Abstract
Background
We sought to investigate left atrial (LA) volume, function, and strain in elite athletes by a meta‐analysis including echocardiographic studies that provided volumetric and strain analysis of LA phasic function.
Methods
The OVID‐MEDLINE, PubMed, and Cochrane CENTRAL databases were searched for English‐language articles without time restriction up to February 2018 through focused and high sensitive search strategies. Studies were identified by crossing the following search terms: “athletes,” “left atrial size,” “left atrial volume,” “atrial function,” “atrial strain,” “atrial strain rate,” “echocardiography,” “2D speckle echocardiography.”
Results
Meta‐analysis included 403 athletes and 314 active but not trained healthy controls from 9 studies. Pooled data showed that average LA volume index was higher in athletes than in healthy controls (28.0 ± 1.0 vs 20.7 ± 0.8 mL/m2, P < 0.001). Global LA longitudinal strain, showing LA reservoir function, was lower in the athletes than in healthy controls with borderline significance (37.0 ± 1.2 vs 38.3 ± 1.5%, P = 0.044). Late diastolic LA strain rate, resembling LA contractile function, was also lower in elite athletes than in control group (−1.56 ± 0.08 vs −1.74 ± 0.09 seconds −1, P = 0.007).
Conclusions
Our meta‐analysis shows that LA volume is higher, while LA reservoir and contractile functions are impaired in elite athletes during active training compared to untrained controls. Whether these changes persist during deconditioning periods remains to be determined. These alterations may be related to the higher risk of arrhythmias, in particular atrial fibrillation, reported among middle/old aged athletes.
Keywords: left atrium, meta‐analysis, phasic function, strain, volume
1. INTRODUCTION
Elite athletes are exposed to intensive training that is associated with hemodynamic adaptation and cardiac remodeling. Most of studies have been focused on left ventricular (LV) changes induced by training. However, an important part of cardiac adjustment to increased cardiac output during effort is played by left atrial (LA) remodeling. There is a consensus regarding LA dilatation in athletes and recent large meta‐analysis confirmed that LA linear dimensions, as well as LA volume, was significantly enlarged in athletes.1, 2
It is more complicated when debate comes to the point of LA function. Namely, LA function determines diastole, which has the key role in athletes.3 Nevertheless, the assessment of LA function is not an easy task because it does not represent evaluation of only one parameter, but the whole set of parameters (total, passive, and active emptying fractions) that resemble LA reservoir, conduit, and active function, respectively.4 LA reservoir function shows the ability of the LA to store pulmonary venous return during left ventricular contraction and isovolumetric relaxation.4 Conduit LA function represent the ability to passively transfer blood into the LV. The LA active function shows the LA contraction during the last diastolic phase that enables up to 30% of LV stroke volume in athletes.5
There is lack of agreement regarding the importance of each of these functions in the athletes. In some of the studies, these LA functions were decreased,6, 7 whereas in some others there was no significant difference between athletes and controls.2 The introduction of strain in echocardiography significantly changed our perspective and improved our knowledge about cardiac mechanics and provided better and more detailed insight into LA mechanics in athletes.8, 9 The strain evaluation provided even more information about each phase of cardiac diastole and therefore LA functions. However, the results obtained by strain‐ and volume‐derived analysis of LA phase function were not always concurrent.
The importance of LA remodeling in athletes is relevant because it is related with overall and cardiovascular mortality in global population,10, 11 and also because of significantly increased risk of atrial fibrillation.12, 13 Considering the fact that regular physical activity is considered as beneficial, it is important to make distinction between this kind of physical activity and extensive effort in elite athletes and to emphasize the fact that elite athletes are under the increased risk of arrhythmias and particularly atrial fibrillation during middle‐age.14 LA remodeling has central role in the later.
This systematic review and meta‐analysis provided comprehensive information on the LA strain in the athletes and summarized current information on this interesting topic.
2. METHODS
The present study was performed according to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines.15 Medical literature was reviewed to identify all articles investigating LA size and function as assessed by echocardiography in athletes. The OVID‐MEDLINE, PubMed, and Cochrane CENTRAL databases were searched for English‐language articles without time restriction up to February 2018 through focused, high‐sensitive search strategies.
Studies were identified by crossing the following search terms: “athletes,” “left atrial size,” “left atrial volume,” “atrial function,” “atrial strain,” “atrial strain rate,” “echocardiography,” “2D speckle echocardiography.”
References from relevant studies were screened for supplementary articles. Any observational (either cross‐sectional or longitudinal) study comparing LA size and function, as assessed by standard and 2D speckle echocardiography, in athletes and in sedentary controls was included in the meta‐analysis, without any restriction about the types of physical training
Inclusion criteria were: (a) full articles published in English in peer‐reviewed journals; (b) studies reporting data on LA strain (SD, SE or confidence interval) by echocardiography in elite/professional athletes; (c) minimum set of data including age, gender, body surface area (BSA), or body mass index (BMI).
Titles and abstracts were screened independently by two authors (CC, EG) who discarded irrelevant studies to the topic. Case reports, reviews, editorials, letters were excluded from qualitative analyses, but screened for potential additional references. Two authors (CC and EG) independently assessed retrieved abstracts and full text of these studies in order to determine eligibility according to inclusion criteria. A third reviewer (CS) solved disagreements on study judgments. Data extraction were performed by one reviewer (CC) and independently verified by another reviewer (CS).
The first literature search identified a total of 159 papers. After the initial screening of titles and abstracts, studies were excluded and were reviewed; of these, nine studies fulfilled the inclusion criteria and contained sufficient details to be included in the final review6, 7, 16, 17, 18, 19, 20, 21, 22 (Figure S1, Supporting Information).
2.1. Statistical analysis
The primary aim of the meta‐analysis was to compare LA size (as assessed by LAV indexed to body surface area) and function, as assessed by 2D speckle tracking echocardiography, both expressed as continuous variables, in competitive high‐trained athletes compared to sedentary controls.
To this purpose, a pooled analysis of the above mentioned continuous variables was performed using fixed or random effects models by Comprehensive Meta‐Analysis Version 2, Biostat, Englewood, New Jersey. Standard means difference (SMD) with 95% confidence interval (CI) was calculated to evaluate the statistical difference of variables between athletes and controls. The limit of statistical significance was set at P < 0.05. Demographic and clinical data provided by selected studies are expressed as absolute numbers, percentage, mean ± SD (SD), mean ± SE (SE). Heterogeneity was estimated by using I‐square, Q, and tau‐square values; random effect model was applied when heterogeneity across studies was high (I2 > 75). Publication bias was assessed by using the funnel plot method according to the trim and fill test. Observed and adjusted values, their lower and upper limits have been calculated.
3. RESULTS
Overall, 697 subjects (403 athletes and 314 active but not trained healthy controls) were included in nine studies (sample size range 40‐240). The majority of cases and controls (56.7%) was examined in a single Italian research center.
The assessment of LA structure and function was the primary aim of eight out of nine studies. Four studies published by D'Ascenzi et al. from 2011 to 20166, 16, 18, 20 and three studies by Gabrielli et al. from 2012 to 201617, 19, 21 investigated different population samples.
3.1. Clinical characteristics of athletes
All cases were highly trained competitive professional athletes from 10 different sports. In order to provide a detailed information on the physical exercise characteristics of the sports included in this analysis each athlete sample was allocated to the following groups based on Mitchell's classification: A1 (low dynamic, low static); A2 (low dynamic, moderate static); A3 (low dynamic, high static); B1 (moderate dynamic, low static); B2 (moderate dynamic, moderate static); B3 (moderate dynamic, high static); C1 (high dynamic, low static); C2 (high dynamic, moderate static); C3 (high dynamic, high static).23
Table 1 shows main characteristics of analyzed studies, including year of publication, type of sport according to Mitchell's classification, sample size, mean age, gender distribution, LAVI, BSA, office systolic/diastolic BP, and LVM indexed to BSA (LVMI) (when available). High dynamic trained athletes with different static component were examined in six out nine studies.
Table 1.
Demographic and clinical characteristics of echocardiographic studies on left atrial strain in athletes
| Author ref. | Year | Sport | Sample (n) | Age (years) | Gender (men/women %) | BSA (m2) | SBP (mm Hg) | DBP (mm Hg) | LAVI (mL/m2) | LVMI (g/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| D'Ascenzi16 | 2011 | PSP | 23 | 26.0 ± 5.0 | M (100%) | 2.0 ± 0.1 | n.a. | n.a. | 26.3 ± 4.3 | n.a. |
| Gabrielli17 | 2012 | PT | 20 | 43.0 ± 4.0 | M (80%) | n. a. | 117 ± 7 | n. a. | 40.0 ± 5.0 | 143.0 ± 12.0 |
| D'Ascenzi18 | 2014 | PVP | 24 | 24.9 ± 4.1 | W (100%) | 1.86 ± 0.11 | n. a. | n.a. | 24.6 ± 3.0 | n. a. |
| Gabrielli19 | 2014 | PHP | 24 | 28.0 ± 4.0 | M (100%) | 2.2 ± 0.17 | n.a. | n.a. | 35.2 ± 8.8 | 116.0 ± 21.0 |
| D'Ascenzi6 | 2015 | PSP,PBP,PVP | 150 | 26.0 ± 8.0 | M (70%) | 2.0 ± 0.1 | 120 ± 20 | 74 ± 10 | 24.6 ± 7.3 | 91.4 ± 22.2 |
| McClean7 | 2015 | EB, PT | 18 | 28.0 ± 8.0 | M (100%) | 1.8 ± 0.5 | 131 ± 7 | 74 ± 7 | 35.0 ± 8.0 | n. a. |
| McClean7 | 2015 | EWL, EA | 18 | 26.0 ± 7.0 | M (100%) | 2.0 ± 0.2 | 134 ± 9 | 74 ± 8 | 26.0 ± 10.0 | n. a. |
| D'Ascenzi20 | 2016 | PBP, PVP | 35 | 22.0 ± 7.0 | M (57%) | 2.1 ± 0.2 | n.a. | n.a. | 27.1 ± 6.6 | 82.2 ± 23.4 |
| Gabrielli 21 | 2016 | CMR, PT | 50 | 37.0 ± 6.0 | M (100%) | 1.9 ± 0.1 | 125 ± 9 | 79 ± 4 | 29.4 ± 9.2 | n. a. |
| Sanchismen 22 | 2017 | CLR | 22 | 38.4 ± 5.8 | M (100%) | 1.9 ± 0.1 | 124 ± 11 | 79 ± 4 | 24.99 ± 6.69 | n.a. |
| Sanchiswomen 22 | 2017 | CLR | 19 | 37.4 ± 6.2 | W (100%) | 1.6 ± 0.1 | 112 ± 12 | 73 ± 8 | 27.06 ± 6.16 | n. a. |
| Total | 403 | |||||||||
Abbreviations: BSA, body surface area; DBP, diastolic blood pressure; LAVI, left atrial volume index; LVMI, left ventricular mass index; SBP, systolic blood pressure.
Type of sport: EA, elite akido (A3); EB, elite boxers (C3); EWL, elite weight lifers (A3); CMR, competitive marathon runners (C1); CLR, competitive long‐distance runners (C1); PBP, professional basket players (C2); PHP, professional handball players (C2); PSP, professional soccer players (C1); PT, professional triathletes (C3); PVP, professional volley players (B1).
Mean age ranged from 22 ± 720 to 43 ± 4.0 years17 (pooled mean 29.7 ± 1.9 years); 73.7% of athletes were men (n = 296). Average BSA ranged from 1.60 ± 0.1022 to 2.20 ± 0.17 m2 19 (pooled mean 1.95 ± 0.03 m2). Average office systolic BP (data from 5 studies) ranged from 112 ± 12 mm Hg22 to 134 ± 9 mm Hg,7 diastolic BP from 73 ± 8 mm Hg22 to 79 ± 4 mm Hg21 (pooled mean values being 123.1 ± 3.0 and 75.8 ± 1.4 mm Hg, respectively). Average LVMI (data from 4 studies) varied from 82 ± 23 g/m2 20 to 143 ± 12 g/m2 17 (pooled mean g/m2 105 ± 9 g/m2).
3.2. Clinical characteristics of controls
All subjects belonging to the control group had never been involved in competition or recreational sports and were not engaged in any regular training program. In non‐trained individuals (68.3% men) mean age ranged from 23.0 ± 6.020 to 45.0 ± 5.0 years17 (pooled mean 29.0 ± 1.8 years) (Table 2). Average BSA ranged from 1.63 ± 0.10 m2 22 to 2.0 ± 0.30 m2 16 (pooled mean 1.83 ± 0.03 m2). Average office systolic BP ranged from 110 ± 13 mm Hg22 to 129 ± 18 mm Hg,7 diastolic BP from 71 ± 9 mm Hg22 to 81 ± 14 mm Hg7 (pooled mean values being 119 ± 1.4 mm Hg and 76.1 ± 1.0 mm Hg, respectively). Average LVMI varied from 63 ± 22 g/m2 20 to 86 ± 10 g/m2 19 (pooled mean 75 ± 5.0 g/m2).
Table 2.
Demographic and clinical characteristics of echocardiographic studies on left atrial strain in non‐trained controls
| Author | Year | Sample(n) | Age (years) | Gender (men/women)%) | BSA (m2) | SBP(mm Hg) | DBP(mm Hg) | LAVI (mL/m2) | LVMI (g/m2) |
|---|---|---|---|---|---|---|---|---|---|
| D'Ascenzi16 | 2011 | 26 | 26.0 ± 6.0 | M (100%) | 2.0 ± 0.30 | n.a. | n.a. | 20.2 ± 3,9 | n.a. |
| Gabrielli17 | 2012 | 20 | 45.0 ± 5.0 | M (80%) | n.a. | 121 ± 9 | n.a. | 25.0 ± 2.0 | 78.0 ± 6.0 |
| D'Ascenzi18 | 2014 | 24 | 24.4 ± 3.2 | W (100%) | 1.68 ± 0.14 | n. a. | n. a. | 20.5 ± 4.9 | n. a. |
| Gabrielli19 | 2014 | 20 | 27.0 ± 4.0 | M (100%) | 1.8 ± 0.12 | n.a. | n.a. | 24.8 ± 4.3 | 86.0 ± 10.0 |
| D'Ascenzi6 | 2015 | 90 | 25.0 ± 4.0 | M (68%) | 1.8 ± 0.2 | 120 ± 8 | 76 ± 10 | 18.4 ± 7.8 | 71.1 ± 15.3 |
| McClean7 | 2015 | 20 | 27.0 ± 8.0 | M (100%) | 1.96 ± 0.13 | 129 ± 18 | 81 ± 14 | 23.0 ± 5.0 | n. a. |
| D'Ascenzi20 | 2016 | 23 | 23.0 ± 6.0 | M (43%) | 1.8 ± 0,2 | n.a. | n.a. | 20.7 ± 4.7 | 62.7 ± 21.9 |
| Gabrielli21 | 2016 | 30 | 35.0 ± 4.0 | M (100%) | 1.9 ± 0.2 | 119 ± 6 | 77 ± 4 | 18.6 ± 4.1 | n.a. |
| Sanchismen 22 | 2017 | 20 | 34.1 ± 5.1 | M (100%) | 1.91 ± 0.15 | 120 ± 7 | 76 ± 5 | 18.6 ± 5,0 | n. a. |
| Sanchiswomen 22 | 2017 | 21 | 37.6 ± 4.3 | W (100%) | 1.63 ± 0.1 | 110 ± 13 | 71 ± 9 | 15.8 ± 4.5 | n. a. |
Abbreviations: BSA, body surface area; DBP, diastolic blood pressure; LAVI, left atrial volume index; LVMI, left ventricular mass index; SBP, systolic blood pressure.
3.3. Echocardiographic methods
LAV was measured in all studies according to current echocardiographic guidelines indications24, 25 by area length method or by disk summation algorithm and indexed to BSA. For all studies, the authors used 2D echocardiographic assessment of LAV. Atrial myocardial deformation was measured offline from 2D echocardiographic images using commercial dedicated software with frame rates between 40 and 90 frames per second. Table S1 summarizes technical data regarding the brand and model of ultrasound system, probe frequency, echocardiographic protocols used to measure LAV and software used to measure LA myocardial deformation. Gating for LA strain assessment was done in two possible ways: the first and larger group of authors used a R‐R gaiting,6, 7, 16, 18, 20 whereas the second group of investigators used P gaiting.17, 19, 22 Both methods are accepted in the guidelines, although R‐R gaiting is preferred. In all studies, LA endocardium was manually traced and corrected if necessary and average longitudinal strain curve was automatically provided by the software. All studies included in this meta‐analysis used GE specific software ‐ Echopac for evaluation of LA strain and strain rates (Table S1).
3.4. Left atrial volume and strain in athletes and controls
Left atrial volume (LAV) was measured in all studies according to current echocardiographic guidelines indications24, 25 by area length method or by disk summation algorithm and indexed to BSA.
Mean LAVI ranged from 24.6 ± 7.3 mL/m2 6 to 40.0 ± 5.0 mL/m2 17 in athletes, and from 15.8 ± 4.5 mL/m2 22 to 25.0 ± 2.0 mL/m2 17 in non‐trained controls. In the pooled athlete population (data from all selected studies), average LAVI value was 28.0 ± 1.0 mL/m2 (CI: 26.0‐30.0); the corresponding value in controls was 20.7 ± 0.8 mL/m2 (CI: 19.0‐22.4). SMD in LAVI was positive in favor of athletes (1.04 ± 0.12, CI: 0.88‐1.19, P < 0.0001) (Figure S2).
Atrial myocardial deformation was measured offline from 2D echocardiographic images using commercial dedicated software with frame rates between 40 and 90 frames per second. Table S1 summarizes technical data regarding the brand and model of ultrasound system, probe frequency, echocardiographic protocols used to measure LAV, and softwares used to measure LA myocardial deformation.
In the present meta‐analysis, the following 2D speckle echocardiographic parameters were considered: global peak atrial longitudinal strain (PALS), global peak atrial contraction strain (PACS), LA peak longitudinal strain rate during ventricular systole (LASRsyst), LA peak longitudinal strain rate during late diastole (LASRlate). Figure 1 shows the assessment of LA strain and strain rate in four‐ and two‐chamber view.
Figure 1.
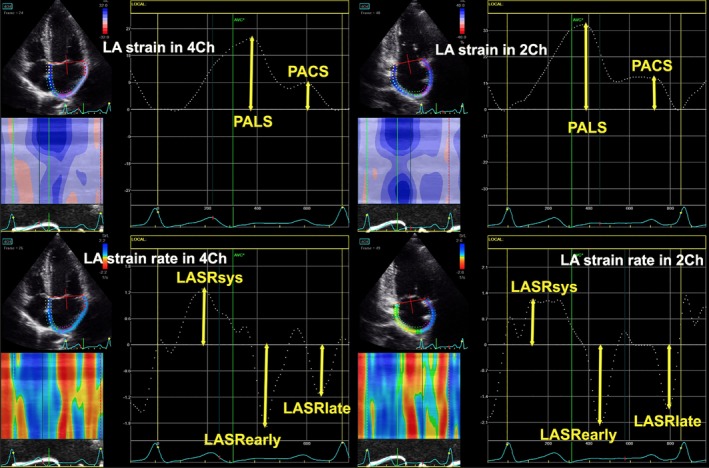
The echocardiographic assessment of left atrial strain and strain rates in four‐ and two‐chamber views. LASRearly, LA peak longitudinal strain rate during early diastole, LASRlate, LA peak longitudinal strain rate during late diastoleLASRsyst, LA peak longitudinal strain rate during ventricular systole; PACS, global peak atrial contraction strain; PALS, global peak atrial longitudinal strain
Pooled average global PALS was 37% ± 1.2% (CI: 34.6%‐39.5%) in athletes and 38.5% ± 1.5% (CI: 35.3%‐41.2%) in controls. Figure 2 reports the results of the meta‐analysis from 8 studies providing data on global PALS in 353 athletes and 267 controls. SMD in global PALS was higher in non‐trained controls (0.16, CI: 0.32‐0.04, P = 0.04).
Figure 2.
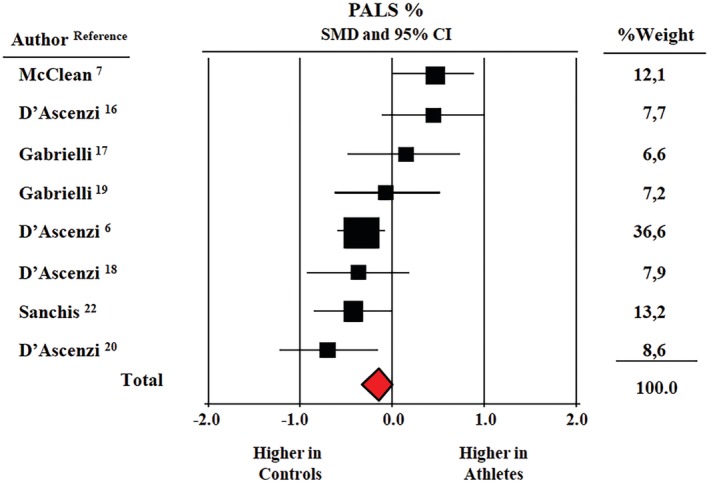
Forest plot for standard means difference (SMD) global peak atrial longitudinal strain (PALS) in elite athletes and healthy non‐athletic controls; data from eight studies (CI, confidence intervals, P = 0.04)
Pooled average global PACS was 12.4% ± 0.4% in athletes and 13.6% ± 0.7% in controls. Figure 3 shows the results of the meta‐analysis from 7 studies reporting data on global PACS in 342 athletes and 236 controls. SMD in global PACS between the two groups was not statistically significant (−0.26, CI: −0.74/+0.22, P = 0.29).
Figure 3.
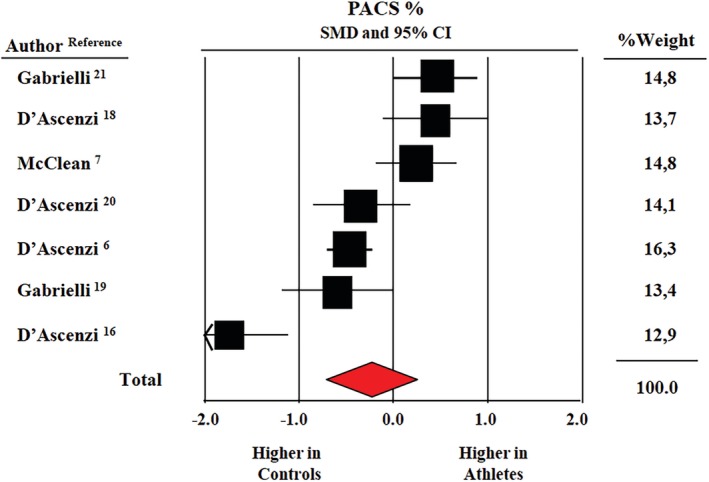
Forest plot for standard means difference (SMD) global peak atrial contraction strain (PACS) in elite athletes and healthy non‐athletic controls; data from seven studies (CI, confidence intervals P = 0.29)
Finally, no differences regarding SMD in LASRsyst (−0.16 CI: −0.43 /+0.11, P = 0.24) were observed between athletes and controls (data from four studies) (Figure S1). This was not the case for LASRlate. Athletes showed a lower LASRlate value (−1.56 ± 0.08, CI: ‐1.70/−1.40 vs 1.74 ± 0.09, CI: −1.93/−1.56) (data from five studies). As shown in Figure S4, SMD was higher in controls (−0.32 CI: −0 to 55/0.09, P = 0.007).
4. DISCUSSION
The present meta‐analysis of nine studies including 403 elite athletes and 297 controls provides a comprehensive information on LA strain in this population. Several important findings should be discussed: (a) athletes had higher LA volume than healthy controls; (b) global LA longitudinal strain (PALS), a marker of LA reservoir function was reduced in athletes with borderline significance; (c) late diastolic LA strain rate, a marker of LA contractile function was lower in athletes (LASRlate); (d) LA strain during LA contraction (PACS) and LA strain rate during LV systole (LASRsyst) were similar in athletes and in controls.
Most studies documented an increase of LA dimension in athletes.6, 7, 8, 16, 17, 18, 19, 20, 21 A large study including 1777 competitive athletes reported a mild increase of LA anteroposterior diameter (>40 mm) in 18% of athletes and a marked LA enlargement (>45 mm) 2% of them.26 The assessment of LA volume provides a more accurate evaluation of LA dimensions than linear measurements, which tend to underestimate LA size. A meta‐analysis of 54 studies including 7189 athletes and 1375 controls, documented that LA dilatation when defined by LA diameter was present in 13% athletes and in up to 30% of athletes when defined by LA volume index.1
LA enlargement is seen as a physiological adaptation to training: LA volume has been reported to gradually increase during lifetime endurance27 and sport season.28 According to D'Ascenzi et al., LA remodeling was closely associated to LV adaptation to exercise and alterations of both cardiac chambers were completely reverted after detraining.28 The increase of LA volume during training,29 has been also reported to be differently affected by types of effort (high dynamic vs low dynamic).7 Of note, a reduction of LA maximal volume during exercise has been observed in endurance athletes.21
LA enlargement in athletes may not be a marker of LA dysfunction. In a large study involving 595 male elite football players, Gjerdalen et al documented that in athletes characterized by increased LA volume, LA function determined by LA strain was fully preserved.2 Similar findings have been reported by McClean et al.7 The CHILD study failed to observe any difference in LA reservoir and contractile function determined by volumetric and strain parameters between pre‐adolescent athletes and controls at baseline.29 The same authors, however, documented a reduced reservoir and contractile LA function after 5 months of intensive supervised training.29 A reduced contractile, but not reservoir function of LA has been also reported.16 Unchanged LA volume‐ and strain‐derived functions during lifetime training, in front of gradual increment in LA conduit and reservoir volumes have been reported by other authors.27 D'Ascenzi et al documented that LA reservoir and contractile functions were reduced in 150 elite athletes6 and the decrease of LA functions was related to the duration of training program in adolescent soccer players.30 According to the same authors, LA reservoir, and contractile functions in female competitive athletes were not different from controls at baseline, but progressively decreased after 16 weeks of supervised intensive training.18
Our meta‐analysis showed that LA global longitudinal strain tended to be reduced in athletes, a finding which is compatible with a reduced reservoir function, whereas no differences in LA contractile function estimated by strain method were found between athletes and controls. It should be pointed out, however, that LA late diastolic strain rate, a marker of LA contractile function, was lower in athletes. These differences are probably related to the small sample size and statistical power of studies investigating elite athletes. Furthermore, at least three different parameters may account for LA function according to strain method: longitudinal strain, strain rate, and velocity. In healthy volunteers, these parameters of LA mechanical function have been reported to be unrelated with echocardiographic parameters of LV diastolic function, LA volume and function.31 To these differences may be related our results on LA atrial contraction strain and late diastolic strain rate in athletes.
Our meta‐analysis revealed that athletes had reduced global LA longitudinal strain, which resembles LA reservoir function. The authors who reported these findings did not provide explanations for their results.19, 22 The reduced LA reservoir function is strong predictor of incident cardiovascular events, atrial fibrillation occurrence and recurrence, and worse outcome in patients with heart failure with preserved ejection fraction.32, 33, 34 Our present findings showed that athletes had reduced LA pump function. The reduction of LA pump function is associated with increased risk of atrial fibrillation,35 which could explain higher incidence of atrial arrhythmias in elite athletes later in life. This is an important clinical implication of our results.
Increased LAVI in our meta‐analysis suggests that LA cavity reacts to the increased hemodynamic pre‐load in elite athletes to adapt the elevated venous return and maintain normal contractile function, efficient emptying function, and stroke volume. However, decreased LA reservoir function together with decreased LA booster pump function in athletes in our meta‐analysis should be also discussed in the reflection of methods used for LA phasic function. Even though strain‐ and volume‐derived parameters used for evaluation of LA phasic function should closely correlate, this is not always the case.36 Longitudinal LA strain showing reservoir function is negatively associated with LAVI,36 whereas LA strains resembling conduit and pump function are not necessary associated with LA volume‐derived equivalent parameters of LA phasic function.36 This could be the reason why we obtained reduced LA reservoir function (with borderline statistical significance) evaluated with LA global longitudinal strain, and other authors obtained different results using LA reservoir emptying fraction.28, 29 Recent study showed that LA pump function, estimated by LA strain, significantly increased immediately after marathon and especially in highly trained athletes.37 The authors reported significant positive correlation between the increase in LA pump function and maximal oxygen consumption measured 1 week before marathon,37 which could indicate that the increment in LA pump function is associated with better cardiorespiratory fitness and may significantly improve performance of athletes.
In pathological conditions, such as arterial hypertension LA remodeling considers impairment of LA reservoir and conduit function as a consequence of increased afterload and elevated LV stiffness.38 An increased LA active contribution to LV filling is the response on elevated LV filling pressure and it maintains normal stroke volume.38 In athlete's heart is expected the dynamic remodeling of the LA which includes an enhanced reservoir and conduit function, while LA contractile function does not change, suggesting a benign adaptation of the LV. However, our meta‐analysis with LA strain data possibly shows that LA remodeling in the athlete is not only a relevant benign contribution to training‐induced cardiovascular remodeling, but could be important substrate for arrhythmias that athletes experience later in the life.35 However, one should be careful in the interpretation of our results regarding LA pump function because strain‐derived LA pump function (PACS) was similar between athletes and controls, whereas strain‐rate‐derived LA pump function showed that LASRlate (parameter of LA pump function) was significantly decreased in elite athletes.
In comparison with recently published large meta‐analysis of normal LA strain, our LA global longitudinal strain in athletes was a bit lower than in normal population (37% vs 39.4%).39 However, our LA strain resembling LA pump function was significantly lower among the athletes than in the normal population (12.4% vs 17.4%).39 This comparison confirms significant LA functional remodeling among elite athletes. The question which remains is if these changes are only benign adaptation to intensive training or they represent initial substrate for further LA changes that could induce adverse cardiovascular outcome in the later course of athletes' life.
Total emptying fraction of LA has been shown to better predict mortality compared to LA volume index in a global population,10 suggesting that LA reservoir function is a more reliable marker of LA remodeling and LV dysfunction than LA enlargement. In a recent study, LA peak longitudinal strain, as a marker of LA reservoir function, has been shown to be the best predictor of atrial fibrillation in male endurance veteran athletes.16 Moreover, LA contractile function has been reported to be an independent predictor of life‐threatening ventricular arrhythmias,35 and possibly the relationship between long‐term endurance exercise and increased risk of cardiac arrhythmias.
Our data suggest that LA function may provide additional information on physiological adaptations to training in elite athletes and differentiate between athlete's heart and cardiac disease. D'Ascenzi et al suggested that the combined evaluation of LA volume and function by volumetric and strain methods may differentiate pathological LA dilatation characterized by reduced reservoir and contractile function from LA enlargement induced by a physiological adaptation to training characterized by preserved LA reservoir and contractile function. Our meta‐analysis findings indicate that both LA reservoir and contractile functions are reduced in elite athletes and raise the question about definition of pathological LA remodeling. The main problem which imposes is if echocardiography is accurate enough to provide precise information. The reported inter‐ and intraobserver variability of LA volumetric and strain parameters were high,36 which confirms the reliability of these data. There is a significant correlation between LAVI obtained by echocardiography and cardiac magnetic resonance (CMR), which still remains the “gold standard “of cardiac imaging.
4.1. Limitations
Limitations and strengths of our meta‐analysis should be underlined. The cross‐sectional nature of all studies included in the meta‐analysis does not allow to assess the causal relationship between training and LA remodeling in elite athletes. Available data regarding parameters of volumetric and LA function are scanty, thus, we could not perform a meta‐analysis of all LA indices. Different authors used various types of LA strain assessment (R‐R and P gating). Predominantly was used R‐R‐gating, but this might be considered as a limitation. Recently published meta‐analysis did not show a significant difference between R‐R and P‐P gating with regard to the practice of measurement of atrial strain.39 For this meta‐analysis, we used aggregated and not original data, which was additional limitation. However, willingness of authors to share their original study is usually limited and therefore analysis of aggregated data represents common and acceptable method. The intra‐ and inter‐observer variability of LA volumes and strain may be also considered as a limitation, but investigations showed that this kind of variability with modern software was unremarkable.36 The number of investigators in this field is very limited, which is understandable due to very specific population of elite athletes who are usually referred to small number of medical centers, and therefore several references originated from the same research group. Considering already limited number of included studies, this limitation was not possible to avoid.
5. CONCLUSION
LA enlargement is a physiological adaptation to training. Whether reduced LA reservoir and contractile function are adaptive changes in athletes remains to be defined. As functional remodeling of LA has been recently related to development of paroxysmal atrial fibrillation in male veteran athletes and normalization of LA changes during deconditioning has been reported, evaluation of LA function by volume and strain analysis is of major importance. All studies involved in this meta‐analysis used GE echocardiographic machine and Echopac software for evaluation of LA strain and strain rates. However, other studies that used other vendors did not fulfilled inclusion criteria for our meta‐analysis. Longitudinal investigations are needed to determine the influence of training‐induced LA remodeling on cardiovascular outcome in elite athletes.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
AUTHOR CONTRIBUTIONS
Cesare Cuspidi and Marijana Tadic contributed to the conception or design of the work, contributed to the acquisition, analysis, or interpretation of data for the work, drafted the manuscript. Carla Sala contributed to the conception or design of the work, contributed to the acquisition, analysis, or interpretation of data for the work and critically revised the manuscript. Elisa Gherbesi contributed to the acquisition, analysis, or interpretation of data. Guido Grassia and Giuseppe Mancia critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Supporting information
FIGURE S1 Schematic flow‐chart for the selection of studies.
FIGURE S2 Forest plot for standard means difference (SMD) of left atrial volume index (LAVI) in elite athletes and healthy non‐athletic controls; data from 9 studies (CI, confidence intervals P < 0.001).
FIGURE S3 Forest plot for standard means difference (SMD) of LA peak longitudinal strain during ventricular systole (LASRsyst) in elite athletes and healthy non‐athletic controls: data from 4 studies (CI, confidence intervals P = 0.24);
FIGURE S4 Forest plot for standard means difference (SMD) of LA peak longitudinal strain during late diastole (LASRlate): data from 5 studies (lower part) (CI, confidence intervals P = 0.007).
TABLE S1 Echocardiographic methodology in studies reporting data on left atrial strain in elite athletes.
Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Left atrial function in elite athletes: A meta‐analysis of two‐dimensional speckle tracking echocardiographic studies. Clin Cardiol. 2019;42:579–587. 10.1002/clc.23180
REFERENCES
- 1. Iskandar A, Mujtaba MT, Thompson PD. Left atrium size in elite athletes. JACC Cardiovasc Imaging. 2015;8:753‐762. [DOI] [PubMed] [Google Scholar]
- 2. Gjerdalen GF, Hisdal J, Solberg EE, Andersen TE, Radunovic Z, Steine K. Atrial size and function in athletes. Int J Sports Med. 2015;36:1170‐1176. [DOI] [PubMed] [Google Scholar]
- 3. Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28:236‐244. [DOI] [PubMed] [Google Scholar]
- 4. Blume GG, Mcleod CJ, Barnes ME, et al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421‐430. [DOI] [PubMed] [Google Scholar]
- 5. Wright S, Sasson Z, Gray T, et al. Left atrial phasic function interacts to support left ventricular filling during exercise in healthy athletes. J Appl Physiol. 2015;119:328‐333. [DOI] [PubMed] [Google Scholar]
- 6. D'Ascenzi F, Pelliccia A, Natali BM, et al. Increased left atrial size is associated with reduced atrial stiffness and preserved reservoir function in athlete's heart. Int J Cardiovasc Imaging. 2015;31:699‐705. [DOI] [PubMed] [Google Scholar]
- 7. McClean G, George K, Lord R, et al. Chronic adaptation of atrial structure and function in elite male athletes. Eur Heart J Cardiovasc Imaging. 2015;16:417‐422. [DOI] [PubMed] [Google Scholar]
- 8. D'Ascenzi F, Caselli S, Solari M, et al. Novel echocardiographic techniques for the evaluation of athletes' heart: a focus on speckle‐tracking echocardiography. Eur J Prev Cardiol. 2016;23:437‐446. [DOI] [PubMed] [Google Scholar]
- 9. D'Ascenzi F, Anselmi F, Focardi M, Mondillo S. Atrial enlargement in the athlete's heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J am Soc Echocardiogr. 2018;31:148‐157. [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cameli M, Lisi M, Focardi M, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264‐269. [DOI] [PubMed] [Google Scholar]
- 12. Sardana M, Lessard D, Tsao CW, et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham Offspring Study. J am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467‐475. [DOI] [PubMed] [Google Scholar]
- 14. Hubert A, Galand V, Donal E, et al. Atrial function is altered in lone paroxysmal atrial fibrillation in male endurance veteran athletes. Eur Heart J Cardiovasc Imaging. 2018;19:145‐153. [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 16. D'Ascenzi F, Cameli M, Zacà V, et al. Supernormal diastolic function and role of left atrial myocardial deformation analysis by 2D speckle tracking echocardiography in elite soccer players. Echocardiography. 2011;28:320‐326. [DOI] [PubMed] [Google Scholar]
- 17. Gabrielli L, Enríquez A, Córdova S, Yáñez F, Godoy I, Corbalán R. Assessment of left atrial function in hypertrophic cardiomyopathy and athlete's heart: a left atrial myocardial deformation study. Echocardiography. 2012;29:943‐949. [DOI] [PubMed] [Google Scholar]
- 18. D'Ascenzi F, Pelliccia A, Natali BM, et al. Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ Cardiovasc Imaging. 2014;7:222‐229. [DOI] [PubMed] [Google Scholar]
- 19. Gabrielli L, Bijnens BH, Butakoff C, et al. Atrial functional and geometrical remodeling in highly trained male athletes: for better or worse? Eur J Appl Physiol. 2014;114:1143‐1152. [DOI] [PubMed] [Google Scholar]
- 20. D'Ascenzi F, Solari M, Biagi M, et al. P‐wave morphology is unaffected by training‐induced biatrial dilatation: a prospective, longitudinal study in healthy athletes. Int J Cardiovasc Imaging. 2016;32:407‐415. [DOI] [PubMed] [Google Scholar]
- 21. Gabrielli L, Bijnens BH, Brambila C, et al. Differential atrial performance at rest and exercise in athletes: potential trigger for developing atrial dysfunction? Scand J Med Sci Sports. 2016;26:1444‐1454. [DOI] [PubMed] [Google Scholar]
- 22. Sanchis L, Sanz‐de La Garza M, Bijnens B, et al. Gender influence on the adaptation of atrial performance to training. Eur J Sport Sci. 2017;17:720‐726. [DOI] [PubMed] [Google Scholar]
- 23. Mitchell JH, Haskell W, Snell P, van Camp SP. Task force 8: classification of sports. J am Coll Cardiol. 2005;45:1364‐1367. [DOI] [PubMed] [Google Scholar]
- 24. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J am Soc Echocardiogr. 2005;18:1440‐1463. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J am Soc Echocardiogr. 2015;28:1‐39e14. [DOI] [PubMed] [Google Scholar]
- 26. Pelliccia A, Maron BJ, Di Paolo FM, et al. Prevalence and clinical significance of left atrial remodleing ling in competitive athletes. J am Coll Cardiol. 2005;46:690‐696. [DOI] [PubMed] [Google Scholar]
- 27. Brugger N, Krause R, Carlen F, et al. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int J Cardiol. 2014;170:419‐425. [DOI] [PubMed] [Google Scholar]
- 28. D'Ascenzi F, Pelliccia A, Natali BM, et al. Training‐induced dynamic changes in left atrial reservoir, conduit, and active volumes in professional soccer players. Eur J Appl Physiol. 2015;115:1715‐1723. [DOI] [PubMed] [Google Scholar]
- 29. D'Ascenzi F, Solari M, Anselmi F, et al. Atrial chamber remodelling in healthy pre‐adolescent athletes engaged in endurance sports: a study with a longitudinal design. The CHILD study. Int J Cardiol. 2016;223:325‐330. [DOI] [PubMed] [Google Scholar]
- 30. D'Ascenzi F, Cameli M, Lisi M, et al. Left atrial remodeling in competetive adolescent soccer players. Int J Sports Med. 2012;33:795‐801. [DOI] [PubMed] [Google Scholar]
- 31. Sun JP, Yang Y, Guo R, et al. Left atrial regional phasic strain, strain rate and velocity by speckle‐tracking echocardiography normal values and effects of aging in a large group of normal subjects. Int J Cardiol. 2013;168:3473‐3479. [DOI] [PubMed] [Google Scholar]
- 32. Chirinos JA, Sardana M, Ansari B, et al. Left atrial phasic function by cardiac magnetic resonance feature tracking is a strong predictor of incident cardiovascular events. Circ Cardiovasc Imaging. 2018;11(12):e007512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mirza M, Caracciolo G, Khan U, et al. Left atrial reservoir function predicts atrial fibrillation recurrence after catheter ablation: a two‐dimensional speckle strain study. J Interv Card Electrophysiol. 2011;31(3):197‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carluccio E, Biagioli P, Mengoni A, et al. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction. Circ Cardiovasc Imaging. 2018;11(11):e007696. [DOI] [PubMed] [Google Scholar]
- 35. Negishi K, Negishi T, Zardkoohi O, et al. Left atrial booster pump function is an independent predictor of subsequent life‐threatening ventricular arrhythmias in non‐ischaemic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2016;17:1153‐1160. [DOI] [PubMed] [Google Scholar]
- 36. Miglioranza MH, Badano LP, Mihăilă S, et al. Physiologic determinants of left atrial longitudinal strain: a two‐dimensional speckle‐tracking and three‐dimensional echocardiographic study in healthy volunteers. J am Soc Echocardiogr. 2016;29(11):1023‐1034.e3. [DOI] [PubMed] [Google Scholar]
- 37. Gabrielli L, Herrera S, Contreras‐Briceño F, et al. Increased active phase atrial contraction is related to marathon runner performance. Eur J Appl Physiol. 2018;118(9):1931‐1939. [DOI] [PubMed] [Google Scholar]
- 38. Tadic M, Cuspidi C, Pencic B, Marjanovic T, Celic V. The association between heart rate variability and biatrial phasic function in arterial hypertension. J am Soc Hypertens. 2014;8(10):699‐708. [DOI] [PubMed] [Google Scholar]
- 39. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J am Soc Echocardiogr. 2017. Jan;30(1):59‐70.e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Schematic flow‐chart for the selection of studies.
FIGURE S2 Forest plot for standard means difference (SMD) of left atrial volume index (LAVI) in elite athletes and healthy non‐athletic controls; data from 9 studies (CI, confidence intervals P < 0.001).
FIGURE S3 Forest plot for standard means difference (SMD) of LA peak longitudinal strain during ventricular systole (LASRsyst) in elite athletes and healthy non‐athletic controls: data from 4 studies (CI, confidence intervals P = 0.24);
FIGURE S4 Forest plot for standard means difference (SMD) of LA peak longitudinal strain during late diastole (LASRlate): data from 5 studies (lower part) (CI, confidence intervals P = 0.007).
TABLE S1 Echocardiographic methodology in studies reporting data on left atrial strain in elite athletes.


