Abstract
Diabetes is a complex metabolic disorder affecting the glucose status of the human body. Chronic hyperglycaemia related to diabetes is associated with end organ failure. The clinical relationship between diabetes and atherosclerotic cardiovascular disease is well established. This makes therapeutic approaches that simultaneously target diabetes and atherosclerotic disease an attractive area for research. The majority of people with diabetes fall into two broad pathogenetic categories, type 1 or type 2 diabetes. The role of obesity, adipose tissue, gut microbiota and pancreatic beta cell function in diabetes are under intensive scrutiny with several clinical trials to have been completed while more are in development. The emerging role of inflammation in both type 1 and type 2 diabetes (T1D and T1D) pathophysiology and associated metabolic disorders, has generated increasing interest in targeting inflammation to improve prevention and control of the disease. After an extensive review of the possible mechanisms that drive the metabolic pattern in T1D and T2D and the inflammatory pathways that are involved, it becomes ever clearer that future research should focus on a model of combined suppression for various inflammatory response pathways.
Keywords: Inflammation, diabetes, obesity, metabolic disorders, adipose tissue, anti-inflammatory treatment
Diabetes is a multifaceted metabolic disorder affecting the glucose status of the human body. Impaired glucose tolerance and hyperglycaemia are the main clinical and diagnostic features and the result of an absolute or relative insulin deficiency or resistance to its action. Chronic hyperglycaemia associated with diabetes can result in end organ dysfunction and failure which can involve the retina, kidneys, nerves, heart and blood vessels.[1] The clinical relationship between diabetes and atherosclerotic cardiovascular disease are well established, with the risk for cardiovascular disease (CVD) being significantly elevated in patients with diabetes.[2,3]
Moreover, CVD typically occurs one to two decades earlier in people with diabetes, with more aggressive, severe and diffuse distribution.[4,5] The first WHO global report on diabetes published in 2016 demonstrates that the number of adults living with diabetes has almost quadrupled since 1980 to 422 million adults and this is expected to rise to 552 million by 2030.[6,7] Thus, the need for effective novel therapeutic approaches for the treatment and/or prevention of diabetes and atherosclerotic disease is crucial.
Traditionally, the majority of cases of diabetes fall into two broad pathogenetic categories, type 1 (T1D) and type 2 (T2D). However, in some people this rigid classification is not applicable because other genetic, immunological or neuroendocrinological pathways are involved in its pathogenesis. T1D is related to an absolute lack of insulin due to a vaguely understood mechanism, where an immune-mediated destruction of pancreatic beta cells is the hallmark of the disorder, with hyperglycaemia only emerging when more than 90% of the beta cells are lost.[8] T2D is the most common form of diabetes, accounting for 90–95% of cases. Its development is secondary to a relative insulin deficiency but the primary defect is insulin resistance.[9]
Various proposals and hypotheses have been developed to describe the mechanisms which are usually involved in the propagation of diabetes, mainly focusing on T2D. The increase in prevalence of the condition has been related to well-recognised risk factors, such as the adoption of a western lifestyle, sedentary lives, lack of physical activity and an energy-dense diet.[10,11] Genetic predisposition, ethnicity and ageing are not modifiable risk factors for T2D, while others, such as being overweight or obese, an unhealthy diet, insufficient physical activity and smoking are modifiable through behavioural and environmental changes. However, increasing evidence has shown that inflammatory pathways are the principal, common pathogenetic mediators in the natural course of diabetes under the stimulus of the risk factors described above.[12]
In this article, we will highlight the emerging role of inflammation in the pathophysiology of diabetes and we will analyse the implicated inflammatory pathways and biomarkers of inflammation in diabetes and metabolic diseases. The focus of this article is to provide an overview of the current state of knowledge on anti-inflammatory therapies for diabetes, along with perspectives on future therapies for the disease.
Historical Perspectives
Observational studies provided the first evidence for the possible association between inflammation and diabetes. Over a century ago, the administration of high doses of sodium salicylate led to decreased glycosuria in people with a suspected or definite diagnosis of diabetes.[13,14] Later studies on the role of inflammation in diabetes, revealed that this hypoglycaemic action was related to the inhibition of the serine kinase IkappaB kinase-beta (IKKbeta), which correlates with the post-receptor action of insulin.[15]
A landmark study to correlate inflammation with diabetes was conducted in animal models by Hotamisiligil et al., in 1993 and it revealed that the role of tumour necrosis factor-alpha (TNF-alpha) in obesity and particularly in insulin resistance and diabetes.[16] Epidemiologic associations of inflammation with obesity and T2D were made when circulating concentrations of markers and mediators of inflammation and acute-phase reactants including fibrinogen, C-reactive protein, interleukin (IL)-6, plasminogen activator inhibitor-1, sialic acid and white cells, have been shown to be elevated in these conditions.[17–21] Over the next decades, numerous studies on human and animal models provided further supporting evidence for the role of inflammation in the initiation and progression of diabetes.[12,22] Accumulative evidence suggests that chronic activation of pro-inflammatory pathways in target cells of insulin action may contribute to obesity, insulin resistance and related metabolic disorders including T2D.[22] The identification of potential pathways connecting inflammation to diabetes has produced growing interest in targeting inflammation to help prevent and control diabetes and related conditions, as well as improving risk stratification for diabetes by using inflammatory biomarkers as potential indexes.[23,24]
Inflammation in Type 1 Diabetes
T1D is an autoimmune disorder characterised by a selective, specific destruction of insulin-producing pancreatic beta cells, without apparent pathological alterations of other Langerhans cells.[25] However, T1D shows significant heterogeneity in regard to the age of onset, severity of autoimmune response and efficacy of therapy, while it has also been demonstrated that both humoral and cellular immunity is involved in the pathogenesis of T1D.[26–28] The first theories about predisposition support that environmental trigger factors in early life, such as infections, nutrition and chemicals that are able to activate self-targeting immune cascades, remain applicable even though the initial event is still unclear.[29,30]
Inflammatory Infiltrates in Type 1 Diabetes
Progress in understanding the pathophysiology of T1D has been made in parallel with the advances in the field of immunology. The predominant theory is that the beta cell pancreatic islets in patients with T1D are inflamed, called insulitis, through the course of T1D. Anderson et al., demonstrated that failure in both central and peripheral immune tolerance mechanisms contribute to the emergence of autoreactive T cells in the periphery of non-obese mice with diabetes.[31] Regulatory T cells (Tregs) have been shown to also be defective in this autoimmune disease setting, along with evidence from animal models demonstrating the participation of both CD4+ and CD8+ T cells (effector T-cells/Teff) in the development of T1D as they target several beta cell autoantigens and related peptide epitopes.[32–35] Moreover, by using adoptive T-cell transfer models of T1D, it has been demonstrated that T-cell subtypes are capable of inducing destructive peri-islet inflammatory infiltrate and overt diabetes.[36,37] This was further depicted in human studies using pancreas samples obtained post mortem from subjects diagnosed with recent-onset T1D.[27,38]
Interestingly, the immune B cell (CD20+) profile also changes during disease progression, as initial studies found they align closely with the migration of CD8+ T cells, following two different patterns, either that of high or low infiltration in islets as reported by Wilcox et al.[27,38] Macrophages are also critical mediators of islet inflammation due to their ability to secrete cytokines, such as Interleukin 1 beta (IL-1beta) and tumour necrosis factor alpha (TNF-alpha) and produce reactive oxygen species (ROS).[27,39] Additional studies have shown that the surrounding pancreatic exocrine tissue is abundant in both lymphocytes and neutrophils in T1D and it is suspected that these cells might also contribute to the evolution of disease.[40,41] In some studies, dendritic cells, natural killer (NK) cells and NKT cells have also been found in the islet infiltrate and may have a partial role in the whole process, however, it seems that overall the interaction among different cell types regulates diabetes progression.[42,43]
Mediators of Inflammation in Type 1 Diabetes
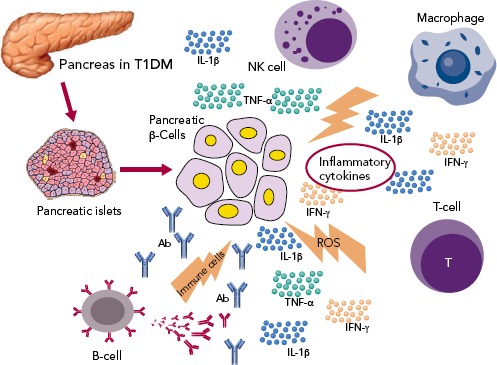
The three cytokines that seem to be implicated in the inflammation of pancreatic beta cells in T1D, are the synergic action of interferon gamma (IFN-gamma) and the innate inflammatory cytokines TNF-alpha and IL-1beta.[44] The combined action of these inflammatory molecules results in the upregulation of inducible nitric oxide synthase (iNOS), with subsequent production of nitric oxide (NO).[45] However, even though ROS plays a role in beta cell destruction, more recent studies have demonstrated that NO is not implicated in the damage of pancreatic beta cell.[46] Furthermore, studies demonstrating that the biology of the beta cell could directly influence the response to an inflammatory environment, through specific gene-guided modulation of beta cell apoptosis induced by IFN-gamma modulated by the PTPN2 gene (Figure 1).[47]
Figure 1: Inflammatory Mediators in Type 1 Diabetes.
Activation of several immune cells are involved in pancreatic beta-cell death through a variety of inflammatory cytokines. Regulatory T cells are defective in this autoimmune disease, while effector T-cells (Teff) participate in the development of type 1 diabetes targeting several beta-cell autoantigens and related peptide epitopes. The profile of immune B cells also changes during disease progression and macrophages are also critical mediators of islet inflammation due to their direct toxicity on beta-cells by reactive oxygen species. Dendritic cells, natural killer cells and natural killer T cells may have a partial role in the process.
The mechanisms mentioned above strongly suggest that multiple pathways may exist which can contribute to pancreatic beta cell death. During this process, the control and regulation of local inflammatory cytokines production are likely to be critical factors in determining the outcome of the autoimmune progression. The disruptive effects of inflammatory and autoimmune-mediated pancreatic islet attack may lead to a vicious cycle where initial cytokine stress may urge the metabolic stress and an additional loss in beta cell function.[48]
Anti-Inflammatory Trials on Type 1 Diabetes
Given the obvious genetic influences in the initiation and progression of T1D, the immune cell type and the pattern that occurs in any given patient offers an important perspective on the design of clinical trials intended to slow or terminate the progression of the disease. Two initial clinical trials with rituximab, a monoclonal anti-CD20 antibody, were only partially successful.[49,50] Furthermore, strategies are being developed targeting the antigen-specific T-cell response, such as the application of plasmid DNA (pDNA) vaccination with promising results.[51] Moreover, two humanised anti-CD3 monoclonal antibody (mAbs), teplizumab and otelixizumab, have been evaluated in people with new and recently diagnosed T1D and showed a reduced rate of loss of beta cell function in the majority of participants.[52]
Cytokines are another promising target for therapy for T1D, given their involvement in the process of beta cell pathology. IL-1beta and TNF-alpha appear to be attractive initial targets for designing clinical trials based on this concept. A pilot study examined the effects of an anti-TNF-alpha therapy, etanercept, on paediatric patients newly diagnosed with T1D and demonstrated an increased endogenous insulin production and better metabolic control.[53] Administration of alpha-1 antitrypsin (AAT), an anti-inflammatory serum protein, to a small group of people with T1D resulted in a reduced IL-1beta response in monocytes and dendritic cells and improved beta cell function.[54] Furthermore, given the broad anti-inflammatory properties of vitamin D, it has also been identified as a potential therapeutic target.[55] However, small studies of vitamin D supplementation in recent onset T1D have only resulted in modest beta cell protection.[56,57]
On a larger scale, interleukin-1 receptor antagonist (IL-1RN) and human monoclonal IL-1beta antibody were employed in two randomised, placebo-controlled trials in people with recent onset T1D.[58] Canakinumab and anakinra were found to be safe but they were not effective as single immunomodulatory drugs in recent-onset T1D and they did not result in preserved beta cell function, as measured by stimulated C-peptide area under the curve (Table 1).
Table 1: Representative Clinical Trials of Anti-inflammatory Treatments on Type 1 Diabetes.
| Mechanism of action | Drug | Main findings | References |
|---|---|---|---|
| Monoclonal anti-CD20 antibody | Rituximab | Rate of C peptide decline ↓, lower insulin requirements, HbA1c ↓ | 49,50 |
| Engineered DNA plasmid encoding proinsulin | BHT-3021 | ↓ CD8+ T cells frequency reactive to proinsulin, C peptide preservation, no change to Interferon-gamma, IL-4, IL-10 | 51 |
| Proinsulin peptide | Human leukocyte antigen-DR4 (DRB1*0401) | ↑ C-peptide, ↑ proinsulin-stimulated IL-10 production, favourable beta-cell stress markers (proinsulin/C-peptide ratio) | 189 |
| TNF antagonism | Etarnecept | HbA1c ↓, endogenous insulin production ↑ | 53 |
| Anti-inflammatory serum protein | Alpha 1 antitrypsin (AAT) | IL-1beta response to monocytes and dendritic monocytes ↓, beta-cell function improvement | 54 |
| Vitamin D analogue | Alfacalcidol | Beta-cell preservation especially in male subjects | 56 |
| Vitamin D analogue | Calcitriol | ↑ in fasting C peptide from diagnosis to 1 year, daily insulin dose ↓ in the treatment group | 190 |
| IL-1 receptor blockade | Anakinra | No C peptide response | 58 |
| IL-1 receptor blockade | Anakinra | ↓ insulin requirements compared with controls, ↓ insulin dose adjusted | 191 |
| IL-1beta antagonism | Canakinumab | No C peptide response | 58 |
| IL-1 receptor blockade IL-1beta antagonism (plasma-induced transcriptional meta-analysis) | Anakinra/canakinumab | Immunomodulation/reverse relationship between inflammation and C peptide stimulation | 192 |
| ALPHAti-CD3 mAbs | Teplizumab/otelixizumab | 52 |
CD20 = cluster of differentiation 20, IL = interleukin, mAbs = monoclonal antibodies, TNF = tumour necrosis factor.
In conclusion, the immunotherapeutic trials that have been completed in human T1D have always focused on patients after clinical onset of diabetes, well after the establishment of targeted adaptive immune responses towards beta cell islets. Targeting these factors is likely to preserve remaining beta cell function, but curative treatments can only be realistically achieved by attempting at the same time to replace part of the beta cell mass that has been lost during the autoimmune process.
Metabolic Disorders and Inflammation in Type 2 Diabetes
Inflammation in Type 2 Diabetes
Several pathophysiological studies have strengthened our understanding of insulin resistance and secretion in the course of disease onset and progression.[59,60] Subjects at risk of T2D display an initial state of insulin resistance compensated by hypersecretion of insulin in the beta cells. However, in the clinical course of the disease this pancreatic functional reserve is eventually unable to cope with the required insulin secretion and by the time diabetes is diagnosed, beta cells are no longer able to secrete enough insulin.[61] Although the relative contribution of beta cell dysfunction and insulin resistance can vary in people with T2D, it is generally accepted that abnormal insulin sensitivity precedes the clinical diagnosis of diabetes by up to 15 years.[62] Therefore, along with mechanistic studies investigating mechanisms forming the basis of insulin resistance, more recent research has also focused on the pathways leading to beta cell failure.[63]
The Role of Adipose Tissue and Obesity
There has been intensive research conducted into the pathophysiology of diabetes and its association with obesity and the biological role of adipose tissue. As addressed before, insulin resistance is a key component in the course of T2D. Liver and muscles have long been recognised as major contributors of systemic insulin resistance.[64] Fat accumulation in the liver (steatosis) precedes overt T2D, is commonly associated with obesity and is considered a major determinant of the reduced hepatic insulin sensitivity resulting in fasting hyperglycaemia.[65–67] Furthermore, it is now well accepted that the accumulation of energy due to excessive calorie intake and the lack of physical activity leads initially to fat accumulation in the subcutaneous tissue and later to other tissue compartments such as the liver, pancreas, muscles, perivascular and pericardium.[67] This fat accumulation increases tissues’ insulin resistance, while pancreatic fat accumulation further determines beta cell dysfunction.[64,68]
Obesity and its associated conditions including metabolic syndrome, hypertension and dyslipidaemia, is positively associated with concentrations of inflammatory biomarkers, which are predictive of insulin resistance and the incidence of T2D and CVD.[69–71] Obesity and metabolic syndrome specifically comprise a cluster of diseases associated with too much food and insufficient physical activity, conditions where sub-acute chronic inflammation is a common and potentially unifying mechanistic cause, accompanied by activation of at least two major inflammatory pathways, stress-activated Jun N-terminal kinases (JNK) and the transcription factor NF-kappaB.[12,16,72–77] This inflammatory state via production of pro-inflammatory cytokines, is further amplified by adipokines, though a number of studies have demonstrated that adipokines stimulate additional inflammatory responses in obesity and promote obesity-induced metabolic and cardiovascular diseases.[78]
Animal studies have demonstrated that brown adipose tissue (BAT) has an important role in regulating energy and glucose homeostasis and is associated with peripheral insulin resistance and glucose levels.[79–81] However, white adipose tissue (WAT) and mainly visceral WAT (around the trunk, upper body or abdomen) appears to be the major source of inflammatory markers in T2D, but also a target of the inflammatory process in people with diabetes. It produces cytokines and several other bioactive substances involved in the inflammatory pathways, such as TNF-alpha, IL-1, IL-6, IL-10, leptin, adiponectin, monocyte chemoattractant protein, angiotensinogen, resistin, chemokines, serum amyloid protein, and many others collectively referred to as adipokines.[82–85] Further infiltration of adipose tissue by macrophages and immune cells (B cells and T cells) trigger local and systemic chronic low-grade inflammation, by producing more cytokines and chemokines that serve as a pathologic link between obesity, insulin resistance and diabetes.[86]
The Role of Gut Microbiota in Type 2 Diabetes
The role of the gut in the pathophysiology of diabetes can be approached from two different viewpoints. Studies have suggested that several mechanisms may be involved in weight loss and diabetes control after bariatric surgery, beyond malabsorption or anatomical restriction.[87] Indeed, complex changes in multiple gut hormones have been shown after bariatric procedures and have been proposed as adjunctive mechanisms for short- and long-term positive metabolic effects, serving as possible novel therapeutic approaches to obesity and insulin resistance.[88,89]
In the past few years, a two-way relationship between the gut microbiome in the host’s energy balance and immune function has been demonstrated.[90] The gut microbiome seems to differ between obese and lean subjects, flora composition influences metabolism and inversely, diet and metabolic status influence the composition of the gut flora, while a faecal microbiome transplantation from lean donors to insulin-resistant subjects results in beneficial metabolic effects.[91–94] It has been postulated that products from the gut microbiome may interact with the immune system inducing a tissue metabolic modification, which feeds the molecular origin of the low-grade inflammation that characterises the onset of obesity and diabetes.[95]
An altered gut microbiota can directly affect immune cells in the gut and indirectly affect immune cells via microbial products including LPS, metabolites and short chain fatty acid (SCFAs), all of which can affect adipogenesis and/or insulin resistance.[96–101] Lipopolysaccharide (LPS) is believed to cause low-grade inflammation mediated by the induction of inflammatory cytokines by immune cells and adipocytes, while SCFAs can modulate gene expression of human monocytes and reduce pro-inflammatory cytokine and chemokine production.[102] SCFAs can also promote regulatory T-cell generation through several pathways, thereby suppressing the function of inflammatory T cells. These are able to block IFN-gamma inducible protein 10 (IP-10) release in human colonic sub-epithelial myofibroblasts, acting not only on immune cells systemically but also on intestinal tissue cells locally.[103,104]
The Role of Pancreatic Beta Cell Failure in Type 2 Diabetes
Independent of the aetiopathogenetic mechanism among the different types of diabetes, the common pathway seems to be the inflammation in the pancreatic Langerhans beta cell islets (insulitis), in the concept of an auto-inflammatory process, which results in reduction in both beta cell number and function.[105] It has been suggested that in people with a genetic predisposition, the ‘stressed’ beta cell may stimulate local inflammation and modify the balance between beta cell mass and function in the islets of Langerhans.[106,107] Several experimental models as well as observational studies in humans have demonstrated that macrophages play a key role in the islet inflammation seen in T2D.[108–111] Inflammasome/IL-1beta signalling is the most common, well-studied and high-impact pathway activated in islets of multiple T2D models that cause beta cell dysfunction.[112,113] It is likely that other immune cell types are involved in islet inflammation in T2D, while islet autoimmunity has also been suggested to contributes to beta cell functional decline during the course of T2D.[114,115]
Among factors that stimulate islet macrophages to secrete IL-1beta in vivo in human islets are amyloid polypeptide, free fatty acids (FFAs) and endocannabinoids.[110,111,116] However, it has been assumed that hyperglycaemia is produced initially in the inflammation in pancreatic beta cells by inducing apoptotic mechanisms.[117] ALPHA particular pathway was proposed by Maedler et al. who showed that hyperglycaemia may induce the production of IL-1beta by stimulating pro-apoptotic receptor FFAs on beta cells.[118]
FFAs can also produce and secrete IL-1beta and IL-1-dependent pro-inflammatory cytokines in pancreatic islets and thus to reduce the inflammation. In addition, after its initial secretion, IL-1beta regulates its production in pancreatic beta cells by auto stimulation, while this process also increases nitric oxide production leading to reduction in ATP concentration in the mitochondria, which can cause further beta cell dysfunction and reduced insulin secretion.[119–122] Oxidative stress may also potentiate the generation of ROS along with other pro-inflammatory cytokines and chemokines in the beta cells that disrupts the blood flow into them and destroys their function.[108,123,124]
Experimental studies have confirmed that IL-6 induces apoptosis in pancreatic islets together with other inflammatory cytokines and acts as a predictor and pathogenic marker for the progression of T2D.[69,124,125] TNF-alpha is also considered to play an essential role, by creating a linkage among insulin resistance, obesity and islet inflammation.[125] Its overproduction in adipose tissue seems to feed the inflammation and beta cell death in pancreatic islets and produces additional insulin resistance in peripheral tissues.[126,127]
Evidence of Inflammation in Other Organs in People with Type 2 Diabetes
Immune system activation is highly related to T2D incidence and progression and adaptive and innate immunity are involved in adipose tissue inflammation. The phenotype switching of macrophages from predominantly anti-inflammatory M2-type to increased proportions of pro-inflammatory M1-type macrophages plays a crucial role in the initiation and amplification of islet inflammation.[128] However, the evidence shows that the recruitment of B cells and T cells precedes adipose tissue infiltration by macrophages.[86]
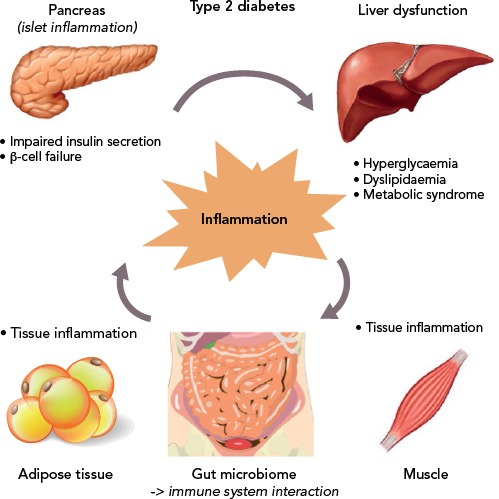
Moreover, several other organs have been reported to participate in the metabolic homeostasis and inflammatory state in T2D, such as the liver, the neural system and possibly skeletal muscle.[129–133] However, more research is needed to support this evidence (Figure 2).
Figure 2: The Vicious Cycle of Inflammation in Various Target Organs in Type 2 Diabetes.
Inflammation has a key role in the pathophysiology of type 2 diabetes and its associated metabolic abnormalities.
Current Knowledge on Diabetes Treatments
Drugs with Pleiotropic Effects
The current therapeutic approaches to T2D have anti-inflammatory properties in addition to their major modes of action. Non-pharmacological therapies, such as lifestyle interventions, but also pharmacological and bariatric surgical approaches for weight loss, appears to reduce inflammation assessed as circulating CRP and IL-6 concentrations, and improves cardiovascular and all-cause mortality.[134–138]
Statins also have anti-inflammatory properties beyond their ability to lower levels of low-density lipoproteins (LDL) cholesterol. The Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) demonstrated that rosuvastatin reduced high-sensitivity CRP along with LDL cholesterol, however the effects of statins on glycaemic control are conflicting, implying that targeting inflammation with statins does not improve glycaemia and therefore does not provide an integrated anti-inflammatory approach for diabetes and CVD.[139–141]
Anti-diabetic agents, including insulin, have intrinsic anti-inflammatory effects associated with their primary mechanisms of action and are also associated with reductions in inflammatory markers. Insulin itself decreases NF-kappaB activity in peripheral blood mononuclear cells which reduces inflammation.[142] The anti-inflammatory actions of thiazolidinediones through binding and activation to the peroxisome proliferator-activated receptor gamma (PPARgamma), seems to be related to trans-repression of NF-kappaB and reduced expression of NF-kappaB targets.[143]
In addition to its metabolic effects, metformin has anti-inflammatory actions that appear to be independent of glycaemia and are most prominent in immune cells and vascular tissues.[144–150] Dipeptidyl peptidase-4 inhibitors (DPP-4) and GLP-1 receptor agonists also have intrinsic anti-inflammatory properties, however, beyond their anti-diabetic effects, the contribution of inflammation reduction to diabetes and cardiovascular improvements remains unknown.[151–153] Finally, a new class of anti-diabetic drugs, sodium–glucose cotransporter-2 inhibitors (SGLT2 inhibitors) acts by increasing renal excretion of glucose. Preliminary data in humans demonstrate a possible improvement on the circulating biomarkers of inflammation by SGLT2-inhibitors; however, more studies are needed.[152]
Anti-Inflammatory Drugs in Type 2 Diabetes
Multiple medical approaches that directly target inflammatory pathways have been studied in the past few years supporting the concept of anti-inflammatory treatment for cardiometabolic diseases, such as diabetes and atherosclerotic CVD.[154–156] For a long time, salicylates, especially aspirin, have been used to treat thrombosis in primary and secondary CVD prevention, as well as to treat rheumatoid diseases.[157,158] They were the first class of drugs reported to lower glucose in diabetes more than a century ago, however, several studies with salicylate products have demonstrated an improved metabolic profile in patients with obesity and diabetes, suggesting a potential efficacy for diabetes prevention and control.[159–166]
Methotrexate is a disease-modifying drug broadly used to treat rheumatic diseases among other conditions, while its efficacy on glycaemic control was demonstrated in a small cohort study.[167] The preliminary data drove the design and conduction of a large clinical trial with methotrexate among patients with previous MI and either T2D or metabolic syndrome, however, methotrexate had neutral findings on IL-1b, IL-6 and CRP levels, while more data are anticipated for the effects on T2D.[168]
Biological Agents as Anti-Inflammatory Therapy in Type 2 Diabetes
Targeting cytokine production and secretion to prevent further activation of inflammation have been proposed with the intention of stopping the initiation and progression of T2D. TNF-alpha antagonists have been used to treat inflammatory conditions and have been associated with improved glycaemic control and decreased incident of diabetes, while more studies on patients with unfavourable cardiometabolic profile did not demonstrate adequate results, with the exception of a randomised 6-month trial.[169–180]
The mechanism of action of IL-1beta is consistent with the pathogenesis and progression of T2D is. Improved beta cell secretory function and glycaemia, as well as reduced inflammatory biomarkers in people with diabetes and pre-diabetes have been demonstrated by IL-1beta antagonists, such as anakinra and gevokizumab.[112,120,181–6] Studies on CVD and atherosclerosis prevention with IL-1beta antagonists have also been conducted. One study showed that canakinumab reduced the inflammatory proteins CRP, IL-6, and fibrinogen in persons with T2D and high cardiovascular risk with no effect on HbA1C, glucose, and insulin at 4 months, while the large randomised trial with canakinumab – Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) – over a median period of 3.7 years did not reduce the incidence of diabetes in patients with prior MI and high-sensitivity CRP (hsCRP) ≥2 mg/l (Table 2).[187,188]
Table 2: Representative Clinical Trials of Anti-Inflammatory Treatments on Type 2 Diabetes – Metabolic Profile.
| Mechanism of Action | Drug | Main Findings | Reference |
|---|---|---|---|
| IL-1 receptor blockade | Anakinra | HbA1c, leukocyte ↓, CR↓ insulin secretion↑ | 182 |
| IL-1 receptor blockade | Anakinra | Sustained CRP ↓, insulin secretion ↑, insulin requirement ↓ | 181 |
| IL-1 receptor blockade | Anakinra | Insulin sensitivity ↑ | 193 |
| IL-1 receptor blockade | Anakinra | Insulin secretion ↑ (first-phase insulin secretion improved) | 194 |
| IL-1 receptor blockade | Anakinra | Insulin secretion↑ | 183 |
| IL-1beta antagonism | Gevokizumab | HbA1c ↓, CRP >↓, insulin secretion ↑ | 112 |
| IL-1beta antagonism | Canakinumab | Insulin secretion ↑, CRP ↓ | 185 |
| IL-1beta antagonism | Canakinumab | CRP ↓, HbA1c ↓, insulin secretion ↑ (not statistically significant) | 184 |
| IL-1beta antagonism | Canakinumab | Significant CRP and IL-6 ↓, 6 month HbA1c ↓, but not consistent Hba1c ↓ long-term | 188 |
| IL-1beta antagonism | Canakinumab | CRP ↓, fibrinogen ↓, IL-6 ↓, no effect on HbA1c, glucose and insulin levels | 187 |
| IL-1beta antagonism | LY2189102 | HbA1c ↓, CRP ↓, insulin secretion ↑ | 186 |
| IKKbeta–NF-kappaB inhibition | Salsalate | FBG ↓, CRP ↓, insulin sensitivity ↑, adiponectin ↑ | 160 |
| IKKbeta–NF-kappaB inhibition | Salsalate | FBG ↓, CRP ↓, adiponectin ↑ | 159 |
| IKKbeta–NF-kappaB inhibition | Salsalate | FBG ↓, insulin ↑, CRP ↓ | 195 |
| IKKbeta–NF-kappaB inhibition | Salsalate | HbA1c ↓, FBG ↓, triglyceride ↓, adiponectin ↑ | 165 |
| IKKbeta–NF-kappaB inhibition | Salsalate | HbA1c ↓, FBG ↓, insulin secretion ↑, triglyceride ↓ | 162 |
| IKKbeta–NF-kappaB inhibition | Salsalate | FBG ↓, adiponectin ↑ | 163 |
| IKKbeta–NF-kappaB inhibition | Salsalate | HbA1c ↓, FBG ↓, triglyceride ↓, leukocyte ↓, uric acid ↓, adiponectin ↑ | 164 |
| TNF antagonism | CDP571 | No effect on insulin sensitivity | 176 |
| TNF antagonism | Single dose of soluble TNF receptor–Fc fusion protein (Ro 45–2081) | No effect on insulin sensitivity | 177 |
| TNF antagonism | Soluble TNF receptor–Fc fusion protein etanercept | CRP ↓, insulin secretion ↑, no effect on insulin sensitivity | 178 |
| TNF antagonism | Soluble TNF receptor–Fc fusion protein etanercept | CRP ↓, adiponectin ↑, LDL ↓, no effect on insulin sensitivity | 179 |
| TNF antagonism | Soluble TNF receptor–Fc fusion protein etanercept | FBG ↓ | 180 |
| TNF antagonism | Infliximab | Fasting glucose improvement, ratio of high molecular weight to total adiponectin ↑, sICAM-1 ↑, no effect on CRP | 170 |
| Decrease in TNF and IL-1beta levels by an unknown mechanism of action | Diacerein | HbA1c ↓, FBG ↓, insulin secretion ↑ | 196 |
| DHFR inhibitor – antimetabolite | Low-dose methotrexate | No effects on CRP, IL-1beta or IL-6 | 168 |
| DHFR inhibitor (DMARD) – combination with sulphasalazine glycocorticosteroids/hydroxychloroquine | Methotrexate | HbA1c ↓ | 167 |
CRP: C-reactive protein; DHFR = dihydrofolate reductase inhibitor; FBG = fasting blood glucose; IKKbeta = IkappaB kinase-beta; IL = Interleukin; mAbs = monoclonal antibodies; NF-kappaB = nuclear factor-kappaB; sICAM-1 = soluble intercellular adhesion molecule-1; TNF = tumour necrosis factor.
Future Perspectives for the Treatment of Diabetes
Novel approaches on T2D to evaluate anti-inflammatory diets and modulate an individual’s microbiome are under study. Clinical trials investigating the effects of vitamin D supplementation on serum levels of inflammatory markers have provided inconsistent results, with no evidence of effects in most trials, or effects on selected markers in others. There are also studies investigating whether antagonists of leukotriene production enzymes – 5-lipoxygenase (5-LO), 5-LO-activating protein and LTA4 hydrolase – or receptor binding BLT1 have cardiometabolic outcome benefits, however these results have not yet been reported. The potential for targeting cholinergic pathways, immune modulation or other mediators of inflammation such as JNK and toll-like receptors (TLRs) are also being researched.
Conclusion
The increasing prevalence of diabetes makes it imperative that research should focus on its prevention as well as its treatment. An improved understanding of the mechanisms linking inflammation to diabetes and related complications has stimulated interest in targeting inflammatory pathways as part of the strategy to prevent or control diabetes and its complications.
T1D is considered to be more of an immunological response rather than a metabolic disorder and the preliminary results of trials using anti-inflammatory and immunomodulatory medication are promising. These treatments in combination with possible use of stem cells to regenerate pancreatic beta cells could potentially be the key to permanent treatment of T1D. Therefore, after a holistic review of the possible mechanisms that lead to T1D and T2D and the numerous already described inflammation pathways that are involved, it becomes more and more clear that future research should focus on simultaneous suppression of various inflammatory response pathways rather than focusing on one pathway at a time.
References
- 1.Inzucchi SE. Diagnosis of diabetes. N Engl J Med. 2013;368:193. doi: 10.1056/NEJMc1212738. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW, Li Y, Wang J et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370:1514–23. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Rönnemaa T et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 4.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J. 2013;34:2444–52. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 6.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Global report on diabetes Geneva WHO. 2016.
- 8.Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol. 2014;126:211–22. doi: 10.1016/B978-0-444-53480-4.00015-1. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes 2018. Diabetes Care. 2018;41((uppl 1)):S13–27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Chan JC, Malik V, Jia W et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 12.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson RT. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. Br Med J. 1901;1:760–2. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid J, Macdougall AI, Andrews MM. Aspirin and diabetes mellitus. Br Med J. 1957;2:1071–4. doi: 10.1136/bmj.2.5053.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–90. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 17.Ogston D, McAndrew GM. Fibrinolysis in obesity. Lancet. 1964;2:1205–7. doi: 10.1016/S0140-6736(64)91042-6. [DOI] [PubMed] [Google Scholar]
- 18.Fearnley GR, Vincent CT, Chakrabarti R. Reduction of blood fibrinolytic activity in diabetes mellitus by insulin. Lancet. 1959;2:1067. doi: 10.1016/S0140-6736(59)91534-X. [DOI] [PubMed] [Google Scholar]
- 19.Kaptoge S, Di Angelantonio E, Lowe G et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridker PM, Cushman M, Stampfer MJ et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 21.Duncan BB, Schmidt MI, Pankow JS et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52:1799–805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 22.Marques-Vidal P, Schmid R, Bochud M et al. Adipocytokines, hepatic and inflammatory biomarkers and incidence of type 2 diabetes. the CoLaus study. PLoS One. 2012;7:e51768. doi: 10.1371/journal.pone.0051768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kengne AP, Batty GD, Hamer M et al. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;35:396–403. doi: 10.2337/dc11-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57:162–7. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atkinson MA, Bluestone JA, Eisenbarth GS et al. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–9. doi: 10.2337/db10-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willcox A, Richardson SJ, Bone AJ et al. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–83. doi: 10.1016/S0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 29.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 30.Hyttinen V, Kaprio J, Kinnunen L et al. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52:1052–5. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 31.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 32.El-Sheikh A, Suarez-Pinzon WL, Power RF, Rabinovitch A. Both CD4+ and CD8+ T cells are required for IFN-gamma gene expression in pancreatic islets and autoimmune diabetes development in biobreeding rats. J Autoimmun. 1999;12:109–19. doi: 10.1006/jaut.1998.0264. [DOI] [PubMed] [Google Scholar]
- 33.Miller BJ, Appel MC, O'Neil JJ, Wicker LS. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988;140:52–8. [PubMed] [Google Scholar]
- 34.Phillips JM, Parish NM, Raine T et al. Type 1 diabetes development requires both CD4+ and CD8+ T cells and can be reversed by non-depleting antibodies targeting both T cell populations. Rev Diabet Stud. 2009;6:97–103. doi: 10.1900/RDS.2009.6.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25:80–92. doi: 10.1007/s12022-014-9297-8. [DOI] [PubMed] [Google Scholar]
- 36.Healey D, Ozegbe P, Arden S et al. In vivo activity and in vitro specificity of CD4+ Th1 and Th2 cells derived from the spleens of diabetic NOD mice. J Clin Invest. 1995;95:2979–85. doi: 10.1172/JCI118006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–8. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 38.Arif S, Leete P, Nguyen V et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63:3835–45. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutchings P, Rosen H, O'Reilly L et al. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1999;348:639–42. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Calvo T, Ekwall O, Amirian N et al. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63:3880–90. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valle A, Giamporcaro GM, Scavini M et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes. 2013;62:2072–7. doi: 10.2337/db12-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dotta F, Censini S, van Halteren AG et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–20. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–13. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 44.Feuerer M, Shen Y, Littman DR et al. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–64. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic beta-cell dysfunction is mediated by beta-cell nitric oxide production. Diabetes. 2002;51:311–6. doi: 10.2337/diabetes.51.2.311. [DOI] [PubMed] [Google Scholar]
- 46.Arif S, Moore F, Marks K et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes. 2011;60:2112–9. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore F, Colli ML, Cnop M et al. PTPN2, a candidate gene for type 1 diabetes, modulates interferon-gamma-induced pancreatic beta-cell apoptosis. Diabetes. 2009;58:1283–91. doi: 10.2337/db08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirot P, Eizirik DL, Cardozo AK. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia. 2006;49:1229–36. doi: 10.1007/s00125-006-0214-7. [DOI] [PubMed] [Google Scholar]
- 49.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pescovitz MD, Greenbaum CJ, Bundy B et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care. 2014;37:453–9. doi: 10.2337/dc13-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roep BO, Solvason N, Gottlieb PA et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci Transl Med. 2013;5:191–ra82. doi: 10.1126/scitranslmed.3006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daifotis AG, Koenig S, Chatenoud L, Herold KC. Anti-CD3 clinical trials in type 1 diabetes mellitus. Clin Immunol. 2013;149:268–78. doi: 10.1016/j.clim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Mastrandrea L, Yu J, Behrens T et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care. 2009;32:1244–9. doi: 10.2337/dc09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb PA, Alkanani AK, Michels AW et al. alpha1-Antitrypsin therapy downregulates toll-like receptor-induced IL-1beta responses in monocytes and myeloid dendritic cells and may improve islet function in recently diagnosed patients with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:E1418–26. doi: 10.1210/jc.2013-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badenhoop K, Kahles H, Penna-Martinez M. Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr Diab Rep. 2012;12:635–42. doi: 10.1007/s11892-012-0322-3. [DOI] [PubMed] [Google Scholar]
- 56.Ataie-Jafari A, Loke SC, Rahmat AB et al. A randomized placebo-controlled trial of alphacalcidol on the preservation of beta cell function in children with recent onset type 1 diabetes. Clin Nutr. 2013;32:911–7. doi: 10.1016/j.clnu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Gabbay MA, Sato MN, Finazzo C et al. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual beta-cell function in new-onset type 1 diabetes mellitus. Arch Pediatr Adolesc Med. 2012;166:601–7. doi: 10.1001/archpediatrics.2012.164. [DOI] [PubMed] [Google Scholar]
- 58.Moran A, Bundy B, Becker DJ et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–15. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saad MF, Knowler WC, Pettitt DJ et al. Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet. 1989;1:1356–9. doi: 10.1016/S0140-6736(89)92804-3. [DOI] [PubMed] [Google Scholar]
- 60.Martin BC, Warram JH, Krolewski AS et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340:925–9. doi: 10.1016/0140-6736(92)92814-V. [DOI] [PubMed] [Google Scholar]
- 61.Jallut D, Golay A, Munger R et al. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism. 1990;39:1068–75. doi: 10.1016/0026-0495(90)90168-C. [DOI] [PubMed] [Google Scholar]
- 62.Tabák AG, Jokela M, Akbaraly TN et al. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. Lancet. 2009;373:2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kahn SE, Prigeon RL, McCulloch DK et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects Evidence for a hyperbolic function. Diabetes. 1993;42:1663–72. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 64.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- 65.Birkenfeld AL, Shulman GI. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–23. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeFronzo RA, E. Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38:387–95. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 67.Sattar N, Gill JM. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:123. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor R. Pathogenesis of type 2 diabetes: tracing the reverse route from cure to cause. Diabetologia. 2008;51:1781–9. doi: 10.1007/s00125-008-1116-7. [DOI] [PubMed] [Google Scholar]
- 69.Pradhan AD, Manson JE, Rifai N et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 70.Thorand B, Löwel H, Schneider A et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study,1984-1998. Arch Intern Med. 2003;163:93–9. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 71.Antonopoulos AS, Tousoulis D. The molecular mechanisms of obesity paradox. Cardiovasc Res. 2017;113:1074–86. doi: 10.1093/cvr/cvx106. [DOI] [PubMed] [Google Scholar]
- 72.Hirosumi J, Tuncman G, Chang Let al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 73.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 74.Olefsky JM, Glass CK. et al. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 75.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishimura S, Manabe I, Nagasaki M et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 78.Takaoka M, Nagata D, Kihara S et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–11. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 79.Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–90. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–86. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–6. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 82.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 83.Kanda H, Tateya S, Tamori Y et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antonopoulos AS, Margaritis M, Coutinho P et al. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–19. doi: 10.2337/db14-1011. [DOI] [PubMed] [Google Scholar]
- 85.Antoniades C, Antonopoulos AS, Tousoulis D, Stefanadis C. Adiponectin: from obesity to cardiovascular disease. Obes Rev. 2009;10:269–79. doi: 10.1111/j.1467-789X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 86.Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239–50. doi: 10.1038/gene.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sandoval D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes (Lond) 2011;35((Suppl 3))::S45–9. doi: 10.1038/ijo.2011.148. [DOI] [PubMed] [Google Scholar]
- 88.Papamargaritis D, Panteliou E, Miras AD, le Roux CW. Mechanisms of weight loss, diabetes control and changes in food choices after gastrointestinal surgery. Curr Atheroscler Rep. 2012;14:616–23. doi: 10.1007/s11883-012-0283-7. [DOI] [PubMed] [Google Scholar]
- 89.Miras AD, le Roux CW. Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery? Int J Obes (Lond) 2014;38:325–33. doi: 10.1038/ijo.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–65. doi: 10.2337/dc14-0769. [DOI] [PubMed] [Google Scholar]
- 91.Turnbaugh PJ, Ley RE, Mahowald MA et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 92.Vrieze A, Van Nood E, Holleman F et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 93.Nicholson JK, Holmes E, Kinross J et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 94.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burcelin R, Garidou L, Pomie C. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol. 2012;24:67–74. doi: 10.1016/j.smim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 96.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes. 2012;3:279–88. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cani PD, Amar J, Iglesias MA et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 98.Hersoug LG, Møller S, Loft S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: implications for inflammation and obesity. Obes Rev. 2016;17:297–312. doi: 10.1111/obr.12370. [DOI] [PubMed] [Google Scholar]
- 99.Remely M, Aumueller E, Merold C et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537:85–92. doi: 10.1016/j.gene.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez-Curto E, Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem Pharmacol. 2016;114:3–13. doi: 10.1016/j.bcp.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 101.Scheithauer TP, Dallinga-Thie GM, de Vos WM et al. Causality of small and large intestinal microbiota in weight regulation and insulin resistance. Mol Metab. 2016;5:759–70. doi: 10.1016/j.molmet.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nastasi C, Candela M, Bonefeld CM et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arpaia N, Campbell C, Fan X et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Inatomi O, Andoh A, Kitamura K et al. Butyrate blocks interferon-gamma-inducible protein-10 release in human intestinal subepithelial myofibroblasts. J Gastroenterol. 2005;40:483–9. doi: 10.1007/s00535-005-1573-4. [DOI] [PubMed] [Google Scholar]
- 105.Brooks-Worrell B, Palmer J. Immunology in the Clinic Review Series; focus on metabolic diseases: development of islet autoimmune disease in type 2 diabetes patients: potential sequelae of chronic inflammation. Clin Exp Immunol. 2012;167:40–6. doi: 10.1111/j.1365-2249.2011.04501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241–54. doi: 10.1373/clinchem.2010.157016. [DOI] [PubMed] [Google Scholar]
- 107.Halban PA, Polonsky KS, Bowden DW et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ehses JA, Perren A, Eppler E et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–70. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 109.Kamata K, Mizukami H, Inaba W et al. Islet amyloid with macrophage migration correlates with augmented beta-cell deficits in type 2 diabetic patients. Amyloid. 2014;21:191–201. doi: 10.3109/13506129.2014.937857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eguchi K, Manabe I, Oishi-Tanaka Y et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–33. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 111.Jourdan T, Godlewski G, Cinar R et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–40. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cavelti-Weder C, Babians-Brunner A, Keller C et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35:1654–62. doi: 10.2337/dc11-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sauter NS, Schulthess FT, Galasso R et al. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–18. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Butcher MJ, Hallinger D, Garcia E et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57:491–501. doi: 10.1007/s00125-013-3116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brooks-Worrell BM, Boyko EJ, Palmer J. Impact of islet autoimmunity on the progressive beta-cell functional decline in type 2 diabetes. Diabetes Care. 2014;37:3286–93. doi: 10.2337/dc14-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta-cell dysfunction. Diabetes. 2014;63:1698–711. doi: 10.2337/db13-0863. [DOI] [PubMed] [Google Scholar]
- 117.Donath MY, Gross DJ, Cerasi E, Kaiser N. Hyperglycemia-induced beta-cell apoptosis in pancreatic islets of Psammomys obesus during development of diabetes. Diabetes. 1999;48:738–44. doi: 10.2337/diabetes.48.4.738. [DOI] [PubMed] [Google Scholar]
- 118.Maedler K, Oberholzer J, Bucher P et al. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–33. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 119.Böni-Schnetzler M, Boller S, Debray S. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–29. doi: 10.1210/en.2009-0543. [DOI] [PubMed] [Google Scholar]
- 120.Böni-Schnetzler M, Thorne J, Parnaud G et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–74. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arafat HA, Katakam AK, Chipitsyna G et al. Osteopontin protects the islets and beta-cells from interleukin-1 beta-mediated cytotoxicity through negative feedback regulation of nitric oxide. Endocrinology. 2007;148:575–84. doi: 10.1210/en.2006-0970. [DOI] [PubMed] [Google Scholar]
- 122.Yang J, Chi Y, Burkhardt BR et al. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68:270–9. doi: 10.1111/j.1753-4887.2010.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423:12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 124.Akash MS, Shen Q, Rehman K, Chen S. Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. J Pharm Sci. 2012;101:1647–58. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 125.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenvinge A, Krogh-Madsen R, Baslund B, Pedersen BK. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scand J Rheumatol. 2007;36:91–6. doi: 10.1080/03009740601179605. [DOI] [PubMed] [Google Scholar]
- 127.Ruan H, Miles PD, Ladd CM et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes. 2002;51:3176–88. doi: 10.2337/diabetes.51.11.3176. [DOI] [PubMed] [Google Scholar]
- 128.Sell HC, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat Rev Endocrinol. 2012;8:709–16. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 129.Kiechl S, Wittmann J, Giaccari A et al. Blockade of receptor activator of nuclear factor-kappaB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19:358–63. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 130.Cai D. Neuroinflammation in overnutrition-induced diseases. Vitam Horm. 2013;91:195–218. doi: 10.1016/B978-0-12-407766-9.00008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varma V, Yao-Borengasser A, Rasouli N et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab. 2009;296:E1300–10. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kampoli AM, Tousoulis D, Briasoulis A et al. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des. 2011;17:4147–58. doi: 10.2174/138161211798764825. [DOI] [PubMed] [Google Scholar]
- 133.Papaoikonomou S, Tousoulis D, Tentolouris N et al. The role of C-reactive protein genetic variability in the onset of carotid artery disease and renal function impairment in patients with diabetes mellitus type 2. Int J Cardiol. 2013;168:4331–2. doi: 10.1016/j.ijcard.2013.05.087. [DOI] [PubMed] [Google Scholar]
- 134.Chow LS, Odegaard AO, Bosch TA et al. Twenty year fitness trends in young adults and incidence of prediabetes and diabetes: the CARDIA study. Diabetologia. 2016;59:1659–65. doi: 10.1007/s00125-016-3969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li G, Zhang P, Wang J et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–80. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 136.Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61:789–807. doi: 10.1007/s00011-012-0473-3. [DOI] [PubMed] [Google Scholar]
- 137.Derosa G, Maffioli P, Sahebkar A. Improvement of plasma adiponectin, leptin and C-reactive protein concentrations by orlistat: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;81:819–34. doi: 10.1111/bcp.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Garvey WT, Ryan DH, Henry R et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37:912–21. doi: 10.2337/dc13-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ridker PM, Danielson E, Fonseca FA et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 140.Rajpathak SN, Kumbhani DJ, Crandall J et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924–9. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tousoulis D, Koniari K, Antoniades C et al. Combined effects of atorvastatin and metformin on glucose-induced variations of inflammatory process in patients with diabetes mellitus. Int J Cardiol. 2011;149:46–9. doi: 10.1016/j.ijcard.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 142.Dandona P, Aljada A, Mohanty P et al. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–65. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 143.Pascual G, Fong AL, Ogawa S et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pradhan AD, Everett BM, Cook NR et al. Effects of initiating insulin and metformin on glycemic control and inflammatory biomarkers among patients with type 2 diabetes: the LANCET randomized trial. JAMA. 2009;302:1186–94. doi: 10.1001/jama.2009.1347. [DOI] [PubMed] [Google Scholar]
- 145.Caballero AE, Delgado A, Aguilar-Salinas CA et al. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: a placebo-controlled, randomized clinical trial. J Clin Endocrinol Metab. 2004;89:3943–8. doi: 10.1210/jc.2004-0019. [DOI] [PubMed] [Google Scholar]
- 146.Dandona P, Aljada A, Ghanim H et al. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab. 2004;89:5043–7. doi: 10.1210/jc.2004-0436. [DOI] [PubMed] [Google Scholar]
- 147.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–8. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 148.Kim J, Kwak HJ, Cha JY et al. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014;289:23246–55. doi: 10.1074/jbc.M114.577908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–72. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- 150.Vasamsetti SB, Karnewar S, Kanugula AK et al. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64:2028–41. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 151.Kim SC, Wu S, Fang X et al. Postconditioning with a CpG containing oligodeoxynucleotide ameliorates myocardial infarction in a murine closed-chest model. Life Sci. 2014;119:1–8. doi: 10.1016/j.lfs.2014.09.029. [DOI] [PubMed] [Google Scholar]
- 152.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 153.Katsi VK, Michalakeas CA, Grassos CE et al. Canagliflozin: a new hope in the antidiabetic armamentarium. Recent Pat Cardiovasc Drug Discov. 2013;8:216–20. doi: 10.2174/1574890108666131213100613. [DOI] [PubMed] [Google Scholar]
- 154.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465–76. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 155.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–7. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guirguis-Blake JM, Evans CV, Senger CA et al. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2016;164:804–13. doi: 10.7326/M15-2113. [DOI] [PubMed] [Google Scholar]
- 158.Frantz B, O'Neill EA. The effect of sodium salicylate and aspirin on NF-kappa B. Science. 1995;270:2017–9. doi: 10.1126/science.270.5244.2017. [DOI] [PubMed] [Google Scholar]
- 159.Goldfine AB, Silver R, Aldhahi W et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1:36–43. doi: 10.1111/j.1752-8062.2008.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31:289–94. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Faghihimani E, Aminorroaya A, Rezvanian H et al. Reduction of insulin resistance and plasma glucose level by salsalate treatment in persons with prediabetes. Endocr Pract. 2012;18:826–33. doi: 10.4158/EP12064.OR. [DOI] [PubMed] [Google Scholar]
- 162.Faghihimani E, Aminorroaya A, Rezvanian H et al. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2013;50:537–43. doi: 10.1007/s00592-011-0329-2. [DOI] [PubMed] [Google Scholar]
- 163.Goldfine AB, Conlin PR, Halperin F et al. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56:714–23. doi: 10.1007/s00125-012-2819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goldfine AB, Fonseca V, Jablonski KA et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159:1–12. doi: 10.7326/0003-4819-159-1-201307020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goldfine AB, Fonseca V, Jablonski KA et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–57. doi: 10.7326/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rumore MM, Kim KS. Potential role of salicylates in type 2 diabetes. Ann Pharmacother. 2010;44:1207–21. doi: 10.1345/aph.1M483. [DOI] [PubMed] [Google Scholar]
- 167.de Rotte MC, de Jong PH, den Boer E et al. Effect of methotrexate use and erythrocyte methotrexate polyglutamate on glycosylated hemoglobin in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:2026–36. doi: 10.1002/art.38652. [DOI] [PubMed] [Google Scholar]
- 168.Ridker PM, Everett BM, Pradhan A et al. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380:752–62. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yazdani-Biuki B, Stelzl H, Brezinschek HP et al. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur J Clin Invest. 2004;34:641–2. doi: 10.1111/j.1365-2362.2004.01390.x. [DOI] [PubMed] [Google Scholar]
- 170.Kiortsis DN, Mavridis AK, Vasakos S et al. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64:765–6. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yazdani-Biuki B, Mueller T, Brezinschek HP et al. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29:1712–3. doi: 10.2337/dc06-0636. [DOI] [PubMed] [Google Scholar]
- 172.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–6. [PubMed] [Google Scholar]
- 173.Huvers FC, Popa C, Netea MG et al. Improved insulin sensitivity by anti-TNFalpha antibody treatment in patients with rheumatic diseases. Ann Rheum Dis. 2007;66:558–9. doi: 10.1136/ard.2006.062323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Marra M, Campanati A, Testa R et al. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20:731–6. doi: 10.1177/039463200702000408. [DOI] [PubMed] [Google Scholar]
- 175.Timper K, Hruz P, Beglinger C, Donath MY. Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care. 2013;36:e90–1. doi: 10.2337/dc13-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ofei F, Hurel S, Newkirk J et al. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–5. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 177.Paquot N, Castillo MJ, Lefèbvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab. 200;85:1316–9. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- 178.Dominguez H, Storgaard H, Rask-Madsen C et al. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42:517–25. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- 179.Bernstein LE, Berry J, Kim S et al. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166:902–8. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stanley TL, Zanni MV, Johnsen S et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E146–50. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Larsen CM, Faulenbach M, Vaag A et al. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663–8. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Larsen CM, Faulenbach M, Vaag A et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 183.van Asseldonk EJ, Stienstra R, Koenen TB et al. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96:2119–26. doi: 10.1210/jc.2010-2992. [DOI] [PubMed] [Google Scholar]
- 184.Hensen J, Howard CP, Walter V, Thuren T. Impact of interleukin-1beta antibody (canakinumab) on glycaemic indicators in patients with type 2 diabetes mellitus: results of secondary endpoints from a randomized, placebo-controlled trial. Diabetes Metab. 2013;39:524–31. doi: 10.1016/j.diabet.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 185.Rissanen A, Howard CP, Botha J et al. Effect of anti-IL-1beta antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab. 2012;14:1088–96. doi: 10.1111/j.1463-1326.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- 186.Sloan-Lancaster J, Abu-Raddad E, Polzer Jet al. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1beta antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36:2239–46. doi: 10.2337/dc12-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ridker PM, Howard CP, Walter V et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 188.Everett BM, Donath MY, Pradhan AD et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71:2392–401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 189.Alhadj Ali M, Liu YF, Arif S et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017;9:eaaf7779. doi: 10.1126/scitranslmed.aaf7779. [DOI] [PubMed] [Google Scholar]
- 190.Pitocco D, Crinò A, Di Stasio E et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset type 1 diabetes (IMDIAB XI). Diabet Med. 2006;23:920–3. doi: 10.1111/j.1464-5491.2006.01921.x. [DOI] [PubMed] [Google Scholar]
- 191.Sumpter KM, Adhikari S, Grishman EK, White PC. Preliminary studies related to anti-interleukin-1beta therapy in children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2011;12:656–67. doi: 10.1111/j.1399-5448.2011.00761.x. [DOI] [PubMed] [Google Scholar]
- 192.Cabrera SM, Wang X, Chen YG et al. Interleukin-1 antagonism moderates the inflammatory state associated with Type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol. 2016;46:1030–46. doi: 10.1002/eji.201546005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.van Asseldonk EJ, van Poppel PC, Ballak DB et al. One week treatment with the IL-1 receptor antagonist anakinra leads to a sustained improvement in insulin sensitivity in insulin resistant patients with type 1 diabetes mellitus. Clin Immunol. 2015;160:155–62. doi: 10.1016/j.clim.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 194.van Poppel PC, van Asseldonk EJ, Holst JJ et al. The interleukin-1 receptor antagonist anakinra improves first-phase insulin secretion and insulinogenic index in subjects with impaired glucose tolerance. Diabetes Obes Metab. 2014;16:1269–73. doi: 10.1111/dom.12357. [DOI] [PubMed] [Google Scholar]
- 195.Koska J, Ortega E, Bunt JC et al. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52:385–93. doi: 10.1007/s00125-008-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ramos-Zavala MG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, González-López R, Santiago-Hernández NJ. Effect of diacerein on insulin secretion and metabolic control in drug-naive patients with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2011;34:1591–4. doi: 10.2337/dc11-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]


