Abstract
Heart failure is a common complication in patients with diabetes, and people with both conditions present a worse prognosis. Sodium–glucose cotransporter 2 inhibitors (SGLT2Is) increase urinary glucose excretion, improving glycaemic control. In type 2 diabetes (T2D), some SGLT2Is reduce major cardiovascular events, heart failure hospitalisations and worsening of kidney function independent of glycaemic control. Multiple mechanisms (haemodynamic, metabolic, hormonal and direct cardiac/renal effects) have been proposed to explain these cardiorenal benefits. SGLT2Is are generally well tolerated, but can produce rare serious adverse effects, and the benefit/risk ratio differs between SGLT2Is. This article analyses the mechanisms underlying the cardiorenal benefits and adverse effects of SGLT2Is in patients with T2D and heart failure and outlines some questions to be answered in the near future.
Keywords: Type 2 diabetes, heart failure, sodium–glucose cotransporter, sodium-glucose cotransporter inhibitors, cardiovascular outcome trials, safety profile
Type 2 diabetes (T2D) remains a major cardiovascular (CV) risk factor[1–5] and it confers an approximately two- to threefold fold excess risk for coronary heart disease, including MI, stroke and heart failure (HF) in patients with and in patients without established cardiovascular disease (CVD).[1,6–8] The prevalence of T2D among patients with HF is as high as 40–45% and that of HF in patients with T2D is reported to be 10–23%.[8] Patients with both conditions – regardless of ejection fraction – present a higher risk of hospitalisation for HF (HHF), all-cause and CV mortality, irrespective of ischaemic/non-ischaemic aetiology.[8–10] The risk is further increased in the presence of diabetic nephropathy. Therefore, new therapeutic strategies that improve symptoms and reduce mortality and hospitalisations are needed for patients with T2D, HF and renal impairment.
For decades, it was hypothesised that glucose-lowering drugs (using HbA1c as a surrogate marker) might improve CV outcomes. However, this glucocentric approach was proved incorrect because firstly, some glucose-lowering drugs (muraglitazar, rosiglitazone) decreased HbA1c levels but worsened CV outcomes, and secondly, the results of the post-trial follow-up of the UK Prospective Diabetes Study (UKPDS),and of a meta-analysis of large glucose-lowering outcome trials,suggested an approximately 15% cardiovascular risk reduction (RR) per 1% decrement in HbA1c.[11,12] The UKPDS recruited low-risk patients with newly diagnosed T2D (only 7.5% had CVD at baseline). During the interventional phase of the study, intensive glucose control using metformin and sulphonylurea-insulin reduced HbA1c by 0.9% for a median of 10 years, but not the risk of death, MI, HF, stroke, or amputations.[11] However, in the 10-year post-trial follow-up, patients originally randomised to intensive therapy achieved a significant reduction in MI (15%) and all-cause mortality (13%) despite an early loss of glycaemic differences between the intensive and conventional therapy groups.[13]
These findings suggested that early and intensive glucose control in newly diagnosed T2D patients could have long-term benefits (‘legacy effect’), irrespective of treatment modality. However, the meta-analysis of randomised controlled trials (RCTs) of more- versus less-intensive glycaemic control in patients with long-standing T2D (8–12 years) and either known CV disease or other risk factors showed that more-intensive glycaemic control (difference in HbA1c 0.9%) was associated with a significant 9% RR for the composite of major adverse cardiovascular events (MACE; CV death, nonfatal stroke or nonfatal MI) during an average follow-up of 4.4 years. This reduction was driven primarily by a 15% RR in MI. However, intensive glucose lowering did not reduce the risk of fatal/nonfatal stroke, peripheral artery disease, hospitalised or fatal HF or CV and all-cause mortality, but increased the risk of severe hypoglycaemia.[12,14]
The differences in outcomes among these studies and the long-term ‘legacy effect’ observed in the UKPDS could be related to important differences in the study populations, HbA1c reduction from baseline, speed of HbA1c lowering, duration of follow-up and background therapies.
Because of the concerns regarding adverse cardiovascular outcomes with antidiabetic agents, in 2008 the US Food and Drug Administration (FDA) mandated sponsors to conduct long-term cardiovascular outcome trials (CVOTs) for ensuring the cardiovascular safety of all new glucose-lowering drugs, with a focus on MACE.[15] Surprisingly, HF was not included as a component of composite endpoints.
Recent CVOTs performed with three sodium–glucose cotransporter 2 inhibitors (SGLT2Is; canagliflozin, empagliflozin and dapagliflozin) demonstrated noninferiority compared with placebo in the MACE primary composite end point and that they reduced the risk of HHF and of progression of renal disease, regardless of the presence of atherosclerotic CVD or HF at baseline.[16] These findings represent a clinical breakthrough in treating T2D as compared with classical glucose-lowering drugs. This article analyses the effects of SGLT2Is in CVOTs, the mechanisms underlying their cardiorenal benefits and their safety profile, together with questions that should be answered in the near future.
SGLT2 Inhibitors
Sodium-dependent glucose cotransporters (SGLTs) are responsible for tissular glucose translocation. SGLT1 is widely expressed in numerous organs (the distal S3 segment of the proximal renal tubule, intestines, heart and skeletal muscles), while SGLT2 is expressed in the luminal surface of the S1 segment of the proximal tubule and alfa-pancreatic cells.[17–19] The active transport of glucose via SGLT2 is linked to Na+ transport maintained by its active extrusion via the Na+/K+ ATPase of the basolateral membrane into the intracellular fluid. Under normal conditions, glucose is freely filtered into the urine at the glomerulus (180 g/day) and reabsorbed in the proximal tubuli by SGLT2 (90%) and SGLT1 (10%).[20]
The plasma glucose concentration above which urinary glucose excretion occurs is approximately 180–200 mg/dl, but under diabetic conditions increases up to 300 mg/dl because of the increased activity of SGLT2. SGLT2Is (canagliflozin, dapagliflozin, empagliflozin and ertugliflozin) shift the renal tubular threshold for glycosuria to 50 mg/dl, reduce the reabsorption of filtered glucose (30–50%) and increase glycosuria, decreasing plasma glucose and HbA1c levels independent of insulin.[17] Because glycosuria occurs only in the presence of hyperglycaemia, the risk of hypoglycaemia with SGLT2Is is low. Additionally, because Na+ is co-transported with glucose, SGLT2Is cause an osmotic diuresis (increased urine output 107–450 ml/day) and a small natriuresis.[21]
Cardiovascular Outcomes Trials with SGLT2Is
Cardioprotective Effects
The effects of empagliflozin, canagliflozin and dapagliflozin were analysed in three CVOTs: EMPAgliflozin cardiovascular outcome event trial in type 2 diabetes mellitus patients – Removing Excess Glucose (EMPA-REG OUTCOME), the CANagliflozin cardioVascular Assessment Study (CANVAS) Program and Dapagliflozin Effect on CardiovascuLAR Events – Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) respectively (Table 1).[22–24]
Table 1: Characteristics of Cardiovascular Outcomes Trials Completed with Sodium–glucose Cotransporter 2 Inhibitors.
| Parameters | EMPA-REG OUTCOME[22] | CANVAS Program[23] † | DECLARE-TIMI 58[24 |
|---|---|---|---|
| Drug | Empagliflozin | Canagliflozin | Dapagliflozin |
| Number of patients/mean age (years) | 7,020/63.1 | 10,142/63.3 | 17,160/63.9 |
| Women (%) | 28.5 | 35.8 | 37.4 |
| White/Asian/black | 72.6/21.5/5.1 | 78.3/12.7/3.3 | 79.4/13.5/3.6 |
| Diabetes duration (years) | 57% >10 | 13.5 | 10.5 |
| HbA1c (%) | 8.0 | 8.2 | 8.3 |
| BMI (kg/m2) | 30.7 | 32 | 32 |
| Established CV disease (%) | 99.5 | 65.6 | 40.6 |
| Coronary artery disease (%) MI (%) Stroke (%) Heart failure (%) Peripheral artery disease (%) |
76 47 23.5 10.1 21 |
57 – 19.3 14.4 20.8 |
33.0 – 7.6 10.0 6.0 |
| Median follow-up time (years) | 3.1 | 2.4 | 4.2 |
| eGFR (ml/min/1.73 m2) | 83.1 | 76.5 | 85.2 |
| eGFR <60 ml/min/1.73 m2 (%) | 25.9 | 20.1 | 7.4 |
| Microalbuminuria (%) | 10.9 | 22.6 | |
| Macroalbuminuria (%) | 28.5 | 7.6 | |
| Prior history of amputations (%) | – | 2.3 | – |
| Primary endpoint | MACE (1) | MACE (1) | MACE (2); a composite of CVD or HHF |
| Three-point MACE: CV death, nonfatal MI, or nonfatal stroke | 0.86 (0.74–0.99) NI, p<0.001 Superiority, p=0.04 |
0.86 (0.75–0.97) NI, p<0.001 Superiority, p=0.02 |
0.93 (0.84–1.03) NI, p<0.001 Superiority, p=0.17 |
| CV death | 0.62 (0.49–0.77)* | 0.87 (0.72–1.06) | 0.98 (0.81–1.17) |
| CV death or hospitalisation for HF | 0.66 (0.55–0.79)* | 0.78 (0.67–0.91)* | 0.83 (0.73–0.95)* |
| All-cause mortality | 0.68 (0.57–0.82)* | 0.87 (0.74–1.01) | 0.93 (0.82–1.04) |
| Hospitalisation for HF | 0.65 (0.50–0.85)* | 0.67 (0.52–0.87)* | 0.73 (0.61–0.88)* |
| MI (fatal or nonfatal) | 0.87 (0.70–1.09) | 0.89 (0.73–1.09) | 0.89 (0.77–1.01) |
| Stroke (fatal or nonfatal) | 1.18 (0.89–1.56) | 0.87 (0.69–1.09) | 1.01 (0.84–1.21) |
| Fatal or hospitalisation for HF | 0.65 (0.50–0.85)* | 0.67 (0.52–0.87)* | 0.83 (0.73-0.95)* |
| Worsening of nephropathy‡ | 0.61 (0.53–0.70)* | 0.60 (0.47–0.77)* | 0.76 (0.67-0.87)* |
| Progression of albuminuria | 0.62 (0.54–0.72)* | 0.73 (0.67–0.79)* | |
| Dose (mg) | 10 and 25 | 100 and 300 | 10 |
| Approved clinical indication | As an adjunct to diet and exercise to improve glycaemic control in adults with T2D | ||
| Reduce the risk of CV death in adult patients with T2D and established CVD | Reduce the risk of MACE in adults with T2D and established CVD | ||
Outcomes reported as HR (95% CI). * Significant. †Pooled data from CANVAS and CANVAS-R. MACE(1): death from cardiovascular causes, nonfatal MI, or nonfatal stroke. MACE(2): CV death, MI, or ischaemic stroke. ‡Worsening nephropathy was defined as doubling of the serum creatinine level and an eGFR of ≤45 ml/min/1.73m2, the need for continuous renal-replacement therapy, or death due to renal events in EMPA-REG OUTCOME; 40% reduction in eGFR, renal-replacement therapy, or death from renal causes in CANVAS; sustained decrease of ≥40% in eGFR to <60 ml/min/1.73m2, new end-stage renal disease, or death from any cause in DECLARE-TIMI 58. CANVAS = CANagliflozin cardioVascular Assessment Study; CV = cardiovascular; CVD = cardiovascular disease; DECLARE-TIMI 58 = Dapagliflozin Effect on CardiovascuLAR Events – Thrombolysis in Myocardial Infarction 58; eGFR = estimated glomerular filtration rate; EMPA-REG = EMPAgliflozin cardiovascular outcome event trial in type 2 diabetes mellitus patients – Removing Excess Glucose; HF = heart failure; MACE = major adverse cardiovascular events; NI = noninferiority; SGLT2I = sodium–glucose cotransporter 2 inhibitor; T2D = type 2 diabetes.
The EMPA-REG OUTCOME trial recruited patients with T2D and established CVD (secondary prevention).[22] Empagliflozin (pooled data of 10 and 25 mg doses) reduced the primary MACE outcome, an effect driven by a marked risk reduction in CV death (38%), without significant effects on atherosclerotic ischaemic events (nonfatal MI and nonfatal stroke). Additionally, empagliflozin significantly reduced all-cause, sudden and HHF. The reduction in HHF was observed in patients with and without documented HF at baseline and was associated to a reduction in the introduction of loop diuretics.[22,25] The benefits were consistent among subgroups defined by baseline characteristics, including age, HbA1c levels, BMI, estimated glomerular filtration rate (eGFR) or patients with versus without HF and across categories of medications to treat diabetes and/or HF.[22,25–27]
The CANVAS Program integrated 2 trials (CANVAS and CANVAS-Renal) recruiting participants with T2D and established CVD (65.6%) or at risk for CV events (primary prevention).[23] Canagliflozin significantly decreased MACE and HHF to a similar extent to empagliflozin. However, none of the three individual components of MACE, nor all-cause mortality, were significantly reduced by canagliflozin.[23] Thus, it is difficult to understand what drives the superiority of canagliflozin for MACE over placebo. The benefit for the primary outcome was abrogated in patients without established CVD, suggesting that the benefit may be mostly in secondary prevention, while the point estimate for HHF was similar in both cohorts, suggesting that this cardiac benefit may extended to diabetic individuals without overt CVD. Interestingly, the benefit on CV death or HHF may be greater in patients with a history of HF at baseline.[28,29]
The DECLARE-TIMI 58 trial recruited patients (40.6%) with established atherosclerotic CVD and with multiple risk factors for atherosclerotic CVD (59.4%).[24] Dapagliflozin met the pre-specified primary safety endpoint of noninferiority for MACE, but in the two primary efficacy analyses, it did not result in a significantly lower rate of MACE than placebo. However, dapagliflozin resulted in a lower rate of the other pre-specified primary efficacy outcome (the composite of CV death or HHF), which reflected a lower rate oh HHF, regardless of a history of atherosclerotic cardiovascular disease or HF.
Thus, SGLT2Is reduce HHF and exert cardioprotective effects in T2D patients, but there were important differences between the CVOTs (Table 1). First, almost all patients in the EMPA-REG OUTCOME trial received secondary prevention of CVD, while the CANVAS Program and DECLARE-TIMI 58 trial included patients who had or were at risk for atherosclerotic CVD (i.e. both primary and secondary prevention). Second, HHF and mortality outcome curves begin to separate within the first 3 months in the EMPA-REG study but later in other CVOTs, i.e. earlier than would be expected from any decrease in atherothrombotic events.[22–24,30,31] Third, only empagliflozin reduced both CV and all-cause mortality, probably because EMPA-REG OUTCOME was a secondary prevention trial and it is presumed that the higher the baseline risk for CV events the better the CV protection, while patients without CVD might require longer drug exposure to observe the benefits.[22] Finally, canagliflozin reduced the risk of nonfatal stroke, while a trend for an increased risk of stroke was observed with empagliflozin, which might be related to the higher CV risk of the population enrolled in EMPA-REG, including more patients with prior stroke (23% versus 19.3%).[32] In a post hoc analysis, this difference was attributed to events occurring >90 days after the last intake of study drug and driven by nonfatal ischaemic stroke, but there were no differences in the risk of recurrent, fatal, or disabling strokes, or transient ischaemic attacks, between empagliflozin and placebo.[32]
Renoprotective Effects
Chronic kidney disease (CKD) affects up to 40% of patients with T2D and increases mortality and morbidity.[33,34] In the CVOTs, mean baseline eGFR ranged between 76 and 85 ml/min/1.73m[2] but there were important differences in the percentage of patients with an eGFR <60 ml/min/1.73 m[2] or with macro/microalbuminuria (Table 1). Canagliflozin, dapagliflozin and empagliflozin showed a favourable effect on renal outcomes and slowed the progression of albuminuria and new onset or worsening nephropathy, even when the components of renal outcomes differ between CVOTs (Table 1).[16,22–24,30,31]
In the EMPA-REG OUTCOME trial, where 25.9% of the population had CKD, the relative reductions in the risk of MACE, CV death, all-cause mortality, and HHF were independent of eGFR down to 30 ml/min/1.73m[2] or albuminuria status at baseline and similar across the two doses of empagliflozin versus placebo.[27] Similarly, in the CANVAS Program (20.1% of patients had CKD), the effects of canagliflozin on MACE, HHF and progression of kidney disease appeared similar across different levels of kidney function down to eGFR levels of 30 ml/min/1.73m[2.[35] These findings require further confirmation in specific, powered trials in patients with diabetic kidney disease.
Interestingly, the curves of renal outcomes start to separate within the first months and were maintained for >3 years, and the renal benefits were observed in patients on renin–angiotensin–aldosterone system (RAAS) inhibitors and with an eGFR >30 ml/min/1.73 m[2, despite the attenuated HbA1c-lowering effects in this setting.[22–24,30,31,36] Because patients with lower eGFR at baseline are at an increased risk of HHF, the renoprotective effects of SGLT2Is may contribute to improved HF outcomes.[30,31,36,37]
Mechanisms of Action
Multiple mechanisms are proposed to explain the early cardiorenal benefits of SGLT2Is[17–20,22,36–73] (Figure 1 and Table 2). The early benefits observed in EMPA-REG OUTCOME and CANVAS Program cannot be explained by the modest changes in HbA1c, blood pressure (BP), weight, visceral adiposity, uricaemia or haematocrit, alone or in combination, suggesting that other glucose-independent mechanisms may contribute to the cardiorenal protective effects of SGLT2Is.[19,30,36,37,41,55] In fact, the reduction in CV events related to glucose control appears only after many years of follow-up[17,37] and in T2D patients antihypertensive therapy takes years to reduce major CV events, including nonfatal stroke and MI which remain unaltered with SGLT2Is.[17,43,44,74]
Figure 1: Potential Mechanisms Involved in the Cardioprotective and Renoprotective Effects of Sodium–glucose Cotransporter 2 Inhibitors.
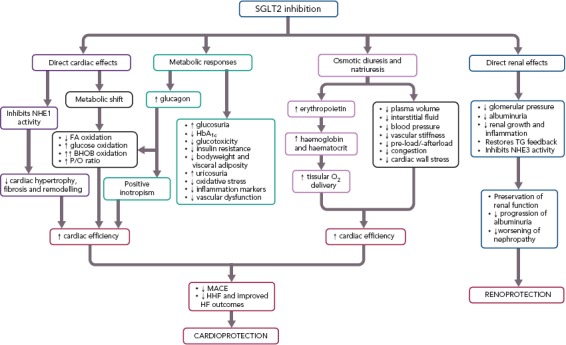
BHOB = 3-beta-hydroxybutyrate; FA = fatty acid; HHF = hospitalisations for HF; MACE = major adverse cardiovascular events; NHE = Na+-H+ exchanger; P/O = ATP yield per oxygen atom consumed of oxidative phosphorylation; SGLT2 = sodium-glucose cotransporter 2; TG = tubuloglomerular.
Table 2: Mechanisms of Action Underlying the Beneficial Effects of Sodium–glucose Cotransporter 2 Inhibitors on Cardiovascular and Renal Outcomes.
| Pharmacological Effect | Cardiovascular and Renal Benefits of SGLT2Is |
|---|---|
| Glycosuria[17–19,36–38] |
|
| Osmotic diuresis and natriuresis[17–19,22,39–41] |
|
| BP reduction[19,39,42–44] |
|
| Decrease arterial stiffness and PVR[40,42,45] |
|
| Decrease body weight and visceral adiposity[17–19,36,37,46] |
|
| Increase in haemoglobin and haematocrit levels[19,39,47] |
|
| Anti-inflammatory and antioxidant effects[48,49] |
|
| A shift in cardiac and renal fuel energetics[41,50–55] |
|
| Metabolic effects[19,36,37,54–56] |
|
| Cardioprotective effects [19,36,37,41,50,55–66] |
|
| Renoprotective effects[18–20,67–71] |
|
BP = blood pressure; FFA = free fatty acids; HHF = hospitalisation for heart failure; LV = left ventricular; NHE = Na+-H+ exchanger; PVR = peripheral vascular resistances; SBP/DBP = systolic/diastolic BP; SGLT2I = sodium-glucose cotransporter 2 inhibitor.
Three hypotheses have been proposed to explain the beneficial CV effects of SGLT2Is – the diuretic hypotheses, the thrifty substrate hypothesis and the NHE (Na+-H+ exchanger) hypothesis.
The Diuretic Hypotheses
The early (<3 months) and significant reduction in HHF and CV mortality produced by empagliflozin in the absence of significant changes in the incidence of MI or stroke suggests that the predominant mechanism may relate to its haemodynamic effects. It has been hypothesised that the reduction in Na+ and water retention, leading to reduced ventricular filling pressure and cardiac workload, could be an important mechanism.[30,75–77] Indeed, an exploratory analysis of the EMPA-REG OUTCOME trial showed that changes in markers of plasma volume were the most important mediators of the reduction in the risk of CV death with empagliflozin versus placebo.[75] However, the diuretic effects of SGLT2Is are quite different from those observed with thiazide or loop diuretics.[77]
The first reason for this is that SGLT2Is act in the proximal tubule, where they inhibit glucose and Na+ reabsorption resulting in osmotic diuresis. However, compared with osmotic diuretics, SGLT2Is do not affect plasma osmolarity.
Second, because SGLT2Is work in the proximal tubule, they increase delivery of fluid and electrolytes to the macula densa, thereby activating tubuloglomerular feedback, an effect that is not achieved by loop and thiazide diuretics because they reduce Na+ flux to the macula densa.[17,69] Third, compared with loop diuretics, SGLT2Is produce a greater fluid clearance from the interstitial fluid space than from the circulation, potentially resulting in better congestion relief with minimal impact on BP, arterial filling and organ perfusion or inducing a neurohumoral activation.[78]
Furthermore, SGLT2Is produce greater electrolyte-free water clearance than loop or thiazide diuretics acting at different sites of the nephron and producing more potent diuresis and natriuresis.[17,20] Finally, loop diuretics reduce HHF but not CV mortality[69] and their long-term use reduces the risk of stroke but can worsen renal function renal function – effects that are not observed with SGLT2Is.[33,69,70,77] Because of these important differences, it is unlikely that SGLT2Is prevent HHF by acting simply as diuretics.[20,37,39]
The Thrifty Substrate Hypothesis
A shift in cardiorenal fuel energetics (the ‘thrifty substrate’ hypothesis). Under physiological conditions, nearly 95% of cardiac energy is derived from mitochondrial oxidative metabolism and fuel is derived from free fatty acids (FAs; 60–70%), glucose (30%) and – to a lesser degree – lactate, ketones and amino acids.[79] In T2D, glucose utilisation decreases while oxidation of FAs markedly increases because of peripheral insulin resistance and inability of insulin to suppress lipolysis.[37,80,81] These changes decrease cardiac efficiency/function because excessive FA oxidation is energetically less efficient, increases oxidative stress and cardiac lipotoxicity and impairs LVF.[37,50,80,81] SGLT2Is increase the hepatic synthesis and decrease the urinary excretion of ketones producing a mild, but persistent, hyperketonaemia.[51] Under these conditions beta-hydroxybutyrate (BHOB) is freely taken up by the heart and kidney and oxidised in preference to FAs and glucose, producing ATP more efficiently. In fact, ATP production/O2 consumption ratio (P/O) favours BHOB (2.50) over FA (2.33), and even when the P/O ratio of BHOD and pyruvate are similar, the combustion of BHOB liberates 31% more calories.[50–53,82]
In rat hearts, BHOB increases external cardiac work and reduces oxygen consumption, thereby improving cardiac efficiency in the diabetic heart.[48–53,82] Thus, it has been hypothesised that the cardiorenal benefits of SGLT2Is might be related to a shift in cardiorenal metabolism away from FAs and glucose oxidation toward ketone bodies, a more energy-efficient fuel, which improves cardiac and renal work efficiency/function, reduces oxygen consumption and exhibits antioxidative and antiarrhythmic properties.[41,50–53] The utilisation of ketones together with an increased oxygen delivery from SGLT2I-induced haemoconcentration and a reduced cardiac load resulting from decreases in intravascular volume and BP could be involved in the early benefits observed in CVOTs.
The NHE Hypothesis
A reduction in intracellular sodium ([Na+]i) by inhibiting the sarcolemmal Na+-H+ exchanger (NHE; the NHE hypothesis).[19,50,55,72] NHE1 is the predominant isoform in the heart and vasculature, while NHE3 is expressed at the apical surface of renal epithelial cells where it co-localises and functionally interacts with SGLT2.[55] In patients with T2D and HF, the activity of NHE1/3 is markedly enhanced. This increase facilitates the accumulation of intracellular Na+ ([Na+]i), which stimulates the reverse activity of the Na+/Ca2+ exchanger (NCX) leading to an increase in [Ca2+]i and cardiomyocyte injury, facilitates mitochondrial Ca2+ extrusion to the cytoplasm and decreases mitochondrial Ca2+ ([Ca2+]m).[55,58–62,83] The reduction in [Ca2+]m impairs Ca2+-induced stimulation of Krebs cycle dehydrogenases and reduces ATP production and mitochondrial antioxidative capacity.[54,55,58,60–63,71]
Even when SGLT2 is not expressed in the heart, SGLT2Is can inhibit cardiac NHE1, possibly through a binding site for SGLT2 on NHE1.[55] The inhibition of NHE1 reduces intracellular Na+ and Ca2+ concentrations and increases [Ca2+]m, which restores mitochondrial function and redox state, activates ATP production in the failing heart and improves systolic function.[55,62] In animal models, SGLT2Is via the inhibition of NHE1 reduce cardiac hypertrophy and fibrosis and ventricular arrhythmias, slow the progression of left ventricular (LV) remodelling and diabetic cardiomyopathy and improve systolic/diastolic function.[55,57–68] In normotensive patients with T2D and established coronary artery disease, but without HF, empagliflozin significantly reduces LV mass and slows the progression of LV hypertrophy versus placebo.[84] This finding suggests that empagliflozin promotes a reverse remodelling, which may contribute to the early cardiovascular and HF benefits observed in the EMPA-REG OUTCOME trial.
However, several questions remain unanswered, including the mechanism underlying the increase in BHOB, the time course of the hyperketonaemia, the relationship between the dose, hyperketonaemia and improvement in cardiac function, or whether hyperketonaemia might increase the risk of diabetic ketoacidosis (DKA).[59,79,83,84] Additionally, an increase in metabolic efficiency and/or NHE inhibition should prove beneficial in myocardial ischaemia and HHF, but in CVOTs these two endpoints were differentially affected by SGLT2Is.[50,79] Thus, at the present time, the ‘thrifty substrate’ hypothesis needs to be demonstrated.[83]
Renal Effects
In patients with T2D, glucose and Na+ reabsorption increases in the proximal tubule via SGLT2 and Na+ delivery to the macula densa decreases, which stimulates renin release by the juxtaglomerular cells and activates the RAAS. This causes, via tubuloglomerular feedback, an afferent arteriolar vasodilation that increases the GFR (‘hyperfiltration’) and contributes to diabetic nephropathy. SGLT2Is reduce Na+ reabsorption in the proximal tubule and increase its delivery to the macula densa.[19,20,69,73,85] This inhibits renin release, activates tubuloglomerular feedback, produces an afferent arteriolar vasoconstriction, normalises the GFR and reduces intraglomerular pressure counteracting hyperglycaemia-induced hyperfiltration – an effect that would be expected to slow the progression of diabetic nephropathy.[17–20,69,70,73,85] However, afferent arteriolar vasoconstriction is present in patients with HF and an enhancement of such vasoconstriction would not be expected to produce favourable renal effects in non-diabetic patients with HF.[55] SGLTIs initially reduce eGFR (~5 ml/min/1.73m[2] and albuminuria (30–40%), but eGFR recovers baseline values after 6–12 months, reflecting a haemodynamic alteration rather than a glomerular damage.[31,84] Additionally, the renoprotective effects of SGLT2Is have been related to a decrease in hyperglycaemia, BP, glomerular capillary pressure and glomerular hyperfiltration, and direct effects on mesangial expansion, tubular growth, and inflammation.[18–20,69,70,85]
Adverse Events
SGLT2Is are generally well tolerated and adverse events (AEs) are considered mild-to-moderate in severity.[18–20,22–24,36,37,83,86–101] However, some serious AEs have been reported in postmarketing surveillance programs (Table 3). In the CANVAS Program, canagliflozin significantly increased the risk of fractures and below-knee lower extremity amputations.[23,95] In the EMPA-REG trial, amputations and fractures were not mentioned in the study protocol, but a post-hoc analysis reported a similar rate of both AEs with empagliflozin or placebo.[26,55] However, EMPA-REG and CANVAS were not powerful enough to detect significant differences in either amputation or fracture among the studied population. Recently, several real-world studies have led to contradictory conclusions on the risk of amputations[90–92,94] and a meta-analysis failed to demonstrate an increase in fracture events with SGLT2Is.[96] Therefore, it remains unclear whether the risk of these AEs extends across the drug class. Early trials raised the concern that SGLT2Is may increase the risk of bladder and breast cancer, and a meta-analysis suggested an increased risk of bladder cancer with empagliflozin.[100] However, given the short-term follow-up and uncertainty of evidence, future long-term prospective studies and postmarketing surveillance studies are warranted.
Table 3: Adverse Effects of Sodium–glucose Cotransporter 2 Inhibitors.
| Adverse Effect | Risk Factors and Recommendations* |
|---|---|
| Infections[22–24,36,37,83,86] |
|
| Volume depletion |
|
| Hypoglycaemia |
|
| Hypotension |
|
| Acute kidney injury[17,36,37,101] |
|
| Diabetic ketoacidosis[19,36,37,87–89] |
|
| Lower-limb amputations[23,28,29,90–94] |
|
| Bone fractures[95–99] |
|
| Increase of LDL cholesterol levels[54,57] |
|
| Cancer100 |
|
*Recommendations according to the FDA and/or EMA.
ACE = angiotensin-converting enzyme; ARBs = angiotensin receptor blockers; BP = blood pressure; CVD = cardiovascular disease; DKA = diabetic ketoacidosis; eGFR = estimated glomerular filtration rate; EMA = European Medicines Agency; FDA = Food and Drug Administration; FGF = fibroblast growth factor; NSAIDs = non-steroidal anti-inflammatory drugs; SGLT2I = sodium–glucose cotransporter 2 inhibitor; T1D = type 1 diabetes; T2D = type 2 diabetes; UTI = urinary tract infection.
Unresolved Issues
Many questions remain to answered in future preclinical studies and carefully designed controlled trials (Table 4).
Table 4: Questions to Address in Future Preclinical and Clinical Research with SGLT2Is.
|
CVD = cardiovascular disease; CVOT = cardiovascular outcome trials; HF = heart failure; SGLT2I = sodium-glucose cotransporter 2 inhibitor; T2D = type 2 diabetes.
What are the mechanisms underlying the early cardiorenal benefits of SGLT2Is? CVOTs were designed to test the safety of SGLT2Is but not the mechanism of action. Therefore, the mechanisms underlying the early separation of the curves of CV mortality, HHF and progression of renal disease and the long-term sustained benefits of SGLT2Is are yet to be elucidated. It is possible that haemodynamic, metabolic, hormonal and direct cardiac and renal mechanisms, possibly unrelated to SGLT2 inhibition, and with different roles over time and in different populations might be involved. So, are the same mechanisms involved in the cardiovascular and renal benefits? A better understanding of the mechanisms of action is the first step to identify the patients who could benefit most from the use of SGLT2Is.
Is the cardiorenal benefit a class effect? A class effect would not be expected if the underlying mechanisms are unrelated to SGLT2 inhibition. There are differences among SGLT2Is in their SGLT2/SGLT1 selectivity (>2,500 for empagliflozin, 1,116 for dapagliflozin, 250 for canagliflozin), pharmacokinetic properties and – possibly – pharmacodynamic off-target properties[17–19,36,37,102] Thus, there is no evidence that the benefits can be a ‘class effect’. Indeed, the FDA and European Medicines Agency approved all SGLT2Is for glycaemic control in adults with T2D. Additionally, empagliflozin is also approved to reduce the risk of CV death in adults with T2D and established CVD, and canagliflozin to reduce the risk of MACE in adults with T2D and established CVD.
How can the marked differences observed in CVOTs among SGLT2Is be explained? Table 1 shows that there are important differences between CVOTs in clinical outcomes related to differences in the recruited population, trial design, concurrent use of cardioprotective drugs, adjudication of CV events or statistical analysis.[20–22] In a recent meta-analysis of 13 clinical trials recruiting 34,533 diabetic patients (60.2% with established atherosclerotic CVD), the most consistent effect of SGLT2Is was to reduce HHF (31%) and progression of renal disease (45%), with a modest reduction in MACE (11%).[14] The reduction in MACE was apparent only in patients with established atherosclerotic CVD, while the reduction in HHF or progression of renal disease was observed regardless of the presence of atherosclerotic CVD, a previous a history of HF and across different levels of kidney function down to eGFR levels of 30 ml/min/1.73 m[2. Are ethnic differences implicated in the response to SGLT2Is? Asian participants (who account for almost half of the world’s population with diabetes)[1] and white participants had better CV benefits than black participants in the EMPA-REG study, whereas canagliflozin was superior in black and white participants.[22,23] These findings suggest that the benefits of SGLT2Is may depend on the population in which they are used.
What is the real benefit of SGLT2Is in patients with HF? CVOTs were not designed to assess the efficacy of SGLT2Is in patients with HF. Indeed, <15% of the patients had HF at baseline, HF phenotyping – including echocardiography or biomarkers (B-type natriuretic peptide, troponin T) – was not performed, and effects of SGLT2Is on LV structure and function or haemodynamics remain to be determined. The significant reduction in HHF observed even in patients without atherosclerotic CVD or a history of HF raises the possibility of using SGLT2Is not only in the primary prevention but also for the treatment of HF patients with reduced and/or preserved ejection fraction.
Can the cardiovascular and renal protection observed in CVOTs be extrapolated to the real world? The observational Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL) and CVD-REAL Nordic trials suggested that initiation of SGLT2Is versus other glucose-lowering drugs was associated with a lower risk of HHF and death regardless of pre-existing CVD, and with reduced CV mortality in patients with T2D and a broad cardiovascular risk profile.[103–106]
Therefore, the benefits observed with empagliflozin and canagliflozin may be a class effect applicable to a broad population of patients with T2D in real-world practice, including in primary prevention. However, because of the observational design, short follow-up and immortal time and time-lag biases, the >50% lower rates of all-cause mortality associated with the use of SGLT2Is in these trials are more likely exaggerated.[107] Additionally, in these trials only 25% of patients presented established CVD, most were treated with canagliflozin and dapagliflozin (not with empagliflozin) and drug safety was not reported. Thus, at the present time there is not enough evidence to extrapolate the data from the CVOTs to the real-world setting.[107]
What is the risk/benefit ratio of SGLT2Is in the real world? Optimal prescription of SGLT2Is requires the understanding of their risk/benefit ratio, but AEs should not overshadow their cardiorenal protective effects. Some serious AEs were not observed in CVOTs, possibly because of the short follow-up and the selection and strict supervision of patients, but they were reported in postmarketing surveillance studies and some of these AEs were unexpected. Are bone fractures and amputations a class effect? What are the mechanisms involved in bone fractures and amputations? What is the clinical meaning of the trend in stroke in the EMPA-REG OUTCOME trial? Further research is needed to identify the risk factors for the development of serious AEs, including infections, Fournier’s gangrene, acute kidney injury, DKA, amputations and bone factures in patients treated with the different SGLT2Is in daily clinical practice. Furthermore, there is little information on drug interactions between SGLT2Is and other treatments prescribed in patients with T2D and HF. In the EMPA-REG OUTCOME trial, the effect of empagliflozin on HHF was reduced by mineralocorticoid receptor antagonists, which only represented 6% of patients in this trial, but are used in >60% of HF patients. Drug–drug interactions should be analysed in long-term RCTs recruiting diabetic and non-diabetic patients with CVD.
The results of several on-going long-term randomised trials should provide key information on the cardiorenal protective effects of SGLT2Is in different patient populations, their safety profile, which patients are at greatest risk for serious AEs, and possible differences in the efficacy/safety profile between drugs of this pharmacological class.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 8th edition. Available at: http://diabetesatlas.org/resources/2017-atlas.html (accessed 08 April 2019)
- 2.Shah AD, Langenberg C, Rapsomaniki E et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–13. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tancredi M, Rosengren A, Svensson AM et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–32. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 4.Bertoluci MC, Rocha VZ. Cardiovascular risk assessment in patients with diabetes. Diabetol Metab Syndr. 2017;9:25. doi: 10.1186/s13098-017-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einarson TR, Acs A, Ludwig C et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17:83. doi: 10.1093/ehjcvp/pvw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–41. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald MR, Petrie MC, Varyani F et al. CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–85. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 8.Seferović PM, Petrie MC, Filippatos GS et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–72. doi: 10.1002/ejhf.1170. [DOI] [PubMed] [Google Scholar]
- 9.Cubbon RM, Adams B, Rajwani A et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diabetes Vasc Dis Res. 2013;10:330–6. doi: 10.1177/1479164112471064. [DOI] [PubMed] [Google Scholar]
- 10.Tajik AA, Dobre D, Aguilar D et al. Database Scientific Committee. A history of diabetes predicts outcomes following myocardial infarction: an analysis of the 28.771 patients in the high-risk MI database. Eur J Heart Fail. 2017;19:635–42. doi: 10.1002/ejhf.797. [DOI] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 12.Turnbull FM, Abraira C, Anderson RJ et al. Control Group. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–98. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Paul SK, Bethel MA et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 14.Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatory-Information/Guidances/ucm071627.pdf Available at: (accessed 08 April 2019)
- 16.Zelniker TA, Wiviott SD, Raz I et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 17.Heerspink HJ, Perkins BA, Fitchett DH et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Ghani MA, Norton L, DeFronzo RA. Role of sodium-glucose cotransporters 2 (SGLT2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. doi: 10.1210/er.2010-0029. [DOI] [PubMed] [Google Scholar]
- 19.Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents DPP-4 and SGLT-2 Inhibitors. Circ Res. 2018;122:1439–59. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia. Diabetologia. 2017;60:215–25. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.List JF, Woo V, Morales E et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7. doi: 10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinman B, Wanner C, Lachin JM et al. EMPA-REG OUTCOME Investigators. EMPA, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 23.Neal B, Perkovic V, Mahaffey KW et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 201;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 24.Wiviott SD, Raz Bonaca MP et al. DECLARE– TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–57. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 25.Fitchett D, Butler J, van de Borne P et al. EMPAREG OUTCOME® Trial Investigators. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39:363–70. doi: 10.1093/eurheartj/ehx511. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Mazer CD, Al-Omran M et al. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA-REG OUTCOME. Circulation. 2018;137:405–7. doi: 10.1161/CIRCULATIONAHA.117.032031. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C, Lachin JM, Inzucchi SE et al. and On behalf of the EMPA-REG OUTCOME Investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–29. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 28.Mahaffey W, Neal B, Perkovic V et al. Canagliflozin for primary and secondary prevention of cardiovascular events. Circulation. 2018;137:323–34. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rådholm K, Figtree G, Perkovic V et al. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018;138:458–68. doi: 10.1161/CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sattar N, McLaren J, Kristensen SL et al. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–9. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rådholm K, Wu JH, Wong MG et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular disease, death and safety outcomes in type 2 diabetes - A systematic review. Diabetes Res Clin Pract. 2018;140:118–28. doi: 10.1016/j.diabres.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 32.Zinman B, Inzucchi SE, Lachin JM et al. Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017;48:1218–25. doi: 10.1161/STROKEAHA.116.015756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Katzmarzyk PT, Horswell R et al. Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int. 2014;85:1192–9. doi: 10.1038/ki.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damman K, Valente MA, Voors AA et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–69. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 35.Neuen BL, Ohkum T, Nela B et al. Cardiovascular and renal outcomes with canagiflozin according to baseline kidney function. Circulation. 2018;138:1537–50. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lytvyn Y, Bjornstad P, Udell JA et al. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017;136:1643–58. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Shao YH, Wang XG et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors as add-on to metformin and sulfonylurea treatment for the management of type 2 diabetes: a meta-analysis. Endocr J. 2018;65:335–44. doi: 10.1507/endocrj.EJ17-0372. [DOI] [PubMed] [Google Scholar]
- 39.Butler J, Harno C, Filipatos G et al. The potential role and rationale for treatment of heart failure with sodium glucose co-transporter 2 inhibitors. Eur J Heart Fail. 2017;19:1390–400. doi: 10.1002/ejhf.933. [DOI] [PubMed] [Google Scholar]
- 40.Baker WL, Smyth LR, Riche DM et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–75. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Flores E, Santos-Gallego CG, Diaz-Mejía N et al. Do the SGLT-2 Inhibitors Offer More than Hypoglycemic Activity? Cardiovasc Drugs Ther. 2018;32:213–22. doi: 10.1007/s10557-018-6786-x. [DOI] [PubMed] [Google Scholar]
- 42.Cherney DZ, Perkins BA, Soleymanlou N et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie X, Atkins E, Lv J et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and metaanalysis. Lancet. 2016;387:435–43. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 44.Emdin CA, Rahimi K, Neal B et al. Blood pressure lowering in type 2 diabetes: a systematic review and metaanalysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 45.Chilton R, Tikkanen I, Cannon CP et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:1180–93. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neeland IJ, Gupta S, Ayers CR et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–7. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano M, Takei M, Shiraishi Y et al. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8:844–7. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: The potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44:457–64. doi: 10.1016/j.diabet.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Prattichizzo F, De Nigris V, Micheloni S et al. A. Increases in circulating levels of ketone bodies and cardiovascular protection with SGLT2 inhibitors: Is low-grade inflammation the neglected component?. Diabetes Obes Metab. 2018;20:2515–22. doi: 10.1111/dom. [DOI] [PubMed] [Google Scholar]
- 50.Bertero E, Prates Roma L, Ameri P et al. Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovasc Res. 2018;114:12–18. doi: 10.1093/cvr/cvx149. [DOI] [PubMed] [Google Scholar]
- 51.Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? Diabetes Care. 2016;39:1115–22. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, Kashiwaya Y, Keon CA et al. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–8. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 53.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a ‘thrifty substrate’ hypothesis. Diabetes Care. 2016;39:1108–14. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 54.Briand F, Mayoux E, Brousseau E et al. Empagliflozin, via switching metabolism toward lipid utilization, moderately increases LDL cholesterol levels through reduced LDL catabolism. Diabetes. 2016;65:2032–8. doi: 10.2337/db16-0049. [DOI] [PubMed] [Google Scholar]
- 55.Packer M, Anker SD, Butler J et al. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol. 2017;2:1025–9. doi: 10.1001/jamacardio.2017.2275. [DOI] [PubMed] [Google Scholar]
- 56.Cheeseman C. Solute carrier family 2, member 9 and uric acid homeostasis. Curr Opin Nephrol Hypertens. 2009;18:428–32. doi: 10.1097/MNH.0b013e32832ee3de. [DOI] [PubMed] [Google Scholar]
- 57.Bays HE, Sartipy P, Xu J et al. Dapagliflozin in patients with type II diabetes mellitus, with and without elevated triglyceride and reduced high-density lipoprotein cholesterol levels. J Clin Lipidol. 2017;11:450 e1–458 e1. doi: 10.1016/j.jacl.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Pessoa TD, Campos LC, Carraro-Lacroix L et al. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25:2028–39. doi: 10.1681/ASN.2013060588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab. 2016;24:200–2. doi: 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 60.Kohlhaas M, Maack C. Adverse bioenergetic consequences of Na+-Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation. 2010;122:2273–80. doi: 10.1161/CIRCULATIONAHA.110.968057. [DOI] [PubMed] [Google Scholar]
- 61.Baartscheer A, Schumacher CA, van Borren MM et al. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–24. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- 62.Baartscheer A, Schumacher CA, Wust RC et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–73. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu T, Takimoto E, Dimaano VL et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a guinea pig model of heart failure. Circ Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura TY, Iwata Y, Arai Y et al. Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure. Circ Res. 2008;103:891–9. doi: 10.1161/CIRCRESAHA.108.175141. [DOI] [PubMed] [Google Scholar]
- 65.Lin B, Koibuchi N, Hasegawa Y et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Leeuw AE, de Boer RA. Sodium-glucose cotransporter 2 inhibition: cardioprotection by treating diabetes-a translational viewpoint explaining its potential salutary effects. Eur Heart J Cardiovasc Pharmacother. 2016;2:244–55. doi: 10.1093/ehjcvp/pvw009. [DOI] [PubMed] [Google Scholar]
- 67.Joubert M, Jagu B, Montaigne D et al. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes. 2017;66:1030–40. doi: 10.2337/db16-0733. [DOI] [PubMed] [Google Scholar]
- 68.Habibi J, Aroor AR, Sowers JR et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16:9. doi: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanton RC. Sodium glucose transport 2 (SGLT2) inhibition decreases glomerular hyperfiltration: is there a role for SGLT2 inhibitors in diabetic kidney disease? Circulation. 2014;129:542–4. doi: 10.1161/CIRCULATIONAHA.113.007071. [DOI] [PubMed] [Google Scholar]
- 70.Staels B. Cardiovascular protection by sodium glucose cotransporter 2 inhibitors: potential mechanisms. Am J Cardiol. 2017;120((1S)):S28–36. doi: 10.1016/j.amjcard.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule. Am J Physiol Renal Physiol. 2015;308:F1343–57. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 73.Ghezzi C, Loo DDF, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–97. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sciarretta S, Palano F, Tocci Get al. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171:384–94. doi: 10.1001/archinternmed.2010.427. [DOI] [PubMed] [Google Scholar]
- 75.McMurray J. EMPA-REG - the “diuretic hypothesis”. J Diabetes Complications. 2016;30:3–4. doi: 10.1016/j.jdiacomp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 76.Inzucchi SE, Zinman B, Fitchett D et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41:356–63. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 77.Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 2016;42:224–33. doi: 10.1016/j.diabet.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 78.Hallow KM, Helmlinger G, Greasley PJ et al. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obesity Metab. 2018;20:479–87. doi: 10.1111/dom.13126. [DOI] [PubMed] [Google Scholar]
- 79.Lopaschuk GD, Ussher JR, Folmes CDL et al. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 80.Fillmore N, Mori J, Lopaschuk GD. Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2080–90. doi: 10.1111/bph.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riggs K, Ali H, Taegtmeyer H et al. The use of SGLT-2 inhibitors in type 2 diabetes and heart failure. Metab Syndr Relat Disord. 2015;13:292–7. doi: 10.1089/met.2015.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot Essent Fatty Acids. 2004;70:309–19. doi: 10.1016/j.plefa.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Lupsa BC, Inzucchi SE. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia. 2018;61:2118–25. doi: 10.1007/s00125-018-4663-6. [DOI] [PubMed] [Google Scholar]
- 84.Verma S, Mazer CD, Yan AT EMPA-HEART CardioLink-6 trial: a randomized trial of empagliflozin on left ventricular structure, function, and biomarkers in people with type 2 diabetes and coronary heart disease. Nov 11, 2018. Presented at: AHA 2018 Chicago, IL.
- 85.Wanner C. EMPA-REG OUTCOME: the nephrologist's point of view. Am J Cardiol. 2017;120((Suppl 1)):S59–67. doi: 10.1016/j.amjcard.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 86.Thong KY, Yadagiri M, Barnes DJ et al. Clinical risk factors predicting genital fungal infections with sodium-glucose cotransporter 2 inhibitor treatment: the ABCD nationwide dapagliflozin audit. Prim Care Diabetes. 2018;12:45–50. doi: 10.1016/j.pcd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 87.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42. doi: 10.2337/dc15-1380. [DOI] [PubMed] [Google Scholar]
- 88.Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376:2300–2. doi: 10.1056/NEJMc1701990. [DOI] [PubMed] [Google Scholar]
- 89.Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017;60:1385–9. doi: 10.1007/s00125-017-4301-8. [DOI] [PubMed] [Google Scholar]
- 90.Khouri C, Cracowski JL, Roustit M. SGLT-2 inhibitors and the risk of lower-limb amputation: Is this a class effect? Diabetes Obes Metab. 2018;20:1531–4. doi: 10.1111/dom.13255. [DOI] [PubMed] [Google Scholar]
- 91.Scheen AJ. Does lower-limb amputation concern all SGLT-2 inhibitors? Nature Rev Endocrinol. 2018;18:326–8. doi: 10.1038/s41574-018-0001-9. [DOI] [PubMed] [Google Scholar]
- 92.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA adverse events reporting system. Lancet Diabetes Endocrinol. 2017;5:680–1. doi: 10.1016/S2213-8587(17)30257-7. [DOI] [PubMed] [Google Scholar]
- 93.Udell JA, Yuan Z, Rush T et al. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World) Circulation. 2018;137:1450–9. doi: 10.1161/CIRCULATIONAHA.117.031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Z, DeFalco FJ, Ryan PB et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes Metab. 2018;20:582–9. doi: 10.1111/dom.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watts NB, Bilezikian JP, Usiskin K et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101:157–66. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang HL, Li DD, Zhang JJ et al. Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2016;18:1199–206. doi: 10.1111/dom.12742. [DOI] [PubMed] [Google Scholar]
- 97.Bilezikian JP, Watts NB, Usiskin K et al. Evaluation of bone mineral density and bone markers in patients with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitors. J Clin Endocrinol Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bays HE, Weinstein R, Law G et al. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014;22:1042–9. doi: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3:8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang H, Dai Q, Shi W et al. SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia. 2017;60:1862–72. doi: 10.1007/s00125-017-4370-8. [DOI] [PubMed] [Google Scholar]
- 101.Singh M, Kumar A. Risks Associated with SGLT2 Inhibitors: An Overview. Curr Drug Saf. 2018;13:84–91. doi: 10.2174/1574886313666180226103408. [DOI] [PubMed] [Google Scholar]
- 102.Grempler R, Thomas L, Eckhardt M et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterization and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 103.Kosiborod M, Birkeland KI, Cavender MA et al. CVD-REALInvestigators and Study Group. Rates of myocardial infarction and stroke in patients initiating treatment with SGLT2-inhibitors versus other glucose-lowering agents in real-world clinical practice: Results from the CVD-REAL study. Diabetes Obes Metab. 2018;20:1983–7. doi: 10.1111/dom.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kosiborod M, Cavender MA, Fu AZ et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Birkeland KI, Jorgensen ME, Carstensen B et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709–17. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 106.Persson F, Nyström T, Jørgensen ME et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: a multinational observational study. Diabetes Obes Metab. 2018;20:344–51. doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41:6–10. doi: 10.2337/dc17-1223. [DOI] [PubMed] [Google Scholar]


