Summary
Human retinal organoids from induced pluripotent stem cells (hiPSCs) can be used to confirm the localization of proteins in retinal cell types and to test transduction and expression patterns of gene therapy vectors. Here, we compared the onset of CRB protein expression in human fetal retina with human iPSC-derived retinal organoids. We show that CRB2 protein precedes the expression of CRB1 in the developing human retina. Our data suggest the presence of CRB1 and CRB2 in human photoreceptors and Müller glial cells. Thus the fetal CRB complex formation is replicated in hiPSC-derived retina. CRB1 patient iPSC retinal organoids showed disruptions at the outer limiting membrane as found in Crb1 mutant mice. Furthermore, AAV serotype 5 (AAV5) is potent in infecting human Müller glial cells and photoreceptors in hiPSC-derived retinas and retinal explants. Our data suggest that human photoreceptors can be efficiently transduced by AAVs in the presence of photoreceptor segments.
Keywords: CRB1, CRB2, organoids, cell polarity, Müller glial cells, photoreceptors, adeno-associated virus, human induced pluripotent stem cells, retina, CRB1 patients
Graphical Abstract
Highlights
-
•
iPSC-derived retinas recapitulate the fetal human Crumbs complex
-
•
CRB2 is expressed earlier than CRB1 in fetal or iPSC-derived retina at the SAR
-
•
CRB1-RP patient iPSC-derived retinas show disruptions at the OLM
-
•
AAV5 infects photoreceptors and Müller glial cells in adult and iPSC-derived retina
Wijnholds and colleagues show that the key Crumbs complex members, CRB1 and CRB2, are recapitulated between human fetal retina and iPSC-derived retinal organoids. CRB2 is expressed earlier than CRB1 in fetal and iPSC-derived human retina. CRB1-RP patient iPSC-derived retinas show a morphological phenotype. In addition, they show that AAV5 infects photoreceptors and Müller glial cells in adult and iPSC-derived human retina.
Introduction
Mutations in the Crumbs homolog-1 (CRB1) gene are linked to an array of retinal dystrophies that exhibit high phenotypic variability and affect approximately 80,000 patients worldwide with an estimated prevalence in the US of 1/86,500 (Alves et al., 2014a, Stone et al., 2017, Talib et al., 2017). A meta-analysis has found that mutations in the CRB1 gene account for 2.7% and 10.1% of autosomal recessive retinitis pigmentosa (RP) and Leber congenital amaurosis (LCA) cases, respectively (Bujakowska et al., 2012). LCA is an early-onset disease, with newborns being blind around birth. However, we are yet to understand the localization of the CRB complex in early human fetal retinal development. No treatment is currently available for CRB1-associated retinal dystrophy in patients, but proof-of-concept gene supplementation studies have shown both morphological and functional rescue in CRB1 RP mouse models (Pellissier et al., 2015). A retrospective cohort study of patients with CRB1-associated RP has shown that gene therapeutic intervention is most likely required within the first three decades of life, but clinical endpoint criteria for a clinical trial need to be established from natural history studies (Talib et al., 2017). Preclinical considerations to be evaluated include the choice of adeno-associated virus (AAV) serotype for delivery of the clinical vector, in terms of potency, tropism, safety, and biodistribution.
Some retinal gene therapies use a serotype of AAV as a delivery vector in clinical trials (MacLaren et al., 2014, Maguire et al., 2008, Reichel et al., 2018) because it restricts vector tropism to specific retinal subpopulations, improves the efficiency of gene delivery, has low immunogenicity and long transgene expression. Cross-species differences in vector tissue tropism between mice and non-human primates have been previously highlighted and must be sufficiently addressed before moving toward clinical trials (Asokan et al., 2012, Ramachandran et al., 2017). We previously showed proof-of-concept for CRB1 gene therapy in Crb1-retinitis pigmentosa-like mice by subretinal application of an AAV9-CMV-CRB2 gene therapy vector, thereby demonstrating the need for transgene expression in both photoreceptors and Müller glial cells (MGCs) (Pellissier et al., 2015). On subretinal application, AAV9 and the AAV6 variant serotype ShH10Y445F are able to efficiently infect mouse photoreceptors, MGCs, and retinal pigment epithelium, whereas AAV5 does not efficiently infect and express in mouse MGCs (Aartsen et al., 2010, Pellissier et al., 2014a). AAV tropism differs between species, therefore the AAV serotype for clinical CRB1 gene therapy in both photoreceptors and MGCs needs to be validated. AAV5 and AAV9 infect non-human primate rod and cone photoreceptors (Boye et al., 2012, Vandenberghe et al., 2013). Human induced pluripotent stem cell (hiPSC)-derived retinal organoids, although in vitro, are a promising alternative or additional/pre-screening tool to animal models for evaluating transgene expression and biological activity (Quinn et al., 2018a). As previously demonstrated by others and ourselves hiPSC-derived retinal organoids and photoreceptors are amenable for the testing of AAV serotype/promoter combinations (Khabou et al., 2018, Quinn et al., 2018a, Wiley et al., 2016). However, for this purpose the hiPSC-derived retinal organoids should suitably recapitulate the human retina and the onset of expression of its proteins.
The core Crumbs complex in mammals is comprised of CRB1-3, PALS1 (also called MPP5), MUPP1, and PATJ. The prototypic CRB protein has a large extracellular domain with epidermal growth factor-like domains and laminin-A globular domains adjacent to a single transmembrane domain. A short C terminus of 37 amino acids contains an FERM protein-binding domain juxtaposing the single transmembrane domain. At the C-terminal end, there is a PDZ protein-binding motif of four amino acids (ERLI) that allows interaction with adaptor proteins such as PALS1 and PAR6 (Bachmann et al., 2001, Bulgakova and Knust, 2009, Lemmers et al., 2004, Roh et al., 2002). In non-human primates, CRB1 and CRB2 proteins localize to the subapical region adjacent to adherens junctions in MGCs and photoreceptors (Quinn et al., 2019). Similarly, in 2-day-old human adult cadaveric retina, both CRB1 and CRB2 are located at the subapical region in MGCs. However, in photoreceptors, the CRB1 protein is detectable at the subapical region near the outer limiting membrane (OLM), but CRB2 is not. In addition, both CRB1 and CRB2 are detected at vesicles in the photoreceptor inner segments at a distance from the OLM (Pellissier et al., 2014b, Pellissier et al., 2015). Thus far, the localization of the CRB complex in early human fetal retinal development is unknown.
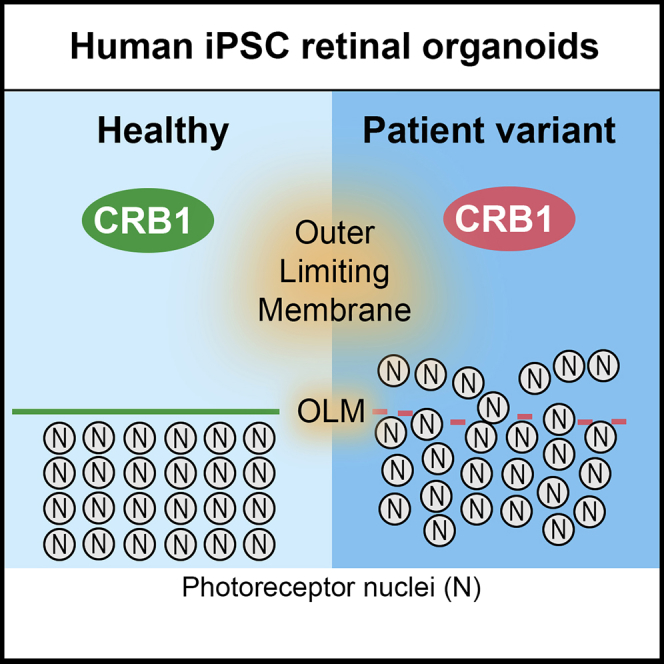
In this study, we show the recapitulation of the CRB complex between the human fetal retina and cultured hiPSC-derived retinal organoids. These studies highlight that CRB2, but not CRB1, is present at the subapical region in human fetal retina during the first trimester of pregnancy. CRB1 is expressed only at later time points from the second trimester onwards, concurring with the birth of differentiated cell types such as photoreceptors and MGCs. These data suggest role(s) for CRB2 but not CRB1 in the first trimester in the earliest human retinal radial glial progenitor cells. The data also suggest that CRB1 patient iPSC-derived retinal organoids develop a retinal phenotype as found in Crb1KO and Crb1KO/C249W mice (van de Pavert et al., 2004, van de Pavert et al., 2007b, van de Pavert et al., 2007a).
In addition, we show higher efficacy of AAV5 and ShH10Y445F over AAV9 serotypes for infection of photoreceptors in cultured human donor retinal explants. We also show the preference of AAV5 and ShH10Y445F over AAV9 to infect MGCs in hiPSC-derived retinal organoids. Overall, our results suggest that the AAV5 serotype, combined with CMV promoter-mediated expression, is suitable for gene therapy for CRB genes into human MGCs and rod and cone photoreceptors.
Results
Retinal Architecture in the Human Fetal Retina and iPSC Retinal Organoids
The human fetal retina, as it transitions from the first to the second trimester of pregnancy, gives rise to all adult retinal cell types as the retina moves from a mitotic to post-mitotic state (Provis et al., 1985). We examined first (weeks 11–13) and second trimester (weeks 16–18) fetal retina and compared these with early (differentiation day 30 [DD30]) and late (DD120–DD240) healthy hiPSC retinal organoids. The retinal architecture in the human fetal retina recapitulated the retinal architecture as found in hiPSC retinal organoids (Figures S1 and S2).
The CRB Complex in the Human Fetal Retina and iPSC Retinal Organoids
We undertook immunohistochemistry studies to delineate the onset of expression of CRB1 and CRB2 in first and second trimester human fetal retina and in early and late stage differentiated retinal organoids.
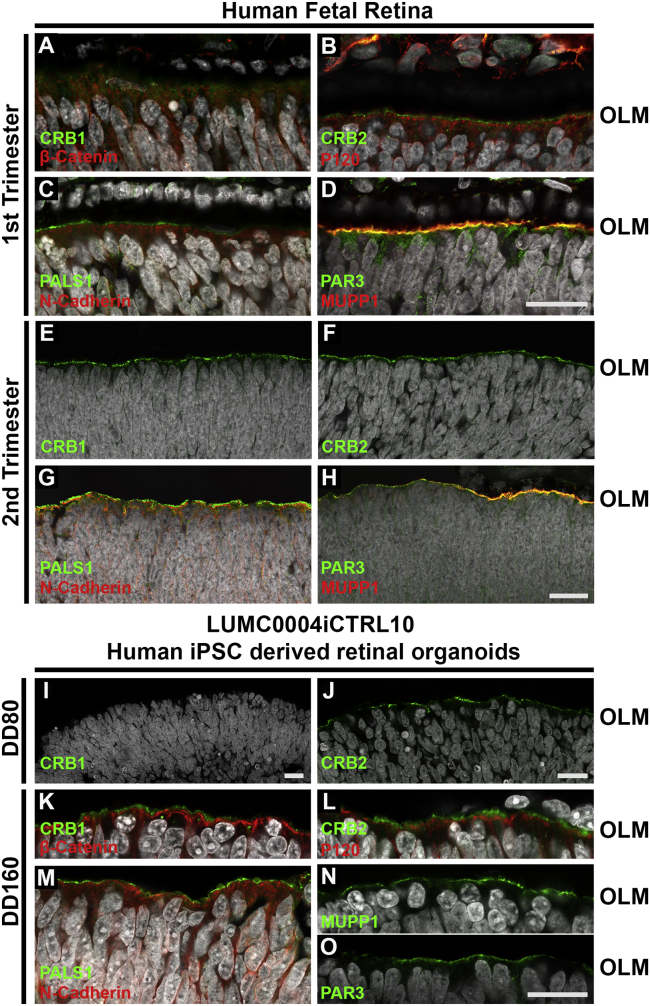
In week 9 human fetal retina we did not detect the typical puncta-like CRB1+ immunostaining at the subapical region adjacent to the adherence junction marker β-catenin (Figures S3A–S3C). However, at week 11 we observed a gradient of CRB1 immunostaining located at the OLM (Figure 1A). In weeks 9 and 11, the human fetal retina stained positive for CRB2 at the subapical region adjacent to the adherence junctions as marked by anti-p120-catenin (Figures 1B and S3D–S3F). Furthermore, in the first trimester fetal retina at week 9 (Figures S3G–S3I), we found PATJ at the OLM and additionally in a subset of anti-Ki67+ cells; this was also seen in the developing mouse retina (Alves et al., 2013). In week 19, the human fetal retina expressed CRB1 (Figure 1E) and CRB2 (Figure 1F) with their prototypic puncta immunostaining pattern. Immunostaining for CRB complex members PALS1, MUPP1, PAR3, and the adherens junction markers β-catenin, p120-catenin, and N-cadherin were detected in both first and second trimester human fetal retina (Figure 1).
Figure 1.
Localization of the CRB Complex at the Outer Limiting Membrane in Human Fetal and iPSC-Derived Human Retina
Immunohistochemistry pictures of first (A–D) and second (E–H) trimester human fetal retina and early (I and J) and late (K–O) LUMC0004iCTRL10 hiPSC-derived retinal organoids. Sections were stained for subapical region markers: CRB1 (A, E, I, and K), CRB2 (B, F, J, and L) PALS1 (C, G, and M), PAR3 (D, H, and O), MUPP1 (D, H, and N) and for adherens junction markers: β-catenin (A and K), p120-catenin (B and L), N-cadherin (C, G, and M). OLM, outer limiting membrane. Scale bars, 20 μm (A–O). See also Figures S1 and S2.
Similarly, In DD28 and DD80 retinal organoids we did not detect typical CRB1+ puncta-like immunostaining (Figures 1I and S3J). Its family member CRB2 was present at DD28 and DD80 (Figures 1J and S3K). PALS1 and MUPP1 were present at the subapical region in DD28 hiPSC-derived retinal organoids (Figures S3L and S3M). Moreover, in retinal organoids at DD30 (Figures S3N–S3P) we found PATJ at the OLM, and additionally in a subset of anti-Ki67+ cells. However, a typical and clear puncta-like staining pattern for CRB1 was detected at DD160 subapical of adherens junctions marker β-catenin (Figure 1K). CRB complex members CRB2, PALS1, MUPP1, and PAR3, and adherens junction markers p120-catenin and N-cadherin, were also detected in DD160 retinal organoids (Figures 1L–1O). CRB1 and CRB2 localization in retinal organoids was also confirmed in two other hiPSC lines: LUMC0080iCTRL12 (Figures S3Q–S3S) and LUMC0044iCTRL44 (Figures 5E–5H and 6E–6H).
Figure 5.
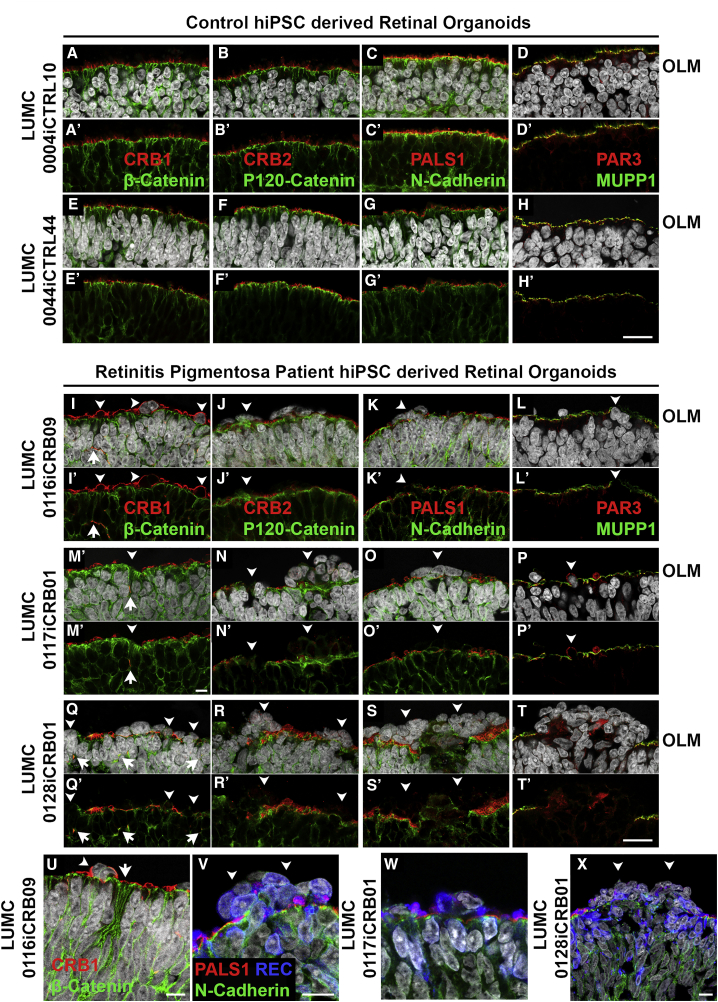
CRB1 Patient Organoids Develop Retinal Degeneration
Immunohistochemistry pictures of healthy: LUMC0004iCTRL10 (A–D and A′–D′) and LUMC0044iCTRL44 (E–H, E′–H′) retinal organoids at DD180; and CRB1 patient: LUMC0116iCRB09 (I–L, I′–L′, U, and V), LUMC0117iCRB01 (M–P, M′–P′, and W), LUMC0128iCRB01 (Q–T, Q′–T, and X) retinal organoids at DD180. Sections were stained for subapical region markers: CRB1 (A, E, I, M, Q, and U), CRB2 (B, F, J, N, and R), PALS1 (C, G, K, O, S, V, W, and X), PAR3 (D, H, L, P, and T), MUPP1 (D, H, L, P, and T); and for adherens junction markers: β-catenin (A, E, I, M, Q, and U), p120-catenin (B, F, J, N, and R), and N-cadherin (C, G, K, O, S, and V). Disruptions of the OLM are seen in patient retinal organoids (I–T and I′–T′) with recoverin+ photoreceptors found displaced (V–X). (A–X) Counterstained with DAPI. Experiments were validated in two differentiations for retinal organoids. OLM, outer limiting membrane; NBL, neurobasal layer. Scale bars (A–X), 20 μm. See also Figure S4.
Figure 6.
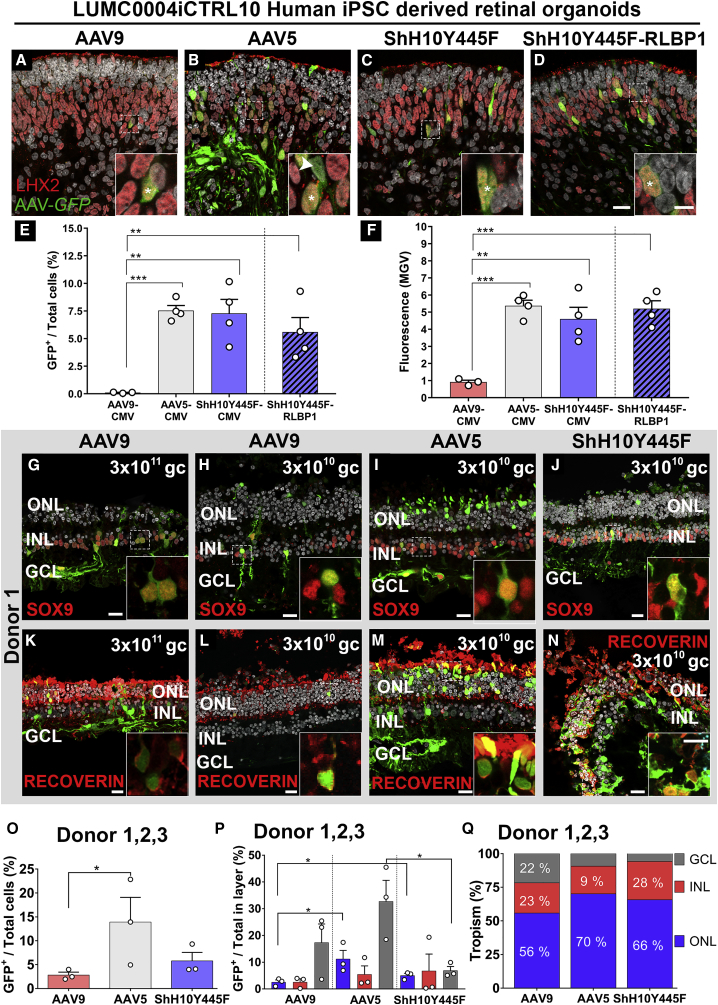
AAV5 and ShH10Y445F Efficiently Infect Müller Glial Cells in Human iPSC Retinal Organoids and Photoreceptors and Müller glial Cells in the Human Adult Retina
(A–F) Infection of 7.5-month-old LUMC0004iCTRL10 hiPSC retinal organoids at 1010 gc with AAV9-CMV-GFP (A), AAV5-CMV-GFP (B), ShH10Y445F-CMV-GFP (C), and ShH10Y445F-RLBP1-GFP (D), and co-staining with the Müller glial cell marker LHX2, the majority of LHX2+ cells were also GFP+ (asterisk). Efficacy of transducing retinal cell types was quantified by measuring the number of GFP+ cells in the total cell population (E) and fluorescence (MGV) (F). AAV5 and ShH10Y445F showed the higher efficacy of transducing retinal cell types at 1010 gc than AAV9, which showed lower efficacy. A total of 1010 gc applied per two organoids, two independent differentiations used, three to four organoids analyzed per vector.
(G–Q) Infection of human retinal explants with AAV9-CMV-GFP at 3 × 1011 gc (G and K) and 3 × 1010 gc (H and L), AAV5-CMV-GFP at 3 × 1010 gc (I and M), and ShH10Y445F-CMV-GFP at 3 × 1010 gc (J and N). Co-staining showed the presence of GFP+/Sox9+ Müller glial cells with AAV9 (G and H), AAV5 (I), and ShH10Y445F (J). Co-staining showed the presence of GFP+/recoverin+ photoreceptors AAV9 (K and L), AAV5 (M), and ShH10Y445F (N). Efficacy of transducing retinal cell types at 3 × 1010 gc was quantified by measuring the number of GFP+ cells in the total cell population (O). When compared for three donors at 3 × 1010 gc, AAV5 showed higher efficacy of transducing retinal cell types than AAV9. The potency of AAV serotypes was compared by analyzing transduction of cell types in each of the ONL, INL, and GCL (P). AAV5 and ShH10Y445F showed higher potency in transduction of photoreceptors in the ONL than AAV9. All serotypes transduced cells in all layers at 3 × 1010 gc (Q). Each dot represents the average of an individual donor (O and P), 10 images were analyzed per donor (O–Q). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer; gc, genome copies; MGV, mean gray value.
Scale bar (A–D, G–N), 20 μm; (A–D, G–N) inserts, 10 μm. Data are presented as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
See also Figures S5–S7.
Ultra-Localization of CRB1 and CRB2 in the Human Fetal Retina and in iPSC Retinal Organoids
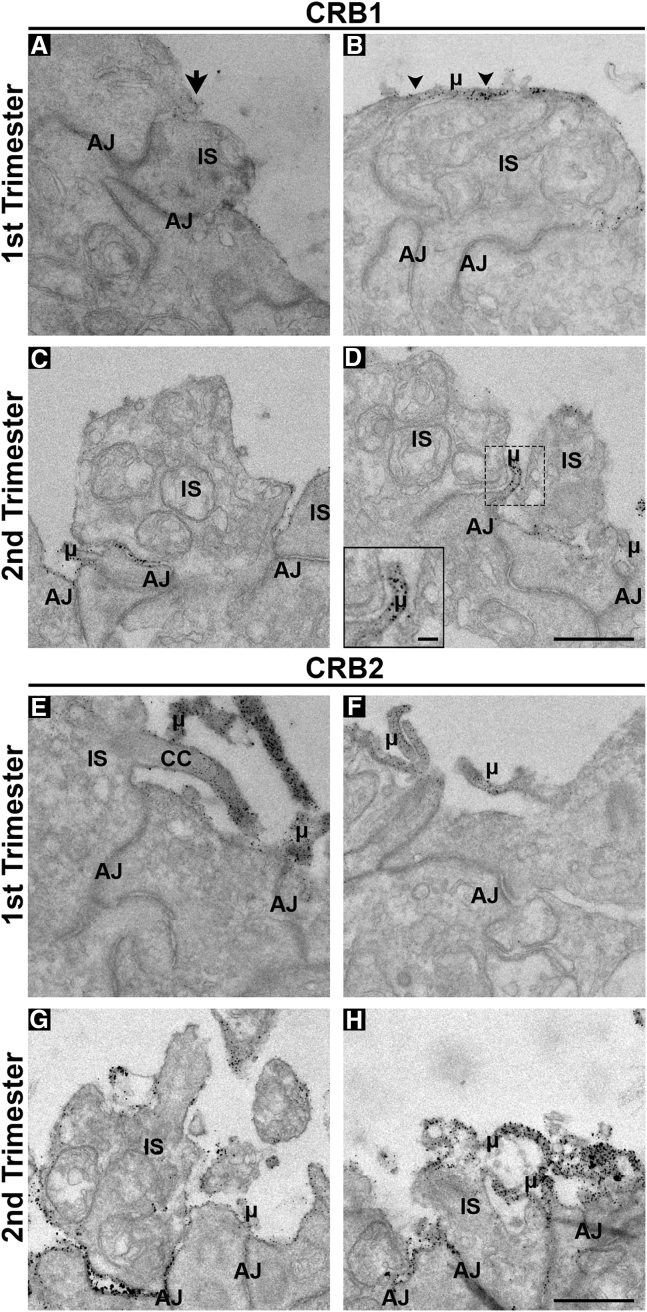
We performed immunoelectron microscopy (immuno-EM) studies to analyze, at ultra-high resolution, the localization of CRB1 and CRB2 in photoreceptor cells (PRCs) and MGCs in first and second trimester human fetal retina and in retinal organoids. Immuno-EM for CRB1 in the first trimester fetal retina showed occasional and limited staining at putative inner segments (Figure 2A) and apical villi (Figure 2B) of radial glial progenitor cells. However, in the second trimester CRB1 labeling could be clearly detected at the subapical region adjacent to adherens junctions between putative photoreceptor inner segments (Figure 2C) and in the apical villi of radial glial progenitor cells/MGCs (Figure 2D). Immuno-EM for CRB2 showed pronounced labeling in first (Figures 3E and 3F) and second trimester (Figures 2G and 2H) fetal retina. CRB2 labeling localized at the plasma membrane and at the subapical region adjacent to adherens junctions between putative photoreceptor inner segments (Figures 2E and 2G) and in the apical villi of radial glial progenitor cells/MGCs (Figures 2F and 2H).
Figure 2.
CRB1 and CRB2 are Located at the Outer Limiting Membrane in Müller Glial Cells and Photoreceptors of First and Second Trimester Human Fetal Retina
Immuno-EM staining showing the localization of CRB1 (A–D) and CRB2 (E–H) in first (A, B, E, and F) and second (C, D, G, and H) trimester human fetal retina. CRB1 was sporadically detected in the first trimester but being found subapically of adherens junctions adjacent to photoreceptor inner segments (A) (arrow) and in Müller glial cell apical villi (B) (arrowheads). In the second trimester, CRB1 was found consistently throughout the outer limiting membrane of human fetal retina subapically of adherens junctions of photoreceptors and Müller glial cells (C and D). CRB2 was localized in both first and second trimester retina subapically of adherens junctions being located in photoreceptor inner segments and apical villi of Müller glial cells. At least two independent samples were analyzed per time point. CC, connecting cilium; AJ, adherens junction; μ, microvilli; IS, inner segment. Scale bar (A–H), 1 μm; insert (D), 500 nm.
Figure 3.
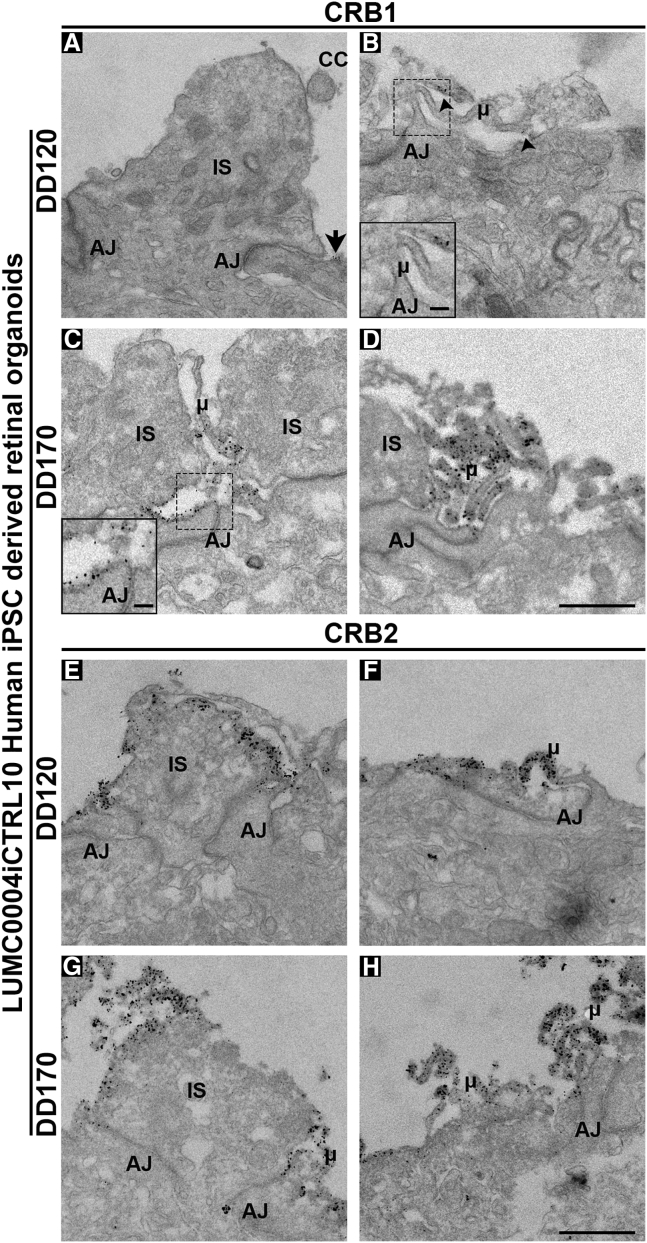
CRB1 and CRB2 are Located at the Outer Limiting Membrane in Müller Glial Cells and Photoreceptors of Human iPSC-Derived Retinal Organoids
Immuno-EM staining showing the localization of CRB1 (A–D) and CRB2 (E–H) in DD120 (A, B, E, and F) and DD170 (C, D, G, and H) LUMC0004iCTRL10 human iPSC-derived retinal organoids. CRB1 was lowly and sporadically detected at DD120 but being found subapically of adherens junctions adjacent to photoreceptor inner segments (A) (arrow) and in Müller glial cell apical villi (B) (arrowheads). At DD170, CRB1 was found consistently throughout the outer limiting membrane of human iPSC-derived retinal organoids subapically of adherens junctions of photoreceptors and Müller glial cells (C and D). CRB2 was localized at both DD120 and DD170 subapically of adherens junctions being located in photoreceptor inner segments and apical villi of Müller glial cells. At least two independent samples were analyzed per time point. CC, connecting cilium; AJ, adherens junction; μ, microvilli; IS, inner segment. Scale bar (A–H), 1 μm; insert (B and C), 500 nm.
Similarly to first trimester human fetal retina, immuno-EM performed on retinal organoids showed sporadic and limited CRB1 labeling at the subapical region adjacent to adherens junctions between putative photoreceptor inner segments (Figure 3A) and in the apical villi of radial glial progenitor cells/MGCs (Figure 3B) in early retinal organoids. However, in late retinal organoids CRB1 labeling localized at the plasma membrane and at the subapical region adjacent to adherens junctions between putative photoreceptor inner segments (Figure 3C) and in the apical villi of radial glial progenitor cells/MGCs (Figure 3D). Immuno-EM for CRB2 showed pronounced labeling in early (Figures 3E and 3F) and late (Figures 3G and 3H) retinal organoids. CRB2 labeling localized at the plasma membrane and at the subapical region adjacent to adherens junctions between putative photoreceptor inner segments (Figures 3E and 3G) and in the apical villi of radial glial progenitor cells/MGCs (Figures 3F and 3H).
Taken together, these data suggest that CRB1 is not required for the localization of CRB2, PALS1, MUPP1, or PATJ in the first trimester of human retinal development. The onset of CRB protein expression in human fetal retina is recapitulated in retinal organoids.
Disruptions at the OLM in Retinal Organoids from CRB1 RP Patients
We generated three hiPSC lines (LUMC0116iCRB, LUMC0117iCRB, and LUMC0128iCRB) from CRB1 RP patients. As with healthy iPSCs (Figure S4A), patient iPSCs were validated via immunostaining with pluripotent and germ layer markers (Figure S4B). LUMC0116iCRB has c.3122T > C p.(Met1041Thr) homozygote missense mutations. LUMC0117iCRB has 2,983G > T p.(Glu995∗) and c.1892A > G, p.(Tyr631Cys) mutations. LUMC0128iCRB has c.2843G > A p.(Cys948Tyr) and c.3122T > C p.(Met1041Thr) missense mutations. The CRB1 gene mutations were re-confirmed in the established iPSCs (Figure S4C).
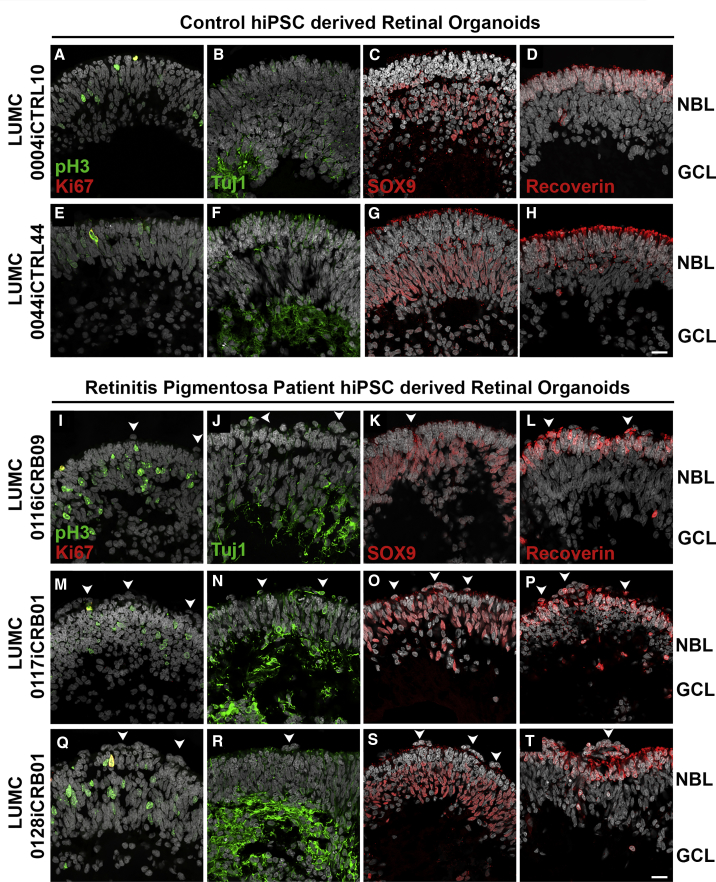
CRB1 patient iPSCs were able to differentiate to DD180 retinal organoids and were compared with healthy retinal organoids (Figure 4). The patient retinal organoids had pH3+ mitotic cells apically at the OLM, with Ki67+ cycling cells less tightly restricted to the middle of the neurobasal layer (NBL) (Figures 4I, 4M, and 4Q). The CRB1 patient iPSCs retinal organoids developed all three retinal layers: a ganglion cell layer marked by Tuj1+ dendrites (Figures 4J, 4N, and 4R), an NBL marked by SOX9+ retinal progenitor cells/MGCs (Figures 4K, 4O, and 4S), and an outer nuclear layer (ONL) marked by recoverin+ PRCs (Figures 4L, 4P, and 4T). However, frequent ectopic cells were found above the OLM (Figures 4I–4T). We observed many areas of funnel-shaped outward protruding recoverin+ PRCs (Figures 4L, 4P, and 4T) and, sporadically, SOX9+ cells above the OLM (Figure 4O).
Figure 4.
CRB1 Patient Retinal Organoids Develop Proper Lamination
Immunohistochemistry pictures of healthy (LUMC0004iCTRL10 and LUMC0044iCTRL44) versus CRB1 patient (LUMC0116iCRB09, LUMC0117iCRB01, and LUMC0128iCRB01) retinal organoids at DD180. Sections were stained for pH3+ mitotic cells and Ki67+ cycling cells (A, E, I, M, and Q); Tuj1+ dendrites marking the GCL (B, F, J, N, and R); SOX9+ NBL cells (C, G, K, O, and S); and recoverin+ PRCs marking the ONL (D, H, L, P, and T). Experiments were validated in two differentiations for retinal organoids. NBL, neurobasal layer; GCL, ganglion cell layer. Scale bars (A–T), 20 μm. See also Figure S4.
All retinal organoids from the three different CRB1 patient lines developed small but frequent disruptions of CRB complex members at the OLM that were not detected in control lines (Figure 5). The adherens junction proteins N-cadherin, p120-catenin, β-catenin, and the subapical region proteins CRB2, PALS1, PAR3, and MUPP1 were localized as in healthy retinal organoids at DD180 (Figure 5). Interestingly, CRB1 variant protein localized similar to the wild-type CRB1 protein at the subapical region above the adherens junctions, but showed a curved and broadened expression pattern (Figures 5I, 5M, 5Q, and 5U) compared with the healthy control lines (Figures 5A and 5E). CRB1 variant protein was also mislocalized in the apical area of the NBL and in the ONL (Figures 5I, 5M, and 5Q). The mislocalized PRCs above the OLM resided at areas of OLM disruptions (Figures 5V, 5W, and 5X). In conclusion, the data from CRB1 patient hiPSC retinal organoids suggest a retinal degeneration phenotype similar to that previously found in mice lacking CRB1 or expressing variant CRB1C249W (van de Pavert et al., 2004, van de Pavert et al., 2007b, van de Pavert et al., 2007a).
Transduction of Human iPSC Retinal Organoids with AAV5, AAV9, and ShH10Y445F
Human iPSC-derived retinal organoids are a promising tool for evaluating transgene expression and biological activity (Quinn et al., 2018a). We have shown the need in Crb1-retinitis pigmentosa-like mice to direct CRB gene therapy to both photoreceptors and MGCs (Pellissier et al., 2015). Choosing the optimal promoter and AAV serotype for the therapeutic vector is therefore crucial to achieving expression in photoreceptors and MGCs. Here, we transduced the hiPSC-derived retinal organoids with AAV9-CMV-GFP, AAV5-CMV-GFP, ShH10Y445F-CMV-GFP, and ShH10Y445F-RLBP1-GFP at 1010 genome copies (gc). The CMV promoter drives expression of GFP in multiple cell types, whereas the hRLBP1 promoter drives expression in MGCs and retinal pigment epithelial cells (Pellissier et al., 2014a). When analyzed at the same laser intensity settings (Figures S5A–S5D) AAV5-CMV-GFP, ShH10Y445F-CMV-GFP, and ShH10Y445F-hRLBP1-GFP significantly outperformed AAV9-CMV-GFP at transducing DD220 hiPSC-derived retinal organoids collected 14 days after infection (Figures 6E and 6F). We quantified both the number of GFP+ cells per total cells (Figure 6E) and the mean gray value (Figure 6F) for each vector. The GFP+ nuclei were mainly located in the inner retina and exhibited radial projections. The GFP+ nuclei co-localized with anti-LHX2 (Figures 6A–6D, inserts) and anti-SOX2 (Figures S5E–S5H and S5E′–S5H′), both transcription factors required for MGC development. Further proof of Müller glial-specific transduction is also seen with the use of ShH10Y445F-RLBP1-GFP (Figure 6D), which drives GFP in MGCs in rat and mouse retinas (Klimczak et al., 2009, Pellissier et al., 2014a). We additionally stained with anti-recoverin and found occasional co-localization between the photoreceptor marker and GFP+ nuclei (Figures S5I–S5L and S5I′–S5L′). In conclusion, these results indicate that AAV5 and ShH10Y445F serotypes are more potent transducers of MGCs than AAV9 in hiPSC-derived retinal organoids.
Transduction of Adult Postmortem Human Retinal Explants with Serotypes AAV5, AAV9, and ShH10Y445F
To verify the results of our transduction studies on hiPSC-derived retinal organoids we also tested AAV9-, AAV5-, and ShH10Y445F-CMV-GFP on human adult retinal explants. Initially, a titration study for AAV9 (Figures S6A–S6C), AAV5 (Figures S6D–S6F), and ShH10Y445F (Figures S6G–S6I) was undertaken to determine what gc level is required to infect photoreceptors and MGCs efficiently. Analysis for total infection (Figures S6P and S6Q), infection per retinal layer (Figures S6R and S6S), and tropism (Figure S6T) in donor 1 indicated 3 × 1010 gc as a suitable level. Similar transduction patterns were found in donor 1 (Figures 6H–6K, 6L–6N, S6B, S6D, and S6G), donor 2 (Figures S6J–S6L), and donor 3 (Figures S6M–S6O) at 3 × 1010 gc. With individual analysis for total infection (Figures S6U, S6V, S6Y, and S6Z), infection per retinal layer (Figures S6W and S6AA), and tropism (Figures S6X and S6BB) also done for donors 2 and 3 at 3 × 1010 gc. Donors 1–3 had similar retinal layer thickness (Figure S6CC) and cells per retinal layer (Figure S6DD). In the inner nuclear layer (INL), GFP co-labeled with MGC marker anti-SOX9 (Figures 6G–6J); and in the ONL, GFP co-labeled with photoreceptor marker anti-recoverin (Figures 6K–6N). When analyzing donors 1–3 together, AAV5 showed the higher efficacy of transducing retinal cell types than AAV9 (14% ± 5% versus 3% ± 1%; Figure 6O), and AAV5 and ShH10Y445F showed higher potency in transduction of photoreceptors in the ONL than AAV9 (11% ± 3% and 5% ± 1% versus 3% ± 1%; Figures 6P and 6Q). Interestingly, we noticed that the photoreceptors of cadaveric human retinal explants were only efficiently infected by AAV9 (Figures S7A and S7B), AAV5 (Figure S7C), or ShH10Y445F (Figure S7D) in the presence of intact photoreceptor segments (Figures S7E–S7G). This suggests an important role for the segments in the photoreceptor uptake of AAV particles. In conclusion, transduction of AAV serotypes in human cadaveric retina was more successful at targeting both photoreceptors and MGCs than in hiPSC-derived retinal organoids. AAV5 at 3 × 1010 gc significantly outperformed AAV9 in the transduction of PRCs.
CRB2 Is Located in the Apical Membrane of iPSC-Derived and Retinal Pigment Epithelium
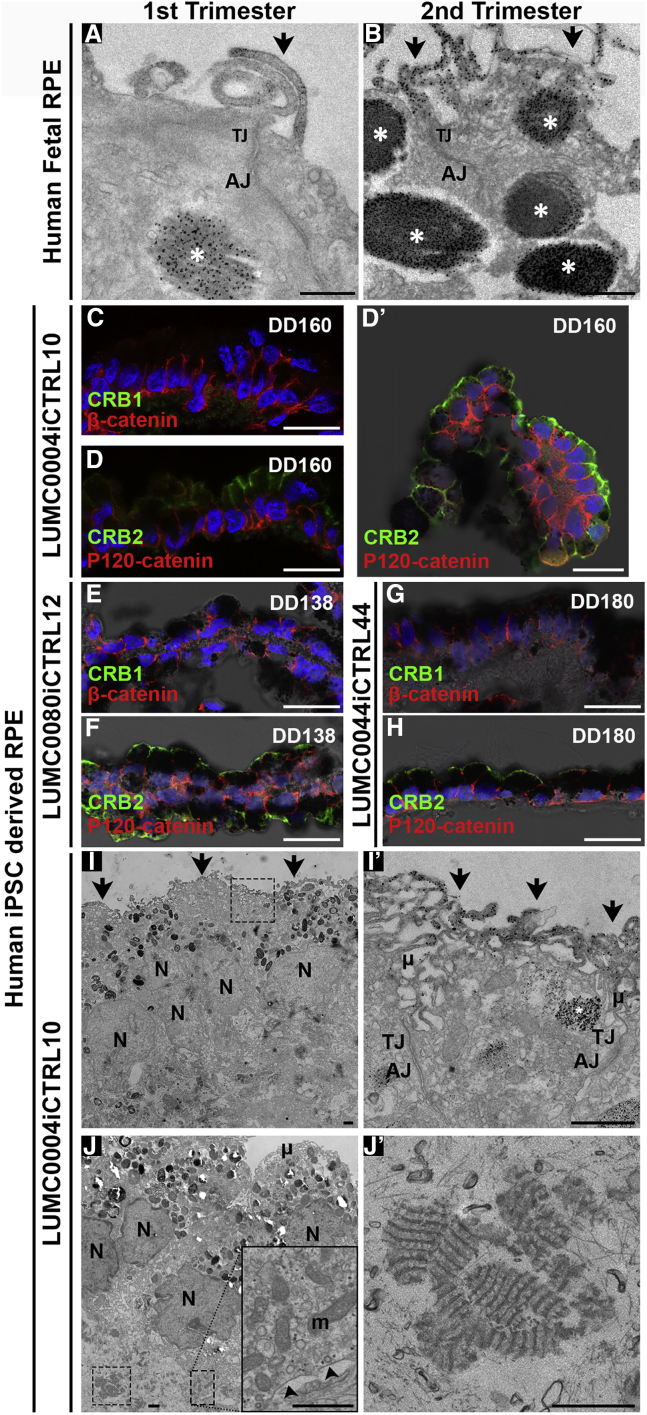
CRB2 but not CRB1 immunostaining was detected in first trimester human fetal retinal pigment epithelium (RPE) (Figures S3D’–S3F′). This was confirmed by immuno-EM in first and second trimester human fetal RPE (Figures 7A and 7B). CRB2 labeling was located above the adherens junctions at and above the tight junctions in the apical membrane and microvilli of human fetal RPE. Spheroids of hiPSC-derived RPE are also generated during the differentiation method used (Liu et al., 2018, Quinn et al., 2018a, Zhong et al., 2014). These RPE spheroids initially attach to the periphery of the retinal organoids but can detach during culturing (Figures 7C, 7D, and 7D′). CRB1 could not be detected apically of β-catenin (Figure 7C) in hiPSC-derived RPE, but CRB2 was found apically of the adherens junction marker p120-catenin in DD160 hiPSC-derived RPE (Figure 7D). This pattern of localization was also found in hiPSC-derived RPE derived from hiPSC lines LUMC0080iCTRL12 (Figures 7E and S7F) and LUMC0044iCTRL44 (Figures 7G and 7H). Immuno-EM of iPSC-derived RPE confirmed the apical staining for CRB2 above adherens junctions at and above the tight junctions in the apical membrane and microvilli. Aspecific staining was detected within melanin granules due to the presence of endogenous peroxidase in these structures (Figures 7I and 7I′). Electron microscopy of hiPSC-derived RPE also showed the presence of melanosomes with pigments, basally located mitochondria and basement membrane (Figure 7J, insert), and fibrous long-spacing collagen (Figures 7J and 7J′). RPE cells were also infected by AAV9, AAV5, and ShH10Y445F (Figures S7H–S7K).
Figure 7.
Human iPSC Retinal Pigment Epithelium Localize CRB2 at and above Tight Junctions in the Apical Membrane and Microvilli
Immuno-EM showed staining for CRB2 above adherens junctions at and above tight junctions in the apical membrane and in the microvilli in first (A) and second (B) trimester in human fetal RPE. Aspecific staining was detected within melanosomes (A and B) (asterisk). Immunohistochemistry did not detect CRB1 apically of β-catenin (C) in hiPSC RPE, but CRB2 was found apically of p120-catenin in LUMC0004iCTRL10 (D and D′), LUMC0080iCTRL12 (E and F), and LUMC0044iCTRL44 (G and H) human iPSC-derived RPE. RPE spheroids were found either attached to retinal organoids or became independent, detached spheroids (D′). Immuno-EM confirmed apical staining for CRB2 above adherens junctions at and above tight junctions in the apical membrane and in the microvilli (I and I′) (arrows). Aspecific staining was detected within pigmented melanosomes (I′) (asterisk). Electron microscopy of hiPSC-derived RPE also showed basally located mitochondria (J) (insert) and basement membrane (J) (insert arrowheads) and fibrous long-spacing collagen (J and J′). AJ, adherens junction; N, nuclei; μ, microvilli; TJ, tight junction; m, mitochondria. Scale bars (C, D, D′, E, F, G, H), 20 μm; (A, B, I, I′, J, J′, insert J), 1 μm.
Discussion
In this study, we showed (1) that in human fetal retina during the first trimester of pregnancy CRB2 is the predominant CRB family member in radial glial progenitor cells; and that CRB1 onset of expression at the subapical region coincides with the maturation of the retina during the second trimester. (2) CRB2 but not CRB1 is expressed in the fetal RPE. (3) The onset of CRB protein expression in human fetal retina and RPE is recapitulated in hiPSC-derived retinal organoids and RPE. (4) CRB1 RP patient retinal organoids develop disruptions at the OLM with misplaced photoreceptors. (5) AAV5 and ShH10Y445F serotypes are more potent than AAV9 serotype in infecting cultured retinal organoids. (6) AAV5-CMV-GFP is more efficient than AAV9-CMV-GFP to express GFP in PRCs in cultured human donor retinal explants. (7) Human PRCs are efficiently transduced only in the presence of photoreceptor segments.
In the human fetal retina, we found that CRB2 is the predominant CRB effector protein in the first trimester of pregnancy. CRB2 is a gene expressed in several tissues, including the cerebral cortex (Dudok et al., 2016), with a crucial role during early development in both mice and humans. Mice lacking Crb2 are embryonic lethal, with a critical role for the CRB2 protein during gastrulation in the epithelial-to-mesenchymal transition (Ramkumar et al., 2016). CRB2 protein variants in humans have been linked to a syndromic phenotype causing kidney and brain dysfunctions and lethality (Ebarasi et al., 2015, Slavotinek et al., 2015) as well as to RP (Chen et al., 2018). In the second trimester, CRB1 and CRB2 localized at the subapical region in apical villi of radial glial progenitor cells/MGCs and at the subapical region above the adherens junctions in the inner segments of PRCs. During the second trimester, the retina undergoes the birth of all adult cell types, and the retina is transitioning from a mitotic to post-mitotic state (Lee et al., 2006, Provis et al., 1985). Retinal organoids go from an early highly cell-cycling state, in which Ki67 marks the entire NBL at DD28, toward a moderate cell-cycling state, in which Ki67 becoming restricted to the mid-NBL at DD120.
Interestingly, here we showed that the onset of CRB1 protein expression coincided with the maturation of the retinal organoids, and this finding is recapitulated in the human fetal retina. In early-stage retinal organoids, we found, as in the first trimester fetal retina, CRB2 but little CRB1 protein expression at the subapical region. In later-stage hiPSC-derived retinal organoids we found CRB2 and CRB1 protein expression at the subapical region as in second trimester fetal retina. We also found a recapitulation of CRB2 expression when comparing first trimester fetal RPE with hiPSC-derived RPE.
We present here the generation and characterization of CRB1 patient-derived hiPSCs differentiated to retinal organoids. We demonstrate that patient retinal organoids give rise to a morphological significant phenotype even though variant CRB1 protein and its interaction partners (MUPP1, PALS1, and CRB2) are detected at the OLM. The data suggest disruptions at the OLM resulting in loss of adhesion between photoreceptors and MGCs. Decreased levels of CRB1 and CRB2 proteins at the OLM exacerbated retinal degeneration in mouse models (Alves et al., 2013, Pellissier et al., 2013, Quinn et al., 2018b). Also, the volcanic-like cell protrusions and OLM disruptions in the patient retinal organoids show striking similarities to the morphological phenotype found in 3-month-old Crb1KO and 8-month-old Crb1KO/C249W RP mice (van de Pavert et al., 2004, van de Pavert et al., 2007b, van de Pavert et al., 2007a). Further studies are needed to elucidate the underlying effects of the variant CRB1 proteins on protein-protein interactions and downstream cell signaling pathways.
We hypothesize that retinal organoids could be a good model for evaluating transgene expression and biological activity due to their close mimicking of human fetal retinal development (Quinn et al., 2018a). Our transduction studies on cadaveric human retinal explants showed a higher potency for AAV5 over AAV9 for transduction of photoreceptors. Also, the data suggest the higher efficacy of AAV5-CMV-GFP than AAV9-CMV-GFP or ShH10Y445F-CMV-GFP to express in PRCs and MGCs. In the absence of photoreceptor segments in the human retinal explants, AAV5-CMV-GFP, ShH10Y445F-CMV-GFP, and AAV9-CMV-GFP showed higher efficacy to express in MGCs than in PRCs. The latter tropism and expression potency data in cultured cadaveric human retinal explants are reproduced in retinal organoids that recapitalize second trimester fetal retina.
Previous subretinal injection studies in which AAV5 was administered in mice at postnatal day 0 (P0) or P30 have shown preferred transduction of P0 cone PRC and MGCs, but only of P30 rod and cone PRCs (Surace et al., 2003). We hypothesize that this preference in transduction patterns of PRC and MGCs in immature versus mature retina is due to the presence or absence of matured photoreceptor segments. In mice, photoreceptor segments seem to be required for the efficient transduction of PRCs with AAV vectors (Petit et al., 2017). A very interesting and clinically relevant finding is that photoreceptors in cultured cadaveric human retinal explants are only efficiently transduced when they have photoreceptor segments. Retinal organoids represent immature fetal retinas that contain PRCs but with yet very immature segments. We hypothesize that PRCs are transduced by AAV5, AAV9, and ShH10Y445F once the PRC segments are formed in sufficient number and size. In the absence of PRC segments, however, there is increased bioavailability of AAV vectors to target less-abundant/preferred receptors for AAV uptake, e.g., on MGCs. Interestingly, dependency on the presence of photoreceptor segments for photoreceptor transduction was observed for all three AAV serotypes (AAV5, AAV9, and ShH10Y445F), suggesting a putative common mechanism of active AAV uptake into photoreceptors. The inner segments are a putative site of receptor-dependent or -independent clathrin- and caveolae-mediated endocytosis (Fuchs et al., 2014).
Our data suggest that for clinical gene therapy with AAV5, AAV9, or ShH10Y445F the target PRCs should have intact photoreceptors to become efficiently transduced. It also implies that the AAV vector particles should be able to reach the PRC segments during clinical surgical application. This condition of accessibility of PRC inner segments in human retina in vivo is met upon subretinal injection, as suggested by AAV5 or AAV9 infection of PRCs in non-human-primate retinas (Boye et al., 2012, Vandenberghe et al., 2011). Our mice lacking CRB1, as well as the mice with reduced levels of CRB2, showed a compromised OLM. We further hypothesize from our previous studies in mice that the retinas of human patients with loss of CRB1, or expressing non-functional CRB1 variants, have a compromised OLM that allows increased passage of AAV viral particles across the adherence junctions to reach the AAV-receptor molecules on MGCs (Pearson et al., 2010).
Experimental Procedures
See further details in the Supplemental Experimental Procedures.
Fetal Human Retinal Tissue
The use and collection of the material was approved by the Medical Ethics Committee of the Leiden University Medical Center (P08.087).
Adult Human Retinal Tissue
Tissue was collected in agreement with the guidelines of the ethics committee of the LUMC. Informed consent was obtained on the basis of the Declaration of Helsinki (World Medical Association).
Cell Culture and Retinal Organoid Differentiation
Human iPSCs (LUMC0004iCTRL10 [Dambrot et al., 2014], LUMC0044iCTRL44 [Chen et al., 2017], LUMC0080iCTRL12 [Figure S4A], LUMC0116iCRB09, LUMC0117iCRB01, and LUMC0128iCRB01 [Figure S4B]) were maintained on Matrigel (BD)-coated plates in mTeSR medium (STEMCELL Technologies) and passaged mechanically. Retinal organoid differentiation was carried out as previously reported (Quinn et al., 2018a, Zhong et al., 2014).
Electron Microscopy
Immuno-EM was performed as described previously (Klooster et al., 2011). In brief, sections were incubated with antibody for 48 h, then incubated with appropriate secondary peroxidase anti-peroxidase for 2 h, then developed in a 2,2-diaminobenzidine solution for 4 min, and then the gold substitute silver peroxidase method applied.
Generation and Purification of the Viral Vectors
The pAAV2-eGFP plasmids were generated previously and consist of the flanking inverted terminal repeats of AAV2, the full-length CMV promoter, or the human RLBP1 promoter, the eGFP cDNA, the Woodchuck posttranscriptional regulatory element, and the bovine growth hormone poly(A) (Aartsen et al., 2010, Alves et al., 2014b, Pellissier et al., 2014a).
In Vitro Transduction of Human Donor Retina and Human Induced Pluripotent Stem Cell-Derived Retinal Organoids
In vitro transduction protocols for (1) human donor retina and (2) hiPSC-derived retinal organoids have been described previously (Buck et al., 2018, Quinn et al., 2018a).
Statistical Method
All statistical analyses were performed using GraphPad Prism version 7 (GraphPad Software). All values are expressed as mean ± SEM. Multiple t tests were performed with the analyzed number of samples indicated in the figure legends. Immunohistochemistry was performed on iPSC-derived retinal organoids from three independent healthy and three independent CRB1-patient iPSC lines from two or more differentiations, with three to six sections examined per organoid. Immunohistochemistry was performed on at least two independent human fetal eyes per time point, with three to six sections examined per eye. Immuno-EM was performed on at least two independent human fetal eyes, iPSC-derived retinal organoids, and RPE for each time point. For AAV transduction studies, between three and six different sections from at least three different human donor retina or hiPSC-derived retinal organoids (one to two organoids from two independent differentiations analyzed) were used for quantification. Between three and five images per organoid and ten images per adult donor retina were analyzed.
Author Contributions
P.M.Q., T.M.B., and J.W. conceived and designed the experiments. P.M.Q., T.M.B., C.O., C.H.A., and A.A.M. performed the experiments. R.M.V. produced virus stocks. M.B., T.v.H., E.H.C.v.D., and M.T. collected study material. H.M.M.M., C.F., R.C.H., M.-J.G., C.J.F.B., A.J.K., and S.M.C.d.S.L. provided study material and/or access to facilities. P.M.Q. and T.M.B. assembled the data. P.M.Q., T.M.B., A.A.M., C.R.J., and J.W. analyzed and interpreted the data. P.M.Q., T.M.B., and J.W. wrote the manuscript. All authors reviewed the manuscript. J.W. provided funding acquisition, supervision, and final approval of manuscript.
Acknowledgments
The Wijnholds Laboratory would like to thank Yacintha van Doorn and Hind Almushattat for differentiation of retinal organoids, André Le Bivic for providing PATJ antibodies, Maaike Nieveen and Fang Wang for collecting study materials, Annelies van der Laan and Joop Wiegant from the LUMC microscope facility, and Anke ’t Jong from the LUMC iPSC facility for advice. We would like to thank the abortion clinic Gynaikon in Rotterdam for the collection of the fetal material. We would also like to thank all our supporters, which include the Foundation Fighting Blindness USA (TA-GT-0313-0607-NIN and TA-GT-0715-0665-LUMC), the Netherlands Organisation for Health Research and Development (ZonMw grant 43200004), the Curing Retinal Blindness Foundation (CRBF), Stichting Retina Fonds, Landelijke Stichting voor Blinden en Slechtzienden (LSBS), Rotterdamse Stichting Blindenbelangen (RSB), Stichting Blindenhulp, and MaculaFonds. The LUMC is the holder of patent number PCT/NL2014/050549, which describes the potential clinical use of CRB2; J.W. is listed as inventor on this patent and is an employee of the LUMC. The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Published: April 4, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.03.002.
Supplemental Information
References
- Aartsen W.M., van Cleef K.W.R., Pellissier L.P., Hoek R.M., Vos R.M., Blits B., Ehlert E.M.E., Balaggan K.S., Ali R.R., Verhaagen J. GFAP-driven GFP expression in activated mouse Müller glial cells aligning retinal blood vessels following intravitreal injection of AAV2/6 vectors. PLoS One. 2010;5:e12387. doi: 10.1371/journal.pone.0012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves C.H., Sanz A.S., Park B., Pellissier L.P., Tanimoto N., Beck S.C., Huber G., Murtaza M., Richard F., Sridevi Gurubaran I. Loss of CRB2 in the mouse retina mimics human retinitis pigmentosa due to mutations in the CRB1 gene. Hum. Mol. Genet. 2013;22:35–50. doi: 10.1093/hmg/dds398. [DOI] [PubMed] [Google Scholar]
- Alves C.H., Pellissier L.P., Wijnholds J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog. Retin. Eye Res. 2014;40:35–52. doi: 10.1016/j.preteyeres.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Alves C.H., Pellissier L.P., Vos R.M., Garcia Garrido M., Sothilingam V., Seide C., Beck S.C., Klooster J., Furukawa T., Flannery J.G. Targeted ablation of Crb2 in photoreceptor cells induces retinitis pigmentosa. Hum. Mol. Genet. 2014;23:3384–3401. doi: 10.1093/hmg/ddu048. [DOI] [PubMed] [Google Scholar]
- Asokan A., Schaffer D.V., Jude Samulski R. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A., Schneider M., Theilenberg E., Grawe F., Knust E. Drosophila stardust is a partner of crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Boye S.E., Alexander J.J., Boye S.L., Witherspoon C.D., Sandefer K.J., Conlon T.J., Erger K., Sun J., Ryals R., Chiodo V.A. The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum. Gene Ther. 2012;23:1101–1115. doi: 10.1089/hum.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck T.M., Pellissier L.P., Vos R.M., van Dijk E.H.C., Boon C.J.F., Wijnholds J. AAV serotype testing on cultured human donor retinal explants. Methods Mol. Biol. 2018;1715:275–288. doi: 10.1007/978-1-4939-7522-8_20. [DOI] [PubMed] [Google Scholar]
- Bujakowska K., Audo I., Mohand-Saïd S., Lancelot M.-E., Antonio A., Germain A., Léveillard T., Letexier M., Saraiva J.-P., Lonjou C. CRB1 mutations in inherited retinal dystrophies. Hum. Mutat. 2012;33:306–315. doi: 10.1002/humu.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgakova N.A., Knust E. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 2009;122:2587–2596. doi: 10.1242/jcs.023648. [DOI] [PubMed] [Google Scholar]
- Chen X., Janssen J.M., Liu J., Maggio I., ’t Jong A.E.J., Mikkers H.M.M., Gonçalves M.A.F.V. In trans paired nicking triggers seamless genome editing without double-stranded DNA cutting. Nat. Commun. 2017;8:657. doi: 10.1038/s41467-017-00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Jiang C., Yang D., Sun R., Wang M., Sun H., Xu M., Zhou L., Chen M., Xie P. CRB2 mutation causes autosomal recessive retinitis pigmentosa. Exp. Eye Res. 2018;180:164–173. doi: 10.1016/j.exer.2018.12.018. [DOI] [PubMed] [Google Scholar]
- Dambrot C., Buermans H.P.J., Varga E., Kosmidis G., Langenberg K., Casini S., Elliott D.A., Dinnyes A., Atsma D.E., Mummery C.L. Strategies for rapidly mapping proviral integration sites and assessing cardiogenic potential of nascent human induced pluripotent stem cell clones. Exp. Cell Res. 2014;327:297–306. doi: 10.1016/j.yexcr.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Dudok J.J., Murtaza M., Henrique Alves C., Rashbass P., Wijnholds J. Crumbs 2 prevents cortical abnormalities in mouse dorsal telencephalon. Neurosci. Res. 2016;108:12–23. doi: 10.1016/j.neures.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Ebarasi L., Ashraf S., Bierzynska A., Gee H.Y., McCarthy H.J., Lovric S., Sadowski C.E., Pabst W., Vega-Warner V., Fang H. Defects of CRB2 cause steroid-resistant nephrotic syndrome. Am. J. Hum. Genet. 2015;96:153–161. doi: 10.1016/j.ajhg.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M., Brandstätter J.H., Regus-Leidig H. Evidence for a Clathrin-independent mode of endocytosis at a continuously active sensory synapse. Front. Cell. Neurosci. 2014;8:1–13. doi: 10.3389/fncel.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabou H., Garita-Hernandez M., Chaffiol A., Reichman S., Jaillard C., Brazhnikova E., Bertin S., Forster V., Desrosiers M., Winckler C. Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight. 2018;3:96029. doi: 10.1172/jci.insight.96029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimczak R.R., Koerber J.T., Dalkara D., Flannery J.G., Schaffer D.V. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Müller cells. PLoS One. 2009;4:e7467. doi: 10.1371/journal.pone.0007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J., Blokker J., ten Brink J.B., Unmehopa U., Fluiter K., Bergen A.A.B., Kamermans M. Ultrastructural localization and expression of TRPM1 in the human retina. Investig. Ophthalmol. Vis. Sci. 2011;52:8356–8362. doi: 10.1167/iovs.11-7575. [DOI] [PubMed] [Google Scholar]
- Lee T.C., Almeida D., Claros N., Abramson D.H., Cobrinik D. Cell cycle-specific and cell type-specific expression of Rb in the developing human retina. Investig. Ophthalmol. Vis. Sci. 2006;47:5590–5598. doi: 10.1167/iovs.06-0063. [DOI] [PubMed] [Google Scholar]
- Lemmers C., Michel D., Lane-Guermonprez L., Delgrossi M.-H., Médina E., Arsanto J.-P., Le Bivic A. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Xie B., Song X., Zheng D., He L., Li G., Gao G., Peng F., Yu M., Ge J. Self-formation of RPE spheroids facilitates enrichment and expansion of hiPSC-derived RPE generated on retinal organoid induction platform. Invest. Ophthalmol. Vis. Sci. 2018;59:5659–5669. doi: 10.1167/iovs.17-23613. [DOI] [PubMed] [Google Scholar]
- MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Reed Clark K., During M.J., Cremers F.P.M., Black G.C.M. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert S.A., Kantardzhieva A., Malysheva A., Meuleman J., Versteeg I., Levelt C., Klooster J., Geiger S., Seeliger M.W., Rashbass P. Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J. Cell Sci. 2004;117:4169–4177. doi: 10.1242/jcs.01301. [DOI] [PubMed] [Google Scholar]
- van de Pavert S.A., Meuleman J., Malysheva A., Aartsen W.M., Versteeg I., Tonagel F., Kamphuis W., McCabe C.J., Seeliger M.W., Wijnholds J. A single amino acid substitution (Cys249Trp) in Crb1 causes retinal degeneration and deregulates expression of pituitary tumor transforming gene Pttg1. J. Neurosci. 2007;27:564–573. doi: 10.1523/JNEUROSCI.3496-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Pavert S.A., Sanz A.S., Aartsen W.M., Vos R.M., Versteeg I., Beck S.C., Klooster J., Seeliger M.W., Wijnholds J. Crb1 is a determinant of retinal apical Müller glia cell features. Glia. 2007;55:1486–1497. doi: 10.1002/glia.20561. [DOI] [PubMed] [Google Scholar]
- Pearson R.A., Barber A.C., West E.L., MacLaren R.E., Duran Y., Bainbridge J.W., Sowden J.C., Ali R.R. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010;19:487–503. doi: 10.3727/096368909X486057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier L.P., Alves C.H., Quinn P.M., Vos R.M., Tanimoto N., Lundvig D.M.S., Dudok J.J., Hooibrink B., Richard F., Beck S.C. Targeted ablation of CRB1 and CRB2 in retinal progenitor cells mimics Leber congenital amaurosis. PLoS Genet. 2013;9:e1003976. doi: 10.1371/journal.pgen.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier L.P., Hoek R.M., Vos R.M., Aartsen W.M., Klimczak R.R., Hoyng S.A., Flannery J.G., Wijnholds J. Specific tools for targeting and expression in Müller glial cells. Mol. Ther. Methods Clin. Dev. 2014;1:14009. doi: 10.1038/mtm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier L.P., Lundvig D.M.S., Tanimoto N., Klooster J., Vos R.M., Richard F., Sothilingam V., Garcia Garrido M., Le Bivic A., Seeliger M.W. CRB2 acts as a modifying factor of CRB1-related retinal dystrophies in mice. Hum. Mol. Genet. 2014;23:3759–3771. doi: 10.1093/hmg/ddu089. [DOI] [PubMed] [Google Scholar]
- Pellissier L.P., Quinn P.M., Alves C.H., Vos R.M., Klooster J., Flannery J.G., Heimel J.A., Wijnholds J. Gene therapy into photoreceptors and Müller glial cells restores retinal structure and function in CRB1 retinitis pigmentosa mouse models. Hum. Mol. Genet. 2015;24:3104–3118. doi: 10.1093/hmg/ddv062. [DOI] [PubMed] [Google Scholar]
- Petit L., Ma S., Cheng S.-Y., Gao G., Punzo C. Rod outer segment development influences AAV-mediated photoreceptor transduction after subretinal injection. Hum. Gene Ther. 2017;28:464–481. doi: 10.1089/hum.2017.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis J.M., van Driel D., Billson F.A., Russell P. Development of the human retina: patterns of cell distribution and redistribution in the ganglion cell layer. J. Comp. Neurol. 1985;233:429–451. doi: 10.1002/cne.902330403. [DOI] [PubMed] [Google Scholar]
- Quinn P.M., Buck T.M., Ohonin C., Mikkers H.M.M., Wijnholds J. Production of iPS-derived human retinal organoids for use in transgene expression assays. Methods Mol. Biol. 2018;1715:261–273. doi: 10.1007/978-1-4939-7522-8_19. [DOI] [PubMed] [Google Scholar]
- Quinn P.M., Alves C.H., Klooster J., Wijnholds J. CRB2 in immature photoreceptors determines the superior-inferior symmetry of the developing retina to maintain retinal structure and function. Hum. Mol. Genet. 2018;27:3137–3153. doi: 10.1093/hmg/ddy194. [DOI] [PubMed] [Google Scholar]
- Quinn P.M., Mulder A.A., Henrique Alves C., Desrosiers M., de Vries S.I., Klooster J., Dalkara D., Koster A.J., Jost C.R., Wijnholds J. Loss of CRB2 in Müller glial cells modifies a CRB1-associated retinitis pigmentosa phenotype into a Leber congenital amaurosis phenotype. Hum. Mol. Genet. 2019;28:105–123. doi: 10.1093/hmg/ddy337. [DOI] [PubMed] [Google Scholar]
- Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I.W., Aleman T.S. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar N., Omelchenko T., Silva-Gagliardi N.F., McGlade C.J., Wijnholds J., Anderson K.V. Crumbs2 promotes cell ingression during the epithelial-to-mesenchymal transition at gastrulation. Nat. Cell Biol. 2016;18:1281–1291. doi: 10.1038/ncb3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel F.F., Peters T., Wilhelm B., Biel M., Ueffing M., Wissinger B., Bartz-Schmidt K.U., Klein R., Michalakis S., Fischer M.D. Humoral immune response after intravitreal but not after subretinal AAV8 in primates and patients. Invest. Ophthalmol. Vis. Sci. 2018;59:1910–1915. doi: 10.1167/iovs.17-22494. [DOI] [PubMed] [Google Scholar]
- Roh M.H., Makarova O., Liu C.-J., Shin K., Lee S., Laurinec S., Goyal M., Wiggins R., Margolis B. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of crumbs and discs lost. J. Cell Biol. 2002;157:161–172. doi: 10.1083/jcb.200109010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavotinek A., Kaylor J., Pierce H., Cahr M., DeWard S.J., Schneidman-Duhovny D., Alsadah A., Salem F., Schmajuk G., Mehta L. CRB2 mutations produce a phenotype resembling congenital nephrosis, Finnish type, with cerebral ventriculomegaly and raised alpha-fetoprotein. Am. J. Hum. Genet. 2015;96:162–169. doi: 10.1016/j.ajhg.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E.M., Andorf J.L., Whitmore S.S., DeLuca A.P., Giacalone J.C., Streb L.M., Braun T.A., Mullins R.F., Scheetz T.E., Sheffield V.C. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology. 2017;124:1314–1331. doi: 10.1016/j.ophtha.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surace E.M., Auricchio A., Reich S.J., Rex T., Glover E., Pineles S., Tang W., O’Connor E., Lyubarsky A., Savchenko A. Delivery of adeno-associated virus vectors to the fetal retina: impact of viral capsid proteins on retinal neuronal progenitor transduction. J. Virol. 2003;77:7957–7963. doi: 10.1128/JVI.77.14.7957-7963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talib M., van Schooneveld M.J., van Genderen M.M., Wijnholds J., Florijn R.J.R.J., Ten Brink J.B.J.B., Schalij-Delfos N.E., Dagnelie G., Cremers F.P.M., Wolterbeek R. Genotypic and phenotypic characteristics of CRB1-associated retinal dystrophies: a long-term follow-up study. Ophthalmology. 2017;124:884–895. doi: 10.1016/j.ophtha.2017.01.047. [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H., Bell P., Maguire A.M., Cearley C.N., Xiao R., Calcedo R., Wang L., Castle M.J., Maguire A.C., Grant R. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H., Bell P., Maguire A.M., Xiao R., Hopkins T.B., Grant R., Bennett J., Wilson J.M. AAV9 targets cone photoreceptors in the nonhuman primate retina. PLoS One. 2013;8:e53463. doi: 10.1371/journal.pone.0053463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley L.A., Burnight E.R., Drack A.V., Banach B.B., Ochoa D., Cranston C.M., Madumba R.A., East J.S., Mullins R.F., Stone E.M. Using patient-specific induced pluripotent stem cells and wild-type mice to develop a gene augmentation-based strategy to treat CLN3 -associated retinal degeneration. Hum. Gene Ther. 2016;27:835–846. doi: 10.1089/hum.2016.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.-H., Peters A., Park T.S., Zambidis E.T., Meyer J.S. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.