Abstract
The μ-opioid receptor (MOR) system has long been thought to underpin the rewarding properties of pleasant touch. Numerous non-human animal studies implicate MORs in social behaviours involving touch, but little is currently known about MOR involvement in human touch reward. Here, we employed a bi-directional pharmacological double-blind crossover design to assess the role of the human MOR system for touch pleasantness and motivation. Forty-nine male volunteers received 10 mg per-oral morphine, 50 mg per-oral naltrexone and placebo before being brushed on their forearm at three different velocities (0.3, 3 and 30 cm/s). In a touch liking task, pleasantness ratings were recorded after each 15 s brushing trial. In a touch wanting task, participants actively manipulated trial duration through key presses. As expected, 3 cm/s was the preferred velocity, producing significantly higher pleasantness ratings and wanting scores than the other stimuli. Contrary to our hypothesis, MOR drug manipulations did not significantly affect either touch pleasantness or wanting. The null effects were supported by post hoc Bayesian analyses indicating that the models with no drug effect were more than 25 times more likely than the alternative models given the data. We conclude that μ-opioid signalling is unlikely to underpin non-affiliative touch reward in humans.
Introduction
The μ-opioid system modulates both reward and pain across species. For instance, stimulating μ-opioid receptor (MOR) with non-sedative doses of opioid drugs leads to an increased preference for sweet and fatty foods in both rats and humans, while opioid antagonists blunt the effect of these foods as positive reinforcers (Fantino et al., 1986; Yeomans and Gray, 1996, 1997; Ziauddeen et al., 2012; Eikemo et al., 2016; Price et al., 2016). The same pattern of MOR effects have been observed in healthy humans in response to monetary (Weber et al., 2016; Eikemo et al., 2017) and social rewards (Chelnokova et al., 2014; Chelnokova et al., 2016; Buchel et al., 2018). Across reward categories, enhanced MOR transmission appears to modulate responses to the most valuable reward option available (Eikemo et al. 2016, 2017), which could mean that the MOR system plays a role in reward saliency—making the richest reward stand out.
Social behaviours that facilitate bonding are typically rewarding in mammals. Behaviours that involve a great deal of touch, such as social playing, grooming and huddling, play a particularly central role in social bonding in mammals (Hertenstein et al., 2006). A large body of psychopharmacological studies implicate the MOR system in social behaviours involving touch in non-human mammals. MOR activation underpins the rewarding properties of social play in juvenile rats (Vanderschuren et al., 1996; Trezza et al., 2011), promoting both motivation for and reward value of social play (Achterberg et al., 2018). Social play is consistently increased by low-dose non-sedative MOR agonists and reduced by MOR antagonist treatment (Beatty and Costello, 1982; Panksepp et al., 1985; Siegel and Jensen, 1985, 1986; Vanderschuren et al., 1995a, 1995b; Vanderschuren et al., 1996; Trezza and Vanderschuren, 2008a, 2008b), with similar patterns found also for social grooming in rats (van Ree and Niesink, 1983; Niesink and van Ree, 1984, 1989) and in juvenile non-human primates (Guard et al., 2002). However, the opposite pattern of MOR drug effects, i.e. increased social motivation following opioid blockade, has been reported more often in the primate literature (Meller et al., 1980; Fabre-Nys et al., 1982; Keverne et al., 1989; Schino and Troisi, 1992; Martel et al., 1995; Graves et al., 2002). We have proposed that these opposing patterns of drug effects can be reconciled by taking into account whether the animal is affectively content or distressed (Løseth et al., 2014).
In humans, little is currently known about MOR involvement in social touch behaviours and central touch-induced MOR responses. Beta-endorphin levels in the blood have been increased by massage (Day et al., 1987; Kaada and Torsteinbo, 1989; Morhenn et al., 2012). These measures might be unrelated to central MOR signalling, however (Fell et al., 2014). One positron emission tomography (PET) study investigated central MOR responses to gentle stroking by a romantic partner and reported ligand binding consistent with reductions in MOR activity during touch (Nummenmaa et al., 2016). While the results appear to go against the hypothesis that touch induces MOR activation, the lack of a non-social touch control condition means the reduction could simply reflect a response to somatosensory stimulation. To our knowledge, only one previous study has investigated the effects of opioid blockade on perceived touch pleasantness in humans (Case et al., 2016). Pharmacological MOR blockade led to ~10% marginally significant increases in average ratings of pleasantness for both fast and slow brushing compared to baseline, consistent with earlier findings in primates but not in rodents.
Here, we used both MOR stimulation with morphine and MOR blockade with naltrexone to assess the role of the human MOR system for touch pleasantness and motivation to receive touch. In a double-blind crossover design, 49 healthy male volunteers received a MOR agonist (10 mg per-oral morphine), antagonist (50 mg per-oral naltrexone) and placebo before completing a series of reward tasks, including two separate tasks assessing touch pleasantness and motivation. Touch was administered as soft brush strokes delivered to the non-dominant forearm at three different velocities (0.3, 3 and 30 cm/s) of which one (3 cm/s) has been shown to elicit the highest pleasantness ratings across participants in a range of studies (Löken et al., 2009; Ackerley et al., 2014; Triscoli et al., 2014; Crucianelli et al., 2016). Light stroking touch at 3 cm/s is also the optimal stimuli for activation of C-tactile (CT) afferents, which is thought to play a particular role in encoding social touch (Morrison, 2012). Touch pleasantness responses were recorded using a visual analogue rating scale. The touch motivation task was designed to allow participants to actively manipulate the duration of each brushing trial through button presses, so that they could work to lengthen the duration of preferred touch and shorten the duration of non-preferred touch velocities.
Based on the extensive literature on MOR modulation of appetitive reward, we hypothesised that MOR manipulation would affect both the experience of touch pleasantness and motivation to receive touch. Specifically, compared to placebo we expected increases in these measures during MOR stimulation with the selective MOR agonist morphine and decreases during MOR blockade with the non-specific opioid antagonist naltrexone. Furthermore, we expected this linear morphine > placebo > naltrexone pattern to be most pronounced for the preferred touch (3 cm/s).
Materials and methods
Study design
The within-subject, double-blind design consisted of three sessions where participants received 10 mg per oral morphine, 50 mg per oral naltrexone or placebo in counterbalanced order, before completing tasks assessing pleasantness (liking) and motivation (wanting) for light stroking touch. These ‘touch liking’ and ‘touch wanting’ tasks were part of a larger study investigating the role of the μ-opioid system in reward behaviours in healthy people (for other results from this study, see: Chelnokova et al., 2014; Chelnokova et al., 2016; Eikemo et al., 2016; Eikemo et al., 2017).
Participants
Forty-nine healthy volunteers completed testing with morphine, placebo and naltrexone in separate sessions. Only males were included to avoid potential interactions between drugs and circulating levels of estradiols and gonadotropin-releasing hormone pulsation in females (Smith et al., 1998). Recruitment and testing was conducted as described in Eikemo et al. (2016), with selective recruitment of carriers of the G allele of OPRM1 in the second data collection wave (N = 17 AG, N = 2 AA carriers in wave 2). In brief, participants had normal or corrected-to-normal vision. Exclusion criteria included a history of or current major psychiatric disorders, chronic or prolonged pain conditions, major medical illness, current use of medication (antihistamines exempt), a history of prolonged opiate drug use, single or repeated use of any strong opiates the past 2 years. Table 1 outlines participant characteristics and self-reported substance use. A fresh urine sample was screened at the beginning of each session to exclude anyone who had recently used opioid medications (using the MOP Opiate300 Test Strip; SureScreen Diagnostics Ltd, Derby, UK). Participants provided informed consent prior to study procedures, which were approved by the regional ethics committee (2011/1337/REK sør-øst D). Participants were instructed not to consume alcohol the day before testing, to abstain from eating and using tobacco at least 1 h before testing commenced and not to drive or operate heavy machinery for 6 h after drug administration. Data were missing from one participants’ naltrexone session; the remaining data were included in the analyses. Due to a computer programming error, touch-liking data was lost for 14 participants, yielding a final sample size of 35 for this task.
Table 1.
Participant characteristics
| Mean | s.d. | ||||
|---|---|---|---|---|---|
| Age (years) | 24.88 | 4.42 | |||
| Weight (kg) | 79.57 | 11.17 | |||
| Height (metre) | 1.84 | 0.07 | |||
| BMI (weight/heigh2) | 23.54 | 2.95 | |||
| Alcohol use | Mean | s.d. | |||
| Units per week* | 7.26 | 5.62 | |||
| Tobacco use | Daily | Occasional | |||
| % | n | % | n | ||
| 26 | 13 | 24 | 12 | ||
| Drug use | Life time | Last year | |||
| % | n | % | n | ||
| Cannabis | 71 | 35 | 31 | 15 | |
| Amphetamines1 | 14 | 7 | 4 | 2 | |
| Cocaine2 | 18 | 9 | 2 | 1 | |
| Opiates3 | 2 | 1 | 0 | 0 | |
| Hallucinogenics4 | 18 | 9 | 6 | 3 | |
| Solvents5 | 0 | 0 | 0 | 0 | |
| GHB and others6 | 10 | 5 | 0 | 0 | |
Participant characteristics and information about substance use. Numbers are reported as percentage (%) and number of group members (n). Categories also include: 1Methamphetamine, phenmetrazine, khat, betel nut; 2crack, freebase, coca leaf; 3heroin and opium; 43,4-methylenedioxymethamphetamine (MDMA), lysergic acid diethylamide (LSD), mescaline, peyote, phencyclidine (PCP), psilocybin; 5Thinner, trichloroethylene, gas, glue; 6γ-hydroxybutyric acid (GHB), anabolic steroids, nitrous oxide, poppers (amyl nitrate) and anticholinergic drugs.
Procedure
Participants attended three experimental sessions separated by a minimum of 7 days to allow complete drug wash out. Baseline subjective state measures were obtained each session before drug administration. Subjects then watched a nature documentary of choice by themselves in a separate room for 1 h, so testing could occur after peak drug uptake. Experimental tasks included tests of monetary reward (Eikemo et al., 2017), face processing (Chelnokova et al., 2014; Chelnokova et al., 2016) and sweet taste reward (Eikemo et al., 2016), in addition to the two touch reward tasks. Tasks were administered in the same pseudo-randomised order, counterbalanced across participants. See Figure 1 for overview of session timeline. To avoid touch satiety, the touch-liking and touch-wanting tasks were separated by at least 15 min. A blood sample was taken at the end of each session to confirm drug uptake. Upon completion of the final session, participants were fully debriefed and reimbursed 400–500 NOK (about 60 US dollars) per session.
Fig. 1.
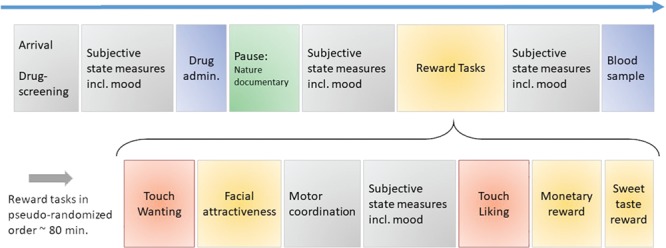
Timeline. Outline of a typical experiment session. The order of reward tasks were pseudo-randomized and counter-balanced between participants.
Drug administration
Morphine is an opioid agonist with high affinity to MOR (Vindenes et al., 2006). To enhance MOR activation with minimal subjective effects, we used 10 mg per oral morphine (Morfin®, Nycomed Pharma, Asker, Norway). The average bioavailability of oral morphine is 30–40%, with a 2–4 h half life (Lugo and Kern, 2002). Naltrexone is a non-specific competitive opioid antagonist with high affinity to μ- and κ-opioid receptors. We used 50 mg per oral naltrexone (Adepend, Orpha-Devel, Purkersdorf, Austria), a standard dosage shown to efficiently block the majority of opioid receptors in the brain (Lee et al., 1988), with only minor side effects in healthy individuals (Miotto et al., 2002; Yeomans and Gray, 2002). Maximal plasma concentration of oral naltrexone is reached at 1 h (Verebey et al., 1976). To ensure stable levels of morphine and naltrexone throughout the session, testing was conducted 60–150 min after drug administration. Placebo tablets visually matched sugar-free cherry-flavoured breath mints. One breath mint was always added to the drug dosages to avoid taste recognition (Chelnokova et al., 2016; Eikemo et al., 2017). Participants were told they might receive an opioid agonist (morphine), antagonist (naltrexone) or placebo at any session. During debriefing, participants were asked to guess which drug they had received each session.
Subjective drug effects and mood
Mood and subjective state (including happiness, anxiety, irritability, feeling good and feeling effect of drug) were collected four times each session, as described previously (Chelnokova et al., 2014; Eikemo et al., 2016). In brief, items were rated on an 11-point electronic visual analogue scale (VAS) anchored by ‘Not at all’ and ‘Very much’, presented on a computer screen (using MatLab version 7.10.0. Natick, Massachusetts; The MathWorks Inc., 2010) before drug administration (t 0), before experiment session (t 60), mid-way through experiment session (t 40) and after completion of all tasks (t 140).
Motor coordination task
As previously described (Eikemo et al., 2016), potential drug effects on motor function and alertness were assessed with an eye–hand coordination test administered mid-way through each session (Bradykinesia Akinesia Incoordination task, Giovannoni et al., 1999). Using their dominant index finger, participants alternated as quickly and accurately as possible between pressing two keys placed 15 cm apart on a standard keyboard for 60 seconds. The Dysmetria score (DS), a weighted index of speed and accuracy reflecting overall task performance (Giovannoni et al., 1999), was used to compare motor function across drug conditions.
Touch tasks
Stimuli
Brush strokes were delivered manually using a soft, 60 mm-wide goat hair artist’s brush, stroking lightly in a proximal-to-distant direction to ~15 cm of the left forearm skin at three different velocities: 0.3, 3 and 30 cm/s. The experimenters were trained to deliver the stimuli prior to the study, focusing on applying the brush strokes with accurate speed and consistent light pressure. Stimuli were administered according to six different sets of counterbalanced pseudorandomised velocity orders. Each default set consisted of 12 brushing trials of 15 s continuous brushing, 4 trials per velocity, with a fixed inter-trial interval of 3 s. The same order set was never delivered twice to the same participant, and the male participants were brushed by the same female experimenter each session. In the touch wanting task, experimenters were guided by a visual meter displayed on a separate screen not visible to the participant. See Figure 2 for experiment setup and Figure 3 for an overview of the task designs.
Fig. 2.
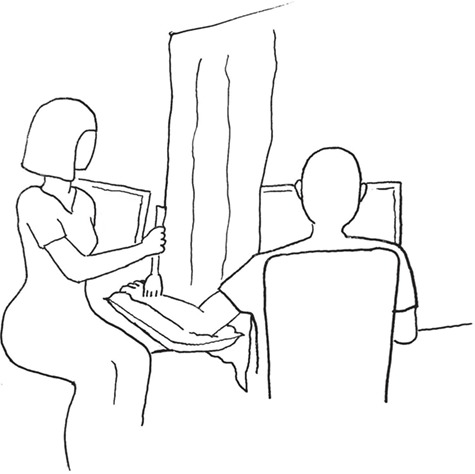
Experiment set-up. Seated in front of a desktop monitor, participants rested their bare left arm on a cushion on the experimenter’s side of a soft curtain, hung from the ceiling to ensure that the participant’s field of view was limited to the screen and did not include the sight of the stimulated limb or the experimenter. On a separate screen, experimenters were guided by a visual meter indicating the brushing speed and remaining trial duration in the touch wanting task.
Fig. 3.
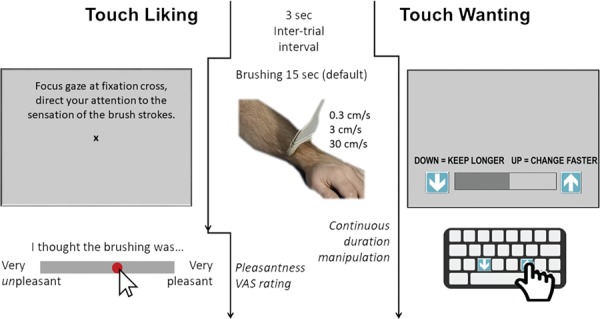
Task designs. Touch liking and touch wanting was measured in two different tasks, separated by a minimum interval of 15 min. Stimuli (brushing strokes of 0.3, 3 and 30 cm/s administered over ~15 cm of the left forearm) and inter-trial intervals were the same in both tasks. Touch liking consisted of 12 trials of 15 s continuous brushing. Participants were instructed to focus on the brushing sensation while resting their gaze at a fixation cross. After each brushing trial, participants rated touch pleasantness by clicking on a VAS scale shown on screen. A 3 s interval followed before the next trial. Touch wanting consisted of brushing trials whose durations could be manipulated by the participant using two buttons—either extending (keep button) or shortening (change button) the duration of each trial. Instructions were displayed on the screen, together with a meter indicating how long was left of the trial. The meter also served as visual feedback on how much they were manipulating the duration. When participants pressed the keep/change buttons on the computer keyboard, the meter would move slower/be brought to a halt/move faster depending on button press activity.
Touch ‘liking’ task
A text appearing on screen for the first 4 s of each brushing trial instructed participants to focus their attention on the sensation of the brush strokes. A fixation cross was then presented mid-screen for the remaining 11 s of the trial. After each brushing trial, participants rated touch pleasantness on a VAS anchored by ‘very unpleasant’ (0) and ‘very pleasant’ (10), with 5 representing neutral pleasantness. E-Prime 2.0® software (Psychology Software Tools Inc., Pittsburg, PA, USA) was used to present visual stimuli and collect subjects’ responses.
Touch ‘wanting’ task
Participants were instructed to manipulate the duration of each ongoing brushing trial according to their own wish, by repeatedly pressing one button to extend trial duration (‘keep’) and another button to shorten it (‘change’). More button presses led to a greater change in duration of the ongoing trial. Participants could monitor the time left of each trial and the effects of their own duration manipulations on a visual meter. Participants were informed that they could only manipulate the trial durations, not the brushing velocity or the total duration of the task. Total task duration was fixed to 216 s. Trials were separated by a 3 s break. In the absence of button presses, the experiment would consist of 12 × 15 s trials. Duration and number of keep presses and change presses for each trial were recorded. MatLab software R2012a (Mathworks, Natic, USA) was used to present the stimuli and collect subjects’ responses.
Behavioural analyses
Control measures
Repeated-measures analyses of variance (rmANOVA) were used to assess drug effects on control measures (mood, subjective drug effect and motor coordination measures). Ratings from the two measurement points closest in time to the touch wanting and liking tasks (t 40 and t 100) were baseline corrected and aggregated as averages. Greenhouse-Geisser correction was used in cases where the assumption of sphericity was violated.
Touch responses
Pre-analysis of Touch Wanting results
The number of trials actually administered during the fixed 216 s task duration was affected by participants’ manipulations of trial durations. Since there was a 3 s fixed interval between trials, total time spent receiving touch thus varied each session as a function of number of trials. Furthermore, the fixed total duration of the task meant that the last trial was ended automatically by the software. To be able to take into account key-pressing activity during all trials, including the final (truncated) trial, we summed up the number of presses to each button for each of the brushing velocities (0.3, 3, 30 cm/s), and then divided each sum by the total touch time available in that session. A composite score was then calculated in which the number of change presses per minute was subtracted from the number of keep presses per minute. This ‘touch wanting’ score is positive when a participant pressed the ‘keep’ button more frequently than the ‘change’ button for a velocity. In contrast, a negative score reflects motivation to end touch of that particular velocity. Using this score has the benefit of avoiding the problems with zero inflation that would occur if the keep presses and change presses were to be analysed separately.
Main analyses
Touch pleasantness ratings and ratio durations were analysed with linear mixed models (LMMs) using the GENLINMIXED command in SPSS (version 22, IBM). Fixed factors relating to the experimental hypothesis and design and recruitment and counterbalancing (such as drug, brushing speed, drug*brushing speed, session and trial order) were always included in the main LMMs. Aside from these variables we aimed for parsimonious models. The remaining covariates such as age, weight and interactions between fixed factors not relating to our hypotheses were removed when they did not significantly affect the outcome and/or did not improve model fit. Adding the random terms for by-subject intercept and slopes improved all LMMs as indicated by lower Bayesian Information Criteria and significant Wald Z statistics. The final models selected for each analysis are described in the result section.
Post hoc analysis of null effects
The lack of a significant result using frequentist statistics does not imply that the null hypothesis is true. To explore the likelihood of a null effect of drug given the data, we performed Bayesian rmANOVAs on aggregated touch pleasantness ratings and on the ‘touch wanting’ scores. Average ratings per brushing speed and drug conditions were calculated and then analysed first with a frequentist rmANOVA, followed up by a Bayesian rmANOVA—both conducted in JASP (version 0.9). For the Bayesian rmANOVA we included brushing speed and subject in the null model, so that these variables were already accounted for—allowing a more direct assessment of the effect of drug and its interaction with brushing speed. The likelihood of this null model given the data (BF01), compared to the alternative models, which also included (i) drug and (ii) drug and the interaction between drug and brushing speed, was assessed with a Jeffreys–Zellner–Siow (JZS) Bayes factor rmANOVA (Rouder et al., 2012) using default prior scales (r scale prior width, 0.5).
Results
Control measures
Subjective effects
As reported in Eikemo et al. (2016), morphine (M) 10 mg and naltrexone (N) 50 mg caused minimal subjective effects compared to placebo (P), and mean changes from baseline for the two drug conditions were less than 1.5 points on the 11-point VAS scale for all subjective state measures. There were no significant effects of drug on mood items (all Fs < 0.4, all Ps > 0.67). Drug condition significantly affected how much the participants reported feeling an effect of the tablet (F2, 96 = 4.60, P = 0.012), reflecting higher ratings on this item in the naltrexone condition compared to the other two [mean (SEM): M = 2.80 (0.36), P = 2.08 (0.35), N = 3.50 (0.38)]. The aggregated ratings of ‘Feeling effect’ from t 40 and t 100 were therefore added to the main analyses to check if the variance in the touch responses explained by the subjective experience of a drug effect was statistically significant. Scores of perceived subjective drug effect did not explain variance in either the touch pleasantness ratings or the touch wanting key press activity to a statistically significant degree and were therefore omitted from the final models.
Drug blinding
At final debriefing, participants were on average able to correctly identify the drug received 33% of the time, indicating successful blinding.
Motor coordination
As reported in Eikemo et al. (2016) there were no significant differences in DSs between drug conditions [mean (SD): M = 1.215 (0.176), P = 1.220 (0.169), N = 1.212 (0.171); F2, 92 = 0.061, P = 0.940, partial η2 = 0.001].
Touch responses
Touch liking—no significant drug effects
The final LMM of pleasantness ratings (N = 35) included the fixed effects: drug, brushing speed, drug*brushing speed, brushing speed order, trial number and session. The random effects structure composed of by-subject random intercept and random slopes for brushing speed, drug and brushing speed*drug, with a variance components covariance structure. The effect of brushing speed was significant [F2, 1220 = 46.17, P < 0.001; estimated mean ratings (SEM): 0.3 cm/s, 4.49 (0.242); 3 cm/s, 7.21 (0.241); 30 cm/s, 5.16 (0.241)], with higher reported pleasantness for 3 cm/s relative to >0.3 cm/s (t1220 = 9.2, P < .001) and 30 cm/s (t1220 = 7.0, P < .001, see Figure 4a). The effect of trial number was also significant (F11, 1220 = 3.76, P < .001). No significant main effect of drug, or interaction between drug and brushing speed, on touch pleasantness ratings was found (Fs < 0.76, all Ps > .555, see Figure 4a).
Fig. 4.
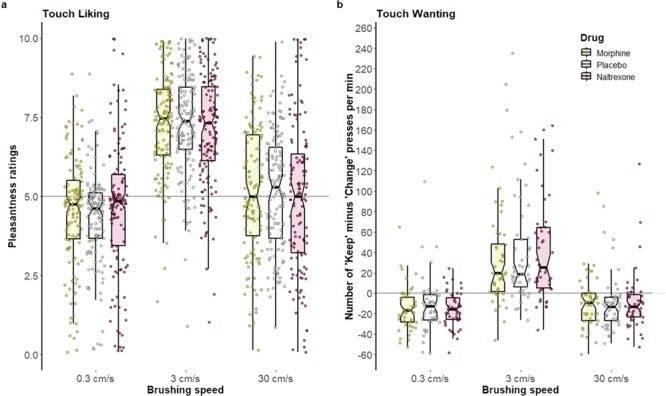
Touch liking and wanting. Notched box plots with scatter plots for (a) pleasantness ratings, and (b) ‘wanting’ scores; horizontal axes set at neutral pleasantness (5) and neutral ‘wanting’ (0). Scatter plot points represent individual raw data points. A random horizontal jitter has been added to enable viewing of all points. For box plots, the lower and upper hinges correspond to the first and third quartiles (the 25th and 75th percentiles). The lower and upper whiskers extend to the smallest and largest values, respectively, no further than 1.5 times the inter-quartile range, which is the distance between the first and third quartiles. The median is indicated by a black band across the boxes, with notches indicating a 95% confidence interval for the median. Non-overlapping notches indicate statistically significant differences at roughly the 95% level (Mcgill et al., 1978). There was a statistically significant main effect of brushing speed on (a) and (b) . There were no statistically significant effects of, or interactions with, drug on (a) or (b) at any level of brushing speed (0.3, 3 and 30 cm/s).
Post hoc analysis of null effect
Note that the following post hoc analyses only include data from 48 participants, due to data missing for one session for one participant. The frequentist rmANOVA yielded comparable results to the main LMM analysis, with a statistically significant main effect of brushing speed following the same pattern (F2, 66 = 47.15, P < .001) and no significant main effect of drug (F2, 66 = 0.17, P = .847) or interaction between drug and brushing speed (F4, 132 = 0.86, P = .481). The JZS Bayes factor rmANOVA (see Table 2) revealed strong evidence supporting the null model in favour of the model including main effects of drug (BF01 = 26.096). Furthermore, there was very strong evidence against including the brushing speed*drug interaction when comparing to the model containing only the main effect of drug in addition to the main effect of brushing speed (BFInclusion = BF interaction model/BF main effects model = 859.191/26.096 = 32.92).
Table 2.
Bayes factor rmANOVA: model comparison
| Touch pleasantness ratings | |||||
|---|---|---|---|---|---|
| Models | P(M) | P(M|data) | BF M | BF 01 | Error, % |
| Null model (brushing speed, subject) | 0.333 | 0.962 | 50.654 | 1 | |
| Drug | 0.333 | 0.037 | 0.077 | 26.096 | 1.696 |
| Drug + drug * brushing speed | 0.333 | 0.001 | 0.002 | 859.191 | 1.663 |
| Touch wanting scores | |||||
| Models | P(M) | P(M|data) | BF M | BF 01 | Error, % |
| Null model (brushing speed, subject) | 0.333 | 0.968 | 61.334 | 1 | |
| Drug | 0.333 | 0.031 | 0.064 | 31.434 | 1.934 |
| Drug + drug * brushing speed | 0.333 | 7.712e-4 | 0.002 | 1255.736 | 1.824 |
Note. All models include brushing speed and subject.
Bayes factor rmANOVA - Model Comparison. (Legend based on Wagenmakers et al., 2018) P(M) indicates prior model probabilities. P (M|data) indicates updated probabilites after having observed the data. BFM indicates the degree to which the data have changed the prior model odds. BF01 shows the Bayes factor for the null model against each model (inverse of BF10 which shows the Bayes factor for each model against the null model). Error % indicates the size of the error in the integration routine relative to the Bayes factor.
Touch wanting—no significant drug effects
The final LMM of touch wanting (N = 49) included the fixed effects: drug, brushing speed, drug*brushing speed, brushing speed order and session. The random effects structure consisted of by-subject random intercept and random slopes for brushing speed, drug and brushing speed*drug, with a scaled identity covariance structure. The effect of brushing speed was significant (F2, 425 = 90.37, P < 0.001; estimated mean ‘touch wanting’ score [mean (SEM): 0.3 cm/s, −14.25 (4.02); 3 cm/s, 37.75 (4.02); 30 cm/s, −9.48 (4.02)], reflecting higher motivation to keep receiving touch at 3 cm/s compared to 0.3 cm/s (t425 = 12.161, P < 0.001) and 30 cm/s (t425 = 11.045, P < 0.001, see Figure 4b). No significant main effect of drug was found (F2, 425 = 0.214, P = 0.807, also Figure 4b), and there was no significant interaction between drug and brushing speed (F4, 425 = 0.788, P = 0.533). Planned pairwise comparisons showed that the expected linear relationship of drugs (M > P > N) at the CT-optimal brushing speed was not found (3 cm/s: N > M: t425 = 1.451, P = 0.442; N > P: t425 = 0.704, P = 0.905; P > M: t425 = 0.752, P = 0.905, see Figure 4b).
Post hoc analysis of null effect
Note that n = 48 for these analyses due to the missing data from one session for one of the participants. The frequentist rmANOVA yielded comparable results to the main LMM analysis, with a statistically significant main effect of brushing speed (F1.33, 62.6 = 48.63, P < 0.001, Greenhouse-Geisser correction for violation of sphericity assumption) and no significant main effect of drug (F2, 97 = 0.57, P = 0.568) or interaction between drug and brushing speed (F3.32, 156.08 = 0.94, P = 0.431, Greenhouse-Geisser correction). The JZS Bayes factor rmANOVA revealed strong evidence supporting the null model in favour of the model including main effects of drug (BF = 31.434). Furthermore, there was very strong evidence against including the brushing speed*drug interaction when comparing to the main effects model (BFInclusion = BF interaction model/BF main effects model = 1255.736/31.434 = 39.95).
Control analyses
Adding ‘task order’ as a fixed factor did not explain a statistically significant amount of variance in the touch liking and wanting tasks and no interactions between ‘task order’ and ‘drug’ were found (all Fs < 0.48, all Ps > .49).
Post hoc sensitivity analyses
To assess the statistical power to detect drug effects specifically to the preferred stimulus (3 cm/s brushing), sensitivity analyses for the liking and wanting responses were conducted post hoc using the software G*Power version 3.1.9 (Faul et al., 2009). The average correlation between the within-subjects measurements for the 3 cm/s brushing was r = 0.681 for touch liking ratings and r = 0.688 for touch wanting scores. With an alpha level of 0.05 we should then have 80% power to detect a small effect of Cohen’s f = 0.147 (Cohen’s d = 0.294) and 90% power to detect an effect of f = 0.166, (d = 0.332).
Discussion
The aim of the present study was to determine the effects of the MOR agonist morphine and the opioid antagonist naltrexone on wanting and liking of gentle caress-like touch in two separate tasks. The findings corroborate previous results showing that CT-optimal brushing at 3 cm/s is consistently rated as more pleasant than brushing at speeds of 0.3 and 30 cm/s, which activate CTs to a lesser degree. Furthermore, the motivational value of CT-optimal brushing was demonstrated in terms of consumption, using a novel touch wanting task where participants engaged in button presses to regulate the amount of each brushing speed they received. Wanting scores for 3 cm/s were significantly higher than those of the very slow or very fast brushing speeds. Contrary to our hypothesis, systemic manipulation of the opioid system with morphine and naltrexone at doses that influenced responses to other reward types (as reported in Chelnokova et al., 2014; Chelnokova et al., 2016; Eikemo et al., 2016; Eikemo et al., 2017) did not significantly affect touch liking or wanting measures. Instead, touch pleasantness ratings were comparable across drug conditions, as were touch wanting scores. Bayesian post hoc analyses supported a rejection of our hypothesis, indicating that the touch pleasantness data were 31 times more likely to occur under the null model than under the model assuming a drug effect. Similarly, the touch wanting data were deemed 26 times more likely under the null model.
Preference for CT-optimal touch evident in both liking and wanting measures
A significant and expected preference for CT-optimal brush stimuli (3 cm/s) was exhibited in both the liking and wanting tasks. Brushing at 3 cm/s was chosen as the main stimulus of interest since it approximates the velocity of naturally occurring affiliative caresses (Croy et al., 2016b) and is an optimal speed for activation of CT-afferents (Löken et al., 2009). We presented the 3 cm/s stimulus in conjunction with brushing at 0.3 and 30 cm/s, which have been consistently rated as less pleasant than more CT-optimal speeds (Löken et al., 2009; Ackerley et al., 2014; Triscoli et al., 2014; Crucianelli et al., 2016). Accordingly, we expected and found that the CT-optimal touch was on average rated as pleasant, while the very slow (0.3 cm/s) and the very fast (30 cm/s) stimuli were rated on average as unpleasant or neutral. A clear preference for CT-optimal touch was similarly exhibited in the wanting task, where participants showed active engagement employing both the ‘keep’ and ‘change’ buttons to manipulate the duration of the touch stimulation. The range of button presses recorded during a single stimulus was 0–597 for the ‘keep’ button and 0–44 for the ‘change’ button. The CT non-optimal speeds both received negative scores on average, reflecting participants’ efforts to receive less of these very slow and very fast brushing stimuli.
No effect of drug on touch liking and wanting
Based on the extensive literature implicating MOR activation in promotion of reward responses in animals as well as humans, we had hypothesised that morphine would increase the pleasantness of touch and motivation to receive touch compared to placebo, while blocking MOR with naltrexone would have the opposite effect—decreasing the experience of pleasure. In line with growing evidence that MOR activation optimises reward behaviour by promoting responses specifically to the most rewarding stimuli available in humans as well as in rodents (Doyle et al., 1993; Giraudo et al., 1993; Mahler and Berridge, 2012; Chelnokova et al., 2014; Chelnokova et al., 2016; Eikemo et al., 2016), we also hypothesised that this linear pattern (morphine > placebo > naltrexone) would be most pronounced for brushing at 3 cm/s. Our results provided no support for the hypothesised involvement of MOR in liking and wanting of the touch stimuli. The null results were corroborated by Bayesian rmANOVAs yielding strong support in favour of the null hypothesis (no effect of drug) over the alternative hypothesis (drug affects touch pleasantness/wanting) given the data. That a dose of 50 mg per-oral naltrexone shown to efficiently block the majority of opioid receptors in the brain (Lee et al., 1988) did not reduce wanting scores or pleasantness ratings for touch in our experiment suggests that endogenous increases in MOR transmission might not be necessary for the experience of light brushing of/on the forearm as pleasant or motivating. The observation that a low dose of morphine did not increase pleasantness ratings or wanting scores further supports the interpretation that MOR activation may not be essential for the enjoyment of or motivation to receive caress-like touch in a laboratory setting. The extent to which one can extrapolate from these findings to other situations and tactile stimuli is unclear.
Considering the animal literature on MOR modulation of social rewards that include touch, the null results were surprising. μ-opioid signalling in the brain is proposed to underpin the pleasant and rewarding properties of social touch in mammals, providing a mechanism that promotes formation and maintenance of long-term relationships for social support (Dunbar, 1980; Panksepp et al., 1980; Seyfarth and Cheney, 1984; Dunbar, 2010; Machin and Dunbar, 2011). Engagement in highly tactile social activities such as social grooming, social play and huddling is associated with MOR functioning in both primates and rodents. Central activation of MOR has been observed in response to social play in juvenile rats (Panksepp and Bishop, 1981; Vanderschuren et al., 1995c, 1995d) and increased cerebrospinal fluid levels of ß-endorphins were measured in monkeys after they received social grooming (Keverne et al., 1989). It is, however, worth noting that the animals in these studies are kept in isolation previous to the social interaction, so the MOR responses could be due to the reinstatement of social contact and may not reflect specifically the tactile aspects of the interaction. Furthermore, some authors highlight opioid contributions to non-hedonic processing (Laurent et al., 2014), although in many cases the lack of a well-matched control condition renders the interpretation unclear (Nummenmaa et al., 2016; Tuulari et al., 2017).
In both animals and humans, touch always happens within a rich multisensory context. As is the case with other rewards, the motivation to engage in specific touch interactions depends on the internal homeostatic and affective state of the animal (Ellingsen et al., 2015; Ellingsen et al., 2016). While touch can be a powerful reward signal that the animal works to achieve, its hedonic value is shaped by surrounding contextual information from other senses (McCabe et al., 2008; Ellingsen et al., 2013; Ellingsen, 2015). Touch pleasantness has previously been successfully modulated under similar experimental conditions as in the current study, by for instance placebo (Ellingsen et al., 2013), smell (Croy et al., 2014) and—in certain contexts only—by oxytocin (Ellingsen et al., 2014;Scheele et al., 2014 ; Kreuder et al., 2017). In these studies, the experimental manipulations altered the context of touch receipt directly through concomitant smell (Croy et al., 2014) by inducing beliefs either about the identity of the toucher (Scheele et al., 2014; Kreuder et al., 2017) or about the participants’ own (increased) ability to experience pleasure (Ellingsen et al., 2013). We speculate that although the MOR system does not appear to be necessary for touch pleasantness, endogenous opioids might nevertheless underpin changes in the reward value of touch as induced for instance by affective relevance, beliefs and expectations (placebo). The majority of psychopharmacological studies on placebo pain relief indicate a substantial contribution of endogenous opioids to expectation-related analgesia (Levine et al., 1978; Amanzio and Benedetti, 1999; Benedetti et al., 1999; Benedetti et al., 2007; Eippert et al., 2009; Rutgen et al., 2015; Rutgen et al., 2017).
The influential finding that opioid microstimulation into ‘hedonic hotspots’ in the ventral pallidum, nucleus accumbens, insula and prefrontal cortex promotes facial ‘liking’ responses (Berridge, 2018) is consistent with a view of the opioid system as the brain’s hedonic regulator (e.g. Koob and Le Moal, 2001). Note that opioid antagonist injections into these regions do not reduce such ‘liking’ responses per se. Instead, opioid signalling in the hotspots only appears necessary for context-induced liking increases (induced by hunger; Smith and Berridge, 2007; Wassum et al., 2009). Accordingly, one might speculate whether previous reports of relatively larger modulations of ratings and behaviour observed for high-value rewards (compared to lower value rewards) by opioid drugs primarily reflect opioid modulation of context effects (i.e. enhanced liking of a very sweet liquid after having had no food or drink for ~2 h; Eikemo et al., 2016) rather than a direct opioid modulation of reward valuation. If this view is correct, one could further argue that the lack of opioid modulation of the highest-value touch stimuli applied here might reflect a smaller or even absent context effect on this primary reward. On the other hand, we cannot exclude the possibility that drug effects on liking and wanting responses to pleasant gentle touch stimuli may be influenced by the rewarding visual, monetary or taste stimuli applied in other tasks during each session (Buchel et al., 2018). Future studies should explore these possibilities, combining touch stimuli and context manipulations.
Methodological considerations
Touch liking
The touch liking task employed here is the most frequently used measurement in the affective touch field (see, e.g. Löken et al., 2009; Case et al., 2016; Sailer et al., 2016; Croy et al., 2016a). While pleasantness ratings reflected the expected effects of brushing speeds, the comparable responses across the three drug conditions yielded no evidence in favour of a role for MOR in the subjective appreciation of brush stimuli to the forearm. A potentially limiting factor in the interpretation of the null effect is the number of stimuli included (only four of each type per session). Nevertheless, weak or unclear effects of MOR drug manipulations on touch liking are also reported by Case et al. (2016), where the effects of naloxone (which increased liking compared to baseline) did not differ significantly from a saline control condition.
Touch wanting
This is, to the best of our knowledge, the first study to demonstrate a motivational value for CT-optimal brushing in terms of self-regulated consumption. This corroborates a previous finding of behavioural preference for CT-activating touch in a task where participants could choose whether the next brush stroke they would receive should be of the same or of a different velocity (Perini et al., 2015). The touch wanting task was based on the wanting task used in Chelnokova et al. (2014), Parsons et al. (2011) and Aharon et al. (2001), where picture-viewing time was upregulated or downregulated with key presses. By enabling participants to engage in instrumental actions to obtain more of the touch they wanted while they were receiving it, we created a measure of consumption of touch reward. Effort exerted to obtain and consume more of a reward is considered to reflect incentive salience—a type of implicit wanting that involves subcortical processing and affects behaviour before and during consumption of a reward, without depending on explicit experiences of desire or liking of the reward (Berridge et al., 2009). Indeed, previous findings indicate that performance on such tasks can reveal dissociations with hedonic ratings in healthy participants (Aharon et al., 2001; Parsons et al., 2011). Hence, our paradigm is likely to tap into such implicit wanting processes.
If the MOR system is mainly involved in the affiliative aspects of touch, the use of a study design, which toned down the social properties of the touch administration (by using a brush and hanging a curtain between the experimenter and participant), could have contributed to the null result. Indeed, the neuroendocrine response to social touch in rodents and primates can vary according to the nature and quality of the relationship between individuals (D'Amato and Pavone, 1993, 1996; D'Amato, 1998; Crockford et al., 2013). Reports of MOR effects on social touch reward in the animal literature typically draw on animals that are housed together and have an already established social relationship. In our case, participants were brushed by experimenters they had no or limited contact with prior to inclusion in the study. Future studies aiming to assess whether MOR also play a role in touch wanting and liking in humans should boost the social significance of the touch provided, by for instance having the experimenter provide certain social cues such as eye contact and facial expressions, administering touch with the hand instead of a brush or including touch provided by someone the participant has a close personal relationship with. Importantly, since the current study only included male participants, results are not immediately generalizable to females. An important avenue for future studies of the neuropharmacology of pleasant touch should be assessment of interactions with variations in hormonal context.
Conclusions
The current investigation explored the involvement of μ-opioid signalling in human touch reward, inspired by evidence implicating MOR in reward across domains and species. We conclude that endogenous opioid signalling is not necessary for the enjoyment of or preference for light stroking touch, when applied in an experimental setting to the forearms of healthy young men. It is possible that endogenous opioids encode the increase of touch pleasantness from contextual cues such as being touched by a loved one, without encoding touch pleasantness per se.
Acknowledgments
We thank I Olsen, L Bachs, V Vindenes and E Øiestad for pharmacological advice; F Willoch for medical advice; and O Chelnokova, H Maurud, J Riegels, LL Knudsen and E Guttormsen for assisting the data collection.
Funding
The project was funded by grant number ES455867 to S.L. from the Research Council of Norway, and grant number 2014100 from the SouthEastern Norway Regional Health Authority to SL.
References
- Achterberg E.J.M., van Swieten M.M.H., Houwing D.J., Trezza V., Vanderschuren L.J.M.J. (2018). Opioid modulation of social play reward in juvenile rats. Neuropharmacology, 10.1016/j.neuropharm.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Ackerley R., Backlund Wasling H., Liljencrantz J., Olausson H., Johnson R.D., Wessberg J. (2014). Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. Journal of Neuroscience, 34(8), 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I., Etcoff N., Ariely D., Chabris C.F., O'Connor E., Breiter H.C. (2001). Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron, 32(3), 537–551. [DOI] [PubMed] [Google Scholar]
- Amanzio M., Benedetti F. (1999). Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. Journal of Neuroscience, 19(1), 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty W.W., Costello K.B. (1982). Naloxone and play fighting in juvenile rats. Pharmacology, Biochemistry, and Behavior, 17(5), 905–907. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Arduino C., Amanzio M. (1999). Somatotopic activation of opioid systems by target-directed expectations of analgesia. Journal of Neuroscience, 19(9), 3639–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Pollo A., Colloca L. (2007). Opioid-mediated placebo responses boost pain endurance and physical performance: is it doping in sport competitions? Journal of Neuroscience, 27(44), 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C. (2018). Evolving concepts of emotion and motivation. Frontiers in Psychology, 9, 1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E., Aldridge J.W. (2009). Dissecting components of reward: 'liking', 'wanting', and learning. Current Opinion in Pharmacology, 9(1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C., Miedl S., Sprenger C. (2018). Hedonic processing in humans is mediated by an opioidergic mechanism in a mesocorticolimbic system. eLife, 7, e39648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case L.K., Ceko M., Gracely J.L., Richards E.A., Olausson H., Bushnell M.C. (2016). Touch perception altered by chronic pain and by opioid blockade. eNeuro, 3(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelnokova O., Laeng B., Eikemo M., et al. (2014). Rewards of beauty: the opioid system mediates social motivation in humans. Molecular Psychiatry, 19(7), 746–747. [DOI] [PubMed] [Google Scholar]
- Chelnokova O., Laeng B., Løseth G., Eikemo M., Willoch F., Leknes S. (2016). The μ-opioid system promotes visual attention to faces and eyes. Social Cognitive and Affective Neuroscience 11(12), 1902–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C., Wittig R.M., Langergraber K., Ziegler T.E., Zuberbühler K., Deschner T. (2013). Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences, 280(1755), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I., D'Angelo S., Olausson H. (2014). Reduced pleasant touch appraisal in the presence of a disgusting odor. PLoS One, 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I., Geide H., Paulus M., Weidner K., Olausson H. (2016a). Affective touch awareness in mental health and disease relates to autistic traits—an explorative neurophysiological investigation. Psychiatry Research, 245, 491–496. [DOI] [PubMed] [Google Scholar]
- Croy I., Luong A., Triscoli C., Hofmann E., Olausson H., Sailer U. (2016b). Interpersonal stroking touch is targeted to C tactile afferent activation. Behavioural Brain Research, 297, 37–40. [DOI] [PubMed] [Google Scholar]
- Crucianelli L., Cardi V., Treasure J., Jenkinson P.M., Fotopoulou A. (2016). The perception of affective touch in anorexia nervosa. Psychiatry Research, 239, 72–78. [DOI] [PubMed] [Google Scholar]
- D'Amato F.R. (1998). Kin interaction enhances morphine analgesia in male mice. Behavioural Pharmacology, 9(4), 369–373. [PubMed] [Google Scholar]
- D'Amato F.R., Pavone F. (1993). Endogenous opioids: a proximate reward mechanism for kin selection? Behavioral and Neural Biology, 60(1), 79–83. [DOI] [PubMed] [Google Scholar]
- D'Amato F.R., Pavone F. (1996). Reunion of separated sibling mice: neurobiological and behavioral aspects. Neurobiology of Learning and Memory, 65(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Day J.A., Mason R.R., Chesrown S.E. (1987). Effect of massage on serum level of beta-endorphin and beta-lipotropin in healthy adults. Physical Therapy, 67(6), 926–930. [DOI] [PubMed] [Google Scholar]
- Doyle T.G., Berridge K.C., Gosnell B.A. (1993). Morphine enhances hedonic taste palatability in rats. Pharmacology Biochemistry and Behavior, 46(3), 745–749. [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M. (1980). Determinants and evolutionary consequences of dominance among female gelada baboons. Behavioral Ecology and Sociobiology, 7(4), 253–265. [Google Scholar]
- Dunbar R.I.M. (2010). The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neuroscience and Biobehavioral Reviews, 34(2), 260–268. [DOI] [PubMed] [Google Scholar]
- Eikemo M., Løseth G., Johnstone T., Gjerstad J., Willoch F., Leknes S. (2016). Sweet taste pleasantness is modulated by morphine and naltrexone. Psychopharmacology (Berlin). [DOI] [PubMed] [Google Scholar]
- Eikemo M., Biele G., Willoch F., Thomsen L., Leknes S. (2017). Opioid modulation of value-based decision-making in healthy humans. Neuropsychopharmacology, 42(9), 1833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Bingel U., Schoell E.D., et al. (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron, 63(4), 533–543. [DOI] [PubMed] [Google Scholar]
- Ellingsen D.M. (2015). How do C-tactile skin afferents contribute to erotic affect? The Journal of Sexual Medicine, 12(7), 1656–1656. [DOI] [PubMed] [Google Scholar]
- Ellingsen D.M., Wessberg J., Eikemo M., et al. (2013). Placebo improves pleasure and pain through opposite modulation of sensory processing. Proceedings of the National Academy of Sciences of the United States of America, 110(44), 17993–17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen D.M., Wessberg J., Chelnokova O., Olausson H., Laeng B., Leknes S. (2014). In touch with your emotions: oxytocin and touch change social impressions while others' facial expressions can alter touch. Psychoneuroendocrinology, 39(1), 11–20. [DOI] [PubMed] [Google Scholar]
- Ellingsen D.M., Leknes S., Kringelbach M.L. (2015). Hedonic value In: Brosch T., Sander D., editors. Handbook of Value: The Affective Sciences of Values and Valuation, Oxford, UK: Oxford University Press. [Google Scholar]
- Ellingsen D.M., Leknes S., Løseth G., Wessberg J., Olausson H. (2016). The neurobiology shaping affective touch: expectation, motivation, and meaning in the multisensory context. Frontiers in Psychology, 6(JAN), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre-Nys C., Meller R.E., Keverne E.B. (1982). Opiate antagonists stimulate affiliative behaviour in monkeys. Pharmacology Biochemistry and Behavior, 16(4), 653–659. [DOI] [PubMed] [Google Scholar]
- Fantino M., Hosotte J., Apfelbaum M. (1986). An opioid antagonist, naltrexone, reduces preference for sucrose in humans. The American Journal of Physiology, 251(1, Part 2), R91–R96. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. (2009). G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fell G.L., Robinson K.C., Mao J., Woolf C.J., Fisher D.E. (2014). Skin β-endorphin mediates addiction to UV light. Cell, 157(7), 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni G., Van Schalkwyk J., Fritz V.U., Lees A.J. (1999). Bradykinesia akinesia incoordination test (BRAIN TEST): an objective computerised assessment of upper limb motor function. Journal of Neurology, Neurosurgery and Psychiatry, 67(5), 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo S.Q., Grace M.K., Welch C.C., Billington C.J., Levine A.S. (1993). Naloxone's anorectic effect is dependent upon the relative palatability of food. Pharmacology Biochemistry and Behavior, 46(4), 917–921. [DOI] [PubMed] [Google Scholar]
- Graves F.C., Wallen K., Maestripieri D. (2002). Opioids and attachment in rhesus macaque (Macaca mulatta) abusive mothers. Behavioral Neuroscience, 116(3), 489–493. [DOI] [PubMed] [Google Scholar]
- Guard H.J., Newman J.D., Lucille Roberts R. (2002). Morphine administration selectively facilitates social play in common marmosets. Developmental Psychobiology, 41(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Hertenstein M.J., Verkamp J.M., Kerestes A.M., Holmes R.M. (2006). The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genetic, Social, and General Psychology Monographs, 132(1), 5–94. [DOI] [PubMed] [Google Scholar]
- Kaada B., Torsteinbo O. (1989). Increase of plasma β-endorphins in connective tissue massage. General Pharmacology, 20(4), 487–489. [DOI] [PubMed] [Google Scholar]
- Keverne E.B., Martensz N.D., Tuite B. (1989). Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology, 14(1–2), 155–161. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. [DOI] [PubMed] [Google Scholar]
- Kreuder A.K., Scheele D., Wassermann L., et al. (2017). How the brain codes intimacy: the neurobiological substrates of romantic touch. Human Brain Mapping, 38(9), 4525–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V., Morse A.K., Balleine B.W. (2014). The role of opioid processes in reward and decision-making. British Journal of Pharmacology, 172, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.C., Wagner H.N. Jr., Tanada S., Frost J.J., Bice A.N., Dannals R.F. (1988). Duration of occupancy of opiate receptors by naltrexone. Journal of Nuclear Medicine, 29(7), 1207–1211. [PubMed] [Google Scholar]
- Levine J.D., Gordon N.C., Fields H.L. (1978). The mechanism of placebo analgesia. Lancet, 2(8091), 654–657. [DOI] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience, 12(5), 547–548. [DOI] [PubMed] [Google Scholar]
- Løseth G., Ellingsen D.M., Leknes S. (2014). State-dependent μ-opioid modulation of social motivation. Frontiers in Behavioral Neuroscience, 8(DEC), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo R.A., Kern S.E. (2002). Clinical pharmacokinetics of morphine. Journal of Pain and Palliative Care Pharmacotherapy, 16(4), 5–18. [DOI] [PubMed] [Google Scholar]
- Machin A.J., Dunbar R.I.M. (2011). The brain opioid theory of social attachment: a review of the evidence. Behaviour, 148(9–10), 985–1025. [Google Scholar]
- Mahler S.V., Berridge K.C. (2012). What and when to "want"? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology, 221(3), 407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel F.L., Nevison C.M., Simpson M.J., Keverne E.B. (1995). Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Developmental Psychobiology, 28(2), 71–84. [DOI] [PubMed] [Google Scholar]
- McCabe C., Rolls E.T., Bilderbeck A., McGlone F. (2008). Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Social Cognitive and Affective Neuroscience, 3(2), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgill R., Tukey J.W., Larsen W.A. (1978). Variations of box plots. The American Statistician, 32(1), 12–16. [Google Scholar]
- Meller R.E., Keverne E.B., Herbert J. (1980). Behavioural and endocrine effects of naltrexone in male talapoin monkeys. Pharmacology Biochemistry and Behavior, 13(5), 663–672. [DOI] [PubMed] [Google Scholar]
- Miotto K., McCann M., Basch J., Rawson R., Ling W. (2002). Naltrexone and dysphoria: fact or myth? American Journal on Addictions, 11(2), 151–160. [DOI] [PubMed] [Google Scholar]
- Morhenn V., Beavin L.E., Zak P.J. (2012). Massage increases oxytocin and reduces adrenocorticotropin hormone in humans. Alternative Therapies in Health and Medicine, 18(6), 11–18. [PubMed] [Google Scholar]
- Morrison I. (2012). CT afferents. Current Biology, 22(3), R77–R78. [DOI] [PubMed] [Google Scholar]
- Niesink R.J., van, Ree J.M. (1984). Neuropeptides and social behavior of rats tested in dyadic encounters. Neuropeptides, 4(6), 483–496. [DOI] [PubMed] [Google Scholar]
- Niesink R.J., Van, Ree J.M. (1989). Involvement of opioid and dopaminergic systems in isolation-induced pinning and social grooming of young rats. Neuropharmacology, 28(4), 411–418. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Tuominen L., Dunbar R., et al. (2016). Social touch modulates endogenous μ-opioid system activity in humans. NeuroImage, 138, 242–247. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Bishop P. (1981). An autoradiographic map of (3H) diprenorphine binding in rat brain: effects of social interaction. Brain Research Bulletin, 7(4), 405–410. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Herman B.H., Vilberg T., Bishop P., DeEskinazi F.G. (1980). Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews, 4(4), 473–487. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Jalowiec J., DeEskinazi F.G., Bishop P. (1985). Opiates and play dominance in juvenile rats. Behavioral Neuroscience, 99(3), 441–453. [DOI] [PubMed] [Google Scholar]
- Parsons C.E., Young K.S., Kumari N., Stein A., Kringelbach M.L. (2011). The motivational salience of infant faces is similar for men and women. PLoS One, 6(5), e20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I., Olausson H., Morrison I. (2015). Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Frontiers in Behavioral Neuroscience, 9(FEB), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price R.C., Christou N.V., Backman S.B., Stone L., Schweinhardt P. (2016). Opioid-receptor antagonism increases pain and decreases pleasure in obese and non-obese individuals. Psychopharmacology, 233(23–24), 3869–3879. [DOI] [PubMed] [Google Scholar]
- Ree J.M., Niesink R.J. (1983). Low doses of beta-endorphin increase social contacts of rats tested in dyadic encounters. Life Sciences, 33(Suppl 1), 611 –614. [DOI] [PubMed] [Google Scholar]
- Rouder J.N., Morey R.D., Speckman P.L., Province J.M. (2012). Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology, 56(5), 356–374. [Google Scholar]
- Rutgen M., Seidel E.M., Silani G., et al. (2015). Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Sciences of the United States of America, 112(41), E5638–E5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgen M., Seidel E.M., Pletti C., et al. (2017). Psychopharmacological modulation of event-related potentials suggests that first-hand pain and empathy for pain rely on similar opioidergic processes. Neuropsychologia, 116, 5–14. [DOI] [PubMed] [Google Scholar]
- Sailer U., Triscoli C., Häggblad G., Hamilton P., Olausson H., Croy I. (2016). Temporal dynamics of brain activation during 40 minutes of pleasant touch. NeuroImage, 139, 360–367. [DOI] [PubMed] [Google Scholar]
- Scheele D., Kendrick K.M., Khouri C., et al. (2014). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology, 39(9), 2078–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G., Troisi A. (1992). Opiate receptor blockade in juvenile macaques: effect on affiliative interactions with their mothers and group companions. Brain Research, 576(1), 125–130. [DOI] [PubMed] [Google Scholar]
- Seyfarth R.M., Cheney D.L. (1984). Grooming, alliances and reciprocal altruism in vervet monkeys. Nature, 308(5959), 541–543. [DOI] [PubMed] [Google Scholar]
- Siegel M.A., Jensen R.A. (1985). The prolonged effects of naloxone on play behavior and feeding in the rat. Behavioral and Neural Biology, 44(3), 509–514. [DOI] [PubMed] [Google Scholar]
- Siegel M.A., Jensen R.A. (1986). The effects of naloxone and cage size on social play and activity in isolated young rats. Behavioral and Neural Biology, 45(2), 155–168. [DOI] [PubMed] [Google Scholar]
- Smith K.S., Berridge K.C. (2007). Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral Pallidum. The Journal of Neuroscience, 27(7), 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y.R., Zubieta J.-K., Carmen M.G.d., et al. (1998). Brain opioid receptor measurements by positron emission tomography in Normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. The Journal of Clinical Endocrinology & Metabolism, 83(12), 4498–4505. [DOI] [PubMed] [Google Scholar]
- Trezza V., Vanderschuren L.J.M.J. (2008a). Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology, 197(2), 217–227. [DOI] [PubMed] [Google Scholar]
- Trezza V., Vanderschuren L.J.M.J. (2008b). Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. European Neuropsychopharmacology, 18(7), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V., Damsteegt R., Achterberg E.J.M., Vanderschuren L.J.M.J. (2011). Nucleus accumbens μ-opioid receptors mediate social reward. The Journal of Neuroscience, 31(17), 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triscoli C., Ackerley R., Sailer U. (2014). Touch satiety: differential effects of stroking velocity on liking and wanting touch over repetitions. PLoS One, 9(11), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari J.J., Tuominen L., de Boer F., et al. (2017). Feeding releases endogenous opioids in humans. The Journal of Neuroscience, 37(34), 8284–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Niesink R.J.M., Spruijt B.M., Van, Ree J.M. (1995a). Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology, 117(2), 225–231. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Niesink R.J.M., Spruijt B.M., Van, Ree J.M. (1995b). μ- and κ-opioid receptor-mediated opioid effects on social play in juvenile rats. European Journal of Pharmacology, 276(3), 257–266. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Stein E.A., Wiegant V.M., Van, Ree J.M. (1995c). Social isolation and social interaction alter regional brain opioid receptor binding in rats. European Neuropsychopharmacology, 5(2), 119–127. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Stein E.A., Wiegant V.M., Van Ree J.M. (1995d). Social play alters regional brain opioid receptor binding in juvenile rats. Brain Research, 680(1–2), 148–156. [DOI] [PubMed] [Google Scholar]
- Vanderschuren L.J.M.J., Spruijt B.M., Hol T., Niesink R.J., Van Ree J.M. (1996). Sequential analysis of social play behavior in juvenile rats: effects of morphine. Behavioural Brain Research, 72(1–2), 89–95. [DOI] [PubMed] [Google Scholar]
- Verebey K., Volavka J., Mule S.J., Resnick R.B. (1976). Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clinical Pharmacology and Therapeutics, 20(3), 315–328. [DOI] [PubMed] [Google Scholar]
- Vindenes V., Handal M., Ripel Å., Boix F., Mørland J. (2006). Conditioned place preference induced by morphine and morphine-6-glucuronide in mice. Pharmacology Biochemistry and Behavior, 85(2), 292–297. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E.J., Love J., Marsman M., et al. (2018). Bayesian inference for psychology. Part II: example applications with JASP. Psychonomic Bulletin and Review, 25(1), 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum K.M., Ostlund S.B., Maidment N.T., Balleine B.W. (2009). Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proceedings of the National Academy of Sciences of the United States of America, 106(30), 12512–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S.C., Beck-Schimmer B., Kajdi M.E., Muller D., Tobler P.N., Quednow B.B. (2016). Dopamine D2/3- and mu-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans. Translational Psychiatry, 6(7), e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans M.R., Gray R.W. (1996). Selective effects of naltrexone on food pleasantness and intake. Physiology & Behavior, 60(2), 439–446. [DOI] [PubMed] [Google Scholar]
- Yeomans M.R., Gray R.W. (1997). Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiology and Behavior, 62(1), 15–21. [DOI] [PubMed] [Google Scholar]
- Yeomans M.R., Gray R.W. (2002). Opioid peptides and the control of human ingestive behaviour. Neuroscience and Biobehavioral Reviews, 26(6), 713. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H., Chamberlain S.R., Nathan P.J., et al. (2012). Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Molecular Psychiatry, 18(12), 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]


