Abstract
Context/Objective: For persons with spinal cord injury, spasticity commonly interferes with activities of daily living such as transfers. Electromyography can be used to objectively measure muscle spasms during transfers, but how electromyographic measures relate to the impact spasticity has on life, or to clinically-rated spasticity, is unclear. We aimed to characterize relationships among spasm duration and magnitude, impact of spasticity on daily life, and a clinical measure of extensor spasticity, as well as to determine reliability of the electromyographic measures.
Design: Participants (N=19) underwent electromyographic measurements of involuntary muscle activity (spasm duration and magnitude) evoked in quadriceps muscles during transfers on two days. Impact of spasticity on daily life was measured with the Spinal Cord Injury Spasticity Evaluation Tool. Clinically-rated spasticity severity was measured with the Spinal Cord Assessment Tool for Spastic reflexes.
Results: No significant associations were found between impact of spasticity and spasm duration, spasm magnitude, or clinical extensor spasticity score. Absolute and normalized spasm duration were positively associated with clinical extensor spasticity score (rho=0.510-0.667, P < 0.05). Spasm measures during transfers had good to excellent day-to-day reliability (rho=0.656-0.846, P < 0.05).
Conclusions: Electromyographic and clinical measures of involuntary activity in the lower extremity do not significantly relate to perceived impact of spasticity on daily life. However, quadriceps spasm duration during transfers is related to clinically-rated extensor spasticity. Electromyography is a reliable method of quantifying quadriceps spasms during transfers. Future investigations should identify factors that influence the impact of spasticity on life, which may help direct treatment strategies to reduce problematic impact.
Keywords: Seated transfers, Electromyography, Spinal cord assessment tool for spastic reflexes, Spinal cord injury spasticity evaluation tool, Quadriceps spasms
Introduction
Spasticity develops in approximately 60–80% of individuals after a spinal cord injury (SCI) and is considered problematic by many of those who experience it.1–4 Spasticity has been defined as “disordered sensori-motor control, resulting from an upper motoneuron lesion, presenting as intermittent or sustained involuntary activation of muscles.”5–7 This definition encompasses clinical signs of spasticity after SCI, including increased tone or stiffness and muscle spasms in response to proprioceptive or exteroreceptive inputs. While spasticity commonly interferes with activities of daily living (ADLs),2,8–11 some individuals find spasticity helpful during specific ADLs (e.g. during transfers,8,11 likely by providing postural stability or body weight support) or as a signal of problems in insensate parts of the body.2,8,9,11–13 One of the most common ADLs with which spasticity interferes is transfer between seating surfaces,8,9 a task that is performed an average of 14 times per day14 and that is critical for independence after SCI.15 Extensor spasms in the lower extremity (knee extension, plantar flexion, and activation of muscles at the hip)9,16–19 is the most common presentation of spasticity after SCI9 and is reported to interfere with transfers in particular8,9 as well as other ADLs.2,8–10
Many researchers have advocated for spasticity research to focus on its problematic aspects,8,11,20,21 however few physiological measures of spasticity that are relevant to ADLs exist, especially for non-ambulatory individuals. A small number of studies19,22–24 have used electromyographic (EMG) recordings from leg muscles paralyzed by SCI to measure involuntary muscle spasms during ADLs. EMG and other measures of involuntary muscle activity fall within the physiological or “body functions” domain of the World Health Organization’s International Classification of Functioning in Disability and Health (ICF).25 The ICF model provides a biopsychosocial framework for understanding and treating health conditions. Characterizing the relationships between body functions and the impacts of spasticity on activities and participation may help identify physiological factors that influence the problematic impact of spasticity on life.
In a recent study by Mayo et al.19 in which EMG was recorded during seated transfers, the authors found associations between the duration and time of agonist-antagonist muscle coactivity and self-reported spasm frequency, as well as the intensity of agonist-antagonist coactivity and self-reported spasm severity. These associations support the utility of EMG measurement during transfers as an objective measure relevant to the subjective experience of spasms. However, EMG measures of spasms during transfers have not been compared with a comprehensive measure that reflects the impact of spasticity on ADLs. We focused on an extensor muscle group (quadriceps) during an ADL (seated transfers) with which extensor spasticity often interferes.8,9 We hypothesized that the duration and magnitude of spasms evoked in a quadriceps muscle during transfers would be strongly associated with more problematic impact of spasticity on transfers and ADLs in general.
Additionally, the day-to-day reliability of EMG recordings during transfers have not yet been evaluated, nor has this measure been compared with a clinical test of spasticity severity. Good day-to-day reliability for EMG measures and a strong relationship between EMG during transfers and clinical spasticity tests would further support the value of EMG recorded during transfers as a tool in a multimodal assessment of spasticity.
Methods
Study design
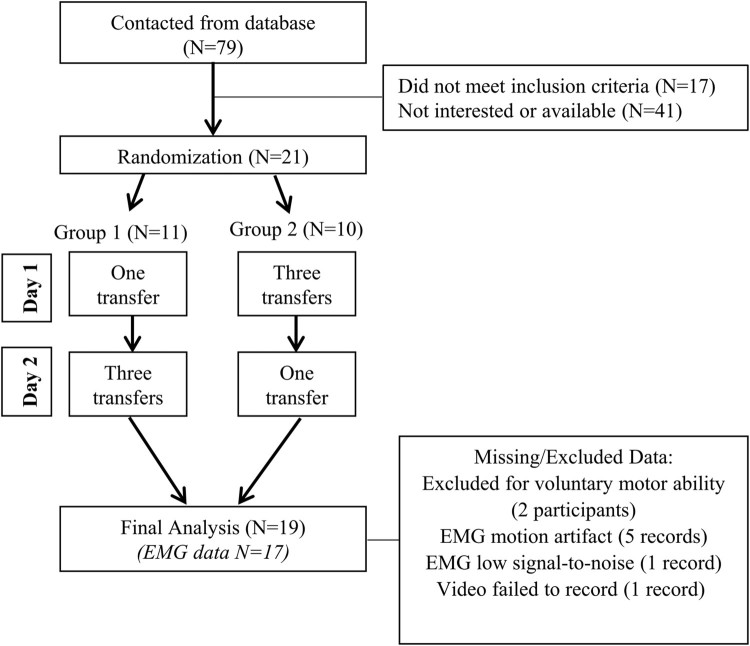
Non-ambulatory participants with SCI and spasticity performed seated pivot transfers on two separate days while spasms (involuntary muscle contractions) were recorded using EMG from a quadriceps muscle. On separate days, participants performed transfers once or three times (Fig. 1). Even though individuals do not perform multiple transfers in succession during ADLs, repeating three trials for a measure is common (e.g. the pendulum test) and allowed us to quantify the stability of repeat measurements. The number of transfers performed on each day was counterbalanced into two groups to prevent potential order effects, and participants were assigned to the groups using a randomization generator.26 Intra-test periods spanned between one and 10 days.
Figure 1.
Study design and participant exclusions.
Participants
Male and female volunteers between the ages of 18 and 65 with chronic SCI (>one year) were recruited over 20 months from The Miami Project’s SCI research volunteer database. Participants had to exhibit knee extensor spasms, either after imposed knee and hip extension during the SCATS test27 or by experimenter observance of extensor spasms evoked by position change. One participant who scored 0 on the SCATS extensor score had visually observable quadriceps spasms invoked by positional change. Potential participants were excluded if they needed lifting assistance to complete seated transfers. Participants were excluded from analysis if they had evidence of voluntary quadriceps function, defined as the ability to elicit any increase in EMG amplitude during intentional voluntary contraction of the quadriceps.
The study was performed at The Miami Project to Cure Paralysis and was approved by the Institutional Review Board of the University of Miami Miller School of Medicine. The investigation was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from each participant.
Measures
Demographic information, injury characteristics and medication information were collected by both examination and interview. The data presented in this article are part of a larger data set.
Transfers
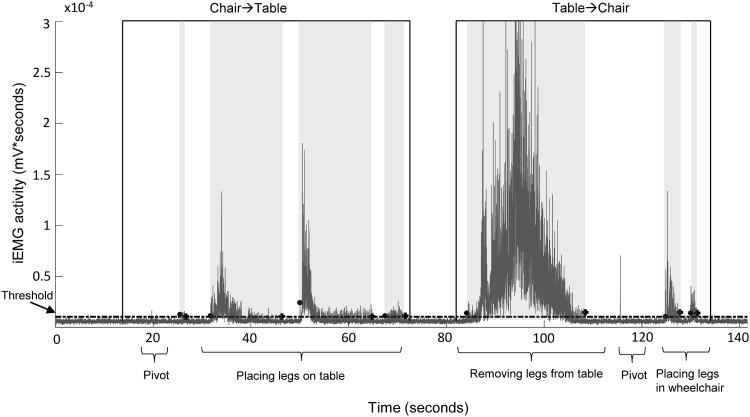
Each participant was given standardized instructions to perform seated pivot transfers between his/her wheelchair and a mat table adjusted to the participant’s preferred height. Participants used their upper extremities to place their feet on the floor and to push from the seating surface and swing their body to the adjacent mat table. Participants then used their upper extremities to place their lower extremities onto and off of the mat table. For data analysis, a single transfer was defined as the movement from wheelchair to the mat table (with legs on table) and then back to the wheelchair (see representative EMG trace in Fig. 2). Transfer duration was defined as the time to complete a single transfer. On the day of three successive transfers, participants rested less than one minute between transfers.
Figure 2.
EMG during transfer. Example record of filtered, rectified, and integrated EMG activity in vastus lateralis during a seated transfer, from wheelchair to mat table and back to wheelchair. Spasms were often evoked when a participant removed his/her legs from the mat table, however, the timing of spasm occurrence was unique to each individual. Individual spasms are highlighted with light gray background for clarity. • Spasm start, + Spasm end. Dotted line represents threshold value to separate EMG from baseline noise.
EMG recordings of spasms
We recorded spasms in the quadriceps, a muscle group involved in extending the knee during extensor spasticity. We chose to record from vastus lateralis based on superior signal quality and innervation zone uniformity compared to rectus femoris.28 To record surface EMG from vastus lateralis, a preamplifier with electrodes (Motion Lab Systems Z03, Baton Rouge, LA, USA) was placed on the vastus lateralis of the leg with the greater SCATS extensor score. The distal electrode was placed approximately 15 cm proximal to the patella at a 20° angle from midline and affixed with medical tape. A 3 cm diameter ground electrode was positioned on the anterior thigh. The electrodes were placed in the same locations on both testing days.
EMG data were sampled at 2000Hz with a Power 1401 interface, DC offset-corrected, and filtered from 30-500Hz using Spike2 software (Cambridge Electronic Design, Cambridge UK). EMG recordings were time-synchronized with video for post hoc evaluation of pivot transfer start/end times and for quality control.
Maximal compound muscle action potentials (M-Max)
M-max was used to normalize EMG amplitude during spasms across our participants. Normalization reconciles biological differences (such as differences in subcutaneous fat) and experimental differences (such as different electrode spacing or placement on muscle) across participants. The M-max of vastus lateralis was elicited by electrical stimulation of the femoral nerve with a DS7A stimulator (Digitimer Ltd, Welwyn Garden City, UK).29 The cathode was placed in the femoral triangle at the site that achieved the greatest evoked EMG at the lowest intensity.30 The current intensity was increased in a step-wise manner until the maximal M-wave amplitude was stable for five pulses (i.e. no further increase in amplitude despite increasing stimulus intensity).
Impact of spasticity on ADLs
Impact of spasticity on daily life was measured with the SCI-Spasticity Evaluation Tool (SCI-SET).13 Participants rated the impact of spasticity from -3 (extremely problematic) to +3 (extremely helpful) on 35 items related to ADLs. The SCI-SET demonstrates internal consistency, test-retest reliability, face validity and construct validity13 and has been recommended based on these psychometric properties to be useful for assessing the impact of spasticity on daily life in people with SCI.31,32 Responses are averaged to provide a measure of the overall impact of spasticity on ADLs for an individual. Correlations were performed separately for participants with SCI-SET scores in the “problematic” and “neutral” range (≤0), from those in the “helpful” range (>0).
Clinical spasticity severity
The Spinal Cord Assessment Tool for Spastic reflexes (SCATS)27 is a clinical spasticity measure with adequate construct validity and inter-rater reliability. Unlike the modified Ashworth scale, it is designed to measure three presentations of spasticity typical in SCI: evoked extensor spasticity, evoked flexor spasticity, and evoked clonus. A trained evaluator applies a stimulus (passive muscle stretch or pinprick) and then scores the response from 0-3 based on the test criteria. For comparisons with vastus lateralis EMG, the SCATS extensor spasticity score for the corresponding leg was utilized throughout this study. The evaluator of the SCATS score was blinded to all of the other assessments, which were performed by a different investigator.
Data analysis
Spasm analysis
All EMG acquired during transfers was considered involuntary, as participants had no perceived control of vastus lateralis, and no visible contraction or increased EMG activity occurred in the muscle during requested maximal voluntary contractions. The methodology of Thomas et al.23 was employed with slight modifications for spasm analysis. Briefly, filtered and rectified EMG was integrated over 10ms. To separate spasms from baseline noise, a threshold was calculated based on the 30s rest period prior to transfer. A spasm was defined as having ≥50ms of above-threshold integrals in a period of at least 100ms, preceded and followed by at least 1s of below-threshold integrals (see Fig. 2). “Spasm duration” was defined as the temporal sum of discrete spasms during a given transfer. “Spasm magnitude” was defined as the total integrated EMG area (mV*s) during spasms for a given transfer, normalized to M-max. When a participant did not experience spasms during a particular transfer (based on EMG activity, N=3), spasm duration and magnitude were “0” for that transfer.
EMG records were visually reviewed for quality. Seven records from five participants were excluded because they had continuous motion artifacts (abnormally high amplitude spikes, N=5), low signal to noise ratio (N=1), or the video failed to record (N=1).
Statistical analysis
An a priori sample size of 19 was determined33 using a two-tailed test with an alpha level of 0.05, a power of 80%, and a correlation coefficient of 0.6. A coefficient of 0.6 would represent a strong correlation between SCI-SET scores and EMG measures, as hypothesized. Data analysis was performed in SPSS 22 (SPSS Inc, Chicago, IL, USA). Shapiro-Wilkes tests revealed transfer and EMG variables (spasm duration, spasm magnitude, and transfer duration) were not normally distributed, therefore nonparametric tests were used to analyse EMG data, as well as ordinal SCATS and SCI-SET data. Specific tests utilized (all 2 tails) are described in the Results. For tests with multiple comparisons, P-values were adjusted with a Bonferroni correction. No significant order effects were found for the four transfer variables (absolute and normalized spasm duration, spasm magnitude, transfer duration) using carryover effects analysis described by Welleck.34 Thus, subject data were combined for all analyses regardless of group assignment (see Fig. 1).
Results
Participants
Nineteen participants were included in the final analysis (17 males, 2 females, Table 1). Mean age was 39.5 years (SD=10.2) and mean time since injury was 15.6 years (SD=11.0). The number of participants contacted and excluded at each stage are noted in Fig. 1.
Table 1. Participants included in final analysis.
| Subject | Group | Sex | Age | Years Post-injury | Injury level | AIS grade | LEMS | SCATS extensor | Prescription antispastics |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | F | 28 | 2 | T2 | A | 0 | 2 | None |
| 2 | 1 | M | 32 | 13 | C7 | A | 0 | 2 | None |
| 3 | 2 | M | 52 | 28 | T9 | C | 0 | 2 | None |
| 4 | 2 | M | 28 | 2 | T2 | B | 0 | 1 | None |
| 5 | 1 | M | 27 | 5 | T1 | B | 0 | 2 | Baclofen 10mg tid, Dantrolene 50mg tid |
| 6 | 1 | M | 46 | 14 | T10 | A | 0 | 3 | None |
| 7 | 2 | M | 41 | 20 | T3 | A | 0 | 2 | None |
| 8 | 1 | M | 40 | 3 | C7 | C | 0 | 1 | Baclofen 20mg qid |
| 9 | 2 | M | 35 | 13 | T8 | A | 0 | 1 | Baclofen 40mg tid |
| 10 | 1 | M | 37 | 20 | T10 | A | 0 | 3 | None |
| 11 | 2 | M | 60 | 41 | T9 | C | 2 | 3 | None |
| 12 | 1 | M | 44 | 14 | T4 | A | 0 | 2 | Baclofen 20mg tid, Oxybutynin 5mg tid |
| 13 | 2 | M | 34 | 16 | T4 | A | 0 | 3 | Solifenacin 20mg prn |
| 14 | 2 | M | 35 | 6 | T4 | A | 0 | 1 | Baclofen 20mg qid, Oxybutynin 5mg tid |
| 15 | 1 | M | 60 | 32 | T5 | A | 0 | 2 | None |
| 16 | 2 | M | 30 | 13 | T3 | A | 0 | 1 | Baclofen 20mg bid, Clonidine 0.1mg sid, Oxybutynin 5mg bid |
| 17 | 2 | M | 32 | 13 | C5 | C | 0 | 1 | Cyclobenzaprine 20mg prn |
| 18 | 1 | F | 39 | 10 | T2 | A | 0 | 0 | Baclofen 20mg tid, Darifenacin 15mg sid |
| 19 | 1 | M | 50 | 32 | T12 | B | 1 | 2 | None |
AIS, american spinal injury association impairment scale; LEMS, lower extremity motor score (in the leg tested, max score=25); SCATS, spinal cord assessment tool for spastic reflexes; qid, four times daily; tid, three times daily; bid, twice daily; sid, once daily; prn, as needed.
Spasm characteristics during transfers
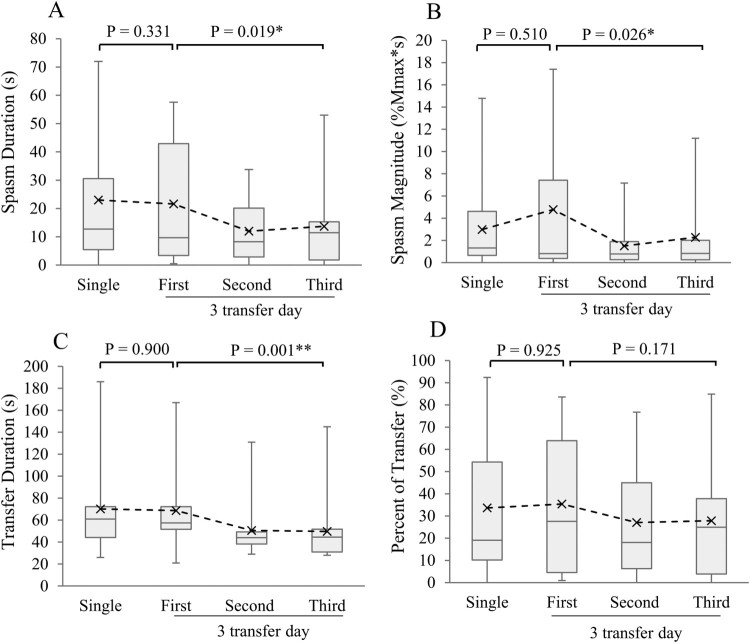
All participants exhibited spasms during at least one of the transfers, although one participant exhibited a brief spasm during only one of the four transfers. On average, participants took 75.4s (SD=47.4) to complete a single transfer and quadriceps spasms were present 23.1s (SD=23.6). When normalized to transfer duration, spasms were present an average of 31.4% (SD=30.3) of the transfer time. Means, medians and ranges for each variable and each transfer are depicted in Fig. 3. No significant differences were detected between a single transfer and the first of three transfers for any of these variables (Wilcoxon signed rank tests, Fig. 3).
Figure 3.
Transfer-related responses from single transfers and repeated transfers. Boxplots represent median, 25th and 75th percentiles for each variable. Whiskers represent ranges and “X”s represent means. (A) Spasm duration, (B) spasm magnitude, (C) transfer duration, and (D) spasm duration as a percent of transfer duration. Wilcoxon signed rank tests performed between single and first of three transfers for each variable. Friedman’s ANOVA performed between first, second and third successive transfers. *P < 0.05, ** P < 0.01. N=14 with EMG data on both days tested.
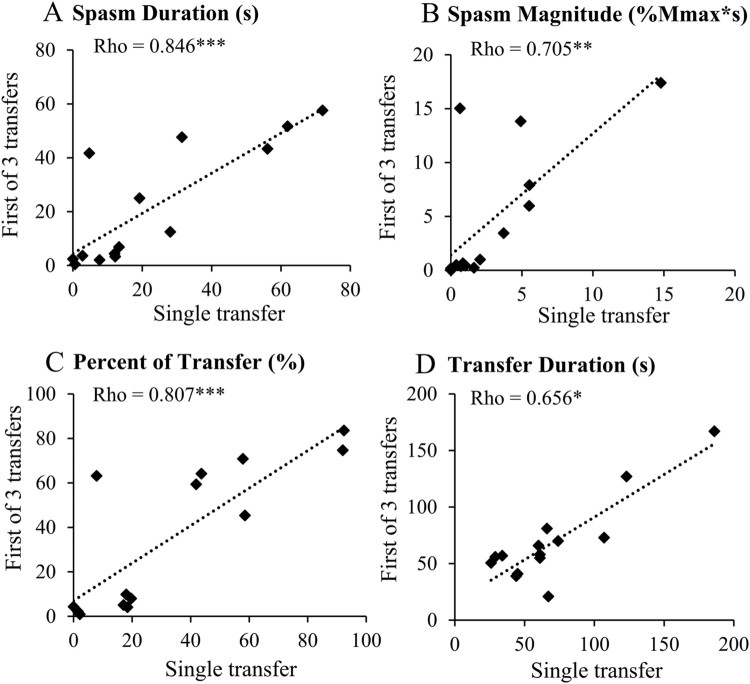
A single transfer and the first of three successive transfers are similar tasks in that they are both a first transfer (whereas the second and third transfers may be influenced by the muscle history of prior transfers). Thus, we compared the reliability of our variables for these two transfers. The single transfer and first of three transfers demonstrated good or excellent test-retest reliability (Spearman’s rho, Fig. 4) for all four variables. Thus, each participant’s values for the single transfer and first of three transfers were averaged over the two testing days to create a single, more robust value for use in correlations to other measures.
Figure 4.
Day-to-day reliability of transfer-related variables. Responses from single transfer plotted against responses from the first of 3 successive transfers. (A) Spasm duration, (B) spasm magnitude, (C) spasm duration as a percent of transfer duration, and (D) transfer duration. Spearman rank correlations; * P <0.05, ** P < 0.01, *** P < 0.001. N=14 with EMG data on both days.
Successive transfers demonstrated good-to-excellent internal consistency for spasm duration, magnitude and transfer duration variables (Cronbach’s α, Table 2). Significant differences were found among three successive transfers for all 4 of these variables (Friedman’s ANOVA by ranks, Fig. 3A, B, and C, respectively), but not when spasm duration was normalized to transfer duration (Fig. 3D).
Table 2. Internal consistency of 3 successive transfers measured by Cronbach’s α.
| α* | 95%CI | |
|---|---|---|
| Spasm Duration | 0.898 | 0.758-0.963 |
| Spasm Magnitude | 0.812 | 0.554-0.932 |
| Transfer Duration | 0.901 | 0.766-0.964 |
| Spasm Duration, % of Transfer Duration | 0.936 | 0.846-0.977 |
Results were calculated from the 15 participants with viable EMG data on 3-transfer day. *all P < 0.001.
Impact of spasticity on ADLs is not associated with measures of spasms evoked during a common ADL
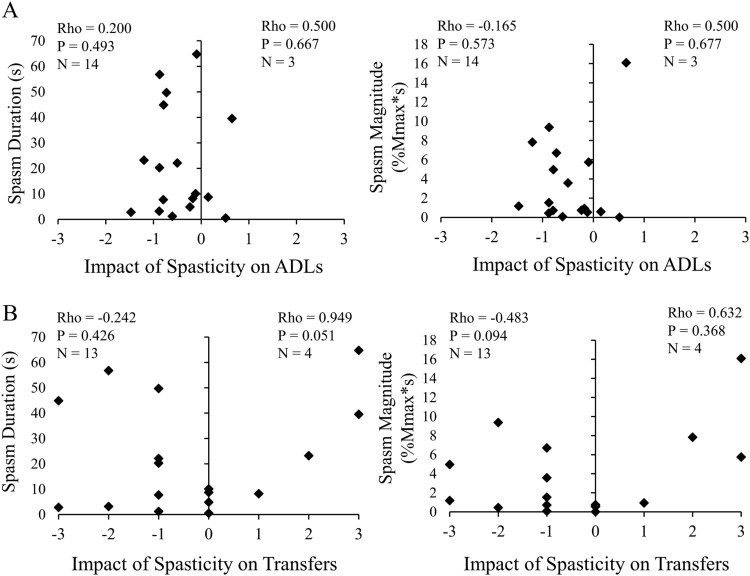
The impact of spasticity on ADLs (mean SCI-SET score) was perceived as “problematic” for 16 participants and “helpful” for three participants. No significant relationships were found between the duration or magnitude of quadriceps spasms during transfers and the perceived problematic or helpful impact of spasticity on ADLs (Spearman’s rho, Fig. 6A). Similarly, spasm duration normalized to transfer duration did not correlate with either problematic or helpful impact (rho = 0.008, P = 0.978, and rho = 0.500, P = 0.677, respectively). Clinically-rated spasticity severity measured by SCATS did not correlate significantly with impact on ADLs for either (unilateral) extensor scores alone or bilateral sum scores, which also included flexor spasms and clonus (rho value range: 0.000 to 0.866; P value range: 0.333 to 1.000).
Figure 6.
Impact of spasticity is not associated with quadriceps spasm EMG during seated transfers. Impact of spasticity on ADLs (SCI-SET mean) plotted against (A) spasm duration (left) and spasm magnitude (right); (B) impact of spasticity on transfers (SCI-SET question #3) plotted against spasm duration (left) and spasm magnitude (right). Participants with “Problematic” and “No Effect” ratings analyzed separately from those with “Helpful” ratings using Spearman rank correlations. X axis values: –3=Extremely Problematic, –2=Moderately Problematic, –1=Mildly Problematic, 0=No Effect, +1=Mildly Helpful, +2=Moderately Helpful, +3=Extremely Helpful.
Specifically regarding the impact of spasticity on transfers (SCI-SET question #3), 15 participants with EMG records considered it problematic or neutral while four considered it helpful. Neither duration nor magnitude of quadriceps spasms during transfers were significantly associated with the perceived impact of spasticity on transfers (Fig. 6B), although spasm duration demonstrated a strong positive correlation with helpful impact that approached significance (rho = 0.949, P = 0.051, Fig. 6B).
A greater time since injury was significantly associated with less problematic impact of spasticity on ADLs overall (rho = 0.501, P = 0.029) and specifically on transfers (rho = 0.568, P = 0.011). Controlling for time since injury did not significantly change correlations between impact scores and transfer-related variables, which remained non-significant. Age, injury level, and dosage of antispastic medications did not have a significant relationship with transfer-related variables or measures related to the impact of spasticity on daily life.
Spasms evoked by transfers correlate with a clinical measure of spasticity severity
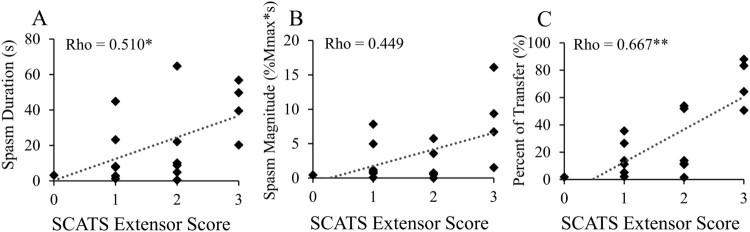
The extensor score of the SCATS was positively associated with average spasm duration (Fig. 5A) and spasm duration normalized to transfer duration (Fig. 5C). Average spasm magnitude did not significantly correlate with SCATS extensor score but the trend was similar to that for spasm duration (Fig. 5B). Transfer duration did not correlate significantly with SCATS extensor score (rho = -0.253, P = 0.327).
Figure 5.
Spasms measured with EMG correlate with a clinical score of spasticity severity. SCATS extensor score plotted against individual two-day averages for each spasm variable. (A) Spasm duration; (B) spasm magnitude; (C) spasm duration as a percent of transfer duration. Spearman rank correlations; *P < 0.05, **P < 0.01. N=17 with EMG data from at least one day.
Discussion
Spasticity is a multidimensional phenomenon,32,35,36 therefore it is important that a combination of self-reported perceptions and objective measures is considered when assessing spasticity or changes in spasticity due to treatment.24,32 This study addressed the lack of characterized associations among measures of involuntary muscle activity and the impact of spasticity on transfers and ADLs in general by investigating relationships among spasm duration and magnitude during transfers, perceived impact of spasticity on ADLs, and clinically-rated spasticity severity. Even though extensor spasticity is often reported to interfere with ADLs,2,8–10 we found no significant relationships between problematic impact scores and quadriceps spasm duration or magnitude measured with EMG or clinically-rated extensor spasticity measured with the SCATS. We found that greater time since injury was associated with less problematic impact of spasticity on both ADLs in general and on transfers, which has been noted in some studies,1,2,8 but not others.20 In another study of individuals with SCI, high self-reported spasm frequency during transfers, pain, and the combined scores for spasm frequency and pain were associated with greater perceived interference with function.19
Interestingly, the impact of spasticity on ADLs for most of our participants was minimally problematic (Fig. 6A). However, for the question related to transfers, participants gave a full range of responses from “extremely problematic” to “extremely helpful” (Fig. 6B). For the four participants reporting a helpful impact of spasticity on transfers, the relationship of impact with spasm duration was strongly positive and nearly significant (Fig. 6B). Helpful influence of spasticity on transfers has been noted in other studies,11,36 as spasms can provide postural stability or body weight support for some individuals during this task.
This study demonstrates that EMG measurement of spasm duration during seated transfers is positively associated with clinically-rated spasticity severity. Other studies have found that EMG activity recorded during a clinical spasticity test correlated with the corresponding score;16,18,27,37 however, our EMG measures were not recorded during the SCATS test itself but instead during seated transfers. The relationships we found provide evidence that the SCATS extensor score has relevance to involuntary muscle activity during a real-life activity. The positive relationship between EMG variables and SCATS extensor score was strongest when spasm duration was normalized to the overall time to complete the transfer. Normalizing spasm duration may have reduced variability due to other factors that influence transfer mobility, such as upper extremity strength, body mass, and transfer technique.38,39
Our EMG measurements of quadriceps spasm duration and magnitude during seated transfers were reliable day-to-day (Fig. 4) and had internal consistency across repeated trials (Table 2), supporting prior evidence that EMG is a useful method for objectively quantifying spasms during transfers.19 Despite adequate internal consistency, both spasm duration and magnitude decreased after the first transfer (Fig. 3A and B). Previous research has demonstrated that both active and passive muscle movement increases post-activation depression and leads to a decrease in spasticity.40,41 Therefore, muscle activation during initial spasms and peripheral stimulation during transfers may have elicited post-activation depression or other sources of segmental inhibitory feedback, resulting in shorter spasm duration during subsequent transfers.
Similar to EMG measures, SCATS scores did not correlate with the impact of spasticity on ADLs. This contrasts with a study by Bravo-Esteban et al.10 In their study, SCATS extensor scores inversely correlated with SCI-SET scores. Differences between studies may be due to differences in participant inclusion -- notably, their inclusion of ambulatory individuals. Extensor spasms strongly interfered with gait function in their study10 and have been associated with slower walking speeds.42 Thus, perceived problematic impact of spasticity may be influenced by gait interference in ambulatory individuals.
This study has some limitations. We recorded EMG responses from a single muscle during one task, and it is possible this task or muscle did not fully reflect the varied nature of spasticity encountered in daily life. In future studies, multiple channel EMG should be used along with the SCI-SET to determine the influence of various muscle groups on perceived impact of spasticity. Further, EMG can reliably capture spasms, but EMG, clinical tests such as the SCATS, and biomechanical tests do not detect common sensations associated with the experience of spasticity (i.e. feeling of muscle tension or pain)8,43 that may influence the problematic impact of spasticity.
The small sample size in this study can be considered a limitation; the present sample size was calculated to detect strong correlations. Due to the small number of participants who reported a helpful impact of spasticity on ADLs (N=3) or transfers (N=4), inferential statistics in this subset should be interpreted with caution. Additionally, we did not include individuals with voluntary quadriceps function to avoid any confounding effects of voluntary activity on EMG recordings. Therefore, results obtained from this sample of participants without voluntary function may not be generalizable to all individuals with SCI. Further research in larger and more diverse study samples are needed to elucidate factors that may influence the perceptions of spasticity and its impact on daily life.
Conclusions
We have demonstrated that the duration and magnitude of quadriceps spasms during transfers are not directly related to the perceived problematic impact of spasticity on transfers or ADLs in general. However, quadriceps spasm duration and magnitude measured with EMG during a seated transfer are reliable, and EMG measures of spasm duration are directly associated with a clinical measure of extensor spasticity. Exploring more complex interactions between body function measures of spasticity and other factors such as pain and psychological characteristics may help identify factors that strongly influence the impact that spasticity has on daily life. Further understanding may help direct treatment strategies to reduce the problematic impact of spasticity in persons with SCI and may also be useful across neurological conditions that involve spasticity, including stroke, multiple sclerosis, cerebral palsy, traumatic brain injury, vasculitis, CNS tumors, and muscular dystrophies.
Acknowledgements
The authors thank Aaisha Sanaullah, Jennifer Tibangin, James Adcock and Maydelis Escalona for their help with experimentation and data processing.
Disclaimer statements
Contributors None
Funding This study was supported by The Miami Project to Cure Paralysis.
Conflict of Interest Statement None.
Ethics approval The protocol was approved by the University of Miami Miller School of Medicine Institutional Review Board and conforms to the guidelines of the Declaration of Helsinki.
ORCID
Jacqueline Tibbetthttp://orcid.org/0000-0002-6807-5696
Eva G. Widerström-Nogahttp://orcid.org/0000-0003-0208-3658
References
- 1.Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG.. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord 1998;36(1):45–50. [DOI] [PubMed] [Google Scholar]
- 2.Sköld C, Levi R, Seiger A.. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil 1999;80(12):1548–57. [DOI] [PubMed] [Google Scholar]
- 3.Levi R, Hultling C, Nash MS, Seiger A.. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia 1995;33(6):308–15. [DOI] [PubMed] [Google Scholar]
- 4.Maynard F, Karunas R, Waring W.. Epidemiology of spasticity following traumatic spinal cord injury. Arch Phys Med Rehabil 1990;71(8):566–9. [PubMed] [Google Scholar]
- 5.Burridge JH, Wood DE, Hermens HJ, Voerman GE, Johnson GR, van Wijck F, et al Theoretical and methodological considerations in the measurement of spasticity. Disabil Rehabil 2005;27(1–2):69–80. [DOI] [PubMed] [Google Scholar]
- 6.Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, et al Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 2005;27(1–2):2–6. [DOI] [PubMed] [Google Scholar]
- 7.Tardieu G, Shentoub S, Delarue R.. [Research on a technic for measurement of spasticity]. Rev Neurol (Paris). 1954;91(2):143–4. [PubMed] [Google Scholar]
- 8.Fleuren JF, Voerman GE, Snoek GJ, Nene AV, Rietman JS, Hermens HJ.. Perception of lower limb spasticity in patients with spinal cord injury. Spinal Cord 2009;47(5):396–400. [DOI] [PubMed] [Google Scholar]
- 9.Little JW, Micklesen P, Umlauf R, Britell C.. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil 1989;68(1):32–6. [DOI] [PubMed] [Google Scholar]
- 10.Bravo-Esteban E, Taylor J, Abián-Vicén J, Albu S, Simón-Martínez C, Torricelli D, et al Impact of specific symptoms of spasticity on voluntary lower limb muscle function, gait and daily activities during subacute and chronic spinal cord injury. NeuroRehabilitation. 2013;33:531–43. [DOI] [PubMed] [Google Scholar]
- 11.Mahoney JS, Engebretson JC, Cook KF, Hart KA, Robinson-Whelen S, Sherwood AM.. Spasticity Experience Domains in Persons With Spinal Cord Injury. Arch Phys Med Rehabil 2007;88(3):287–94. [DOI] [PubMed] [Google Scholar]
- 12.Manella KJ, Torres J, Field-Fote EC.. Restoration of walking function in an individual with chronic complete (AIS A) spinal cord injury. J Rehabil Med 2010;42(8):795–8. [DOI] [PubMed] [Google Scholar]
- 13.Adams MM, Ginis KM, Hicks AL.. The Spinal Cord Injury Spasticity Evaluation Tool: Development and Evaluation. Arch Phys Med Rehabil 2007;88(9):1185–92. [DOI] [PubMed] [Google Scholar]
- 14.Finley MA, McQuade KJ, Rodgers MM.. Scapular kinematics during transfers in manual wheelchair users with and without shoulder impingement. Clin Biomech 2005;20(1):32–40. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon D, Nadeau S, Noreau L, Eng JJ, Gravel D.. Trunk and upper extremity kinematics during sitting pivot transfers performed by individuals with spinal cord injury. Clin Biomech 2008;23:279–90. [DOI] [PubMed] [Google Scholar]
- 16.Schmit BD, Benz EN.. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp Brain Res 2002;145(4):520–7. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Hornby TG, Hilb J, Schmit BD.. Extensor spasms triggered by imposed knee extension in chronic human spinal cord injury. Exp Brain Res 2005;162(2):239–49. [DOI] [PubMed] [Google Scholar]
- 18.Sherwood AM, Graves DE, Priebe MM.. Altered motor control and spasticity after spinal cord injury: Subjective and objective assessment. J Rehabil Res Dev 2000;37(1):41–52. [PubMed] [Google Scholar]
- 19.Mayo M, DeForest BA, Castellanos M, Thomas CK.. Characterization of involuntary contractions after spinal cord injury reveals associations between physiological and self-reported measures of spasticity. Front Integr Neurosci 2017;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Cooten IP, Snoek GJ, Nene AV, de Groot S, Post MW.. Functional hindrance due to spasticity in individuals with spinal cord injury during inpatient rehabilitation and 1 year thereafter. Spinal Cord 2015;53(9):663–7. [DOI] [PubMed] [Google Scholar]
- 21.Voerman GE, Gregoric M, Hermens HJ.. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 2005;27(1–2):33–68. [DOI] [PubMed] [Google Scholar]
- 22.Winslow J, Martinez A, Thomas CK.. Automatic Identification and Classification of Muscle Spasms in Long-Term EMG Recordings. IEEE J Biomed Heal Informatics 2015;19(2):464–70. [DOI] [PubMed] [Google Scholar]
- 23.Thomas CK, Dididze M, Martinez A, Morris RW.. Identification and classification of involuntary leg muscle contractions in electromyographic records from individuals with spinal cord injury. J Electromyogr Kinesiol 2014;24(5):747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voerman GE, Fleuren JFM, Kallenberg LAC, Rietman JS, Snoek GJ, Hermens HJ.. Patient ratings of spasticity during daily activities are only marginally associated with long-term surface electromyography. J Neurol Neurosurg Psychiatry 2009;80(2):175–81. [DOI] [PubMed] [Google Scholar]
- 25.Organization WH International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization; 2001. [Google Scholar]
- 26.Urbaniak GC, Plous S.. Research Randomizer. 2013. [Google Scholar]
- 27.Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD.. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil 2005;86(1):52–9. [DOI] [PubMed] [Google Scholar]
- 28.Rainoldi A, Melchiorri G, Caruso I.. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J Neurosci Methods 2004;134(1):37–43. [DOI] [PubMed] [Google Scholar]
- 29.Crone C, Hultborn H, Mazières L, Morin C, Nielsen J, Pierrot-Deseilligny E.. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 1990;81(1):35–45. [DOI] [PubMed] [Google Scholar]
- 30.Manella KJ, Roach KE, Field-Fote EC.. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol 2013;109(11):2666–79. [DOI] [PubMed] [Google Scholar]
- 31.Balioussis C, Hitzig S, Flett H, Noreau L, Craven B.. Identifying and Classifying Quality of Life Tools for Assessing Spasticity After Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2014;20(3):208–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh JTC, Wolfe DL, Miller WC, Curt A.. Spasticity outcome measures in spinal cord injury: psychometric properties and clinical utility. Spinal Cord 2008;46(2):86–95. [DOI] [PubMed] [Google Scholar]
- 33.Hulley S, Cummings S, Browner W, Grady D, Newman T.. Designing clinical research: an epidemiologic approach. 4th ed Philadelphia: Lippincott Williams & Wilkins; 2013. 79 p. [Google Scholar]
- 34.Wellek S, Blettner M.. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2012;109(15):276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priebe MM, Sherwood AM, Thornby JI, Kharas NF, Markowski J.. Clinical assessment of spasticity in spinal cord injury: a multidimensional problem. Arch Phys Med Rehabil 1996;77(7):713–6. [DOI] [PubMed] [Google Scholar]
- 36.Sköld C.Spasticity in spinal cord injury: Self- and clinically rated intrinsic fluctuations and intervention-induced changes. Arch Phys Med Rehabil 2000;81(2):144–9. [DOI] [PubMed] [Google Scholar]
- 37.Sköld C, Harms-Ringdahl K, Hultling C, Levi R, Seiger A.. Simultaneous Ashworth measurements and electromyographic recordings in tetraplegic patients. Arch Phys Med Rehabil 1998;79(8):959–65. [DOI] [PubMed] [Google Scholar]
- 38.Nyland J, Quigley P, Huang C, Lloyd J, Harrow J, Nelson A.. Preserving transfer independence among individuals with spinal cord injury. Spinal Cord 2000;38(11):649–57. [DOI] [PubMed] [Google Scholar]
- 39.Gagnon D, Nadeau S, Gravel D, Noreau L, Larivière C, Gagnon D.. Biomechanical analysis of a posterior transfer maneuver on a level surface in individuals with high and low-level spinal cord injuries. Clin Biomech 2003;18(4):319–31. [DOI] [PubMed] [Google Scholar]
- 40.Trompetto C, Marinelli L, Mori L, Pelosin E, Currà A, Molfetta L, et al Pathophysiology of Spasticity: Implications for Neurorehabilitation. Biomed Res Int 2014;2014:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y-J, Liang J-N, Hsu M-J, Lien H-Y, Fang C-Y, Lin C-H.. Effects of continuous passive motion on reversing the adapted spinal circuit in humans with chronic spinal cord injury. Arch Phys Med Rehabil 2013;94(5):822–8. [DOI] [PubMed] [Google Scholar]
- 42.Manella KJ, Field-Fote EC.. Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor Neurol Neurosci 2013;31(5):633–46. [DOI] [PubMed] [Google Scholar]
- 43.Lechner HE, Frotzler A, Eser P.. Relationship Between Self- and Clinically Rated Spasticity in Spinal Cord Injury. Arch Phys Med Rehabil 2006;87(1):15–9. [DOI] [PubMed] [Google Scholar]