Abstract
Amyloid β (Aβ) has been reported to have an important role in the cognitive deficits of Alzheimer's disease (AD), as oligomeric Aβ promotes synaptic dysfunction and triggers neuronal death. Recent evidence has associated an endocytosis protein, endophilin 1, with AD, as endophilin 1 levels have been reported to be markedly increased in the AD brain. The increase in endophilin 1 levels in neurons is associated with an increase in the activation of the stress kinase JNK, with subsequent neuronal death. In the present study, whole-cell patch-clamp recording demonstrated that oligomeric Aβ caused synaptic dysfunction and western blotting revealed that endophilin 1 was highly expressed prior to neuronal death of cultured hippocampal neurons. Furthermore, RNA interference and electrophysiological recording techniques in cultured hippocampal neurons demonstrated that knockdown of endophilin 1 prevented synaptic dysfunction induced by Aβ. Thus, a potential role for endophilin 1 in Aβ-induced postsynaptic dysfunction has been identified, indicating a possible direction for the prevention of postsynaptic dysfunction in cognitive impairment and suggesting that endophilin may be a potential target for the clinical treatment of AD.
Keywords: amyloid β, endophilin 1, synaptic dysfunction, Alzheimer's disease
Introduction
Alzheimer's disease (AD) is a degenerative brain disease and the most common cause of dementia (1,2). At the microscopic level, the hallmarks of AD are amyloid β (Aβ) deposition-induced senile plaques (SP), abnormal accumulation of Tau protein-induced neurofibrillary tangles and extensive neuronal loss (3–5). A key observation changing the diagnostic and therapeutic approaches to AD is that the pathological changes underlying brain degeneration and cognitive loss in patients with AD begin 10–20 years prior to the onset of dementia (6,7). Furthermore, the cognitive dysfunction of AD patients may present years before the pathological changes can be detected. Studies have demonstrated that the Aβ oligomer-induced synaptic dysfunction is a major factor associated with early cognitive dysfunction in AD (8,9).
Aβ is derived from a larger protein, amyloid precursor protein (APP), and includes two main forms in vivo: Amyloid β-peptide 1–42 (Aβ1-42) and amyloid β-peptide 1–40 (Aβ1-40) (9). Of these two proteins, the proportion of Aβ1–42 is lower than that of Aβ1-40, but Aβ1–42 polymerizes more readily and is more toxic (6). Furthermore, soluble Aβ1–42 oligomers are also more neurotoxic than Aβ1–40 (10–12). Studies on mouse hippocampal neurons overexpressing the APP gene revealed that excitatory neuronal synaptic transmission was impaired, reflected in the significant attenuation of miniature excitatory postsynaptic currents (mEPSCs) (13). Synaptic dysfunction caused by Aβ oligomers is an important cause of the cognitive decline observed in AD, and synaptic dysfunction may affect synaptic plasticity events associated with learning and memory, namely long-term potentiation (LTP) and long-term depression (LTD) (14). Soluble Aβ1-42-perfused hippocampal sections and in vivo experiments demonstrated that Aβ, and its oligomers, can suppress the formation of LTP in the hippocampus (15–17) or facilitate LTD (18), potentially leading to an early decrease in cognitive function in AD. Furthermore, inhibitors of Aβ oligomers can alleviate the damage of LTP caused by Aβ (19). Therefore, identifying factors that can alleviate synaptic dysfunction is crucial for the prevention and treatment of AD.
Endophilin A, which is composed of three homologous cytosolic proteins (endophilin A1-3, also termed endophilin 1–3) is highly expressed in the nervous system. Endophilin 1 is only expressed in the brain, endophilin 2 is found in multiple tissues and endophilin 3 is predominantly expressed in the brain and testis (20,21). Endophilin A has been reported to have a key role in endocytosis, including synaptic vesicle endocytosis and member receptor endocytosis (22–25). In the study of Ren et al (26), endophilin 1 was found to be highly expressed in the brain of patients with AD and AD transgenic mice. An increase in endophilin 1 levels in neurons is associated with an increase in the activation of the stress kinase JNK, with subsequent neuronal death (26). The present study demonstrated that soluble Aβ can cause synaptic dysfunction in cultured hippocampal neurons, and endophilin 1 is highly expressed prior to the death of neurons. By coupling RNA interference and electrophysiological recording techniques in cultured hippocampal neurons, it was shown that knockdown of endophilin 1 prevents synaptic dysfunction induced by Aβ.
Materials and methods
Plasmids and RNA interference
Total RNA was extracted from the brain of 1 1-month-old male Sprague-Dawley (SD) rat (purchased from the Laboratory Animal Center of Sun Yat-Sen University and immediately sacrificed upon arrival) using an RNeasy Mini Kit (Qiagen, Inc.). The first-strand cDNA was generated using Superscript II reverse transcriptase (Thermo Fisher Scientific, Inc.) and RT was performed according to the manufacturer's protocol. Target DNA fragments were amplified by PCR using the following primers designed and synthetized according to the GenBank Database: Forward 5′-ATAGAATTCATGTCGGTGGCGGGG-3′ (containing an EcoRI target sequence) and reverse, 5′-TAAGTCGACCTAATGGGGCAGAGC-3′ (containing a SalI target sequence). PCR was conducted as follows: 95°C for 3 min, followed by 34 cycles of 95°C for 50 sec, 58°C for 50 sec and 72°C for 2 min, and finally 72°C for 15 min. The full-length endophilin 1 cDNA fragment was inserted into pEGFP-C1 plasmid (Clontech Laboratories, Inc.). The construct was verified by sequencing. The methods used for constructing cDNA plasmids were previously described in detail (27,28). Validated endophilin 1 small interfering RNA (Endo1 siRNA; 5′-GGGCTAAACTCAGTATGAT-3′) and negative control (NC; 5′-AGCTAGGCATATGACTGTA-3′) were synthesized by Shanghai GenePharma Co., Ltd.
Preparation of Aβ1–42 oligomers
Aβ1–42 (human) was purchased from Tocris Bioscience. The lyophilized powder was solubilized in 50 mM Tris Buffer to 200 µM and stored at −20°C according to the manufacturer's instructions. Prior to use, stock solutions were thawed and incubated at 37°C for 24 h to induce peptide aggregation and then diluted to the final concentration (1 or 50 µM) in culture medium as previously described (29).
Cell culture and transfection
293 cells (a gift from Dr Mingtao Li, Zhongshan School of Medicine, Sun Yat-Sen University, Guangzhou, China) culture was performed as described previously (27,28). Cells were maintained in DMEM (Gibco, Thermo Fisher Scientific, Inc.) supplied with 10% FBS (Gibco, Thermo Fisher Scientific, Inc.) and penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) in a 5% CO2 and 37°C incubator. To determine the efficacy and specificity of Endo1 siRNA, 100 pmol Endo1 siRNA or NC together with 2 µg endophilin 1-pEGFP-C1 plasmid were co-transfected into 293 cells using calcium phosphate (Beyotime Institute of Biotechnology). The calcium phosphate/DNA precipitate were maintained in the culture for 4 h and washed with fresh transfection media. Then fresh culture medium was added and further analysis was performed 24–48 h after transfection.
Western blotting
After co-transfection for 24–48 h, the 293 cells were lysed in lysis buffer [150 mM NaCl, 20 mM Tris, 1 mM EDTA, 1% Triton X-100, and protease inhibitors cocktail (Sigma-Aldrich; Merck KGaA)] on ice for 30 min. The lysates were centrifuged at 20,000 × g for 15 min at 4°C. The supernatants were then recovered and quantified using a bicinchoninic acid protein quantification assay (Pierce; Thermo Fisher Scientific, Inc.). Proteins were separated by SDS-PAGE, with ~30 µg protein loaded per lane of 10% gels, which was then electrophoretically transferred onto PVDF membranes (Pierce; Thermo Fisher Scientific, Inc.). The membranes were blocked with 5% non-fat milk in TBS and 0.1% Tween-20 at room temperature for 1 h, and then incubated with endophilin 1 antibody (cat. no. sc-10874; Santa Cruz Biotechnology, Inc.) at a dilution of 1:1,000 overnight at 4°C. After 3–4 washes with TBS and 0.1% Tween-20, the membranes were incubated with horseradish peroxidase-conjugated Donkey anti Goat IgG (H + L) secondary antibody (cat. no. AS031; ABclonal Biotech Co., Ltd.) at a dilution of 1:1,000 for 2 h at room temperature. The protein bands were detected after developing the blots using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce; Thermo Fisher Scientific, Inc.). Western blot bands were quantified by densitometric analysis using Image-Pro Plus 6.0 (Media Cybernetics, Inc.)
Hippocampal neuronal culture and transfection
Rat hippocampal neurons were cultured as described previously (24). A total of 50 postnatal SD rat pups (days 0 to 1) were purchased from the Laboratory Animal Center of Sun Yat-Sen University and immediately sacrificed upon arrival. Hippocampi were dissected from SD rat pups, and dissociated hippocampal neurons were obtained using 0.125% trypsin at 37°C for 10 min and plated at a density of 1×104 cells/cm2 onto poly-D-lysine coated glass coverslips. Cultures were maintained in Neurobasal A medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 2% B27 (Invitrogen; Thermo Fisher Scientific, Inc.) and 0.5 mM glutamine supplement (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2 humidified incubator, and half of the culture media was replaced every 3 days. The neurons were cultured in vitro in 24-well culture plates for 8–10 days prior to transfection. To transfect neurons in 24-well tissue culture plates, 100 pmol of Endo1 siRNA or its control was combined with 37 µl of 2 M CaCl2 solution in sterile, deionized water to a final volume of 300 µl, and then mixed well with 300 µl of 2X HEPES-buffered saline. The mixtures were vortexed and incubated at room temperature for ~4 min. In each well, 30 µl mixture was added drop-wise to the cells. Additionally, 1 µg/well GFP plasmid was co-transfected with the siRNA to mark the transfected cells. The calcium phosphate/DNA precipitate were maintained in the culture for 40 min and washed with fresh transfection media twice. At 24–48 h after transfection, 1 µM Aβ1–42 oligomer was added to the culture medium.
Fluorescence immunostaining
Three days after Endo1 siRNA transfection, the hippocampal neurons were fixed with 4% paraformaldehyde at room temperature for 1 h (Sigma-Aldrich; Merck KGaA). Immunostaining was then performed using a standard protocol, as described previously (26). The primary anti-endophilin 1 antibody (cat no. sc-10874; Santa Cruz Biotechnology, Inc.) was used at a dilution of 1:100, and Alexa Fluor® 594 AffiniPure Donkey anti-Goat IgG (H + L; cat no. 705-585-147; Jackson ImmunoResearch Laboratories, Inc.) was used at a dilution of 1:500. After staining, the cells were mounted on glass slides using Fluoro Gel II with DAPI (Electron Microscopy Sciences) and imaged with a Carl Zeiss LSM 710 confocal microscope (Carl Zeiss AG). Images were acquired with the same optical slice thickness in every channel using a 63× oil objective and a resolution of 1,024×1,024 pixels. The RNA interference efficiency in hippocampal neurons was determined by calculating the percentage of endophilin 1-positive cells, as previously described (30).
Determination of neuronal survival rate
To assess the survival rate of hippocampal neuronal, hippocampal neurons were exposed to 0, 1 and 50 µM Aβ. At 24 h after exposure, the hippocampal neurons were fixed with 4% paraformaldehyde at room temperature for 1 h (Sigma-Aldrich; Merck KGaA) and nuclear staining with DAPI was performed as described above. After staining, the cells were mounted on glass slides using Fluoro Gel II without DAPI (Electron Microscopy Sciences) and imaged with Nikon Eclipse Ti-S fluorescence microscope (Nikon Corporation). The number of live cells (as assessed by DAPI staining; small bright blue nuclei indicated dead cells) per coverslip at the completion of the experiment was counted. As a small number of glial cells are mixed in the cultured neurons, only the death of neurons was counted by also viewing cells under bright field microscopy. The neuronal survival rate was calculated as the number of living cells divided the total number of neurons. The experiment was performed five times with three fields analyzed per view.
Electrophysiology
Whole-cell patch-clamp recordings of mEPSCs were obtained from transfected cultured hippocampal neurons treated with Aβ oligomers on 8–12 days in vitro (DIV). During the recordings, the cells were bathed in an external solution (pH 7.3) containing 128 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 15 mM glucose, 20 mM HEPES, 1 mM tetrodotoxin, and 100 µM picrotoxin. Recording pipettes were filled with the intracellular solution containing 147 mM KCl, 5 mM Na2-phosphocreatine, 2 mM EGTA, 10 mM HEPES, 2 mM MgATP and 0.3 mM Na2GTP. Recordings were performed at room temperature in voltage clamp mode, at a holding potential of −70 mV, using a Multiclamp 700 B amplifier (Molecular Devices, LLC) and Clampex 10.5 software (Axon Instruments; Molecular Devices, LLC). The series resistance was <30 MΩ, and data were acquired at 10 kHz and filtered at 1 kHz. mEPSCs were analyzed using MiniAnalysis software (Synaptosoft, Inc.), and the experiments were repeated at least three times.
Statistical analysis
Data are presented as the mean ± standard error. The statistical significance of the differences between two groups was analyzed using Student's t-test and comparisons between more than two groups were performed using one-way analysis of variance with Newman-Keuls post hoc tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Oligomeric Aβ causes synaptic dysfunction in cultured hippocampal neurons
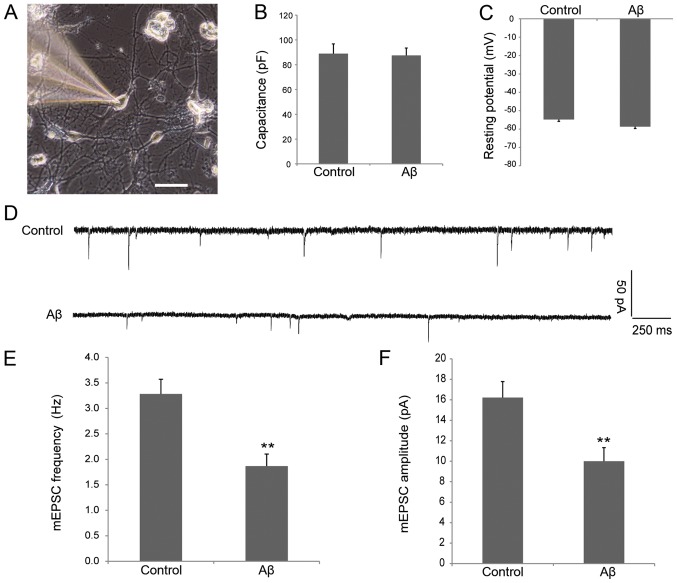
Data from several studies has demonstrated that Aβ causes synaptic dysfunction. For example, overexpression of APP in hippocampal neurons depresses excitatory transmission (13). In addition, when brain slices were incubated with 1 µM Aβ1–42 oligomers, the frequency and amplitude of mEPSCs were markedly reduced (13,16). To determine whether oligomeric Aβ can cause synaptic dysfunction in cultured hippocampal neurons, cultured DIV8 hippocampal neurons were incubated with 1 µM Aβ1–42 oligomers or control solvent for 24 h, then mEPSCs were recorded using the whole-cell patch-clamp technique. The neurons selected for electrophysiological examination in the two groups are shown in Fig. 1A. The cells were healthy and exhibited similar membrane capacitance (88.86±7.94 vs. 87.44±5.99 pF; Fig. 1B) and resting potential (−54.8±2.06 vs. −58.7±1.83 mV; Fig. 1C) in control and Aβ groups, indicating that soluble Aβ does not affect the intrinsic electrophysiological properties of neurons. As shown in Fig. 1D-F, the frequency (3.28±0.29 vs. 1.87±0.24 Hz; P<0.01; Fig. 1E) and amplitude (16.22±1.56 vs. 10.00±1.32 pA; P<0.01; Fig. 1F) of the mEPSCs in neurons processed with oligomeric Aβ decreased significantly when compared with the control group. These data suggest that oligomeric Aβ can cause synaptic dysfunction in cultured hippocampal neurons.
Figure 1.
Electrophysiology of cultured hippocampal neurons treated with oligomeric 1 µM Aβ. (A) Image of a neuron obtained from patch recording captured using an inverted fluorescence microscope. Scale bar, 50 µm. Bar plots represent the mean values of resting (B) capacitance and (C) membrane potentials of patched neurons in the control and oligomeric Aβ-processed groups. n=10 cells per group. (D) mEPSC tracings are shown for control and oligomeric Aβ-treated neurons. (E) mEPSC frequency in control and oligomeric Aβ-treated neurons. (F) mEPSC amplitude in control and oligomeric Aβ-treated neurons. n=16 cells per group, **P<0.01 vs. control. Aβ, amyloid β; mEPSC, miniature excitatory postsynaptic current.
Endophilin 1 is highly expressed during synaptic dysfunction induced by Aβ
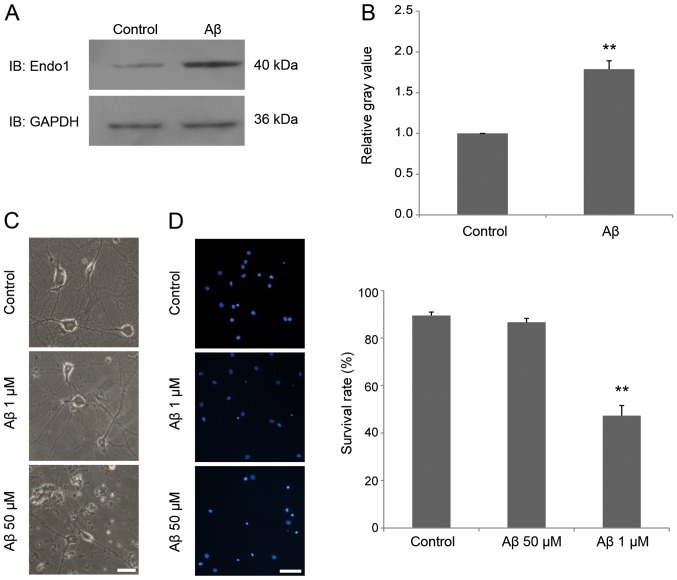
It was previously demonstrated that endophilin 1 is highly expressed in patients with AD patients and an AD mouse model (26). To investigate whether the expression of endophilin 1 is also altered in Aβ-treated neurons, cultured mature neurons were incubated in 1 µM Aβ1–42 for 24 h, and then the expression of endophilin 1 was detected by western blot analysis. The results demonstrated that the expression of endophilin 1 in the Aβ-treated group was significantly higher than in the control group (P<0.01; Fig. 2A and B). Furthermore, to assess whether 1 µM Aβ1–42 induced the death of neurons, hippocampal neurons were treated with 0, 1 and 50 µM oligomeric Aβ1–42 for 24 h. As shown in Fig. 2C, neurons treated with 1 µM Aβ1–42 were healthy, as in the control group, whereas neurons treated with 50 µM Aβ1–42 exhibited broken cell bodies and collapsed protrusions. DAPI staining further confirmed that there was no neuronal death in the group treated with 1 µM Aβ1–42 for 24 h (Fig. 2D). These results indicate that oligomeric Aβ promotes endophilin 1 expression before it causes neuronal death.
Figure 2.
Increased expression of endophilin 1 in neuronal synaptic dysfunction induced by oligomeric Aβ. (A) Hippocampal neurons were treated with Aβ. After 48 h, endophilin 1 and GAPDH were probed using the designated antibodies. (B) Relative expression of endophilin 1 in the two groups n=4. (C) Photomicrographs illustrating hippocampal neurons treated with 0, 1 and 50 µM oligomeric Aβ. Scale bar, 25 µm. (D) DAPI staining assay showing survival of hippocampal neurons treated with 0, 1 and 50 µM oligomeric Aβ. Scale bar, 50 µm. n=5 per group. **P<0.01 vs. control. Aβ, amyloid β; IB, immunoblot; Endo 1, endophilin 1.
Interfering with the expression of endophilin 1 attenuates the synaptic dysfunction caused by Aβ
To elucidate whether endophilin 1 is involved in Aβ-induced synaptic dysfunction, RNA interference and electrophysiological recording techniques were performed using cultured hippocampal neurons.
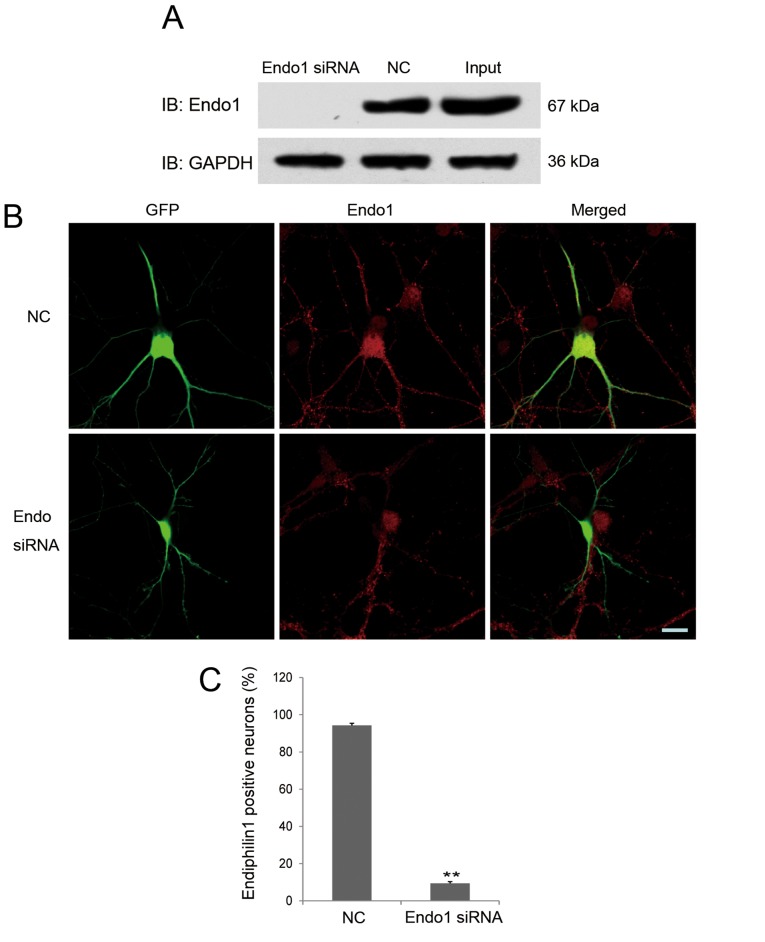
An siRNA targeting endophilin 1 was designed, and its interference efficacy was determined by immunoblotting through transfecting Endo1 siRNA or NC together with endophilin1-pEGFP-C1 plasmids into 293 cells. As shown in Fig. 3A, endophilin 1 expression was barely detectable in 293 cells co-transfected with the Endo1 siRNA, indicating that the interference fragment was effective.
Figure 3.
Verification of the efficacy of endophilin 1 siRNA. (A) 293 cells were co-transfected with endophilin 1-pEGFP-C1 plasmids and Endo1 siRNA or NC. After 48 h, endophilin 1 and GAPDH as a control in the cell lysates were probed by the designated antibodies. (B) Images of neurons co-transfected with green fluorescent protein and NC or Endo1 siRNA. Cultures were fixed after transfection for 2–3 days and stained using endophilin 1 antibody (red). Scale bar, 20 µm. (C) Percentage of endophilin 1-positive neurons in the two groups. n=4, **P<0.01. Endo1, endophilin 1; siRNA, small interfering RNA; NC, negative control; IB, immunoblot; GFP, green fluorescent protein; Input, endophilin 1-pEGFP-C1 plasmid transfection only.
The effectiveness of Endo1 siRNA in cultured hippocampal neurons was also confirmed. As shown in Fig. 3B, compared with NC, the expression of endogenous endophilin 1 was markedly knocked down in Endo1 siRNA-transfected neurons. As shown in Fig. 3B, endophilin 1 was expressed in both hippocampal neurons transfected with NC (green and red co-localized fluorescence) as well as untransfected neurons (red fluorescence only), and red fluorescence was evenly distributed in the cell body and processes of each neuron. However, invisible or very weak red fluorescence was distributed in the cell body of neurons transfected with Endo1 siRNA (identified by GFP fluorescence). These results indicate that the expression of endogenous endophilin 1 was reduced by the specific siRNA. To further elucidate the effect of Endo1 siRNA on endogenous endophilin 1, the experiment was repeated four times and the number of endophilin 1-positive neurons in the NC and Endo1 siRNA groups were counted; the result demonstrated that 9.47±0.91% neurons transfected with Endo1 siRNA were positive for endophilin 1 staining, while 94.24±1.19% neurons transfected with NC were positive for endophilin 1 staining. These results indicate that Endo1 siRNA effectively reduced the expression of endogenous endophilin 1 in cultured hippocampal neurons. This result was consistent with previous validation results for subtype-specific siRNAs targeting endophilin (30).
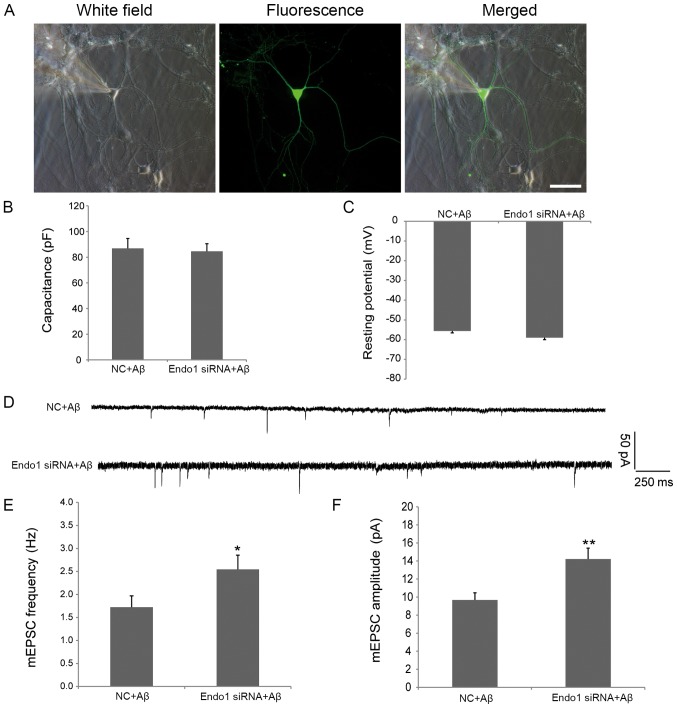
Finally, to explore the role of endophilin 1 in Aβ-induced synaptic dysfunction, cultured DIV8 hippocampal neurons were transfected with NC or Endo1 siRNAs for 48 h and incubated with 1 µM Aβ1–42 oligomers for a further 24 h. Subsequently, the mEPSCs of neurons transfected with NC and Endo1 siRNA were measured using electrophysiological recording techniques. As shown in Fig. 4A, healthy cells with the same membrane capacitance and resting potential were selected to determine the intrinsic electrophysiological properties of transfected neurons (Fig. 4B and C). As shown in Fig. 1, the administration of oligomeric Aβ reduced both the frequency and amplitude of mEPSCs. When endophilin 1 was silenced in cells treated with Aβ, the frequency (2.55±0.31 vs. 1.72±0.25 Hz; P<0.05; Fig. 4E) and amplitude (14.22±1.21 vs. 9.68±0.79 pA; P<0.01; Fig. 4F) of mEPSCs were increased significantly compared with the NC group. Of note, in the absence of Aβ, there were no significant differences between the frequency or amplitude of mEPSCs in NC- and Endo1 siRNA-transfected cells (data not shown), indicating that the effects of knockdown of endophilin1 on synaptic function were dependent on the presence of Aβ. Therefore, the results suggested that knockdown of endophilin 1 expression can reduce oligomeric Aβ-induced synaptic dysfunction.
Figure 4.
Electrophysiology of endophilin 1 knockdown neurons processed by oligomeric Aβ. (A) Image of a neuron obtained from patch recording. Scale bar, 50 µm. Mean values of (B) capacitance and (C) resting membrane potentials of patched neurons in NC + Aβ and Endo1 siRNA + Aβ groups. n=10 cells per group. (D) mEPSC tracings from NC + Aβ and Endo1 siRNA + Aβ groups. (E) mEPSC frequency and (F) amplitude in NC + Aβ and Endo1 siRNA + Aβ group, n=16 cells per group, *P<0.05, **P<0.01 vs. NC + Aβ. NC, negative control; Aβ, amyloid β; Endo1, endophilin 1; siRNA, small interfering RNA; mEPSC, miniature excitatory postsynaptic current.
Discussion
Accumulating evidence indicates that oligomeric Aβ has an important role in the cognitive impairment of patients with AD (3,4). During the early stages of AD, before a large number of neurons are lost, oligomeric Aβ can induce synaptic dysfunction, which is an important cause of cognitive decline in patients with AD (6). Therefore, using various methods to prevent synaptic damage and rescuing damaged synapses may help prevent AD-related cognitive impairment, and improve learning and memory ability.
Synaptic disorders include changes in synaptic numbers, abnormal synaptic transmission and synaptic plasticity. The present study demonstrated that 1 µM Aβ1–42 can cause synaptic dysfunction in cultured hippocampal neurons, which is manifested by a decrease in the frequency and amplitude of mEPSCs, which is consistent with the results of a previous study on APP overexpression in mouse hippocampal neurons (13). Alteration of mEPSCs is closely associated with the inhibition of presynaptic vesicle transport and postsynaptic glutamate receptor transport induced by Aβ (13). The effect of Aβ on postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors has a more important role (13). It has been demonstrated that Aβ can inhibit the induction and maintenance of LTP by directly or indirectly interfering with the insertion and localization of AMPA receptors in the postsynaptic membrane (17,31). Furthermore, Aβ can enhance LTD by promoting endocytosis and enzymatic hydrolysis of AMPA receptors (18,32). The frequency and amplitude of mEPSCs mediated by AMPA receptors in hippocampal sections was reported to be markedly attenuated following perfusion with 1 µM Aβ1–42 (12).
Endophilin is an important contributor to clathrin-dependent endocytosis, and includes three subtypes, endophilins 1–3 (20,21). Among the three endophilin subtypes, which exhibit high homology, endophilin 1 is specifically expressed in the central nervous system and distributed in neuronal cell bodies and at synaptic sites (21,25). Previous studies have demonstrated that endophilin 1 has an important role in the regulation of synaptic vesicle endocytosis (30,33). Knockdown or mutation of endophilin 1 inhibits neuronal synaptic vesicle endocytosis, resulting in synaptic transmission disorders (30,33). In recent years, Ren et al (26) reported that endophilin 1 is highly expressed in patients with AD and AD transgenic mice. The increase in endophilin 1 levels in neurons is associated with an increase in the activation of the stress kinase JNK, with subsequent neuronal death (26). The present study demonstrated that endophilin 1 is highly expressed when synaptic dysfunction occurs. We hypothesize that synaptic dysfunction occurs earlier than neuronal death, because endophilin 1 is mainly distributed at the synaptic site (25). The initial increase in endophilin 1 caused by Aβ may induce synaptic transmission disorder via regulation of postsynaptic receptors. With the increased neurotoxicity, the increasing endophilin 1 level will alter JNK activation, with the subsequent death of neurons. However, the more detailed mechanisms via which endophilin 1 causes synaptic inhibition and neuronal death require further research.
Endophilin has been reported to have an important role in endocytosis of postsynaptic AMPA receptor (25). AMPA receptor endocytosis is mainly achieved via a clathrin-dependent pathway (34). Two subtypes of endophilin A (endophilin 2 and endophilin 3) have been demonstrated to be involved in AMPA receptor endocytosis mediated by the immediate early gene activity regulated cytoskeleton associated protein (Arc/Arg3.1). Endocytosis proteins, such as endophilin 2/3 and dynamin, form complexes with Arc/Arg3.1 to mediate AMPA receptor endocytosis (25). However, none of these proteins were shown to have a direct interaction with AMPA receptor (25). It was subsequently demonstrated that endophilin 2 interacts with glutamate ionotropic receptor AMPA type subunit 1, which is one of the subunits of the AMPA receptor, to mediate AMPA receptor endocytosis (35). In addition, endophilin 1 and endophilin 2 are predominantly found as stable dimers interacting via a coiled-coil domain in their conserved NH2-terminal moiety (36), and they have similar effects on calcium-dependent interactions with other proteins and synaptic vesicle endocytosis (30). Therefore, it was hypothesized that endophilin 1, which has a similar function to endophilin 2, may be involved in Aβ-mediated AMPA receptor endocytosis. Endophilin B also has been reported be associated with AD; however, unlike endophilin 1, loss of the endophilin B1 subunit exacerbates AD pathology. In mouse primary cortical neuron cultures, overexpression of the neuron-specific endophilin B1 isoform protected against Aβ-induced apoptosis and mitochondrial dysfunction (37). These studies suggest that isoform specificity results in different roles within the mechanism of AD. The current study demonstrated the effect of endophilin 1 knockdown on the frequency and amplitude of mEPSCs in cultured neurons incubated with Aβ, and concluded that knockdown of endophilin 1 prevents synaptic dysfunction induced by Aβ. However, further research is required to determine whether this process is due to inhibition of AMPA receptor endocytosis.
In summary, the present study revealed that silencing of endophilin 1 expression alleviates oligomeric Aβ-mediated synaptic dysfunction. These findings may provide experimental evidence for identifying targets for AD prevention and treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 31300885, 81571191 and 81771144), the Natural Science Foundation of Guangdong Province, China (grant no. 2017B030311002), the Science and Technology Planning Project of Guangdong Province (grant no. 2013B021800036), the Medical Research Foundation of Guangdong Province, China (grant no. A2017154) and the Guangzhou Institute of Pediatrics/Guangzhou Women and Children's Medical Center (grant no. IP-2018-010).
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YY, CC, JZ and GG conceived and designed the study. YY and CC performed the experiments on hippocampal neuronal culture, whole-cell patch-clamp recordings and analyzed the data. FW, JL, SL and XZ performed the western blotting, fluorescence immunostaining, 293 cell culture and transfection. JZ drafted the manuscript. GG reviewed and edited the manuscript. All the authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
All animal procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (38). The protocol was approved by the Institutional Animal Care and Use Committee at Jinan University. All efforts were made to minimize suffering and the number of animals used.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.delEtoile J, Adeli H. Graph theory and brain connectivity in Alzheimer's disease. Neuroscientist. 2017;23:616–626. doi: 10.1177/1073858417702621. [DOI] [PubMed] [Google Scholar]
- 2.Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Wärmländer SK, Graslund A, Abrahams JP. Cross-interactions between the alzheimer disease amyloid-β peptide and other amyloid proteins: A further aspect of the amyloid cascade hypothesis. J Biol Chem. 2016;291:16485–16493. doi: 10.1074/jbc.R116.714576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamasbi E, Wade JD, Separovic F, Hossain MA. Amyloid Beta (Aβ) Peptide and factors that play important roles in Alzheimer's disease. Curr Med Chem. 2016;23:884–892. doi: 10.2174/0929867323666160229113911. [DOI] [PubMed] [Google Scholar]
- 5.Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB. Brain atrophy in Alzheimer's disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: The challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parameshwaran K, Sims C, Kanju P, Vaithianathan T, Shonesy BC, Dhanasekaran M, Bahr BA, Suppiramaniam V. Amyloid beta-peptide Abeta(1–42) but not Abeta(1–40) attenuates synaptic AMPA receptor function. Synapse. 2007;61:367–374. doi: 10.1002/syn.20386. [DOI] [PubMed] [Google Scholar]
- 13.Ting JT, Kelley BG, Lambert TJ, Cook DG, Sullivan JM. Amyloid precursor protein overexpression depresses excitatory transmission through both presynaptic and postsynaptic mechanisms. Proc Natl Acad Sci USA. 2007;104:353–358. doi: 10.1073/pnas.0608807104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer's disease: An emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Y, Zhao D, Xie CW. Neurotrophins enhance CaMKII activity and rescue amyloid-β-induced deficits in hippocampal synaptic plasticity. J Alzheimers Dis. 2010;21:823–831. doi: 10.3233/JAD-2010-100264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid AW, Freir DB, Herron CE. Inhibition of LTP in vivo by beta-amyloid peptide in different conformational states. Brain Res. 2008;1197:135–142. doi: 10.1016/j.brainres.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 17.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, Hartley DM, Selkoe DJ. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural A beta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringstad N, Nemoto Y, De Camilli P. The SH3p4/Sh3p8/SH3p13 protein family: Binding partners for synaptojanin and dynamin via a Grb2-like Src homology 3 domain. Proc Natl Acad Sci USA. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giachino C, Lantelme E, Lanzetti L, Saccone S, Bella Valle G, Migone N. A novel SH3-containing human gene family preferentially expressed in the central nervous system. Genomics. 1997;41:427–434. doi: 10.1006/geno.1997.4645. [DOI] [PubMed] [Google Scholar]
- 22.Gad H, Ringstad N, Löw P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman MH, et al. Fission and uncoating of synaptic clathrin-coated vesicles are perturbed by disruption of interactions with the SH3 domain of endophilin. Neuron. 2000;27:301–312. doi: 10.1016/S0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 23.Huttner WB, Schmidt A. Lipids, lipid modification and lipid-protein interaction in membrane budding and fission-insights from the roles of endophilin A1 and synaptophysin in synaptic vesicle endocytosis. Curr Opin Neurobiol. 2000;10:543–551. doi: 10.1016/S0959-4388(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 24.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camilli P. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y, Xu HW, Davey F, Taylor M, Aiton J, Coote P, Fang F, Yao J, Chen D, Chen JX, et al. Endophilin I expression is increased in the brains of Alzheimer disease patients. J Biol Chem. 2008;283:5685–5691. doi: 10.1074/jbc.M707932200. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Fan J, Tian Q, Song Z, Zhang J, Chen Y. Characterization of two distinct modes of endophilin in clathrin-mediated endocytosis. Cell Signal. 2012;24:2043–2050. doi: 10.1016/j.cellsig.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Tian Q, Zhang J, Fan J, Song Z, Chen Y. Endophilin isoforms have distinct characteristics in interactions with N-type Ca2+ channels and dynamin I. Neurosci Bull. 2012;28:483–492. doi: 10.1007/s12264-012-1257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J. Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β. Neurobiol Aging. 2013;34:226–237. doi: 10.1016/j.neurobiolaging.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Tan M, Yin Y, Ren B, Jiang N, Guo G, Chen Y. Distinct functions of endophilin isoforms in synaptic vesicle endocytosis. Neural Plast. 2015;2015:371496. doi: 10.1155/2015/371496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/S0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- 34.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Yin Y, Ji Z, Cai Z, Zhao B, Li J, Tan M, Guo G. Endophilin2 Interacts with GluA1 to Mediate AMPA receptor endocytosis induced by oligomeric amyloid-β. Neural Plast. 2017;2017:8197085. doi: 10.1155/2017/8197085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringstad N, Nemoto Y, De Camilli P. Differential expression of endophilin 1 and 2 dimers at central nervous system synapses. J Biol Chem. 2001;276:40424–40430. doi: 10.1074/jbc.M106338200. [DOI] [PubMed] [Google Scholar]
- 37.Wang DB, Kinoshita Y, Kinoshita C, Uo T, Sopher BL, Cudaback E, Keene CD, Bilousova T, Gylys K, Case A, et al. Loss of endophilin-B1 exacerbates Alzheimer's disease pathology. Brain. 2015;138:2005–2019. doi: 10.1093/brain/awv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Washington (DC): National Academies Press (US); 2011. Care NRCU, Animals AUOL. Guide for the care use of laboratory animals. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.