Abstract
Introduction
Stem cell therapies represent a promising new frontier for the treatment of refractory diabetic erectile dysfunction (DED). The use of stem cell-derived extracellular vesicles (EVs) is a novel strategy for cell-free stem cell therapy. We have reported that urine-derived stem cells (USCs) can improve DED; however, the therapeutic effects of EVs secreted by USCs (USC-EVs) remain unknown.
Aim
To determine the therapeutic effects of USC-EVs on DED in a rat model.
Methods
USC-EVs were isolated from conditioned medium by ultracentrifugation. DED was induced in male Sprague–Dawley rats via an intraperitoneal injection of streptozotocin. Sixteen DED rats were divided into phosphate-buffered saline (PBS) and USC-EV groups. Eight normal rats served as the normal control group. PBS or USC-EVs were transplanted into the corpora cavernosa in the corresponding groups.
Main Outcome Measure
Intracavernosal pressure (ICP), mean arterial pressure (MAP), expression of endothelial markers (CD31), endothelial nitric oxide synthase (eNOS), phospho-eNOS, and neural nitric oxide synthase (nNOS) were assessed in each group. Masson’s trichrome staining was used to determine the collagen deposition and ratio of smooth muscle cells to collagen. The microRNA (miRNA) cargo of USC-EVs was characterized by high-throughput RNA sequencing.
Results
Recovery of erectile function was observed in the USC-EV group, as represented by improved ICP and ICP/MAP ratio. CD31, eNOS, phospho-eNOS, and nNOS expression in the penis was significantly improved in the USC-EV group. In addition, the ratio of smooth muscle to collagen was significantly increased in the USC-EV group. RNA sequencing revealed that USC-EVs were enriched for distinct classes of miRNA (miR-21-5p, let-7 family, miR-10 family, miR-30 family, and miR-148a-3p) that promote angiogenesis.
Conclusion
USC-EV transplantation can ameliorate DED in rats. Its mechanism may involve the delivery of proangiogenic miRNA.
Ouyang B, Xie Y, Zhang C, et al. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex Med 2019;7:241–250.
Key Words: Erectile Dysfunction, Sexual Dysfunction, Stem Cells, Extracellular Vesicles, Diabetes, Endothelial Dysfunction
Introduction
Erectile dysfunction (ED) is one of the main complications of diabetes mellitus, affecting an estimated 35%–90% of diabetic men.1 ED is more severe in diabetic men than in nondiabetic men. The management of diabetic ED (DED) is complex and challenging. Many men with DED are refractory to phosphodiesterase type 5 inhibitor, a generally successful first-line therapy. The primary reason for this refractory response may be the complex pathophysiology of DED, which includes endothelial dysfunction, decreased smooth muscle volume, neural degeneration and fibrosis.2 Among these factors, endothelial dysfunction is recognized as a mainstay in the pathophysiology of the disease.3 Large clinical trials in men with diabetes have found that early hyperglycemia can promote disease progression and late complications, perpetuating ED despite the normalization of glucose levels.4 This phenomenon, known as “metabolic memory,” has also been reported in endothelial cells in diabetic rats and contributes to the refractory nature of DED.5
Stem cell therapies represent a promising new frontier for the treatment of refractory DED. A growing body of research is demonstrating the therapeutic effects of numerous types of stem cells.6, 7, 8, 9, 10 We previously reported that adipose-derived stem cells may correct DED11 and cavernous nerve injury–induced erectile dysfunction (CNIED).12 Urine-derived stem cells (USCs) isolated from human voided urine have many characteristics of mesenchymal stem cells (MSCs) and are capable of differentiating into multiple cell lines, including endothelial and smooth muscle cells.13 We also have demonstrated that USCs can ameliorate DED14 and CNIED15 in a rat model. Interestingly, USCs can be obtained abundantly and noninvasively.
However, despite attracting considerable attention, stem cell therapy also faces many realistic obstacles. The therapeutic effect of stem cells might not correlate with engraftment, differentiation, and cell fusion.16 Accordingly, many scientists place greater emphasis on paracrine-secreted factors of stem cells. Extracellular vesicles (EVs), including microvesicles and exosomes, are key components in the paracrine secretion of stem cells.17
Numerous studies have reported that EVs from stem cells yield similar therapeutic benefits as stem cells in various disease models.17 Exosomes derived from adipose-derived stem cells and bone marrow–derived MSCs have been reported to alleviate CNIED.18, 19 It also has been reported that EVs from stem cells can ameliorate DED via antifibrotic and antiapoptotic mechanisms.20, 21 Exosomes secreted by human USCs can prevent diabetic kidney complications in rats.22 Compared with stem cells, EVs are easier to store and manage, precluding the risk of tumor formation. Furthermore, it has been suggested that EVs can be harnessed to treat endothelial metabolic memory by delivering microRNA (miRNA).23 In summary, EV fusion may be more feasible and advantageous than stem cells and represent a safer therapeutic approach. There is no evidence of the efficacy of EVs from USCs (USC-EVs) for treating DED in a rat model based on published reports.
In the present study, we characterized USC-EVs and investigated the therapeutic effects of USC-EVs in a streptozotocin (STZ)-induced DED rat model.
Methods
Our animal experiment protocol was approved by the Committee for Animal Care and Use of Guangzhou First People's Hospital. Human urine samples were collected from 3 healthy adult volunteers. The protocol for use of human urine and informed consent was approved by Guangzhou First People's Hospital Health Sciences Institutional Review Board. Written informed consent was obtained from each urine donor.
Establishment of STZ-Induced DED Rat Model and Study Design
Thirty-one Sprague–Dawley male rats (approximately 200–260 g) were obtained from the Animal Center of Sun Yat-sen University (Guangzhou, China) and maintained under standard laboratory conditions. The rats were given food and UV-sterilized tap water ad libitum. After a 1-week acclimatization period, 23 rats were injected intraperitoneally (IP) with 1 dose of STZ (50 mg/kg). Random blood glucose levels were measured at 3 days after STZ injection by tail tip snipping with a glucose meter (One Touch; Johnson & Johnson, New Brunswick, NJ, USA). A blood glucose level >300 mg/dL was considered to indicate diabetes. Apomorphine (100 mg/kg; Sigma-Aldrich, St Louis, MO, USA) was used to identify the DED rats at 7 weeks, as reported previously.14 Sixteen out of 23 rats successfully developed DED, and the remaining 7 rats that failed to develop DED were not used in this study.
Eight normal rats served as a standard control. The 16 DED rats were divided at random into phosphate-buffered saline (PBS; n = 8) and USC-EV (n = 8) groups, which received intracavernous (IC) injections with PBS and USC-EVs, respectively. Erectile function was evaluated in all animals from both groups at 28 days postinjection, and penises were harvested for histological analysis and measurement of expression of endothelial markers (CD31), endothelial function protein (eNOS and phospho-eNOS), and neural protein (nNOS) in the corpora cavernosa.
USC Culture in Vitro
A total of 11 sterile voided urine samples (approximately 4.5–5 L) were collected from 3 healthy volunteers age 22–30 years. Midstream and last stream urine samples were collected and centrifuged. Cell pellets were washed with PBS and then plated in 24-well tissue culture plates at approximately 500 cells per well with a mixed medium composed of keratinocyte serum-free medium and progenitor cell medium in a 1:1 ratio as reported previously.14
High-Density Cell Culture in a Hollow Fiber Bioreactor for EV Collection
A hollow fiber bioreactor (FiberCell Systems, New Market, MD, USA) equipped with a cartridge with a molecular weight cutoff of 20 kD (#C2011), was used to culture cells at high density for EV collection as described previously.24 The cartridge was primed sequentially with PBS, serum-free Dulbecco's modified Eagle medium (DMEM), and high-glucose DMEM with 10% exosome-free fetal bovine serum and 1% penicillin/streptomycin for 24 hours for each agent. Then 1.8 × 108 USCs were seeded into the cartridge. The glucose level of the medium was monitored daily to evaluate cell growth rate, and the medium was changed once the glucose level decreased to half of the original level. When the glucose level dropped to 50% within 1 day, the medium was changed to mixed medium composed of keratinocyte serum-free medium and progenitor cell medium in a 1:1 ratio. Afterward, EV-containing medium was collected from the cartridge daily for approximately 30 days.
Isolation and Purification of USC-EVs
The conditioned medium was collected from the hollow fiber bioreactor and centrifuged at 300 × g for 10 minutes at 4 °C. After centrifugation, the supernatant was filtered with a 0.22-μm Steritop sterilized filter unit (MilliporeSigma, Burlington, MA, USA) to remove the remaining cells and cellular debris. Then the supernatant was ultracentrifuged (Optima XE-100 ultracentrifuge; Beckman Coulter Life Sciences, Indianapolis, IN, USA) as indicated in the flow diagram in Figure 1B.25 Finally, the USC-EV pellet was resuspended in 200 μL of PBS. The USC-EVs were stored at −80 °C or used for other downstream experiments.
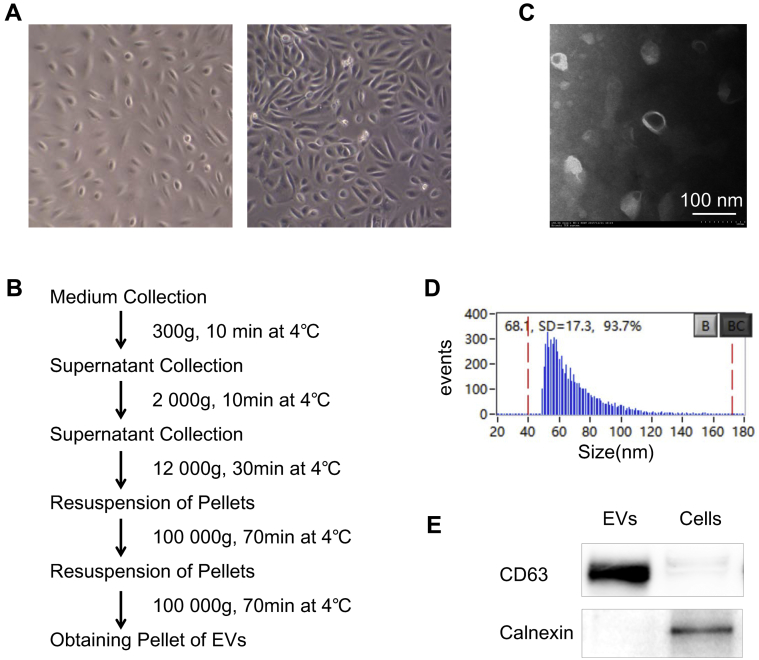
Figure 1.
Characterization of isolated USC-EVs. A, Characteristic of human urine–derived stem cells in vitro (left, P1; right, P3). B, Flow diagram of EV collection from conditioned medium using ultracentrifugation. C, Size and the spheroid morphology of EVs on TEM. (Scale bar: 100 nm.) D, Diameter quantitation of EVs performed with high- sensitivity flow cytometry. Mean size of USCEVs, 68.1 ± 17.3 nm (B, blank, BC, blank clean). E, Western blot analysis showing that the collected USC-EVs express EVs-specific surface markers CD63 and do not express the endoplasmic reticulum-specific marker calnexin.
Characteristics of USC-EVs
The number and size of EVs were quantified by high-sensitivity flow cytometry as reported previously.26 Transmission electron microscopy (TEM) was performed using a Hitachi transmission electron microscope system (Hitachi, Tokyo, Japan). Western blot analysis was used to detect the positive EV marker CD63 and negative EV marker calnexin in isolated EVs.
In Vivo Implantation
At 8 weeks after STZ injection, each rat in the PBS and USC-EVs groups received an injection of 0.1 mL of PBS and USC-EVs (100 μg in 0.1 mL of PBS), respectively. Rats were anesthetized with sodium pentobarbital (40 mg/kg IP). The penis was exposed, and a rubber band was placed around the base to facilitate delivery of the implanted cells. The middle of the penis was punctured with a needle. The needle was left in place for 1 minute to allow diffusion of the injected PBS or EVs into the cavernosal tissue. The elastic band was removed at 1 minute after the injection.
Determination of Erectile Function
The intracavernous pressure (ICP)/main arterial pressure (MAP) ratio was used to evaluate erectile function at 28 days after IC injection. All animals were anesthetized with sodium pentobarbital (40 mg/kg, IP). A PE-50 catheter filled with 250 units/mL of heparinized saline was cannulated to the left carotid artery and connected to a pressure transducer (Taimeng, Chengdu, China) to monitor arterial blood pressure (ABP). A low abdominal incision was used to expose the right major pelvic ganglion and identify the ipsilateral cavernous nerve. The right corporal body was cannulated with a 23 G butterfly. Continual monophasic rectangular pulses generated by a signal generator (Taimeng, Chengdu, China) were used to stimulate the right cavernous nerve via a bipolar stainless steel hook electrode. Stimulus parameters were 5 V, 20 Hz, 0.2-ms pulse width, and 60-second duration.
Protein Contents of USC-EVs and Penile Tissue
USC-EV protein content was determined with a bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions. The absorbance was read at 562 nm using a microplate reader (Thermo Fisher Scientific).
Western Blot Analysis of USC-EVs and Penile Tissue Protein
The identity of USC-EVs was confirmed by the presence of the specific surface proteins CD63 and the negative expression of the cell protein calnexin. Protein was prepared and run as reported previously. The presence of protein was tested by exposing the membranes to primary rabbit polyclonal anti-CD63 (Abcam, Cambridge, UK; ab59479, 1:1,000), anti-calnexin (Abcam; ab133615, 1:1,000), rabbit monoclonal anti-CD31 (Abcam; ab222783, 1:1,000), mouse monoclonal anti-eNOS (Abcam; ab76198, 1:1,000), rabbit polyclonal anti phospho-eNOS (p-eNOS, Ser1177; Cell Signaling Technology, Danvers, MA, USA; 9571, 1:1,000), and rabbit monoclonal anti-nNOS (Cell Signaling; 4231, 1:1,000). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a loading control. Signals were obtained in the linear range of detection and quantified on a Quantity One bioimaging system (Bio-Rad, Hercules, CA, USA). Data are presented as the relative density of each protein compared with GAPDH.
Masson’s Trichrome Staining
Rat penile tissues were harvested at 28 days. Masson’s trichrome staining was used to determine collagen deposition and the ratio of cells to collagen within the cavernous tissues at 28 days.
RNA Sequencing for miRNA Cargo of USC-EVs
RNA was isolated from EV pellets using Trizol (TaKaRa, Tokyo, Japan). RNA sequencing was performed as described previously.24
Statistical Analysis
Data are reported as mean ± SD and were analyzed using SPSS version 20.0 (IBM, Armonk, NY, USA). The ICP, ICP/MAP ratio, and Western blot analysis results were compared among the 3 groups using one-way ANOVA followed by a Student–Newman–Keuls post hoc comparison. A P value <.05 was considered to indicate a statistically significant difference.
Results
Characterization of Isolated USC-EVs
To harvest EVs, human USCs were isolated from 4.5–5 L of fresh human urine, expanded, and passaged to the third generation in vitro for further study (Figure 1A). Approximately 25 stem cells were retrieved in 1 L of urine. Then EVs were isolated by serial centrifugation25 (Figure 1B) and measured by TEM and high-sensitivity flow cytometry, which showed that the collected spherical membrane-bound EVs were homogeneous with a mean diameter of 68.1 ± 17.3 nm (Figure 1C and D). In addition, Western blot analysis showed that the collected USC-EVs expressed the EV-specific surface marker CD63 and did not express the endoplasmic reticulum-specific marker calnexin (Figure 1E). These results demonstrate that the isolated vesicles have the characteristics of EVs, as reported in previous studies.25
Assessment of Erectile Function
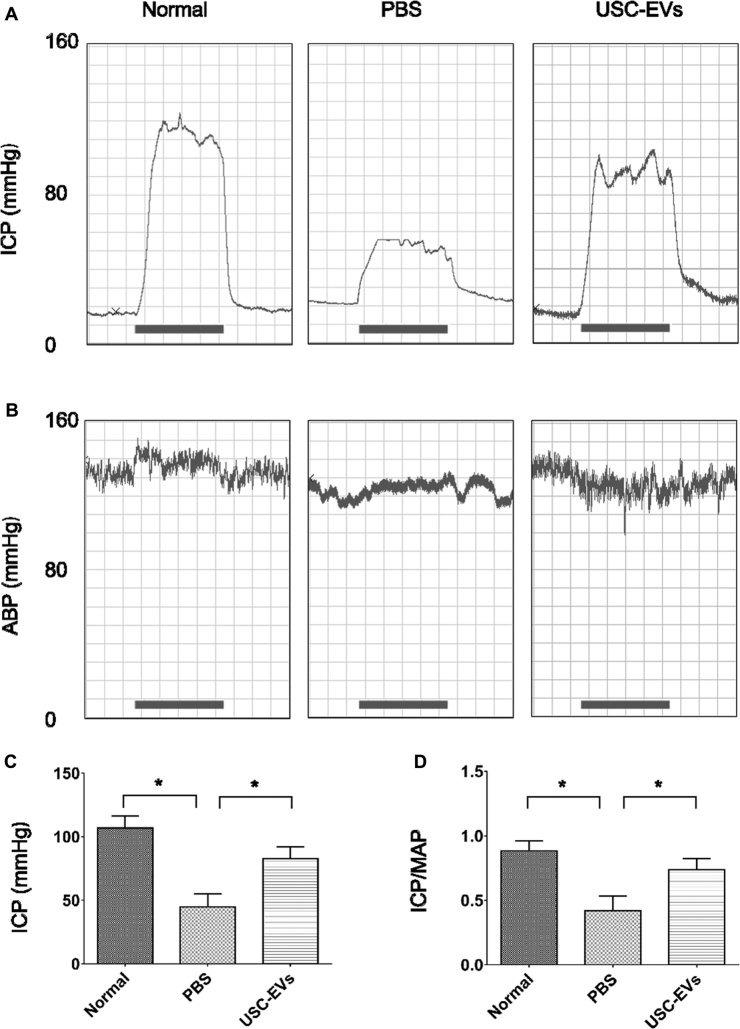
ICP and MAP values at week 4 were used to assess erectile function. Representative ICP and ABP tracing responses to the stimulation of the cavernous nerve were recorded in each group at 4 weeks after IC injection (Figure 2A and B). Control rats displayed normal ICP curves and significantly higher ICP (Figure 2C) and ICP/MAP ratios (Figure 2D) compared with those in PBS-treated diabetic rats (P < .05). After treatment with USC-EVs, ICP and the ICP/MAP ratio were significantly increased (P < .05) in diabetic rats; however, the values were still significantly lower compared with those in normal rats (P < .05).
Figure 2.
USC-EVs improved erectile function in diabetic rats. A and B, Representative ICP (A) and ABP (B) tracing responses to the stimulation of the cavernous nerve in age-matched normal rats or diabetic rats at 4 weeks treated with PBS or USC-EVs (n = 8 in each group). C, Effects of treatment with USC-EVs on the increase in ICP. D, Ratio of total ICP to MAP calculated for each group. *P < .05.
Expression of CD31, eNOS, p-eNOS, and nNOS in Corpora Cavernosa Tissue
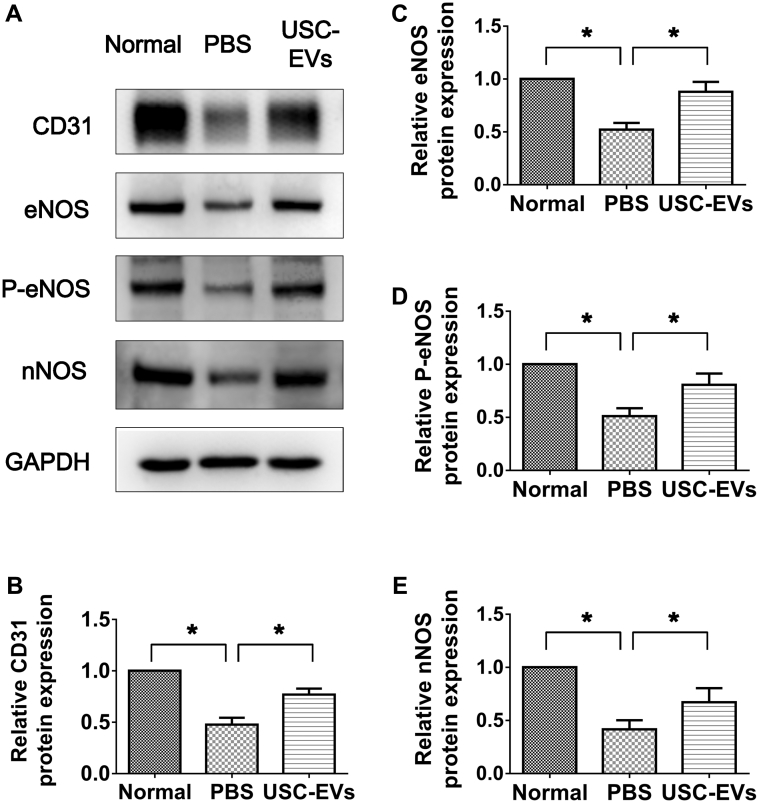
The levels of endothelial protein (CD31) (Figure 3A and B) were significantly lower within the corpora cavernosa in PBS-treated DED rats compared with normal rats (P < .05) based on Western blot analysis results. However, endothelial content within the cavernous tissue was significantly restored after USC-EV injection (P < .05).
Figure 3.
USC-EVs increased expression of CD31, eNOS, p-eNOS, and nNOS in corpora cavernosa. A, Western blot analysis. B, C, D, E, Quantitative analysis for Western blot analysis showing significantly increased expression of CD31, eNOS, P-eNOS, and nNOS in USC-EV–treated diabetic rats. *P < .05.
The expression of eNOS and p-eNOS within corpora tissue was significantly decreased in PBS-treated DED rats compared with age-matched normal controls, as confirmed by semiquantitative Western blot analysis (P < .05; Figure 3A, C, and D). USC-EVs showed significantly increased eNOS and p-eNOS expression (P < .05; Figure 3A, C, and D); however, the values were still significantly lower than those seen in normal rats (P < .05).
The expression of nNOS within the corpora cavernosa was significantly lower in PBS-treated DED rats compared with the age-matched normal rats (P < .05; Figure 3A and E). After IC injection with USC-EVs, expression of nNOS within corporal tissue was significantly increased but was still lower than that in the age-matched controls (P < .05; Figure 3A and E).
Collagen Deposition
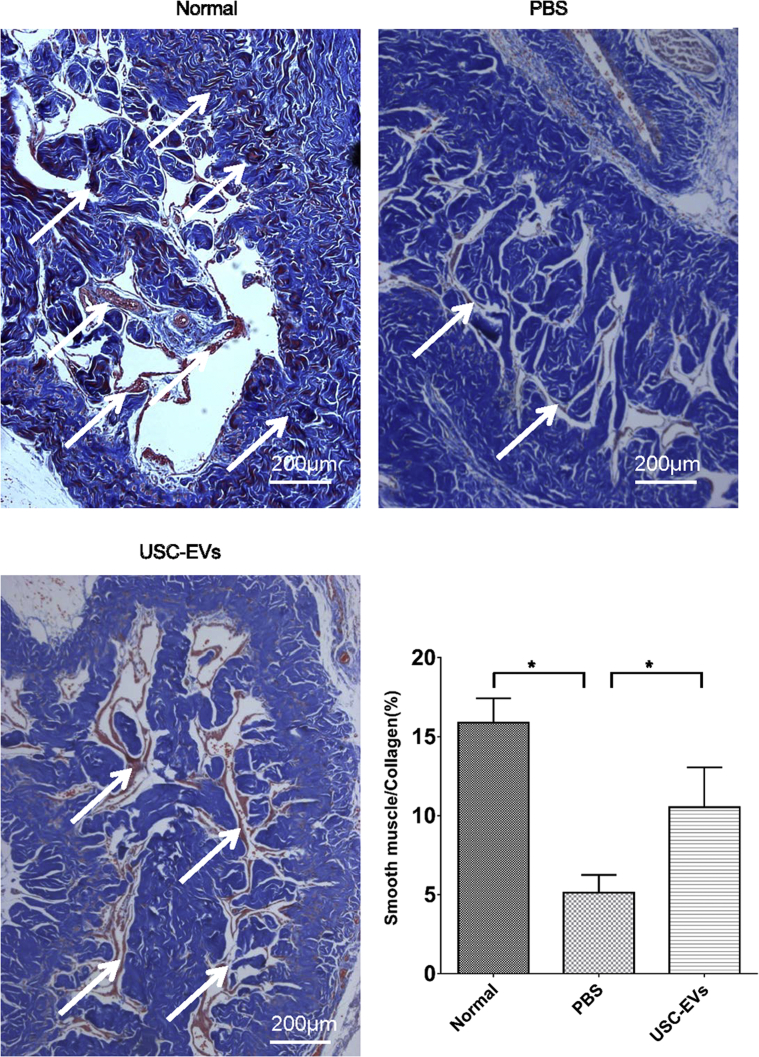
Masson's trichrome staining was used to assess the cell/collagen ratio. As shown in Figure 4, abundant collagen deposition was observed in the PBS group. However, in animals receiving USC-EV treatment, red-staining areas expanded and blue-staining areas shrank, demonstrating smooth muscle restoration (P < .05; Figure 4).
Figure 4.
Results of histological analysis of collagen deposition. A, Masson's trichrome staining to assess collagen deposition, which indicated tissue scarring. Smooth muscles are stained in red; while collagen fibers, in blue. (Scale bar: 200 μm.) B, Quantitative analysis of the ratio of smooth muscles to collagen fibers. n = 8. *P < .05.
miRNA Cargo of Isolated USC-EVs
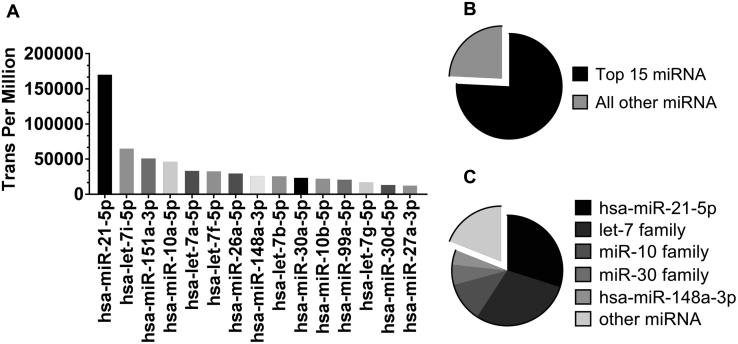
RNA sequencing detected 465 human miRNAs (hsa-miRNAs) in USC-EVs. Analysis of miRNA species revealed that 15 annotated miRNAs (75.8%) were enriched in the USC-EVs. 11 proangiogenesis miRNAs accounted for 81% of these 15 miRNAs (hsa-miR-21-5p, 29.9%; let-7 family, 29.3%; miR-10 family, 11.6%; miR-30 family, 5.9%; and hsa-miR-148a-3p, 4.3%) (Figure 5A and C).
Figure 5.
Enriched miRNA in the USC-EVs. A and B, The 15 most-enriched miRNAs, which account for 75.8% of all the identified miRNAs in the USC-EVs. C, 11 proangiogenesis miRNAs (hsa-miR-21-5p, let-7 family, miR-10 family, miR-30 family, and hsa-miR-148a-3p) account for 81% of the 15 miRNAs.
Discussion
Our results show that IC injection of USC-EVs significantly corrected erectile dysfunction by ameliorating endothelial functional, promoting penile neural regeneration, smooth muscle regeneration, and reduced collagen deposition. Endothelial dysfunction is one of the most essential pathophysiologic mechanisms in DED, which functionally refers to the inability of the endothelium to produce vasodilatory messengers, such as nitric oxide (NO), and to maintain vasodilatory and vascular homeostasis.27 eNOS is pivotal to the production of NO, and kinase-mediated protein phosphorylation plays a critical role in post-translational regulation mechanisms of eNOS.28 In this study, the expression levels of endothelial marker (CD31), eNOS, and phospho-eNOS were significantly restored by IC injection of USC-EVs. Thus, the enhancement of erectile function, which is indicated by increased ICP and ICP/MAP ratio, is due to the improved function of endothelium and smooth muscles. The therapeutic benefits of USC-EVs are similar to those of USCs.
The isolation of EVs from stem cells is another challenge. Although currently available commercial exosome extraction reagents yield high numbers of exosomes, the products still require purification because they contain non-EV contaminants, such as lipoproteins.29 Ultracentrifugation requires a large amount of cells to yield the conditioned medium.17 It has been reported that growing cell cultures within a hollow fiber bioreactor generates large quantities of miRNA-enriched EVs that can be concentrated by tangential flow filtration.30 In this study, the hollow fiber bioreactor was used to continuously collect the conditioned medium of USCs, and copious EVs were subsequently isolated from the medium for the downstream experiments.
As a key component of paracrine secretion from stem cells, EVs can be released by various types of stem cells and contain genetic information, including messenger RNAs, miRNAs, and other noncoding RNAs. EVs horizontally transfer this genetic information between stem cells and tissue-injured cells and then modify the function of the receptor cells and tissues to exert the beneficial action of stem cells. By binding to the 3'- untranslated regions of target messenger RNAs, miRNAs can modulate gene expression posttranscriptionally.31 In this study, 11 proangiogenesis hsa-miRNAs (miR-21-5p, let-7i-5p, let-7a-5p, let-7f-5p, let-7b-5p, let-7g-5p, miR-10a-5p, miR-10b-5p, miR-30a-5p, and miR-148a-3p) were enriched in the USC-EVs. MiR-21 is involved in angiogenesis.32 As one of the proangiogenic miRNAs, the let-7 family plays crucial roles in angiogenesis by targeting angiogenesis-related genes.33, 34 It also has been demonstrated that miR-10 regulates angiogenic behavior in a Notch-dependent manner.35 The miRNA-30 family can also modulate endothelial cell behavior during angiogenesis.36 By promoting the expression of thrombospondin-4, miRNA-148a-3p enhances angiogenesis.37 The proangiogenic potency of these miRNAs indicates the potential mechanism of the therapeutic effects of USC-EVs.
This study has several limitations. The use of only a single dose limited the assessment of the dose-dependent therapeutic effects of EVs. The mechanism responsible for the improvement of neural regeneration and cavernous fibrosis remains unclear. The miRNA cargo of the EVs involved in the recovery of endothelial function requires further verification. The expression of the target genes of miRNA in the rats is also unclear, and the synergism among multiple miRNAs and their regulatory effects remains largely unknown. More research is needed to clarify the exact mechanism of cell-free stem cell therapy with EVs.
In summary, our data indicate that USC-EVs enhance the expression of endothelial cell markers, reduce collagen deposition, and improve the neurogenic-mediated erectile response in DED rats. The improvement in DED in a rodent model after administration of USC-EVs is similar to that seen after cell therapy with USCs or other types of MSCs. The cargo of proangiogenic miRNA may play an important role in endothelial repair.
Statement of authorship
Category 1
-
(a)Conception and Design
- Bin Ouyang; Yun Xie; Yuanyuan Zhang; Guihua Liu; Junhong Deng; Chunhua Deng
-
(b)Acquisition of Data
- Bin Ouyang; Yun Xie; Chi Zhang; Cuncan Deng; Linyan Lv; Jiahui Yao; Yuanyuan Zhang; Guihua Liu
-
(c)Analysis and Interpretation of Data
- Bin Ouyang; Yun Xie; Chi Zhang; Cuncan Deng; Linyan Lv; Jiahui Yao; Yuanyuan Zhang; Guihua Liu; Junhong Deng; Chunhua Deng
Category 2
-
(a)Drafting the Article
- Bin Ouyang; Yun Xie; Chi Zhang; Cuncan Deng; Linyan Lv; Jiahui Yao; Yuanyuan Zhang; Guihua Liu; Junhong Deng; Chunhua Deng
-
(b)Revising It for Intellectual Content
- Bin Ouyang; Yun Xie; Chi Zhang; Cuncan Deng; Linyan Lv; Jiahui Yao; Yuanyuan Zhang; Guihua Liu; Junhong Deng; Chunhua Deng
Category 3
-
(a)Final Approval of the Completed Article
- Bin Ouyang; Yun Xie; Chi Zhang; Cuncan Deng; Linyan Lv; Jiahui Yao; Yuanyuan Zhang; Guihua Liu; Junhong Deng; Chunhua Deng
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
Funding: Supported by grants from the Natural Science Foundation of Guangdong Province (2014A030310359, 2015A030313730, and 2016A030313460) and the National Natural Science Foundation of China (81671834 and 81671449).
References
- 1.Giugliano F., Maiorino M., Bellastella G. Determinants of erectile dysfunction in type 2 diabetes. Int J Impot Res. 2010;22:204–209. doi: 10.1038/ijir.2010.1. [DOI] [PubMed] [Google Scholar]
- 2.Thorve V.S., Kshirsagar A.D., Vyawahare N.S. Diabetes-induced erectile dysfunction: Epidemiology, pathophysiology and management. J Diabetes Complicat. 2011;25:129–136. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Castela A., Costa C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat Rev Urol. 2016;13:266–274. doi: 10.1038/nrurol.2016.23. [DOI] [PubMed] [Google Scholar]
- 4.Holman R.R., Paul S.K., Bethel M.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A. The emerging challenge in diabetes: The “metabolic memory”. Vasc Pharmacol. 2012;57:133–138. doi: 10.1016/j.vph.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Vakalopoulos I., Memmos D., Mykoniatis I. Stem cell therapy in erectile dysfunction: Science fiction or realistic treatment option? Hormones. 2018;17:315–320. doi: 10.1007/s42000-018-0050-4. [DOI] [PubMed] [Google Scholar]
- 7.Alwaal A., Zaid U.B., Lin C. Stem cell treatment of erectile dysfunction. Adv Drug Deliver Rev. 2015;82-83:137–144. doi: 10.1016/j.addr.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Al Demour S., Jafar H., Adwan S. Safety and potential therapeutic effect of two intracavernous autologous bone marrow–derived mesenchymal stem cell injections in diabetic patients with erectile dysfunction: An open-label phase I clinical trial. Urol Int. 2018;101:358–365. doi: 10.1159/000492120. [DOI] [PubMed] [Google Scholar]
- 9.Sun X., Luo L., Feng L. B cell lymphoma-2–modified bone marrow–derived mesenchymal stem cell transplantation for the treatment of diabetes mellitus–induced erectile dysfunction in a rat model. Urol Int. 2017;98:358–366. doi: 10.1159/000452253. [DOI] [PubMed] [Google Scholar]
- 10.Li M., Li H., Ruan Y. Stem cell therapy for diabetic erectile dysfunction in rats: A meta-analysis. PloS One. 2016;11:e154341. doi: 10.1371/journal.pone.0154341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G., Sun X., Bian J. Correction of diabetic erectile dysfunction with adipose-derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PloS One. 2013;8:e72790. doi: 10.1371/journal.pone.0072790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X., Yang Q., Zheng T. Neurotrophic effect of adipose tissue-derived stem cells on erectile function recovery by pigment epithelium–derived factor secretion in a rat model of cavernous nerve injury. Stem Cells Int. 2016;2016:1–12. doi: 10.1155/2016/5161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., McNeill E., Tian H. Urine-derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–2233. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang B., Sun X., Han D. Human urine-derived stem cells alone or genetically modified with FGF2 improve type 2 diabetic erectile dysfunction in a rat model. PloS One. 2014;9:e92825. doi: 10.1371/journal.pone.0092825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Q., Chen X., Zheng T. Transplantation of human urine-derived stem cells transfected with pigment epithelium–derived factor to protect erectile function in a rat model of cavernous nerve injury. Cell Transplant. 2016;25:1987–2001. doi: 10.3727/096368916X691448. [DOI] [PubMed] [Google Scholar]
- 16.Da Silva Meirelles L., Caplan A.I., Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 17.Han C., Sun X., Liu L. Exosomes and their therapeutic potentials of stem cells. Stem Cells Int. 2016;2016:1–11. doi: 10.1155/2016/7653489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang X., Han X., Chen Z. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res Ther. 2018;9:246. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M., Lei H., Xu Y. Exosomes derived from mesenchymal stem cells exert therapeutic effect in a rat model of cavernous nerves injury. Andrology. 2018;6:927–935. doi: 10.1111/andr.12519. [DOI] [PubMed] [Google Scholar]
- 20.Chen F., Zhang H., Wang Z. Adipose-derived stem cell–derived exosomes ameliorate erectile dysfunction in a rat model of type 2 diabetes. J Sex Med. 2017;14:1084–1094. doi: 10.1016/j.jsxm.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L.L., Huang X., Yu W. Transplantation of adipose tissue–derived stem cell–derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50:e12871. doi: 10.1111/and.12871. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Z., Liu Y., Niu X. Exosomes secreted by human urine–derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24. doi: 10.1186/s13287-016-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prattichizzo F., Giuliani A., De Nigris V. Extracellular microRNAs and endothelial hyperglycaemic memory: A therapeutic opportunity? Diabetes Obes Metab. 2016;18:855–867. doi: 10.1111/dom.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson D.C., Bayik D., Srivatsan A. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. doi: 10.1016/j.biomaterials.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao J., Zheng J., Cai J. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2018:201800131R. doi: 10.1096/fj.201800131RR. [DOI] [PubMed] [Google Scholar]
- 26.Tian Y., Ma L., Gong M. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12:671–680. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 27.Musicki B., Burnett A.L. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 28.Kukreja R.C., Xi L. eNOS phosphorylation: A pivotal molecular switch in vasodilation and cardioprotection? J Mol Cell Cardiol. 2007;42:280–282. doi: 10.1016/j.yjmcc.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witwer K.W., Buzás E.I., Bemis L.T. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo K.W., Li N., Makani V. Large-scale preparation of extracellular vesicles enriched with specific microRNA. Tissue Eng Part C Methods. 2018;24:637–644. doi: 10.1089/ten.tec.2018.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eulalio A., Huntzinger E., Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Chang C., Yen M., Liao S. Dual role of MiR-21-mediated signaling in HUVECs and rat surgical flap under normoxia and hypoxia condition. Int J Mol Sci. 2017;18:1917. doi: 10.3390/ijms18091917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S., Yu W., Qu X. Argonaute 2 promotes myeloma angiogenesis via microRNA dysregulation. J Hematol Oncol. 2014;7:40. doi: 10.1186/1756-8722-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otsuka M., Zheng M., Hayashi M. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Ling C.C., Li L. MicroRNA-10a/10b represses a novel target gene mib1 to regulate angiogenesis. Cardiovasc Res. 2016;110:140–150. doi: 10.1093/cvr/cvw023. [DOI] [PubMed] [Google Scholar]
- 36.Bridge G., Monteiro R., Henderson S. The microRNA-30 family targets DLL4 to modulate endothelial cell behavior during angiogenesis. Blood. 2012;120:5063–5072. doi: 10.1182/blood-2012-04-423004. [DOI] [PubMed] [Google Scholar]
- 37.Ge H., Shrestha A., Liu C. MicroRNA 148a-3p promotes Thrombospondin-4 expression and enhances angiogenesis during tendinopathy development by inhibiting Kruppel-like factor 6. Biochem Biophys Res Commun. 2018;502:276–282. doi: 10.1016/j.bbrc.2018.05.167. [DOI] [PubMed] [Google Scholar]