Abstract
Aging and senescence in plants has a major impact on agriculture, such as in crop yield, the value of ornamental crops, and the shelf life of vegetables and fruits. Senescence represents the final developmental phase of the leaf and inevitably results in the death of the organ. Still, the process is completely under the control of the plant. Plants use their protein degradation systems to maintain proteostasis and transport or salvage nutrients from senescing organs to develop reproductive parts. Herein, we present an overview of current knowledge about the main protein degradation pathways in plants during senescence: The proteasome and autophagy. Although both pathways degrade proteins, autophagy appears to prevent aging, while the proteasome functions as a positive regulator of senescence.
Keywords: aging, senescence, autophagy, proteasome, plants
1. Introduction
The growth and development of plants is governed by proteins that act developmental phase-specific to control processes like cell division, differentiation, organ growth, and cell death. The regulation of protein levels relies on the one hand on de novo synthesis and on the other on the regulated breakdown of unwanted or damaged proteins, together referred to as proteostasis. Especially when considering aging and senescence, it is of pivotal importance to remove damaged proteins to maintain cellular integrity. In animals, the accumulation of damaged proteins and the demise of proteostasis is a hallmark of aging [1]. Although aging is often associated with deterioration [2], plant cells fully control and initiate their own senescence and death over the course of the life cycle.
Wear and tear on proteins is in part imposed by the environment in which they act and the biochemical processes they control, which can result in misfolding, aggregation, or mistargeting. As a prerequisite, cells monitor the state of each protein through either chaperones or components of diverse proteolytic pathways [3,4]. Still, potentially the largest fraction of the proteins that are removed through proteostasis systems are actually undamaged. Based on the variable physiological demands of proteins during cellular processes, unwanted proteins are removed. For instance, this is required to control cell proliferation and cell differentiation, which are sequential processes that involve specific protein sets during the course of development. In addition, many proteins have a short half-life, which potentially minimizes the risk of protein damage to prevent the accumulation of aberrant and damaging proteins [5].
Protein turnover is performed mostly through the ubiquitin–proteasome system (UPS) and autophagy. Proteins targeted for degradation by the UPS are labeled with ubiquitin to enable recognition by the proteasome. Subsequently, recognized proteins are unfolded in an ATP-dependent manner and degraded by the proteasome. Whereas the UPS typically removes single proteins, autophagy removes complete protein complexes, protein aggregates, cytosol, and organelles [6]. Still, autophagy only operates inside the cytosol, while the UPS is also present in the nucleus. Considering the differences between UPS and autophagy, one would also expect different functions for both protein clearing systems during plant aging, the onset of leaf senescence, and the progression of senescence. One would expect that the proteasome mainly affects the timing and onset of senescence, while autophagy is highly relevant for bulk protein degradation during the progression of senescence.
2. Protein Degradation Pathways
The UPS and autophagy constitute two evolutionarily conserved protein degradation mechanisms in eukaryotic cells. While the proteasome uses its own protease activity to degrade target proteins, autophagy works as an intracellular vesicle trafficking system that moves cargo to specialized vacuolar compartments where digestive proteolysis of the cargo occurs through different types of proteases. Below, we briefly introduce the molecular components of the different systems in plants.
2.1. The Ubiquitin–26S Proteasome System
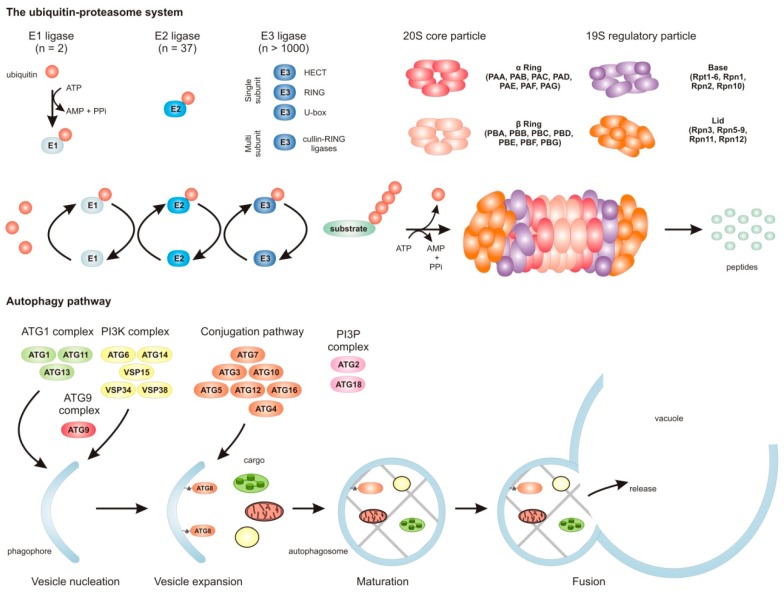
The 26S proteasome is a 2.5 MDa ATP-dependent protease complex composed of two particles, a 19S regulatory particle (RP) and a 20S core particle (CP) (Figure 1) [7]. The internal chamber of the CP houses the protease active sites [8]. The entrance to this chamber is spatially restricted to ensure the entry of only deliberately unfolded and treaded substrates. The recognition and unfolding of substrates is regulated by the RP. The base of the RP contains six AAA-ATPases (Rpt1–6) that control protein unfolding, and three non-ATPase subunits (Rpn1–2 and Rpn10). The lid of the RP consists of eight subunits (Rpn3, Rpn5–9, and Rpn11–12) and is responsible for substrate recognition and deubiquitination [1]. The 26S proteasome specifically recognizes ubiquitinated substrates (Figure 1). Ubiquitination of target proteins is highly controlled and specifically mediated by the concerted action of three enzymes [9]. The E1 activating enzyme covalently binds ubiquitin (UBQ) in an ATP-dependent manner and passes it on to an E2 UBQ conjugating enzyme. The E2 assembles with an E3 UBQ ligase that transfers the UBQ moiety to specific substrate proteins. Since plants contain more than 1000 different E3 ligases [10], it is clear that proteins can be targeted for degradation with surgical precision by the cell. Moreover, this also underlines why the UPS is suitable for the selective degradation of master regulators to guide developmental processes such as, for instance, the onset of senescence.
Figure 1.
Overview of the main cellular protein clearance mechanisms, the ubiquitin–proteasome system (UPS), and the autophagy pathway. Protein degradation by the UPS is initiated by the specific labeling of target proteins with ubiquitin. Attachment of a ubiquitin molecule requires the action of three enzymes, an ATP-dependent ubiquitin-activating enzyme (E1), a subsequent ubiquitin-conjugating enzyme (E2), and finally a ubiquitin ligase (E3) that transfers the ubiquitin from E2 to a target protein. After (poly)ubiquitination, the target protein is recognized and degraded by the 26S proteasome. Proteasomes contain a 19S regulatory particle and a catalytic active core particle (20S). The 19S regulatory particle recognizes the substrates, deubiquitinates the substrates, and unfolds them at the expense of ATP. The unfolded protein is translocated into the active chamber of the 20S particle to be degraded by the different proteases. The lower part of the figure represents the autophagy pathway. Autophagosomes are initiated by the formation of a phagophore at the outer surface of the ER through the action of the ATG1 complex, ATG9, and the PI3K complex. The growing phagophore surrounds cellular targets in either a selective or nonselective manner. Especially ATG8 plays a major role in substrate recognition for both the selective and nonselective pathways. A mature autophagosome moves toward the vacuole and fuses with it to release its cargo for proteolytic processing.
2.2. Autophagy
Autophagy (from ancient Greek, meaning self-eating) is a key intracellular trafficking and degradation pathway that is evolutionarily conserved between eukaryotes [11]. Autophagy functions as a recycling machine to preserve cellular homeostasis and is triggered upon stress and specific developmental phases such as senescence. The initiation of autophagy occurs with the formation of a phagophore (Figure 1), a membrane cisterna that eventually expands to sequester specific cargo, including portions of the cytoplasm, large protein complexes, or organelle parts. The origin of the membrane for the formation of the phagophore has long been undisclosed, but recent findings have suggested that most phagophores are formed using endoplasmic reticulum-derived membrane material [12]. As soon as the growing phagophore is sealed around the target cargo, an autophagosome is formed (Figure 1). The autophagosome matures and eventually fuses with the vacuolar membrane to deliver its cargo for degradation. In principle, two types of autophagy occur, macro- and microautophagy [13]. In macroautophagy, cytoplasmic castoff (such as organelles) are sequestered by a double membrane-bound vesicle and transported to the vacuole. In contrast, microautophagy involves the engulfment of cytoplasm by vacuolar invagination. Here, the macroautophagy pathway is discussed and described.
The formation of an autophagosome and trafficking to the vacuole relies on the activity of autophagy-related (ATG) proteins that are largely conserved between all eukaryotes [14]. The genes that encode the different ATG genes can be divided into five functional classes [15]. The first group of ATG proteins form the ATG1 kinase complex (ATG1, ATG11, ATG13), which in plants is not required for the initiation of phagophores as it is in yeast, but rather acts during autophagosome enclosure [16]. The second group of proteins forms a class III phosphatidylinositol 3-kinase (PI3K) complex (VPS15, VPS34, VPS38, ATG6, and ATG14) that seems to mark the phagophore to distinguish it from other endomembranes in plants [13,17]. ATG9 represents the third class of ATG proteins, and is a transmembrane protein that appears to cycle between endomembranes and the phagophore assembly site (PAS) to ensure nucleation of the phagophore [18]. The class four members ATG2 and ATG18 tether pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation with ATG9 in plants [19]. Expansion, autophagosome maturation, and targeting rely on two ubiquitin-like conjugation pathways, that of ATG8 and ATG12 [13]. The conjugation pathways involve the E1 ligase-like ATG7, E2 ligase-like ATG3, and E3 ligase-like ATG12–ATG5–ATG16 complex. ATG8 is a ubiquitin-like protein that is conjugated to phosphatidylethanolamine (creating ATG8-PE). ATG8-PE is found on the phagophore and autophagosome membranes, and is delivered to the vacuole together with the engulfed cargo [14]. In contrast with the previous idea that autophagy is a nonselective protein degradation pathway, it has become apparent that the ATG8 protein serves as a cargo specifier by interacting with so-called cargo-receptors to enable selective autophagy [13]. One of these cargo-receptors is Rpn10, which initiates the targeting of inactive 26S proteasomes for destruction by autophagy in plants [20]. Thus, the UPS and autophagy system are interconnected, suggesting a complex regulation to maintain cellular proteostasis during development and stress.
2.3. Proteases
Next to the central protein clearance systems, the plant genome encodes for an additional 800 proteases that process or remove a vast variety of proteins [21]. Here, we do not explicitly discuss proteases, but would like to refer to several interesting observations made in relation to senescence. Proteases can be divided into three types based on the position of the peptide bonds they act on in target proteins; Internal endopeptidases, C-terminal carboxypeptidases, or N-terminal aminopeptidases [21]. Peptidases are classified based on the catalytic mechanism they employ, resulting in six catalytic types: Serine, cysteine, threonine, aspartic, glutamic, and metalloproteases [22,23]. Protease abundance during development is highly regulated, and many accumulate in specific subcellular compartments.
Serine and cysteine proteases are especially strongly upregulated at the expression level during leaf senescence [24,25,26]. SAG12, a marker for the onset of senescence, belongs to the Papain-like cysteine proteases (PLCPs) [27]. In addition, vacuolar processing enzymes (VPEs), another class of cysteine proteases, are also highly expressed during leaf senescence in Arabidopsis [28]. Activity-based protein profiling (ABPP) to detect specific protease activities during senescence has shown that only several PLCPs show an increase in activity during senescence, while VPE activity even decreases, despite the increase in transcript abundance [26]. In addition, the activity of SAG12 increases during senescence: However, the sag12 mutant does not show a visible senescence-related phenotype, indicating that it is dispensable in senescence or acts redundantly to other proteases. Still, accumulating molecular evidence does indicate that SAG12 participates in protein degradation and N remobilization during senescence [29,30]. As autophagy is a protein trafficking system that relies on the proteolytic activity of proteases in the vacuole, it is expected that many senescence-induced proteases might be part of this pathway. In addition, several ATG genes encode proteases, with ATG4 encoding a cysteine protease that is required for the processing of ATG8. It would be interesting to determine if senescence-associated proteases cooperate with autophagy in the recycling of cellular constituents during senescence.
3. Impact of the Proteasome and Autophagy on Aging and the Onset of Senescence
As main pathways for the degradation of unwanted or misfolded proteins and dysfunctional organelles, the proteasome and autophagy pathways play important roles in the adaption of organisms during stress and ensure their survival and longevity.
The terms aging and senescence are somewhat differently maintained in human and plant biology [2]. Aging and senescence often refers to a process of deterioration in human biology, while in plant biology these terms do not reflect simply a decline in viability, but rather a distinct developmental phase that may lead to the controlled death of an organ or organism. Senescence is no longer only a hallmark of animal aging, but has recently been uncovered as a positive cellular response that triggers tissue remodeling and minimizes the damage caused by stresses [31,32]. In animals, the activity of the proteasome declines with age [1], resulting in the accumulation of misfolded proteins, which is linked to multiple age-related diseases such as Alzheimer’s, Parkinson’s, or Huntington’s disease. Indeed, the maintenance of proteasome activity with age has been suggested as a key feature in promoting the longevity of naked mole-rats compared to mice [33]. Next to the proteasome, autophagy also promotes longevity in animals [34]. Considering the conservation of both proteolytic pathways, one might expect that the proteasome and autophagy have a similar role in promoting longevity in plants.
In plants, abiotic stress and senescence result in the upregulation of ATG gene expression [35], suggesting a specific role for autophagy during these processes. It has been shown that defective autophagy (through the disruption of different ATG genes, including atg2, atg4a/4b, atg5, atg7, atg9, atg10, and atg18a) causes early senescence in Arabidopsis [36,37,38,39,40,41]. Especially under nutrient-limiting conditions (low nitrate), atg mutants display earlier leaf senescence and lower rosette biomass [42,43]. Thus, like in animals, autophagy prevents early aging and the precocious onset of senescence in plants. Recently, this was further demonstrated by the fact that constitutive overexpression of ATG5 or ATG7 results in a significantly delayed onset of leaf senescence [44]. For a long period of time, autophagy has been a synonym for nonselective bulk protein degradation: However, an increasing number of studies have demonstrated the selective autophagic-dependent degradation of cell components, including mitochondria [45], peroxisomes [46], chloroplasts [47], ribosomes [48], the proteasome [20], and the endoplasmic reticulum [49]. During senescence, the delivery of chloroplast material is in part regulated by ATG8-INTERACTING PROTEIN1 (ATI1), a transmembrane protein present in the ER and plastid membrane [50]. The loss of ATI1 causes premature senescence during stress, suggesting that coordinated removal of specific plastid proteins during stress is required to prevent a premature collapse of the complete photosynthetic apparatus. Like plastids, mitochondria are also salvaged during plant development. During senescence, ATG11 is required for mitophagy, the selective breakdown of mitochondria-resident proteins and mitochondrial vesicles [45]. Recently, a new role for ATG8 was found in the regulation of leaf senescence. A multidrug and toxic compound extrusion (MATE) transporter called ABNORMAL SHOOT3 (ABS3) was shown to promote senescence under natural and carbon deprivation conditions in Arabidopsis [51]. The senescence-promoting ABS3 pathway functions in concert with the longevity-promoting autophagy pathway to balance plant senescence and survival. ABS3 interacts with ATG8 to promote senescence and protein degradation. Thus, on the one hand, autophagy appears to clearly prevent senescence, but when senescence occurs, autophagy seems important for the controlled salvaging of organelles. This notion is especially underlined by the fact that precocious senescence in atg mutants can be prevented by blocking SA-induced cell death [36]. Autophagy counteracts instantaneous cell death by controlling NPR1-dependent salicylic acid (SA) signaling, implying that autophagy has a dual role (executioner and procrastinator) in the onset and progression of senescence to maximize the salvaging of remaining nutrients.
In contrast with the global upregulation of ATG genes during senescence, only a fraction of the proteasome subunit genes increase their expression [52]. In senescing leaves of oilseed rape and wheat, the proteasome is highly active [29,53], in contrast with the observation that proteasome activity declines with age in animals [1]. Apart from the difference in senescence-induced gene expression, the more interesting point is that knocking down/out subunit genes of the proteasome in Arabidopsis often causes a delay in the onset of senescence [54]. Thus, it seems that autophagy and the proteasome influence aging and the onset of senescence differentially in plants. Loss of the regulatory particle subunit RPN10 significantly delays the onset of senescence [54], while the overexpression of RPN5a promotes premature senescence [55]. Knockouts in other subunits are often lethal or cause pleiotropic developmental defects [8]. In addition, the use of proteasome inhibitors is able to delay the onset of senescence [56]. Importantly, the loss of autophagy causes early senescence, which has been linked to SA accumulation [36]. However, as the proteasome is removed by autophagy, a potential accumulation of proteasomes might also cause early senescence [57]. Indeed, proteasome activity is increased in atg mutants, indicating that proteasome activity might be implicated in the early senescence phenotype of autophagy mutants, still it might also represent a compensation for the decreased proteolytic activity that occurs.
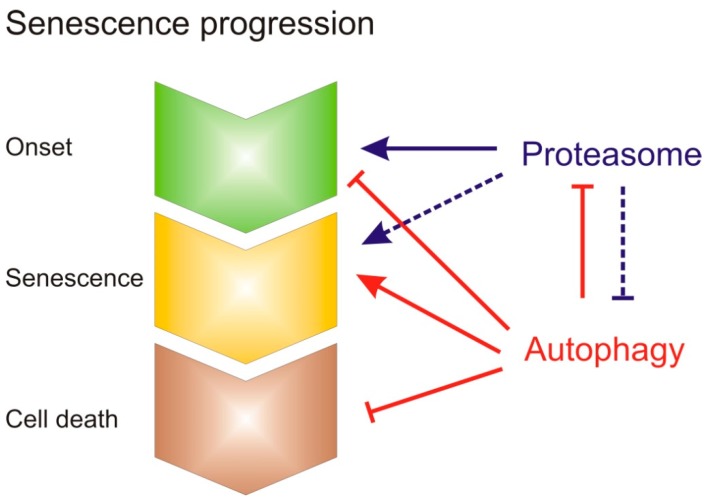
The proteasome is considered to be a requirement for the degradation of short-lived proteins, which often encompass regulatory proteins, while autophagy remains a bulk protein salvage pathway for more long-lived proteins and protein complexes [58]. Bulk protein degradation is a hallmark of leaf senescence to salvage and remobilize nitrogen and carbon compounds for other developing parts of the plant. This task clearly fits the function of the autophagy pathway. We speculate that the proteasome plays a crucial role in determining the onset of leaf senescence. The specific degradation of regulatory proteins, especially those connected to hormone signaling pathways (see below), has a major impact on the onset of senescence. Still, as autophagy seems to delay senescence, and the proteasome appears to promote senescence, these two pathways need to balance their actions during senescence to optimize nutrient recovery (Figure 2). As indicated above, the number of proteasome complexes is under the control of the autophagy pathway [20]. Interestingly, research in animals has shown that autophagy receptors, which regulate selective autophagy, undergo ubiquitination and thereby become targets of the proteasome [59]. Strikingly, a number of studies have reported that the inhibition of proteasome activity results in the compensatory activation of autophagy [60,61]. Thus, the roles of the proteasome and autophagy pathways during senescence appear to be complex and interconnected. However, we can speculate about the order of events (Figure 2): (i) First, the activity of the proteasome is temporarily enhanced during the onset of senescence to degrade potential negative regulators of senescence, including those of specific hormone pathways; (ii) proteins destined for degradation are initially mainly processed by the proteasome during the onset of senescence; and (iii) cellular dismantling during senescence overloads the proteasome, and more focus is placed on autophagy, the bulk degradation system, to degrade protein complexes and salvage organelles. In addition, autophagy prevents premature programmed cell death (PCD) during the senescence process to ensure that cell death is only initiated during the final phase of leaf senescence.
Figure 2.
Model depicting the role of the 26S proteasome and autophagy pathways during the onset and progression of senescence. The proteasome acts as a positive regulator of the onset of senescence, while autophagy appears to act as a negative regulator. During the senescence phase, the autophagy pathway is essential for nutrient recovery and the prevention of precocious cell death. Potentially, the proteasome and autophagy pathways affect each other’s functions.
4. Transcriptional Regulation of 26S Proteasome
Interestingly, inactive proteasomes are targeted for degradation by autophagy [20], indicating that the level of the 26S proteasome is adjusted to the demands of the cell. When higher proteasome activity is required, the expression of all core and most accessory factors is coordinately upregulated. In several species, key transcription factors have been identified that control the expression of the whole regulon. In yeast, the C2H2-type zinc-finger transcription factor Rpn4 has been shown to recognize a specific cis-element (proteasome-associated control element) found upstream of most proteasome subunit genes [62]. Rpn4 accumulates when proteasome activity is impaired, as it itself represents a protein target with a short half-life [63]. The subsequent activation of proteasome gene expression restores proteasome activity and leads to the degradation of Rpn4, establishing an autoregulatory system to control proteasome activity. In mammalian cells, two Nuclear factor erythroid-derived 2-related factor (Nrf) transcription factors have been shown to control the expression of the proteasome regulon [64,65]. Nrf1 and Nrf2 are basic leucine zipper TFs that were originally found to act during oxidative stress by binding to the antioxidant response element (ARE) in the promoters of their target genes [66,67]. In proteasome subunit promoters, these factors also recognize the ARE motif. Like the yeast transcriptional regulator, both Nrf proteins have short half-lives and are readily degraded. Interestingly, Nrf1 is localized to the endoplasmic reticulum membrane and protected from degradation in its inactive form [68]. Upon proteotoxic stress, Nrf1 is deglycosylated, and its N-terminus is cleaved, resulting in the release of Nrf1 and its translocation to the nucleus.
In plants, two membrane-bound NO APICAL MERISTEM/ARABIDOPSIS TRANSCRIPTION ACTIVATION FACTOR1/CUP-SHAPED COTYLEDONS2 (NAC) transcription factors have been identified as major regulators of the expression of proteasome subunit genes [69,70,71]. ANAC053 and ANAC078 are able to form a heterodimer and act redundantly to protect the plant from proteotoxic stress. Interestingly, both transcription factors are present in an inactive form in the plasma membrane. The treatment of plants with the senescence-inducing hormone abscisic acid (ABA) or a proteasome inhibitor results in the processing of ANAC053 and the release of its transcriptionally active form to the nucleus [72]. Moreover, during drought stress, anac053 mutants show a delayed senescence, indicating that the transcriptional regulation of the proteasome is vital to the onset of senescence during stress [72]. Still, an in-depth analysis of the role of both regulators of the proteasome during senescence is so far lacking. To understand if other transcription factors might also control the expression of proteasomal subunit genes in plants, we screened publicly available ChIP-Seq data. Surprisingly, hormone-related transcription factors such as ABA INSENSITIVE 3 (ABI3), ABI5, ETHYLENE INSENSITIVE 3 (EIN3), and BRASSINAZOLE-RESISTANT 2 (BZR2) do not associate with any proteasomal gene, while BZR1 has been found to interact with only 4 out of 55 genes [73,74,75,76,77]. A negative regulator of cell expansion, ILI1 BINDING BHLH 1 (IBH1), associates with 11 proteasomal genes [78]. Overexpression of IBH1 results in a severe dwarf phenotype and delayed development, including senescence [79]. IBH1 acts antagonistically to PHYTOCHROME-INTERACTING FACTOR4 (PIF4), which is another transcription factor with potentially 7 proteasomal subunit genes as direct downstream targets [78,80]. Moreover, PIFs have been shown to act as positive regulators of senescence [81], fitting with the antagonistic relation to IBH1. Although a number of transcriptional regulators of the proteasome have been identified, it is not known which specifically regulate the expression of the proteasome during the onset of senescence.
5. Post-Translational Regulation of the 26S Proteasome
Post-translational modifications (PTMs) play vital roles in cellular processes and can affect protein function, activity, structure, and stability, which increases proteome diversity, complexity, and functionality [82,83]. To date, more than 345 PTMs classified into 11 types have been identified as occurring on different proteasome subunits in yeast [84]. Compared to plants, information on proteasomal PTMs in animals and yeast is much more abundant. In addition, the roles of several proteasome PTMs have been revealed. PTMs affect all major regulatory steps, from the synthesis of the proteasome to its destruction. For instance, phosphorylation of the Rpt6 ATPase subunit affects the assembly of the 26S proteasome in yeast [85]. In addition, phosphorylation of the proteasome by protein kinase A at more than 20 subunits, including the catalytic ones, results in increased proteolytic activity [86]. In addition, phosphorylation impacts substrate affinity, 26S stability, and ATPase activity [84,85,86,87,88]. Other modifications, such as O-GlcNAcylation (O-linked N-acetylglucosamine attachment) of Rpt2, reduce proteasome activity [89], while acetylation on the α6 and β3,6,7 subunits upregulates proteasome activity in mice [90]. The ubiquitin-like domain-containing C-terminal domain phosphatase 1 (UBLCP1) dephosphorylates the Rpt1 subunit to block its ATPase activity and thereby disrupts the assembly of the 26S proteasome in human cells [91]. S-glutathiolation of the free 20Sproteasome not only increases its proteolytic activity but also promotes the entrance of oxidized proteins [92]. Interestingly, relocalization of the proteasome from the nucleolus to the cytoplasm in yeast has been linked to N-myristoylation of the proteasome subunit Rpt2 [93]. Furthermore, aged proteasomes are post-translationally tagged prior to disposal through phosphorylation of the Rpn3 subunit in mice cells [94]. These findings suggest that PTMs might regulate the stability and function of the proteasome during aging.
Until now, the role of PTMs on proteasome subunits in plants has not been well understood. Directed proteome studies of the proteasome in plants have revealed several subunits that can be post-translationally modified [53,95]. Here, we screened the Plant PTM Viewer database [83] to obtain a complete overview of currently detected PTMs on proteasome subunits (Table S1). In total, 207 PTMs have been detected on proteasomal subunits in Arabidopsis, including carbonylation, lysine acetylation, lysine 2-hydroxyisobutyrylation, lysine malonylation, lysine methylation, lysine succinylation, lysine SUMOylation, lysine ubiquitination, methionine oxidation, myristoylation, N-terminal acetylation, N-glycosylation, N-terminus proteolysis, N-terminal ubiquitination, O-GlcNAcylation, phosphorylation, reversible cysteine oxidation, S-Glutathionylation, and S-nitrosylation.
Although the biological significance of these modifications and their roles have hardly been explored, there is an overlap with the PTMs found in yeast, suggesting a possible functional conservation. Proteasome inhibitors are known to cause the ubiquitination of many proteasome subunits, which attracts RPN10, which subsequently can activate proteophagy [20]. In rice, the C2 subunit (PAF2) has been identified as a phosphorylation target of a casein kinase, causing (most likely) nuclear translocation [96].
Phosphorylation of the proteasome might be highly relevant during the onset of senescence, as (for instance) mitogen-activated kinase cascades have been shown to affect the timing of the senescence process in Arabidopsis [97]. In all cases, a better understanding of the role of PTMs in the proteasome is needed.
6. Interactions Between Phytohormones and the 26S Proteasome
Phytohormones and the 26S proteasome are major regulators during the lifespan of a plant, including in seedling development, maturation, and senescence. Inevitably, positive and negative cross-regulation between them happens. Mass spectrometric analysis has revealed ubiquitylated master regulatory proteins involved in brassinosteroid, auxin, abscisic acid, and ethylene signaling [98], but also for other hormone pathways proteasome-dependent degradation is well known [99,100]. Herein, we focus on the relationship between phytohormones, the 26S proteasome, and senescence.
6.1. Ethylene
Ethylene was one of the first hormones known to promote senescence, which it does in an age-dependent manner [101,102]. Upon senescence, the abundance of ethylene-related transcripts increases, while photosynthesis-related transcripts decline [103]. The senescence-promoting effect of ethylene requires so-called age-related changes (ARCs) [101], such as the end of the cell expansion phase, for the leaf to perceive and integrate internal and external signals to activate senescence. The proteasome is known to control both ethylene biosynthesis and signaling by regulating the turnover of key components. Both type 2 and type 3 1-aminocyclopropane-1-carboxylic acid synthase (ACS) proteins, enzymes controlling the rate-limiting steps during the biosynthesis of ethylene, are targeted for degradation by the proteasome [104,105]. The master activator of ethylene responses, ETHYLENE-INSENSITIVE 3 (EIN3), is degraded by the proteasome through the action of EIN3-BINDING F-BOX (EBF) proteins [106]. Thus, proteasome degradation of ACS proteins and EIN3 largely blocks ethylene responses. Still, ethylene signaling is also controlled by the proteasome. Upon ethylene perception, EBF1 and EBF2 are targeted for proteasomal destruction, stabilizing EIN3 [107]. EIN3 has been identified as a senescence-promoting transcription factor [108], while EBF1 and EBF2 are negative regulators of the onset of senescence [109]. Before the onset of senescence, key components of ethylene biogenesis and transcription factors that control ethylene-related genes need to be stabilized, while at the same time negative regulators of ethylene signaling need to be removed by the proteasome. The specificity of the proteasome is largely controlled by E3-ligases, suggesting a shift in E3-ligase activity or substrate recognition during the aging of the plant to activate ethylene signaling.
6.2. Abscisic Acid
ABA has a stimulating effect on the onset of senescence [110]. Interestingly, ABA has been shown to inhibit the UPS during germination at high temperatures [111]. Considering the low level of ABA during vegetative growth and the dramatic increase in senescent leaves, it is possible that ABA might control the activity of the UPS during senescence. Still, activation of ABA signaling requires the removal of protein phosphatase 2C (PP2C) proteins such as ABA-INSENSITIVE 1 (ABI1) [112]. ABA-bound PYR/PYL/RCAR ABA receptors (PYLs) can only interact with PP2C proteins in the presence of ABA. The bound PP2Cs are recognized by Plant U-box (PUB) E3 ligases and targeted for proteasomal degradation. The ABA receptors are positive regulators of senescence. Loss of PYL9 delays senescence, while overexpression promotes it [113]. A 12-fold pyl/pyr knockout has shown dramatic impacts on plant development and ABA-induced senescence [114]. The SENESCENCE-ASSOCIATED E3 UBIQUITIN LIGASE 1 (SAUL1) has been shown to prevent premature ABA-induced senescence [115]. Thus, similarly to ethylene signaling, the turnover of key components in the ABA signaling pathway can either prevent or induce senescence. On the one hand, ABA might restrict proteasome activity, which is certainly worth analyzing during senescence. On the other hand, for ABA-induced senescence, the proteasome needs to remove repressors of ABA signaling to induce downstream senescence-related genes.
6.3. Salicylic Acid
Recently, the regulation of SA signaling through selective protein turnover has been uncovered. Upon SA accumulation, NON-EXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) moves into the nucleus, where it acts as a transcriptional coactivator to modulate SA responses [116]. The Cullin-RING LIGASE 3 (CRL3) has long been implicated in controlling SA responses, but no direct interaction with NPR1 has been found [117]. Instead, the SA-binding proteins NPR3 and NPR4 turned out to act as scaffolds for CRL3 to mediate the degradation of NPR1 by the proteasome [118]. NPR4 has a high affinity for SA and only binds NPR1 when it is free of SA. NPR3 has a low affinity for SA and can only interact with NPR1 once it has incorporated SA. Through this mechanism, plants can set the level of NPR1. Arabidopsis plants with defects in SA signaling pathways, such as npr1, or plants that suffer from low SA levels due to the NahG transgene, show delayed yellowing and reduced necrosis during developmental senescence [119]. Interestingly, SA and ABA could antagonistically affect NPR1 protein levels via the CRL3/NPR3/NPR4 module [120]. Whereas SA results in the accumulation of NPR1, ABA results in the degradation of NPR1. Potentially, this mechanism is relevant during leaf senescence, as ABA could repress SA signaling and PCD during the early phases of leaf development by modulating the proteasomal degradation of NPR1.
6.4. Auxin
It is well known that the negative regulators of auxin signaling AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) proteins are degraded by the SCFTIR1 complex upon auxin treatment [121,122]. Interestingly, auxin treatment has been shown to suppress the activity of the 26S proteasome by stimulating translocation of a protein named PROTEASOME REGULATOR 1 (PTRE1) from the nucleus to the plasma membrane in Arabidopsis [123]. The rapid degradation of AUX/IAA proteins mediated by TIR1 upon auxin treatment is counterbalanced by the simultaneous translocation of PTRE1 from the nucleus to limit the activity of the 26S proteasome. Thus, auxin modulates total nuclear proteasome activity. Auxin flow in plants is controlled by PIN proteins, which show a high turnover rate. Ubiquitylation of PIN2 causes endocytosis from the plasma membrane and subsequent targeting for proteolytic processing [124]. Auxin is known to delay senescence [125,126]: However, the mechanism through which this occurs is so far not known. Potentially, the repression of the activity of the 26S proteasome by auxin might be causal to its delaying effect on senescence.
6.5. Cytokinin
The plant hormone cytokinin is, in relation to senescence, mainly known as a stay-green hormone [127,128]. Abiotic stresses such as salt or drought stress result in leaf senescence, which is preceded by a decline in the concentration of active cytokinin [129,130]. Cytokinin signaling, such as ethylene signaling, involves the stabilization of positive regulators and the degradation of negative regulators. In this case, type B ARABIDOPSIS RESPONSE REGULATORS (ARRs) act as promoting factors for cytokinin signaling, while type A ARRs act as negative regulators [131,132]. Interestingly, the loss of the proteasomal subunit RPN12 causes impaired cytokinin responses, indicating a major role of the proteasome in the regulation of cytokinin signaling [133]. During the aging of the leaf and the onset of senescence, an increase in the number of oxidized (carbonylated) proteins is observed [134]. Proteins marked with an oxidative modification are primed for proteolysis. Interestingly, cytokinin inhibits the proteasome-mediated degradation of carbonylated proteins [135]. These findings clearly demonstrate that cytokinin signaling relies on the proteasome, but also that the effect of cytokinin on the proteasome might explain how this hormone can prevent the onset of senescence.
6.6. Strigolactone
Mutants in strigolactone signaling were originally identified as late senescence mutants, with the oresara9 (ore9) mutant as a novel F-Box protein in Arabidopsis and its orthologue DWARF3 in rice [136,137]. Treatment of plants with a synthetic strigolactone has been shown to promote senescence [138]. In addition, the strigolactone biosynthesis genes MORE AXILLARY BRANCHES 1/3/4 are upregulated in senescent leaves [139]. Furthermore, the senescence-related WRKY53 transcription factor induces the expression of MAX2/ORE9 during leaf aging. ORE9 targets the central brassinosteroid (BR) signal regulators BRASSINAZOLE-RESISTANT 1 (BZR1) and BRI1-EMS-SUPPRESSOR 1 (BES1) for degradation by the proteasome [140]. As BRs repress senescence, the role of ORE9 is to relieve this inhibitory effect. Thus, leaves undergoing senescence not only enhance the level of strigolactone but also recruit the UPS to aid in the perception of the hormone and target negative regulators of senescence for degradation.
6.7. Jasmonic Acid
Jasmonic acid (JA) is known as a senescence-promoting plant hormone that is also involved in defense responses and chlorophyll breakdown [141,142,143]. In addition, JA biosynthesis is increased during senescence, as the level of JA during senescence increases at least four-fold [141]. The induction of JA signaling and biosynthesis relies on the removal of jasmonate ZIM-domain (JAZ) proteins in a UPS-dependent manner [144]. JAZ proteins act as repressors of JA responses and have been shown to function as negative regulators of senescence [145,146]. The notion that JA promotes senescence and relies on the removal of JAZ proteins to become active indicates that the proteasome is required for JA-induced leaf senescence.
6.8. Gibberellic Acid
Impaired GA biosynthesis delays senescence, while increased GA responsiveness results in precocious senescence [147]. Plant growth is restricted by DELLA proteins [148]. GA promotes growth by causing the removal of the DELLA proteins via the 26S proteasome pathway. Removal of certain DELLA proteins not only promotes growth, but also results in the activation of senescence-promoting transcription factors of the WRKY family [149,150]. Moreover, overexpression of several DELLA proteins delays senescence [147,149]. Thus, similarly to JA-promoted senescence, the removal of specific DELLA proteins by the 26S proteasome is essential to trigger leaf senescence. Still, several lines of evidence indicate that GA might also inhibit senescence [151,152]. Therefore, the effect of GA on senescence is not completely clarified: However, the removal of DELLA proteins appears to be crucial.
6.9. Brassinosteroids
Application of brassinosteroids (BRs) results in a delay of senescence through the action of downstream transcription factors [153,154,155]. The BRASSINOSTEROID INSENSITIVE2 (BIN2) kinase phosphorylates BZR1, resulting in its degradation by the proteasome [156]. BIN2 itself is removed upon BR accumulation, releasing the brakes on BR responses [157]. Not surprisingly, bin2 mutants display delayed senescence, while bzr1 mutants show accelerated senescence [153,154]. Thus, the removal of BR-related transcription factors during the aging of the leaf by the proteasome prepares the leaf for senescence [155].
Taken together, the overview presented in this section indicates that the UPS may regulate senescence through its control over multiple hormone signaling pathway components. It would therefore be highly interesting to determine which hormone-related components are removed during the aging of the leaf by the proteasome to promote the onset of leaf senescence.
7. Transcriptional Regulation of Autophagy
Initial transcriptome studies on senescing leaves revealed that several senescence-associated genes encode components of the autophagy pathway, indicating that autophagy is activated during senescence [158]. Genes such as ATG7, ATG8a, ATG8b, ATG8c, and ATG9 were among the first identified as being induced during senescence [159]. Mutations in ATG7 and ATG9 were already known to cause premature senescence [38]. This might be in part due to an altered sink–source relationship within the leaf, as nutrients are no longer efficiently managed, or it might point out that autophagy prevents the early aging of plants.
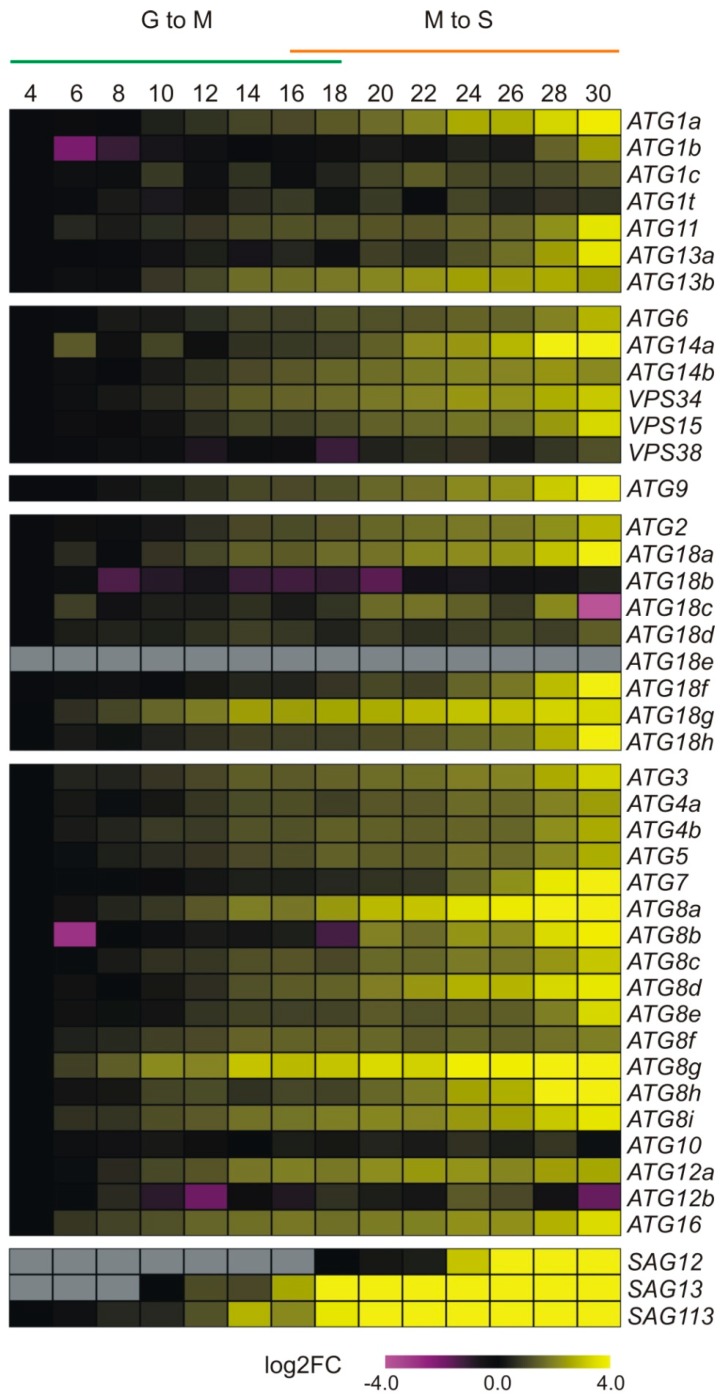
Here, we made use of an extensive RNA-SEQ dataset to monitor the expression of core ATG genes [159]. Among the genes encoding for the ATG1 complex, mainly ATG1a, ATG11, and ATG13a show a strong upregulation during the final phase of leaf senescence (Figure 3). Other genes are already upregulated during the maturation phase and are not further induced during senescence, such as ATG1c and ATG13b. Most of the genes encoding the phospatidylinositol 3-kinase (PI3K) complex show a steady increase in transcript levels from eight days onwards. Only ATG14a shows a strong additional induction during leaf senescence. The ATG9 complex also displays a clear steady increase in transcript levels with age, whereby its transcript level is further induced during senescence. Genes encoding for the phospatidylinositol 3 phosphate (PI3P) complex subunits ATG2 and ATG18 show a varying transcriptional response during leaf growth. ATG2 levels rise with age and show an additional induction during the later phases of senescence. Among the genes encoding the ATG18 subunit, only ATG18a, ATG18f, and ATG18h are also induced during the final phase of leaf senescence. The other isoforms are either not induced or reach their maximal expression at the onset of senescence (ATG18g). Finally, genes encoding conjugation pathway components follow a largely similar expressional behavior to those of the ATG18 subunit. However, several genes encoding conjugation components do not show an upregulation in expression during senescence, including the ATG12-conjugating enzyme ATG10, which is essential to the formation of autophagic bodies [40]. In addition, ATG12b is downregulated during senescence, while ATG12a transcript levels are not induced during senescence. Potentially, the ATG8-directed conjugation pathway might be more prevalent during senescence. That said, atg10 and atg12a/b double mutants display early senescence, especially upon nitrogen starvation [40,160]. Taken together, a general upregulation of ATG genes with leaf age and senescence occurs, implicating the importance of this pathway for the nutrient recovery of senescing leaves.
Figure 3.
Expression pattern of ATG genes during leaf development. Data from a previous transcriptome analysis (on the fourth rosette leaf at 2-day (d) intervals from 4 to 30 d after emergence from Arabidopsis) were used [159]. The leaf lifespan was divided into two stages, growth-to-maturation (G-to-M) and maturation-to-senescence (M-to-S). Expression data are represented as a heatmap based on the log2FC change in Fragments Per Kilobase Million (FPKM) values compared to day 4 for each transcript. Next to the ATG genes, three SAG genes are also shown as representative markers for the onset of senescence.
The strong upregulation of ATG genes during senescence, but also during nutrient starvation, abiotic stress, and biotic stress suggests the existence of demand-driven transcriptional regulation of ATG genes. Still, direct transcriptional regulators of ATG genes appear to be lacking thus far. In animals, the target of rapamycin (TOR) kinase has long been known as a negative regulator of autophagy [161]. Only recently, a similar role for TOR in the regulation of autophagy has been uncovered in plants [162]. Under nutrient-sufficient conditions, TOR is active, while Snf1-related protein kinases (SnRKs) that act as energy sensors are inactive [163]. SnRK1.1 has been shown to activate autophagy in plants, indicating that both central energy-sensing mechanisms modulate cellular autophagy activity in plants [164]. SnRK1.1 inactivates the TOR pathway through the phosphorylation of RAPTOR [165], releasing TOR repression of the autophagy pathway. Furthermore, SnRK1.1 can interact with ATG1a and ATG13a, and it enhances the phosphorylation of ATG1a, suggesting that SnRK1.1 regulates autophagy by modulating the activity of the ATG1 kinase complex [166].
Interestingly, reprogramming by TOR and SnRK kinases occurs in part through the modulation of transcription factor activity. Therefore, it is highly likely that downstream targets of these pathways are direct transcriptional regulators of autophagy. SnRK1 activates basic leucine zipper (bZIP) transcription factors through phosphorylation, such as, for instance, bZIP63, which initiates a low energy response in plants [167]. Next to that, SnRK1.1 is able to interact with the NAC transcription factor ATAF1 [168], which causes early senescence when overexpressed. In contrast, TOR activates BZR1, which represses several NAC transcription factors to repress the onset of senescence [154,155]. Interestingly, stabilization of BZR1 by TOR protects BZR1 from degradation through autophagy but not by the 26S proteasome [169]. Sugar signaling mediated by TOR therefore controls the accumulation of the BR-signaling transcription factor BZR1. The repression of autophagy by TOR might actually be in part mediated by BZR1, as it associates with at least eight ATG genes [75]. Taken together, it can be expected that the transcription factors acting downstream of TOR and SnRK kinases are direct transcriptional regulators of autophagy in plants.
8. Interaction Between Autophagy and Hormones
Autophagy is upregulated throughout maturation and senescence, while it is repressed during the growth phase of a leaf. This clear distinction nicely reflects the sink to source transition that occurs during leaf development [170]. Leaves start off as heterotrophic sink tissues that rely on nutrients and resources from other parts of the plant to develop. During the expansion phase, active photosynthesis results in an autotrophic tissue that efficiently assimilates carbon and nitrogen [2,171] and starts to function as a source tissue. Based upon the expressional behavior of the different ATG genes, one would expect that growth-related hormones repress autophagy, while stress and senescence-inducing hormones might activate autophagy.
Cytokinin is a potent inhibitor of leaf senescence through the maintenance of a sink signature in the tissues where it accumulates [172]. Cytokinin accumulation promotes the activation of extracellular invertases and hexose transporters [173]. Interestingly, genes upregulated in the atg5 mutant are also upregulated in triple cytokinin receptor mutants [35], suggesting that autophagy is needed for proper cytokinin signaling. However, both atg mutants and cytokinin receptor mutants show early senescence phenotypes. Therefore, the transcriptional overlap might also represent an early aging signature, which does not necessarily imply the interaction of both pathways. As indicated above, the growth-promoting BR hormone appears to repress autophagy during the growth phase, but loses this control with the age of the leaf as its levels decline [155]. Still, reports in tomatoes have indicated that BR can also promote autophagy in leaves [174]. Therefore, the exact interaction between BR and autophagy requires further studies to resolve when BR represses or activates autophagy during leaf development.
Interestingly, even under nutrient-sufficient conditions, atg mutants undergo early senescence [36]. A phytohormone analysis of atg5 plants has revealed that they accumulate salicylic acid and to a minor extent jasmonic acid, which are both known to promote senescence [102]. The introduction of the NahG transgene into the atg2 or atg5 mutant completely suppresses the early senescence phenotype, indicating that this phenotype is related to the accumulation of SA [36]. In contrast, the generation of double mutants with JA or ethylene signaling mutants do not block the early senescence phenotype of atg2 and atg5, indicating that these hormone signaling pathways are not required for the initiation of senescence in atg mutants. Other atg mutants (atg7 and atg18a) also display increased JA levels and increased expression of the JA-regulated PDF1.2 defensin gene under control conditions [175]. However, upon infection with Botrytis, no further induction has been observed, and the atg mutants display impaired pathogen resistance. In addition to SA and JA, a link between autophagy and ABA has also been established. A FYVE domain protein required for endosomal sorting 1 (FREE1), which acts as a phosphatidylinositol-3-phosphate-binding protein, and VSP23 have been shown to interact with ABA receptor proteins [176,177]. Thus, sensitivity toward ABA in plants is modulated by both the proteasome and autophagy pathways. It still remains to be analyzed if the activation of ABA responses during senescence depends on the activity of the proteasome or that of autophagy.
9. Perspectives
The described opposite roles of autophagy and the proteasome during leaf aging and the onset of senescence indicates that plants, in contrast with animals, differentially use their protein degradation machineries during aging. Autophagy is clearly required for delaying the onset of PCD to allow for efficient nutrient recovery of senescing leaves. Still, how autophagy is regulated during senescence at the transcriptional and post-transcriptional level is largely unknown. While autophagy prevents precocious leaf senescence, mutations in proteasome subunits result in a delay in the onset of senescence. The delay can be explained by the idea that the proteasome is required to remove negative regulators of senescence, such as, for instance, hormone signaling components. However, the specific proteins targeted by the proteasome to initiate senescence are unknown, although they might be components of the different hormone pathways, as indicated above. The identification of such targets and transcriptional and post-transcriptional regulators of the proteasome and autophagy pathways are expected to enable the targeted manipulation of senescence processes in plants. Such knowledge will allow for the breeding of crops with improved senescence properties to boost their yield in the field.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/4/267/s1, Table S1: Detected PTMs on proteasome subunits of Arabidopsis.
Funding
Haojie Wang would like to thank the China Scholarship Council for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Vilchez D., Saez I., Dillin A. The role of protein clearance mechanisms in organismal ageing and age-related diseases. Nat. Commun. 2014;5:5659. doi: 10.1038/ncomms6659. [DOI] [PubMed] [Google Scholar]
- 2.Thomas D. Senescence, ageing and death of the whole plant. New Phytol. 2013;197:696–711. doi: 10.1111/nph.12047. [DOI] [PubMed] [Google Scholar]
- 3.Esser C., Alberti S., Höhfeld J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim. Biophys. Acta. 2004;1695:171–188. doi: 10.1016/j.bbamcr.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Amm I., Sommer T., Wolf D.H. Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim. Biophys. Acta. 2014;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen K.T., Mun S.H., Lee C.S., Hwang C.S. Control of protein degradation by N-terminal acetylation and the N-end rule pathway. Exp. Mol. Med. 2018;50:91. doi: 10.1038/s12276-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber A., Peter M. Substrate recognition in selective autophagy and the ubiquitin-proteasome system. Biochim. Biophys. Acta. 2014;1843:163–181. doi: 10.1016/j.bbamcr.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Livneh I., Cohen-Kaplan V., Cohen-Rosenzweig C., Avni N., Ciechanover A. The life cycle of the 26S proteasome: from birth, through regulation and function, and onto its death. Cell Res. 2016;8:869–885. doi: 10.1038/cr.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vierstra R.D. The ubiquitin–26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009;10:385–397. doi: 10.1038/nrm2688. [DOI] [PubMed] [Google Scholar]
- 9.Chen L., Hellmann H. Plant E3 ligases: flexible enzymes in a sessile world. Mol. Plant. 2013;6:1388–1404. doi: 10.1093/mp/sst005. [DOI] [PubMed] [Google Scholar]
- 10.Shu K., Yang W. E3 ubiquitin ligases: Ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol. 2017;58:1461–1476. doi: 10.1093/pcp/pcx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Üstün S., Hafren A., Hofius D. Autophagy as a mediator of life and death in plants. Curr. Opin. Plant Biol. 2017;40:122–130. doi: 10.1016/j.pbi.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Lamb C.A., Yoshimori T., Tooze S.A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 13.Michaeli S., Galili G., Genschik P., Fernie A.R., Avin-Wittenberg T. Autophagy in plants–what’s new on the menu? Trends Plant Sci. 2016;21:134–144. doi: 10.1016/j.tplants.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Kang S., Shin K.D., Kim J.H., Chung T. Autophagy-related (ATG) 11, ATG9 and the phosphatidylinositol 3-kinase control ATG2-mediated formation of autophagosomes in Arabidopsis. Plant Cell Rep. 2018;37:653–664. doi: 10.1007/s00299-018-2258-9. [DOI] [PubMed] [Google Scholar]
- 15.Shibutani S.T., Yoshimori T. A current perspective of autophagosome biogenesis. Cell Res. 2014;24:58–68. doi: 10.1038/cr.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suttangkakul A., Li F., Chung T., Vierstra R.D. The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell. 2011;23:3761–3779. doi: 10.1105/tpc.111.090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F., Hu W., Vierstra R.D. The vacuolar protein Sorting-38 subunit of the Arabidopsis phosphatidylinositol-3-kinase complex plays critical roles in autophagy, endosome sorting, and gravitropism. Front. Plant Sci. 2018;9:781. doi: 10.3389/fpls.2018.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang X., Chung K.P., Cui Y., Lin W., Gao C., Kang B.H., Jiang L. ATG9 regulates autophagosome progression from the endoplasmic reticulum in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2017;114:E426–E435. doi: 10.1073/pnas.1616299114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotani T., Kirisako H., Koizumi M., Ohsumi Y., Nakatogawa H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. USA. 2018;115:10363–10368. doi: 10.1073/pnas.1806727115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall R.S., Li F., Gemperline D.C., Book A.J., Vierstra R.D. Autophagic degradation of the 26S proteasome is mediated by the dual ATG8/Ubiquitin receptor RPN10 in Arabidopsis. Mol. Cell. 2015;58:1053–1066. doi: 10.1016/j.molcel.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Hoorn R.A. Plant proteases: from phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- 22.López-Otín C., Bond J.S. Proteases: multifunctional enzymes in life and disease. J. Biol. Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlings N.D., Barrett A.J., Thomas P.D., Huang X., Bateman A., Finn R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018;46:D624–D632. doi: 10.1093/nar/gkx1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts I.N., Caputo C., Criado M.V., Funk C. Senescence-associated proteases in plants. Physiol. Plant. 2012;145:130–139. doi: 10.1111/j.1399-3054.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- 25.Díaz-Mendoza M., Velasco-Arroyo B., González-Melendi P., Martínez M., Díaz I. C1A cysteine protease–cystatin interactions in leaf senescence. J. Exp. Bot. 2014;65:3825–3833. doi: 10.1093/jxb/eru043. [DOI] [PubMed] [Google Scholar]
- 26.Pružinská A., Shindo T., Niessen S., Kaschani F., Tóth R., Millar A.H., van der Hoorn R.A. Major Cys protease activities are not essential for senescence in individually darkened Arabidopsis leaves. BMC Plant Biol. 2017;17:4. doi: 10.1186/s12870-016-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohman K.N., Gan S., John M.C., Amasino R.M. Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol. Plant. 2014;92:322–328. doi: 10.1111/j.1399-3054.1994.tb05343.x. [DOI] [Google Scholar]
- 28.Bhalerao R., Keskitalo J., Sterky F., Erlandsson R., Björkbacka H., Birve S.J., Karlsson J., Gardeström P., Gustafsson P., Lundeberg J. Gene expression in autumn leaves. Plant Physiol. 2003;131:430–442. doi: 10.1104/pp.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poret M., Chandrasekar B., van der Hoorn R.A., Avice J.C. Characterization of senescence-associated protease activities involved in the efficient protein remobilization during leaf senescence of winter oilseed rape. Plant Sci. 2016;246:139–153. doi: 10.1016/j.plantsci.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 30.James M., Poret M., Masclaux-Daubresse C., Marmagne A., Coquet L., Jouenne T., Chan P., Trouverie J., Etienne P. SAG12, a major cysteine protease involved in nitrogen allocation during senescence for seed production in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:2052–2063. doi: 10.1093/pcp/pcy125. [DOI] [PubMed] [Google Scholar]
- 31.Muñoz-Espín D., Cañamero M., Maraver A., Gómez-López G., Contreras J., Murillo-Cuesta S., Rodríguez-Baeza A., Varela-Nieto I., Ruberte J., Collado M., et al. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Munoz-Espin D., Serrano M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 33.Pérez V.I., Buffenstein R., Masamsetti V., Leonard S., Salmon A.B., Mele J., Andziak B., Yang T., Edrey Y., Friguet B., et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masclaux-Daubresse C., Clément G., Anne P., Routaboul J.M., Guiboileau A., Soulay F., Shirasu K., Yoshimoto K. Stitching together the multiple dimensions of autophagy using metabolomics and transcriptomics reveals impacts on metabolism, development, and plant responses to the environment in Arabidopsis. Plant Cell. 2014;26:1857–1877. doi: 10.1105/tpc.114.124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshimoto K., Jikumaru Y., Kamiya Y., Kusano M., Consonni C., Panstruga R., Ohsumi Y., Shirasu K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doelling J.H., Walker J.M., Friedman E.M., Thompson A.R., Vierstra R. The APG8/12-activating enzyme APG7 is required for proper nutrient recycling and senescence in Arabidopsis thaliana. J. Biol. Chem. 2002;277:33105–33114. doi: 10.1074/jbc.M204630200. [DOI] [PubMed] [Google Scholar]
- 38.Hanaoka H., Noda T., Shirano Y., Kato T., Hayashi H., Shibata D., Tabata S., Ohsumi Y. Leaf senescence and starvation-induced chlorosis are accelerated by the disruption of an Arabidopsis autophagy gene. Plant Physiol. 2002;129:1181–1193. doi: 10.1104/pp.011024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimoto K., Hanaoka H., Sato S., Kato T., Tabata S., Noda T., Ohsumi Y. Processing of ATG8s, ubiquitin-like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell. 2004;16:2967–2983. doi: 10.1105/tpc.104.025395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips A.R., Suttangkakul A., Vierstra R.D. The ATG12-conjugating enzyme ATG10 Is essential for autophagic vesicle formation in Arabidopsis thaliana. Genetics. 2008;178:1339–1353. doi: 10.1534/genetics.107.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong Y., Contento A.L., Bassham D.C. AtATG18a is required for the formation of autophagosomes during nutrient stress and senescence in Arabidopsis thaliana. Plant J. 2005;42:535–546. doi: 10.1111/j.1365-313X.2005.02397.x. [DOI] [PubMed] [Google Scholar]
- 42.Guiboileau A., Yoshimoto K., Soulay F., Bataillé M.P., Avice J.C., Masclaux-Daubresse C. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol. 2012;194:732–740. doi: 10.1111/j.1469-8137.2012.04084.x. [DOI] [PubMed] [Google Scholar]
- 43.Guiboileau A., Avila-Ospina L., Yoshimoto K., Soulay F., Azzopardi M., Marmagne A., Lothier J., Masclaux-Daubresse C. Physiological and metabolic consequences of autophagy deficiency for the management of nitrogen and protein resources in Arabidopsis leaves depending on nitrate availability. New Phytol. 2013;199:683–694. doi: 10.1111/nph.12307. [DOI] [PubMed] [Google Scholar]
- 44.Minina E.A., Moschou P.N., Vetukuri R.R., Sanchez-Vera V., Cardoso C., Liu Q., Elander P.H., Dalman K., Beganovic M., Lindberg Yilmaz J., et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 2018;69:1415–1432. doi: 10.1093/jxb/ery010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li F., Chung T., Vierstra R.D. AUTOPHAGY-RELATED11 plays a critical role in general autophagy-and senescence-induced mitophagy in Arabidopsis. Plant Cell. 2014;26:788–807. doi: 10.1105/tpc.113.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shibata M., Oikawa K., Yoshimoto K., Kondo M., Mano S., Yamada K., Hayashi M., Sakamoto W., Ohsumi Y., Nishimura M. Highly oxidized peroxisomes are selectively degraded via autophagy in Arabidopsis. Plant Cell. 2013;25:4967–4983. doi: 10.1105/tpc.113.116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izumi M., Ishida H., Nakamura S., Hidema J. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell. 2017;29:377–394. doi: 10.1105/tpc.16.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillwig M.S., Contento A.L., Meyer A., Ebany D., Bassham D.C., MacIntosh G.C. RNS2, a conserved member of the RNase T2 family, is necessary for ribosomal RNA decay in plants. Proc. Natl. Acad. Sci. USA. 2011;108:1093–1098. doi: 10.1073/pnas.1009809108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang X., Srivastava R., Howell S.H., Bassham D.C. Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J. 2016;85:83–95. doi: 10.1111/tpj.13091. [DOI] [PubMed] [Google Scholar]
- 50.Michaeli S., Honig A., Levanony H., Peled-Zehavi H., Galili G. Arabidopsis ATG8-INTERACTING PROTEIN1 is involved in autophagy-dependent vesicular trafficking of plastid proteins to the vacuole. Plant Cell. 2014;26:4084–4101. doi: 10.1105/tpc.114.129999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia M., Liu X., Xue H., Wu Y., Shi L., Wang R., Chen Y., Xu N., Zhao J., Shao J., et al. Noncanonical ATG8–ABS3 interaction controls senescence in plants. Nat. Plants. 2019 doi: 10.1038/s41477-018-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Y., Gan S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012;35:644–655. doi: 10.1111/j.1365-3040.2011.02442.x. [DOI] [PubMed] [Google Scholar]
- 53.Roberts I., Murray P.F., Passeron S., Barneix A.J. The activity of the 20S proteasome is maintained in detached wheat leaves during senescence in darkness. Plant Physiol Biochem. 2002;40:161–166. doi: 10.1016/S0981-9428(01)01349-3. [DOI] [Google Scholar]
- 54.Lin Y.L., Sung S.C., Tsai H.L., Yu T.T., Radjacommare R., Usharani R., Fatimababy A.S., Lin H.Y., Wang Y.Y., Fu H. The defective proteasome but not substrate recognition function is responsible for the null phenotypes of the Arabidopsis proteasome subunit RPN10. Plant Cell. 2011;23:2754–2773. doi: 10.1105/tpc.111.086702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Book A.J., Smalle J., Lee K.H., Yang P., Walker J.M., Casper S., Holmes J.H., Russo L.A., Buzzinotti Z.W., Jenik P.D., et al. The RPN5 subunit of the 26S proteasome is essential for gametogenesis, sporophyte development, and complex assembly in Arabidopsis. Plant Cell. 2009;21:460–478. doi: 10.1105/tpc.108.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pak C., van Doorn W.G. Delay of Iris flower senescence by protease inhibitors. New Phytol. 2005;165:473–480. doi: 10.1111/j.1469-8137.2004.01226.x. [DOI] [PubMed] [Google Scholar]
- 57.Havé M., Balliau T., Cottyn-Boitte B., Dérond E., Cueff G., Soulay F., Lornac A., Reichman P., Dissmeyer N., Avice J.C., et al. Increases in activity of proteasome and papain-like cysteine protease in Arabidopsis autophagy mutants: back-up compensatory effect or cell-death promoting effect? J. Exp. Bot. 2018;69:1369–1385. doi: 10.1093/jxb/erx482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lilienbaum A. Relationship between the proteasomal system and autophagy. Int. J. Biochem. Mol. Biol. 2013;4:1–26. [PMC free article] [PubMed] [Google Scholar]
- 59.Deng Z., Purtell K., Lachance V., Wold M.S., Chen S., Yue Z. Autophagy receptors and neurodegenerative diseases. Trends Cell Biol. 2017;27:491–504. doi: 10.1016/j.tcb.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Zhu K., Dunner K., Jr., McConkey D.J. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010;29:451–462. doi: 10.1038/onc.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laussmann M.A., Passante E., Düssmann H., Rauen J.A., Würstle M.L., Delgado M.E., Devocelle M., Prehn J.H., Rehm M. Proteasome inhibition can induce an autophagy-dependent apical activation of caspase-8. Cell Death Differ. 2011;18:1584–1597. doi: 10.1038/cdd.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mannhaupt G., Schnall R., Karpov V., Vetter I., Feldmann H. Rpn4p acts as a transcription factor by binding to PACE, a nonamer box found upstream of 26S proteasomal and other genes in yeast. FEBS Lett. 1999;450:27–34. doi: 10.1016/S0014-5793(99)00467-6. [DOI] [PubMed] [Google Scholar]
- 63.Ju D., Wang L., Mao X., Xie Y. Homeostatic regulation of the proteasome via an Rpn4-dependent feedback circuit. Biochem. Biophys. Res. Commun. 2004;321:51–57. doi: 10.1016/j.bbrc.2004.06.105. [DOI] [PubMed] [Google Scholar]
- 64.Radhakrishnan S.K., Lee C.S., Young P., Beskow A., Chan J.Y., Deshaies R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steffen J., Seeger M., Koch A., Krüger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 66.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myhrstad M.C., Husberg C., Murphy P., Nordström O., Blomhoff R., Moskaug J.O., Kolstø A.B. TCF11/Nrf1 overexpression increases the intracellular glutathione level and can transactivate the gamma-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim. Biophys. Acta. 2001;1517:212–219. doi: 10.1016/S0167-4781(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 68.Sha Z., Goldberg A.L. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr. Biol. 2014;24:1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yabuta Y., Osada R., Morishita T., Nishizawa-Yokoi A., Tamoi M., Maruta T., Shigeoka S. Involvement of Arabidopsis NAC transcription factor in the regulation of 20S and 26S proteasomes. Plant Sci. 2011;181:421–427. doi: 10.1016/j.plantsci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen H.-M., Schippers J.H.M., Gõni-Ramos O., Christoph M.P., Dortay H., van der Hoorn R.A., Mueller-Roeber B. An upstream regulator of the 26S proteasome modulates organ size in Arabidopsis thaliana. Plant J. 2013;74:25–36. doi: 10.1111/tpj.12097. [DOI] [PubMed] [Google Scholar]
- 71.Gladman N.P., Marshall R.S., Lee K.H., Vierstra R.D. The proteasome stress regulon is controlled by a pair of NAC transcription factors in Arabidopsis. Plant Cell. 2016;28:1279–1296. doi: 10.1105/tpc.15.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S., Seo P.J., Lee H.J., Park C.M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012;70:831–844. doi: 10.1111/j.1365-313X.2012.04932.x. [DOI] [PubMed] [Google Scholar]
- 73.Mönke G., Seifert M., Keilwagen J., Mohr M., Grosse I., Hähnel U., Junker A., Weisshaar B., Conrad U., Bäumlein. H., et al. Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res. 2012;40:8240–8254. doi: 10.1093/nar/gks594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee K.P., Piskurewicz U., Turečková V., Carat S., Chappuis R., Strnad M., Fankhauser C., Lopez-Molina L. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev. 2012;26:1984–1996. doi: 10.1101/gad.194266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu X., Li L., Zola J., Aluru M., Ye H., Foudree A., Guo H., Anderson S., Aluru S., Liu P., et al. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant J. 2011;65:634–646. doi: 10.1111/j.1365-313X.2010.04449.x. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y., Fan X.Y., Cao D.M., Tang W., He K., Zhu J.Y., He J.X., Bai M.Y., Zhu S., Oh E., et al. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Dev. Cell. 2010;19:765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang K.N., Zhong S., Weirauch M.T., Hon G., Pelizzola M., Li H., Huang S.S., Schmitz R.J., Urich M.A., Kuo D., et al. Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. Elife. 2013;2:e00675. doi: 10.7554/eLife.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhiponova M.K., Morohashi K., Vanhoutte I., Machemer-Noonan K., Revalska M., Van Montagu M., Grotewold E., Russinova E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA. 2014;111:2824–2829. doi: 10.1073/pnas.1400203111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang L.Y., Bai M.Y., Wu J., Zhu J.Y., Wang H., Zhang Z., Wang W., Sun Y., Zhao J., Sun X., et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell. 2009;21:3767–3780. doi: 10.1105/tpc.109.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oh E., Zhu J.Y., Wang Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012;14:802–809. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakuraba Y., Jeong J., Kang M.Y., Kim J., Paek N.C., Choi G. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat. Commun. 2014;14 doi: 10.1038/ncomms5636. [DOI] [PubMed] [Google Scholar]
- 82.Friso G., van Wijk K.J. Posttranslational protein modifications in plant metabolism. Plant Physiol. 2015;2015 169:1469–1487. doi: 10.1104/pp.15.01378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willems P., Horne A., Goormachtig S., De Smet I., Botzki A., Van Breusegem F., Gevaert K. The Plant PTM Viewer, a central resource exploring plant protein modifications. From site-seeing to protein function. bioRxiv. 2018;1:415802. doi: 10.1111/tpj.14345. [DOI] [PubMed] [Google Scholar]
- 84.Hirano H., Kimura Y., Kimura A. Biological significance of co-and post-translational modifications of the yeast 26S proteasome. J. Proteomics. 2016;134:37–46. doi: 10.1016/j.jprot.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 85.Satoh K., Sasajima H., Nyoumura K.I., Yokosawa H., Sawada H. Assembly of the 26S proteasome is regulated by phosphorylation of the p45/Rpt6 ATPase subunit. Biochemistry. 2001;40:314–319. doi: 10.1021/bi001815n. [DOI] [PubMed] [Google Scholar]
- 86.Lu H., Zong C., Wang Y., Young G.W., Deng N., Souda P., Li X., Whitelegge J., Drews O., Yang P.Y., et al. Revealing the dynamics of the 20 S proteasome phosphoproteome a combined CID and electron transfer dissociation approach. Mol. Cell Proteomics. 2008;7:2073–2089. doi: 10.1074/mcp.M800064-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iwafune Y., Kawasaki H., Hirano H. Electrophoretic analysis of phosphorylation of the yeast 20S proteasome. Electrophoresis. 2002;23:329–338. doi: 10.1002/1522-2683(200202)23:2<329::AID-ELPS329>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 88.Suchira B.O., Stratford F.L., Broadfoot K.I., Mason G.G., Rivett A.J. Phosphorylation of 20S proteasome alpha subunit C8 (α7) stabilizes the 26S proteasome and plays a role in the regulation of proteasome complexes by γ-interferon. Biochem. J. 2004;378:177–184. doi: 10.1042/BJ20031122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang F., Su K., Yang X., Bowe D.B., Paterson A.J., Kudlow J.E. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/S0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 90.Wang D., Fang C., Zong N.C., Liem D.A., Cadeiras M., Scruggs S.B., Yu H., Kim A.K., Yang P., Deng M., et al. Regulation of acetylation restores proteolytic function of diseased myocardium in mouse and human. Mol. Cell Proteomics. 2013;12:3793–3802. doi: 10.1074/mcp.M113.028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun S., Liu S., Zhang Z., Zeng W., Sun C., Tao T., Lin X., Feng X.H. Phosphatase UBLCP1 controls proteasome assembly. Open Biol. 2017;7:170042. doi: 10.1098/rsob.170042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silva G.M., Netto L.E., Simoes V., Santos L.F., Gozzo F.C., Demasi M.A., Oliveira C.L., Bicev R.N., Klitzke C.F., Sogayar M.C., et al. Redox control of 20S proteasome gating. Antioxid. Redox Signal. 2012;16:1183–1194. doi: 10.1089/ars.2011.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kimura A., Kurata Y., Nakabayashi J., Kagawa H., Hirano H. N-Myristoylation of the Rpt2 subunit of the yeast 26S proteasome is implicated in the subcellular compartment-specific protein quality control system. J. Proteomics. 2016;130:33–41. doi: 10.1016/j.jprot.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 94.Tomita T., Hirayama S., Sakurai Y., Ohte Y., Yoshihara H., Saeki Y., Hamazaki J., Murata S. Specific modification of aged proteasomes revealed by tag-exchangeable knock-in mice. Mol. Cell Biol. 2018;39 doi: 10.1128/MCB.00426-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shibahara T., Kawasaki H., Hirano H. Identification of the 19S regulatory particle subunits from the rice 26S proteasome. Eur. J. Biochem. 2002;269:1474–1483. doi: 10.1046/j.1432-1033.2002.02792.x. [DOI] [PubMed] [Google Scholar]
- 96.Umeda M., Manabe Y., Uchimiya H. Phosphorylation of the C2 subunit of the proteasome in rice (Oryza sativa L.) FEBS Lett. 1997;403:313–317. doi: 10.1016/S0014-5793(97)00073-2. [DOI] [PubMed] [Google Scholar]
- 97.Zhou C., Cai Z., Guo Y., Gan S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol. 2009;150:167–177. doi: 10.1104/pp.108.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aguilar-Hernández V., Kim D.Y., Stankey R.J., Scalf M., Smith L.M., Vierstra R.D. Mass spectrometric analyses reveal a central role for ubiquitylation in remodeling the Arabidopsis proteome during photomorphogenesis. Mol. Plant. 2017;10:846–865. doi: 10.1016/j.molp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santner A., Estelle M. The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 2010;61:1029–1040. doi: 10.1111/j.1365-313X.2010.04112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim H.J., Chiang Y.H., Kieber J.J., Schaller G.E. SCFKMD controls cytokinin signaling by regulating the degradation of type-B response regulators. Proc. Natl. Acad. Sci. USA. 2013;110:10028–10033. doi: 10.1073/pnas.1300403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jing H.C., Schippers J.H.M., Hille J., Dijkwel P.P. Ethylene-induced leaf senescence depends on age-related changes and OLD genes in Arabidopsis. J. Exp. Bot. 2005;56:2915–2923. doi: 10.1093/jxb/eri287. [DOI] [PubMed] [Google Scholar]
- 102.Schippers J.H.M., Jing H.C., Hille J., Dijkwel P.P. Developmental and hormonal control of leaf senescence. In: Gan S., editor. Senescence Processes in Plants. Volume 26. Blackwell Publishing; Oxford, UK: 2007. pp. 145–170. [Google Scholar]
- 103.Breeze E., Harrison E., McHattie S., Hughes L., Hickman R., Hill C., Kiddle S., Kim Y.S., Penfold C.A., Jenkins D., et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell. 2011;23:873–894. doi: 10.1105/tpc.111.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshida H., Nagata M., Saito K., Wang K.L., Ecker J.R. Arabidopsis ETO1 specifically interacts with and negatively regulates type 2 1-aminocyclopropane-1-carboxylate synthases. BMC Plant Biol. 2005;5 doi: 10.1186/1471-2229-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lyzenga W.J., Booth J.K., Stone S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012;71:23–34. doi: 10.1111/j.1365-313X.2012.04965.x. [DOI] [PubMed] [Google Scholar]
- 106.Potuschak T., Lechner E., Parmentier Y., Yanagisawa S., Grava S., Koncz C., Genschik P. EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell. 2003;115:679–689. doi: 10.1016/S0092-8674(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 107.An F., Zhao Q., Ji Y., Li W., Jiang Z., Yu X., Zhang C., Han Y., He W., Liu Y., et al. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell. 2010;22:2384–2401. doi: 10.1105/tpc.110.076588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Z., Peng J., Wen X., Guo H. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. Plant Cell. 2013;25:3311–3328. doi: 10.1105/tpc.113.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gagne J.M., Smalle J., Gingerich D.J., Walker J.M., Yoo S.D., Yanagisawa S., Vierstra R.D. Arabidopsis EIN3-binding F-box 1 and 2 form ubiquitin-protein ligases that repress ethylene action and promote growth by directing EIN3 degradation. Proc. Natl. Acad. Sci. USA. 2004;101:6803–6888. doi: 10.1073/pnas.0401698101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang K., Gan S.S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves. Plant Physiol. 2012;158:961–969. doi: 10.1104/pp.111.190876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiu R.S., Pan S., Zhao R., Gazzarrini S. ABA-dependent inhibition of the ubiquitin proteasome system during germination at high temperature in Arabidopsis. Plant J. 2016;88:749–761. doi: 10.1111/tpj.13293. [DOI] [PubMed] [Google Scholar]
- 112.Kong L., Cheng J., Zhu Y., Ding Y., Meng J., Chen Z., Xie Q., Guo Y., Li J., Yang S., et al. Degradation of the ABA co-receptor ABI1 by PUB12/13 U-box E3 ligases. Nat. Commun. 2015;20 doi: 10.1038/ncomms9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao Y., Chan Z., Gao J., Xing L., Cao M., Yu C., Hu Y., You J., Shi H., Zhu Y., et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA. 2016;113:1949–1954. doi: 10.1073/pnas.1522840113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao Y., Zhang Z., Gao J., Wang P., Hu T., Wang Z., Hou Y.J., Wan Y., Liu W., Xie S., et al. Arabidopsis duodecuple mutant of PYL ABA receptors reveals PYL repression of ABA-independent SnRK2 activity. Cell Rep. 2018;23:3340–3351. doi: 10.1016/j.celrep.2018.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raab S., Drechsel G., Zarepour M., Hartung W., Koshiba T., Bittner F., Hoth S. Identification of a novel E3 ubiquitin ligase that is required for suppression of premature senescence in Arabidopsis. Plant J. 2009;59:39–51. doi: 10.1111/j.1365-313X.2009.03846.x. [DOI] [PubMed] [Google Scholar]
- 116.Rochon A., Boyle P., Wignes T., Fobert P.R., Despres C. The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell. 2006;18:3670–3685. doi: 10.1105/tpc.106.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Furniss J.J., Spoel S.H. Cullin-RING ubiquitin ligases in salicylic acid-mediated plant immune signaling. Front. Plant Sci. 2015;13:154. doi: 10.3389/fpls.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu Z.Q., Yan S., Saleh A., Wang W., Ruble J., Oka N., Mohan R., Spoel S.H., Tada Y., Zheng N., et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morris K., Mackerness S.A., Page T., John C.F., Murphy A.M., Carr J.P., Buchanan-Wollaston V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000;23:677–685. doi: 10.1046/j.1365-313x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 120.Ding Y., Dommel M., Mou Z. Abscisic acid promotes proteasome-mediated degradation of the transcription coactivator NPR 1 in Arabidopsis thaliana. Plant J. 2016;86:20–34. doi: 10.1111/tpj.13141. [DOI] [PubMed] [Google Scholar]
- 121.Gray W.M., Kepinski S., Rouse D., Leyser O., Estelle M. Auxin regulates SCF TIR1-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 122.Zenser N., Ellsmore A., Leasure C., Callis J. Auxin modulates the degradation rate of Aux/IAA proteins. Proc. Natl. Acad. Sci. USA. 2001;98:11795–11800. doi: 10.1073/pnas.211312798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang B.J., Han X.X., Yin L.L., Xing M.Q., Xu Z.H., Xue H.W. Arabidopsis PROTEASOME REGULATOR1 is required for auxin-mediated suppression of proteasome activity and regulates auxin signalling. Nat. Commun. 2016;25 doi: 10.1038/ncomms11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leitner J., Petrášek J., Tomanov K., Retzer K., Pařezová M., Korbei B., Bachmair A., Zažímalová E., Luschnig C. Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc. Natl. Acad. Sci. USA. 2012;109:8322–8327. doi: 10.1073/pnas.1200824109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lim P.O., Lee I.C., Kim J., Kim H.J., Ryu J.S., Woo H.R., Nam H.G. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J. Exp. Bot. 2010;61:1419–1430. doi: 10.1093/jxb/erq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J.I., Murphy A.S., Baek D., Lee S.W., Yun D.J., Bressan R.A., Narasimhan M.L. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2011;62:3981–3992. doi: 10.1093/jxb/err094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gan S., Amasino R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 128.Lara M.E., Garcia M.C., Fatima T., Ehneß R., Lee T.K., Proels R., Tanner W., Roitsch T. Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell. 2004;16:1276–1287. doi: 10.1105/tpc.018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janečková H., Husičková A., Ferretti U., Prčina M., Pilařová E., Plačková L., Pospíšil P., Doležal K., Špundová M. The interplay between cytokinins and light during senescence in detached Arabidopsis leaves. Plant Cell Environ. 2018;41:1870–1885. doi: 10.1111/pce.13329. [DOI] [PubMed] [Google Scholar]
- 130.Hönig M., Plíhalová L., Husičková A., Nisler J., Doležal K. Role of cytokinins in senescence, antioxidant defence and photosynthesis. Int. J. Mol. Sci. 2018;14 doi: 10.3390/ijms19124045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li Y., Kurepa J., Smalle J. AXR1 promotes the Arabidopsis cytokinin response by facilitating ARR5 proteolysis. Plant J. 2013;74:13–24. doi: 10.1111/tpj.12098. [DOI] [PubMed] [Google Scholar]
- 132.Kurepa J., Li Y., Smalle J.A. Cytokinin signaling stabilizes the response activator ARR 1. Plant J. 2014;78:157–168. doi: 10.1111/tpj.12458. [DOI] [PubMed] [Google Scholar]
- 133.Smalle J., Kurepa J., Yang P., Babiychuk E., Kushnir S., Durski A., Vierstra R.D. Cytokinin growth responses in Arabidopsis involve the 26S proteasome subunit RPN12. Plant Cell. 2002;14:17–32. doi: 10.1105/tpc.010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Havé M., Leitao L., Bagard M., Castell J.F., Repellin A. Protein carbonylation during natural leaf senescence in winter wheat, as probed by fluorescein-5-thiosemicarbazide. Plant Biol. 2015;17:973–979. doi: 10.1111/plb.12315. [DOI] [PubMed] [Google Scholar]
- 135.Jain V., Kaiser W., Huber S.C. Cytokinin inhibits the proteasome-mediated degradation of carbonylated proteins in Arabidopsis leaves. Plant Cell Physiol. 2008;49:843–852. doi: 10.1093/pcp/pcn060. [DOI] [PubMed] [Google Scholar]
- 136.Woo H.R., Chung K.M., Park J.H., Oh S.A., Ahn T., Hong S.H., Jang S.K., Nam H.G. ORE9, an F-box protein that regulates leaf senescence in Arabidopsis. Plant Cell. 2001;13:1779–1790. doi: 10.1105/tpc.13.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan H., Saika H., Maekawa M., Takamure I., Tsutsumi N., Kyozuka J., Nakazono M. Rice tillering dwarf mutant dwarf3 has increased leaf longevity during darkness-induced senescence or hydrogen peroxide-induced cell death. Genes Genet. Syst. 2007;82:361–366. doi: 10.1266/ggs.82.361. [DOI] [PubMed] [Google Scholar]
- 138.Yamada Y., Umehara M. Possible roles of strigolactones during leaf senescence. Plants. 2015;4:664–677. doi: 10.3390/plants4030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ueda H., Kusaba M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015;169:138–147. doi: 10.1104/pp.15.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Y., Sun S., Zhu W., Jia K., Yang H., Wang X. Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev. Cell. 2013;27:681–688. doi: 10.1016/j.devcel.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 141.He Y., Fukushige H., Hildebrand D.F., Gan S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002;128:876–884. doi: 10.1104/pp.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jiang Y., Liang G., Yang S., Yu D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell. 2014;26:230–245. doi: 10.1105/tpc.113.117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tsuchiya T., Ohta H., Okawa K., Iwamatsu A., Shimada H., Masuda T., Takamiya K. Cloning of chlorophyllase, the key enzyme in chlorophyll degradation: finding of a lipase motif and the induction by methyl jasmonate. Proc. Natl. Acad. Sci. USA. 1999;96:15362–15367. doi: 10.1073/pnas.96.26.15362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chico J.M., Chini A., Fonseca S., Solano R. JAZ repressors set the rhythm in jasmonate signaling. Curr. Opin. Plant Biol. 2008;11:486–494. doi: 10.1016/j.pbi.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 145.Yu J., Zhang Y., Di C., Zhang Q., Zhang K., Wang C., You Q., Yan H., Dai S.Y., Yuan J.S., et al. JAZ7 negatively regulates dark-induced leaf senescence in Arabidopsis. J. Exp. Bot. 2016;67:751–762. doi: 10.1093/jxb/erv487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Monte I., Franco-Zorrilla J.M., García-Casado G., Zamarreño A.M., García-Mina J.M., Nishihama R., Kohchi T., Solano R. A single JAZ repressor controls the jasmonate pathway in Marchantia polymorpha. Mol. Plant. 2019;12:185–198. doi: 10.1016/j.molp.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 147.Chen M., Maodzeka A., Zhou L., Ali E., Wang Z., Jiang L. Removal of DELLA repression promotes leaf senescence in Arabidopsis. Plant Sci. 2014;219:26–34. doi: 10.1016/j.plantsci.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 148.Gao X.H., Xiao S.L., Yao Q.F., Wang Y.J., Fu X.D. An updated GA signaling ‘relief of repression’ regulatory model. Mol. Plant. 2011;4:601–606. doi: 10.1093/mp/ssr046. [DOI] [PubMed] [Google Scholar]
- 149.Chen L., Xiang S., Chen Y., Li D., Yu D. Arabidopsis WRKY45 interacts with the DELLA protein RGL1 to positively regulate age-triggered leaf senescence. Mol. Plant. 2017;10:1174–1189. doi: 10.1016/j.molp.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y., Liu Z., Wang X., Wang J., Fan K., Li Z., Lin W. DELLA proteins negatively regulate dark-induced senescence and chlorophyll degradation in Arabidopsis through interaction with the transcription factor WRKY6. Plant Cell Rep. 2018;37:981–992. doi: 10.1007/s00299-018-2282-9. [DOI] [PubMed] [Google Scholar]
- 151.Saks Y., Van Staden J., Smith M.T. Effect of gibberellic acid on carnation flower senescence: evidence that the delay of carnation flower senescence by gibberellic acid depends on the stage of flower development. Plant Growth Regul. 1992;11:45–51. doi: 10.1007/BF00024432. [DOI] [Google Scholar]
- 152.Li J.R., Yu K., Wei J.R., Ma Q., Wang B.Q., Yu D. Gibberellin retards chlorophyll degradation during senescence of Paris polyphylla. Biol Plant. 2010;54:395–399. doi: 10.1007/s10535-010-0072-5. [DOI] [Google Scholar]
- 153.De Assis-Gomes M.D., Pinheiro D.T., Bressan-Smith R., Campostrini E. Exogenous brassinosteroid application delays senescence and promotes hyponasty in Carica papaya L. leaves. Theor. Exp. Plant Physiol. 2018;30:193–201. doi: 10.1007/s40626-018-0114-5. [DOI] [Google Scholar]
- 154.Lozano-Duran R., Macho A.P., Boutrot F., Segonzac C., Somssich I.E., Zipfel C. The transcriptional regulator BZR1 mediates trade-off between plant innate immunity and growth. Elife. 2013;2 doi: 10.7554/eLife.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schippers J.H.M. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015;27:77–83. doi: 10.1016/j.pbi.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 156.He J.X., Gendron J.M., Yang Y., Li J., Wang Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2002;99:10185–10190. doi: 10.1073/pnas.152342599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peng P., Yan Z., Zhu Y., Li J. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation. Mol. Plant. 2008;1:338–346. doi: 10.1093/mp/ssn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., et al. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 2005;42:567–585. doi: 10.1111/j.1365-313X.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 159.Woo H.R., Koo H.J., Kim J., Jeong H., Yang J.O., Lee I.H., Jun J.H., Choi S.H., Park S.J., Kang B., et al. Programming of plant leaf senescence with temporal and inter-organellar coordination of transcriptome in Arabidopsis. Plant Physiol. 2016;171:452–467. doi: 10.1104/pp.15.01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Thompson A.R., Doelling J.H., Suttangkakul A., Vierstra R.D. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138:2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng Y., Yao Z., Klionsky D.J. How to control self-digestion: transcriptional, post-transcriptional, and post-translational regulation of autophagy. Trends Cell Biol. 2015;25:354–363. doi: 10.1016/j.tcb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Y., Bassham D.C. TOR is a negative regulator of autophagy in Arabidopsis thaliana. PLoS ONE. 2010;5:e11883. doi: 10.1371/journal.pone.0011883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Broeckx T., Hulsmans S., Rolland F. The plant energy sensor: evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016;67:6215–6252. doi: 10.1093/jxb/erw416. [DOI] [PubMed] [Google Scholar]
- 164.Soto-Burgos J., Bassham D.C. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS ONE. 2017;12:e0182591. doi: 10.1371/journal.pone.0182591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nukarinen E., Nägele T., Pedrotti L., Wurzinger B., Mair A., Landgraf R., Börnke F., Hanson J., Teige M., Baena-Gonzalez E., et al. Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci. Rep. 2016;6 doi: 10.1038/srep31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen L., Su Z.Z., Huang L., Xia F.N., Qi H., Xie L.J., Xiao S., Chen Q.F. The AMP-activated protein kinase KIN10 is involved in the regulation of autophagy in Arabidopsis. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mair A., Pedrotti L., Wurzinger B., Anrather D., Simeunovic A., Weiste C., Valerio C., Dietrich K., Kirchler T., Nägele T., et al. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. Elife. 2015;11 doi: 10.7554/eLife.05828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kleinow T., Himbert S., Krenz B., Jeske H., Koncz C. NAC domain transcription factor ATAF1 interacts with SNF1-related kinases and silencing of its subfamily causes severe developmental defects in Arabidopsis. Plant Sci. 2009;177:360–370. doi: 10.1016/j.plantsci.2009.06.011. [DOI] [Google Scholar]
- 169.Zhang Z., Zhu J.Y., Roh J., Marchive C., Kim S.K., Meyer C., Sun Y., Wang W., Wang Z.Y. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr. Biol. 2016;26:1854–1860. doi: 10.1016/j.cub.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Masclaux C., Valadier M.H., Brugière N., Morot-Gaudry J.F., Hirel B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta. 2000;211:510–518. doi: 10.1007/s004250000310. [DOI] [PubMed] [Google Scholar]
- 171.Schippers J.H.M., Schmidt R., Wagstaff C., Jing H.C. Living to die and dying to live: the survival strategy behind leaf senescence. Plant Physiol. 2015;169:914–930. doi: 10.1104/pp.15.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hwang I., Sheen J., Müller B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- 173.Ehness R., Roitsch T. Co-ordinated induction of mRNAs for extracellular invertase and a glucose transporter in Chenopodium rubrum by cytokinins. Plant J. 1997;11:539–548. doi: 10.1046/j.1365-313X.1997.11030539.x. [DOI] [PubMed] [Google Scholar]
- 174.Wang Y., Cao J.J., Wang K.X., Xia X.J., Shi K., Zhou Y.H., Yu J.Q., Zhou J. BZR1 mediates brassinosteroid-induced autophagy and nitrogen starvation tolerance in tomato. Plant Physiol. 2019;179:671–685. doi: 10.1104/pp.18.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lai Z., Wang F., Zheng Z., Fan B., Chen Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. 2011;66:953–968. doi: 10.1111/j.1365-313X.2011.04553.x. [DOI] [PubMed] [Google Scholar]
- 176.Gao C., Zhuang X., Cui Y., Fu X., He Y., Zhao Q., Zeng Y., Shen J., Luo M., Jiang L. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl. Acad. Sci. USA. 2015;112:1886–1891. doi: 10.1073/pnas.1421271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yu F., Lou L., Tian M., Li Q., Ding Y., Cao X., Wu Y., Belda-Palazon B., Rodriguez P.L., Yang S., et al. ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation. Mol. Plant. 2016;5:1570–1582. doi: 10.1016/j.molp.2016.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.