Abstract
The toxicity of heavy metals such as Hg++ is a serious risk for human health. We evaluated whether 90 days of nutritional supplementation (d90, n = 16) with Chlorella vulgaris (CV) and Fucus sp extracts in conjunction with aminosulphurate (nutraceuticals) supplementation could detox heavy metal levels in patients with long-term titanium dental implants (average: three, average: 12 years in mouth) and/or amalgam fillings (average: four, average: 15 years) compared to baseline levels (d0: before any supplementation, n = 16) and untreated controls (without dental materials) of similar age (control, n = 21). In this study, we compared levels of several heavy metals/oligoelements in these patients after 90 days (n = 16) of nutritional supplementation with CV and aminozuphrates extract with their own baseline levels (d0, n = 16) and untreated controls (n = 21); 16 patients averaging 44 age years old with long-term dental amalgams and titanium implants for at least 10 years (average: 12 years) were recruited, as well as 21 non-supplemented controls (without dental materials) of similar age. The following heavy metals were quantified in hair samples as index of chronic heavy metal exposure before and after 90 days supplementation using inductively coupled plasma-mass spectrometry (ICP-MS) and expressed as μg/g of hair (Al, Hg++, Ba, Ag, Sb, As, Be, Bi, Cd, Pb, Pt, Tl, Th, U, Ni, Sn, and Ti). We also measured several oligoelements (Ca++, Mg++, Na+, K+, Cu++, Zn++, Mn++, Cr, V, Mo, B, I, P, Se, Sr, P, Co, Fe++, Ge, Rb, and Zr). The algae and nutraceutical supplementation during 90 consecutive days decreased Hg++, Ag, Sn, and Pb at 90 days as compared to baseline levels. The mercury levels at 90 days decreased as compared with the untreated controls. The supplementation contributed to reducing heavy metal levels. There were increased lithium (Li) and germanium (Ge) levels after supplementation in patients with long-term dental titanium implants and amalgams. They also (d90) increased manganesum (Mn++), phosphorum (P), and iron (Fe++) levels as compared with their own basal levels (d0) and the untreated controls. Finally, decreased SuperOxide Dismutase-1 (SOD-1) activity (saliva) was observed after 90 days of supplementation as compared with basal levels (before any supplementation, d0), suggesting antioxidant effects. Conversely, we detected increased SOD-1 activity after 90 days as compared with untreated controls. This SOD-1 regulation could induce antioxidant effects in these patients. The long-term treatment with algae extract and aminosulphurates for 90 consecutive days decreased certain heavy metal levels (Hg++, Ag, Sn, Pb, and U) as compared with basal levels. However, Hg++ and Sn reductions were observed after 90 days as compared with untreated controls (without dental materials). The dental amalgam restoration using activated nasal filters in conjunction with long-term nutritional supplementation enhanced heavy metals removal. Finally, the long-term supplementation with these algae and aminoazuphrates was safe and non-toxic in patients. These supplements prevented certain deficits in oligoelements without affecting their Na+/K+ ratios after long-term nutraceutical supplementation.
Keywords: algae, Chlorella, Fucus, detoxification, environmental pollution, antioxidants, heavy metals, selenium, SOD-1, neurotoxicology, aminoazuphrates, clinical medicine, nutrition, neuropathology
1. Introduction
Humans are exposed to pollutants, xenobiotics, and heavy metals that can be accumulated in the body when detox mechanisms are defective. Heavy metals can affect metallothionein and glutathione levels (its reduced form labeled herein as GSH) as well as SuperOxide Dismutase-1 (SOD-1) enzymatic activity [1,2,3,4]. Selenium (Se) is a crucial element for heavy metal removal by conjugation with GSH [2]. These xenobiotics can provoke hypertension and other clinical alterations in patients [5,6]. Mercury may cause neurodevelopmental disorders as autism spectrum disorders. Dental amalgams contain 50% of mercury (Hg), 41% of silver (Ag), Tin (Sn: 5–8%), Zn++, and Cu++ as minority oligoelements; titanium dental implants contain Ti-6Al-4V alloy [7]. Levels of heavy metals/oligoelements can be measured by inductively coupled plasma-mass spectrometry (ICP-MS) [8] in human samples such as urine, plasma, or hair [9,10]. The toxicity of heavy metals (e.g., mercury, cadmium) depends on the route, the concentration [11], and the exposure time and mixtures of heavy metals [12]. The function of oligoelements in odontology is still little studied (to review its functions, consult reference number [13].
The increasing concern of health problems associated with environmental pollutants is a serious one in humans because aluminum (Al) [14], lead (Pb), mercury (Hg++), cadmium (Cd), arsenic (As), nickel (Ni), copper (Cu++), iron (Fe++), chromium (Cr), and cobalt (Co) could provoke health problems in the case of heavy metal accumulation [14,15,16], which could be reduced by microalgae [17]. The occupational exposure to Cd and Hg++ are associated with antropometric activities, cremation, plastics, glass, and metal alloys. These heavy metals are also present in electrode material, nickel-cadmium batteries, water, and cigarette smoke [18,19].
Detoxification is the ability to remove drugs, mutagens, and other harmful agents from the body. The detoxification takes place in the intestinal tract, the liver, and the kidneys by microbiota able to chelate several heavy metals [20,21]. For instance, increased blood lead, mercury, and zinc levels were associated with Sarcopenia in the elderly population [6]. In addition, increased hair mercury levels (but not urinary levels) were correlated with the elevated title for the Lupus Eritematose marker in women (nuclear antigen: ANA) [10]. Several metabolic pathways in food-derived compounds are involved in detoxification [21]. Thus, clinical protocols able to prevent heavy metal accumulation are necessary in patients in conjunction with long-term nutritional supplementation. Antioxidants contribute to chelating reactive oxygen species (ROS) by removing heavy metals; thus, the screening of new antioxidants from plants is important from a clinical view point.
Some microalgae can remove heavy metals from wastewater. Chlorella vulgaris (CV) is a unicellular marine algae rich in chlorophyll (1–4%) that contains 55–67% protein, 9–18% dietary fiber, minerals, vitamins, and several oligoelements [17,22]. The CV algae are considered to be highly resistant to heavy metals and are widely used as a food supplement in Japan [17,23]. The Chlorella sorokiniana can promote antioxidant responses under zinc tolerance by increasing antioxidant enzymatic activities and increasing flavonoids, polyphenols, tocopherols, glutathione, and ascorbate (ASC) levels [24]. The CV extract can also excrete dioxin [25] and remove Cd levels by inducing metallotionein-like proteins. The biosorption of Pb2+ and Cd2+ have been described on a fixed bed column with immobilized Chlorella algae biomass [26]. Chlorella protothecoides algae promote heavy metal detoxification in chlordecone poisoned-treated rats by reducing the half-life of the toxin from 40 to 19 days. In addition, the Fucus spiralis is a marine brown alga (spiral wrack) that contains phlorotannins (antioxidant) [22]. The phytochelatins are short produced peptides from plants, algae, and fungi in response to heavy metal exposure, which detoxificate heavy metals by its high cysteine-content. Fucus versiculosus also may chelate Zn++ [22,27]. Phytochelatins are a natural source of novel angiotensin-I converting enzyme (ACE) inhibitors [28].
Our hypothesis is that the use of nasal filters (active carbon) in conjunction with long-term algae extract (Chlorella and Fucus sp) and aminosulphurates supplementation for 90 consecutive days contributes to the removal of heavy metals (Hg++, Ag, Sn, Pb) in patients with long-term dental titanium implants and amalgam fillings restorations.
2. Aim
We evaluated whether dietary chronic supplementation with CV and aminoazuphrates during 90 consecutive days could contribute to detoxificating heavy metals and/or prevent certain oligoelement deficits in patients with long-term dental titanium implants and amalgam fillings restorations. Therefore, the study was conducted to investigate whether long-term dietary CV contributed to the prevention of heavy metal accumulation after 90 days of supplementation (d90) in patients with long-term dental titanium implants and amalgam fillings restorations as compared with their own baseline levels (before any nutritional supplementation: d0) as well as untreated controls (without dental materials).
3. Materials and Methods
3.1. Patients
All selected patients were 49–68 years old (average: 58.5 years). The percentage of smokers was 7%, and their sociocultural states were medium-high levels (higher school education: 70%). Similar untreated (non-supplemented) control patients were included in this study. Their average age was similar to the rest of the patients. These untreated controls did not receive nutritional supplementation with the formulations. They did not have dental materials in their mouths (n = 21 controls).
The average number of dental amalgam fillings was 4, and there were 3 dental titanium implant alloys on average. All selected patients had a dental filling at least 10 years in their mouths (average: 15 years) and long-term titanium dental alloys for at least 10 years as well (average: 12 years). We selected 16 patients who had at least two or more long-term dental amalgams.
The number of enrolled patients suitable according to inclusion criteria was 21 untreated controls as well as 16 patients.
They received nutritional algae extracts (Chlorella/Fucus sp) and aminoazuphrates supplementation during 90 consecutive days (d90). Their heavy metal/oligoelement levels were compared at day 90 (d90, n = 16) with their basal levels (d0: before any supplementation, baseline, n = 16) as well as untreated controls (without dental materials in mouth, n = 21). These controls did not receive supplementation.
Their dental amalgams fillings were progressively replaced by composites (bisphenol A free) every 20 days following a clinical safe protocol by using active carbon filters (@InspiraHealth, Barcelona, Spain) and nutritional supplementation [29]. There are four quadrants in the mouth, and these amalgams were progressively replaced by each quadrant (each session within 20 days). Thus, some patients still had dental fillings during the 90 consecutive days of supplementation before their complete removal. The patients took supplements by oral intake (formulations) from the beginning (day 0, baseline levels) until the end of supplementation (d90: 90 consecutive days). We also compared levels of heavy metals/oligoelements after 90 days of supplementation with untreated controls (control, n = 21) and baseline levels (day zero, d0: before any supplementation). The nutritional supplementation took place during the time of dental amalgam restoration by composites. It is noteworthy that all dental materials were progressively replaced by composites at the time of collecting hair samples (90 days of supplementation). The fish consumption was 1–2 times per week in all recruited patients, including the controls. The basal heavy metals/oligoelements levels were taken at the initial visit to the dental clinic (CIROM: Centro de Implantología y Rehabilitación Oral Multidisciplinaría, https://clinicacirom.com/) before taking any nutritional supplementation, which were termed d0 (day 0) patients in the present study.
All supplemented patients received nutritional treatment by oral intake during 90 consecutive days with the following formulations: GREEN-FLOR (2-0-2; 4 capsules/day: Chlorella and Fucus algae extract), ERGYTAURINE (1-0-1; 2 capsule/day), and ERGYLIXIR formulations (Laboratorios Nutergia) during 90 consecutive days [from the initial day that patients visited the dental clinic (day 0) until the end of nutritional supplementation (d90: day 90)]. Controls without dental materials did not receive these treatments.
All ICP-MS heavy metal or oligoelements data were evaluated as percentiles (median, 25% and 75%) for non-parametric data (Kruskal-Wallis) and expressed as μg/g of hair in all cases (except vadanium). The ANOVA (analysis of variance) evaluated differences for vanadium levels, which were expressed as mean values ± standard error media (S.E.M). S.E.M was the variance divided by root square, and n was the size simple. The size sample was n = 16 patients at d90 and d0 and n = 21 untreated controls; the following heavy metals and oligoelements, respectively, were quantified by ICP-MS in the hair samples (Al, Hg++, Ba, Sn, Ag, Sb, As, Be, Bi, Cd, Pb, Pt, Tl, Th, U, Ni, Sn, Ti); (Ca++, Mg++, Na+, K+, Cu++, Zn++, Mn++, Cr, V, Mo, B, I, P, Se, Sr, P, Co, Fe, Ge, Rb, Zr).
These heavy metal/oligoelements were compared after 90 days of nutritional supplementation (d90) with their own basal levels (d0: before any supplementation) as well as untreated controls without dental materials (control, n = 21, non-supplemented). These untreated controls did not receive supplements and they did not carry dental materials. Saliva samples were taken at these study times for SOD-1 determination.
The limitation of the present study was the size sample (pilot study) and the absence of a placebo group. However, we included untreated controls (without dental materials and non-supplemented). This placebo group in patients with long-term dental implants and amalgam fillings could be not ethically justifiable since it is not possible to keep dental amalgam fillings, which release mercury in patients [9]. The implementation of this protocol in the Caucasian population (Spaniards) was the other limitation.
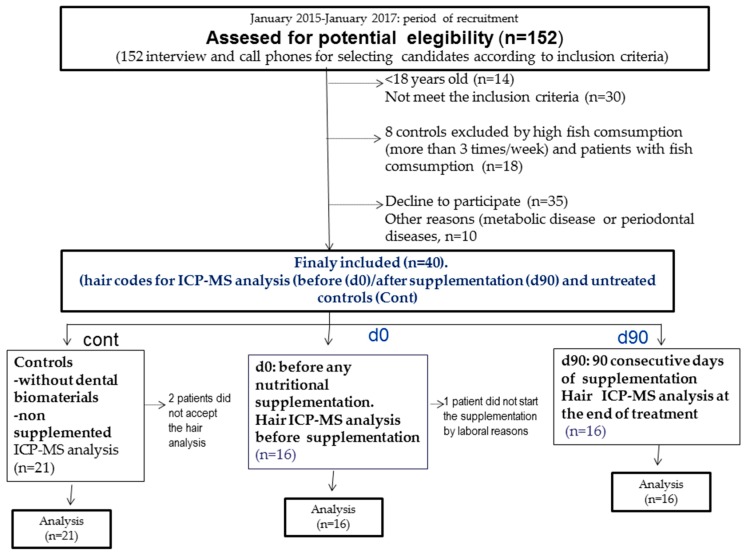
A dentist assessed 152 interviews or call phones to select potentially eligible patients. There were 35 patients who declined to participate and 30 patients who did not meet the inclusion criteria. We also excluded patients with fish consumption higher than 2 times by week. We did not enroll patients with periodontal disease or metabolic alterations. Thus, we selected 40 patients, and 37 participated in the end, which comprised the untreated controls (n = 21) and the baseline patients [before taking supplements (d0, n = 16) and after 90 days of nutritional supplementation (d90, n = 16), see study groups in Figure 1]. All statistical analyses were evaluated in 37 patients and 53 hair samples for each heavy/metal oligoelement determination here.
Figure 1.
Study groups.
3.2. Inclusion Criteria
This study followed the Declaration of Helsinki (1974, updated 2000), and it was approved by the Institutional Review Board from CIROM (Murcia, #2016/014). All subjects were properly instructed by signing the appropriate consent paperwork. In addition, all efforts were made to protect patient privacy and anonymity. The CIROM Center was approved and certified by AENOR Spain (Spain; CIROM CERTIFICATE for dentist services, CD-2014-001 number; ER-0569/2014 following UNE-EN ISO 9001: 2008 as well as UNE 179001-2001 Directive from Spain). We selected 16 patients who had at least two or more long-term dental amalgams. They had long-term dental amalgam fillings for at least 10 years in their mouths (average: 15 years) and long-term titanium dental implants for at least 10 years (average: 12 years). The fish consumption was 1-2 times per week in all recruited patients. The average number of dental amalgam fillings was 4, and the average number of dental titanium implant alloys was 3.
Controls were selected after clinical examination. They did not have dental materials in their mouths, nor did they show signs of periodontal diseases. We excluded patients who had fish consumption higher than 2 times by week.
3.3. Exclusion Criteria
Physically handicapped patients who had metabolic diseases [diabetes, metabolic syndrome, liver/kidney disease, systemic inflammation, lupus/autoimmune disease, thyroid disease, adrenal disease, or neuropsychiatry disorders Diagnostic and Statistical manual of Mental disorders (4th Edition, DSM IV)] [30], were excluded in the present study. Patients taking regular medication or stimulants, anticonvulsants, atypical antipsychotic drugs, or those who had history of liver/kidney disease or DMSA (dimercaptosuccinic acid) prescribed (or chelators) patients were also excluded. Particularly, hypertensive patients and those who had periodontal disease tattoos or were taking nutritional supplements were excluded in the present study. Finally, patients who had orthodontic devices were not included here. The correct diagnostic of periodontal disease was based on several parameters, such as visual exploration (palpation), presence of dental calculus, radiographic evaluation, dental mobility, and oclusal exploration (pathological eroding facets). Periodontal disease was also a cause of exclusion, which was identified by following several criteria by an expert dentist, such as a deep dental probe higher than 3 mm, loss of bone (radiography), possible bleeding, and dental mobility [31].
3.4. Composition of Nutritional Supplementation (Algae and Other Bioactive Phytomolecules)
All patients took the following nutritional supplementation during 90 consecutive days (oral intake): GREEN-FLOR (2-0-2), ERGYTAURINE (1-0-1), and ERGYLIXIR formulation (Nutergia, 1 bottle/month) following the patterns of their antioxidants properties (see Table 1). The controls of the intakes were registered by dentists every 20 days, and we administered the following dosages according our previous clinical experience.
Table 1.
Composition (nutraceuticals) of formulations.
| GREEN-FLOR (Formulation-1) | |
| Nutritional supplementation during 90 consecutive days (4 capsules/day 2-0-2) | dosage (mg/day) |
| Chlorella: 80 mg/capsule | 320 mg/day |
| Spirulina: 80 mg/capsule | 320 mg/day |
| Kelp of Pacific: 60 mg/capsule | 240 mg/day |
| Fucus: 30 mg/capsule | 120 mg/day |
| Cardille: 25 mg/capsule | 100 mg/day |
| Pectine of apple: 60 mg/capsule | 240 mg/day |
| Acerole (rich in vitamin C): 50 mg/capsule | 200 mg/day |
| Fructooligosacarides: 280 mg | 1120 mg/day |
| Scolymus hispanicus: 60 mg/capsule | 240 mg/day |
| ERGYTAURINE (Formulation-2) | |
| Treatment: 90 consecutive days (2 capsules/day; 1-0-1) | dosage (mg or μg/day) |
| Selenium (Se): 25 μg/capsule | 50 μg/day |
| Vitamin B6: 0.8 μg/capsule | 1.6 μg/day |
| Folic Acid (B-9): 100 μg/capsule | 200 μg/day |
| Zinc (Zn++): 3.5 mg/capsule | 50 μg/day |
| Taurine: 120 mg/capsule | 240 mg/day |
| Extract of Raphanus niger L. 15 mg/capsule | 30 mg/day |
| ERGYLIXIR (Formulation-3) | |
| Extracts from: | dosage: mg/month |
| Cynara scolymus (artichoe) | 1440 mg |
| Raphanus niger | 900 mg |
| Taraxacum officinale | 400 mg |
| Arctium lappa | 320 mg |
| Vaccinium macrocarpo | 228 mg |
| Solidago virgaurea | 200 mg |
| Rosmarinus officinalis | 640 mg |
| Sambucus niger (elderberry: antocianines) | 200 mg |
| Sodium Moligdate | 50 μg |
| Selenium | 50 μg |
3.5. Inductible Coupled Mass Spectromery Analysis (ICP-MS)
In the weight of the dental amalgam fillings, mercury (Hg) was 50%, and silver (Ag) was 41%, Sn was approximately 5–8%, and Cu++ and Zn++ levels were in the minority. Hair samples close to the scalp were taken from all subjects (0.25 g from the occipital area) to measure a plethora of heavy metals/oligoelements by ICP-MS (Doctor’s Data, USA). Doctor’s Data is a pioneer laboratory specializing in the toxicology of heavy metals with over 35 years of experience, and they provide analytical tests for healthcare practitioners. ICP-MS values for heavy metals were expressed in μg/g of hair.
3.6. Super Oxide Dismutase-1 (SOD-1 Activity)
The saliva SOD-1 activity was measured following a modified protocol by [9] Cabaña-Muñoz et al., 2015. Briefly, the buffer assay contained 0.1 mM EDTA (Ethylenediaminetetraacetic acid), 50 mM sodium carbonate, and 96 mM of nitro blue tetrazolium (NBT). Then, 470 µL of the above mixture was added to 100 µL of saliva, and the auto-oxidation of hydroxylamine was observed by adding 0.05 mL of hydroxylamine hydrochloride (pH 6.0). Finally, SOD-1 activity was measured by the change in optical density at 560 nm for 2 min at 30/60 s intervals and normalized as optical density (D.O) by protein [9].
3.7. Statistical Analysis
All data were analyzed by SPSS software (v17.0) (U.C.M: Universidad Complutense, Madrid, Spain) and Sigma Plot (v11.0, U.C.M, Madrid, Spain). Mean 25%, 75%, and median values (μg/g of hair) were estimated for heavy metals/oligoelements in the hair samples. Non-parametric tests were applied in cases without homogeneity of variance (Mann Whitney/Kruskal Wallis). The Bonferroni tests were applied for multiple comparisons when there was homogeneity of variance (e.g., vanadium, V). All results were expressed as percentiles 25%, 75%, and median (μg/g of hair) according to Kruskal Wallis values (H value) and Mann Whitney (MW) and Dunn’s post hoc test in the case of non-parametric data between (d0: n = 16, d90: n = 16) and controls (control: n = 21). The Levene test identified whether or not there was homogeneity of variance depending on its significance. Correlations between variables were performed by Spearman’s rank correlation. Differences were considered statistically significant if p < 0.05 and highly significant when p < 0.01.
4. Results
4.1. SOD-1 Activation Reflects Antioxidants Responses in Patients after Long-Term Supplementation with Algae Extract and Aminoazuphrates Compared with Untreated Controls
There was a statistically significant effect for SOD-1 activity in the Kruskal Wallis analysis (H = 45.1, p ≤ 0.001) for SOD-1 activity (saliva). The parametric Dunn’s non-analysis revealed decreased SOD-1 activity after 90 days of supplementation (d90) compared to their basal levels (d0: before any supplementation, p < 0.05); Conversely, increased activity SOD-1 activity was detected before any supplementation as compared with untreated controls (without dental materials, p < 0.05, Table 2).
Table 2.
Regulation of SuperOxide Dismutase-1 (SOD-1) by long-term algae and aminosulphurate supplementation and SOD-1 activity.
| Group | Median | 25% | 75% |
| SOD-1 activity (control) | 100 | 100 | 100 |
| SOD-1 activity (d0) | 143.5 | 139.500 * | 149 |
| SOD-1 activity (d90) | 121 | 119.000 *,# | 125 |
| SOD-1 Activity | Difference of Ranks | Q | p < 0.05? |
| Control vs. d0 (before treatment) | 34.000 | 6.7 | Yes |
| d0 vs. d90 (after 90 days) | 16.000 | 2.920 | Yes |
| d90 vs. Cont: controls | 18.500 | 3.600 | Yes |
* p < 0.05 vs. control, # p < 0.05 d90 vs. d0; control: controls without dental materials and non-supplemented (n = 21); d0: patients with long-term titanium implants and dental amalgam fillings restorations before any nutritional supplementation (d0, n = 16); d90: patients with long-term titanium implants and dental amalgam fillings restorations after 90 days of supplementation (d90, n = 16).
4.2. Reduced Mercury (Hg++) and Silver (Ag) Levels after 90 Days of Nutritional Supplementation (d90) as Compared with Their Baseline Levels (d0) as ell as Untreated Controls (Without Dental Materials and non Supplemented, cont)
We compared levels of heavy metals (Hg++, Ag, Sn) as well as titanium alloys (Ti-6Al-4V) in patients with long-term dental titanium implants and amalgams fillings after 90 days of nutritional supplementation as compared with their own basal levels (before any supplementation, d0) as well with non-supplemented controls (without dental materials, controls). The Kruskal Wallis and Mann Whitney post hoc analyses revealed mercury (Hg++) and tin (Sn) reductions after 90 days of supplementation as compared with their own basal levels (d0) without reaching a significant effect in Zn++, Co, Ni, or Cu++ levels (Table 3).
Table 3.
Heavy metals/oligoelements of dental materials.
| Heavy Metals from Dental Amalgams | ||||
| Hg++ | Median | 25% | 75% | H |
| Control | 1.6 | 1.25 | 2.3 | H = 13.85, p < 0.001. |
| d0 | 1.9 | 1.9 | 3.7 * | MW (* p < 0.005). |
| d90 | 1.15 | 0.34 | 2.1 *,# | MW (# d90 vs. d0, p = 0.049); * p < 0.05 d90 vs. Cont |
| Ag | Median | 25% | 75% | H |
| Control | 0.03 | 0.02 | 0.06 | H = 9.3, p = 0.01. |
| d0 | 0.1 | 0.03 | 0.155 # | MW (* p = 0.005 d0 vs. control). (# d90 vs. d0, p = 0.031) |
| d90 | 0.055 | 0.025 | 0.075 | |
| Sn | Median | 25% | 75% | H |
| Control | 0.045 | 0.02 | 0.095 | H = 6.27, p = 0.43. |
| d0 | 0.11 | 0.04 | 0.20 * | MW (* d0 vs. Cont, p = 0.023). |
| d90 | 0.03 | 0.02 | 0.105 # | MW (# d90 vs. d0, p = 0.047). |
| Zn++ | Median | 25% | 75% | H |
| Control | 195 | 180 | 230 | H = 5, p = 0.078 |
| d0 | 245 | 208 | 275 * | MW (* p < 0.05 d0 vs. control). |
| d90 | 210 | 180 | 242 | |
| Cu++ | Median | 25% | 75% | H |
| Control | 13.5 | 10.5 | 35.5 | H = 1.01, p = 0.6, n.s |
| d0 | 15 | 11 | 31 | |
| d90 | 13.5 | 10 | 19.5 | |
| Materials from Dental Titanium Alloys (Cr, Ni, Co) | ||||
| Al | Median | 25% | 75% | H |
| Control | 2.9 | 2.05 | 5.6 | H = 4.6, p = 0.1, n.s. |
| d0 | 3 | 1.6 | 4.6 | |
| d90 | 1.6 | 1.5 | 2.4 * | MW (* d90 vs. control, p = 0.029). |
| Cr | Median | 25% | 75% | H |
| Control | 0.35 | 0.31 | 0.39 | H=9.64, p = 0.008 |
| d0 | 0.35 | 0.35 | 0.39 | |
| d90 | 0.41 | 0.36 | 0.45 *,# | MW or Dunn’s.* p < 0.05 vs. cont, # p < 0.05 d90 vs. d0 |
| Co | Median | 25% | 75% | H |
| Control | 0.004 | 0.004 | 0.010 | H = 4.97, p = 0.083, n.s. |
| d0 | 0.017 | 0.04 | 0.035 * | MW * p < 0.05 d0 vs. control. |
| d90 | 0.06 | 0.035 | 0.012 | |
| Ni | Median | 25% | 75% | H |
| Control | 0.055 | 0.04 | 0.10 | H = 3.07, p = 0.21, n.s. |
| d0 | 0.09 | 0.08 | 0.16 * | MW (* p < 0.05, d0 vs. Cont). |
| d90 | 0.11 | 0.04 | 0.16 | |
| V | Media | S.E.M | F | |
| Control | 0.04 | 0.003 | F (2.50) = 2.73, p = 0.07, n.s. | |
| d0 | 0.031 | 0.004 * | Bonferroni (p = 0.043, alpha (α) = 0.05, beta (β) = 0.42). * p < 0.05 vs. control |
|
| d90 | 0.041 | 0.0035 # | (# p < 0.05, d90 vs. d0) | |
| * p < 0.05 vs. Cont | # p < 0.05 d90 vs. d0 | n.s: non significant effect (p > 0.05, n.s). | ||
Percentiles analysis for heavy metals/oligoelement levels in Kruskal-Wallis (H) between patients with long-term titanium implant and dental fillings after 90 days of supplementation (d90, n = 16) as compared with their own basal levels (d0: before any supplementation, n = 16) and untreated (non-supplemented) controls without dental materials (control, n = 21). All heavy metals and oligoelements were expressed as μg/g of hair. H is the Krukal-Wallis analysis and F is ANOVA data. MW = Mann Whitney, S.E.M = standard error media; Control: controls without dental materials and non-supplemented (n = 21); d0: patients with long-term titanium implants and dental amalgam fillings restorations before any supplementation (d0, n = 16); d90: patients with long-term titanium implants and amalgams after 90 days of supplementation (n = 16); n.s: not significant effect, p > 0.05. * p < 0.05 vs. Control; # p < 0.05 d90 vs. d0.
Finally, Ag levels decreased after 90 days as compared to their basal levels (d0, before any supplementation) without reaching a significant effect as compared to untreated the controls.
The aluminium (Al) levels decreased after 90 days of supplementation (d90) as compared with untreated controls (p < 0.05); increased d90 vanadium (V) levels were observed as compared with basal levels (d0, p < 0.05). There were no effects in Ti or Co levels by treatment (p > 0.05, non-supplemented, Table 3).
4.3. Levels of Oligoelements Involved in Metabolic Functions (Se, Mn++, Li, Mg++, Ge, S, P, I, Ca2+, Sr, Na+, K+)
Patients with long-term titanium implants and amalgam fillings increased germanium (Ge), manganesum (Mn++), chromium (Cr), vanadium (V), phosphorum (P), and lithium levels (Li) after 90 days of supplementation (day 90) as compared with untreated controls (control, n = 21). In addition, after 90 days (d90, n = 16), their selenium (Se) levels decreased in comparison to their basal levels (d0, n = 16, p < 0.05, μg/g of hair); however, they were higher than the control values (Table 4, p < 0.05). These supplements could promote antihypertensive effects by rising certain oligoelements. Finally, there were no effects in other oligoelements (Ca2+, Mg2+, I, Sr, B, Rb) or for Be, Bi, Tl, To (data not shown, p > 0.05, n.s).
Table 4.
Percentiles for oligoelements involved in metabolic functions in patients with long-term titanium implant and dental fillings after 90 days of supplementation (d90, n = 16) and their basal levels (d0: before any supplementation, n = 16) and non-supplemented controls (control: without dental materials and non-supplemented, n = 21). All heavy oligoelements were expressed as μg g/g of hair.
| Se | Median | 25% | 75% | H |
| Control | 0.66 | 0.59 | 0.73 | H = 10.91, p = 0.004. |
| d0 | 0.6 | 0.47 | 0.67 * | MW (* d0 vs. control, p = 0.05). |
| d90 | 0.55 | 0.48 | 0.62 * # | MW (# d90 vs. d0, p = 0.039; * p < 0.05 vs. cont). |
| Mo | Median | 25% | 75% | H |
| Control | 0.034 | 0.0140 | 0.0032 | H = 14.5, p < 0.001. |
| d0 | 0.022 | 0.0079 | 0.023 * | MW or Dunn’s (* p < 0.05 d0 vs. control). |
| d90 | 0.020 | 0.0084 | 0.150 * | MW or Dunn’s: * p < 0.05 d90 vs. control |
| Mn++ | Median | 25% | 75% | H |
| Control | 0.075 | 0.06 | 0.10 | H = 5.42, p = 0.066, n.s. |
| d0 | 0.085 | 0.04 | 0.11 | |
| d90 | 0.115 | 0.07 | 0.18 * # | MW (* d90 vs. control, p = 0.05); MW (d90 vs. d0, p < 0.05; * p < 0.05 vs. cont) |
| Li | Median | 25% | 75% | H |
| Control | 0.008 | 0.006 | 0.012 | H = 1.45, p < 0.001. |
| d0 | 0.005 | 0.0045 | 0.0075 * | MW (* d0 vs. control, p = 0.03). |
| d90 | 0.023 | 0.010 | 0.010 * # | MW (* d90 vs. control, p < 0.05); MW (# d90 vs. d0, p = 0.05). |
| Ge | Median | 25% | 75% | H |
| Control | 0.031 | 0.024 | 0.033 | H = 13.1, p = 0.01. |
| d0 | 0.024 | 0.021 | 0.032 | MW (* d0 vs. control, p = 0.1, n.s). |
| d90 | 0.023 | 0.032 | 0.035 # | MW or Dunn’s (# p < 0.05 d90 vs. d0). |
| S | Median | 25% | 75% | H |
| Control | 47700 | 47200 | 49250 | H = 3.97, p = 0.13, n.s. |
| d0 | 46900 | 46350 | 47600 * | MW (* d0 vs. control, p < 0.05). |
| d90 | 46850 | 45600 | 49950 | |
| P | Median | 25% | 75% | H |
| Control | 185 | 155 | 197 | H = 8.88, p = 0.012. |
| d0 | 153 | 134 | 160 * | MW (* d0 vs. control, p < 0.05). |
| d90 | 170 | 154 | 179 # | MW (# d90 vs. d0, p = 0.004). |
| I | Median | 25% | 75% | H |
| Control | 0.58 | 0.37 | 2.05 | H=3.67, p = 0.15, n.s |
| d0 | 0.39 | 0.29 | 0.5 | |
| d90 | 0.47 | 0.31 | 0.77 | |
| Ca++ | Median | 25% | 75% | H |
| Control | 488 | 285 | 705 | H = 1.37, p = 0.5, n.s |
| d0 | 785 | 410 | 1262 | |
| d90 | 676 | 380 | 1060 | |
| Sr | Median | 25% | 75% | H |
| Control | 2.7 | 0.93 | 6.55 | H = 4.41, p = 0.11, n.s |
| d0 | 7 | 2.97 | 11.7 | d0 vs. control, p = 0.064, n.s. |
| d90 | 11.81 | 1.9 | 17.75 | d90 vs. control, p = 0.099, n.s |
| B | Median | 25% | 75% | H |
| Control | 0.5 | 0.41 | 0.87 | H = 1.5, p = 0.46, n.s. |
| d0 | 0.63 | 0.56 | 1.1 | |
| d90 | 0.71 | 0.52 | 0.8 | |
| Na+ | Median | 25% | 75% | H |
| Control | 35 | 14.5 | 73.25 | H = 6, p =0.05. |
| d0 | 62.5 | 52 | 140 * | MW or Dunn’s (* d0 vs. control, p = 0.046). |
| d90 | 48 | 33 | 75 | d90 vs. control, p = 0.1, n.s. |
| K+ | Median | 25% | 75% | H |
| Control | 13.5 | 4 | 31.5 | H = 1.3, p = 0.52, n.s |
| d0 | 5.5 | 3.5 | 44 | |
| d90 | 8.5 | 3 | 15 | |
| Mg++ | Median | 25% | 75% | H |
| Control | 50 | 32.5 | 94 | H = 3.63, p = 0.16, n.s. |
| d0 | 99 | 43.5 | 184.5 | |
| d90 | 137 | 57 | 345 | MW (d90 vs. control, p = 0.088, n.s). |
| Rb | Median | 25% | 75% | H |
| Control | 0.015 | 0.0045 | 0.031 | H = 2.72, p = 0.25, n.s. |
| d0 | 0.014 | 0.0040 | 0.019 | |
| d90 | 0.011 | 0.0030 | 0.013 | |
| Fe++ | Median | 25% | 75% | H |
| Control | 6.7 | 6.2 | 7.7 | H = 3.7, p = 0.15, n.s. |
| d0 | 6.6 | 6.4 | 7.4 | |
| d90 | 7.7 | 6.5 | 8.4 # | MW (d90 vs. d0, # p = 0.022). |
| * p < 0.05 vs. control | # p < 0.05 d90 vs. d0 |
* p < 0.05 vs. control; # p < 0.05 d90 vs. d0; controls (without dental materials and non-supplement; control, n = 21); d0: patients with long-term titanium implants and dental amalgam fillings restorations before any supplementation (d0, n = 16); d90: patients with long-term titanium implants and dental amalgams after 90 days of supplementation (d90, n = 16); (n.s: not significant effect, p > 0.05; * p < 0.05 vs. Control; # p < 0.05 d90 vs. d0).
4.4. Metals of Environmental Exposure
The algae extract and aminoazuphrates supplements decreased lead (Pb) levels after 90 days of supplementation (day 90) as compared with baseline levels (d0: before any supplementation); the aluminium (Al) levels were reduced after 90 days in comparison to untreated controls (see Table 5).
Table 5.
Decreased lead (Pb) levels after 90 days of supplementation as compared with baseline levels.
| Ba | Median | 25% | 75% | H |
| Control | 0.17 | 0.1 | 0.33 | H = 7.73, p = 0.021. |
| d0 | 0.54 | 0.25 | 0.84 * | MW (* d0 vs. control, p = 0.05). |
| d90 | 0.28 | 0.2 | 0.36 | MW (d90 vs. control, p = 0.11, n.s). |
| Pb | Median | 25% | 75% | H |
| Control | 0.11 | 0.07 | 0.3 | H = 3.41, p = 0.18, n.s. |
| d0 | 0.14 | 0.09 | 0.21 | |
| d90 | 0.085 | 0.05 | 0.14 # | MW (# d90 vs. d0, p = 0.047). |
| Cd | Median | 25% | 75% | H |
| Control | 0.009 | 0.009 | 0.010 | H = 4.73, p = 0.094, n.s. |
| d0 | 0.009 | 0.009 | 0.009 | |
| d90 | 0.009 | 0.009 | 0.009 | MW (d90 vs. control, p = 0.08, n.s). |
| Sb | Median | 25% | 75% | H |
| Control | 0.01 | 0.01 | 0.018 | H = 3.5, p = 0.16, n.s. |
| d0 | 0.01 | 0.01 | 0.01 | |
| d90 | 0.01 | 0.01 | 0.01 | |
| As | Median | 25% | 75% | H |
| Control | 0.038 | 0.01 | 0.018 | H = 0.9, p = 0.62, n.s. |
| d0 | 0.028 | 0.022 | 0.042 | |
| d90 | 0.028 | 0.023 | 0.052 | |
| * p < 0.05 vs. Cont | # p < 0.05 d90 vs. d0 |
These tables show percentile values (median, 25%, and 75%) in Kruskal Wallis analysis for several heavy metals/oligoelements (Hg++, Ag, Sn, Zn++, Cu++, Al, Cr, V, Co, and Ni), metabolic oligoelements (Se, Mo, Mn++, Li, Ge, S, P, I, Ca++, Sr, B, Na+, K+, Mg++, Rb, B, and Fe++), and metals of environmental exposure in Table 6 (Ba, As, Pt, Sb, Tl, To, Cd, Be, Bi, Zr, Pb, Cd, As, and U). The ANOVA data for V are shown by mean values ± S.E.M [the root square divided by n; n was the size sample; n = 16 (d90), n = 16 (d0), n = 21 controls]. Post hoc differences were evaluated by the Mann Whitney or Dunn’s method; Control: controls without dental materials and non-supplemented (n = 21); d0: patients with long-term titanium implants and dental amalgam fillings restorations (d0, n = 16); d90: patients with long-term titanium implants and dental amalgams after 90 days of supplementation (n = 16); n.s: not significant effect, p > 0.05). * p < 0.05 vs. Control; # p < 0.05 d90 vs. d0).
There was a lack of effect in several heavy metals (As, Ti, Pt, Sb, Tl, To, Cd, Be, Bi, Zr, p > 0.05, n.s) and oligoelements by treatment (Zn++, Cu++, Ca++, Sr, B, I, K+, Mg++, Rb) after 90 days of supplementation as compared with their baseline (d0) and control levels.
4.5. Effects on Selenium (Se) Ratios and Heavy Metals after 90 Days of Nutritional Supplementation
For example, we found decreased Se/Hg++, and increased Se/Al, and Mo/Hg++ ratios after 90 days of supplementation (d90) compared to their respective basal levels (before any treatment, d0, p < 0.05); However, these Se/Hg++, Se/Ag, and Mo/Hg++, and Na+/K+ ratios decreased before any treatment (d0) as compared with non-supplemented patients (controls, p < 0.05, Table 6).
Table 6.
Effects on Se/Hg++, Se/Ag, Se/Al, Se/Pb, Mo/Hg++, Na+/K+ before/after nutritional supplementation and untreated controls.
| Se/Hg++ Ratio | Median | 25% | 75% | H |
| Control | 2.21 | 1.6 | 3.2 | H = 31.42, p < 0.001. |
| d0 | 0.23 | 0.17 | 0.31 * | MW (* d0 vs. control, p < 0.001). |
| d90 | 0.28 | 0.24 | 0.56 * # | MW (# d90 vs. d0, p = 0.05. * p < 0.05 vs. cont). |
| Se/Ag ratio | Median | 25% | 75% | H |
| Control | 22.3 | 12.26 | 37.7 | H = 6.25, p = 0.044 |
| d0 | 7.16 | 4.12 | 20.5 | MW (* d0 vs. control, p = 0.04). |
| d90 | 11 | 6.8 | 18.2 * | MW (* d90 vs. control, p = 0.032). |
| Se/Al ratio | Median | 25% | 75% | H |
| cont | 0.26 | 0.1 | 0.39 | H = 3.76, p = 0.15, n.s. |
| d0 | 0.14 | 0.07 | 0.29 | |
| d90 | 0.34 | 0.19 | 0.36 # | MW (# d90 vs. d0, p = 0.05). |
| Se/Pb | Median | 25% | 75% | H |
| Control | 4.57 | 1,79 | 9.1 | H = 1.12, p = 0.57, n.s |
| d0 | 3.81 | 2.2 | 6.9 | |
| d90 | 5.72 | 3.76 | 8.24 | |
| Mo/Hg++ | Median | 25% | 75% | H |
| Control | 0.018 | 0.011 | 0.03 | H = 13.51, p = 0.001 |
| d0 | 0.0089 | 0.0067 | 0.011 * | MW or Dunn’s, * d0 vs. Cont, p = 0.001 |
| d90 | 0.026 | 0.011 | 0.112 * # | MW or Dunn’s, # d90 vs. d0, p < 0.001, * p < 0.05 vs. control |
| Na+/K+ | Median | 25% | 75% | H |
| Control | 3.57 | 4.46 | 0.94 | H = 2.59, p = 0.2, n.s. |
| d0 | 7.74 | 16.3 | 0.82 * | MW (* p < 0.05, d0 vs. control). |
| d90 | 8 | 13.6 | 0.79 | MW (d90 vs. control, p = 0.065, n.s). |
| * p < 0.05 vs. control | # p < 0.05 d90 vs. d0 |
Control: controls without dental materials and non-supplemented (n = 21); d0: patients with long-term titanium implants and dental amalgam fillings restorations (d0, n = 16); d90: patients with long-term titanium implants and dental amalgams after 90 days of supplementation (n = 16); n.s: not significant effect, p > 0.05; * p < 0.05 vs. Control; # p < 0.05 d90 vs. d0.
4.6. Correlations between Selenium (Se) and Heavy Metals Ratios after 90 Days of Nutritional Supplementation
The r Spearman correlations between selenium and heavy metal ratios are shown in Table S1. For example, there was a strong correlation between the Se/Hg++ (d90) ratio and Se levels after 90 days of supplementation [Se (d90), r = −0.76, p = 0.004] as well as with Mo/Hg++ (d90) ratio after 90 days (d90, r = 0.6, p = 0.02). Two outlier values were excluded for statistical analysis herein (Table S1, see Supplementary Materials). Table S2 showed other correlations between heavy metals and oligoelements (see Supplementary Materials).
5. Discussion
This section discusses the effects of dental amalgam restoration in mercury reduction in patients with long-term titanium implants and dental amalgam restorations using carbon active (nasal filters) and long-term algae and aminoazuphrates supplementation.
The exposure derived from amalgam fillings exceeds that from food, air, or beverages. Chronic nutritional supplementation contributes to preventing mercury release peaks caused by dental amalgam restoration (replacement by biocompatible materials like Bisphenol A free composites). A study of 12 patients demonstrated that the long-term presence of dental amalgam (at least five years) did not result in any remarkable changes in mercury or tin levels in the pulp tissue after comparing 12 restored amalgams and 12 non-restored patients. However, elevated blood mercury levels were observed even five years after the placement of the restoration [32]. These data suggest that mercury release is important even after complete dental amalgam restoration with composites, because five years after its restoration, mercury is still present in the blood [32]. Bergerow et al. reported that within 12 months after removing dental amalgam fillings (restoration by composites), patients showed substantially lower urinary mercury levels [33]. In the present study, the period of supplementation was shorter (three months: 90 days), which minimized mercury release by using carbon active (nasal filters) during dental restorations [29]. The synergic algae and aminoazuphrates treatment contributed to activating the detoxification because the mercury reached peaks shortly at 24 h after replacement with composites until 3–7 days later [34].
5.1. Detoxification of Heavy Metals in Patients with Long-Term Amalgam Fillings and Titanium Dental Implants
We determined that chronic nutritional Chlorella and Fucus algae extract supplementation in conjunction with aminosulphurates lowered certain heavy metal levels in patients with long-term titanium implants and dental amalgams restoration using activated carbon active nasal filter as well as the nutraceuticals. Preclinical findings suggest a role of Chlorella vulgaris as a heavy chelator in preventing toxicity of certain xenobiotics and accelerating dioxin excretion in rats [25,35]. The mercury and tin reduction after 90 days in patients agreed with enhanced heavy metal removal by Chlorella sp [36,37,38]. However, the exact mechanism by which chronic algae consumption removes heavy metals has not been tested yet in humans. Our aim is to develop a clinical and practical protocol to chelate heavy metals with a mixture of bioactive nutraceuticals such as algae extracts and aminosulphurates that could act independently of signaling pathways involved in detoxification.
Supplementation with Chlorella sp promoted detoxification of heterocyclic amines (carcinogenic chemical) in six young Korean adults [39]. This randomized, double blind, placebo-controlled crossover study was performed in six female supplemented-patients; the nutritional period of three months in our study was longer than in the Korean study. Our findings also reflected enhanced removal of certain heavy metals, including lead (a metal of environmental exposure). Our patients’ Hg++, Sn, and Pb accumulations were strongly reduced after 90 days of consecutive nutritional supplementation as compared with basal levels (before any supplementation). Interestingly, mercury and Sn levels reductions were observed after 90 days as compared with untreated controls (without dental materials and non-supplemented).
5.2. SOD-1 Activity in Patients with Long-Term Dental Titanium Implants and Amalgams Restorations
Although it was not possible to elucidate the exact nutraceutical involved in SOD-1 activation here, we must consider that SOD-1 activation decreased after 90 days as compared with their basal levels (d0, before any supplementation). Conversely, higher SOD-1 activity was observed after long-term supplementation (day 90) compared with untreated controls; this suggests algae and aminoazuphates treatment may activate SOD-1. In addition, increased Mn++ levels could suggest enhanced antioxidant responses after 90 days of supplementation. In fact, Ala16Val MnSOD-2 polymorphism has been described in cells exposed to methylmercury [40]. We have previously observed higher SOD-1 activation in women with long-term dental amalgams only (without titanium dental alloys) as compared with controls (without dental materials) [9]. Our clinical findings were in consonance with the detoxification induced by Ag nanoparticles through inducing SOD, peroxidase, catalase, and glutamine synthetase enzymatic activities [41,42]. The silver (Ag) reduction after 90 days of supplementation as compared with the patients’ baseline levels (before any supplementation) agreed with the enhanced removal of heavy metals. However, silver levels after 90 days did not differ with controls.
Heavy metals detox requires (i) a healthy gut microbiome state [20], (ii) the induced-activation of endogenous hepatic I-II-enzyme, which can be activated by phytonaturals in these formulations [43], and (iii) the chelation and excretion of these heavy metals [44]. Steps (i) and (ii) are activated by natural products from these formulations. The ERGYLIXIR formulation contains synergic depurative bioactive compounds from extracts such as Cinara scolymus (artichoke) [45], Raphanus niger [46], Taraxacum officinale [47], Arctium lappa (dandelion root) [48], Vaccinium macrocarpo [49], Solidago virgaurea (quercitin, afzelin) [50], Rosmarinus officinallis [51], Scolymus hispanicus [52], and Sambucus nigra (elderberry with antocianines) [53]. In addition, sulfur-rich extracts such as garlic acid (Allium sativa in the ERGYTAURINE formulation) may enhance heavy metal removal by inducing antioxidant activities [54,55,56]. Apple pectin [56] and acerole (very rich in vitamin C) also contribute to heavy metals removal [57]. In addition, Sambucus nigra (elderberry) contains antocyanines that supply 87% of the daily vitamin C levels necessary for humans [57]. Vitamins B6, B-9, and B-12, as well as Se, Zn++, and Mg++ (ERGYTAURINE formulation) [58] are necessary for certain enzymatic activities.
5.3. Possible Role of Selenium (Se) in Detox after Long-Term Chlorella CV Supplementation in Patients
Because Se levels decreased after 90 days of supplementation, we cannot exclude the possibility that selenomercurials reflect the Se-heavy metal complex formation in order to prevent mercury toxicity (or other metals) in patients with long-term dental amalgams and titanium alloys. As the Na+/K+ ratio did not differ after 90 days as compared with their basal levels (before any supplementation), we can confirm that chronic algae and aminoazuphrates supplementation are safe and non-toxic for humans. The increased Se/Hg++ ratios suggest enhanced detoxification after 90 days compared to their basal levels (before any supplementation) as well as untreated controls. Surprisingly, a toxic effect has been demonstrated in autistic children who had elevated hair selenium levels [59]. These lower Se levels observed in conjunction with the lack of effect on the Na+/K+ and Se/Pb ratios could prevent mercury accumulation at 90 consecutive days of supplementation. In fact, antagonistic interaction between selenomethionine enantiomers and methylmercury toxicity was described with Chlorella sorokiniana [60]. Mercury loss with Chlorella vulgaris is largely influenced by amino acids, cysteine being the most effective in promoting the detoxification of mercury (Hg2+)− in Chlorella sp exposed to this metal [61]. The amino acid taurine (ERGYTAURINE formulation) is derived from cysteine [62] and also contributes to heavy metal detoxification. In fact, increased oxidative stress and low systemic taurine levels were demonstrated in patients with long-term dental amalgam fillings and/or titanium alloys [63]. This indirect evidence agreed with a study in which selenocystine (SeCys2) reduced MeHg cytotoxicity in Hepatic HepG2 cells by inducing MeHg-glutathione (GSH) and also formed MeHg-cysteine (Cys) complex in vitro [64]. These indirect findings suggest that selenium contributed to detoxification in the present clinical study. Uchikawa et al. (2011) described the enhanced removal of tissue methylmercury in (BP) Parachlorella beijerinckii-fed mice; this continuous BP intake (10%) accelerated MeHg excretion and subsequently decreased tissue mercury accumulation by inducing the GSH metabolism [65].
Other metals such as Pb, Cd, and U that are associated with occupational exposure were significantly decreased after three consecutive months of supplementation compared with their basal levels (before any supplementation) without affecting the untreated controls (without dental materials). The biosorption of Pb2+ and Cd2+ was detected using a fixed bed column analysis with immobilized Chlorella algae biomass [66]. Pb levels decreased after 90 days of supplementation, agreeing with the 56% Pb reduction at four days of algae Chlorella sp supplementation, 69% at eight days, and 77% at 12 days of treatment [26]. Although U levels were within the normal detection range in our patients, their decrease after 90 days of supplementation was crucial. As selenium-enriched spirulina formulation reduces the development radiation that is pneumonitis-induced [67], the lower U levels after chronic algae supplementation are important from a clinical view point. In addition, a glutathione-dependent detoxification pathway has been described in Chlorella algae exposed to U [68,69,70].
5.4. The Nutritional Supplementation after 90 Days Prevented Certain Oligoelements Deficit in Patients with Long-Term Titanium Implants and Dental Amalgam Restorations
These polyphenols from Azorean brown algae (Fucus spiralis or Fucus vesiculosus in GREEN-FLOR formulation) may enhance heavy metal removal in patients with long-term dental fillings and titanium alloys. In fact, the marine algae Ulva lactuca and Fucus vesiculosus can sequester Cd and Cu++ [70], which explained the induced-detoxification here. The phlorotannins have potential impact on public health, particularly in hypertensive patients [71,72]. The in situ determination of trace elements in fucoids by field-portable-X-ray fluorescence (FP-XRF) provides a rapid monitoring environmental contamination [73]. Increased mercury levels can provoke hypertension, and Se may exhibit a protective effect against cardiovascular disease [6]. Long-term nutritional supplementation could increase germanium (Ge) levels in patients with long-term dental amalgam fillings and titanium implants, seemingly by reflecting antihypertensive effects. However, a direct causal relationship between antihypertensive effects and Cr and Ge elevations was not conclusive in the present study. The Sn-Se correlation observed in conjunction with Ge, Li, Cr, P and I elevations after 90 days of supplementation could be explained by the high oligoelement content (10–15%) in the supplement, resulting from its marine origin [74]. The detoxification of Hg++ and Cd levels here agreed with the enhanced Hg++, Cd++, and Pb removal by Fucus from contaminated salt waters exposed to heavy metals for seven days [74]. The Fucus sp algae is also traditionally used to prevent obesity or gastrointestinal diseases. As Fucus vesiculosus extracts reduced the blood glucose peak in mice fed with a normal diet [75], the possibility that chromium Cr and Ge elevation could contribute to these antihypertensive effects should not be excluded here. These oligoelements also increased after 90 days of supplementation as compared with untreated controls. The increased Li levels suggest a better regulation of gut microbiota after treatment with these formulations, since the host serotonine biosynthesis is regulated by intestinal microbiota [76]. In fact, the strong r Spearman correlation together with the Se/Li ratio and Li correlation suggest a better state of gut microbiota in treated patients at 90 days of supplementation as compared with their basal and control levels. Finally, the augmented phosphorous (P) levels described here may have been a consequence of chronic spirulina supplementation (GREEN-FLOR). Since undernourished children receiving Spirulina platensis plus Misola extract treatment have a better hematocrite that those taking Misola alone [77], the chronic algae-supplementation could prevent iron deficit. These synergic supplementations contribute to heavy metal removal in these patients. Moreover, increased systemic malondialdehyde levels and lower Mo/Co and Mo/Fe2+ ratios have been described in patients with long-term dental titanium implants and dental amalgams [74]. Further studies should evaluate detoxification pathways by which long-term supplementation Chlorella or Fucus vesiculosus treatment contribute to the removal of heavy metals in patients with long-term dental amalgam fillings and titanium implants. The absence of placebo, the non-RCT (randomised controlled trials), the size sample (pilot study), as well as the Caucasian population (Spaniards) are limitations in this study.
6. Conclusions
The aminosulphurates and Chlorella and Fucus sp algae supplementation enhanced detoxification of heavy metals by reducing Hg++, Ag, Sn, and Pb levels in patients with long-term dental amalgam filling and titanium implants. The chronic nutritional supplementation with algae extract reduced Hg++ and Sn levels in patients with long-term titanium implants and dental amalgam restorations as compared with untreated controls (without dental materials). In addition, increased Mn++, Li, Ge, Cr and lower U levels, and decreased Se levels were observed after 90 days of supplementation as compared to their basal levels (before any supplementation). These findings suggest that these nutraceuticals promote beneficial effects in patients. The safety of long-term algae and aminoazuphrates supplementation were confirmed by the lack of effect in Ka+/K+ and Se/Pb ratios after 90 days compared to their basal levels (before any supplementation) and untreated controls. The SOD-1 activity could explain antioxidant and enhanced detoxification of certain heavy metals by nutritional supplementation in the present study.
Acknowledgments
We thank Laboratorios Nutergia (Basque Country) the support of this research project to Jose Joaquin Merino. We also thank InspiraHealth® (Barcelona) for the supplying of nasal filters. GREEN-FLOR (Nutergia), ERGYLIXIR (Nutergia), ERGYTAURINE (Nutergia) were supplied by Nutergia Laboratories. We also thank all enrolled patients from CIROM (Murcia, Spain) in the present study. The principal researcher of this project Jose Joaquin Merino thanks the support of Nutergia laboratories and CIROM Clinic (Murcia, Spain). IP Research project: ¨Detoxification of heavy metals by long-term algae and aminoazuphrate supplementation in patients with long-term dental amalgam fillings and titanium implants to Jose Joaquin Merino.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/8/4/101/s1, Table S1: correlations between Se, Mo and heavy metal ratios, Table S2: correlations between heavy metals and oligoelements (r Spearman).
Author Contributions
J.J.M., M.E.C.-M.: writing the manuscript; M.E.C.-M., J.M.P.-I.: performed the samples collection and clinical data; J.J.M., J.M.P.-I., A.T.G., M.E.C.-M.: statistical analysis and experimental design; J.J.M., J.M.P.-I., A.T.G., M.E.C.-M. planned and supervised and the final revision.
Funding
Funds # 20151602 from CIROM (Murcia, Spain), Article processing charge (APC) supported by Nutergia Laboratories (San Sebastian).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dierickx P.J. In vitro interaction of organic mercury compounds with soluble glutathione s-transferases from rat liver. Pharmacol. Res. Commun. 1985;17:489–500. doi: 10.1016/0031-6989(85)90084-0. [DOI] [PubMed] [Google Scholar]
- 2.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazúr M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Farina M., Aschner M., Rocha J.B.T. Oxidative stress in MeHg induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011;256:405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyaeva E.A., Sokolova T.V., Emelyanova L.V., Zakharova I.O. Mitochondrial Electron Transport Chain in Heavy Metal-Induced Neurotoxicity: Effects of Cadmium, Mercury, and Copper. Sci. J. 2012;2012:1–14. doi: 10.1100/2012/136063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu X.F., Eccles K.M., Chan H.M. High selenium exposure lowers the odds ratios for hypertension, stroke, and myocardial infarction associated with mercury exposure among Inuit in Canada. Environ. Int. 2017;102:200–206. doi: 10.1016/j.envint.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Yoo J., II, Ha Y.-C., Lee Y.-K., Koo K.-H. High levels of heavy metals increases the prevalence of sarcopenia in the Ederly population. J. Bone Metab. 2016;23:101–109. doi: 10.11005/jbm.2016.23.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joska L., Fojt J., Cvrcek L., Březina V. Properties of titanium-alloyed DLC layers for medical applications. Biomatter. 2014;4:e29505. doi: 10.4161/biom.29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puchyr R.F., Bass D.A., Gajewski R., Calvin M., Marquardt W., Urek K., Druyan M.E., Quig D. Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS) Biol. Elem. Res. 1998;62:167–182. doi: 10.1007/BF02783969. [DOI] [PubMed] [Google Scholar]
- 9.Cabaña-Muñoz M.E., Parmigiani-Izquierdo J.M., Bravo-González L.A., Kyung H.M., Merino J.J. Increased Zn/Glutathione Levels and Higher Superoxide Dismutase-1 Activity as Biomarkers of Oxidative Stress in Women with Long-Term Dental Amalgam Fillings: Correlation between Mercury/Aluminium Levels (in Hair) and Antioxidant Systems in Plasma. PLoS ONE. 2015;10:e0126339. doi: 10.1371/journal.pone.0126339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somers E.C., Ganser M.A., Warren J.S., Basu N., Wang L., Zick S.M., Park S.K. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Perspect. 2015;123:792–798. doi: 10.1289/ehp.1408751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López E., Arce C., Oset-Gasque M., Cañadas S., González M. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Cobbina S.J., Chen Y., Zhou Z., Wu X., Zhao T., Zhang Z., Feng W., Wang W., Li Q., Wu X., et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015;294:109–120. doi: 10.1016/j.jhazmat.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Soetan K.O., Olaiya C.O., Otewole O.E. The importance of mineral elements for humans, domestic animals and plants. A review. Afr. J. Food Sci. 2010;4:200–222. [Google Scholar]
- 14.Fattoretti P., Bertoni-Freddari C., Balietti M., Giorgetti B., Solazzi M., Zatta P., Bertoni-Freddari C. Chronic Aluminum Administration to Old Rats Results in Increased Levels of Brain Metal Ions and Enlarged Hippocampal Mossy Fibers. Ann. N. Y. Acad. Sci. 2004;1019:44–47. doi: 10.1196/annals.1297.010. [DOI] [PubMed] [Google Scholar]
- 15.Vig E.K., Hu H. Lead toxicity in older adults. J. Am. Geriatr. Soc. 2000;48:1501–1506. doi: 10.1111/jgs.2000.48.11.1501. [DOI] [PubMed] [Google Scholar]
- 16.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travieso L., Cañizares R.O., Borja R., Benitez F., Domínguez A.R., Dupeyrón R., Valiente V. Heavy metals removal by microalgae. Bull. Environ. Contam. Toxicol. 1999;62:144–151. doi: 10.1007/s001289900853. [DOI] [PubMed] [Google Scholar]
- 18.Waisberg M., Joseph P., Hale B., Beyersmann D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology. 2003;192:95–117. doi: 10.1016/S0300-483X(03)00305-6. [DOI] [PubMed] [Google Scholar]
- 19.Pacyna J.M., Pacyna E.G., Aas W. Changes of emissions and atmospheric deposition of mercury, lead, and cadmium. Atmos. Environ. 2009;43:117–127. doi: 10.1016/j.atmosenv.2008.09.066. [DOI] [Google Scholar]
- 20.Monachese M., Burton J.P., Reid G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodges R.E., Minich D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015;2015:1–23. doi: 10.1155/2015/760689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jervis L., Rees-Naesborg R., Brown M. Biochemical responses of the marine macroalgae Ulva lactuca and Fucus vesiculosus to cadmium and copper-from sequestration to oxidative stress. Biochem. Soc. Trans. 1997;25:63. doi: 10.1042/bst025063s. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Bassat D., Mayer A.M. Volatilization of mercury by algae. Pshysiol. Plant. 1975;33:128–132. doi: 10.1111/j.1399-3054.1975.tb03779.x. [DOI] [Google Scholar]
- 24.Hamed S.M., Zinta G., Klöck G., Asard H., Selim S., AbdelGawad H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017;140:256–263. doi: 10.1016/j.ecoenv.2017.02.055. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.H., Hwang Y.K., Lee Y.W., Yun J.Y., Hwang J.M., Yoo J.D. Effect of Chlorella diet supplementation on blood and urine cadmium levels in cadmium poisoned rats. J. Biomed. Lab. Sci. 2003;9:133–137. [Google Scholar]
- 26.Cantu V., Garza-González M.T., de la Rosa J.R., Loredo-Medrano J.A. Biosorption of Pb2+ and Cd2+ in a fixed bed column with immobilised Chorella sp. biomass. J. Nutr. 1999;129:1731–1736. doi: 10.2166/wst.2008.451. [DOI] [PubMed] [Google Scholar]
- 27.Castro L., Blázquez M.L., González F., Muñoz J.A., Ballester A. Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017;598:856–866. doi: 10.1016/j.scitotenv.2017.04.138. [DOI] [PubMed] [Google Scholar]
- 28.Isuru W., Se-Kwon K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs. 2010;8:1080–1093. doi: 10.3390/md8041080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.APA. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC, USA: 2006. [Google Scholar]
- 30.Cabaña-Muñoz M.E., Parmigiani-Izquierdo J.M., Merino J.J. Safe renoval of dental amalgams by using nasal filtres and phytoteraphy. IJSR Int. J. Sci. Res. 2015;4:2391–2395. [Google Scholar]
- 31.Salvi G.E., Lindhe J., Lang N.P. Examination of patients with periodontal disease. In: Lindh J., Lan N.P., Karring T., editors. Clinical Periodontology and Implants Dentistry. 5th ed. Wiley; Oxford, UK: 2008. pp. 573–586. [Google Scholar]
- 32.Saghiri M.A., Banava S., Sabzian M.A., Gutmann J.L., Asatourian A., Ramezani G.H., García-Godoy F., Sheibani N. Correlation between long-term in vivo amalgam restorations and the presence of heavy elements in the dental pulp. J. Elem. Med. Biol. 2014;28:200–204. doi: 10.1016/j.jtemb.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Begerow J., Zander D., Freier I., Dunemann L. Long-term mercury excretion in urine after removal of amalgam fillings. Int. Arch. Occup. Environ. Health. 1994;66:209–212. doi: 10.1007/BF00380782. [DOI] [PubMed] [Google Scholar]
- 34.Kremers L., Halbach S., Willruth H., Mehl A., Welzl G., Wack F.-X., Greim H., Wack F., Hickel R. Effect of rubber dam on mercury exposure during amalgam removal. Eur. J. Oral Sci. 1999;107:202–207. doi: 10.1046/j.0909-8836.1999.eos1070307.x. [DOI] [PubMed] [Google Scholar]
- 35.Morita K., Matsueda T., Iida T., Hasegawa T. Chlorella Accelerates Dioxin Excretion in Rats. J. Nutr. 1999;129:1731–1736. doi: 10.1093/jn/129.9.1731. [DOI] [PubMed] [Google Scholar]
- 36.Mahltig B., Soltmann U., Haase H. Modification of algae with zinc, copper and silver ions for usage as natural composite for antibacterial applications. Mater. Sci. Eng. C. 2013;33:979–983. doi: 10.1016/j.msec.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 37.Rai U.N., Singh N.K., Upadhyay A.K., Verma S. Chromate tolerance and accumulation in Chlorella vulgaris. A role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour. Technol. 2013;136:604–609. doi: 10.1016/j.biortech.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y., Purchase D., Jones H., Garelick H. Effects of arsenate (As5+) on growth and production of glutathione (GSH) and phytochelatins (PCS) in Chlorella vulgaris. Int. J. Phytoremediation. 2011;13:834–844. doi: 10.1080/15226514.2010.525560. [DOI] [PubMed] [Google Scholar]
- 39.Lee I., Tran M., Evans-Nguyen T., Stickle D., Kim S., Han J., Park J.Y., Yang M. Detoxification of chlorella supplement on heterocyclic amines in Korean young adults. Environ. Toxicol. Pharmacol. 2015;39:441–446. doi: 10.1016/j.etap.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Algaerve T.D., Barbisan F., Ribeiro E.E., Duarte M.M., Manica-Cattani M.F., Mostardeiro C.P., Lenz A.F., da Cruz I.B. In vitro effects of Ala16Val manganese superoxide dismutase gene polymorphism on human white blood cells exposed to methylmercury. Genet. Mol. Res. 2014;12:5133–5144. doi: 10.4238/2013.October.29.7. [DOI] [PubMed] [Google Scholar]
- 41.Mohseniazar M., Barin M., Zarredar H., Alizadeh S., Shanehbandi D. Potential of Microalgae and Lactobacilli in Biosynthesis of Silver Nanoparticles. Bioimpacts. 2011;1:149–152. doi: 10.5681/bi.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian H., Zhu K., Lu H., Lavoie M., Chen S., Zhou Z., Deng Z., Chen J., Fu Z. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses. Sci. Total Environ. 2016;572:1213–1221. doi: 10.1016/j.scitotenv.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 43.Hinson J.A., Forkert P.G. Phase II enzymes and bioactivation. Can. J. Physiol. Pharmacol. 1995;73:1407–1413. doi: 10.1139/y95-196. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Bassat D., Mayer A.M. Reduction of mercury chloride by Chlorella: Evidence for a reducing agent. Physiol. Plant. 1977;40:157–162. doi: 10.1111/j.1399-3054.1977.tb04049.x. [DOI] [Google Scholar]
- 45.Walker A.F., Middleton R.W., Petrowicz O. Artichoke leaf extract reduces symptoms of irritable bowel síndrome in a post-marketing surveillance study. Phytother. Res. 2001;15:58–61. doi: 10.1002/1099-1573(200102)15:1<58::AID-PTR805>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 46.N’jai A.U., Kemp M.Q., Metzger B.T., Hanlon P.R., Robbins M., Czuyprynski C., Barnes D.M. Spanish Black Radish (Raphanus sativus L. var niger) diet enhances clearance of DMBA and diminishes toxic effects on bone marrow progenitor cells. Nutr. Cancer. 2012;64:1038–1048. doi: 10.1080/01635581.2012.714831. [DOI] [PubMed] [Google Scholar]
- 47.Wei S., Wang S., Zhou Q., Zhan J., Ma L., Wu Z., Sun T., Prasad M.N. Potential of Tarazacum mongolium hand-mazz for accelerating phytoextration of cadmium in combination with eco-friendly amendments. J. Hazard. Mater. 2010;15:480–484. doi: 10.1016/j.jhazmat.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 48.Chan Y.S., Cheng L.N., Wu J.H., Chan E., Kwan Y.W., Lee S.M.Y., Leung G.P.H., Yu P.H., Chan S.W. A review of the pharmacological effects of Arctium lappa (burbock) Inflammapharmacology. 2010;19:245–254. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- 49.Yan X., Murphy B.T., Hammond G.B., Vinson J.A., Neto C.C. Antioxidant Activities and Antitumor Screening of Extracts from Cranberry Fruit (Vaccinium macrocarpon) J. Agric. Chem. 2002;50:5844–5849. doi: 10.1021/jf0202234. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z., Kim J.H., Jang Y.S., Lee J.Y., Lim S.S. Anti-obesity effect of Solidago virgaurea vs. Gigantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mic (C57BL/6N) Food Nutr. Res. 2017;13:1273479. doi: 10.1080/16546628.2016.1273479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lara M.S., Gutierrez J.I., Timón M., Andrés A.I. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L). as antioxidants in cooked pork patties packed in MPA. Meat Sci. 2011;88:481–488. doi: 10.1016/j.meatsci.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 52.Kirimer N., Tunalier Z., Başer K.C., Cingi I. Antispasmodic and Spasmogenic Effects of Scolymus hispanicus and Taraxasteryl Acetate on Isolated Ileum Preparation. Planta Med. 1997;63:556–558. doi: 10.1055/s-2006-957765. [DOI] [PubMed] [Google Scholar]
- 53.Viapiana A., Wesolowski M. The phenolic contents and antioxidant activities of infussions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017;72:82–87. doi: 10.1007/s11130-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawal A.O., Lawal A.F., Ologundudu A., Adeniran O.Y., Omonkhua A., Obi F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J. Toxicol. Sci. 2011;36:549–557. doi: 10.2131/jts.36.549. [DOI] [PubMed] [Google Scholar]
- 55.Yun H.M., Ban J.O., Park K.R., Lee C.K., Jeong H.S., Han S.B., Hong J.T. Potential terapeutic effects of functional active compounts from garlic. Pharmacol. Ther. 2013;142:183–195. doi: 10.1016/j.pharmthera.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Khotimchenko M., Serguschenko I., Khotimchenko Y. Lead Absorption and Excretion in Rats Given Insoluble Salts of Pectin and Alginate. Int. J. Toxicol. 2006;25:195–203. doi: 10.1080/10915810600683291. [DOI] [PubMed] [Google Scholar]
- 57.Sato Y., Uchida E., Aoki H., Hanamura T., Nagamine K., Kato H., Koizumi T., Ishigami A. Acerola (Malpica emarginarta DC) juice intake supress UVB-induced skin pigmentation in SMP30/GNL knockout hairless mice. PLoS ONE. 2017;23:e0170438. doi: 10.1371/journal.pone.0170438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dean C. The Magnesium Miracle. Ballantine books; New York, NY, USA: 2007. [Google Scholar]
- 59.El-Ansary A., Bjorklund G., Tinkow A.A., Skalny A.V. Relationship between selenium, lead, and mercury in red blood cells of Saudi austistic children. Metab. Brain Dis. 2017;32:1073–1080. doi: 10.1007/s11011-017-9996-1. [DOI] [PubMed] [Google Scholar]
- 60.Moreno F., García-Barrera T., Gómez-Jacinto V., Gómez-Ariza J.L., Garbayo-Nores I., Vilchez-Lobato C. Antagonistic interaction of selenomethionine enantiomers on methylmercury toxicity in the microalgae Chlorella sorokiniana. Metallomics. 2014;6:347. doi: 10.1039/c3mt00296a. [DOI] [PubMed] [Google Scholar]
- 61.Mohapatra D.K., Mohanty L., Mohanty R.C., Mohapatra P.K. Biotoxicity of mercury to Chlorella vulgaris as influenced by amino acids. Acta Biol. Hung. 1997;48:497–504. [PubMed] [Google Scholar]
- 62.Ripps H., Shen W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012;18:2673–2686. [PMC free article] [PubMed] [Google Scholar]
- 63.Cabaña-Muñoz M.E., Parmigiani-Izquierdo J.M., Camacho-Alonso F., Merino J.J. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. J. Clin. Med. 2019;8:86. doi: 10.3390/jcm8010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi C., Zhou X., Zhang J., Wang J., Xie H., Wu Z. Alpha lipoic acid protects against the cytotoxicity and oxidative stress induced by cadmum in HepG2 cells through regeneration of glutathione by glutathione reductase via Nrf-2/ARE signaling pathway. Environ. Toxicol. Pharmacol. 2016;45:274–281. doi: 10.1016/j.etap.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 65.Uchikawa T., Kumamoto Y., Maruyama I., Kumamoto S., Ando Y., Yasutake A. The enhanced elimination of tissue methylmercury in Parachlorella beijerinckii-fed mice. J. Toxicol. Sci. 2011;36:121–126. doi: 10.2131/jts.36.121. [DOI] [PubMed] [Google Scholar]
- 66.Kumar R.M., Frankilin J., Raj S.P. Accumulation of heavy metals (Cu, Cr, Pb and Cd) in freshwater micro algae (Chlorella sp.) J. Environ. Sci. Eng. 2013;55:371–376. [PubMed] [Google Scholar]
- 67.Bai Y., Wang D., Cui X., Yang Z., Zhu M., Zhang Z., Xia G., Gong Y. Preventive effects of selenium-enriched spiruline (SESP) on radiation pneumonitis. J. Environ. Pathol. Toxicol. Oncol. 1998;17:159–163. [PubMed] [Google Scholar]
- 68.Evseeva T.I., Maĭstrenko T.A., Geras’kin S.A. An assessment of relative contribution of DNA reparation and glutathione-dependent pathway of detoxification in response of Chlorella algae to uranium. Radiats Biol. Radioecol. 2013;53:236–245. doi: 10.7868/s0869803113020033. [DOI] [PubMed] [Google Scholar]
- 69.Horikoshi T., Nakajima A., Sakaguchi T. Update of uranium by various cell fractions of Chlorella vulgaris. Radioisotopes. 1979;28:485–488. doi: 10.3769/radioisotopes.28.8_485. [DOI] [PubMed] [Google Scholar]
- 70.Simmons D.B.D., Hayward A.R., Hutchinson T.C., Emery R.J.N. Identification and quantification of glutathione and phytochelatins from Chlorella vulgaris by RP-HPLC ESI-MS/MS and oxygen-free extraction. Anal. Bioanal. Chem. 2009;395:809–817. doi: 10.1007/s00216-009-3016-1. [DOI] [PubMed] [Google Scholar]
- 71.Paiva L., Lima E., Neto A.I., Baptista J. Angiotensin I-converting enzyme (ACE) inhibitory activity of Fucus spiralis macroalgae and influence of the extracts storage temperature—A short report. J. Pharm. Biomed. Anal. 2016;131:503–507. doi: 10.1016/j.jpba.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 72.Lopes G., Andrade P.B., Valentão P., McPhee D.J. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules. 2016;22:56. doi: 10.3390/molecules22010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turner A., Poon H., Taylor A., Brown M.T. In situ determination of trace elements in Fucus sp by field-portabel-XRF. Sci. Total Environ. 2017;593–594:227–235. doi: 10.1016/j.scitotenv.2017.03.091. [DOI] [PubMed] [Google Scholar]
- 74.Henriques B., Lopes C.B., Figueira P., Rocha L.S., Duarte A.C., Vale C., Pardal M.A., Pereira E. Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere. 2017;171:208–222. doi: 10.1016/j.chemosphere.2016.12.086. [DOI] [PubMed] [Google Scholar]
- 75.Gabbia D., Dall’Acqua S., Di Gangi I.M., Bogialli S., Caputi V., Albertoni L., Marsilio I., Paccagnella N., Carrara M., Giron M.C., et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs. 2017;15:41. doi: 10.3390/md15020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell. 2015;163:258. doi: 10.1016/j.cell.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simpore J., Kabore F., Zongo F., Dansou D., Bere A., Pignatelli S., Biondi D.M., Ruberto G., Musumeci S. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr. J. 2006;5:3. doi: 10.1186/1475-2891-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.