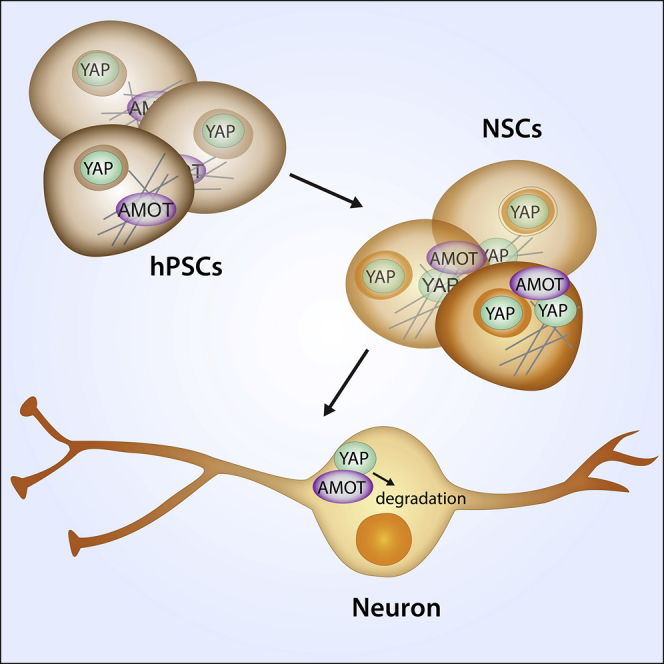
Summary
Leveraging the extraordinary potential of human pluripotent stem cells (hPSCs) requires an understanding of the mechanisms underlying cell-fate decisions. Substrate elasticity can induce differentiation by signaling through the transcriptional coactivator Yes-associated protein (YAP). Cells cultured on surfaces mimicking brain elasticity exclude YAP from their nuclei and differentiate to neurons. How YAP localization is controlled during neural differentiation has been unclear. We employed CRISPR/Cas9 to tag endogenous YAP in hPSCs and used this fusion protein to identify YAP's interaction partners. This engineered cell line revealed that neural differentiation promotes a change in YAP interactors, including a dramatic increase in angiomotin (AMOT) interaction with YAP. AMOT regulates YAP localization during differentiation. AMOT expression increases during neural differentiation and leads to YAP nuclear exclusion. Our findings that AMOT-dependent regulation of YAP helps direct hPSC fate provide insight into the molecular mechanisms by which the microenvironment can induce neural differentiation.
Keywords: YAP, mechanosensing, neuronal differentiation, angiomotin, human pluripotent stem cells, embryonic stem cells, neural progenitor cells, ubiquitination, CRISPR genome engineering, proteomics
Graphical Abstract
Highlights
-
•
Endogenous tagging reveals YAP interactors in hPSCs
-
•
AMOT-YAP complex concentration increases during neural differentiation
-
•
AMOT regulates YAP localization in hPSCs
-
•
hPSC cytoskeleton influences YAP localization via AMOT
Kiessling and colleagues employed CRISPR/Cas9 to generate an hPSC line in which YAP is tagged, thus facilitating affinity purification of endogenous-level YAP complexes for analysis. This strategy uncovered proteins that interact with YAP during self-renewal and differentiation and identified angiomotin as a key regulator of YAP localization during neural differentiation.
Introduction
Human pluripotent stem cells (hPSCs) integrate multiple signals to determine cell fate. These signals include soluble factors, such as growth factors and small molecules, neighboring cells, and insoluble cues, such as the extracellular matrix composition and rigidity (Klim et al., 2010, Murphy et al., 2014, Wrighton et al., 2014). Matrix rigidity influences the cell fate of adult stem cells (Dupont et al., 2011, Engler et al., 2006), and the elasticity (or rigidity) of an hPSC's microenvironment can potently signal self-renewal or differentiation (Musah et al., 2012, Musah et al., 2014, Sun et al., 2014). We previously showed that when hPSCs are cultured in microenvironments with elasticities comparable with human brain tissue, they efficiently differentiate to neurons—without soluble neurogenic factors (Musah et al., 2014). The transcriptional coactivator Yes-associated protein (YAP) can mediate responses to substrate stiffness in mesenchymal stem cells (Dupont et al., 2011) and hPSCs. When hPSCs are cultured on a rigid surface, such as polystyrene or glass, YAP localizes to the nucleus and hPSCs self-renew (Musah et al., 2012). In contrast, when cells are cultured on soft substrates or with small-molecule inhibitors of F-actin polymerization, YAP translocates out of the nucleus and the cells differentiate to neurons (Musah et al., 2014). Knockdown of YAP in cells on rigid surfaces induces neuronal differentiation, phenocopying cell growth on soft surfaces or in the presence of compounds that disrupt F-actin polymerization.
Beyond mechanotransduction, YAP participates in an array of cellular processes including Hippo signaling, tissue homeostasis, cancer stem cell reprogramming, and growth factor signaling (Piccolo et al., 2014). The regulation of YAP has been characterized in the contexts above but not in hPSC fate. This latter context is relevant as YAP helps maintain the mammalian stem cell pluripotency. In mouse embryonic stem cells (ESCs), knockdown of YAP led to the loss of pluripotency factors OCT4 and SOX2 and consequent differentiation (Lian et al., 2010). In hPSCs, YAP/TAZ-SMAD2/3 complexes engage with TEA domain (TEAD) transcription factors and OCT4 (Beyer et al., 2013). YAP also helps promote the self-renewal of neural stem cells (NSCs), although how it does so is not well understood. In the developing chick neural tube, YAP gain of function significantly expanded the neural progenitor pool in a TEAD-dependent manner (Cao et al., 2008). Repression of YAP/TEAD target genes led to cell-cycle exit and premature neuronal differentiation. Similar results were observed for NSCs in the mouse embryonic brain (Han et al., 2015). YAP induction also can reprogram terminally differentiated neurons into NSCs (Panciera et al., 2016). Transient YAP expression was sufficient to induce NSCs and promote self-renewal.
We sought to understand better the molecular mechanisms regulating YAP subcellular localization in hPSCs during self-renewal and differentiation. Prior studies have characterized YAP interactors in immortalized cell lines (Couzens et al., 2013, Kohli et al., 2014, Wang et al., 2014), but how YAP interactions change during differentiation is unclear. We employed CRISPR/Cas9 to generate an hPSC line in which YAP is tagged, thus facilitating affinity purification of endogenous-level YAP complexes for analysis. This strategy uncovered proteins that interact with YAP during self-renewal and differentiation and pointed to angiomotin (AMOT) as a critical regulator of YAP localization during neural differentiation.
Results
CRISPR/Cas9 Generation of an hPSC Line with Tagged YAP
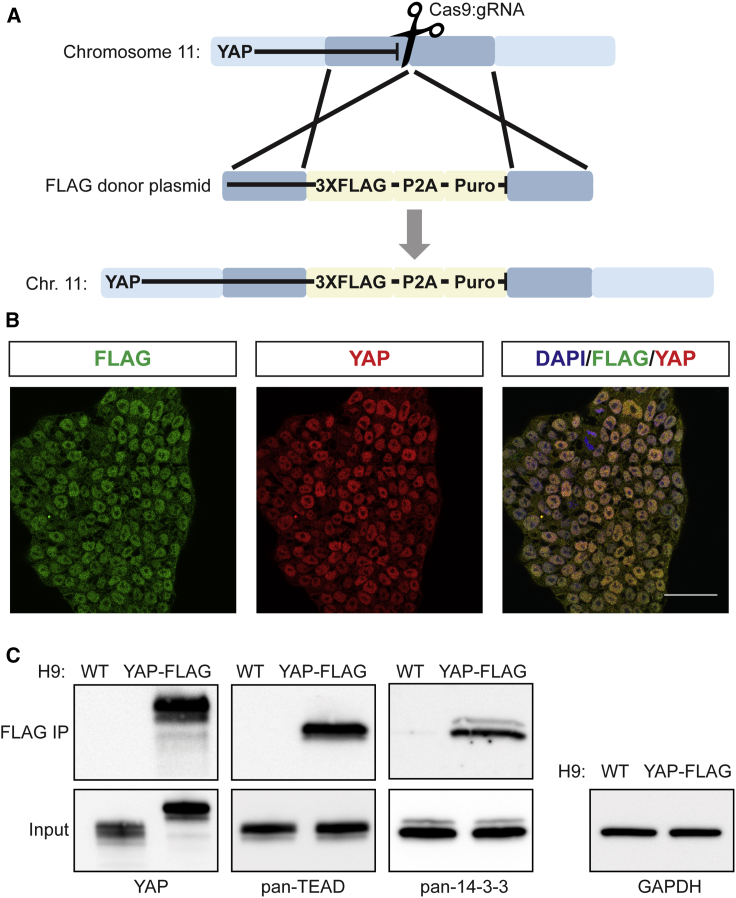
We employed CRISPR/Cas9 and took advantage of homology-directed repair to engineer the H9 human ESC line to produce a C-terminal 3xFLAG-tagged YAP (Figure 1A). After puromycin selection and clone screening, we isolated a clone in which the introduced tag was present in both alleles (Figure S1A). In this YAP-FLAG cell line, the FLAG tag was universally expressed and entirely coincident with YAP staining (Figure 1B). As expected, this engineered cell line expressed the pluripotency markers NANOG and OCT4 (Figure S1B). The fusion protein also retained proper localization in response to mechanical cues: YAP-FLAG was present in the nucleus on rigid surfaces; the fusion protein was detected in the cytoplasm on soft surfaces (Figure S1C). Treatment with latrunculin A (lat-A), an F-actin polymerization inhibitor that elicits a cellular response akin to that caused by culture on soft surfaces, also resulted in YAP-FLAG detection in the cytoplasm (Figure S1B). The YAP-FLAG cell line allowed for highly specific affinity purification of YAP with anti-FLAG magnetic beads (Figure 1C). Moreover, YAP copurified with well-characterized binding partners, including the TEAD transcription factors and 14-3-3 adaptor proteins (Figure 1C). Therefore, application of CRISPR/Cas9 afforded an hPSC line in which a tagged YAP is produced from its endogenous genomic locus; this cell line enables the affinity purification of endogenous-level YAP complexes. Together, these results indicated that the YAP-FLAG cell line could reveal relevant YAP interactors.
Figure 1.
CRISPR/Cas9-Mediated Editing Enables Generation of an hPSC Line in which FLAG-Tagged YAP Is Produced from Its Native Chromosomal Locus
(A) Scheme for the generation of the 3xFLAG-tagged YAP hPSC line (H9) using CRISPR/Cas9 and homology-directed repair.
(B) YAP subcellular localization in H9 YAP-FLAG cell line. Scale bar, 50 μm.
(C) Top: immunoblots from FLAG coimmunoprecipitation (IP) from H9 wild-type (WT) and H9 YAP-FLAG cell line lysates. Bottom: IP input.
See also Figure S1.
Enhancement of the YAP-AMOT Interaction during Neural Differentiation
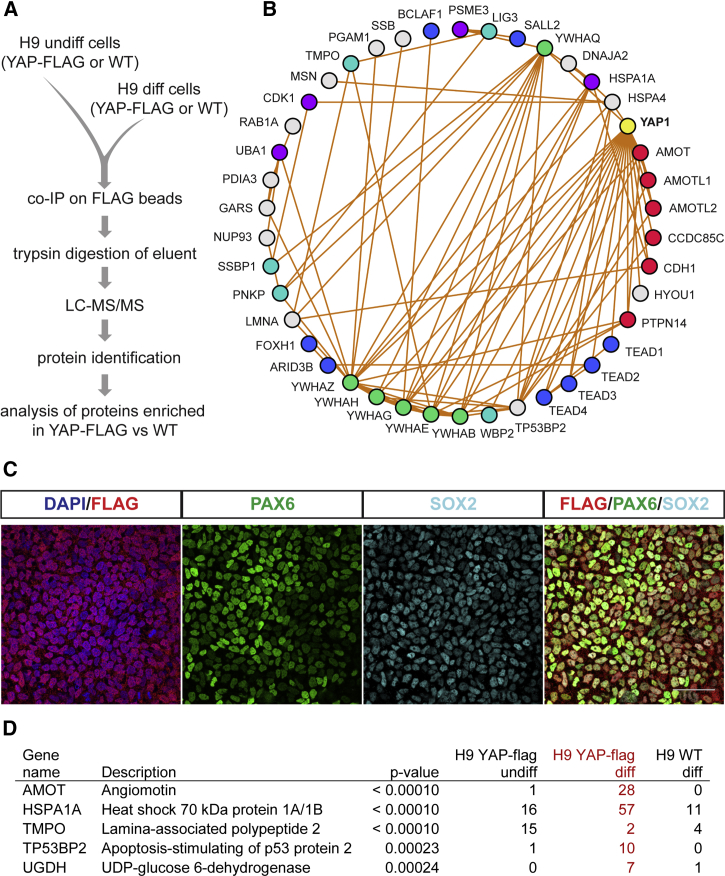
The YAP-FLAG cell line was used for affinity purification-mass spectrometry (AP-MS) to characterize the YAP interactors within hPSCs (Figure 2A). As expected, immunoprecipitation from undifferentiated H9 cell lysates with anti-FLAG magnetic beads afforded YAP as the protein most highly enriched in YAP-FLAG cells relative to wild-type cells (Table S1). Previously characterized YAP binding partners (e.g., PTPN14, TEAD1–4, and 14-3-3 proteins) (Yu and Guan, 2013) were also enriched. We additionally detected several novel interactors (e.g., ARID3B and SALL4) (Figure 2B and Table S1) and noted an absence of enrichment for the Hippo kinases LATS1/2.
Figure 2.
AMOT Interaction with YAP Increases during Neural Differentiation
(A) Diagram of affinity purification-mass spectrometry for YAP-FLAG. coIP, coimmunoprecipitation; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
(B) Graphic representations of the top proteins enriched in FLAG coIP from H9 YAP-FLAG cells. Edges denote previously characterized interactions. Node colors represent protein groupings as follows: yellow, bait (YAP-FLAG); red, junction proteins; blue, transcription factors; teal, DNA binding proteins; green, 14-3-3 proteins; purple, ubiquitin-proteasome pathway.
(C) H9 YAP-FLAG cells differentiated to PAX6 and/or SOX2+ neural progenitor cells. Scale bar, 50 μm.
(D) Top 5 protein interactions with YAP-FLAG that significantly increased or decreased during neural differentiation (H9 YAP-FLAG diff versus H9 YAP-FLAG undiff, H9 WT diff is control).
To determine those interactors that regulate YAP localization during neural differentiation, we examined which partners change as cells differentiate. YAP is expressed in NSCs but not in neurons, so we monitored cells for NSC markers in the early stages of neuronal differentiation (Davis-Dusenbery et al., 2014). After 6 days, the majority of the cells expressed PAX6 and/or SOX2, markers of NSC state (Figure 2C). We then used AP-MS to compare YAP's binding partners in differentiated and undifferentiated cells. During the differentiation, we found an increase among YAP's protein interaction network with cytoplasmic proteins (e.g., HSPA1A and UGDH), including with members of the ubiquitin-proteasome pathway (e.g., HUWE1 and UBA1) (Figure 2D and Table S2). The increase in cytoplasmic interactions was accompanied by a decrease in YAP interaction with nuclear proteins (e.g., TMPO, ARID3B, and SALL4). The most significant increase among YAP's protein interaction network was with the tight junction (TJ) protein AMOT (Figure 2D and Table S2). These results suggest that AMOT is a regulator of YAP during neural differentiation.
AMOT Upregulation and YAP Downregulation during Neural Differentiation
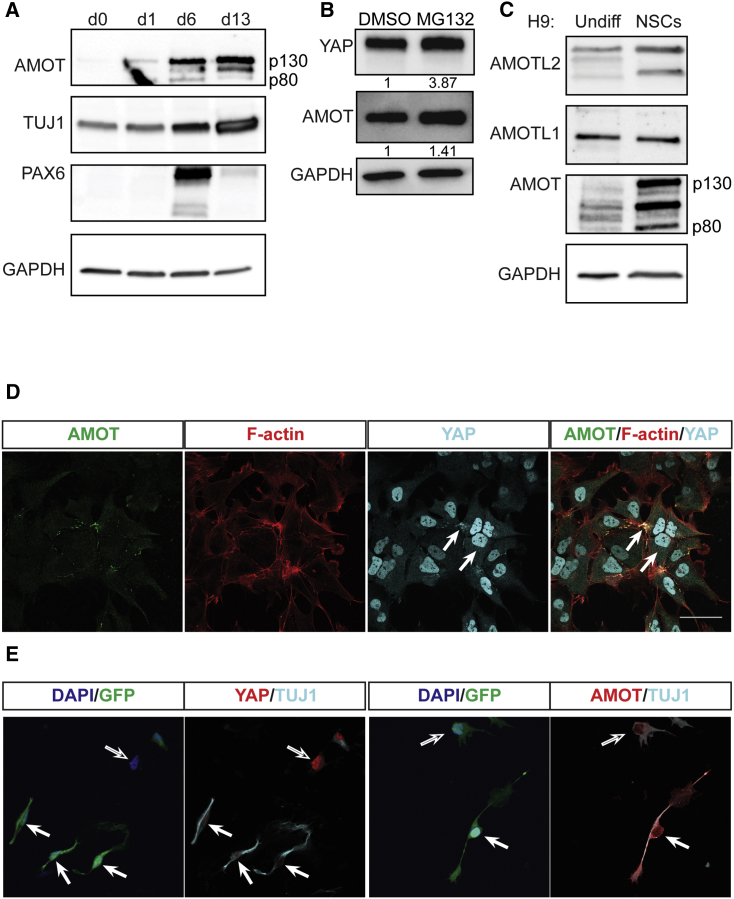
The increase in YAP-AMOT complexes during differentiation prompted us to examine whether cellular concentrations of AMOT increase. AMOT levels are low in hPSCs but progressively escalate as cells differentiate to the NSC and neuronal states (Figure 3A). During neural differentiation we found an increase in interactions with YAP and proteins of the ubiquitin-proteasome pathway; therefore, we examined whether this pathway influences YAP levels. During neuronal differentiation, YAP mRNA levels did not change substantially, but YAP protein levels decreased significantly (Figure S2A). When the proteasome was inhibited with MG-132 during neural differentiation, the levels of both YAP and AMOT were enhanced (Figure 3B). These data indicate that the proteasome can downregulate YAP.
Figure 3.
AMOT Expression Increases while YAP Is Downregulated during Neuronal Differentiation
(A) Protein levels of AMOT, neuronal marker β-tubulin III (TUJ1), and NSC marker PAX6 during neuronal differentiation from H9 cells. d, day.
(B) Protein levels of YAP and AMOT following treatment for 1 h with DMSO or 1 μM MG-132 at day 6 of neural differentiation.
(C) Protein levels of AMOT, AMOTL1, and AMOTL2 in undifferentiated H9 cells versus H9-derived NSCs.
(D) Subcellular localization of AMOT, F-actin, and YAP in H9-derived NSCs. Filled arrows indicate colocalization. Scale bar, 50 μm. (also applies to E).
(E) Expression of YAP and TUJ1 (left) or AMOT and TUJ1 (right) in Neurog2-T2A-GFP H9 cells, 12 h after induction. Filled arrows indicate GFP+ cells, and empty arrows cells with low or absent GFP expression.
See also Figure S2.
We further explored the link between AMOT and YAP in neural differentiation. AMOT has two paralogs, angiomotin-like 1 and angiomotin-like 2 (AMOTL1 and AMOTL2). AMOTL1 and AMOTL2 are also expressed in H9-derived NSCs, albeit at lower levels than AMOT. AMOT was the protein that exhibited the most significant increase in expression in NSCs relative to undifferentiated cells (Figure 3C). In NSCs, AMOT colocalized with both F-actin and YAP at TJs (Figures 3D and S2B). As expected due to its role in promoting NSC self-renewal, YAP was present in the nucleus of NSCs. To examine AMOT and YAP dynamics during neuronal differentiation, we generated a stable H9 cell line harboring an inducible system in which expression of Neurog2 drives neuronal differentiation (Figure S2C) (Zhang et al., 2013). At 12 h following doxycycline addition, cells with low levels of Neurog2 induction remained undifferentiated. These cells produced low levels of AMOT, and YAP was robustly detected in the nuclei. In contrast, differentiated neuronal cells upregulated AMOT while YAP levels decreased (Figures 3D, S2E, and S2F). Accordingly, expression of CTGF—a well-characterized YAP target gene (Yu and Guan, 2013), including in hPSCs (Musah et al., 2014)—significantly decreased (Figure S2E).
Our detection of AMOT upregulation during neuronal differentiation of hPSCs led us to hypothesize that a similar upregulation could occur during the differentiation of adult mammalian NSCs. One of the main sites of adult neurogenesis is in the subgranular zone (SGZ) of the dentate gyrus (Eriksson et al., 1998). NSCs in the SGZ differentiate to intermediate neural progenitors, which migrate and eventually give rise to hippocampal granule neurons (Yu et al., 2014). A subset of non-neuronal cells in the SGZ of the mouse dentate gyrus expressed YAP. In differentiated cells expressing the neuronal marker MAP2, we detected lower YAP levels and increased AMOT levels (Figure S2F). The accumulated data indicate that AMOT expression increases as cells differentiate to NSCs and neurons, and YAP is excluded from nuclei and downregulated by the proteasome.
AMOT Regulates YAP Localization in hPSCs
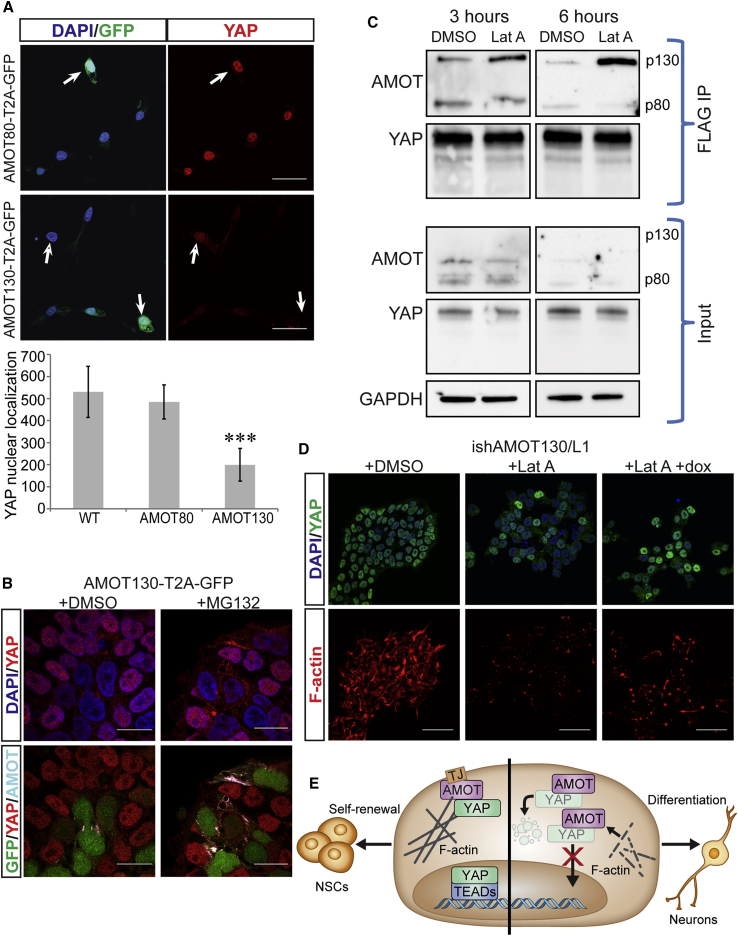
During differentiation, increases in AMOT expression coincide with YAP nuclear exclusion; therefore, we examined AMOT's role in this process. AMOT has two main isoforms: p130, which contains binding sites for F-actin and YAP, and p80, which lacks these sites (Zhao et al., 2011). Forced expression of p130, but not p80, resulted in YAP nuclear exclusion (Figures 4A and S4A). The low levels of cytoplasmic YAP prompted us to assess whether YAP undergoes proteasomal degradation upon forced expression of AMOT-p130. When we added the proteasome inhibitor MG-132, we detected an increase in YAP and AMOT colocalization in the cytoplasm (Figure 4B). Since F-actin depolymerization triggers YAP nuclear exclusion (Figure S1B) and promotes neuronal differentiation (Musah et al., 2014), we hypothesized that a reduction in F-actin would increase the AMOT-p130 interaction with YAP. Treatment with lat-A had no effect on YAP interactions with the p80 AMOT isoform, but we detected a time-dependent increase in the interaction of AMOT-p130 with YAP (Figure 4C).
Figure 4.
AMOT-p130 Control of YAP Localization in Response to the Actin Cytoskeleton
(A) Top panels: subcellular localization of YAP in AMOT130-T2A-GFP H9 cells (top) or AMOT80-T2A GFP H9 cells (bottom), 24 h after induction. Filled arrows indicate cells producing AMOT/GFP, and hollow arrows indicate cells that fail to produce AMOT/GFP. Bottom panel: quantification of YAP nuclear localization (n = 30 cells over three independent experiments); y axis is fluorescence intensity of YAP in the nucleus. Data are presented as mean ± SEM; ∗∗∗p < 0.0001 by Student's t test.
(B) Subcellular localization of YAP and AMOT following forced expression of AMOT-p130 and treatment with DMSO (left) or 1 h of 1 μM MG-132 (right).
(C) Top: immunoblots of FLAG coimmunoprecipitation (IP) after 3 and 6 h of DMSO or 1 μM lat-A treatment. Bottom: IP input.
(D) Subcellular localization of YAP (top) or F-actin staining (bottom) after treatment with DMSO (left), 6 h of 1 μM lat-A (middle), or doxycycline (dox) induction of AMOT-p130 and AMOTL1 shRNAs (right).
(E) Proposed mechanism for AMOT-mediated regulation of YAP during neural differentiation. In NSCs, AMOT sequesters YAP at TJ, and a pool of nuclear YAP remains where it regulates self-renewal of NSCs. During initiation of neuronal commitment, TJs are downregulated and AMOT expression further increases. In soft microenvironments, F-actin polymerization is reduced and AMOT liberated. These events enable AMOT to sequester YAP in the cytoplasm where YAP undergoes proteasomal degradation. An absence of YAP from the nucleus lifts repression of neuronal differentiation.
Scale bars, 50 μm. See also Figures S3 and S4.
From the initial data, we devised a model: a decrease in F-actin polymerization increases AMOT interaction with YAP and promotes YAP sequestration in the cytoplasm where YAP can be targeted for proteasomal degradation. We tested this model by targeting AMOT using conditional RNA interference. Because hPSCs express both AMOT and AMOTL1, and both contain F-actin and YAP binding sites, we employed inducible short hairpin RNAs (shRNAs) to downregulate both proteins. The expression of YAP target gene CTGF was used as a readout of YAP coactivator function and therefore indicative of nuclear YAP. Knockdown of either AMOT-p130 or AMOTL1 increased CTGF expression, indicating an increase in YAP's nuclear activity. Alternatively, knockdown of AMOT-p80 had no effect (Figure S3A). We also explored the link between F-actin polymerization and AMOT-YAP interaction. Our model indicates that in the absence of F-actin polymerization, AMOT enables YAP's sequestration in the cytosol. Accordingly, knockdown of AMOT should rescue YAP nuclear localization in cells treated with lat-A. Lat-A treatment afforded the expected decrease in YAP levels in the nucleus, and concomitant knockdown of AMOT-p130 and AMOTL1 rescued YAP nuclear localization and transcriptional control (Figures 4D and S3B). In contrast, we observed no change upon knockdown of AMOT-p80, which cannot bind YAP (Figure S3B). These results indicate that AMOT regulates YAP localization in hPSCs in response to the status of the cell's cytoskeleton.
Modulating the levels of AMOT-p130 and AMOTL1 influences YAP localization in hPSCs. We hypothesized that altering the levels of these YAP interactors during neuronal differentiation would augment or inhibit differentiation, depending upon YAP's absence or presence in the nucleus, respectively. To this end, the forced expression of AMOT-p130 led to a decrease in YAP nuclear localization (Figures 4A and S4A) and promoted neuronal differentiation (Figure S4B). Since concomitant knockdown of AMOT-p130 and AMOTL1 increased YAP localization in the nucleus (Figure S3A) and YAP promotes NSC self-renewal, we anticipated that AMOT knockdown during neuronal differentiation would augment the ratio of progenitor cells to differentiated cells. Indeed, following the induction of AMOT-p130 and AMOTL1 knockdown, we detected increased expression of progenitor genes HES5 and OLIG2 and decreased expression of the neuronal maturation gene NEUROD1. These results provide additional evidence that AMOT regulates YAP localization during neural differentiation (Figure 4E).
Discussion
We previously reported that a decrease in F-actin polymerization effected either by culture on a pliable surface or by lat-A treatment induces neuronal differentiation of hPSCs and nuclear exclusion of YAP. These data link F-actin polymerization status to YAP subcellular localization, but the mechanism underlying the influence of the actin cytoskeleton on YAP localization was unclear. In the present study, we found that during neural differentiation AMOT protein levels increase as does AMOT binding to YAP and that these changes coincide with a decrease in YAP nuclear translocation. Blocking F-actin polymerization results in neuronal differentiation, and we also detect an increase in AMOT-YAP interactions. These results suggest that during neural differentiation AMOT acts as a link between the state of the actin cytoskeleton and YAP subcellular localization.
Our identification of AMOT as a regulator of YAP during neural differentiation was facilitated by performing AP-MS on endogenously tagged YAP. The approach is highly selective for YAP; in contrast, many antibodies used for immunoprecipitation also recognize the paralogous TAZ protein. Thus, the contribution of YAP versus TAZ to a given process can be difficult to discern. Exogenous expression of a tagged YAP construct can result in expression levels that differ from those of the endogenous protein and thus may not be representative of physiologically relevant interactions. Furthermore, YAP has eight alternatively spliced isoforms (Gaffney et al., 2012), and picking just one isoform for expression can miss isoform-specific interactions, which have been documented for YAP (Finch-Edmondson et al., 2016). Our system overcomes these challenges by tagging the endogenous protein, including all of its isoforms, and capturing it using magnetic beads conjugated to a highly specific antibody against the FLAG tag (Brizzard et al., 1994). Finally, compared with transformed cell lines (Couzens et al., 2013, Kohli et al., 2014, Wang et al., 2014), there are advantages to investigating YAP interactions in a cell line such as the one we utilize, which is non-transformed, diploid, and clonal.
AMOT has previously been shown to regulate YAP in other contexts. In epithelial and endothelial cells, AMOT, with its paralogs AMOTL1 and AMOTL2, localizes to TJs and regulates apical-basal polarity (Moleirinho et al., 2014). All three AMOT proteins, except for AMOT-p80, contain PPXY motifs that can bind to the WW domains of YAP (Zhao et al., 2011). These AMOT proteins include an F-actin binding domain that links the actin cytoskeleton to YAP subcellular localization in immortalized cell lines (Mana-Capelli et al., 2014). AMOT can also regulate YAP localization in endothelial cells in response to shear stress (Nakajima et al., 2017), but a role for AMOT in sensing substrate elasticity was not known. We previously reported that hPSCs on pliant surfaces exhibit low levels of F-actin and exclude YAP from the nucleus, effects that can be mimicked with lat-A (Musah et al., 2012, Musah et al., 2014). The observed increase in YAP-AMOT complexes following lat-A treatment suggests a novel role for AMOT in regulating YAP subcellular localization during hPSC mechanosensing, as well as in differentiation.
Although AMOT has not been previously implicated in neural differentiation, it is expressed in mammalian brain tissue (Ernkvist et al., 2006). Intriguingly, a recent study demonstrated that AMOT-p130 controls dendritic spine maturation in rat neurons (Wigerius et al., 2018). Together with our observations, these findings indicate that an increase in AMOT-p130 expression during differentiation is vital for promoting both neuronal differentiation and maturation.
Our observations in mouse brain tissue suggest that AMOT regulates YAP during adult neurogenesis. These observations could extend to brain tumorigenesis, as AMOT has been linked to neural cancers. AMOT—the p130 isoform in particular—is upregulated in dormant versus fast-growing glioblastomas (Almog et al., 2009). YAP also is upregulated in human brain cancers and promotes glioblastoma growth (Orr et al., 2011). Our observations of AMOT-YAP interactions are consistent with a model in which concurrent upregulation of AMOT in dormant glioblastomas results in AMOT sequestration of YAP out of the nucleus, thereby inhibiting proliferation.
Several novel YAP interactors that we identified in undifferentiated cells point to a role for nuclear YAP in promoting self-renewal and in actively inhibiting differentiation. Specifically, during neural differentiation we detected fewer YAP complexes containing either the ARID3B or SALL4 transcription factors. ARID3B belongs to an AT-rich interaction domain family of DNA binding proteins and controls several genes responsible for pluripotency in hPSCs (Liao et al., 2016). In cancer cells, ARID3B expression results in the downregulation of genes associated with neuron development (Bobbs et al., 2015). SALL4 is a member of the spalt-like (SALL) C2H2-type zinc-finger transcription factors and was shown in ESCs to contribute to maintaining pluripotency by preventing activation of neural development genes (Miller et al., 2016). In addition to its role as a coactivator, YAP can also function as a transcriptional corepressor (Kim et al., 2015). Therefore, YAP's interaction with these transcription factors suggests a mechanism by which nuclear YAP represses neuronal differentiation in hPSCs.
We observed that increased AMOT levels during neural differentiation corresponded to an absence of YAP from the nucleus and a decrease in total YAP protein levels. YAP can be targeted for degradation in the cytoplasm by the ubiquitin-proteasome pathway (Yu and Guan, 2013). In undifferentiated cells, YAP interacts with several components of the ubiquitin-proteasome system, and those interactions increase during differentiation. In cancer cells, AMOT-p130 can modulate cytoplasmic YAP stability by acting as a scaffold and recruiting a ubiquitin ligase that targets YAP for proteasomal degradation (Adler et al., 2013a, Adler et al., 2013b). In hPSCs, proteasomal inhibition increased colocalization of YAP and AMOT in the cytoplasm. Thus, proteasome-mediated degradation of YAP may serve as an additional mode of regulation to prevent YAP from translocating to the nucleus and repressing the neuronal differentiation program.
In summary, we developed an engineered hPSC line and used it to identify that AMOT-YAP interactions can sequester YAP from the nucleus and thereby promote neural differentiation. We anticipate that this cell line will facilitate the elucidation of YAP interactions in response to other perturbagens in pluripotent cells and their differentiated progeny.
Experimental Procedures
Cell Culture and Differentiation
H9 human ESC maintenance and neuronal differentiation were performed as described by Wrighton et al. (2014).
Immunoprecipitation and AP-MS
Affinity purification of YAP-FLAG was performed using anti-FLAG M2 magnetic beads (Sigma-Aldrich). Analysis of protein samples by MS is described in Supplemental Experimental Procedures.
Immunoblotting, Immunostaining, and qPCR
Methods for immunoblotting, immunostaining, and qPCR have been described (Musah et al., 2014). Antibodies and primers are listed in Supplemental Experimental Procedures.
Author Contributions
Y.Z. designed the study. Y.Z., S.M., and J.J.B. performed the experiments, and Y.Z. and L.L.K. interpreted the results. L.L.K. supervised the study. Y.Z. and L.L.K. wrote the manuscript.
Acknowledgments
This work was supported by National Institutes of Health grant R01 GM049975 (to L.L.K.); the University of Wisconsin (UW)-Madison Biochemistry Stefaniak Fellowship and the UW-Madison Stem Cell and Regenerative Medicine Center Training Award (to Y.Z.); and the UW-Madison Hilldale Fellowship (to J.J.B.). We thank G. Sabat at the UW-Madison Biotechnology Center for MS analysis. We thank Dr. A.G. Fitzpatrick and B. Gray (UW-Madison Research Animal Resource Center) for mouse tissue sections. We acknowledge C. Isabella, Dr. J.R. Klim, and D. Mahbuba for helpful feedback.
Published: April 18, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.03.009.
Supplemental Information
References
- Adler J.J., Heller B.L., Bringman L.R., Ranahan W.P., Cocklin R.R., Goebl M.G., Oh M., Lim H.S., Ingham R.J., Wells C.D. Amot130 adapts atrophin-1 interacting protein 4 to inhibit yes-associated protein signaling and cell growth. J. Biol. Chem. 2013;288:15181–15193. doi: 10.1074/jbc.M112.446534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler J.J., Johnson D.E., Heller B.L., Bringman L.R., Ranahan W.P., Conwell M.D., Sun Y., Hudmon A., Wells C.D. Serum deprivation inhibits the transcriptional co-activator YAP and cell growth via phosphorylation of the 130-kDa isoform of angiomotin by the LATS1/2 protein kinases. Proc. Natl. Acad. Sci. U S A. 2013;110:17368–17373. doi: 10.1073/pnas.1308236110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almog N., Ma L., Raychowdhury R., Schwager C., Erber R., Short S., Hlatky L., Vajkoczy P., Huber P.E., Folkman J. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- Beyer T.A., Weiss A., Khomchuk Y., Huang K., Ogunjimi A.A., Varelas X., Wrana J.L. Switch enhancers interpret TGF-beta and Hippo signaling to control cell fate in human embryonic stem cells. Cell Rep. 2013;5:1611–1624. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Bobbs A., Gellerman K., Hallas W.M., Joseph S., Yang C., Kurkewich J., Cowden Dahl K.D. ARID3B directly regulates ovarian cancer promoting genes. PLoS One. 2015;10:e0131961. doi: 10.1371/journal.pone.0131961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzard B.L., Chubet R.G., Vizard D.L. Immunoaffinity purification of FLAG epitope-tagged bacterial alkaline phosphatase using a novel monoclonal antibody and peptide elution. Biotechniques. 1994;16:730–735. [PubMed] [Google Scholar]
- Cao X., Pfaff S.L., Gage F.H. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens A.L., Knight J.D., Kean M.J., Teo G., Weiss A., Dunham W.H., Lin Z.Y., Bagshaw R.D., Sicheri F., Pawson T. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci. Signal. 2013;6:rs15. doi: 10.1126/scisignal.2004712. [DOI] [PubMed] [Google Scholar]
- Davis-Dusenbery B.N., Williams L.A., Klim J.R., Eggan K. How to make spinal motor neurons. Development. 2014;141:491–501. doi: 10.1242/dev.097410. [DOI] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Eriksson P.S., Perfilieva E., Bjork-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernkvist M., Aase K., Ukomadu C., Wohlschlegel J., Blackman R., Veitonmaki N., Bratt A., Dutta A., Holmgren L. p130-angiomotin associates to actin and controls endothelial cell shape. FEBS J. 2006;273:2000–2011. doi: 10.1111/j.1742-4658.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Finch-Edmondson M.L., Strauss R.P., Clayton J.S., Yeoh G.C., Callus B.A. Splice variant insertions in the C-terminus impairs YAP's transactivation domain. Biochem. Biophys. Rep. 2016;6:24–31. doi: 10.1016/j.bbrep.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney C.J., Oka T., Mazack V., Hilman D., Gat U., Muramatsu T., Inazawa J., Golden A., Carey D.J., Farooq A. Identification, basic characterization and evolutionary analysis of differentially spliced mRNA isoforms of human YAP1 gene. Gene. 2012;509:215–222. doi: 10.1016/j.gene.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Byun S.H., Park S., Kim J., Kim I., Ha S., Kwon M., Yoon K. YAP/TAZ enhance mammalian embryonic neural stem cell characteristics in a TEAD-dependent manner. Biochem. Biophys. Res. Commun. 2015;458:110–116. doi: 10.1016/j.bbrc.2015.01.077. [DOI] [PubMed] [Google Scholar]
- Kim M., Kim T., Johnson R.L., Lim D.S. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep. 2015;11:270–282. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Klim J.R., Li L., Wrighton P.J., Piekarczyk M.S., Kiessling L.L. A defined glycosaminoglycan-binding substratum for human pluripotent stem cells. Nat. Methods. 2010;7:989–994. doi: 10.1038/nmeth.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli P., Bartram M.P., Habbig S., Pahmeyer C., Lamkemeyer T., Benzing T., Schermer B., Rinschen M.M. Label-free quantitative proteomic analysis of the YAP/TAZ interactome. Am. J. Physiol. Cell Physiol. 2014;306:C805–C818. doi: 10.1152/ajpcell.00339.2013. [DOI] [PubMed] [Google Scholar]
- Lian I., Kim J., Okazawa H., Zhao J., Zhao B., Yu J., Chinnaiyan A., Israel M.A., Goldstein L.S., Abujarour R. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao T.T., Hsu W.H., Ho C.H., Hwang W.L., Lan H.Y., Lo T., Chang C.C., Tai S.K., Yang M.H. let-7 modulates chromatin configuration and target gene repression through regulation of the ARID3B complex. Cell Rep. 2016;14:520–533. doi: 10.1016/j.celrep.2015.12.064. [DOI] [PubMed] [Google Scholar]
- Mana-Capelli S., Paramasivam M., Dutta S., McCollum D. Angiomotins link F-actin architecture to Hippo pathway signaling. Mol. Biol. Cell. 2014;25:1676–1685. doi: 10.1091/mbc.E13-11-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Ralser M., Kloet S.L., Loos R., Nishinakamura R., Bertone P., Vermeulen M., Hendrich B. Sall4 controls differentiation of pluripotent cells independently of the Nucleosome Remodelling and Deacetylation (NuRD) complex. Development. 2016;143:3074–3084. doi: 10.1242/dev.139113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleirinho S., Guerrant W., Kissil J.L. The angiomotins—from discovery to function. FEBS Lett. 2014;588:2693–2703. doi: 10.1016/j.febslet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat. Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S., Morin S.A., Wrighton P.J., Zwick D.B., Jin S., Kiessling L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musah S., Wrighton P.J., Zaltsman Y., Zhong X., Zorn S., Parlato M.B., Hsiao C., Palecek S.P., Chang Q., Murphy W.L. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc. Natl. Acad. Sci. U S A. 2014;111:13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Yamamoto K., Agarwala S., Terai K., Fukui H., Fukuhara S., Ando K., Miyazaki T., Yokota Y., Schmelzer E. Flow-dependent endothelial YAP regulation contributes to vessel maintenance. Dev. Cell. 2017;40:523–536.e6. doi: 10.1016/j.devcel.2017.02.019. [DOI] [PubMed] [Google Scholar]
- Orr B.A., Bai H., Odia Y., Jain D., Anders R.A., Eberhart C.G. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J. Neuropathol. Exp. Neurol. 2011;70:568–577. doi: 10.1097/NEN.0b013e31821ff8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panciera T., Azzolin L., Fujimura A., Di Biagio D., Frasson C., Bresolin S., Soligo S., Basso G., Bicciato S., Rosato A. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S., Dupont S., Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- Sun Y., Yong K.M., Villa-Diaz L.G., Zhang X., Chen W., Philson R., Weng S., Xu H., Krebsbach P.H., Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 2014;13:599–604. doi: 10.1038/nmat3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Li X., Huang J., Feng L., Dolinta K.G., Chen J. Defining the protein-protein interaction network of the human hippo pathway. Mol. Cell. Proteomics. 2014;13:119–131. doi: 10.1074/mcp.M113.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigerius M., Quinn D., Diab A., Clattenburg L., Kolar A., Qi J., Krueger S.R., Fawcett J.P. The polarity protein angiomotin p130 controls dendritic spine maturation. J. Cell Biol. 2018;217:715–730. doi: 10.1083/jcb.201705184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrighton P.J., Klim J.R., Hernandez B.A., Koonce C.H., Kamp T.J., Kiessling L.L. Signals from the surface modulate differentiation of human pluripotent stem cells through glycosaminoglycans and integrins. Proc. Natl. Acad. Sci. U S A. 2014;111:18126–18131. doi: 10.1073/pnas.1409525111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D.X., Marchetto M.C., Gage F.H. How to make a hippocampal dentate gyrus granule neuron. Development. 2014;141:2366–2375. doi: 10.1242/dev.096776. [DOI] [PubMed] [Google Scholar]
- Yu F.X., Guan K.L. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lu Q., Wang L.H., Liu C.Y., Lei Q., Guan K.L. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.