Abstract
Objective: Neurogenic bladder dysfunction, including neurogenic detrusor overactivity (NDO) is one of the most clinically significant problems for persons with spinal cord injury (SCI), affecting health and quality of life. Genital nerve stimulation (GNS) can acutely inhibit NDO-related reflex bladder contractions and increase bladder capacity. However, it is unknown if GNS can improve urinary continence or help meet individuals’ bladder management goals during sustained use, which is required for GNS to be clinically effective.
Design: Subjects maintained voiding diaries during a one-month control period without stimulation, one month with at-home GNS, and one month after GNS. Urodynamics and quality of life assessments were conducted after each treatment period, and a satisfaction survey was taken at study completion.
Setting: Subject screening and clinical procedures were conducted at the Louis Stokes Cleveland VA Medical Center. Stimulation use and voiding diary entries were conducted in subjects’ homes.
Participants: Subjects included five men with SCI and NDO.
Interventions: This study tested one month of at-home portable non-invasive GNS.
Outcome Measures: The primary outcome measure was leakage events per day. Secondary outcome measures included self-reported subject satisfaction, bladder capacity, and stimulator use frequency.
Results: GNS reduced the number of leakage events from 1.0 ± 0.5 to 0.1 ± 0.4 leaks per day in the four subjects who reported incontinence data. All study participants were satisfied that GNS met their bladder goals; wanted to continue using GNS; and would recommend it to others.
Conclusions: Short term at-home GNS reduced urinary incontinence and helped subjects meet their bladder management goals. These data inform the design of a long-term clinical trial testing of GNS as an approach to reduce NDO.
Keywords: Neurogenic bladder, Electrical stimulation, Spinal cord injury, Genital nerve stimulation
Introduction
Spinal cord injury (SCI) frequently results in neurogenic bladder dysfunction. Neurogenic bladder dysfunction is one of the most clinically significant problems in the lives of persons with SCI, resulting in lost independence, social isolation, and medical complications. Neurogenic detrusor overactivity (NDO) is the most common presentation of neurogenic bladder dysfunction following SCI.1 NDO results in spontaneous reflex bladder contractions at lower bladder volumes, causing urinary incontinence, feelings of urgency, and low bladder capacity. Approximately two thirds of individuals with SCI have upper motor neuron injuries2 that are associated with bladder reflexes and the distribution depends on spinal level.3 These bladder contractions can be a trigger for autonomic dysreflexia (AD), causing dangerously elevated blood pressure. If dysfunction of the urethra or bladder outlet prevents urinary bladder emptying, as with detrusor-sphincter dyssynergia, bladder contractions may result in ureteric reflux and kidney damage.
Current management strategies for NDO and the urinary incontinence stemming from it are limited. Pharmacological approaches can be used to inhibit unwanted bladder contractions, but these drugs are not always effective and the systemic side effects may not be well tolerated. Indwelling catheters, increased intermittent catheterization frequency, or fluid restriction can interfere with quality of life or cause further medical complications. External or Foley catheters may be used, but not all persons with SCI are in favor of being dependent on continuous catheterization. Surgical approaches may be used, but these measures are irreversible and more invasive than other techniques. There remains an unmet need to provide an alternative therapy for suppressing unwanted bladder contractions.
Electrical stimulation of the genital nerves (GNS) has the potential to reduce NDO. GNS can modulate inhibitory spinal reflex circuits, causing increased sympathetic activity as recorded in the hypogastric nerve and decreased parasympathetic activity to the bladder as recorded in the pelvic nerve in animals.4–6 The genital nerves are located subcutaneously, can be stimulated bilaterally with a single electrode, and can be stimulated with no activation of other nerves. Multiple human studies have demonstrated the acute effectiveness of genital nerve stimulation to acutely inhibit reflex bladder activity, resulting in acute improvements in bladder capacity.7–17 A recent meta-analysis indicated that subject characteristics, such as sex, injury level, injury completeness, and time since injury, do not predict the effectiveness of acute GNS to inhibit bladder activity, suggesting that this approach has the potential to be effective for any individual with neurogenic detrusor overactivity whose bladder reflex pathways remain intact.18 GNS can be tolerated and effective in persons with skin sensation.19 GNS does not correlate with increased blood pressure, and thus does not appear to be a risk for autonomic dysreflexia.20
Evidence that chronic, at-home GNS is feasible and effective is required to advance GNS as a clinical therapy. Evaluating whether habituation occurs and whether it is safe to use for an extended period is required. Only limited chronic work been conducted to date and there remains little evidence that GNS can reduce incontinence over weeks of use. GNS improved bladder capacity and voiding volumes in 11 subjects over 3 days of GNS use21and improved bladder capacity and bladder compliance in 6 subjects after approximately one month of use.22 GNS reduced incontinence in one subject over 3 weeks of use22 and in 2 of 9 subjects over six weeks of use.23
In addition to the physiologic benefits, GNS may have a positive impact on the daily lives of the people who use it. Although previous GNS studies have noted the importance of user acceptance of the technology21 and investigating quality of life,22 the impact of GNS on routine bladder management and quality of life has not been well explored. User acceptance depends on not only effectiveness and impact on quality of life, but also on minimizing encumbrance and discomfort. GNS users have reported concerns regarding encumbrance and technical difficulty with the device;14,23 leg spasms during stimulation, though not interfering with function or comfort;22 inconvenience with electrode application;14,21 and discomfort with the electrical stimulation.21,23 Evaluation of issues related to user satisfaction will improve feasibility and approach implementation.
The primary goal of this pilot study was to determine the feasibility of at-home GNS to inform the design of a clinical trial by reporting on the ways in which subjects used a portable, user-controlled stimulator system and on the challenges that they faced. The secondary goal of this study was to add evidence to the literature in support of the effectiveness of at-home GNS to improve urinary continence, bladder capacity, quality of life and user satisfaction. The literature reports on urinary continence improvement in three subjects across two studies and this study adds to those data. This work improves our understanding of at-home GNS by reporting urinary continence data, user satisfaction, and impact on quality of life. These data provide justification for testing GNS over a one-year period measuring urinary continence and user satisfaction and inform study design of a one-year study.
Methods
This study was conducted with human participants at the Louis Stokes Cleveland VA Medical Center and approved by the Institutional Review Board. Study participants provided written informed consent to be included in this study. Thirty-six individuals with spinal cord injury were screened for this study. Inclusion criteria included suprasacral spinal lesion; neurologically stable; no active sepsis; no active pressure sores in the pelvic region; no ureteric reflux; and no significant urethral trauma. Before enrolling into the chronic study, subjects completed an initial urodynamics assessment to determine if subjects had NDO and if acute GNS inhibited bladder contractions. Five men whose neurogenic detrusor overactivity was acutely inhibited by GNS participated in this 3-month study (Table 1).
Table 1. Study participants.
| Subject | Age | Spinal cord lesion | Time since injury (yrs) | Bladder management |
|---|---|---|---|---|
| 1 | 65 | AIS D, C7, Brown Sequard | 15 | Voluntary voiding with urgency |
| 2 | 53 | AIS A, T12 | 29 | Condom catheter with some sensation of urgency |
| 3 | 76 | AIS D, C6 | 53 | Voluntary voiding with urgency |
| 4 | 62 | AIS D, C2 | 1 | Voluntary voiding with urgency |
| 5 | 74 | AIS C, C5 | 30 | Condom catheter with some sensation of urgency |
Study design
Subjects underwent three one-month testing periods – Control, Stimulation, and Post-stimulation. The subjects used a portable stimulator (Empi, St Paul, MN) for at-home GNS only during the Stimulation period. Data were collected during a Post-stimulation period to measure potential carry-over effects on bladder activity from the Stimulation period. During each testing period, the subjects filled out daily voiding diaries to record voids, voiding volumes, leakage episodes and fluid intake.
At the end of each of the three testing periods, a follow-up urodynamics test was performed, measuring bladder capacity as the volume at the first reflex bladder contraction. Stimulus thresholds were recorded, noting the threshold to sense stimulation; the threshold to evoke the pudendo-anal reflex; the tolerance limit (if the subject was sensate); and the amplitude used for bladder inhibition. Quality of life and user feedback were obtained.
Initial evaluation: Acute GNS bladder inhibition
Before enrolling into the chronic study, subjects completed an initial urodynamics assessment to determine if subjects had reflexive bladder contractions and if GNS inhibited bladder contractions. The first control cystometric fill was performed without stimulation to determine if the subject had NDO, producing a sustained bladder pressure rise of at least 35 cmH2O in response to filling. If the subject had NDO, then we measured the pudendo-anal reflex and determined the appropriate amplitude for GNS. Additional cystometric fills were conducted to determine if spontaneous reflex bladder contractions were inhibited with GNS. Up to six additional cystometric fills were performed, randomizing fill trials conducted with or without GNS. Stimulation pulses were monophasic, cathodic, 200 microsecond pulse width square waves (Digitimer DS7a, Hertfordshire, UK).
Urodynamics
Cystometrograms were conducted using a clinical urodynamics system (Laborie, Williston, VT). A dual lumen intraurethral catheter was placed, with one lumen used for filling the bladder with room temperature normal saline and the other lumen used for measuring vesicular pressure. A rectal balloon catheter measured abdominal pressure and detrusor pressure was calculated as the difference between vesicular and abdominal pressures. Pelvic floor electromyograms were recorded with surface electrodes at the external anal sphincter. The bladder was filled with normal room temperature saline at a rate of 50 cc/min. Pressures were measured using inline fluidic transducers (Utah Medical Inc., Midvale, UT). Data were recorded using a data acquisition system running custom LabVIEW software (National Instruments, Austin, TX).
Electrical stimulation
Figure 1 illustrates the electrode locations for genital nerve stimulation. GNS was applied across two round surface electrodes, 2 cm in diameter (Natus, Middleton, WI). Both male and female subjects participated in the initial evaluation for potential inclusion in this study. In men, the cathode was placed on the proximal, dorsal penile shaft and the anode placed approximately 2 cm distally. In women, the cathode was placed directly superior to the clitoris and the anode was placed on the left lateral labia majora. Alternatively, a 5 × 10 cm surface electrode (Natus, Middleton, WI) was sometimes used as the anode, placed on the mons pubis or inner thigh. Biphasic, charge-balanced, cathodic-leading pulses with 0.2 ms pulse width were delivered at 20 Hz (Empi, St Paul, MN). The amplitude was set to twice the threshold required to evoke the pudendo-anal reflex.7–16 If the subject had neither a pudendo-anal reflex nor pelvic sensation, then the amplitude was set to 40 mA. If the subject had pelvic sensation and the tolerance limit was less than twice the pudendo-anal reflex, then the amplitude was set to the tolerance limit. GNS was well-tolerated by subjects with pelvic sensation.19
Figure 1.
Genital nerve stimulation. Individuals don a pair of 1-cm surface electrodes on the dorsum of the penis (in men) or the labia majora (in women) to stimulate the genital nerves (shown under electrodes) bilaterally. The genital nerves are located subcutaneously, can be stimulated bilaterally with a single electrode, and can be stimulated with no activation of other nerves. Electrodes are connected to a battery-powered stimulator. The user is able to control the stimulation amplitude and start and stop stimulation on demand.
During the at-home Stimulation period, subjects with pelvic sensation (subjects 1, 3, and 4) used the stimulator when they felt bladder urgency to prevent incontinence (on-demand). Subjects without pelvic sensation (subjects 2 and 5) used the stimulator continuously to prevent incontinence. Stimulation electrode locations were not changed. Subjects were instructed to set the stimulation amplitude based on their feelings of comfort and to achieve effective bladder inhibition. Stimulation amplitude and duration were recorded in the voiding diary.
User satisfaction
A quality of life questionnaire was administered at the end of each of the three testing periods and a satisfaction survey was administered at the end of the three-month study. The Qualiveen quality of life survey measured the impact of urinary problems on quality of life.24 It has five indices scaled from 0 (no impact) to 4 (high impact) in the categories of Inconvenience, Restrictions, Fears, Impact on Daily Life, and the Specific Impact of Urinary Problems (SIUP). It also includes a general quality of life index scaled from -2 (very negative outlook) to + 2 (very positive outlook), with 0 being neutral. The satisfaction survey included key questions of subjective user feedback: whether subjects benefited from GNS; whether subjects would keep using it; and whether subjects would recommend it to others. It also provided an opportunity for subjects to give open feedback on their experience.
Data analysis
The primary outcome variables were the average number of daily incontinence episodes recorded in the voiding diaries and the bladder capacities recorded during acute urodynamics trials. A chi-square goodness-of-fit test was used to compare the number of incontinence episodes that occurred between treatment periods, reporting the median values. An analysis of variance (ANOVA) was used to determine if subject was a factor that contributed to the variance in incontinence episodes. An ANOVA was also used to determine if treatment group or subject (individual subject tested) were contributing factors in other outcome measures, such as bladder capacity from the urodynamics tests; voiding volumes, fluid intake, and voiding frequency from the voiding diaries; and Qualiveen questionnaire scores. The values for these secondary outcome measures are expressed as mean and standard deviation. The median was used to report additional outcome measures, including the number of voids per day, stimulation times per day, and stimulation days per week. A p-value less than 0.05 was considered statistically significant for all tests. Responses to the satisfaction survey questions are reported qualitatively.
The stimulator was typically only used during the day, therefore only daytime voids were analyzed. The first void of the day was not included for subjects 2 and 5 who used a condom catheter and bed bag overnight and demonstrated large bag volumes from overnight collection that were not representative of their voiding patterns. For subjects who used GNS on condition of feelings of urgency (subjects 1, 3, and 4), voiding data from the Control periods were taken during the times corresponding to use of GNS during the Stimulation period. For example, if a subject only used at-home stimulation between noon and going to bed for the night, then voids during the Control period that occurred in the morning were not included.
Incontinence episodes were not included for subject 2 who wore a condom catheter and did not report any episodes of incontinence. Incontinence episodes from subject 5 who wore a condom catheter are included because subject 5 was able to report incontinence episodes as uncontrolled voiding events that were distinct from controlled, intentional voiding events.
Some data were not available for analyses. Subject 5 did not record voiding volumes. Subject 5 also had a urinary tract infection during the last week of the Stimulation period, so data from that week were not included in the analyses.
Results
Recruitment
Thirty-six individuals, including men and women with spinal cord injury, met study inclusion criteria and were screened for this study. Twenty-seven of those individuals demonstrated neurogenic detrusor overactivity, and in 22 of those individuals, detrusor overactivity was acutely inhibited by GNS (Figure 2). Those 22 individuals were invited to participate in this study and 16 consented. Four subjects did not respond to attempts to contact them after the initial screening. Two dropped out for unrelated medical or personal reasons prior to stimulation. Four subjects were dissatisfied with the cumbersome stimulator or electrodes and did not begin stimulation. One subject began stimulation and withdrew due to an unrelated hospitalization. Five male subjects began stimulation and completed the study (Table 1) and data from these five subjects are included in this analysis. None of the five subjects used anticholinergics for bladder management. No subjects stopped participation because of a lack of effect from GNS.
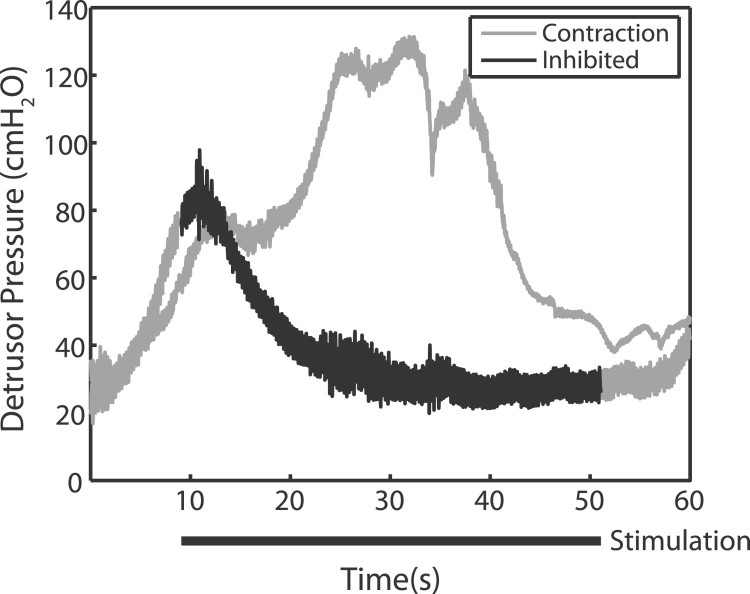
Figure 2.
Genital nerve stimulation (GNS) acutely inhibits bladder contractions. In response to filling, the bladder reflexively contracts (grey line: contraction). When GNS is applied (black bar: stimulation), the reflex bladder contraction is inhibited (dark line). Subjects who demonstrated reflex bladder contractions that were inhibited by GNS were considered appropriate for testing at-home GNS. Example traces taken from subject 3 and are typical of observed responses across subjects.
GNS effects on urinary continence
The median number of leaks per day significantly decreased from the Control period to the Stimulation period from 1.0 ± 0.5 to 0.1 ± 0.4 leaks per day (Figure 3, chi-square test, P < 0.001, n = 30). The median number of leaks per day significantly decreased individually in all 4 subjects: from Control period 1.1 ± 0.8, 1 ± 0, 1 ± 0.2 and 1.3 ± 0.4 to Stimulation period 0.1 ± 0.1, 0 ± 0, 0.2 ± 0.2 and 1.1 ± 0.5 in subjects 1, 3, 4 and 5, respectively. Subject 5 did not experience as large of a decrease in incontinence episodes as the other subjects did, but it was statistically significant (chi-square test, P = 0.016, n = 6). Subject 5 reported that the leaks were smaller in volume and less urgent, which helped the subject to keep the skin around the pelvis dry. Thirty observations accounts for four subjects reporting incontinence events for four weeks in two treatment groups, and one datum per treatment group is omitted because subject 5 had a urinary tract infection during the last week of the Stimulation period (see Methods). There was only marginal confidence that subject was not a factor accounting for variance in incontinence data (ANOVA, P = 0.052, n = 30).
Figure 3.
One month of genital nerve stimulation (GNS) at home decreased urinary incontinence episodes in each subject. Fewer median leaks per day were recorded during the Stimulation period than during the Control period (chi-square test, P < 0.001, n = 30). This decrease from 1.0 ± 0.5 leaks per day during the Control period to 0.1 ± 0.4 leaks per day with GNS suggests that subjects used GNS to inhibit neurogenic detrusor overactivity that would normally result in urinary incontinence. Incontinence increased to 0.4 ± 0.8 leaks per day during the Post-stimulation period. This incontinence which was significantly different from both the Control and Stimulation periods (chi-square test, P < 0.001, n = 30), suggesting a gradual return to baseline incontinence with GNS. Incontinence episodes were pooled by treatment period.
Median incontinence episodes increased during the Post-stimulation period to 0.4 ± 0.8 leaks per day, and this increase was significantly different from both the Control and Stimulation periods (chi-square test, P < 0.001, n = 30), suggesting some potential carryover effect and not a return to normal incontinence. Voiding diary data and subject reporting did not indicate any changes in lifestyle, daily activities, or behavior that would account for changes in urinary continence. Therefore, voiding diary variability and lifestyle variability were not thought to contribute to changes in urinary continence.
GNS effects on bladder capacity
Bladder capacity without stimulation measured during acute urodynamics did not change significantly across treatment groups, with average bladder capacities of 347 ± 168, 313 ± 232, and 424 ± 153 mL for Control, Stimulation, and Post-stimulation periods, respectively (ANOVA, P = 0.14, n = 28). Bladder capacity increased acutely during each urodynamics in response to GNS by 156 ± 70 mL (ANOVA, P < 0.001, n = 14), regardless of treatment period. Subject was a significantly contributing factor accounting for variance in the changes in bladder capacity (ANOVA, P < 0.001, n = 28).
The mean volume per void was 233 ± 50 mL, 241 ± 74 mL, and 218 ± 63 mL during Control, Stimulation, and Post-stimulation periods respectively, and did not change significantly (ANOVA, P = 0.13, n = 30). For all subjects, the mean time that elapsed between voids (2.5 ± 0.6 hours); the median number of voids per day (8.2 ± 3.0); and the mean daily fluid intake (2.0 ± 0.63 liters) did not significantly change from the Control period.
Routine use of stimulator
Subjects routinely used the stimulator to improve bladder control. They used the stimulator on average 5.3 ± 1.3 days per week. Subjects 1, 3, and 4 used the stimulator “on demand” when feeling urgency to void. Upon activating GNS, subjects reported that the urgency would diminish, suggesting that bladder activity was inhibited, and they would then find a bathroom and switch the stimulator off to void. These subjects would usually opt to find a bathroom within minutes of turning GNS on, however they would sometimes postpone voiding for up to approximately 5-30 minutes. These subjects used the stimulator on average 3.6 ± 2.8 times per day. Subjects 2 and 5 kept GNS on continuously during the daytime. These two subjects turned GNS off to facilitate efficient voiding when urgency was sufficiently high or when they chose a voiding time that was convenient for them.
Stimulation amplitudes were consistent during the Stimulation period and for each monthly urodynamics experiment. Subjects 1, 2, and 5 reported stimulation amplitude data in their voiding diaries. They set their at-home stimulation amplitudes to 13 ± 2 (12-18) mA, 29 ± 16 (14-55) mA, and 28 ± 7 (12-38) mA, respectively. Subjects reported that they got used to stimulation within the first week and then opted to increase stimulation amplitudes in subsequent weeks to improve bladder inhibition. Stimulation amplitudes did not show a trend of increasing during the stimulation period in those subsequent weeks. During monthly urodynamics experiments, GNS sensation threshold was 7 ± 4 mA and did not change significantly (ANOVA, P = 0.21, n = 14). Subject was a significant factor accounting for the variance of sensation threshold (ANOVA, P = 0.004, n = 14). Stimulation amplitudes during urodynamics experiments were 32 ± 14 mA and did not change significantly between urodynamics experiments (ANOVA, P = 0.09, n = 14).
User impact
GNS helped subjects meet their goals for bladder management. All subjects had some urgency sensation, which they would use as a feedback signal to know when to turn the stimulator on. Urgency itself was not a concern for these subjects, but it was an indicator that the bladder was contracting and that they might leak. Subjects 2 and 5 reported that they could not always tell when they were about to have an incontinence episode, and thus used a condom catheter and leg bag. Subjects 1, 2, 3, and 4 wanted enough time to get to the bathroom upon urgency sensation without leaking. In these subjects, GNS successfully delayed the need to void sufficiently to get to a bathroom without leaking. Subject 5 wanted to keep the skin around the pelvis dry due to concern about skin damage from urine leakage. Subject 5 reported that the leakage episodes were significantly diminished and the skin stayed dry. Subjects 2 and 5 wanted to stop using a condom catheter and leg bag. GNS inhibited their bladder activity, but additional interventions are required to meet this goal, particularly a reliable feedback mechanism. These subjects reported that they did not stop using the condom catheter because they were not confident in their ability to know when they had to get to a bathroom, sometimes voiding without feeling bladder fullness or urgency.
All participants felt that they benefited from GNS, were satisfied by it, wished to keep using it, and would recommend it to others (Table 2). However, subjects did report dissatisfaction with the stimulator itself, including bulkiness and long electrode lead wires. Subjects 1 and 2 enrolled into a year-long chronic study of GNS. Subject 3 did not want to continue participating in urodynamics examinations. Subjects 3 and 4 said that they would enroll in another study if the stimulator were less bulky. All five subjects expressed interest in having an implanted system.
Table 2. User satisfaction.
| Subject | Bladder goals | Wish to continue | Satisfied | Interested in implant | Bladder goals met |
|---|---|---|---|---|---|
| 1 | Get to bathroom without leaks | Strongly Agree | Agree | Yes | Yes |
| 2 | Get to bathroom without leaks and go off condom catheter | Strongly Agree | Strongly Agree | Yes | Yes/No* |
| 3 | Get to bathroom without leaks | Agree | Strongly Agree | Yes | Yes |
| 4 | Get to bathroom without leaks | Agree | Agree | Yes | Yes |
| 5 | Reduce moisture on skin and go off condom catheter | Agree | Agree | Yes | Yes/No* |
*Subjects 2 and 5 were both able to meet their bladder goals of getting to a bathroom without leaks and maintaining dry skin around the pelvis, respectively. These subjects did not meet their bladder goals of ceasing use of their condom catheters because they could not sense when their bladder was full or contracting (see Discussion).
Qualiveen questionnaires, completed at the end of each of the treatment periods, did not show statistically significant changes in any metrics of quality of life as a function of treatment group. Overall subjects were content. Inconvenience, Restrictions, Fears, Impact, and SIUP indices were 1.2 ± 1.2, 1.3 ± 1.1, 1.0 ± 0.8, 1.0 ± 1.2, and 1.1 ± 1.1, respectively, and all well below the maximum negative impact of 4. The Quality of Life Index was 0.9 ± 1.2, which is better than a neutral 0 and almost a maximum of 2.
Discussion
Genital nerve stimulation has been shown to acutely inhibit bladder activity in almost 100 subjects,18 however GNS has only been shown to improve urinary continence in three subjects.14,23 This pilot study examined the feasibility for users to incorporate at-home GNS into their bladder management strategies and the effectiveness of at-home GNS on clinically relevant measures of urinary continence and user satisfaction in five subjects. Subjects controlled the application of GNS and their voiding diaries provided important information about potential effects of GNS on continence, lifestyle habits, and stimulator usage. Subjects used GNS routinely; GNS helped meet subjects’ bladder management goals; GNS reduced incontinence; and subjects reported satisfaction with GNS. This study provides important information to power a randomized, controlled clinical trial; inform study design; and identify potential challenges and opportunities for translating this approach clinically.
At-home, short-term GNS effectiveness
GNS reduced urinary incontinence (Figure 3). This improved urinary continence is consistent with previous work14,23 and more than doubles the number of reported subjects in which GNS improved urinary continence. The decrease in the number of leak episodes during the Stimulation period suggests that GNS repeatedly inhibited reflex bladder contractions as has been demonstrated acutely.18 Three of four subjects were able to reduce their incontinence from an average of once per day to almost zero per day. This substantial improvement suggests that GNS may be an effective chronic approach for these individuals. These data support extended testing of GNS as an approach to reduce neurogenic detrusor overactivity following SCI.
Bladder capacity is commonly reported as a primary outcome measure instead of incontinence in GNS studies. Our subjects identified urinary incontinence as a primary concern, but not bladder capacity. With GNS, subjects were able to postpone voiding by approximately 5-30 minutes, depending primarily on their strategy. Some subjects preferred to get to the bathroom to void as soon as they felt urgency and merely used GNS to maintain bladder control until they got to the bathroom. Subjects reported that the improved urinary incontinence afforded them to go on long car rides; function at work without having to be immediately near a bathroom; and generally feeling more confident about being dry. Future studies testing chronic GNS and advancing this approach should therefore examine the effectiveness of GNS to improve urinary continence.
GNS inhibited bladder activity and allowed the bladder to fill to greater volumes, but was not associated with ureteral reflux. There is no evidence that urine passively flows back up the ureters and the bladder pressure is not sufficient for this reflux to occur with GNS. Therefore, there is little concern of ureteral reflux with GNS and, indeed, GNS may prevent ureteral reflux by inhibiting bladder activity.
We did not observe increases in bladder capacity, though others have reported increased bladder capacity with short-term chronic GNS.22,23 Bladder capacity was measured in only a few urodynamics experiments per subject, limiting the number of samples for this measurement. The voiding volumes recorded in voiding diaries also remained unchanged, as did the voiding frequency and volume intake, which suggests that bladder capacity did not change and that subject habits of daily living also remained unchanged. However, the number of voids per day did appear to decrease during the Stimulation period, though this decrease was not statistically significant. The total duration of one month or the short durations of GNS may not have been long enough to affect bladder capacity.
Habituation of GNS effectiveness was not observed during one month of home use. In general, electrical activation of sensory neurons as a mechanism to inhibit motor efferents raises the concern of habituation to the stimulation. Previous reports on conditional GNS have raised the possibility that habituation could occur and render GNS less effective over time.10–12,15,22 In our study, three subjects (1, 3, and 4) used the portable, at-home stimulator for on-demand GNS typically lasting only 5-30 minutes every few hours, thus providing little concern of habituation with this intermittent use. Two subjects (2 and 5) used GNS continuously for hours during the day, several days per week. There was no evidence for habituation to GNS. GNS remained effective without requiring significant changes to stimulation amplitudes. The threshold at which subjects could sense GNS and the amplitude used to effectively inhibit reflex bladder contractions did not change over time. Typical stimulation amplitudes for chronic GNS were approximately 12-55 mA, which is consistent with the literature.7–10,12,25 Though we did not observe habituation, the potential time course under which habituation might occur is unclear. GNS should be tested for a longer period with habituation as one of the concerns to watch for.
There was some evidence for carryover effects during the Post-stimulation period following one month of GNS use. The number of incontinence episodes increased from the Stimulation period to the Post-stimulation period, but the incontinence did not return to the levels observed during the Control period. Subjects reported a return of incontinence episodes within the first week of the Post-stimulation period, immediately after returning their stimulators.
User experience
Users’ bladder management goals were largely met using GNS as part of their bladder control strategy (Table 2). These subjects had already strategized to minimize leaks, yet we were still able to observe significant improvements in bladder control. Subjects were interested in being able to get to the bathroom without leaking and reported greater confidence in their ability to control their bladders and remain dry.
Of the five subjects who tested at-home GNS, they all were very satisfied with GNS. These five subjects wished to continue to use GNS at home after the study; expressed an interest in an implanted version; and reported that they would recommend the system to others. These subjects reported a beneficial impact on their quality of life and a positive user experience. No one was concerned about lack of effectiveness or discomfort. The high level of user satisfaction is encouraging. These data justify testing GNS over an extended chronic period and developing an implanted system.
Subject satisfaction data were limited to those subjects that used GNS at home. We cannot speculate on the opinions of the three subjects who chose not to participate due to unrelated medical reasons or the four subjects who did not respond after consenting. Four subjects expressed dissatisfaction with the large (size: 12 cm x 7 cm x 2.5 cm), limited stimulation system available for this study, however we are unable to determine their opinions on GNS since GNS was not tested in those subjects. Some subjects were concerned with the commitment involved in the study design, including multiple visits to the medical center and daily voiding diaries. GNS should be provided on an easy-to-use system and future studies should include supporting technology and study design to promote subject compliance.
Most, but not all of the subject bladder goals were met. The primary goal for most subjects was to be able to get to the bathroom without leaks, which 4/4 subjects achieved. The on-demand GNS approach that three subjects used was effective at preventing incontinence once subjects began to sense the onset of leakage and allowing them to get to the bathroom in time. Subjects 2 and 5 did not meet their goals of stopping condom catheter use. Subject 2 was concerned that leaks would still occur without a reliable feedback method for knowing when to turn the stimulator on.
Qualiveen questionnaires did not show a difference in quality of life measures across treatment periods. Perhaps the biggest factor affecting the significance of this questionnaire was the generally positive disposition of the subjects. These subjects started with relatively positive outlooks and thus did not show a significant change.
Nocturia was not improved. Subject 3 experienced improved bladder control during the day, but nocturia was not improved with GNS likely due to low bladder capacity and incomplete bladder emptying during nighttime voids. Subject 4 similarly attempted to use GNS to relieve nocturia without success. GNS for nocturia may be limited to subjects with sufficient bladder capacity to get through the night without voiding or leaking.
Keeping a daily voiding diary for 3 months proved to be challenging for some subjects. Subjects were interested in potential ways to automatically record high priority data without the need for maintaining a user-completed diary.
Clinical application and translation
This pilot study informs the design of a chronic clinical trial. The small sample size of our study and short chronic duration represent important study limitations. Owing to the challenges of conducting such a study, three previous studies of short-term chronic GNS had similarly small sample sizes of 6, 1, and 9 total subjects in each study. In the study of 9 subjects, subjects were not screened for acute responsiveness to GNS before enrollment, so only 2 subjects showed a clinical benefit. When combined with data from the literature, data from this study provides supporting evidence that at-home GNS can improve urinary continence. A one-year clinical trial determining feasibility and effectiveness of GNS in a larger cohort of subjects is required to support continued development and for comparison to clinically standard approaches. This work provides justification and the empirical data required to conduct a randomized clinical trial that includes more subjects testing GNS for a longer period.
Heterogeneity in subject injury level or completeness is not a concern for effectiveness of GNS to inhibit bladder activity. Previous work has already demonstrated that subject AIS score, injury level, age, and sex do not predict acute effectiveness of GNS to inhibit neurogenic detrusor overactivity.18 The predictive factor is whether subjects retain bladder reflexes below the lesion level. Therefore, any subject with neurogenic detrusor overactivity that is acutely inhibited by GNS would likely have intact spinal reflexes affecting bladder function and would be appropriate for such a clinical study. However, subject heterogeneity should be considered in future clinical trials because there may be variations in subject goals and bladder management strategies that will affect outcome measures, as we found in this study. For example, individuals without bladder sensation might leave the stimulator on continuously, rather than only use GNS when they feel bladder urgency. These subjects may use a condom catheter, instead of intermittent catheterization or voluntary voiding, and not report incontinence but instead report on alternative goals. This study has provided this insight that a future clinical trial will need to be designed to consider the differences in bladder management strategies and how incorporation of GNS might impact those strategies and routines.
Subjects with bladder sensation successfully applied GNS on demand, using it only when needed to reduce feelings of urgency and/or to prevent incontinence. An implanted pressure sensor might be able to provide that feedback for insensate individuals as part of a closed loop bladder neuroprosthesis.26–28
Both user experience and at-home GNS would benefit from an improved stimulation system. 4/16 subjects were interested in participating, but chose not to participate due to dissatisfaction with the cumbersome stimulator or electrodes. Tested subjects felt that the stimulator was unnecessarily bulky with long electrode leads that could get caught on clothing or assistive devices such as wheelchairs. The surface electrodes sometimes came off and had to be reapplied. Previous reports have cited subjects’ concerns regarding electrode stability and cumbersome stimulator devices with long cables as potential barriers to user acceptance of a GNS system.12,14,21,23 The purpose of this study was to test genital nerve stimulation, not the limited stimulator that was available to deliver stimulation. Subjects felt that the portable devices used in this study should have had an easier-to-operate, wireless on/off button and stimulus amplitude control with larger indicator numbers.
An implanted system would eliminate hand function issues, would not be cumbersome, or require daily application of surface electrodes. All subjects expressed interest in an implanted system and the data reported here justify advancing an implanted approach.
Conclusion
Genital nerve stimulation was feasible and effective for inhibiting unwanted bladder activity and improving urinary continence in subjects during a one-month at-home study with a portable stimulator and surface electrodes. GNS helped meet bladder management goals and subjects were satisfied with GNS; wanted to keep using it; and would recommend it to others. This work indicates the next step in the development of GNS as clinical trials and extended at-home use, evaluating it as an approach to reduce neurogenic detrusor overactivity following SCI.
Funding Statement
This work was supported by the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service RX000822, RX000960 and RX001962. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Acknowledgements
The authors thank Melissa Schmitt, RN, for her assistance during experiments and subject follow-up throughout this chronic study. They also thank Dr. Mary Ann Richmond, MD, Kesley Aamoth, Katherine Berry, Hongfei Di, MD, Elizabeth Harpster, MD, Michael Jacobson, and Janel Montfort for assistance with experiments.
Disclaimer statements
Contributors None.
Conflict of interest The authors have no conflicts of interest to report.
Ethics approval None.
Declaration of interest The authors report no declarations of interest.
References
- 1.New PW, Dillon L.. Neurogenic bladder and urodynamic outcomes in patients with spinal cord myelopathy. Top Spinal Cord Inj Rehabil 2015;21(3):250–6. doi: 10.1310/sci2103-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Z, Sun S, Deng R, Cai D, Yuan X, Du G, et al. The assessment of bladder and urethral function in spinal cord injury patients. J Huazhong Univ Sci Technolog Med Sci 2009;29(5):609–13. doi: 10.1007/s11596-009-0515-4 [DOI] [PubMed] [Google Scholar]
- 3.Doherty JG, Burns AS, O’Ferrall DM, Ditunno JF.. Prevalence of upper motor neuron vs lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J Spinal Cord Med 2002;25(4):289–92. doi: 10.1080/10790268.2002.11753630 [DOI] [PubMed] [Google Scholar]
- 4.de Groat WC, Ryall RW.. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol 1969;200(1):87–108. doi: 10.1113/jphysiol.1969.sp008683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundin T, Carlsson CA, Kock NG.. Detrusor inhibition induced from mechanical stimulation of the anal region and from electrical stimulation of pudendal nerve afferents. An experimental study in cats. Invest Urol 1974;11(5):374–8. [PubMed] [Google Scholar]
- 6.Walter JS, Wheeler JS, Robinson CJ, Wurster RD.. Inhibiting the hyperreflexic bladder with electrical stimulation in a spinal animal model. Neurourol Urodyn 1993;12(3):241–52; discussion 253. doi: 10.1002/nau.1930120306 [DOI] [PubMed] [Google Scholar]
- 7.Wheeler JS, Walter JS, Zaszczurynski PJ.. Bladder inhibition by penile nerve stimulation in spinal cord injury patients. J Urol 1992;147(1):100–3. doi: 10.1016/S0022-5347(17)37145-8 [DOI] [PubMed] [Google Scholar]
- 8.Previnaire J, Soler J, Perrigot M, Boileau G, Delahaye H, Schumacker P, et al. Short-term effect of pudendal nerve electrical stimulation on detrusor hyperreflexia in spinal cord injury patients: importance of current strength. Paraplegia 1996;34:95–9. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham APS, Shah NC, Knight SL, Shah PJR, Craggs MD.. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord 2001;39:420–8. doi: 10.1038/sj.sc.3101177 [DOI] [PubMed] [Google Scholar]
- 10.Dalmose AL, Rijkhoff NJM, Kirkeby HJ, Nohr M, Sinkjaer T, Djurhuus JC.. Conditional stimulation of the dorsal penile/clitoral nerve may increase cystometric capacity in patients with spinal cord injury. Neurourol Urodyn 2003;22(2):130–7. doi: 10.1002/nau.10031 [DOI] [PubMed] [Google Scholar]
- 11.Hansen J, Media S, Nøhr M, Biering-Sørensen F, Sinkjaer T, Rijkhoff NJM.. Treatment of neurogenic detrusor overactivity in spinal cord injured patients by conditional electrical stimulation. J Urol 2005;173(6):2035–9. doi: 10.1097/01.ju.0000158160.11083.1b [DOI] [PubMed] [Google Scholar]
- 12.Opisso E, Borau A, Rodríguez A, Hansen J, Rijkhoff NJM.. Patient controlled versus automatic stimulation of pudendal nerve afferents to treat neurogenic detrusor overactivity. J Urol 2008;180:1403–8. doi: 10.1016/j.juro.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 13.Horvath EE, Yoo PB, Amundsen CL, Webster GD, Grill WM.. Conditional and continuous electrical stimulation increase cystometric capacity in persons with spinal cord injury. Neurourol Urodyn 2010;29(3):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YH, Creasey GH.. Self-controlled dorsal penile nerve stimulation to inhibit bladder hyperreflexia in incomplete spinal cord injury: A case report. Arch Phys Med Rehabil 2002;83:273–7. doi: 10.1053/apmr.2002.28817 [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Kim JM, Im HT, Lee K, Kim SH, Hur DM.. Semiconditional electrical stimulation of pudendal nerve afferents stimulation to manage neurogenic detrusor overactivity in patients with spinal cord injury Ann Rehabil Med. 2011;35:605–12. doi: 10.5535/arm.2011.35.5.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman HB, Amundsen CL, Mangel J, Grill J, Bennett M, Gustafson KJ, et al. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn 2008;27:499–503. doi: 10.1002/nau.20544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farag FF, Martens FMJ, Rijkhoff NJM, Heesakkers JPFA.. Dorsal genital nerve stimulation in patients with detrusor overactivity: a systematic review. Curr Urol Rep 2012;13(5):385–8. doi: 10.1007/s11934-012-0273-x [DOI] [PubMed] [Google Scholar]
- 18.Bourbeau DJ, Creasey GH, Sidik S, Brose SW, Gustafson KJ.. Genital nerve stimulation increases bladder capacity after SCI: A meta-analysis. J Spinal Cord Med 2017;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brose SW, Bourbeau DJ, Gustafson KJ.. Genital nerve stimulation is tolerable and effective for bladder inhibition in sensate individuals with incomplete SCI. J Spinal Cord Med 2017;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y-H, Creasey GH, Lim H, Song J, Song K, Kim J.. Detrusor and blood pressure responses to dorsal penile nerve stimulation during hyperreflexic contraction of the bladder in patients with cervical cord injury. Arch Phys Med Rehabil 2003;84:136–40. doi: 10.1053/apmr.2003.50075 [DOI] [PubMed] [Google Scholar]
- 21.Opisso E, Borau A, Rijkhoff NJM.. Subject-Controlled stimulation of dorsal genital nerve to treat neurogenic detrusor overactivity at home. Neurourol Urodyn 2013;32(7):1004–9. doi: 10.1002/nau.22359 [DOI] [PubMed] [Google Scholar]
- 22.Lee Y-H, Kim S-H, Kim JM, Im HT, Choi IS, Lee KW.. The effect of semiconditional dorsal penile nerve electrical stimulation on capacity and compliance of the bladder with deformity in spinal cord injury patients: a pilot study. Spinal Cord 2012;50:289–93. doi: 10.1038/sc.2011.141 [DOI] [PubMed] [Google Scholar]
- 23.Wheeler J, Walter J, Sibley P.. Management of incontinent SCI patients with penile stimulation: Preliminary results. J Am Paraplegia Soc 1994;17(2):55–9. doi: 10.1080/01952307.1994.11735917 [DOI] [PubMed] [Google Scholar]
- 24.Costa P, Perrouin-Verbe B, Colvez A, Didier J, Marquis P, Marrel A, et al. Quality of life in spinal cord injury patients with urinary difficulties. Development and validation of qualiveen. Eur Urol 2001;39(1):107–13. doi: 10.1159/000052421 [DOI] [PubMed] [Google Scholar]
- 25.Fjorback MV, Rijkho N, Petersen T, Nohr M, Sinkjaer T.. Event driven electrical stimulation of the dorsal penile / clitoral nerve for management of neurogenic detrusor overactivity in multiple sclerosis. Neurourol Urodyn 2006;25:349–55. doi: 10.1002/nau.20170 [DOI] [PubMed] [Google Scholar]
- 26.Majerus SJA, Garverick SL, Suster M a., Fletter PC, Damaser MS.. Wireless, ultra-low-power implantable sensor for chronic bladder pressure monitoring. ACM J Emerg Technol Comput Syst 2012;8(2):1–13. doi: 10.1145/2180878.2180883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majerus SJ, Fletter PC, Damaser MS, Garverick SL.. Low-power wireless micromanometer system for acute and chronic bladder-pressure monitoring. IEEE Trans Biomed Eng 2011;58(3):763–7. doi: 10.1109/TBME.2010.2085002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karam R, Bourbeau DJ, Majerus S, Makovey I, Goldman H, Damaser M, et al.Real-time classification of bladder events for effective diagnosis and treatment of urinary incontinence. IEEE Trans Biomed Eng 2015;63(4):721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]