Abstract
Brugada syndrome (BrS) is an inherited disease with an increased risk of sudden cardiac death (SCD). However, testing identifies genetic disorders in only 20–30% of patients analysed, indicating a gap in knowledge of its genetic aetiology. Diagnosis relies on ECG, and risk stratification in BrS patients is challenging, primarily because of the complexity of the issue. As a result, clinicians fail to provide the appropriate strategy for SCD prevention for many patients. Several variables and interventions are being studied to improve diagnostics and maximise patient protection. In addition, the scientific community must increase efforts to provide patient care according to knowledge and research for improving stratification of risk. In this article, the authors summarise contemporary evidence on clinical variables and provide an overview of future directions in risk stratification and SCD prevention.
Keywords: Brugada syndrome, sudden cardiac death, ECG, risk stratification, patient management
Brugada syndrome (BrS) is an inherited disease with an increased risk of sudden cardiac death (SCD). Despite the wide spectrum of clinical manifestations, young and apparently healthy individuals are the most frequently affected by this devastating event.[1,2] During the last 20 years, the genetic basis of Brugada syndrome has been extensively investigated, leading to major changes in gene encoding of the alpha-subunit of the Nav1.5 (SCN5A, driving the fast depolarising sodium current), the gene most frequently associated with functional abnormalities underlying arrhythmogenicity. However, testing identifies genetic disorders in only 20–30% of the patients analysed, indicating a big gap in knowledge and understanding of the genetic aetiology of the syndrome.[3,4] Indeed, diagnosis relies on conventional ECG, with a record of the type 1 BrS pattern – occurring either spontaneously or provoked with a sodium channel blocker – being the necessary condition for diagnosis (Figure 1).[5,6]
Figure 1: Example of Different Types of Brugada Pattern.
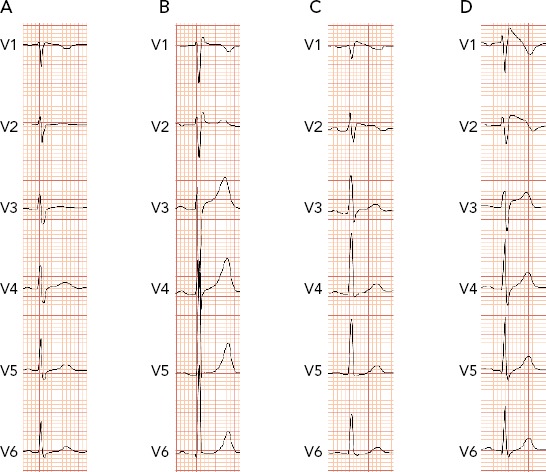
A: Normal ECG; B: Type 3 Brugada pattern; C: Type 2 Brugada pattern; D: Type 1 Brugada pattern. Adapted from: García Iglesias et al. 2018.36 Used with permission from MDPI.
The first investigation into a plausible relationship between the characteristic ECG tracing and the risk of SCD was performed in the late 1980s.[7] However, it was not until 1992 that the Brugada brothers popularised the syndrome and provided strong evidence for a distinct clinical entity causing SCD in patients with apparently normal heart structure.[8] Since the first descriptions reporting high mortality, the incidence of SCD has been declining and remains a source of debate. It is now clear that the population of patients with BrS is heterogeneous. Some patients accumulate the highest risk for developing malignant ventricular arrhythmias, while others follow a benign course with a long life expectancy. This may be because BrS is a dynamic entity, with ECG patterns alternating between the different types and normal records in the same patient. This situation creates uncertainty because, if conditions are changing, our risk stratification may need to be recalculated with time. What makes the heart of a patient with BrS change from periodic rhythmicity to the sudden turbulence of fibrillation that renders the heart unable to pump blood? Can we predict that transition and take preventative measures? In such a complex scenario, risk stratification emerges as the key issue in clinical practice. In this article, we summarise contemporary evidence on clinical variables and provide an overview of future directions in risk stratification and SCD prevention.
Syncope and Sudden Cardiac Arrest
Prior cardiac arrest is the strongest factor predicting recurrence of ventricular arrhythmias and SCD. There is no doubt about the convenience of an ICD in this clinical scenario. However, conclusions about the link with syncope – believed to be the consequence of self-limited ventricular arrhythmias in a significant proportion of patients – are less certain. In some studies, history of syncope was strongly associated with an increased risk of SCD, with about four-times greater risk compared with asymptomatic patients.[3,9] The clinical presentation of the syncope may help to distinguish between malignant syncope as a result of polymorphic ventricular tachycardia or fibrillation, and neuromediated syncope, which is the most frequent cause of syncope in BrS and confers a benign prognosis.[1] Evaluation of symptoms associated with the syncope event, prodromals, situational circumstances and witness description, is fundamental in the clinical management of syncope in BrS patients.[10] For those patients with malignant syncope, the ICD is a well-established recommendation, whereas patients with neuromediated syncope require general recommendations, similar to those provided for the general population. However, a gray zone exists, consisting of those patients in who clinicians cannot distinguish between a benign and a malignant pattern. General recommendations are not conclusive, but intense monitoring, i.e. implantable loop recorders and close follow-up, is usually indicated. This should include alerting the patient to the relevance of syncope as an alarm symptom that must be followed by immediate admission to an accident and emergency department for acute evaluation.[2]
Asymptomatic Brugada Syndrome Patients
Asymptomatic patients with BrS tend to display a more benign disease course than those with symptoms. In several studies and registries the incidence of arrhythmic events or sudden cardiac arrest (SCA) in asymptomatic patients was between 0.8% and 1.0% per year.[3,11] However, some studies demonstrate that most of the BrS patients experiencing a SCA were previously asymptomatic and were classified as at low-risk of arrhythmic events.[12] In addition, the risk seems to be cumulative with time. Some clinical series demonstrated that the cumulative risk at 10 years of follow-up progressed linearly up to 10%.[13] Such a situation remains unacceptable from a clinical point of view and suggests a need for better methods of risk stratification to predict low-risk arrhythmic events, which have tremendous clinical relevance. Thus, the more up to date the risk stratification of asymptomatic BrS patients is, the more relevant it is for decision making in clinical practice.
Two combined strategies might provide efficient clinical management. One strategy is effective lifestyle measures, including advising patients to avoid situations with increased risk for arrhythmic events – for example, avoid drugs with potential adverse effects and promptly seek treatment for any episodes of fever – and reminding them of the role of syncope as an alarm symptom to which they should pay special attention. We have previously suggested that patients without an ICD experiencing a SCA have better surveillance if they previously had a premonitory syncope.[2] Time-to-contact with medical services should not be delayed in cases of newly detected syncope because these patients would benefit from urgent evaluation.
Another strategy is the employment of multi-parameter scores in an attempt to improve stratification of risk and detect special cohorts of asymptomatic patients with increased risk. Based on some risk variables – aborted SCD, syncope, male gender, spontaneous type 1 pattern, family history of SCD, sinus node dysfunction or VF induction – the incidence of SCD in these patients can be reasonably well predicted. Although these scores may have future importance, they are not yet validated in an external sample, so their usefulness in clinical practice is still unknown. Another criticism is that none of the models were specifically constructed from cohorts of asymptomatic patients, so validation will require additional efforts.[14]
This article will now provide evidence regarding individual variables in the risk stratification of BrS patients.
Spontaneous Versus Induced Type 1 Brugada ECG Pattern
The presence of spontaneous type 1 BrS pattern is a cornerstone in the process of risk stratification. It confers a 2.98- to 4.20-fold increase in the risk of SCD, compared with patients with the drug-induced pattern.[3,9] However, it must be considered that the relevance of this variable in predicting SCD is strongly modulated by additional factors.
First, in patients with prior cardiac arrest, it has not been demonstrated that the spontaneous versus induced type 1 pattern influences prognosis. As such, clinical decision making regarding ICD implantation is not affected. Second, the presence of malignant syncope in a patient with spontaneous type 1 ECG seems to confer a high risk of SCD. On the contrary, asymptomatic patients with spontaneous type 1 ECG seem to follow a benign clinical course unless their clinical status changes because of the occurrence of new episodes of syncope. Third, we must consider the dynamic nature of the ECG patterns. As the type 1 pattern may be intermittent, it is not known if patients who continuously express the type 1 pattern are at higher risk of SCD compared with those with an intermittent manifestation.
This point introduces some uncertainty in how often the ECG screening must be done in patients with drug-induced type 1 pattern. We have previously demonstrated that clinical follow-up in the absence of intensive screening, i.e. intense Holter monitoring, may render an 8% reclassification of patients.[5] In the absence of standardised recommendations, we believe that BrS patients with drug-induced type 1 ECG must be followed up at least annually for reclassification of risk and reminders of general measures to control their risk of SCD – that is, lifestyle measures, avoidance of drugs with potential adverse effects, prompt treatment of fever episodes and considering new episodes of syncope to be an alarm symptom. The relationship between the type 1 BrS pattern and fever, or some drugs, is clear, highlighting the importance of patient education on preventative measures.
Gender, Family History and Genetic Testing
The incidence of SCD peaks in young men, with the highest risk in those under 40 years old. Women and elderly patients are usually considered to be at lower risk. Overall, women have a lower incidence of BrS and lower risk of SCD than men.[15–17] Despite this, the influence of gender and age as variables for risk stratification continues to be a matter of discussion, probably because of the wide variety of precipitating factors and circumstances that surround episodes of SCD in patients with BrS.[2] In fact, age and gender have never been proven as an indication for ICD implantation.[1]
The role of information provided by relatives and genetic testing in risk stratification is also not clear. To date, a positive family history of BrS or SCD is not consistently associated with an increased risk of SCD in BrS patients. However, some authors propose that those variables should be considered for clinical decision making as they might modulate the risk in combination with other variables. That is the case for programmed ventricular stimulation (PVS) attempting VF induction, which seems to increase predictability in BrS patients with a positive family history of SCD.[18] In the same way, the effect of mutations in different genes that have causative relationships with BrS – including SC5NA mutations – display contradictory results in the literature.[11,19,20] Overall, this has been an extensive area of research with disappointing results; first, because of the low incidence of causative mutations – not exceeding 30% of patients – and second, because of the high heterogeneity in the mutations found and in the patients’ profiles, which makes it difficult to draw general conclusions.
Programmed Ventricular Stimulation
The role of PVS for risk stratification has been debated since its first description in the late 1990s. The potential benefits of the technique are not agreed upon, with some authors describing VF induction as a strong predictor of SCD,[21] while others failed to confirm any significant association.[3] Sustained VF can be induced in up to 40% of BrS patients (Figure 2A), which is significantly higher than the induction rate found in the general population.[1,22,23] However, many factors seem to influence inducibility, from the number of induction attempts and the aggressiveness of the pacing protocol to particular conditions of the patient that would make VF inducibility more or less easy at different times. This is reflected by the fact that the same patient may change from inducible to non-inducible during sequential PVS procedures, which may be particularly important in interpreting results. Some questions arise in light of these results, for example, even if non-inducibility is a protective state for a particular patient, how long can that condition apply during follow-up? Should we periodically reassess the risk by repeated PVS procedures? If yes, how often should risk be reassessed? Also, there is no consensus about how the PVS should be done (number of pacing sites, number of extra stimuli, and so on), as the more aggressive the protocol is the higher the rate of false positive results and the lower the specificity we achieve.[24] A two-extra-stimuli PVS protocol would probably be the most accurate for risk stratification.[1] Moreover, VF induction may be dependent on the basal risk profile of the patient. In symptomatic patients with malignant syncope, VF induction does not seem to add additional value for stratification of risk.
Figure 2: Example of VF Induction.
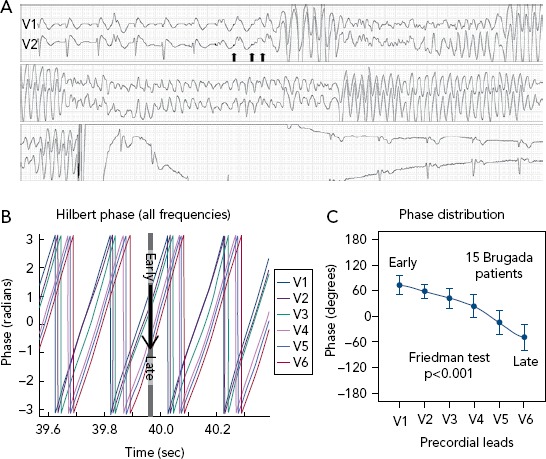
A: Example of VF induction during a programmed ventricular stimulation with three coupled extra-stimuli (arrows). It lasts 17 seconds and needs an external cardiac defibrillator shock to finish. B: Example of Hilbert transform of VF signal in a patient with Brugada syndrome. It provides the time course of the phases along the precordial locations. C: Cumulative data of phases in a subset of patients with Brugada syndrome. B and C show that the phase distribution of the signals denotes a sequence from early in V1 to late in V6 (black arrow, 95% CI). Overall, organisation of the phase-frequency content during VF points to the outflow tracts as the source of high-frequency inputs maintaining VF. Source: Calvo et al. 2015.43 Adapted with permission from Wolters Kluwer Health.
For asymptomatic BrS patients, the interpretation is contradictory, with some groups suggesting a relevant role in deciding on ICD implantation in primary prevention,[25] while others do not support that conclusion.[3] In a meta-analysis, VF inducibility correlated with SCD, with an inverse relationship between the number of extra stimuli needed to induce VF and the risk of SCD.[21] However, it was also found that non-inducibility does not protect enough patients against SCD, which highlights the role of other clinical variables – such as syncope and spontaneous type 1 BrS pattern – for risk stratification. In addition, an expert consensus document stated that the association between VF inducibility and SCD/ventricular arrhythmias is not statistically significant in asymptomatic BrS patients.[26]
While testing VF induction has been the main goal during PVS, the clinical significance of other findings during the electrophysiological study may be of interest. The PRogrammed ELectrical stimUlation preDictive valuE (PRELUDE) study evaluated the role of refractory periods of the ventricle in predicting SCD/ventricular arrhythmias during follow-up.[3] The authors found that a refractory period of less than 200 ms effectively predicted events. Other authors reported the role of sinus node dysfunction or the length of the HV interval with similar results. However, confirmation of the role of these variables in systematic stratification of BrS patients requires additional studies.
Future Directions in Risk Stratification and Prevention of Sudden Cardiac Death
There is a need for improved sensitivity and specificity in the diagnosis of BrS. Provocative testing with drugs blocking the sodium channel provides a sensitivity of around 80%. Ajmaline looks superior to flecainide and may help to clarify diagnosis in patients with suspected false negative responses to flecainide.[5] The time for monitoring seems to influence the result of provocative testing with flecainide, with extended monitoring time allowing detection of late positive responses.[6]
In addition, routine performance of ECG recording, ECG recording during fever episodes and alternative provocative testing, such as the full stomach test and exercise testing, may also be recommended for borderline cases in which a false negative response is suspected and cardiac syncope alerts to a risky clinical situation.[27–30] This displays a complex scenario that calls for urgent definition of the appropriate standards in the diagnostic work flow to avoid false negative responses.
A variety of ECG findings might also help in discerning diagnosis and at-risk patients. For the last 10 years, several authors have focused on fragmentation of the QRS, association with early repolarisation syndrome, increased Tpeak–Tend intervals, quantitative measurements on the terminal R wave in lead V1 and the extension of the PR interval as ECG markers denoting either increased clinical suspicion or increased risk profile.[31–35] However, for most of these features there were a limited number of patients considered, which precludes extensive and conclusive implementation in the clinic. While promising, more studies are needed for characterisation. We have demonstrated that the frequency domain analysis of the surface ECG may provide additional insights.[36] In a wide population of patients at risk of ventricular arrhythmias, including BrS patients, the spectral properties of the high-frequency content behave distinctly compared with controls at low risk (Figure 3). Those frequency components might reflect a substrate for conduction delay or even voltage gradients promoting phase-two re-entry, which leads some investigators to associate their presence with an increased risk of SCD. Usually demonstrated with a signal-averaged ECG, other novel techniques are now under investigation.[36–38] However, validity in the BrS population still requires confirmation.
Figure 3: High-frequency Content Analysis Along the QRS.
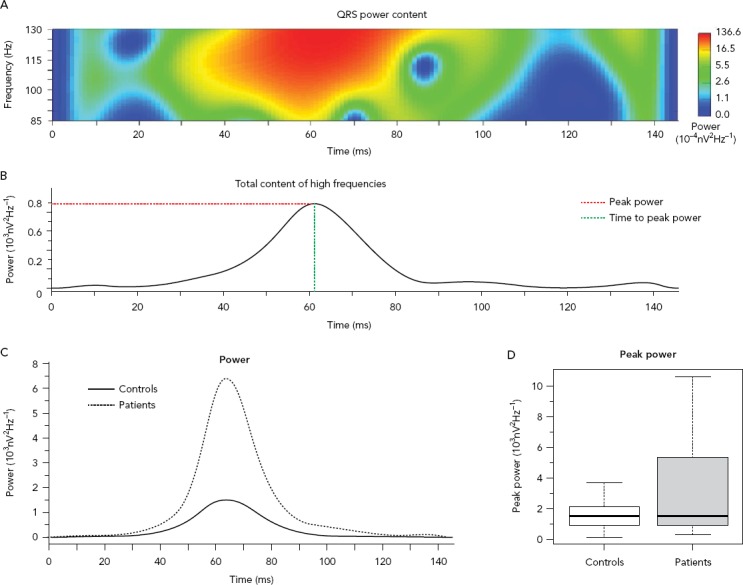
High-frequency content analysis along the QRS with the wavelet analysis for comparison between patients with and without prior history of arrhythmic events. A: Power spectrum of the QRS; B: Cumulative power of the content at each each epoch (the red dotted line marks the peak power and the green dotted line marks time time to peak power); C: Time distribution of the high-frequency content for each group at each time epoch; D: Boxplot comparing the peak power. Source: García Iglesias et al. 2018.36 Adapted with permission from MDPI.
The role of drugs in preventing arrhythmic events in BrS patients is an area of interest. In particular, those drugs with a blockade effect in transient outward potassium current may have a special role because its differential expression explains action potential dispersion leading to endo-epicardial voltage gradients.[39] Notably, quinidine has demonstrated a reduction in VF inducibility during a PVS protocol and significantly ameliorates VF recurrence during follow-up.[40] However, studies report the main limitation with the use of quinidine is the gastrointestinal intolerance, which leads to therapy discontinuation in an important number of patients.
Another promising therapy seems to be ablation of the arrhythmogenic substrate at the epicardium of the ventricular outflow tracts, where areas of abnormal potentials during sinus rhythm have been related to VF inducibility.[41,42] Also VF in BrS patients seems to organise in well-demarcated sequences, pointing to the outflow tract as the preferential location of VF sources (Figures 2B and 2C).[43] The ablation of abnormal potentials at the outflow tract is able to revert the expression of the type 1 BrS pattern and normalise the ECG, which paves the way for interventional procedures aimed at substrate modification to improve patient prognosis.[41] Despite this approach providing exciting perspectives, no work has yet demonstrated benefits in the long term. In the coming years prospective studies will clarify any potential role.
Conclusion
Risk stratification in BrS patients is challenging. Implantation of an ICD is generally accepted if the estimated 5-year risk is higher than about 6.0% or the annual risk is higher than about 1.2%.[9,27,28] Stratification of risk currently relies on a set of limited clinical variables that provide the best results according to evidence-based medicine (Figure 4). However, the predictive values of stratification procedures are far from perfect and for many patients we fail to provide the appropriate strategy for SCD prevention. Many different variables and interventions are being studied to increase the diagnostic yield and to maximise patient protection. Meanwhile, the scientific community has to increase efforts in providing patient care according to knowledge and research for improving stratification of risk.
Figure 4: Suggested Risk Stratification for Primary Prevention in Brugada Syndrome Patients.
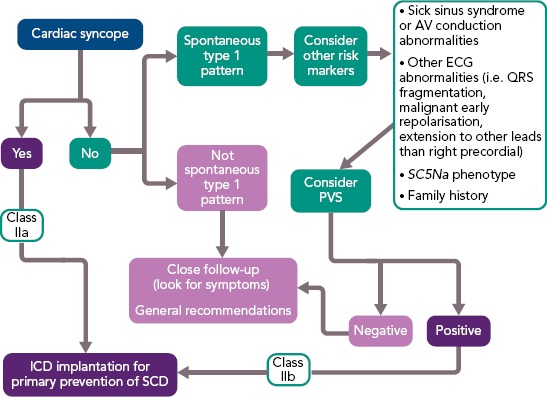
AV = atrioventricular; PVS = programmed ventricular stimulation; SCD = sudden cardiac death.
References
- 1.Antzelevitch C, Yan G-X, Ackerman MJ et al. J-Wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace. 2017;19:665–94. doi: 10.1093/europace/euw235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo D, Flórez JP, Valverde I et al. Surveillance after cardiac arrest in patients with Brugada syndrome without an implantable defibrillator: An alarm effect of the previous syncope. Int J Cardiol. 2016;218:69–74. doi: 10.1016/j.ijcard.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Gasparini M, Napolitano C et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Kapplinger JD, Tester DJ, Alders M et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pablo Flórez J, García D, Valverde I et al. Role of syncope in predicting adverse outcomes in patients with suspected Brugada syndrome undergoing standardized flecainide testing. Europace. 2018;20:f64–71. doi: 10.1093/europace/eux315. [DOI] [PubMed] [Google Scholar]
- 6.Calvo D, Rubín JM, Pérez D et al. Time-dependent responses to provocative testing with flecainide in the diagnosis of Brugada syndrome. Heart Rhythm. 2015;12:350–7. doi: 10.1016/j.hrthm.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Martini B, Nava A, Thiene G et al. Ventricular fibrillation without apparent heart disease: description of six cases. Am Heart J. 1989;118:1203–9. doi: 10.1016/0002-8703(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 8.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome: A multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 9.Sroubek J, Probst V, Mazzanti A et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome. Circulation. 2016;133:622–30. doi: 10.1161/CIRCULATIONAHA.115.017885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brignole M, Moya A, de Lange FJ et al. ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;2018;39:1883–948. doi: 10.1093/eurheartj/ehy037. [DOI] [PubMed] [Google Scholar]
- 11.Probst V, Veltmann C, Eckardt L et al. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–43. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 12.Raju H, Papadakis M, Govindan M et al. Low prevalence of risk markers in cases of sudden death due to Brugada syndrome. J Am Coll Cardiol. 2011;57:2340. doi: 10.1016/j.jacc.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 13.Sacher F, Probst V, Maury P et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome. Circulation. 2013;128:1739–47. doi: 10.1161/CIRCULATIONAHA.113.001941. [DOI] [PubMed] [Google Scholar]
- 14.Letsas KP, Asvestas D, Baranchuk A et al. Prognosis, risk stratification, and management of asymptomatic individuals with Brugada syndrome: A systematic review. Pacing Clin Electrophysiol. 2017;40:1332–45. doi: 10.1111/pace.13214. [DOI] [PubMed] [Google Scholar]
- 15.Milman A, Gourraud J-B, Andorin A et al. Gender differences in patients with Brugada syndrome and arrhythmic events: data from a survey on arrhythmic events in 678 patients. Heart Rhythm. 2018;15:1457–65. doi: 10.1016/j.hrthm.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Yuan M, Tian C, Li X et al. Gender differences in prognosis and risk stratification of Brugada syndrome: a pooled analysis of 4,140 patients from 24 clinical trials. Front Physiol. 2018;9:1127. doi: 10.3389/fphys.2018.01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthome P, Tixier R, Briand J et al. Clinical presentation and follow-up of women affected by Brugada syndrome. Heart Rhythm. 2019;16:260–7. doi: 10.1016/j.hrthm.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Delise P, Allocca G, Marras E et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur Heart J. 2011;32:169–76. doi: 10.1093/eurheartj/ehq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagata K, Horie M, Aiba T et al. Genotype-Phenotype Correlation of SCN5A Mutation for the Clinical and Electrocardiographic Characteristics of Probands With Brugada Syndrome: A Japanese Multicenter Registry. Circulation. 2017;135:2255–70. doi: 10.1161/CIRCULATIONAHA.117.027983. [DOI] [PubMed] [Google Scholar]
- 20.Priori SG, Napolitano C, Gasparini M et al. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation. 2002;105:1342–7. doi: 10.1161/hc1102.105288. [DOI] [PubMed] [Google Scholar]
- 21.Brugada P, Brugada R, Mont L et al. Natural history of Brugada syndrome: the prognostic value of programmed electrical stimulation of the heart. J Cardiovasc Electrophysiol. 2003;14:455–7. doi: 10.1046/j.1540-8167.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- 22.Triedman JK. Brugada and short QT syndromes. Pacing Clin Electrophysiol. 2009;32:S58–62. doi: 10.1111/j.1540-8159.2009.02386.x. [DOI] [PubMed] [Google Scholar]
- 23.Gehi AK, Duong TD, Metz LD et al. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–83. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Fauchier L, Isorni MA, Clementy N et al. Prognostic value of programmed ventricular stimulation in Brugada syndrome according to clinical presentation: an updated meta-analysis of worldwide published data. Int J Cardiol. 2013;168:3027–9. doi: 10.1016/j.ijcard.2013.04.146. [DOI] [PubMed] [Google Scholar]
- 25.Sieira J, Conte G, Ciconte G et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ Arrhythm Electrophysiol. 2015;8:777–84. doi: 10.1161/CIRCEP.114.002647. [DOI] [PubMed] [Google Scholar]
- 26.Kusumoto FM, Bailey KR, Chaouki AS et al. Systematic review for the 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e253–74. doi: 10.1016/j.hrthm.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Shimeno K, Takagi M, Maeda K et al. Usefulness of multichannel Holter ECG recording in the third intercostal space for detecting type 1 Brugada ECG: comparison with repeated 12-lead ECGs. J Cardiovasc Electrophysiol. 2009;20:1026–31. doi: 10.1111/j.1540-8167.2009.01490.x. [DOI] [PubMed] [Google Scholar]
- 28.Adler A, Topaz G, Heller K et al. Fever-induced Brugada pattern: how common is it and what does it mean? Heart Rhythm. 2013;10:1375–82. doi: 10.1016/j.hrthm.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda T, Abe A, Yusu S et al. The full stomach test as a novel diagnostic technique for identifying patients at risk of Brugada syndrome. J Cardiovasc Electrophysiol. 2006;17:602–7. doi: 10.1111/j.1540-8167.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 30.Makimoto H, Nakagawa E, Takaki H et al. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J Am Coll Cardiol. 2010;56:1576–84. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 31.Morita H, Kusano KF, Miura D et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 32.Takagi M, Aonuma K, Sekiguchi Y et al. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: multicenter study in Japan. Heart Rhythm. 2013;10:533–9. doi: 10.1016/j.hrthm.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Maury P, Sacher F, Gourraud J-B et al. Increased Tpeak-Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm. 2015;12:2469–76. doi: 10.1016/j.hrthm.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Sugrue A. New electrocardiographic criteria to differentiate the type-2 Brugada pattern from electrocardiogram of healthy athletes with r'-wave in leads V1/V2. Europace. 2015;17:504–5. doi: 10.1093/europace/euu348. [DOI] [PubMed] [Google Scholar]
- 35.Migliore F, Testolina M, Zorzi A et al. First-degree atrioventricular block on basal electrocardiogram predicts future arrhythmic events in patients with Brugada syndrome: a long-term follow-up study from the Veneto region of Northeastern Italy. Europace. 2019;21:322–31. doi: 10.1093/europace/euy144. [DOI] [PubMed] [Google Scholar]
- 36.García Iglesias D, Roqueñi Gutiérrez N, De Cos JF, Calvo D. Analysis of the high-frequency content in human QRS complexes by the continuous wavelet transform: an automatized analysis for the prediction of sudden cardiac death. Sensors. 2018;18:E560. doi: 10.3390/s18020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gramatikov B, Iyer V. Intra-QRS spectral changes accompany ST segment changes during episodes of myocardial ischemia. J Electrocardiol. 2015;48:115–22. doi: 10.1016/j.jelectrocard.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gramatikov B, Brinker J, Yi-Chun S, Thakor NV. Wavelet analysis and time-frequency distributions of the body surface ECG before and after angioplasty. Comput Methods Programs Biomed. 2000;62:87–98. doi: 10.1016/s0169-2607(00)00060-2. [DOI] [PubMed] [Google Scholar]
- 39.Brodie OT, Michowitz Y, Belhassen B. Pharmacological Therapy in Brugada Syndrome. Arrhythmia Electrophysiol Rev. 2018;7:135–42. doi: 10.15420/aer.2018.21.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andorin A, Gourraud J-B, Mansourati J et al. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm. 2017;14:1147–54. doi: 10.1016/j.hrthm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Brugada J, Pappone C, Berruezo A et al. Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ Arrhythm Electrophysiol. 2015;8:1373–81. doi: 10.1161/CIRCEP.115.003220. [DOI] [PubMed] [Google Scholar]
- 42.Pappone C, Ciconte G, Manguso F et al. Assessing the malignant ventricular arrhythmic substrate in patients with Brugada syndrome. J Am Coll Cardiol. 2018;71:1631–46. doi: 10.1016/j.jacc.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Calvo D, Atienza F, Saiz J et al. Ventricular tachycardia and early fibrillation in patients with Brugada syndrome and ischemic cardiomyopathy show predictable frequency-phase properties on the precordial ECG consistent with the respective arrhythmogenic substrate. Circ Arrhythm Electrophysiol. 2015;8:1133–43. doi: 10.1161/CIRCEP.114.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]


