Abstract
Hepatocellular carcinoma (HCC) has become a global public health problem. Therefore, the development of novel and effective therapeutic agents for the treatment of HCC is considered an emergency. Avicularin, a bio-active flavonoid from plants, has been reported to exhibit diverse pharmacological properties. The aim of the present study was to investigate the role of avicularin in HCC and the underlying mechanism of action. Huh7 cells were treated with avicularin in a concentration-dependent manner, and the cell proliferation was examined using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay kit. The cell migration and invasion abilities were detected using wounding-healing assays and Transwell assays. Flow cytometric analysis was performed to investigate the cell cycle distribution and cell apoptosis. The activity of nuclear factor (NF)-κB (p65), cyclooxygenase-2 (COX-2) and peroxisome proliferator-activated receptor γ (PPAR-γ) were measured by reverse transcription-quantitative polymerase chain reaction and western blot analyses, respectively. The results indicated that avicularin treatment markedly decreased cell proliferation concentration-dependently in HCC, and inhibited cell migration and invasion in Huh7 cells. It was also found that the treatment of avicularin markedly inhibited the G0/G1-phase cells and decreased the accumulation of S-phase cells in the cell cycle and induced cell apoptosis. In addition, it was confirmed that the anticancer efficacy of avicularin in HCC was dependent on the regulation of NF-κB (p65), COX-2 and PPAR-γ activities. In conclusion, the findings suggested that avicularin serves an antineoplastic role in HCC and may provide a potential therapeutic strategy for the treatment of HCC.
Keywords: hepatocellular carcinoma, avicularin, cell proliferation, nuclear factor-κB, cyclooxygenase-2, peroxisome proliferator-activated receptor γ
Introduction
Human hepatocellular carcinoma (HCC) is one of the most common types of cancer and the third leading cause of cancer-associated mortality worldwide (1). The incidence of HCC is highest in sub-Saharan Africa and East Asia (2). HCC is a multistep process mainly associated with alcohol, aflatoxin and persistent infection with hepatitis B virus or hepatitis C virus (3). Curative treatments, including locoregional ablation, surgical resection and liver transplantation, are only appropriate for a small number of patients diagnosed at an early stage; the majority of patients are diagnosed with HCC at an advanced disease stage, resulting in a high mortality rate of HCC (4). At present, sorafenib is the unique drug that has been approved by the U.S. Food and Drug Administration (FDA) for patients with advanced HCC (5). However, the majority of patients are unable to afford the high expense of sorafenib, therefore, there is an urgent need for novel therapies.
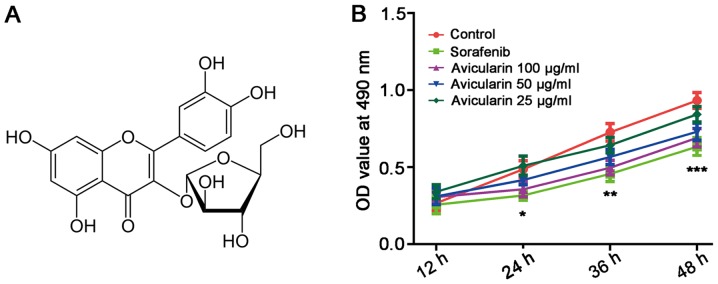
Several dietary flavonoids, abundant in various fruits and vegetables, are regarded as bioactive components with particular benefit to the health of the body. Accumulating evidence has suggested that flavonoids exert effective biological activity, including anticancer, anti-inflammatory and antiviral effects against infection, in addition to potential protective activity against liver damage (6–8). Avicularin (quercetin-3-α-L-arabinofuranoside; Fig. 1A), a glycoside of quercetin, is a type of flavonoid which has been reported to inhibit obesity, inflammation and multidrug resistance (9–11). In addition, previous studies have indicated that the quercetin avicularin can induce cytotoxicity in cancer lines and tumor tissues by activating the intrinsic pathway of apoptosis (12). However, the anticancer activity of avicularin itself has been not fully elucidated.
Figure 1.
Avicularin inhibits Huh7 cell proliferation in hepatocellular carcinoma. (A) Chemical structure of avicularin. (B) Proliferation of Huh7 cells was determined using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay. OD values at 490 nm were observed at 12, 24, 36 and 48 h, respectively. Data are presented as the mean ± standard deviation of three experiments. *P<0.05, **P<0.01 and ***P<0.001, vs. control. OD, optical density.
The aim of the present study was to investigate the effects of avicularin on HCC and its potential mechanism of action in Huh7 cells. It was hypothesized that avicularin has a positive influence on HCC, and that avicularin may be a potential effective and safe therapy for HCC.
Materials and methods
Cell culture
The Huh7 HCC cells were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum (FBS; both Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1% antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin) and 2 mM glutamine at 37°C in a humidified atmosphere of 5% CO2. The cells were treated with sorafenib (5 µmol/l; BioVision, Inc.) as the positive control, and treated with different concentrations (100, 50 and 25 µg/ml) of avicularin (Aladdin Biochemical Technology, Co., Ltd., Shanghai, China) as the treatment groups.
Cell viability assay
The cell viability assay was performed using a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, Merck KGaA) assay. Briefly, the Huh7 cells were seeded in 96-well plates at a density of 1×104 cells/well and incubated overnight at 37°C. Following incubation, cells were treated with sorafenib/avicularin in 10% FBS-supplemented medium for 12, 24, 36 and 48 h at 37°C, respectively. The medium was replaced with MTT (0.5 mg/ml) for 4 h at 37°C. The resulting formazan crystals were then dissolved in DMSO. The absorbance was determined at 490 nm.
Cell migration assays
The cells were incubated at 5×105 per dish with sorafenib or avicularin and grown to confluence for 48 h. Subsequently, a pipette tip was used to generate a wound, vertical to the parallel line (interval of 2–3 mm) marked on the bottom of the dish. After 0 and 48 h, the migration distance was analyzed using Image J software (version 1.46; National Institutes of Health, Bethesda, MD, USA). The experiments were performed in triplicate.
Transwell invasion assay
The invasion assay of cells was assessed using Transwell cell culture chambers (8 µm pore size; EMD Millipore, Billerica, MA, USA). Briefly, 600 µl medium containing 10% FBS was added to the lower 24-well chamber. The treated cells were resuspended in serum-free medium, and then cultured in the upper chamber coated with 50 µl of 1 g/l Matrigel (BD Biosciences). After 24 h, the upper side of the membrane was gently wiped with a cotton swab to remove cells whose upper surface did not penetrate, and the membrane was stained with 1% crystal violet for 20 min at room temperature, followed by washing with PBS. The invasive cells were counted at ×200 magnification by an Olympus light microscope (BX43; Olympus Corporation, Tokyo, Japan) in five randomly selected fields per well. Each experiment was performed in triplicate.
Reserve transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated with TRIzol (Invitrogen, Thermo Fisher Scientific, Inc.) and complementary DNA was then synthesized with PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan) using the following conditions: Initial incubation at 37°C for 15 min, followed by incubation at 85°C for 5 sec. Subsequently, qPCR was performed by FastStart Universal SYBR-Green Master (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocols. The qPCR thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. The relative mRNA expression was calculated using the 2−∆∆Cq method (13), and normalized using GAPDH as the reference gene. The following primers were used for the experiment: Nuclear factor (NF)-κB (p65) forward, 5′-ACGATCTGTTTCCCCTCATCT-3′ and reverse, 5′-TGCTTCTCTCCCCAGGAATA-3′; peroxisome proliferator-activated receptor γ (PPAR-γ) forward, 5′-AACTGCAGGGTGAAACTCTGGGAGATTCTCC-3′ and reverse, 5′-GGATTCAGCAACCATTGGGTCAGCTCT-3′; cyclooxygenase-2 (COX-2), forward, 5′-CTGAGGGGTTACCATTCCA-3′ and reverse, 5′-TGAGCAAGTCCGTGTTCAAG-3′; GAPDH forward, 5′-GTGAGGAGGGGAGATTCAG-3′ and reverse, 5′-GCATCCTGGGCTACACTG-3′.
Western blot analysis
The cells were lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4; 0.15 M NaCl; 1% Nonidet P-40, 0.1% sodium dodecyl sulfate and 0.5% sodium deoxycholate) containing protease and phosphatase inhibitors (Sigma-Aldrich, Merck KGaA), and the protein concentration was measured using a BCA protein assay kit (Pierce, Thermo Fisher Scientific, Inc.). The same amount (20 µg) of proteins were separated on 10% SDS-PAGE gels and transferred onto Immobilon polyvinylidene difluoride membranes (EMD Millipore), and then blocked in 5% skim milk in TBST for 1 h at room temperature. The primary antibodies against COX-2 (1:500; cat. no. ab23672), PPAR-γ (1:500; cat. no. ab45036), NF-κB (p65) (1:2,000; cat. no. ab86299) and GAPDH (1:2,500; cat. no. ab9485; Abcam, Cambridge, UK) were added for overnight incubation at 4°C. Following washing three times with 1X TBST buffer, a secondary antibody coupled to horseradish peroxidase (1:2,000; cat. no. sc-2030) from Santa Cruz Biotechnology, Inc. was incubated with the membranes for 1.5 h at room temperature, and immunoactivity was detected using an ECL-Plus kit (GE Healthcare Life Sciences, Pollards Wood, UK). Band intensity on scanned films was quantified using ImageJ software (version 1.46; National Institutes of Health, Bethesda, MD, USA) and expressed as relative intensity compared with control. The intensity of the protein bands was normalized to GAPDH and the relative intensity was calculated.
Flow cytometric analysis
Following treatment for 48 h, the cells were harvested and washed in cold PBS. For cell cycle analysis, the cells were fixed in 70% ethanol at −20°C overnight. Following washing and centrifugation at 1,000 × g for 5 min at 4°C, the cells were treated with RNase A and stained with propidium iodide (PI) working solution for 30 min in the dark. Analysis was determined using a FACS Caliber flow cytometer and CellQuest software (version 5.1; BD Biosciences). For the analysis of cell apoptosis, the Annexin-V-FITC Apoptosis Detection kit I (BD Pharmingen) was used, according to the manufacturer's protocol. Briefly, the cells were resuspended with 1X binding buffer, following which the cells were stained with 5 µl Annexin-V-FITC and 5 µl PI, and then incubated in the dark for 15 min. Subsequently, the appropriate 1X binding buffer was added and apoptosis was analyzed by the FACSCalibur flow cytometer.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). The results are expressed as the mean ± standard deviation from at least three independent experiments. Statistical significance was analyzed using a two-tailed Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Avicularin inhibits the proliferation of Huh7 cells
The physiological effect of avicularin on HCC was investigated by observing the proliferation of Huh7 cells treated with avicularin. As shown in Fig. 1B, sorafenib, an oral multikinase inhibitor approved for advanced HCC (5), resulted in marked inhibition of cell proliferation. Avicularin significantly inhibited cell proliferation in a concentration-dependent manner. With the growth of Huh7 cells cultured with avicularin, the suppressing effect of avicularin on cell proliferation was more marked.
Avicularin repress the migration and invasion of Huh7 cells
To confirm that avicularin functions as tumor suppressor in HCC, the present study investigated the influence of avicularin on the migration and invasion of Huh cells. The results suggested that the wounding healing of Huh7 cells was markedly retarded in the presence of sorafenib and avicularin (Fig. 2A). Therefore avicularin suppressed cell migration following treatment at concentrations of 100 and 50 µg/ml, and was concentration-dependent. Additionally, the invasive ability of Huh7 cells cultured under sorafenib/avicularin was observed through a Transwell assay. As shown in Fig. 2B, compared with the control cells, the cell counts were markedly decreased following sorafenib and avicularin treatment. Avicularin resulted in a marked inhibition of cell invasion at the concentrations of 100 and 50 µg/ml. As a result, avicularin effectively suppressed the migration and invasion of Huh7 cells in HCC.
Figure 2.
Avicularin repress the migration and invasion of Huh7 cells. (A) Wounding-healing assays and (B) Transwell assays were used to investigate the changes in the migratory and invasive abilities of Huh7 cells following treatment with sorafenib and avicularin. All results were quantified. Magnification, ×200. Data are presented as the mean ± standard deviation of three experiments. *P<0.05 and ***P<0.001, vs. control; #P<0.05 and ###P<0.001, vs. sorafenib.
Avicularin promotes cell apoptosis and regulates cell cycle progression
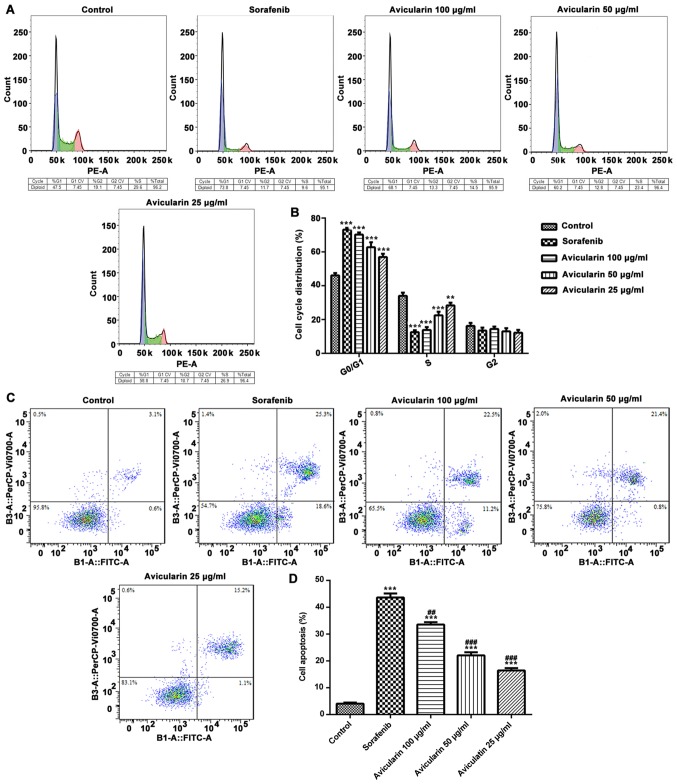
To determine the effects of avicularin on cell cycle, the cells were treated with different concentrations (25, 50 and 100 µg/ml) of avicularin for 48 h, and the percentage of cells in different cell cycle phases were analyzed. As shown in Fig. 3A and B, compared with the control cells, sorafenib and avicularin induced notable G0/G1 arrest in the Huh7 cells. Additionally, there was a marked decrease in the accumulation of S-phase cells in the avicularin-treated group, similar to sorafenib, particularly at the concentration of 100 µg/ml. These data suggested that the inhibition of cell proliferation by avicularin was connected with an increase in the G0/G1 phase and a decrease in the S phase of the cell cycle.
Figure 3.
Cell were stained with propidium iodide and the cell cycle distributions and cell apoptosis were measured by flow cytometry. (A) Effects of avicularin on the cycle distribution of Huh7 cells. (B) Quantification of cells in each phase of the cell cycle. (C) Cell apoptosis was analyzed and (D) quantified. Data are presented as the mean ± standard deviation of three experiments. **P<0.01 and ***P<0.001, vs. control; ##P<0.01 and ###P<0.001, vs. sorafenib.
In order to assess whether avicularin inhibited cell proliferation through an apoptotic mechanism in HCC, flow cytometric analysis was performed in Huh7 cells. As shown in Fig. 3C and D, the percentage of cell apoptosis was increased in the sorafenib-treated cells. It was confirmed that avicularin enhanced cell apoptosis, which occurred in a concentration-dependent manner. The above results demonstrated that avicularin regulates cell cycle progression and promotes cell apoptosis, which may lead to the inhibition of cell proliferation in HCC.
Avicularin serves an antineoplastic role through the regulation of NF-κB (p65), COX-2 and PPAR-γ
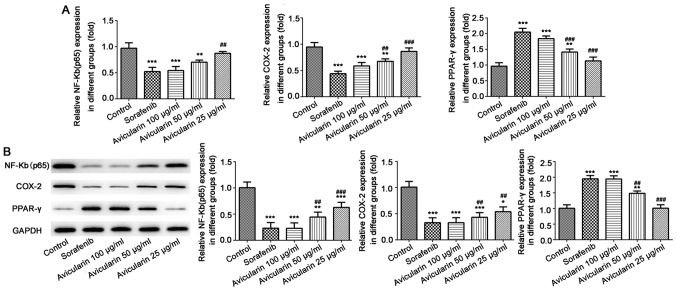
Subsequently, to gain further insight into the mechanism underlying the antiproliferative activity of avicularin in HCC, the present study aimed to determine whether avicularin-induced cell apoptosis and cell cycle arrest involves NF-κB (p65), COX-2 and PPAR-γ, which have been known to be involved in cell cycle progression and proliferation. As shown in Fig. 4A and B, sorafenib resulted in significant inhibition of NF-κB (p65) and COX-2, not only at the mRNA level, but also at the protein level. The mRNA level and protein expression levels of PPAR-γ were increased. Similarly, avicularin resulted in the same effects on NF-κB (p65), COX-2 and PPAR-γ in a concentration-dependent manner. Taken together, these results suggest that avicularin regulates cell apoptosis and cell cycle via the downregulation of NF-κB (p65) and COX-2 and the upregulation of PPAR-γ.
Figure 4.
Expression of NF-κB (p65), COX-2 and PPAR-γ. (A) Following treatment with sorafenib and avicularin, the mRNA levels of NF-κB (p65), COX-2 and PPAR-γ was measured by reverse transcription-quantitative polymerase chain reaction analysis. (B) Protein expression of NF-κB (p65), COX-2 and PPAR-γ was measured by western blot analysis and quantified. Data is presented as the mean ± standard deviation of three experiments. *P<0.05, **P<0.01 and ***P<0.001, vs. control; ##P<0.01 and ###P<0.001, vs. sorafenib. NF-κB, nuclear factor-κB; COX-2, cyclooxygenase-2; PPARγ, peroxisome proliferator-activated receptor γ.
Discussion
HCC is a global public health problem that accounts for a high rate of morality. Currently, liver resection, liver transplantation and ablation remain the mainstream treatment, which are only appropriate for those patients at an early stage and can easily result in adverse effects. Therefore, to overcome these deficiencies, sorafenib, approved by the FDA, has been shown to be effective against several solid tumors and is a standard treatment for HCC (14). Previous studies have reported that sorafenib exerts its antitumor activities by triggering cell apoptosis and inhibiting tumor angiogenesis through the Raf, c-kit, vascular endothelial growth factor, platelet-derived growth factor, fms-like tyrosine kinase 3 and mitogen-activated protein kinase pathways (5,15). In addition, it is reported that sorafenib can reduce Alzheimer's disease pathology by reducing neuroinflammation through inhibition of the expression of NF-κB and COX-2 (16). In the present study, it was found that sorafenib also targets NF-κB and COX-2 to exert its antineoplastic role. However, as the high cost of sorafenib makes is difficult to afford, it remains essential to develop more effective, safe and appropriate methods for the treatment of HCC. In the present study, a novel therapy against HCC was investigated and its mechanism of anticancer activity was examined. It was found that avicularin inhibited the proliferation, migration and invasion of Huh7 cells in HCC through influencing cell apoptosis and cycle distribution on the regulation of NF-κB (p65), COX-2 and PPAR-γ.
In oriental culture, numerous plants have been used in folk medicine for thousands of years. Among abundant plants, the flavonoid family, as a group of plant secondary metabolites with multiple phenolic structures, is of particular interest and is usually found in vegetables and fruits (17). It has been reported that numerous flavonoids can enhance the efficacy and alleviate the adverse effects of tumor therapies. Baicalein and silymarin, extracts of Scutellaria baicalensis, have been shown to exert a suppressive effect on tumors. The combination of baicalein and silymarin can suppress HepG2 cell proliferation by increasing the percentages of cells in the G0/G1 phase and decreasing those in the S phase (18). Amentoflavone, a flavonoid compound extracted from Selaginella tamariscina Spring, reportedly exerts antineoplastic activity via the induction of cell apoptosis and inhibition of glycolysis in HCC (19). Additionally, baicalein (20), tectorigenin (21) and other flavonoids have been shown to protect cells against cancer progression though the activation of proapoptotic and antiproliferative pathways or other pathways (22). Avicularin (quercetin-3-α-L-arabinofuranoside) belongs to a group of flavonoid glycosides. It has been reported that avicularin has a protective effect against human gastric cancer through inducing apoptosis dependent on Bax and BCL-2-related ovarian killer (11). The present study focused on the efficacy of avicularin on HCC. The data showed that avicularin exerted marked anticancer activity in Huh7 cells in a concentration-dependent manner. Avicularin at 100 µg/ml had a marked suppressive effect on cell proliferation, migration and invasion, similar to sorafenib. However, at a concentration at 25 µg/ml, avicularin had no effect on HCC.
NF-κB is a protein complex that controls the transcription of DNA (23). It is a key transcription factor that is closely associated with the proliferation and apoptosis of cancer, and it also serves an important role in the cell cycle, which is vital in determining the degree of cellular proliferation and apoptosis. In the present study, the cell population was increased in the G0/G1 phase but decreased in the S phase. Cell apoptosis was improved following treatment with avicularin. The mRNA level and protein expression levels of p65 (a subunit of NF-κB) were significantly inhibited by avicularin, which indicates that avicularin suppressed cell cycle progression and promoted cell apoptosis via the inhibition of NF-κB activity. The involvement of COX-2 in tumorigenesis in HCC has been widely reported in several studies, and is also closely linked with NF-κB. The promoter regions of the COX-2 gene in human and mice harbor binding sites for NF-κB. Therefore the expression of COX-2 can be mediated via NF-κB (24). Accordingly, the results of the present study showed that avicularin markedly downregulated the mRNA and protein expression levels of COX-2 in Huh7 cells. Therefore, it is possible that avicularin exerts its anticancer activity on the inhibition of COX-2 through NF-κB.
PPAR-γ is a member of the nuclear hormone receptor superfamily. It is involved in the control of biological processes associated with differentiation, growth, apoptosis and cell cycle (25). The activity of PPAR-γ has been shown to be inhibited in HCC (26). The overexpression of PPAR-γ can inhibit cell proliferation in HCC, and the downregulation of PPAR-γ has been associated with differentiation and poor prognosis in patients in HCC (27). PPAR-γ is also reported to be correlated with cell cycle arrest through p53 and p21 (28). Additionally, PPAR-γ promotes cell apoptosis, which resulted in the inhibition of cancer (29). Based on these results, the present data showed that the expression of PPAR-γ was significantly increased when the cells were treated with sorafenib and avicularin. The anticancer activity of avicularin involved in the inhibition of cell proliferation, migration and invasion, and changes in cell apoptosis and cell cycle may partly depend on the upregulation of PPAR-γ.
In conclusion, the results demonstrate an antineoplastic role of avicularin of HCC in vitro. It was clearly demonstrated that avicularin can inhibit cell proliferation, migration and invasion in HCC through inducing apoptosis and suppressing cell cycle progression. Additionally, the decreased activity of NF-κB and COX-2 and increased activity of PPAR-γ suggest that avicularin has an antineoplastic effect through the regulation of these, and that NF-κB, COX-2 and PPAR-γ are vital factors predicting the anticancer effect in HCC. Overall, the results of the present study suggests that avicularin may be a valuable therapeutic agent in the treatment of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health and Family Planning Commission of Zhejiang Province (grant no. GZS2012031)
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JS was involved in the design of the experiment. ZM, FL and YQ performed the experiments and analyzed the data. ZM and YQ wrote the manuscript. JS reviewed and edited the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lu T, Seto WK, Zhu RX, Lai CL, Yuen MF. Prevention of hepatocellular carcinoma in chronic viral hepatitis B and C infection. World J Gastroenterol. 2003;19:8887–8894. doi: 10.3748/wjg.v19.i47.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X, Feng K, Zhang Y, Li Z, Zhou F, Dou H, Wang T. Sorafenib and DE605, a novel c-Met inhibitor, synergistically suppress hepatocellular carcinoma. Oncotarget. 2015;6:12340–12356. doi: 10.18632/oncotarget.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui X, Wang Y, Kokudo N, Fang D, Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. Biosci Trends. 2010;4:39–47. [PubMed] [Google Scholar]
- 7.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Handoussa H, Osmanova N, Ayoub N, Mahran L. Spicatic acid: A 4-carboxygentisic acid from Gentiana spicata extract with potential hepatoprotective activity. Drug Discov Ther. 2009;3:278–286. [PubMed] [Google Scholar]
- 9.Fujimori K, Shibano M. Avicularin, a plant flavonoid, suppresses lipid accumulation through repression of C/EBPα-activated GLUT4-mediated glucose uptake in 3T3-L1 cells. J Agric Food Chem. 2013;61:5139–5147. doi: 10.1021/jf401154c. [DOI] [PubMed] [Google Scholar]
- 10.Vo VA, Lee JW, Chang JE, Kim JY, Kim NH, Lee HJ, Kim SS, Chun W, Kwon YS. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 macrophages. Biomol Ther (Seoul) 2012;20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo XF, Liu JP, Ma SQ, Zhang P, Sun WD. Avicularin reversed multidrug-resistance in human gastric cancer through enhancing Bax and BOK expressions. Biomed Pharmacother. 2018;103:67–74. doi: 10.1016/j.biopha.2018.03.110. [DOI] [PubMed] [Google Scholar]
- 12.Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, Arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Lang L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology. 2008;134:379. doi: 10.1053/j.gastro.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Hahn O, Stadler W. Sorafenib. Curr Opin Oncol. 2006;18:615–621. doi: 10.1097/01.cco.0000245316.82391.52. [DOI] [PubMed] [Google Scholar]
- 16.Echeverria V, Burgess S, Gamble-George J, Zeitlin R, Lin X, Cao C, Arendash GW. Sorafenib inhibits nuclear factor kappa B, decreases inducible nitric oxide synthase and cyclooxygenase-2 expression, and restores working memory in APPswe mice. Neuroscience. 2009;162:1220–1231. doi: 10.1016/j.neuroscience.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 18.Chen CH, Huang TS, Wong CH, Hong CL, Tsai YH, Liang CC, Lu FJ, Chang WH. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 cells. Food Chem Toxicol. 2009;47:638–644. doi: 10.1016/j.fct.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Liu B, Yu S. Amentoflavone suppresses hepatocellular carcinoma by repressing hexokinase 2 expression through inhibiting JAK2/STAT3 signaling. Biomed Pharmacother. 2018;107:243–253. doi: 10.1016/j.biopha.2018.07.177. [DOI] [PubMed] [Google Scholar]
- 20.Liang RR, Zhang S, Qi JA, Wang ZD, Li J, Liu PJ, Huang C, Le XF, Yang J, Li ZF. Preferential inhibition of hepatocellular carcinoma by the flavonoid Baicalein through blocking MEK-ERK signaling. Int J Oncol. 2012;41:969–978. doi: 10.3892/ijo.2012.1510. [DOI] [PubMed] [Google Scholar]
- 21.Jiang CP, Ding H, Shi DH, Wang YR, Li EG, Wu JH. Pro-apoptotic effects of tectorigenin on human hepatocellular carcinoma HepG2 cells. World J Gastroenterol. 2012;18:1753–1764. doi: 10.3748/wjg.v18.i15.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia ER, Gutierrez EA, de Melo FCSA, Novaes RD, Goncalves RV. Flavonoids effects on hepatocellular carcinoma in murine models: A systematic review. Evid Based Complement Alternat Med. 2018;2018:6328970. doi: 10.1155/2018/6328970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore TD. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Na HK, Pak YK, Lee YS, Lee SJ, Moon A, Surh YJ. Roles of ERK and p38 mitogen-activated protein kinases in phorbol ester-induced NF-kappaB activation and COX-2 expression in human breast epithelial cells. Chem Biol Interact. 2008;171:133–141. doi: 10.1016/j.cbi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Koeffler HP. Peroxisome proliferator-activated receptor gamma and cancers. Clin Cancer Res. 2003;9:1–9. [PubMed] [Google Scholar]
- 26.Vara D, Morell C, Rodriguez-Henche N, Diaz-Laviada I. Involvement of PPARgamma in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 2013;4:e618. doi: 10.1038/cddis.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Li J, Fei BY, Shao D, Pan Y, Mo ZH, Sun BZ, Zhang D, Zheng X, Zhang M, et al. MiR-27a promotes hepatocellular carcinoma cell proliferation through suppression of its target gene peroxisome proliferator-activated receptor γ. Chin Med J (Engl) 2015;128:941–947. doi: 10.4103/0366-6999.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galli A, Ceni E, Mello T, Polvani S, Tarocchi M, Buccoliero F, Lisi F, Cioni L, Ottanelli B, Foresta V, et al. Thiazolidinediones inhibit hepatocarcinogenesis in hepatitis B virus-transgenic mice by peroxisome proliferator-activated receptor gamma-independent regulation of nucleophosmin. Hepatology. 2010;52:493–505. doi: 10.1002/hep.23669. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Shen B, Chu ES, Teoh N, Cheung KF, Wu CW, Wang S, Lam CN, Feng H, Zhao J, et al. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology. 2010;51:2008–2019. doi: 10.1002/hep.23550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.