Abstract
Orthopedic trauma is a significant military problem, causing several of the most disabling conditions with high rates of separation from duty and erosion of military readiness. The objective of this report is to summarize the findings of case series of a non-opioid therapy—percutaneous peripheral nerve stimulation (PNS) – and describe its potential for postoperative analgesia, early opioid cessation, and improved function following orthopedic trauma. Percutaneous PNS has been evaluated for the treatment of multiple types of pain, including two case series on postoperative pain following total knee replacement (n = 10 and 8, respectively) and a case series on postamputation pain (n = 9). The orthopedic trauma induced during TKR is highly representative of multiple types of orthopedic trauma sustained by Service members and frequently produces intense, prolonged postoperative pain and extended opioid use following surgery. Collectively, the results of these three clinical studies demonstrated that percutaneous PNS can provide substantial pain relief, reduce opioid use, and improve function. These outcomes suggest that there is substantial potential for the use of percutaneous PNS following orthopedic trauma.
Keywords: Postsurgical pain, neurostimulation, non-opioid, total joint replacement
INTRODUCTION
Orthopedic trauma is a significant problem in the military and Veteran populations, causing several of the most disabling conditions that contribute to separation from duty (e.g., post-traumatic osteoarthritis (OA), joint replacement, amputation).1 Combat-related extremity injuries in particular have been associated with increased inpatient stays and a majority of findings of “unfit for duty,” with estimated disability costs near $2 billion.2 Traumatic injuries (e.g., high-energy injuries; blunt/penetrating/perforating trauma) may involve damage of multiple tissue types, including skin, muscle, bone, joint, and nerve (Figs 1,2). While injuries caused by orthopedic trauma can cause considerable pain, the surgeries to treat orthopedic trauma can also cause substantial long-lasting postoperative pain. Postoperative pain following orthopedic surgeries such as bone fracture repair, amputation, limb salvage, and joint replacement, is moderate to severe in up to 90% of patients, and uncontrolled acute postoperative pain can transition to chronic pain, frequently lasting multiple years.3–5
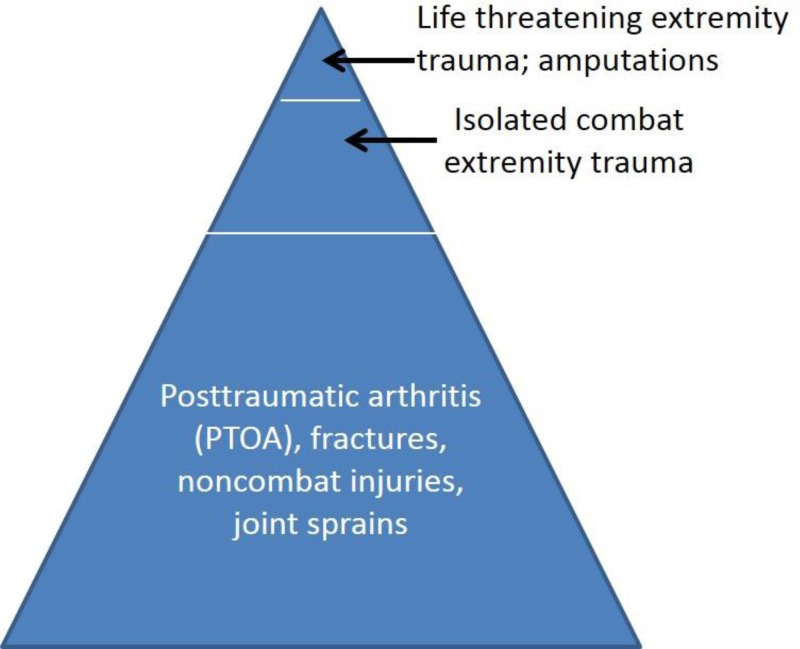
FIGURE 1.
The “iceberg” of orthopedic injuries (adapted from Owens and Cameron).68 The severe orthopedic traumatic injuries at the “tip” (e.g., amputation) receive a large share of attention from the media, researchers, and funding sources. Orthopedic injuries at the “base” receive less attention but cause a larger burden of injury and disease on the military and its health system. This is due in part to the surgeries to correct the injuries (e.g., joint replacement), which often induce trauma to soft tissue and bone and result in severe postoperative pain, prolonged opioid use, and delayed functional recovery.
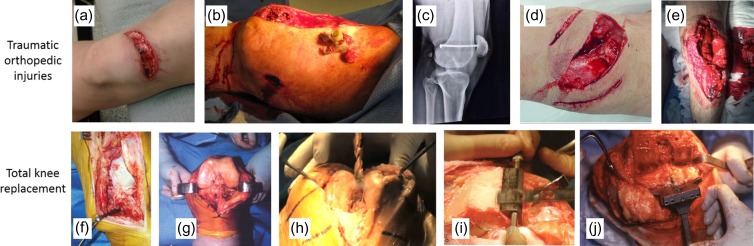
FIGURE 2.
Traumatic orthopedic injuries and total knee replacements result in trauma to soft tissue and bone. Top: traumatic injuries, including (A) lacerations,70 (B) shotgun blast injury,71 (C) nail through femur and patella,72 and (D, E) open knee fractures.73,74 Bottom: total knee replacement: (F) cut through soft tissue (i.e., skin, muscle, ligaments) to expose bone.75 (G) Joint exposed by severing ligaments/meniscus.75 (H–I) Drilling and sawing through bone.76,77 (J) Flat surfaces cut in bone and holes drilled through bone in preparation for joint implant.77
Following orthopedic surgery, pain is a primary source of disability that inhibits rehabilitation, limits a return to normal function, and correlates negatively with return to duty or employment.6–8 Poor function following major orthopedic surgery can persist for ≥1 years in 25–50% of patients, resulting in high rates of medical separation from military service and failure to return to work.9–12 An inability to participate effectively in rehabilitation after trauma or surgery due to pain can lead to volumetric muscle loss, a disqualifying problem that further prevents return to duty.13
One of the primary treatments for postoperative pain is opioids, which can result in misuse, addiction, and debilitating side effects that often interfere with function, activities of daily living, and physical rehabilitation.14 In addition, patients who undergo the most painful orthopedic surgeries often use opioids for several weeks following surgery.15–17 Such long-term use of opioids increases the risks of addiction, dependence, use of illicit substances (e.g., heroin), overdose, and death.14,18
Anesthetic nerve blocks can provide postoperative pain relief while recovering from surgery in the hospital, but they are not compatible with extended use outside of the hospital. Analgesia from injections last ≤24 hours and cannot be delivered outside the clinical setting. Continuous nerve blocks are seldom used for more than 4–7 days due to the risk of catheter dislodgement and infection.19,20 Nerve blocks also carry the risk of muscle weakness and reduced proprioception due to inadvertent block of motor and sensory fibers that increase the risk of falling.
Traditional methods of electrical stimulation avoid some complications of medications and nerve blocks and have been used successfully to treat chronic pain. However, traditional methods of stimulation (e.g., spinal cord stimulation) require invasive surgery to implant the permanent electrodes and stimulator. As a result, they are not practical as a temporary postoperative therapy. The objective of the present report is to describe the use of a novel modality of neurostimulation – percutaneous peripheral nerve stimulation (PNS) – for postoperative analgesia in orthopedic indications that are common in military and Veteran populations.
METHODS
Percutaneous Peripheral Nerve Stimulation
Percutaneous PNS utilizes a fine wire open-coil stimulation lead temporarily implanted percutaneously to target peripheral nerves that innervate the region of pain. The lead is connected to an external stimulator, and the therapy is designed to deliver selective stimulation of pain-relieving fibers to avoid the induction of unwanted muscle contractions, muscle weakness, and reduced proprioception.
Percutaneous PNS has received United States (U.S.) Food and Drug Administration (FDA) 510(k) clearance for the treatment of chronic pain and acute pain, including postoperative and post-traumatic pain, for up to 60 days in the back and/or extremities (SPRINT PNS System, SPR Therapeutics, Inc, Cleveland, OH, USA). The lead is designed to remain indwelling for an extended duration with minimal risk of infection and lead migration. The leads consist of a 0.1-mm diameter, 7-strand stainless steel wire insulated with a fluoropolymer and wound into an open helical coil with the distal tip forming an anchor (Fig. 3). The lead is preloaded into a 20-gauge needle and implanted percutaneously using ultrasound guidance towards the target nerve, leveraging approaches similar to those used for local anesthetic injections and other ultrasound-guided procedures. For example, when used to treat lower limb pain, the lead may be implanted near the femoral crease to target the femoral nerve. Similarly, a lead may target the sciatic nerve using an approach similar to one used for local anesthetic-based sciatic nerve blocks (e.g., transgluteal). After the introducer is withdrawn and the lead is deployed, it is connected to an external stimulator (Fig. 4).
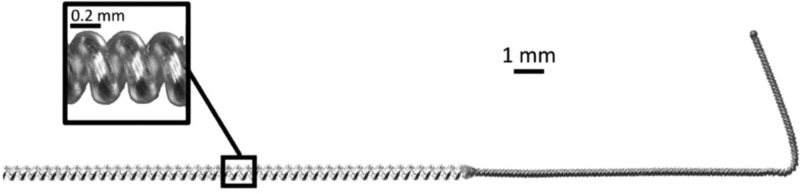
FIGURE 3.
A small-diameter (<0.3 mm) open-coiled, helical electrical lead with an anchoring wire (MicroLead, SPR Therapeutics, Inc, Cleveland, OH, USA; figure used with permission from SPR Therapeutics).
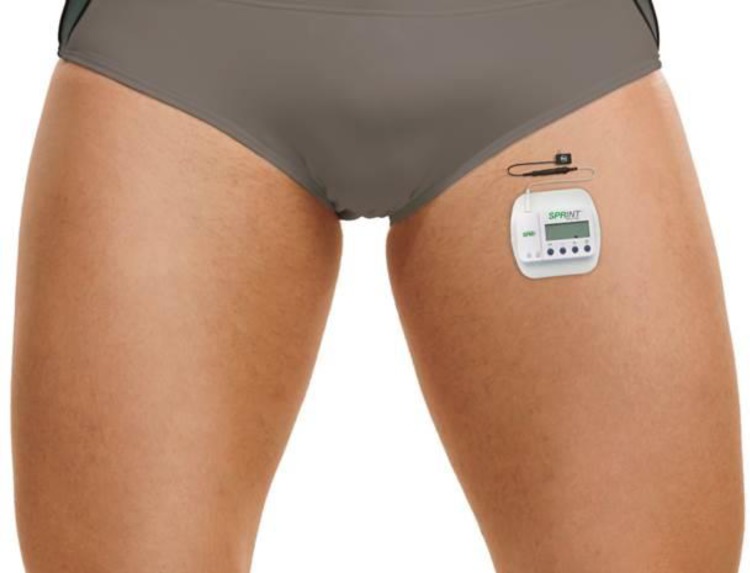
FIGURE 4.
A stimulator attached to the surface return electrode (SPR Therapeutics, Inc., Cleveland, OH, USA; figure used with permission from SPR Therapeutics).
Device-related adverse events in clinical studies have been consistently mild (95%) or moderate (5%), anticipated, non-serious, and have required little to no intervention to resolve. The most common adverse events were limited to skin irritation, erythema, a blister, or a mild skin tear.21–28 No infections have been reported to date in over 330 leads when used to treat pain and left indwelling for up to 60 days (compared to 1.5% with continuous nerve block catheters), and the lead has a risk of infection of less than 1 per 30,000 indwelling days.20,29 Removal of the lead at the end of therapy does not require surgery and is performed similar to the removal of a continuous nerve block catheter. In addition, several hundred similar open-coil leads have been used safely in other non-pain indications30–33, creating an established safety profile for the open-coil lead.
Evaluation of Percutaneous PNS in a Proxy Population That Is Highly Representative of Multiple Types of Orthopedic Trauma Sustained by Service Members
To prepare for major military conflicts that would be expected to produce a large volume of traumatic combat-related orthopedic injuries, studies of postoperative pain treatments should be conducted in patients that have experienced orthopedic trauma. However, orthopedic trauma presents unique challenges in clinical investigations due to a high degree of patient variability. Certain types of orthopedic trauma cause unpredictable severity and location of injury (e.g., high-energy injuries); confounding secondary health issues (e.g., compartment syndrome, infection); and variability in the degree of postoperative pain, opioid use, and disability.17,34,35 Also, surgeries must often be performed immediately following orthopedic trauma to stabilize the injury and save life or limb36–38, and enrollment of this patient population into clinical studies is often challenging (e.g., unpredictable surgery date, obtaining informed consent in vulnerable population, low/variable numbers of patients with combat-related trauma available prior to major military conflicts).
To address these issues, percutaneous PNS has been investigated in a well-defined proxy population representing the major characteristics of the target military population with traumatic orthopedic injuries. The proxy population consisted of individuals scheduled to undergo total knee replacement (TKR). While TKR has a high long-term success rate, it has a high incidence of long-lasting severe postoperative pain, extended opioid use (i.e., median time to opioid cessation of 45–60 days), and prolonged rehabilitation.15,16,35,39–43 Although joint replacements are used to correct joint damage (e.g., degeneration, injury), the surgeries themselves involve major injury to the joint and surrounding tissue to remove and replace the joint. TKR requires an incision through soft tissue and drilling and sawing through major weight-bearing bones, with potential for injury of other tissues and connective structures (Fig. 2). Unlike combat-related trauma, TKR induces orthopedic trauma in a predictable (controlled) manner, which is expected to reduce variability across study participants.
Functional recovery following TKR is often slow and impacted by postoperative pain. Patients who can undergo early and intense rehabilitation can minimize volumetric muscle loss and knee stiffness that typically occur following TKR, resulting in greater long-term functional outcomes.44–49 However, physical rehabilitation is delayed and greatly limited by both existing pain and the fear of inducing additional pain, which impedes recovery.45,50 As a result, it is common for activities of daily living to remain difficult and painful for a year or more following surgery9,10,45, and one-third of the general population fail to return to work after joint replacement.51 These challenges associated with postoperative recovery following TKR are comparable or worse compared to other surgeries for orthopedic trauma.
Unlike combat-related trauma or orthopedic injuries following accidents, TKR is an elective procedure with a predictable surgery date scheduled weeks in advance, which facilitates enrollment, provides patients adequate time to consider their participation in the study, and allows for informed consent. Also, the high volume of TKR procedures suggests that the proxy population will be large (>700,000 TKR procedures per year in the USA).52
In addition, joint replacement surgeries are highly relevant to the military population. Pain and activity limitations following joint replacement surgery result in medical separation of approximately 18% of military Service members.53 Long-lasting pain following joint replacement is correlated with younger age54, indicating that Service members are at a higher risk of disability and failure to return to duty. In a study examining the disabling conditions of the U.S. Army before and during Operation Iraqi Freedom and Operation Enduring Freedom, only amputation and total joint replacement were in the top 10 orthopedic conditions associated with the highest percent disability in both peacetime and war; and during war, total joint replacement was the single condition with the highest average percent disability.1
Further, TKR and other joint replacement surgeries are used to treat OA, one of the most common and disabling orthopedic war injuries. OA is one of the leading unfitting conditions among Service members with orthopedic war injuries, and many of these cases can be associated with a traumatic initiating event that leads to post-traumatic OA (PTOA)55,56 (Fig. 1). OA affects millions of U.S. adults,57 and Service members and Veterans have even higher rates of OA and report more activity limitations than the general population.58,59 As a result, orthopedic trauma and joint replacement place a large burden on the military and its health system. The following studies were conducted by authors of the present study to explore the possibility of treating postoperative pain, decreasing opioid use, and improving functional recovery following orthopedic trauma using percutaneous PNS.
RESULTS
Three case series studies were conducted investigating the use of percutaneous PNS following orthopedic trauma (total n = 27). In Series 1, 10 individuals experiencing postoperative pain following surgically-induced orthopedic trauma (i.e., TKR) enrolled in a case series study.22,23 Six subjects were tested <14 days following surgery (range: 6–13 days), and the other four subjects were tested >40 days following surgery (range: 41–97 days). Fine-wire percutaneous leads were placed using ultrasound guidance to target the femoral and/or sciatic nerves. The leads were connected to external stimulators, and stimulation parameters were programmed to generate comfortable sensations in the regions of pain. Stimulation immediately reduced pain at rest compared to stimulation off by ≥50% in 9 subjects (90%) with an average reduction of 75%, and 5 subjects (50%) experienced complete pain relief. Stimulation did not subjectively impair motor function, and subjects were able to flex their knee without assistance. After testing, all leads were removed safely, and no serious device-related adverse events were reported.
A second prospective case series study was conducted evaluating percutaneous PNS for postoperative analgesia after TKR (Series 2).60 Eight individuals scheduled to undergo primary unilateral TKR enrolled at a single site (University of California, San Diego). Percutaneous leads were placed prior to surgery using ultrasound guidance to target the femoral and sciatic nerves. The leads were connected to external stimulators, and stimulation parameters were programmed to generate comfortable sensations in the region anticipated to be painful following surgery (i.e., knee and surrounding regions). Immediately prior to surgery, the leads were disconnected from the stimulators. A single-injection adductor canal block was administered (ropivacaine 0.5% and epinephrine; 20 mL), and spinal or general anesthetic was used to provide surgical anesthesia. Within 20 hours after TKR, stimulators were reconnected to the leads and turned on. During and following hospital discharge, subjects continued using stimulation for up to 6 weeks. There were no reports of impaired sensory/motor function during stimulation, and subjects were able to use stimulation during physical therapy and daily activities. The leads were left indwelling for a median duration of 45 days (range: 8–51 days) and removed at end of therapy. No falls, motor block, lead infections, or other serious device-related adverse events were reported.
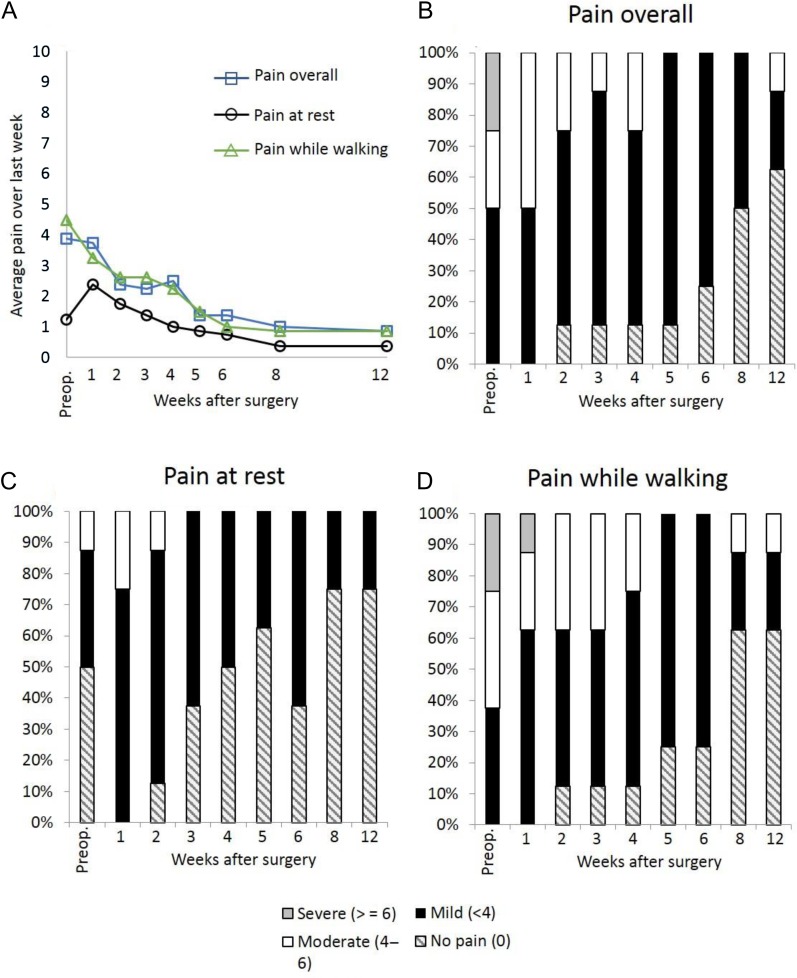
Most subjects had well-controlled postoperative pain with percutaneous PNS following TKR. Subjects recorded their average daily pain in a diary using a 0–10 numerical rating scale. The average of daily pain scores for pain at rest, while walking, and overall was mild (<4/10)61 in a majority of subjects during the first week (Fig. 5). Average pain over the previous week was also assessed and was well-controlled during subsequent weeks; and, by 12 weeks following TKR, 7 of 8 subjects (88%) had well-controlled pain, and 5 subjects (63%) were pain free (Fig. 6). Four of the 8 subjects had well-controlled pain and discontinued opioid use within one week. The median time to opioid cessation across all 8 subjects was 16.5 days.
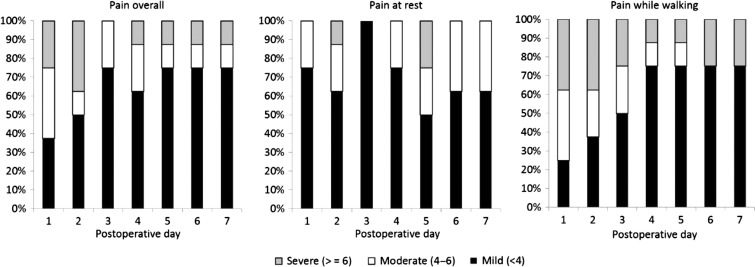
FIGURE 5.
Percentage of subjects (n = 8) with mild, moderate, and severe postoperative pain (“Average pain over the last 24 hours”) overall [left], at rest [center], and during ambulation [right] through postoperative day 7 (Series 2).
FIGURE 6.
Average pain over the past week. Panel (A) Pain averaged across subjects (n = 8). Panels (B–D) Percentage of subjects with no pain, mild pain, moderate pain, and severe pain (B) overall, (C) at rest, and (D) while walking (Series 2).
Walking speed and endurance were assessed using the Six Minute Walk Test (6MWT), which measures the distance that a subject can walk in 6 minutes.62 All 8 subjects completed the 6MWT test preoperatively (average = 336 ± 56 m); and by 2 weeks following surgery, 6 of 7 subjects (86%) that were able to perform the 6MWT had returned approximately to preoperative levels (≥95% of preoperative distance) (average distance = 312 ± 87 m). By 12 weeks following surgery, 7 of 8 subjects (88%) had improved on the 6MWT by ≥10% compared to preoperative distances (average distance = 410 ± 56 m), with an average improvement of 24%.
Functional outcomes improved following surgery compared to before surgery as measured by the Western Ontario & McMaster University Osteoarthritis Index (WOMAC; assesses pain, stiffness, and difficulty with activities of daily living). By 6 weeks following surgery, all 8 subjects (100%) had achieved clinically significant improvements in WOMAC of at least 33%63, with an average improvement of 76%. By 12 weeks following surgery, the average improvement relative to before surgery was 86%.
The results of this case series study compare favorably to studies described in the Methods reporting outcomes following TKR using standard techniques of postoperative analgesia (i.e., oral medications, local anesthetic-based nerve blocks). Recent studies showed that more than 80% of patients continued to use opioids at 2 weeks following TKR, more than 70% of patients continued to use opioids at 4 weeks following TKR, and the median time to opioid cessation was approximately 45–60 days.15,16,35,39,40 Also, published studies assessing the 6MWT at 1 year following surgery reported average distances across studies of only 116% compared to preoperative distances (i.e., 16% improvement; range = 99–130%).44–46,64–67 Comparisons to historical controls from previous studies should be considered cautiously; nonetheless, the ability of percutaneous PNS to reduce opioid use and accelerate functional recovery following orthopedic surgery is promising.
Additionally, percutaneous PNS was investigated previously by some of the authors of the present study as a method to provide chronic pain relief in amputees.21 In a case series feasibility study (Series 3), nine subjects with lower limb amputations enrolled and received 2 weeks of stimulation. All subjects reported clinically significant68 (≥30%) reduction in pain at end of treatment (average reduction = 76%). Also, all 9 subjects reported improved function with an average of 82% reduction in pain interference on daily activities, including 4 (44%) subjects who reported 100% improvement (i.e., no pain interference) at the end of the stimulation period.
CONCLUSIONS
Collectively, these prospective case series studies suggest that percutaneous PNS may relieve pain, reduce opioid use, and improve function following orthopedic trauma. The pain indications for which percutaneous PNS may benefit Service members, Veterans, their families, and other military health system beneficiaries include pain following the most severe orthopedic injuries (tip of the “iceberg”, Fig. 1) and the surgeries used to treat the most common orthopedic injuries that place an immense burden on the military and its health system (middle and base of “iceberg”, Fig. 1).69 A limitation of these studies is the small sample sizes, and randomized controlled trials in patients following orthopedic trauma are in progress and are expected to further elucidate the relative risks and benefits of percutaneous PNS for postoperative pain.
Acknowledgments
The authors would like to thank all of the investigators and clinical staff involved in the clinical studies described in this manuscript.
Previous Presentation
Presented as a poster at the 2017 Military Health System Research Symposium, Kissimmee, FL (abstract MHSRS-17–1450).
Funding
Funding for this work provided by the National Institute on Aging [Grant number R44AG052196] and the National Institute on Neurological Disorders and Stroke [Grant number R43NS066523] of the National Institutes of Health, and SPR Therapeutics (Cleveland, OH). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding entities. This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.
References
- 1. Patzkowski JC, Rivera JC, Ficke JR, Wenke JC: The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg 2012; 20: S23–S30. [DOI] [PubMed] [Google Scholar]
- 2. Masini BD, Waterman SM, Wenke JC, et al. : Resource utilization and disability outcome assessment of combat casualties from Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma 2009; 23: 261–6. [DOI] [PubMed] [Google Scholar]
- 3. Lavand’homme P: The progression from acute to chronic pain. Curr Opin Anaesthesiol 2011; 24: 545–50. [DOI] [PubMed] [Google Scholar]
- 4. Wylde V, Hewlett S, Learmonth ID, Dieppe P: Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011; 152: 566–72. [DOI] [PubMed] [Google Scholar]
- 5. Chan EY, Blyth FM, Nairn L, Fransen M: Acute postoperative pain following hospital discharge after total knee arthroplasty. Osteoarthritis Cartilage 2013; 21: 1257–63. [DOI] [PubMed] [Google Scholar]
- 6. Whyte AS, Carroll LJ: A preliminary examination of the relationship between employment, pain and disability in an amputee population. Disabil Rehabil 2002; 24: 462–70. [DOI] [PubMed] [Google Scholar]
- 7. Ponsford J, Hill B, Karamitsios M, Bahar-Fuchs A: Factors influencing outcome after orthopedic trauma. J Trauma 2008; 64: 1001–9. [DOI] [PubMed] [Google Scholar]
- 8. Sayer NA, Cifu DX, McNamee S, et al. : Rehabilitation needs of combat-injured service members admitted to the VA Polytrauma Rehabilitation Centers: the role of PM&R in the care of wounded warriors. Pm R 2009; 1: 23–8. [DOI] [PubMed] [Google Scholar]
- 9. Alzahrani K, Gandhi R, Debeer J, Petruccelli D, Mahomed N: Prevalence of clinically significant improvement following total knee replacement. J Rheumatol 2011; 38: 753–9. [DOI] [PubMed] [Google Scholar]
- 10. Singh JA, O’Byrne M, Harmsen S, Lewallen D: Predictors of moderate–severe functional limitation after primary Total Knee Arthroplasty (TKA): 4701 TKAs at 2-years and 2935 TKAs at 5-years. Osteoarthritis Cartilage 2010; 18: 515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starr AJ: Fracture repair: successful advances, persistent problems, and the psychological burden of trauma. J Bone Joint Surg Am 2008; 90(1): 132–7. [DOI] [PubMed] [Google Scholar]
- 12. Doukas CWC, Hayda CRA, Frisch HM, et al. : The Military Extremity Trauma Amputation/Limb Salvage (METALS) study: outcomes of amputation versus limb salvage following major lower-extremity trauma. J Bone Joint Surg Am 2013; 95: 138–45. [DOI] [PubMed] [Google Scholar]
- 13. Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR: Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev 2015; 52: 785–92. [DOI] [PubMed] [Google Scholar]
- 14. Benyamin R, Trescot AM, Datta S, et al. : Opioid complications and side effects. Pain Physician 2008; 11: S105–20. [PubMed] [Google Scholar]
- 15. Carroll I, Barelka P, Wang CK, et al. : A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg 2012; 115: 694–702. [DOI] [PubMed] [Google Scholar]
- 16. Hah JM, Mackey S, Barelka PL, et al. : Self-loathing aspects of depression reduce postoperative opioid cessation rate. Pain Med 2014; 15: 954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Namba RS, Inacio MC, Pratt NL, et al. : Postoperative opioid use as an early indication of total hip arthroplasty failure. Acta Orthop 2016; 87(1): 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dasgupta N, Creppage K, Austin A, et al. : Observed transition from opioid analgesic deaths toward heroin. Drug Alcohol Depend 2014; 145: 238–41. [DOI] [PubMed] [Google Scholar]
- 19. Ilfeld BM: Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg 2011; 113: 904–25. [DOI] [PubMed] [Google Scholar]
- 20. Capdevila X, Bringuier S, Borgeat A: Infectious risk of continuous peripheral nerve blocks. Anesthesiology 2009; 110: 182–8. 10.1097/ALN.0b013e318190bd5b. [DOI] [PubMed] [Google Scholar]
- 21. Rauck RL, Cohen SP, Gilmore CA, et al. : Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation 2014; 17: 188–97. [DOI] [PubMed] [Google Scholar]
- 22. Ilfeld BM, Gilmore CA, Grant SA, et al. : Ultrasound-guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res 2017; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilfeld BM, Grant SA, Gilmore CA, et al. : Neurostimulation for post-surgical analgesia: a novel system enabling ultrasound-guided percutaneous peripheral nerve stimulation. Pain Pract 2016; 17: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chae J, Yu DT, Walker ME, et al. : Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil 2005; 84: 832–842. [DOI] [PubMed] [Google Scholar]
- 25. Yu DT, Chae J, Walker ME, Fang ZP: Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil 2001; 82: 20–5. [DOI] [PubMed] [Google Scholar]
- 26. Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW: Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract 2013; 13: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J: Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil 2011; 92: 837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wilson RD, Gunzler DD, Bennett ME, Chae J: Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil 2014; 93: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ilfeld BM, Gabriel RA, Saulino MF, et al. : Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2016; 17: 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knutson JS, Naples GG, Peckham PH, Keith MW: Electrode fracture rates and occurrences of infection and granuloma associated with percutaneous intramuscular electrodes in upper-limb functional electrical stimulation applications. J Rehabil Res Dev 2002; 39: 671–83. [PubMed] [Google Scholar]
- 31. Matzel KE, Kamm MA, Stosser M, et al. : Sacral spinal nerve stimulation for faecal incontinence: multicentre study. Lancet 2004; 363: 1270–76. [DOI] [PubMed] [Google Scholar]
- 32. Shimada Y, Matsunaga T, Misawa A, et al. : Clinical application of peroneal nerve stimulator system using percutaneous intramuscular electrodes for correction of foot drop in hemiplegic patients. Neuromodulation 2006; 9: 320–7. [DOI] [PubMed] [Google Scholar]
- 33. Onders RP, Elmo M, Khansarinia S, et al. : Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc 2009; 23: 1433–40. [DOI] [PubMed] [Google Scholar]
- 34. Gadsden J, Warlick A: Regional anesthesia for the trauma patient: improving patient outcomes. Local Reg Anesth 2015; 8: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernandez NM, Parry JA, Taunton MJ: Patients at risk: large opioid prescriptions after total knee arthroplasty. J Arthroplasty 2017; 32: 2395–8. [DOI] [PubMed] [Google Scholar]
- 36. Wise R, Higginson I, Benger J, Rawlinson N: Lower limb amputation with CPR in progress: recovery following prolonged cardiac arrest. Emerg Med J 2006; 23: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsai YH, Huang TJ, Hsu RW, et al. : Necrotizing soft-tissue infections and primary sepsis caused by Vibrio vulnificus and Vibrio cholerae non-O1. J Trauma 2009; 66: 899–905. [DOI] [PubMed] [Google Scholar]
- 38. Pape H-C, Giannoudis P, Krettek C: The timing of fracture treatment in polytrauma patients: relevance of damage control orthopedic surgery. Am J Surg 2002; 183: 622–9. [DOI] [PubMed] [Google Scholar]
- 39. Bedard NA, Pugely AJ, Westermann RW, et al. : Opioid use after total knee arthroplasty: trends and risk factors for prolonged use. J Arthroplasty 2017; 32: 2390–4. [DOI] [PubMed] [Google Scholar]
- 40. Goesling J, Moser SE, Zaidi B, et al. : Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016; 157: 1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Page M.G, Katz J, Romero Escobar EM, et al. : Distinguishing problematic from nonproblematic postsurgical pain: a pain trajectory analysis after total knee arthroplasty. Pain 2015; 156: 460–8. [DOI] [PubMed] [Google Scholar]
- 42. Fitzgerald JD, Orav EJ, Lee TH, et al. : Patient quality of life during the 12 months following joint replacement surgery. Arthritis Rheum 2004; 51: 100–9. [DOI] [PubMed] [Google Scholar]
- 43. Hamel MB, Toth M, Legedza A, Rosen MP: Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch Intern Med 2008; 168: 1430–40. [DOI] [PubMed] [Google Scholar]
- 44. Bade MJ, Kohrt WM, Stevens-Lapsley JE: Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010; 40: 559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bade MJ, Stevens-Lapsley JE: Early high-intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther 2011; 41: 932–41. [DOI] [PubMed] [Google Scholar]
- 46. Mizner RL, Petterson SC, Clements KE, et al. : Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty 2011; 26: 728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bandholm T, Kehlet H: Physiotherapy exercise after fast-track total hip and knee arthroplasty: time for reconsideration? Arch Phys Med Rehabil 2012; 93: 1292–4. [DOI] [PubMed] [Google Scholar]
- 48. Thomas AC, Stevens-Lapsley JE:: Importance of attenuating quadriceps activation deficits after total knee arthroplasty. Exerc Sport Sci Rev 2012; 40: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME: Total joint arthroplasties: current concepts of patient outcomes after surgery. Rheum Dis Clin North Am 2007; 33: 71–86. [DOI] [PubMed] [Google Scholar]
- 50. Ryu J, Saito S, Yamamoto K, Sano S: Factors influencing the postoperative range of motion in total knee arthroplasty. Bull Hosp Jt Dis 1993; 53: 35–40. [PubMed] [Google Scholar]
- 51. Kievit AJ, van Geenen RC, Kuijer PP, et al. : Total knee arthroplasty and the unforeseen impact on return to work: a cross-sectional multicenter survey. J Arthroplasty 2010; 29: 1163–8. [DOI] [PubMed] [Google Scholar]
- 52. Centers for Disease Control and Prevention/National Center for Health Statistics National Hospital Discharge Survey, 2010. Available at http://www.cdc.gov/nchs/data/nhds/4procedures/2010pro4_numberprocedureage.pdf; accessed: April 25, 2018.
- 53. Belmont PJ Jr, Heida K, Keeney JA, et al. : Return to work and functional outcomes following primary total knee arthroplasty in U.S. military servicemembers. J Arthroplasty 2015; 30: 968–72. [DOI] [PubMed] [Google Scholar]
- 54. Singh JA, Gabriel S, Lewallen D: The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res 2008; 466: 2717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rivera JC, Wenke JC, Buckwalter JA, Ficke JR, Johnson AE: Posttraumatic osteoarthritis caused by battlefield injuries: the primary source of disability in warriors. J Am Acad Orthop Surg 2012; 20(1): S64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patzkowski JC, Rivera JC, Ficke JR, Wenke JC: The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg 2012; 20(1): S23–30. [DOI] [PubMed] [Google Scholar]
- 57. Neogi T, Zhang Y: Epidemiology of osteoarthritis. Rheum Dis Clin North Am 2013; 39: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ: Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum 2011; 63: 2974–82. [DOI] [PubMed] [Google Scholar]
- 59. Dominick KL, Golightly YM, Jackson GL: Arthritis prevalence and symptoms among US non-veterans, veterans, and veterans receiving Department of Veterans Affairs Healthcare. J Rheumatol 2006; 33: 348–54. [PubMed] [Google Scholar]
- 60. Ilfeld BM, Ball ST, Gabriel RA, et al. : A feasibility study of percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty. Neuromodulation 2018. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W: Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011; 107: 619–26. [DOI] [PubMed] [Google Scholar]
- 62. Ko V, Naylor JM, Harris IA, Crosbie J, Yeo AE: The six-minute walk test is an excellent predictor of functional ambulation after total knee arthroplasty. BMC Musculoskelet Disord 2013; 14: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hmamouchi I, Allali F, Tahiri L, et al. : Clinically important improvement in the WOMAC and predictor factors for response to non-specific non-steroidal anti-inflammatory drugs in osteoarthritic patients: a prospective study. BMC Res Notes 2012; 5: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carli F, Clemente A, Asenjo JF, et al. : Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block. Br J Anaesth 2010; 105: 185–95. [DOI] [PubMed] [Google Scholar]
- 65. Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D: Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord 2005; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM: Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012; 92: 210–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stevens-Lapsley JE, Petterson SC, Mizner RL, Snyder-Mackler L: Impact of body mass index on functional performance after total knee arthroplasty. J Arthroplasty 2010; 25: 1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dworkin RH, Turk DC, Wyrwich KW, et al. : Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Pain 2008; 9: 105–21. [DOI] [PubMed] [Google Scholar]
- 69. Owens BD, Cameron KL: Chapter 1. In: Musculoskeletal Injuries in the Military. Edited by Cameron KL and Owens BD. Springer, 2016. 3–10.
- 70.“Kneelaceration”. Westfield High School Athletic Training. Available at atplacement.com/westfield/media/Kneelaceration.jpg; accessed March 27, 2018.
- 71.DiverDave. “Shotgun Wound”. Wikimedia Commons. Available at commons.wikimedia.org/wiki/File:Shotgun_wound.JPG; accessed October 22, 2010.
- 72.Docbastard. X-ray of nail embedded into femur and patella. docbastard.net. Available at www.docbastard.net/2015/09/safety-mechanisms.html; accessed September 27, 2015.
- 73. Brinker MR: “Complex-Fracture-Knee1”. drbrinker.com.drbrinker.com/attachments/wysiwyg/5/Complex-Fracture-Knee1.jpg
- 74. Delniotis I. “Open fracture of proximal tibia”. I.D. Orthopaedics. www.id-orthopaedics.com/open-fractures-treatment/
- 75.Innomed Orthopaedic Instruments. Available at http://www.innomed.net/knee_rets_standard.htm
- 76. Turnage KL: Screenshot from "Total Knee Replacement Surgery Part 2 - Update 2011”. Youtube. Available at https://www.youtube.com/watch?v=vJGJJOA1Me0; accessed October 10, 2010.
- 77. Scott RD: Total Knee Arthroplasty, Ed 2, Elsevier Saunders, Philadelphia, 2015. [Google Scholar]