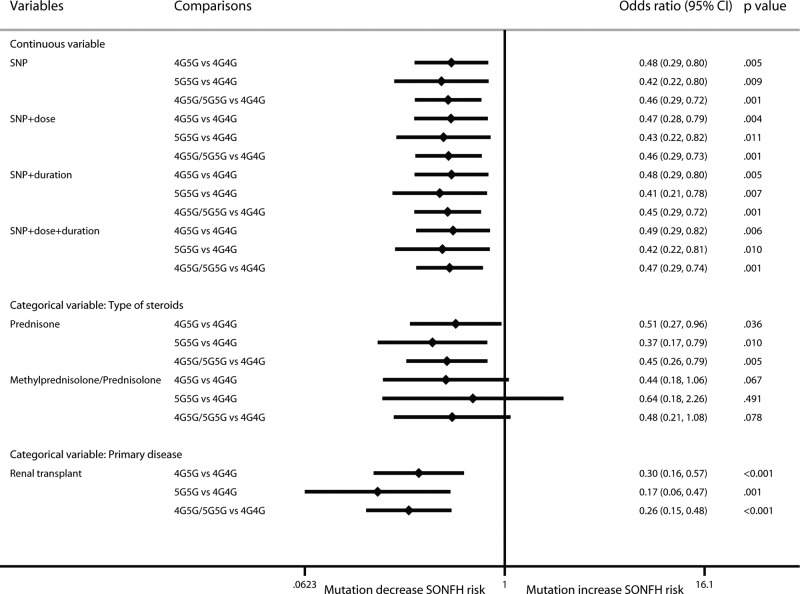
For PAI-1 rs1799768, 5G mutation had a protective effect against SONFH (4G5G vs 5G5G: OR: 0.48; 95% CI: 0.29–0.80; P=0.005; 5G5G vs 4G4G: OR: 0.42; 95% CI: 0.22–0.80; P=0.009; 4G5G/5G5G vs 4G4G: OR: 0.46; 95% CI: 0.29–0.72; P=0.001) (Figure 8). These results differed from previous results because in previous research a random-effect model was used due to large heterogeneity, and a mixed-effect model was adopted in this research. The results did not change after considering cumulative dose and treatment duration. In the categorical analysis, the protective effect existed in the prednisone-use and renal transplant population. However, there was no significant correlation in the methylprednisolone/prednisolone-use population (4G5G vs 5G5G: OR: 0.44; 95% CI: 0.18–1.06; P=0.067; 5G5G vs 4G4G: OR: 0.64; 95% CI: 0.18–2.26; P=0.491; 4G5G/5G5G vs 4G4G: OR: 0.48; 95% CI: 0.21–1.08; P=0.078).
Figure 8. Forest plot of PAI-1 rs1799768 mutation on SONFH risk by the multilevel mixed-effects logistic regression model.
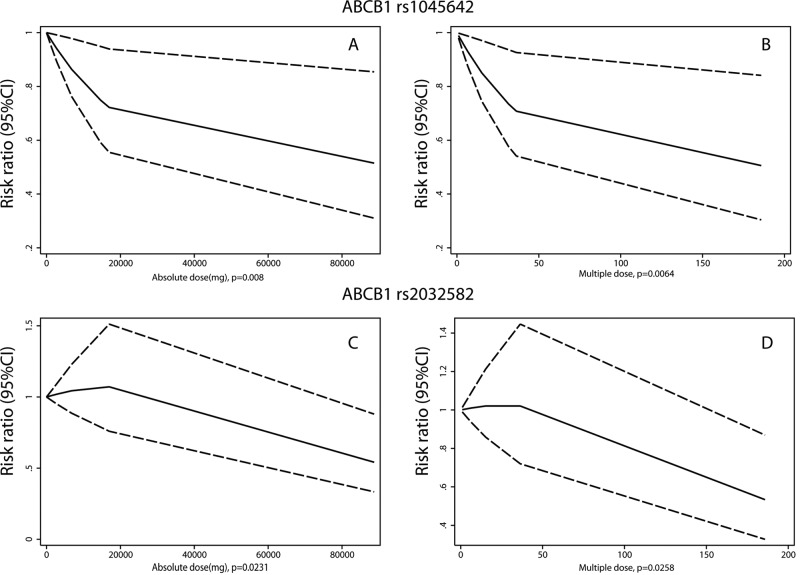
Furthermore, we sought to analyze the cumulative dosage and SONFH risk in mutation carriers by a dose-response meta-analysis. SONFN/ONFH incidence in mutation carriers cannot be obtained when steroids are not used. Therefore, the minimum dose must be used as a reference, and whether increasing the dose affects SONFH incidence must be assessed. Two dose assessment methods were used: an absolute dose and multiple doses. For ABCB1 rs1045642, there was a correlation between the cumulative dose and the occurrence of SONFH in the mutation carriers. As the dose increased, the risk of SONFH decreased (absolute dose: P=0.008; multiple dose: P=0.0064) (Figure 9A,B). This negative correction also persists in homozygous mutation (TT genotype, absolute dose: P=0.0368; multiple dose: P=0.0435) and heterozygous mutation patients (CT genotype, absolute dose: P<0.001; multiple dose: P<0.001) (Supplementary Figure S1A–D). In wild-type populations, there was no correlation between the dose and the risk of SONFH (absolute dose: P=0.098; multiple dose: P=0.098). Although there was no significant correlation between the ABCB1 rs2032582 mutation and the risk of SONFH, the cumulative dose in the mutant population was correlated with the occurrence of SONFH. As the dose increased, the risk of SONFH decreased (absolute dose: P=0.0231; multiple dose: P=0.0258) (Figure 9C,D). This negative correction mainly persists in heterozygous mutation patients (GA/GT genotype, absolute dose: P=0.0286; multiple dose: P=0.0293), but not in other mutation patients (AA/AT/TT genotype, absolute dose: P=0.7103; multiple dose: P=0.7716) (Supplementary Figure S1E–H). In wild-type populations, there was no correlation between the dose and the risk of SONFH (absolute dose: P=0.2821; multiple dose: P=0.285). These findings may suggest that mutation carriers still have a risk of SONFH when using steroids, similar to the wild-type population. If SONFH does not occur after small-dose steroid application, increasing the steroid dose in mutation carriers will not increase but rather decrease the risk of SONFH.
Figure 9. Dose-response analyses of cumulative steroid dose and SONFH risk in mutation carriers.
(A) Analysis of absolute dose in ABCB1 rs1045642 mutation carriers; (B) analysis of multiple dose in ABCB1 rs1045642 mutation carriers; (C) analysis of absolute dose in ABCB1 rs2032582 mutation carriers; (D) analysis of multiple dose in ABCB1 rs2032582 mutation carriers.
For ApoB rs693, although SONFH was correlated with the dose in the mutation carriers (absolute dose: P=0.0064; multiple dose: P=0.0064), a correlation was also found in the wild-type population (absolute dose: P=0.0177; multiple dose: P=0.0013). For ApoB rs1042031, there was no correlation between the cumulative dose and SONFH risk in the mutation carriers (absolute dose: P=0.662; multiple dose: P=0.7078) or in the wild-type population (absolute dose: P=0.3106; multiple dose: P=0.0719). ApoB rs693 and rs1042031 have too few homozygous mutation patients to analyze separately. We did not perform a dose-response analysis for MTHFR rs1801133 and PAI-1 rs1799768 due to the small number of included studies.
Discussion
ABCB1 rs1045642 mutations have a protective effect against SONFH, and mutations in ApoB rs693 and rs1042031 increase the SONFH risk. The present study further analyzed the effects of each specific characteristic, such as the primary disease of the included patients, the types of steroids, the cumulative dosage, and the treatment duration, on outcomes. However, with the increase in variable factors, the results will be more dependent on the results of single studies, which will reduce the stability of the results. However, the analyses performed here can provide additional clues and research directions for further basic and clinical research studies. In previous research, we analyzed the associations between SNPs and SONFH by traditional meta-analysis, and more than three SNPs were included. Ultimately, ABCB1 rs1045642, rs2032582, ApoB rs693, rs1042031, MTHFR rs1801133, and PAI-1 rs1799768 were included.
In previous meta-analyses, one study analyzed the association between ABCB1 gene polymorphism and SONFH and the 3435T (rs1045642) and 2677T/A (rs2032582) alleles of ABCB1 were identified, which may reduce the risk of GC-induced ONFH [6]. However, our study included more reports and found that rs2032582 had no correlation with SONFH. Our result is the same as that from another meta-analysis that analyzed the correlation between gene polymorphism and SONFH occurrence with the GRADE method to assess the level of evidence. The results showed that ABCB1 rs1045642 could reduce the occurrence of SONFH but had a very low level of evidence. ApoB rs693 and rs1042031 mutations increased the risk of SONFH and had a moderate level of evidence [46]. The results of our study are essentially the same as those of the above two studies, except for rs2032582. However, our work further analyzed the associations between SNP and SONFH based on steroid type, primary disease, drug dose, and applied duration using a mixed-effects logistic regression model. We also conducted a dose-response meta-analysis to analyze the cumulative dosage and SONFH risk in mutation carriers. Our work expanded the research results and found that cumulative steroid dosage and treatment duration had little effect on the results. There was a dose-effect correlation in ABCB1 rs1045642 and rs2032582 mutation carriers. Another study analyzed the associations between ONFH and genetic polymorphisms of vascular endothelial growth factor rs2010963, endothelial nitric oxide synthase rs2070744, and ABCB1 rs1045642. However, the included studies contained SONFH- and ONFH-related studies. The results showed that the mutations of rs2010963 and rs1045642 increased the risk of ONFH, and the mutation of rs2070744 increased the risk of ONFH in an allelic model. There were errors in the present study, as the authors proposed that the mutation of ABCB1 rs1045642 increased the risk of ONFH, but the data showed that it reduced the risk [47]. Our study analyzed SONFH risk, but not ONFH, which is different from the above study.
In the present study, there were significant correlations between the ABCB1 rs1045642 mutant and SONFH in the prednisone-use and methylprednisolone/prednisone-use populations. The ABCB1 rs2032582 mutant homozygote had a protective effect in the methylprednisolone/prednisolone renal transplant population. For ApoB rs693, mutation increased the incidence of SONFH in the prednisone-use and methylprednisolone/prednisolone-use populations and renal transplant patients. For ApoB rs1042031, mutation increased the risk of SONFH in the prednisone-use population. The PAI-1 rs1799768 mutation had a protective effect in the SONFH risk prednisone-use and renal transplant population.
There were different outcomes for the different types of steroid-use populations, possibly because the results were based on even fewer studies. These differences may also indicate that different types of steroids have subtle differences in affecting the risk of SONFH. It is generally believed that prednisone must be transformed to an active form through the liver, while prednisolone does not require this change, which may be the reason for the different results of the two drugs in ABCB1-related mutation states. In addition, more evidence is needed to determine whether the different results for the types of steroids in ApoB and PAI-1 gene polymorphisms were due to different effects on adipocytes.
Currently, a registered study is analyzing the pharmacokinetics of different steroids in children, but the results have not yet been reported (NCT02252237). An earlier study also reported that the cumulative dosage of methylprednisolone, but not prednisone, is correlated with avascular necrosis incidence [48]. Therefore, defined steroid types should be evaluated in further genetic polymorphism- and SONFH-related studies.
Although there are reports about the effect of primary disease on SONFH risk, most have focussed on the influence of steroids used in disease treatment. It was also reported that in renal transplant patients, cyclosporine versus tacrolimus as well as gender factors may be independent risk factors for avascular necrosis [49]. A meta-analysis of the avascular necrosis risk in SLE patients also showed, that in addition to hormonotherapy, arthritic, cushingoid, hypertension, cytotoxic drugs, etc., also affect the occurrence of SONFH [50]. Therefore, the primary disease and the treatment strategy are also possible risk factors for SONFH incidence.
It is generally believed that the occurrence of SONFH is due to long-term and/or high-dosage steroid application. A dose-response meta-analysis showed that the incidence of osteonecrosis is correlated with both the cumulative dose and treatment duration [51]. However, there is still no related research on specific mutant carriers. First, the present study found that the ABCB1 rs1045642 mutation has a protective effect on SONFH, and the risk will be further reduced with increasing cumulative steroid dosage. It can also be concluded that some mutant carriers will have SONFH when the cumulative dosage is low; however, if SONFH does not occur, the risk of SONFH will not increase when the cumulative dose of steroids is increased. Therefore, for mutant carriers, the critical time period for prevention and diagnosis of SONFH is during the early stage of steroid application, not the late stage. A negative correlation was also found in ABCB1 rs2032582 mutation carriers, even though the mutations were not significantly associated with SONFH in general.
Limitation
The present study still has several limitations. First, our study was performed at the study level instead of the individual level. Second, in stratified analyses, with the increase in variable factors, the results become more dependent on the results of single studies, which will reduce the stability of the results. Third, in dose-response analyses, SONFN/ONFH incidence in mutation carriers without steroid application cannot be calculated; therefore, the minimum dose must be used as a reference. Fourth, our study only analyzed SNPs that were examined in more than three studies, but with emerging research and evidence, more SNPs may be studied in the future. Therefore, the present study was limited by the research available at the time.
Supporting information
Supplementary Figure S1.
Supplementary Table S1. Search queries in each database.
Supplementary Table S2. NOS score details for each included study.
Supplementary Table S3. Details of the included studies for the analysis of each group in the meta-analysis.
Abbreviations
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- SNP
single-nucleotide polymorphism
- SONFH
steroid-induced osteonecrosis of the femoral head
Author Contribution
Y.J. designed the study; performed statistical analysis; edited and reviewed the manuscript; guarantor of integrity of the entire study. Y.X.G. performed literature research and data acquisition and analysis. J.M. performed literature research and data acquisition and analysis; prepared and reviewed the manuscript. All authors approved the final manuscript.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
References
- 1.Luo P., Gao F., Han J., Sun W. and Li Z. (2018) The role of autophagy in steroid necrosis of the femoral head: a comprehensive research review. Int. Orthop. 42, 1747–1753 10.1007/s00264-018-3994-8 [DOI] [PubMed] [Google Scholar]
- 2.Johnson E.O., Soultanis K. and Soucacos P.N. (2004) Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthop. Clin. North Am. 35, 285–291, viii 10.1016/j.ocl.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 3.He W. and Li K. (2009) Incidence of genetic polymorphisms involved in lipid metabolism amongst Chinese patients with osteonecrosis of the femoral head. Acta Orthop. 80, 325–329 10.3109/17453670903025378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang A., Ren M. and Wang J. (2018) The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene 671, 103–109 10.1016/j.gene.2018.05.091 [DOI] [PubMed] [Google Scholar]
- 5.Gong L.L., Fang L.H., Wang H.Y., Peng J.H., Si K., Zhu J.. et al. (2013) Genetic risk factors for glucocorticoid-induced osteonecrosis: a meta-analysis. Steroids 78, 401–408 10.1016/j.steroids.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z., Hua Y., Liu J., Zuo D., Wang H., Chen Q.. et al. (2015) Association of ABCB1/MDR1 polymorphisms in patients with glucocorticoid-induced osteonecrosis of the femoral head: evidence for a meta-analysis. Gene 569, 34–40 10.1016/j.gene.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 7.Li Z., Zhao D. and Wang B. (2014) ABCB1 gene polymorphisms and glucocorticoid-induced avascular necrosis of the femoral head susceptibility: a meta-analysis. Med. Sci. Monit. 20, 2811–2816 10.12659/MSM.891286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo F.Q. and Deng M. (2017) Correlation between steroid-induced osteonecrosis of the femoral head and hepatic CYP3A activity: a systematic review and meta-analysis. J. Invest. Surg. 2017, 1–9 10.1080/08941939.2017.1385663 [DOI] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D.. et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 10.Berlin J.A., Longnecker M.P. and Greenland S. (1993) Meta-analysis of epidemiologic dose-response data. Epidemiology 4, 218–228 10.1097/00001648-199305000-00005 [DOI] [PubMed] [Google Scholar]
- 11.Yu M., Yang Z., Zhu Y. and Lu N. (2014) Efficacy of glucocorticoids in rodents of severe acute pancreatitis: a meta-analysis. Int. J. Clin. Exp. Pathol. 7, 3647–3661 [PMC free article] [PubMed] [Google Scholar]
- 12.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13.Wang Q., Ke J., Song Q., Hu W., Lu X., Wang Z.. et al. (2015) The SNP rs931794 in 15q25.1 is associated with lung cancer risk: a hospital-based case-control study and meta-analysis. PLoS ONE 10, e0128201 10.1371/journal.pone.0128201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Ruan W., Peng Y. and Li W. (2018) Sleep duration and the risk of osteoporosis amongst middle-aged and elderly adults: a dose-response meta-analysis. Osteoporos. Int. 29, 1689–1695 10.1007/s00198-018-4487-8 [DOI] [PubMed] [Google Scholar]
- 15.Orsini N., Bellocco R. and Greenland S. (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J. 6, 40–57 10.1177/1536867X0600600103 [DOI] [Google Scholar]
- 16.Zhao Z., Xue Y., Hong D., Zhang H., Hu Z., Fan S.. et al. (2017) Polymorphisms in the glucocorticoid receptor gene and associations with glucocorticoid-induced avascular osteonecrosis of the femoral head. Genet. Test Mol. Biomarkers 21, 322–327 10.1089/gtmb.2016.0260 [DOI] [PubMed] [Google Scholar]
- 17.Plesa M., Gagne V., Glisovic S., Younan M., Sharif-Askari B., Laverdiere C.. et al. (2017) Influence of BCL2L11 polymorphism on osteonecrosis during treatment of childhood acute lymphoblastic leukemia. Pharmacogenomics J. 14, A94. [DOI] [PubMed] [Google Scholar]
- 18.Karol S.E., Yang W., Van Driest S.L., Chang T.Y., Kaste S., Bowton E.. et al. (2015) Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood 126, 1770–1776 10.1182/blood-2015-05-643601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X.D., Liang J.C., Zeng P., Qin G. and Lv L.Q. (2015) The correlation between apolipoprotein gene polymorphisms and steroid-induced osteonecrosis of the femoral head. Guangdong Med. J. 36, 1364–1368, [In Chinese] [Google Scholar]
- 20.Zhang Y., Kong X., Wang R., Li S., Niu Y., Zhu L.. et al. (2014) Genetic association of the P-glycoprotein gene ABCB1 polymorphisms with the risk for steroid-induced osteonecrosis of the femoral head in Chinese population. Mol. Biol. Rep. 41, 3135–3146 10.1007/s11033-014-3173-y [DOI] [PubMed] [Google Scholar]
- 21.Xue Y., Zhao Z.Q., Hong D., Zhang H.J., Chen H.X. and Fan S.W. (2014) MDR1 gene polymorphisms are associated with glucocorticoid-induced avascular necrosis of the femoral head in a Chinese population. Genet. Test Mol. Biomarkers 18, 196–201 10.1089/gtmb.2013.0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Y., Kaisaierjiang A., Cao P., Wu Z.Y. and Lv Q. (2014) Association of apolipoprotein A5 genetic polymorphisms with steroid-induced osteonecrosis of femoral head in a Chinese Han population. Diagn. Pathol. 9, 229 10.1186/s13000-014-0229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng P., Liang J.C., Wei X.D. and Wei B.F. (2014) Relationship between apolipoprotein B gene polymorphism and tendon-vessel stagnation syndrome of steroid induced osteonecrosis of the femoral head. Shandong Med. J. 54, 1–3, [In Chinese] [Google Scholar]
- 24.Zhang Y., Wang R., Li S., Kong X., Wang Z., Chen W.. et al. (2013) Genetic polymorphisms in plasminogen activator inhibitor-1 predict susceptibility to steroid-induced osteonecrosis of the femoral head in Chinese population. Diagn Pathol. 8, 169 10.1186/1746-1596-8-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Zhang Y., Kong X., Li S., Hu Y., Wang R.. et al. (2013) Association of a polymorphism in PON-1 gene with steroid-induced osteonecrosis of femoral head in Chinese Han population. Diagn. Pathol. 8, 186 10.1186/1746-1596-8-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., He W., Zhang Q.W., Luo Y.Z., Chen Q.L. and Zhou C. (2012) Association between polymorphisms of steroid induced osteonecrosis of the femoral head and traditional Chinese medicine syndrome types. J. New Chin. Med. 44, 87–89, [In Chinese] [Google Scholar]
- 27.Wei B.F. (2011) Relationship between gene polymorphisms and syndrome types in steroid- induced femoral head osteonecrosis. J. Shandong Med. College 33, 321–325, [In Chinses] [Google Scholar]
- 28.Wei B.F. (2011) Relationship between gene polymorphisms and hereditary susceptibility of steroid-induced femoral head osteonecrosis in Linyi City of Shandong Province. J. Clin. Rehabilitative Tissue Eng. Res. 15, 7403–7406 [Google Scholar]
- 29.Bond J., Adams S., Richards S., Vora A., Mitchell C. and Goulden N. (2011) Polymorphism in the PAI-1 (SERPINE1) gene and the risk of osteonecrosis in children with acute lymphoblastic leukemia. Blood 118, 2632–2633 10.1182/blood-2011-05-355206 [DOI] [PubMed] [Google Scholar]
- 30.He W. and Li K. (2009) Incidence of genetic polymorphisms involved in lipid metabolism amongst Chinese patients with osteonecrosis of the femoral head. Acta Orthop. 80, 325–329 10.3109/17453670903025378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He W. and Li K. (2009) Genetic susceptibility of corticosteroid-induced osteonecrosis of femoral head. Chin. Bone Joint Surg. 2, 284–289, [In Chinese] [Google Scholar]
- 32.Kuribayashi M., Fujioka M., Takahashi K.A., Arai Y., Hirata T., Nakajima S.. et al. (2008) Combination analysis of three polymorphisms for predicting the risk for steroid-induced osteonecrosis of the femoral head. J. Orthop. Sci. 13, 297–303 10.1007/s00776-008-1244-4 [DOI] [PubMed] [Google Scholar]
- 33.French D., Hamilton L.H., Mattano L.A. Jr., Sather H.N., Devidas M., Nachman J.B.. et al. (2008) A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood 111, 4496–4499 10.1182/blood-2007-11-123885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Wei M., Han Y., Zhang K., He L., Yang Z.. et al. (2008) Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-Cov infection: a case-control study. BMC Infect. Dis. 8, 27 10.1186/1471-2334-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamura K., Nakajima S., Hirota Y., Takahashi K.A., Fujioka M., Kubo T.. et al. (2007) Genetic association of a polymorphism of the cAMP-responsive element binding protein-binding protein with steroid-induced osteonecrosis after kidney transplantation. J. Bone Miner. Metab. 25, 320–325 10.1007/s00774-007-0770-z [DOI] [PubMed] [Google Scholar]
- 36.Yang X.Y. and Xu D.H. (2007) MDR1(ABCB1) gene polymorphisms associated with steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus. Pharmazie 62, 930–932 [PubMed] [Google Scholar]
- 37.Hirata T., Fujioka M., Takahashi K.A., Asano T., Ishida M., Akioka K.. et al. (2007) Low molecular weight phenotype of Apo(a) is a risk factor of corticosteroid-induced osteonecrosis of the femoral head after renal transplant. J. Rheumatol. 34, 516–522 [PubMed] [Google Scholar]
- 38.Hirata T., Fujioka M., Takahashi K.A., Arai Y., Asano T., Ishida M.. et al. (2007) ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J. Orthop. Sci. 12, 199–206 10.1007/s00776-007-1110-9 [DOI] [PubMed] [Google Scholar]
- 39.Ekmekci Y., Keven K., Akar N., Egin Y., Sengul S., Kutlay S.. et al. (2006) Thrombophilia and avascular necrosis of femoral head in kidney allograft recipients. Nephrol. Dial. Transplant. 21, 3555–3558 10.1093/ndt/gfl400 [DOI] [PubMed] [Google Scholar]
- 40.Celik A., Tekis D., Saglam F., Tunali S., Kabakci N., Ozaksoy D.. et al. (2006) Association of corticosteroids and factor V, prothrombin, and MTHFR gene mutations with avascular osteonecrosis in renal allograft recipients. Transplant. Proc. 38, 512–516 10.1016/j.transproceed.2005.12.062 [DOI] [PubMed] [Google Scholar]
- 41.Relling M.V., Yang W., Das S., Cook E.H., Rosner G.L., Neel M.. et al. (2004) Pharmacogenetic risk factors for osteonecrosis of the hip amongst children with leukemia. J. Clin. Oncol. 22, 3930–3936 10.1200/JCO.2004.11.020 [DOI] [PubMed] [Google Scholar]
- 42.Asano T., Takahashi K.A., Fujioka M., Inoue S., Ueshima K., Hirata T.. et al. (2004) Relationship between postrenal transplant osteonecrosis of the femoral head and gene polymorphisms related to the coagulation and fibrinolytic systems in Japanese subjects. Transplantation 77, 220–225 10.1097/01.TP.0000101433.99651.96 [DOI] [PubMed] [Google Scholar]
- 43.Asano T., Takahashi K.A., Fujioka M., Inoue S., Satomi Y., Nishino H.. et al. (2003) Genetic analysis of steroid-induced osteonecrosis of the femoral head. J. Orthop. Sci. 8, 329–333 10.1007/s10776-003-0646-7 [DOI] [PubMed] [Google Scholar]
- 44.Asano T., Takahashi K.A., Fujioka M., Inoue S., Okamoto M., Sugioka N.. et al. (2003) ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics 13, 675–682 10.1097/00008571-200311000-00003 [DOI] [PubMed] [Google Scholar]
- 45.Ferrari P., Schroeder V., Anderson S., Kocovic L., Vogt B., Schiesser D.. et al. (2002) Association of plasminogen activator inhibitor-1 genotype with avascular osteonecrosis in steroid-treated renal allograft recipients. Transplantation 74, 1147–1152 10.1097/00007890-200210270-00016 [DOI] [PubMed] [Google Scholar]
- 46.Chen X., Zhang L., Liang D., Li J., Liu F. and Ma H. (2018) Lipid transporter activity-related genetic polymorphisms are associated with steroid-induced osteonecrosis of the femoral head: an updated meta-analysis based on the GRADE guidelines. Front. Physiol. 9, 1684 10.3389/fphys.2018.01684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Z.C., Gu S.Z., Wu J. and Liang Q.W. (2015) VEGF, eNOS, and ABCB1 genetic polymorphisms may increase the risk of osteonecrosis of the femoral head. Genet. Mol. Res. 14, 13688–13698 10.4238/2015.October.28.31 [DOI] [PubMed] [Google Scholar]
- 48.Bradbury G., Benjamin J., Thompson J., Klees E. and Copeland J. (1994) Avascular necrosis of bone after cardiac transplantation. Prevalence and relationship to administration and dosage of steroids. J. Bone Joint Surg. Am. 76, 1385–1388 10.2106/00004623-199409000-00014 [DOI] [PubMed] [Google Scholar]
- 49.Schachtner T., Otto N.M. and Reinke P. (2018) Cyclosporine use and male gender are independent determinants of avascular necrosis after kidney transplantation: a cohort study. Nephrol. Dial. Transplant. 33, 2060–2066 10.1093/ndt/gfy148 [DOI] [PubMed] [Google Scholar]
- 50.Zhu K.K., Xu W.D., Pan H.F., Zhang M., Ni J., Ge F.Y.. et al. (2014) The risk factors of avascular necrosis in patients with systemic lupus erythematosus: a meta-analysis. Inflammation 37, 1852–1864 10.1007/s10753-014-9917-y [DOI] [PubMed] [Google Scholar]
- 51.Zhao R., Wang H., Wang X. and Feng F. (2017) Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos. Int. 28, 1027–1034 10.1007/s00198-016-3824-z [DOI] [PMC free article] [PubMed] [Google Scholar]