Abstract
In the setting of prior myocardial infarction, the oral antiplatelet ticagrelor added to aspirin reduced the risk of recurrent ischemic events, especially, in those with diabetes mellitus. Patients with stable coronary disease and diabetes are also at elevated risk and might benefit from dual antiplatelet therapy. The Effect of Ticagrelor on Health Outcomes in diabEtes Mellitus patients Intervention Study (THEMIS, NCT01991795) is a Phase 3b randomized, double‐blinded, placebo‐controlled trial of ticagrelor vs placebo, on top of low dose aspirin. Patients ≥50 years with type 2 diabetes receiving anti‐diabetic medications for at least 6 months with stable coronary artery disease as determined by a history of previous percutaneous coronary intervention, bypass grafting, or angiographic stenosis of ≥50% of at least one coronary artery were enrolled. Patients with known prior myocardial infarction (MI) or stroke were excluded. The primary efficacy endpoint is a composite of cardiovascular death, myocardial infarction, or stroke. The primary safety endpoint is Thrombolysis in Myocardial Infarction major bleeding. A total of 19 220 patients worldwide have been randomized and at least 1385 adjudicated primary efficacy endpoint events are expected to be available for analysis, with an expected average follow‐up of 40 months (maximum 58 months). Most of the exposure is on a 60 mg twice daily dose, as the dose was lowered from 90 mg twice daily partway into the study. The results may revise the boundaries of efficacy for dual antiplatelet therapy and whether it has a role outside acute coronary syndromes, prior myocardial infarction, or percutaneous coronary intervention.
Keywords: antiplatelet therapy, clinical trials, diabetes mellitus, general clinical cardiology/adult, ischemic heart disease
1. INTRODUCTION
Type 2 diabetes mellitus (DM) is a highly prevalent risk factor for coronary artery disease, with an incidence that is increasing worldwide. In those who have established atherosclerosis, the presence of DM further increases future risk of ischemic events in a synergistic fashion.1 DM multiplies cardiovascular (CV) risk not only in those with prior ischemic events, but also in those with stable coronary artery disease.
Heightened platelet activity appears to be present in atherothrombotic patients with DM. Long‐term dual antiplatelet therapy (DAPT) reduces CV event rates in patients with acute coronary syndromes, both in the short‐ and long term.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Dual antiplatelet therapy appears to have a particular benefit in patients with prior myocardial infarction (MI) and diabetes.12, 13, 14, 15 Whether that benefit extends to patients with diabetes and stable coronary artery disease without a history of prior MI remains a major unanswered question.
The Effect of Ticagrelor on Health Outcomes in diabEtes Mellitus patients Intervention Study (THEMIS) trial was designed to evaluate the potential benefits and risks of dual antiplatelet therapy with ticagrelor plus low dose aspirin vs placebo plus aspirin in patients with established stable coronary artery disease and DM treated with medications.
2. METHODS
THEMIS (NCT01991795) is a Phase 3b randomized, double‐blinded, placebo‐controlled trial of ticagrelor vs placebo, on top of low dose aspirin (75‐150 mg) unless contraindicated or not tolerated. The primary hypothesis of the trial is that twice daily ticagrelor when added to aspirin will reduce the risk of CV death, MI, or stroke in patients with DM and stable coronary artery disease. Patients were initially randomized to 90 mg twice daily of ticagrelor or matching placebo (Figure 1). Partway through the trial, the dose in the ticagrelor arm was lowered to 60 mg twice daily to be consistent with updated product labeling of ticagrelor in response to external clinical trial data in patients with an MI more than 1 year previously.14 Specifically, in PEGASUS, the efficacy profiles of ticagrelor 90 mg twice daily and 60 mg twice daily administered with low‐dose ASA were similar to each other.15 The lower dose had a better tolerability profile with regard to dyspnea, less risk of bleeding, and led to fewer discontinuations from study drug. Patients with diabetes did not have a different efficacy and safety profile to that of the overall study population with respect to the two dosing strategies of ticagrelor.14 Hence, to use the lowest effective dose and taking the overall benefit‐risk into consideration, the dose in THEMIS was changed to ticagrelor 60 mg twice daily. A protocol amendment enabling a dose reduction was finalized in May 2015, resulting in that approximately 25% of the randomized patients started on ticagrelor 60 mg twice daily or matching placebo. Because the lower dose was introduced rather early in the study, it is expected that at end of the study, at least 75% of the exposure time will be on 60 mg twice daily. The first patient was randomized on February 10, 2014 and the last patient on May 24, 2016. A total of 1315 sites in 42 countries were involved in the study.
Figure 1.
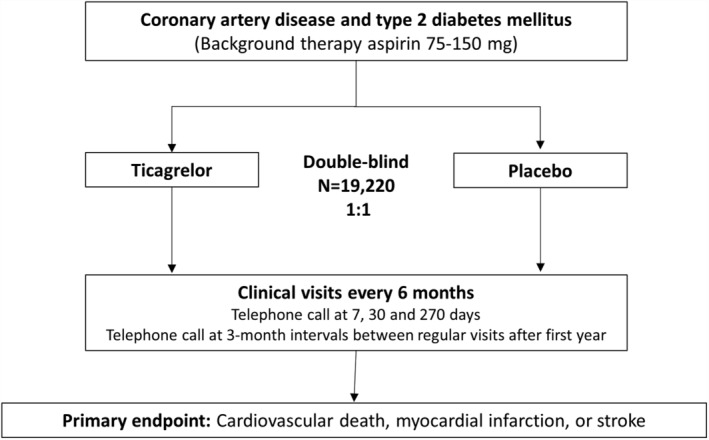
THEMIS study design. Data as of Feb 8, 2019
Patients ≥50 years with DM receiving anti‐diabetic medications for at least 6 months with stable coronary artery disease as determined by a history of previous percutaneous coronary intervention, coronary artery bypass grafting, or angiographic stenosis of ≥50% of at least one coronary artery were enrolled. Patients with known prior MI or stroke were excluded. Detailed inclusion and exclusion criteria are listed in Table 1. If a clinical indication arose, open‐label use of DAPT was allowed in the trial and patients came off blinded study drug for that duration.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: ADP, adenosine diphosphate; BP, blood pressure; CV, cardiovascular; CYP, cytochrome P450; MI, myocardial infarction; T2DM, type 2 diabetes mellitus; TIA, transient ischemic attack.
Previous MI is a documented hospitalization with a final diagnosis of spontaneous MI.
The primary efficacy endpoint is a composite of CV death, myocardial infarction, or stroke. The primary safety endpoint is Thrombolysis in Myocardial Infarction major bleeding. Table 2 provides further details about the pre‐specified hierarchical testing of secondary endpoints. Appendix S1, Supporting Information provides the definitions. All endpoints were adjudicated by a blinded academic clinical endpoint committee. Partway through the trial, given an increasing appreciation of the importance of peripheral artery ischemic endpoints, these were also adjudicated by a blinded academic clinical endpoint committee.15, 16, 17 To ensure data integrity, there was a firewall maintained between the clinical endpoint committee (CEC) and the data monitoring committee (DMC). Specifically, the DMC statistical data analysis center (SDAC), handling all unblinded study data, was located at Duke Clinical Research Institute (DCRI).
Table 2.
Primary and secondary efficacy variables
| Primary efficacy variable | Secondary efficacy variables (in hierarchical order) |
|---|---|
| Time from randomization to the first occurrence of any event from the composite of CV death, MI, or stroke (ischemic, hemorrhagic, or unknown etiology) | Time from randomization to death of CV cause |
| Time from randomization to the first occurrence of MI | |
| Time from randomization to the first occurrence of ischemic stroke | |
| Time from randomization to death of any cause |
Abbreviations: CV, cardiovascular; MI, myocardial infarction.
The primary efficacy endpoint will be tested at a 4.96% significance level (two‐sided), adjusted for 1 planned efficacy interim analysis with family‐wise error controlled at 5%. The one planned efficacy interim analysis occurred on the 29th of March 2017. The recommendation from the DMC was to continue the study according to the protocol. The estimated annual event rate in the placebo arm was 2.5%. An effect size of 16% relative risk reduction was hypothesized, with 1385 primary endpoint events needed to provide 90% power. This resulted in an estimated sample size of 19 000 patients, randomized in a 1:1 ratio, with an average follow‐up time of 40 months (maximum 58 months). Even with the ticagrelor dosage switch, a power of 90% in the study is maintained, as the main analysis is based on randomization to ticagrelor irrespective of dose.
There are no sub‐studies planned, but predefined subgroup analyses will explore efficacy and safety according to baseline characteristics, such as revascularization history, single vs multivessel coronary artery disease, duration of diabetes, glycemic control, anti‐diabetic medications, age groups, and renal function.
3. RESULTS
A total of 20 108 patients were enrolled. Of these, 19 271 were randomized, and because of closure of a single site secondary for inadequate adherence to good clinical practice in a different trial, 19 220 patients are expected to be available for analysis (Figure 2).
Figure 2.
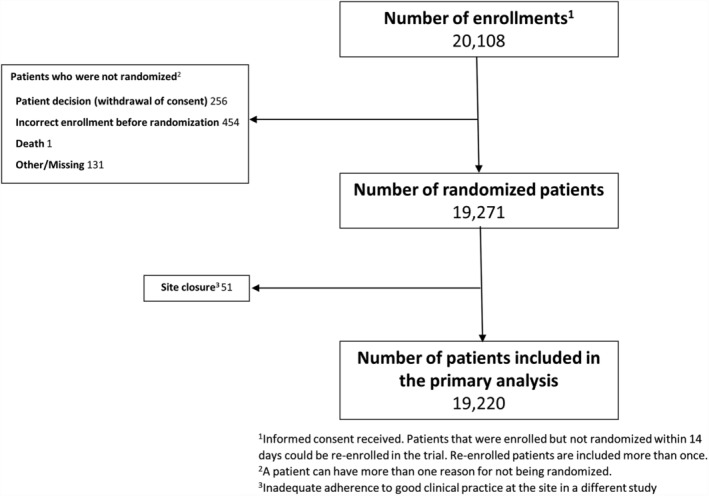
THEMIS study flow diagram. Data as of Feb 8, 2019
The baseline characteristics and medications are described in Table 3. The randomized population had a median age of 66 years and 31.4% were women. As expected in patients with DM, there was a high prevalence of concomitant hypertension and dyslipidemia. The prevalence of multivessel coronary artery disease was 62.1%, although 20.2% had no prior revascularization. The median duration of DM was 10.0 years, the hemoglobin A1c at baseline was 7.1%, and 28.7% of the patients were treated with insulin. At baseline, the population was very well‐treated, with 89.8% on statins and 78.6% on an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker.
Table 3.
Baseline characteristics. (not final data; data as of Feb 8, 2019)
| Characteristic | Randomized patients (N = 19 220)a |
|---|---|
| Age (years), median (IQR) | 66.0 (61.0‐72.0) |
| Male, n (%) | 13 189 (68.6) |
| BMI (kg/m2), median (IQR) | 29.0 (26.0‐32.7) |
| Current smoker, n (%) | 2094 (10.9) |
| Race, n (%) | |
| Asian | 4406 (22.9) |
| Black or African American | 403 (2.1) |
| Other | 715 (3.7) |
| White | 13 696 (71.3) |
| Geographic region, n (%) | |
| Asia and Australia | 4288 (22.3) |
| Central and South America | 2169 (11.3) |
| Europe, Middle East, and South Africa | 9768 (50.8) |
| North America | 2995 (15.6) |
| Disease history | |
| Hypertension, n (%) | 17 776 (92.5) |
| Dyslipidemia, n (%) | 16 753 (87.2) |
| Angina pectoris, n (%) | 10 801 (56.2) |
| Multi‐vessel coronary artery disease (>1 vessel), n (%) | 11 935 (62.1) |
| Revascularization status, n (%) | |
| Previous PCI only | 9808 (51.0) |
| Previous CABG only | 4191 (21.8) |
| Previous PCI and CABG | 1346 (7.0) |
| No previous revascularizationb | 3875 (20.2) |
| Time since most recent PCI (years), median (IQR) | 3.3 (1.5‐6.6) |
| Time since most recent CABG (years), median (IQR) | 4.3 (1.5‐9.2) |
| History of peripheral artery disease, n (%) | 1687 (8.8) |
| History of poly‐vascular diseasec, n (%) | 2579 (13.4) |
| Duration of diabetes (years), median (IQR) | 10.0 (5.0‐16.0) |
| History of any diabetes complicationsd, n (%) | 4910 (25.5) |
| HbA1c at baseline (%), median (IQR) | 7.1 (6.4‐8.1) |
| eGFR (MDRD) at baseline (mL/min/1.73 m2), median (IQR) | 75.0 (60.5‐89.6) |
| Medication use at baselinee | |
| Aspirin, n (%) | 19 104 (99.4) |
| Aspirin dose (mg), median (IQR) | 100 (80‐100) |
| Statin, n (%) | 17 266 (89.8) |
| Proton pump inhibitor, n (%) | 4901 (25.5) |
| ACE‐inhibitor or ARB, n (%) | 15 113 (78.6) |
| ACE‐inhibitor | 8145 (42.4) |
| ARB | 7211 (37.5) |
| Beta‐blocker, n (%) | 14 192 (73.8) |
| Insulin, n (%) | 5508 (28.7) |
| Any diabetes medications, n (%) | 19 156 (99.7) |
| 1 | 8609 (44.8) |
| 2 | 6911 (36.0) |
| 3 | 2892 (15.0) |
| >3 | 744 (3.9) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; IQR, interquartile range; MDRD, modification of diet in renal disease; PCI, percutaneous coronary intervention; PAD, peripheral artery disease.
N is the total number of randomized patients, patients that have been randomized more than once are only included according to their first randomization. Patients that are randomized but will not be included in the primary analysis are not included in this table;
significant stenosis on coronary angiography but no revascularization;
Defined as arterial obstructive disease involving at least 2 vascular beds where vascular bed involvement is characterized by either 1) CAD (defined as CAD, PCI or CABG), 2) PAD, 3) carotid artery stenosis or cerebral revascularization;
Defined as at least one of retinopathy, autonomic neuropathy, peripheral neuropathy, and nephropathy;
Medications used within 30 days of randomization, aspirin use is captured on day of randomization.
4. DISCUSSION
The THEMIS trial was designed to address an important question in the management of patients with DM and stable coronary artery disease but no prior MI—does intensification of the antiplatelet regimen beyond aspirin reduce the risk of CV events? The trial is fully enrolled and well‐powered to detect moderate relative risk reductions in the overall population studied. The sample size will hopefully allow examination of clinically logical and well‐defined subgroups even if the overall trial does not meet its primary endpoint or shows marginal net clinical benefit. The evidence from THEMIS will complement that of several other trials which assessed the value of DAPT in patients with acute coronary syndromes or prior myocardial infarction.2, 7, 11, 12, 15
When added to aspirin, ticagrelor specifically provides superior benefit compared with clopidogrel in patients with acute coronary syndromes7 and compared with placebo in high‐risk patients with prior myocardial infarction.15 There is lack of clear benefit of ticagrelor monotherapy, compared with clopidogrel in peripheral artery disease18 and compared with aspirin in ischemic stroke.19 In the context of patients undergoing coronary stenting, there was no clear benefit to a strategy of DAPT for a month followed by ticagrelor monotherapy over a more conventional strategy of DAPT for a year followed by aspirin monotherapy, although additional study is ongoing in this regard.20, 21, 22
There is evidence that platelet aggregation is enhanced in patients with DM compared with those without DM and that aspirin may have reduced efficacy in DM.23 This appears to be because of several factors, including accelerated platelet turnover. Indeed, there is some evidence that twice daily administration of aspirin is more effective at inhibition of generation of thromboxane than once a day administration.24, 25 In addition, enteric coated aspirin is widely used and appears to be somewhat less effective than non‐enteric coated aspirin in pharmacodynamic and pharmacokinetic analyses.26 Recent data suggest that aspirin has only a very modest effect for primary prevention CV events in patients with DM.27, 28, 29
Similarly, there is also evidence of reduced pharmacodynamic and pharmacokinetic efficacy of clopidogrel among patients with DM, in part because of less efficient metabolism of clopidogrel as a prodrug.30, 31, 32, 33 However, the clinical implications of these observations are not entirely clear since clopidogrel did not appear less effective in patients with DM in a large outcome trial of clopidogrel vs aspirin.34
With more effective oral ADP receptor antagonists than clopidogrel, such as prasugrel or ticagrelor, there is no evidence of reduced efficacy in patients with DM who present with acute coronary syndromes or long‐term post‐MI.14, 35, 36 In particular, some of the concerns regarding the efficacy of aspirin are related to short plasma residence time of the drug, while there is accelerated platelet generation during the 24‐hour cycle. As ticagrelor is a reversible inhibitor of the P2Y12 receptor, it is given twice daily. This results in persistent plasma levels that may be able to antagonize the P2Y12 receptor in newly formed platelets around the entire circadian cycle. These observations led to the hypothesis of the THEMIS trial that DAPT with ticagrelor plus aspirin would be superior to aspirin alone even in DM patients with stable coronary artery disease.14
The baseline characteristics of the THEMIS population suggest that it is very representative of daily practice in the care of DM patients. Thus, the results of THEMIS should be quite generalizable to the large number of patients with DM and stable coronary artery disease worldwide. Furthermore, the background medical therapy, such as statin use, is excellent, and therefore any observed benefits would truly be an incremental advance.
Furthermore, THEMIS is the largest randomized trial of patients with DM performed to date. Beyond the randomized question being addressed, the THEMIS database will allow several important hypotheses to be tested in observational analyses using a large, well‐characterized population of patients with coronary artery disease and DM.
Limitations include the lowering of dose that occurred partway through the trial from ticagrelor 90 mg twice daily to ticagrelor 60 mg twice daily, which reduces the statistical power to examine either dose alone. Given the overall similar efficacy but trend towards more bleeding and less tolerability of ticagrelor 90 mg twice daily compared with ticagrelor 60 mg twice daily in PEGASUS, the lowering of the dose in THEMIS should impact efficacy less but could overestimate the overall bleeding risk, which is a conservative approach.37, 38, 39 Multiple subgroup analyses have been prespecified, such as patients with multivessel coronary artery disease and prior percutaneous or surgical revascularization, but despite its size, the trial may not be well powered for subgroup analysis. Some peripheral ischemic events may not have been captured, as adjudication of these endpoints were added partway through the trial, though the trial was still blinded when this occurred, thus any assessment should be unbiased.
5. CONCLUSION
Approaches to reduce CV morbidity further in patients with DM with stable atherosclerosis are urgently needed. More intense antiplatelet therapy is a promising approach. The THEMIS trial is assessing whether DAPT with ticagrelor and low‐dose aspirin provides a significant reduction in ischemic events with an acceptable increase in bleeding. If the trial is positive overall, it will change the treatment paradigm for patients with diabetes. If the trial is clearly negative overall and in all major subgroups, it will provide a clear risk level below which more intense antiplatelet therapy is not indicated. Thus, irrespective of the exact results, THEMIS should greatly refine our understanding of the role of DAPT in patients across the atherothrombotic spectrum.
CONFLICT OF INTEREST
Dr. Deepak L. Bhatt discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca (including for his role as co‐Chair of THEMIS), Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braun wald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. Dr Kim Fox receives fees, honoraria and/or travel expenses from Servier, TauRx, CellAegis, Celixir and Broadview Ventures. He is a director of Vesalius Trials Ltd. Dr Harrington discloses research grants/contracts from the National Heart Lung and Blood Institute, Duke, AstraZeneca, CSL‐Behring, Glaxo Smith Kline, Merck, Portola, Regado, Sanofi‐Aventis, and The Medicines Company, and consulting/advisory for Adverse Events, Amgen, Element Science, Gilead, Merck, MyoKardia, The Medicines Company, VidaHealth, and WebMD. Dr. Lawrence Leiter discloses the following relationships: research grants from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion, GSK, Kowa, Novo Nordisk, Sanofi, The Medicines Company; speaking or consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, Sanofi. Dr. Shamir Mehta discloses the following relationships: Institutional research grants from Abbott Vascular, AstraZeneca, Boston Scientific and Sanofi and consulting fees from AstraZeneca and Sanofi. Dr Tabassome Simon discloses the following relationships: research grants from AstraZeneca, Daiichi‐Sankyo, Eli‐Lilly, GSK, MSD, Novartis, Sanofi, speaking or consulting fees from AstraZeneca, BMS, Novartis Sanofi. Dr Claes Held discloses the following relationships: Institutional research grants from AstraZeneca, GlaxoSmith Kline, BristolMyers Squibb, Pfizer and Merck. Advisory board ‐ AstraZeneca, Bayer, Boehringer Ingelheim, Idorsia and Coala Life. Speaking or consulting fees from AstraZeneca, Bayer. Dr. Ph. Gabriel Steg discloses the following relationships: research grants from Amarin, Bayer, Merck, Sanofi, and Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Idorsia, Lilly, Merck, Novartis, Novo‐Nordisk, Pfizer, Regeneron, Sanofi, Servier. Dr Anders Himmelmann, Dr Wilhelm Ridderstråle, and Marielle Andersson report being employees of AstraZeneca.
Supporting information
Appendix S1. Supplementary Appendix to Bhatt et al., Rationale, Design, and Baseline Characteristics of THEMIS: Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study
ACKNOWLEDGMENTS
We would like to thank Jayne Prats PhD for assistance with editing (limited to collation of author comments and formatting), with funding from AstraZeneca.
Executive Committee
Deepak L. Bhatt MD MPH (co‐Chair and co‐Principal Investigator), Kim Fox MD, Robert A. Harrington MD, Lawrence A. Leiter MD, Shamir R. Mehta MD MSc, Tabassome Simon, MD, PhD, Marielle Andersson MSc, Anders Himmelmann MD, PhD, Ph. Gabriel Steg MD (co‐Chair and co‐Principal Investigator).
Steering Committee
Rafael Diaz (Argentina), John Amerena (Australia), Kurt Huber (Austria), Peter Sinnaeve (Belgium), José Carlos Nicolau (Brazil), José Francisco Kerr Saraiva (Brazil), Ivo Petrov (Bulgaria), Lawrence A. Leiter (Canada), Shamir R. Mehta (Canada), Ramón Corbalán (Chile), Junbo Ge (China), Qiang Zhao (China), Rodrigo Botero (Colombia), Petr Widimský (Czech Republic), Steen Dalby Kristensen (Denmark), Juha Hartikainen (Finland), Nicolas Danchin (France), Harald Darius (Germany), Tse Hung Fat (Hong Kong), Robert Gabor Kiss (Hungary), Prem Pais (India), Eli Lev (Israel), Leonardo De Luca (Italy), Shinya Goto (Japan), Gabriel Arturo Ramos López (Mexico), Jan Hein Cornel (Netherlands), Frederic Kontny (Norway), Felix Medina (Peru), Noe Babilonia (Philippines), Grzegorz Opolski (Poland), Dragos Vinereanu (Romania), Dmitry Zateyshchikov (Russia), Mikhail Ruda (Russia), Omer Elamin (Saudi Arabia), František Kovář (Slovakia), Anthony John Dalby (South Africa), Myung Ho Jeong (South Korea), Héctor Bueno (Spain), Stefan James (Sweden), Chern‐En Chiang (Taiwan), Damras Tresukosol (Thailand), Zeki Ongen (Turkey), Kausik Ray (UK), Alexander Parkhomenko (Ukraine), Darren McGuire (USA), Mikhail Kosiborod (USA), Tuan Quang Nguyen (Vietnam).
Data Monitoring Committee
Lars Wallentin MD, PhD (Chair), Keith A. A. Fox MB ChB, John W. Eikelboom MBBS, Jaakko Tuomilehto MD, Kerry L. Lee PhD; Hussein R. al‐Khalidi PhD and Stephen J. Ellis PhD (non‐voting independent statisticians, DCRI).
Clinical Endpoint Committee
Uppsala Clinical Research Centers members
Claes Held MD, PhD (CEC Chair), Emil Hagström MD, PhD (CEC co‐chair), Pernilla Holmgren (CEC Project Manager), Ulrika Heldestad (CEC Coordinator Consultant), Theresa Hallberg (CEC Coordinator), Karin Renlund Grausne (CEC coordinator), Cristina Alm (CEC monitor), Åsa Michelgård Palmquist (CEC monitor), Camilla Svanberg (CEC administrator).
Colorado Prevention Center (CPC) members
Warren H. Capell MD (CEC Co‐chair), Mark R. Nehler MD (CEC Co‐chair), William R. Hiatt MD (Senior Scientific Lead), Marc P. Bonaca MD (Senior Scientific Lead), Stacey Houser (CEC Operations Manager), Susie Bachler (CEC Coordinator), Nicole Jaeger (Executive Project Manager).
AstraZeneca
Marielle Andersson MSc (Statistician), Maria Aunes MD (Safety physician), Åsa Brusehed (Project management lead), Jersey Chen MD (Study physician), Björn Dahlöf (Programming), Jitka Doležalová (Site Management and monitoring), Maciej Domzol (Study leader), Magdalena Findley (Study management), Anders Himmelmann MD, PhD (Clinical lead physician), Niclas Holmberg (Programming), Marianne Jahreskog RN (Safety scientist), Mikael Knutsson PhD (Statistician), Jakub Kruszewski (Enablement), Maria Leonsson‐Zachrisson MD, PhD (Senior physician), Wilhelm Ridderstråle MD, PhD (Study physician), Maj Stark (Clinical Development), Elin Winder (Data management).
Countries Participating in THEMIS
Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, Chile, China, Colombia, Czech Republic, Denmark, Finland, France, Germany, UK, Hong Kong, Hungary, India, Israel, Italy, Japan, Mexico, Netherlands, Norway, Peru, Philippines, Poland, Republic of Korea, Romania, Russia, Saudi Arabia, Slovakia, South Africa, Spain, Sweden, Taiwan, Thailand, Turkey, Ukraine, USA, Vietnam.
Bhatt DL, Fox K, Harrington RA, et al. Rationale, design and baseline characteristics of the effect of ticagrelor on health outcomes in diabetes mellitus patients Intervention study. Clin Cardiol. 2019;42:498–505. 10.1002/clc.23164
Drs. Ph. Gabriel Steg and Deepak L. Bhatt contributed equally to this study.
The trial is sponsored by AstraZeneca.
Funding information AstraZeneca
Contributor Information
Deepak L. Bhatt, Email: dlbhattmd@post.harvard.edu.
Philippe Gabriel Steg, Email: gabriel.steg@aphp.fr.
on behalf of the THEMIS Steering Committee:
R. Diaz, J. Amerena, K. Huber, P. Sinnaeve, J.C. Nicolau, J.F.K. Saraiva, I. Petrov, L.A. Leiter, S.R. Mehta, R. Corbalán, J. Ge, Q. Zhao, R. Botero, P. Widimský, S.D. Kristensen, J. Hartikainen, N. Danchin, H. Darius, T.H. Fat, R.G. Kiss, P. Pais, E. Lev, L.D. Luca, S. Goto, G.A.R. López, J.H. Cornel, F. Kontny, F. Medina, N. Babilonia, G. Opolski, D. Vinereanu, D. Zateyshchikov, M. Ruda, O. Elamin, F. KováŘ, A.J. Dalby, M.H. Jeong, H. Bueno, S. James, C.‐E. Chiang, D. Tresukosol, Z. Ongen, K. Ray, A. Parkhomenko, D. McGuire, M. Kosiborod, and T.Q. Nguyen
REFERENCES
- 1. Cavender MA, Steg PG, Smith SC Jr, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation. 2015;132(10):923‐931. [DOI] [PubMed] [Google Scholar]
- 2. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345(23):1716. [DOI] [PubMed] [Google Scholar]
- 3. Mehta SR, Yusuf S, Peters RJG, et al. Effects of pretreatment with clopidogrel and aspirin followed by long‐term therapy in patients undergoing percutaneous coronary intervention: the PCI‐CURE study. Lancet. 2001;358(9281):527‐533. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001‐2015. [DOI] [PubMed] [Google Scholar]
- 5. Roe MT, Armstrong PW, Fox KA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367(14):1297‐1309. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, White HD, Ohman EM, et al. Prasugrel versus clopidogrel for patients with unstable angina or non‐ST‐segment elevation myocardial infarction with or without angiography: a secondary, prespecified analysis of the TRILOGY ACS trial. Lancet. 2013;382(9892):605‐613. [DOI] [PubMed] [Google Scholar]
- 7. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045‐1057. [DOI] [PubMed] [Google Scholar]
- 8. Cannon CP, Harrington RA, James S, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double‐blind study. Lancet. 2010;375(9711):283‐293. [DOI] [PubMed] [Google Scholar]
- 9. Steg PG, James S, Harrington RA, et al. Ticagrelor versus clopidogrel in patients with ST‐elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a platelet inhibition and patient outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131‐2141. [DOI] [PubMed] [Google Scholar]
- 10. Steg PG, Harrington RA, Emanuelsson H, et al. Stent thrombosis with ticagrelor versus clopidogrel in patients with acute coronary syndromes: an analysis from the prospective, randomized PLATO trial. Circulation. 2013;128(10):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 11. Udell JA, Bonaca MP, Collet JP, et al. Long‐term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta‐analysis of randomized trials. Eur Heart J. 2016;37(4):390‐399. [DOI] [PubMed] [Google Scholar]
- 12. Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706‐1717. [DOI] [PubMed] [Google Scholar]
- 13. Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982‐1988. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Bonaca MP, Bansilal S, et al. Reduction in ischemic events with Ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS‐TIMI 54. J Am Coll Cardiol. 2016;67(23):2732‐2740. [DOI] [PubMed] [Google Scholar]
- 15. Bonaca MP, Bhatt DL, Cohen M, et al. Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791‐1800. [DOI] [PubMed] [Google Scholar]
- 16. Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719‐2728. [DOI] [PubMed] [Google Scholar]
- 17. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;391(10117):219‐229. [DOI] [PubMed] [Google Scholar]
- 18. Jones WS, Baumgartner I, Hiatt WR, et al. Ticagrelor compared with Clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135(3):241‐250. [DOI] [PubMed] [Google Scholar]
- 19. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 20. Vranckx P, Valgimigli M, Jüni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug‐eluting stent: a multicentre, open‐label, randomised superiority trial. Lancet. 2018;392(10151):940‐949. [DOI] [PubMed] [Google Scholar]
- 21. Bhatt DL. Aspirin‐still the GLOBAL LEADER in antiplatelet therapy. Lancet. 2018;392(10151):896‐897. [DOI] [PubMed] [Google Scholar]
- 22. Baber U, Dangas G, Cohen DJ, et al. Ticagrelor with aspirin or alone in high‐risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am Heart J. 2016;182:125‐134. [DOI] [PubMed] [Google Scholar]
- 23. Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123(7):798‐813. [DOI] [PubMed] [Google Scholar]
- 24. Capodanno D, Patel A, Dharmashankar K, et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ Cardiovasc Interv. 2011;4(2):180‐187. [DOI] [PubMed] [Google Scholar]
- 25. Dillinger JG, Drissa A, Sideris G, et al. Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am Heart J. 2012;164(4):600‐606.e1. [DOI] [PubMed] [Google Scholar]
- 26. Bhatt DL, Grosser T, Dong JF, et al. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J Am Coll Cardiol. 2017;69(6):603‐612. [DOI] [PubMed] [Google Scholar]
- 27. McNeil JJ, Nelson MR, Woods RL, et al. Effect of aspirin on all‐cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ASCEND Study Collaborative Group , Bowman L, Mafham M. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;18(16):1529‐1539. [DOI] [PubMed] [Google Scholar]
- 29. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2018;392(10152):1036‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Angiolillo DJ, Bernardo E, Ramírez C, et al. Insulin therapy is associated with platelet dysfunction in patients with type 2 diabetes mellitus on dual oral antiplatelet treatment. J Am Coll Cardiol. 2006;48(2):298‐304. [DOI] [PubMed] [Google Scholar]
- 31. Angiolillo DJ, Fernandez‐Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54(8):2430‐2435. [DOI] [PubMed] [Google Scholar]
- 32. Angiolillo DJ, Jakubowski JA, Ferreiro JL, et al. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol. 2014;64(10):1005‐1014. [DOI] [PubMed] [Google Scholar]
- 33. Feldman L, Tubach F, Juliard JM, et al. Impact of diabetes mellitus and metabolic syndrome on acute and chronic on‐clopidogrel platelet reactivity in patients with stable coronary artery disease undergoing drug‐eluting stent placement. Am Heart J. 2014;168(6):940‐7.e5. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90(6):625‐628. [DOI] [PubMed] [Google Scholar]
- 35. James S, Angiolillo DJ, Cornel JH, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2010;31(24):3006‐3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel‐thrombolysis in myocardial infarction 38. Circulation. 2008;118(16):1626‐1636. [DOI] [PubMed] [Google Scholar]
- 37. Franchi F, Rollini F, Aggarwal N, et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (optimizing antiplatelet therapy in diabetes mellitus)‐4 study. Circulation. 2016;134(11):780‐792. [DOI] [PubMed] [Google Scholar]
- 38. Storey RF, Angiolillo DJ, Bonaca MP, et al. Platelet inhibition with Ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS‐TIMI 54 trial. J Am Coll Cardiol. 2016;67(10):1145‐1154. [DOI] [PubMed] [Google Scholar]
- 39. Thomas MR, Angiolillo DJ, Bonaca MP, et al. Consistent platelet inhibition with ticagrelor 60 mg twice‐daily following myocardial infarction regardless of diabetes status. Thromb Haemost. May 3, 2017;117(5):940‐947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary Appendix to Bhatt et al., Rationale, Design, and Baseline Characteristics of THEMIS: Effect of Ticagrelor on Health Outcomes in Diabetes Mellitus Patients Intervention Study


