ABSTRACT
Background: Evidence from recent years suggests most cases of hemoptysis to be caused by non-malignant etiologies, but the long-term outcome for these patients has been less thoroughly investigated. Objective: We aimed to assess the rates of hemoptysis recurrence, new lung cancer diagnoses and death within 5 years of initial referral for hemoptysis. Design: In this retrospective study, we reviewed clinical records of consecutive patients referred to evaluation for hemoptysis with no malignancy suspected on chest computed tomography at Aalborg University Hospital, Denmark, in a seven-year period from 2006 to 2012.
Results: A total of 609 patients (mean age 56.7 ± 14.1 years, 60.6% male) were included in the study. The etiology was cryptogenic in 81% of patients and no patients had malignant disease. In the following 5 years, lung cancer developed in 1.5% of patients. Median time to diagnosis was 26 (IQR 18–33) months. Nine percent of patients had at least one recurrence of hemoptysis, and the 5-year mortality rate was 12% with median time to death 31 (IQR 13–43) months.
Conclusions: Lung cancer developed in less than 2% within 5 years after referral for hemoptysis with no malignancy suspected on chest computed tomography. Further research is needed to identify risk factors for the development of lung cancer in these patients.
KEYWORDS: Hemoptysis, cryptogenic hemoptysis, bronchoscopy, computed tomography of the thorax, follow-up, mortality, recurrence, prognosis
Introduction
Hemoptysis is defined as coughing up or spitting of blood originating from the lower airways [1]. Although often associated with serious disease by the patient and, to some extent, health care professionals, evidence from later years points to low proportions of lung cancer and other serious conditions as etiologies of hemoptysis, while common respiratory tract infections and cryptogenic hemoptysis are more frequent [2–6].
The long-term outcomes in hemoptysis patients have been less frequently studied than the immediate etiologies. Studies from recent years have found very low incidences of lung cancer in the years after initial referral for hemoptysis [6–10], even though a 3-year incidence of 10% was reported in one larger study [5]. Reported mortality rates in hemoptysis patients range from 4% at 1 year [11] to around 20% at 2–3 years after initial referral for hemoptysis [5,9,12], while reports on recurrence rates of hemoptysis are difficult to compare but also vary considerably from 0.3% at 1 year to 16.3% at 3 years [5,7,8,11]. The long-term prognosis of patients with hemoptysis is not well known regarding diagnoses such as Chronic Obstructive Pulmonary Disease (COPD), asthma and bronchiectasis.
Guidelines recommend evaluation with fiberoptic bronchoscopy and chest computed tomography (CT) in patients with hemoptysis, but no consensus exists regarding the necessity of follow-up in these patients. This may be explained by the scarcity of evidence when it comes to the natural history of hemoptysis and the patient prognoses.
Hypothesis and aims
We hypothesize that patients with hemoptysis and no malignancy suspected on chest CT do not have recurrent episodes of hemoptysis and do not develop lung cancer within 5 years.
Thus, primary outcomes are the proportion of this patient group experiencing recurrent hemoptysis and the proportion diagnosed with lung cancer within 5 years of initial referral for hemoptysis.
Secondary outcomes are the etiologies in recurrent hemoptysis and the rates of new diagnoses of COPD, asthma, bronchiectasis and interstitial lung disease within 5 years of referral. Lastly, we will determine the 5-year mortality rate in this patient group.
Materials and methods
Setup and study population
The patients presented in this study comprise part of the study population presented in another study from our group [13]. Said report is currently in submission.
We retrospectively reviewed clinical records of patients referred to evaluation for hemoptysis at the Department of Respiratory Diseases, Aalborg University Hospital, Denmark. Study subjects were identified by the ICD-10 code for hemoptysis, DR042. Consecutive patients referred in a 7-year period from 1 January 2006 to 31 December 2012 were eligible for inclusion to allow for a full 5-year follow-up.
The following exclusion criteria were applied: <18 years of age; previous evaluation for hemoptysis; cancellation of the clinical evaluation for any reason; bleeding not originating from the lower airways as assessed on first visit; no chest CT or chest CT older than two months at referral; and CT suspicious of pulmonary malignancy.
Data collection and definitions
From the patients’ case records from the first visit upon referral, the following data were collected: gender and age at referral; present somatic diseases (diagnoses applicable to the patient at initial referral); previous somatic diseases (diagnoses no longer applicable to the patient at initial referral); and smoking history (never, ex-smoker [>6 months absence], smoker). If not noted independently, present somatic diseases were estimated from the medication listed in the case record. Smoking history in smokers and ex-smokers was quantified in pack years (≤30/>30) [14].
Results of the chest CT were obtained from the radiological description. Any abnormality of the lungs, including bronchiectasis but excluding emphysematic shape of the thorax and normal anatomical variations, was considered a positive finding. Bronchoscopy results were recorded as assessed by the procedure description from the investigating physician. Bronchoscopy was considered macroscopically positive if: the site of bleeding was identified; a tumor or foreign body was identified; a substantially vulnerable mucosa was described; or sequelae after previous surgery were described.
Results of histological, cytological and microbiological samples were obtained from the respective integrated applications of the patients’ case records. Histology and cytology were considered positive if suggestive of dysplasia or malignancy. Metaplasia and inflammation were not considered positive, unless the pattern of inflammation suggested interstitial lung disease. As to microbiology, any growth not described as normal flora was considered positive, as was positive polymerase chain reaction (PCR) for mycobacteria.
Etiologies of hemoptysis were defined as the diagnoses stated or acted upon by the physician at the end of initial diagnostic workup. Etiologies were grouped into primary lung cancers, metastatic cancer to the lungs, mycobacterial infections, fungal infections, other respiratory tract infection (excluding mycobacterial and fungal infections) and interstitial lung disease. All other etiologies were recorded individually. In patients with more than one diagnosis, all were recorded as etiologies. Cases with no established etiology were recorded as cryptogenic.
Follow-up data were collected from clinical records dated from a 5-year period after the initial referral for hemoptysis and included: number of new episodes of hemoptysis, and, for the first of these, etiology and time from initial referral; new diagnoses of lung cancer, COPD, asthma, bronchiectasis or interstitial lung disease and time from initial referral; death and time from initial referral. Recurrent hemoptysis was recorded for all new contacts with the ICD-10 diagnosis of hemoptysis that were not directly connected to the initial referral for hemoptysis.
Data management, statistics and approvals
Study data were collected and managed using REDCap electronic data capture tools hosted at Aalborg University Hospital [15].
Descriptive statistics were carried out using the integrated ‘Data Exports, Reports, and Stats’ module of REDCap and IBM SPSS Statistics for Macintosh, Version 25.0, the latter of which was also used for hypothesis testing and multivariable regression. Normally distributed data were presented as mean and standard deviation (SD), while data with non-normal distribution were presented as median and interquartile range (IQR). Frequencies were presented as absolute numbers and percentages of cases with relevant data available. Cox proportional hazards regression was applied to identify independent risk factors during follow-up. Hazard ratios were presented along with confidence intervals and p-values. A significance level of 95% applied to all tests.
The study was approved by the Danish Patient Safety Authority with case ID 3-3013-2350/1/and by the Danish Data Protection Agency under the umbrella application for the North Denmark Region (2008-58-0028) with project ID 2017-60.
Results
Study population and baseline characteristics
A total of 802 patients were referred to our clinic with hemoptysis during the study period from 1 January 2006 to 31 December 2012. Of these, 193 met one or more exclusion criteria, leaving 609 patients to be included in the study. The inclusion process is depicted in Figure 1.
Figure 1.
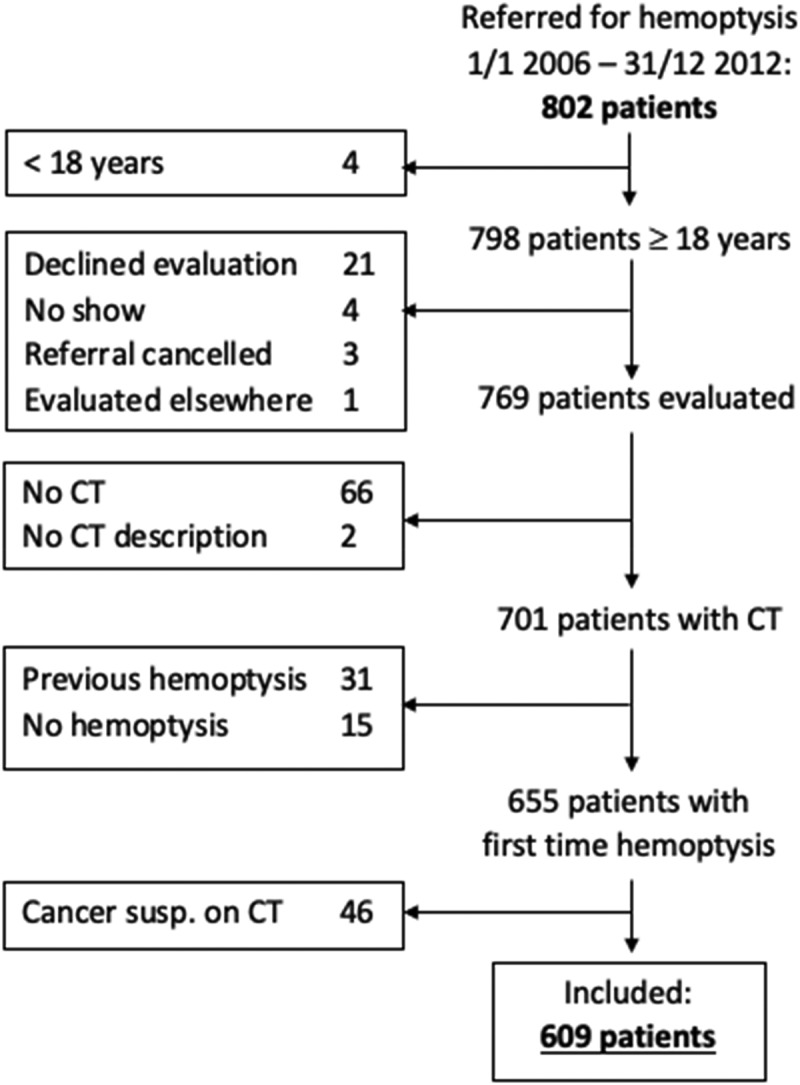
Inclusion process and excluded patients by exclusion criterium.
The majority of patients were male, mean age was 56.7 years and 76.7% were current or previous smokers. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics in absolute numbers and percentages of patients with relevant data available, where not stated otherwise.
| Mean age (SD) (n = 609) | 56.7 (14.1) |
| Male (n = 609) | 369 (60.6) |
| Smoking (n = 600) | 460 (76.7) |
| Current (n = 600) | 258 (43.0) |
| Previous (n = 600) | 202 (33.7) |
| Pack years >30 (n = 577) | 155 (26.9) |
| Anticoagulant treatment (n = 592) | 162 (27.4) |
| Amount of hemoptysis (n = 394) | |
| Mild | 340 (86.3) |
| Moderate–severe | 49 (12.4) |
| Massive | 5 (1.3) |
| Episodes (n = 546) | |
| 1 | 108 (19.8) |
| 2–5 | 191 (35.0) |
| >5 | 247 (45.2) |
| Current/prev. lung disease (n = 601) | 153 (25.5) |
At initial referral, no present somatic disease was known in 42.9% of patients. COPD was known in 13.5% of patients, 8.3% had asthma, and 1.3% already had a diagnosis of bronchiectasis.
A previous history of lung cancer was known in 2.5% and of non-pulmonary cancer in 7.4%. Present and previous health data are presented in Figure 2.
Figure 2.
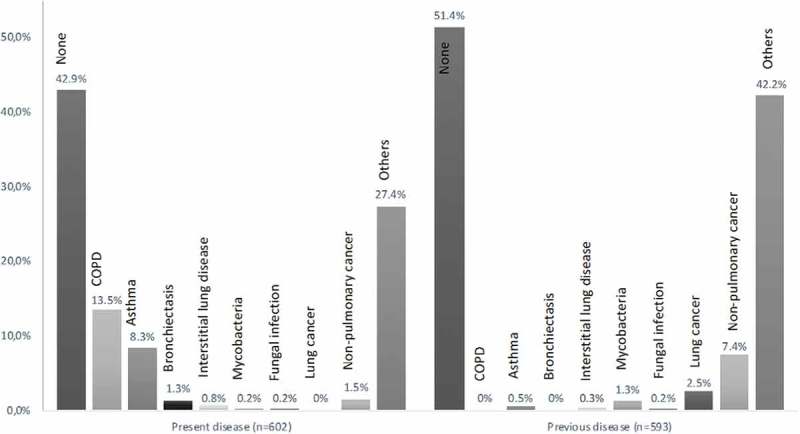
Patients with somatic disease diagnoses applicable at referral (present) and no longer applicable at referral (previous), as percentage of patients with relevant data available (n).
Results of initial diagnostic workup
All 609 patients had computed tomography performed, and 28.7% had a radiological finding of bronchiectasis. In total, 70.3% of CT scans were positive. Bronchoscopy was performed in 92.8% of patients and macroscopically positive in 38.7%. Microbiological sampling was performed in 64.5% of patients, and 24.9% of these were positive. Cytological evaluation was done in 58.4% of patients with 1.4% of these turning out positive, while histological evaluation of 7.4% of patients was positive in 7% of evaluated cases.
Cryptogenic hemoptysis was concluded in 80.5% patients. No patients were diagnosed with primary or metastatic cancer to the lungs. The most frequent etiology was respiratory tract infection (13.6%) followed by bronchiectasis (3.8%). Five patients had a double diagnosis of bronchiectasis and respiratory tract infection. All etiologies are shown in Figure 3.
Figure 3.
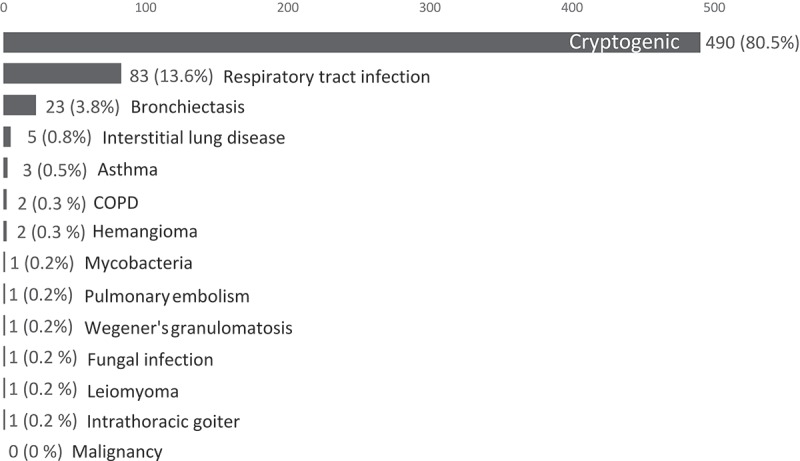
Patients according to etiology established at initial diagnostic workup.
Five-year follow-up
In the follow-up period, 53 (8.7%) patients had at least one recurrence of hemoptysis. Median time to first recurrence was 15 (IQR 9–32) months. Of these, 89% had only one recurrence. Most frequent etiologies at first recurrence were cryptogenic hemoptysis (71%) and infection (15%), while two patients (4%) were diagnosed with lung cancer. Radiological findings of bronchiectasis were not found to be an independent risk factor for recurrence, and neither was age, gender, current or previous lung disease or smoking history.
A total of nine (1.5%) patients, including the two mentioned above with recurrent hemoptysis, were diagnosed with lung cancer (three small-cell carcinomas, three adenocarcinomas, two non-specified non-small-cell carcinomas and one unknown subtype). Median time to diagnosis was 26 (IQR 18–33) months. Earliest diagnosis was at 5 months and latest diagnosis at 52 months after initial referral. One patient was 40 years old at initial referral and the remaining eight patients 66–78 years old. Six were female, and eight were current or previous smokers, while smoking history was unknown in one patient. Two patients were previously treated for lung cancer and one was previously treated for a non-pulmonary cancer. One had COPD. At initial diagnostic workup, three had a normal CT, and seven underwent bronchoscopy, four of which were macroscopically positive. Histological evaluation was performed in one patient and cytologic evaluation in four patients. All turned out negative. Eight had cryptogenic hemoptysis, while one had a respiratory tract infection. All nine patients died during follow-up.
In total, 5.3% of patients had a new diagnosis of chronic lung disease during follow-up. All new diagnoses are presented in Table 2.
Table 2.
Diagnoses during follow-up and median time to diagnosis.
| Diagnosis within 5 years | Patients diagnosed (%) | Median time to diagnosis, months (IQR) |
|---|---|---|
| Cancer | 9 (1.5) | 26 (18–33) |
| COPD | 6 (1) | 38 (11–47) |
| Bronchiectasis | 7 (1.1) | 9 (5–54) |
| Asthma | 5 (0.8) | 22 (11–23) |
| Interstitial lung disease | 5 (0.8) | 18 (10–21) |
The 5-year mortality rate was 12%. Median time from initial referral to death was 31 (IQR 13–43) months. From Cox proportional hazards regression, independent risk factors for death during follow-up were increasing age (HR 1.07, CI 1.05–1.10, p < 0.001), current or previous lung disease (HR 1.97, CI 1.18–3.30, p = 0.01), previous lung cancer (HR 3.44, CI 1.46–8.10, p = 0.005) and current or previous smoking history (HR 2.26, CI 1.08–4.76, p = 0.031). Gender was not found to be an independent risk factor (p = 0.296).
Discussion
In this study, we found a recurrence rate in hemoptysis of 8.7% in 5 years, which is comparable to the study by Delage et al., who found a recurrence rate of 8% at a mean follow-up of 5 years [7], while others found higher recurrence rates ranging from 11% to 20% at approximately 1 year’s follow-up [5,8,11]. We did not identify any independent risk factors for recurrence. Notably, radiological findings of bronchiectasis were not significantly associated with recurrence. This has not been investigated in previous reports. Most cases comprised benign causes, but 4% of first recurrences were caused by lung cancer, indicating that patients with recurrence should be investigated thoroughly as the risk of cancer is higher than for incidence cases of hemoptysis.
The rate of lung cancer diagnosed in the 5 years following initial referral was 1.5%. This is comparable to results from previous studies, although these all have shorter follow-up periods than our study. Thirumaran et al. reported 0.4% of hemoptysis patients with normal chest x-ray to develop lung cancer during a mean follow-up of three years [6], while Tsoumakidou et al. found no new lung cancers in 189 hemoptysis patients during an average follow-up of 2.7 years [9]. Bønløkke et al. found no lung cancers within two years of initial referral for hemoptysis in a group of 78 patients with no pathology on CT [2]. A larger French study evaluated the three-year outcomes of around 30,000 hemoptysis patients and estimated 4% of patients with cryptogenic hemoptysis to be diagnosed with lung cancer during follow-up [5].
All but one patient diagnosed with cancer in our follow-up were verified smokers, while one did not have data available on smoking. Eight out of nine were substantially older than the average patient in our population. Correspondingly, Herth et al., who reported a lung cancer rate of 6% at a mean follow-up of 6.6 years, found all cancers during follow-up to occur in smokers over 40 years [10]. Also comparable to our findings, these cases were diagnosed before 3 years. With the earliest cancer diagnosis in our study established at 5 months and median time to diagnosis 26 months, the question could be raised if these represent missed cases of cancer at initial diagnostic workup. However, the histologic and cytologic evaluations performed in these patients were all negative.
In our study, one third of patients with a new cancer diagnosis were previously treated for cancer, two of which were lung cancers, indicating that these patients may comprise a group of particular interest with regard to follow-up after incident cases of hemoptysis. Statistical assessment in the form of Cox proportional hazards regression to identify individual risk factors would be beneficial, but due to the low number of cancer cases, this was not deemed meaningful [16].
The low rate of new diagnoses during follow-up (5%) indicates that the initial workup is effective in diagnosing chronic lung conditions. This has not previously been investigated systematically and may reflect the fact that a large proportion of patients already have chronic lung diseases before the incident case of hemoptysis.
Seven patients had a new diagnosis of bronchiectasis within 5 years, although bronchiectasis was identified radiologically in 123 patients at initial diagnostic workup but only recorded as the etiology of hemoptysis in 23 patients. Thus, the apparent underreporting of bronchiectasis as the etiology may either question the clinical significance of these radiological findings or indicate a lack of awareness of the diagnosis.
The mortality rate of 12% at 5 years is low compared to similar studies. Reports from Greece and Turkey found markedly higher mortality rates although their follow-up period was shorter [9,12]. This may in part be explained by the high frequencies of lung cancer at initial workup compared to our study. Interestingly, the French study reported three-year mortality rates of around 20% when excluding deaths from lung cancer [5], representing a rather large, unexplained difference to our study. More similar to our findings, Herth et al. reported 13% of patients with cryptogenic hemoptysis to be dead at 6.6 years [10].
The independent risk factors for death identified in this study (increasing age, current or previous lung disease, current or previous smoking history and previous lung cancer) have not been systematically assessed in previous studies, which may explain the differences in results.
This study is among the largest studies regarding long-term outcome of hemoptysis. This allowed statistical analysis of infrequent variables and outcomes, although some outcomes were too rare to yield meaningful statistical assessment. The large number of patients with cryptogenic hemoptysis, in whom one might suspect overlooked etiologies, enabled us to show that even in these patients, severe disease is rarely seen in the years following hemoptysis.
The retrospective design has several built-in limitations, not least missing data and the need to interpret clinical records. This also limited the assessment of some variables, e.g. the quantification of pack-years as ≤/>30 which is an arbitrary limit. With regard to selection bias, some patients were excluded because they declined evaluation, which may be due to very poor health and thereby comprises a possible bias of our results. However, the proportion of patients excluded was small and we do not believe this to influence our results significantly.
Conclusion
Recurrence of hemoptysis was seen in 8.7% within 5 years and 4% of these were caused by lung cancer. Only 1.5% of hemoptysis patients with no malignancy suspected on CT developed lung cancer within 5 years of initial referral. Although the number of cancer cases in our study was limited, our data indicate smokers and patients previously treated for cancer to be of particular interest with regard to establishing follow-up programs for hemoptysis patients.
Biographies
Christian Lund Petersen, MS, is a medical student at Aalborg University, Aalborg, Denmark. With a special interest in pulmonology, he has worked as a locum doctor at the Department of Respiratory Diseases, Aalborg University Hospital, Aalborg, Denmark, alongside his studies and finished his master’s thesis in the field of pulmonology.
Ulla Møller Weinreich, MD, PhD, is a consultant at the Department of Respiratory Diseases, Aalborg University Hospital, Aalborg, Denmark and Assistant Professor at Aalborg University. She is a specialist in Pulmonology and her main research focus is in COPD and bronchiectasis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Lenner R, Schilero GJ, Lesser M.. Hemoptysis: diagnosis and management. Compr Ther. 2002;28(1):7–6. [DOI] [PubMed] [Google Scholar]
- [2].Bønløkke S, Guldbrandt LM, Rasmussen TR. Bronchoscopy in patients with haemoptysis and normal computed tomography of the chest is unlikely to result in significant findings. Dan Med J. 2015;62(8):A5123. [PubMed] [Google Scholar]
- [3].Nielsen K, Gottlieb M, Colella S, et al. Bronchoscopy as a supplement to computed tomography in patients with haemoptysis may be unnecessary. Eur Clin Respir J. 2016;3:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arooj P, Bredin E, Henry MT, et al. Bronchoscopy in the investigation of outpatients with hemoptysis at a lung cancer clinic. Respir Med. 2018;139:1–5. [DOI] [PubMed] [Google Scholar]
- [5].Abdulmalak C, Cottenet J, Beltramo G, et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J. 2015August;46(2):503–511. [DOI] [PubMed] [Google Scholar]
- [6].Thirumaran M, Sundar R, Sutcliffe IM, et al. Is investigation of patients with haemoptysis and normal chest radiograph justified? Thorax. 2009;64(10):854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Delage A, Tillie-Leblond I, Cavestri B, et al. Cryptogenic hemoptysis in chronic obstructive pulmonary disease: characteristics and outcome. Respiration. 2010;80(5):387–392. [DOI] [PubMed] [Google Scholar]
- [8].Lee YJ, Lee S-M, Park JS, et al. The clinical implications of bronchoscopy in hemoptysis patients with no explainable lesions in computed tomography. Respir Med. 2012March;106(3):413–419. [DOI] [PubMed] [Google Scholar]
- [9].Tsoumakidou M, Chrysofakis G, Tsiligianni I, et al. A prospective analysis of 184 hemoptysis cases: diagnostic impact of chest X-ray, computed tomography, bronchoscopy. Respiration. 2006;73(6):808–814. [DOI] [PubMed] [Google Scholar]
- [10].Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest. 2001;120(5):1592–1594. [DOI] [PubMed] [Google Scholar]
- [11].Abal AT, Nair PC, Cherian J. Haemoptysis: aetiology, evaluation and outcome–a prospective study in a third-world country. Respir Med. 2001July;95(7):548–552. [DOI] [PubMed] [Google Scholar]
- [12].Uzun O, Atasoy Y, Findik S, et al. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Respir J. 2010;4(3):131–138. [DOI] [PubMed] [Google Scholar]
- [13].Petersen CL, Weinreich UM. Hemoptysis with no malignancy suspected on chest computed tomography rarely requires bronchoscopy (Manuscript submitted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Prignot J. Quantification and chemical markers of tobacco-exposure. Eur J Respir Dis. 1987January;70(1):1–7. [PubMed] [Google Scholar]
- [15].Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710–718. [DOI] [PubMed] [Google Scholar]


