Abstract
In recent years, many attempts have been made to enhance the drug bioavailability and therapeutic effectiveness of oral dosage forms. In this context, various gastroretentive drug delivery systems (GRDDS) have been used to improve the therapeutic efficacy of drugs that have a narrow absorption window, are unstable at alkaline pH, are soluble in acidic conditions, and are active locally in the stomach. In this review, we discuss the physiological state of the stomach and various factors that affect GRDDS. Recently applied gastrointestinal technologies such as expandable, superporous hydrogel; bio/mucoadhesive, magnetic, ion-exchange resin; and low- and high-density-systems have also been examined along with their merits and demerits. The significance of in vitro and in vivo evaluation parameters of various GRDDS is summarized along with their applications. Moreover, future perspectives on this technology are discussed to minimize the gastric emptying rate in both the fasted and fed states. Overall, this review may inform and guide formulation scientists in designing the GRDDS.
Keywords: gastroretentive drug delivery systems, gastric retention time, narrow absorption window, bioavailability, polymer
1. Introduction
Oral drug delivery systems have dominated other drug delivery systems for human administration due to their various advantages including ease of administration, flexibility in formulation, cost-effectiveness, easy storage and transport, and high patient compliance. However, oral drug delivery systems face challenges such as low bioavailability due to the heterogeneity of the gastrointestinal system, pH of the commensal flora, gastric retention time of the dosage form, surface area, and enzymatic activity [1]. Conventional drug delivery systems may not overcome the issues imposed by the gastrointestinal tract (GIT) such as incomplete release of drugs, decrease in dose effectiveness, and frequent dose requirement. Therefore, the failure of conventional drug delivery systems to retain drugs in the stomach may lead to the development of GRDDS. These systems offer several benefits such as prolonged gastric residence time (GRT) of dosage forms in the stomach up to several hours, increased therapeutic efficacy of drugs by improving drug absorption, and suitability for targeted delivery in the stomach. In addition, GRDDS can enhance the controlled delivery of drugs by continuously releasing the drug for an extended period at the desired rate and to the desired absorption site until the drug is completely released from the dosage form [1,2].
GRDDS are feasible for drugs that have low absorption in the lower part of the GIT, are unstable and poorly soluble at alkaline pH, have a short half-life, and show local activity at the upper part of the intestine for eradication of Helicobacter pylori [3,4,5,6,7,8,9,10,11,12,13,14,15]. Several formulation strategies have been used to design successful controlled release GRDDS including superporous hydrogel, bio/mucoadhesive, raft-forming, magnetic, ion-exchange, expandable, and low- and high-density systems [3,4,5,6,7,8,9].
Various formulation-related factors such as polymer types (nonionic, cationic, and anionic polymers), polymer composition in dosage form, viscosity grade, molecular weight of the polymer, and drug solubility can affect the quality of the gastroretentive dosage form [9]. Moreover, the physicochemical nature of excipients plays an important role in various GRDDS. For instance, density of excipients and composition of effervescent agents are critical factors in effervescent floating systems. In the case of superporous hydrogel systems, high swelling excipients such as crospovidone and sodium carboxymethylcellulose are required to form a superporous hydrogel [9,16]. Likewise, process variables can also influence the quality of the gastroretentive dosage form, as the density of a tablet can be altered by the compression pressure during tableting [9].
The main purpose of this review is to provide information on various GRDDS that have been developed to date, as well as the physiological state of the stomach, suitable drug candidates for GRDDS, factors affecting GRDDS, and in vitro and in vivo characterization of GRDDS. In addition, challenges and future perspectives on GRDDS are discussed.
2. Physiology of Stomach
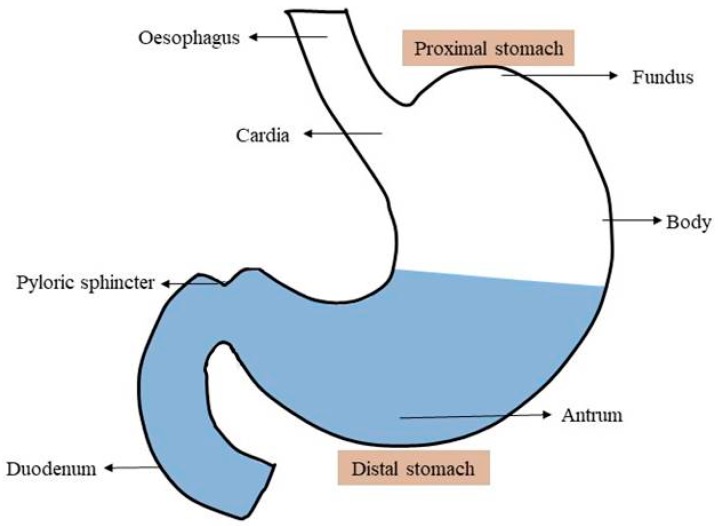
In the GRDDS, the stomach has a crucial role; therefore, a good understanding of the anatomy and physiology of the stomach is a prerequisite for successful development of the gastroretentive dosage form. Anatomically, the stomach is divided into two parts: the proximal stomach, which consists of the fundus and body; and the distal stomach, which consists of the antrum and the pylorus as shown in Figure 1. The main role of the stomach is to store the food temporarily, grind it, and then slowly release it into the duodenum [17]. The fundus and body primarily act as reservoirs for undigested food, whereas the antrum acts as a pump to assist in gastric emptying by a propelling action [17,18]. The mobility pattern of the stomach is termed as the migrating myoelectric complex (MMC); the different phases of the MMC are presented in Table 1. Gastric emptying occurs in both the fed and fasted states, but the pattern of gastric emptying drastically varies between both states. In the fasted state, an interdigestive sequence of electrical events follows in a cyclic manner through both the stomach and the small intestine every 90–120 min [17]. During the interdigestive phase, the diameter of the pylorus increases up to approximately 19 mm [1,19]. As a result, particles smaller than the diameter of pyloric sphincter can easily evacuate from the pylorus to the duodenum during the interdigestive phase [19,20]. However, in the fed state, motor activity is generated 5–10 min after ingestion of a meal and continues as long as the food remains in the stomach, which can delay the gastric emptying rate.
Figure 1.
Schematic view on the anatomy of stomach.
Table 1.
| Phase | Comments | Duration |
|---|---|---|
| Phase 1 | Quiescent period with rare contractions. | 30–60 min |
| Phase 2 | Intermittent action potentials and contraction that gradually increases in intensity and frequency as the phase progresses. | 20–40 min |
| Phase 3 | Short periods of intense, large, regular contractions. This phase is termed as “housekeeper wave” as it enables all undigested materials to be swept out of the stomach and down to the small intestine. | 10–20 min |
| Phase 4 | Occurs between phase 3 and phase 1 of two consecutive cycles in a brief transitional phase. | 0–5 min |
3. Application of GRDDS
Suitable drug candidates for GRDDS are introduced in Table 2. Even though various types of GRDDS are reported in the literature, floating and mucoadhesive systems are the most popular gastroretentive dosage forms in pharmaceutical companies and contribute the most to the market. Table 3 presents the commercially available gastroretentive dosage forms.
Table 2.
Suitable drug candidates for gastroretentive drug delivery systems (GRDDS).
| Bioavailability Challenges | Drug | Therapeutic Indications | References |
|---|---|---|---|
| Local activity | Ranitidine, Amoxicillin, Levofloxacin, Metronidazole | Peptic ulcer and reflux esophagitis, eradication of H. pylori | [1,13,21,22,23,24] |
| Plasma fluctuations | Ciprofloxacin, Clarithromycin |
Urinary tract, respiratory, and GI infections | [1,25,26,27,28] |
| Low solubility at alkaline pH | Ofloxacin | Urinary tract, respiratory, and GI infections | [1,10] |
| Cinnarizine | Nausea, vertigo, and motion sickness | [15] | |
| Narrow absorption window | Riboflavin | Essential nutrients, mouth ulcer and sore throat | [29,30] |
| Cilostazol | Inhibits platelet aggregation | [4] | |
| Pregabalin | Fibromyalgia, diabetic peripheral neuropathy, post-herpetic neuralgia, and adjunctive therapy for partial onset seizures | [5,31] | |
| Short half-life, narrow absorption window | Levodopa | Parkinson’s disease | [32] |
| Metformin | Type II diabetes mellitus | [7,9,33,34] | |
| Poor absorption from lower GIT | Atenolol | Hypertension | [1] |
| Lafutidine | Gastric and duodenal ulcers | [35] | |
| Unstable at alkaline pH | Verapamil, Captopril | Hypertension | [1,11,14] |
Table 3.
| Delivery Systems | Brand Name | Active Ingredient | Manufacturing Company |
|---|---|---|---|
| Bioadhesive tablets | Xifaxan® | Rifampicin | Lupin, India |
| Bilayer floating capsule | Cytotec® | Misoprostol | Pfizer, UK |
| Coated multi-layer & swelling system | Baclofen GRS® | Baclofen | Sun Pharma, India |
| Colloidal gel forming floating system | Conviron® | Ferrous sulphate | Ranbaxy, India |
| Effervescent floating system | Zanocin OD® | Ofloxacin | Ranbaxy, India |
| Riomet OD® | Metformin hydrochloride | Ranbaxy, India | |
| Cifran OD® | Ciprofloxacin | Ranbaxy, India | |
| Effervescent floating liquid alginate preparation | Liquid Gaviscon® | Alginic acid and sodium bicarbonate | Reckitt Benckiser Healthcare, UK |
| Effervescent and swelling based floating system | Prazopress XL® | Prazosin hydrochloride | Sun Pharma, Japan |
| Erodible matrix based system | Cipro XR® | Ciprofloxacin hydrochloride and betaine | Bayer, USA |
| Expandable system (unfolding) | Accordion Pill® | Carbidopa/levodopa | Intec Pharma, Israel |
| Raft forming system | Topalkan® | Aluminum magnesium | Pierre Fabre Medicament, France |
| Almagate FlatCoat® | Aluminium-magnesium antacid | Pierre Fabre Medicament, France | |
| Floating system—controlled release capsule | Madopar HBS® | Levodopa and benserzide | Roche, UK |
| Prolopa HBS® | Levodopa and benserzide hydrochloride | Roche, UK | |
| Valrelease® | Diazepam | Roche, UK | |
| Foam based floating system | Inon Ace Tables® | Simethicone | Sato Pharma, Japan |
| Gastroretention with osmotic system | Coreg CR® | Carvedilol | GlaxoSmithKline, UK |
| Minextab Floating®—floating and swelling system | Metformin HCl | Metformin hydrochloride | Galanix, France |
| Cafeclor LP | Cefaclor | Galanix, France | |
| Tramadol LP | Tramadol | Galanix, France | |
| Polymer based swelling technology: AcuFormTM | Gabapentin GR | Gabapentin | Depomed, USA |
| proQuin XR | Ciprofloxacin | Depomed, USA | |
| Glumetza | Metformin hydrochloride | Depomed, USA | |
| Metfromin GRTM | Metformin hydrochloride | Depomed, USA |
4. Critical Factors Affecting GRDDS Efficacy
There are various factors that affect the performance of gastroretentive dosage forms. These factors are mainly categorized into pharmaceutical factors, physiological factors, and patient-related factors.
4.1. Pharmacutical Factors
For the successful design of GRDDS, it is important to understand the role of excipients and polymers on various types of GRDDS [9]. For instance, in the mucoadhesive system, polymers with high mucoadhesion strength, such as carbopol and hydroxypropyl methylcellulose (HPMC) may be required for successful design of the mucoadhesive dosage form. Likewise, with the expandable system, polymers with high swelling properties are more desirable. Moreover, the molecular weight, viscosity, and physiochemical properties of polymers can also affect the dosage form. Other formulation components such as gas generating agents in an effervescent floating tablet, high swelling excipients of sodium croscarmellose, and crospovidone for superporous hydrogels may be required.
Moreover, the shape and size of the dosage unit is also important [38]. Garg and Sharma reported that ring shape and tetrahedron-shape dosage forms have a longer GRT compared to other shapes [39]. In most cases, the GRT of the dosage form is proportionately dependent on the size. An increase in the size of the dosage form could prevent its passage through the pyloric antrum in the intestine due to the size of the dosage form being larger than the pyloric sphincter diameter (mean, 12.8 ± 7 mm) [40]. Similarly, the density of the dosage form is also an important factor for low- and high-density systems. In low-density systems, the density of the dosage forms should be lower than that of the gastric fluid (1.004 g/cm3) in order to float in the gastric environment [41,42]. Increasing the floating capacity can improve the GRT of the low-density system; however, this effect is decreased in the presence of food. Moreover, the floating force of the dosage form decreases as a function of time, which could be due to the hydrodynamic equilibrium [43]. On the other hand, in high-density systems, the density of the dosage form should be greater than that of the gastric fluid so that it can sink in the bottom of the stomach and prevent gastric emptying. An increase in density of the dosage form greater than 2.500 g/cm3 enhances the GRT [44].
4.2. Physiological Factors
Several studies have reported that various extrinsic factors including the nature of meal, caloric content (caloric density and nature of the calories), frequency of ingestion, posture, sleep, and physical activity can affect the GRTs of drugs in the stomach [1,38,45,46]. In fasting states, gastrointestinal motility is represented by the MMC that occurs every 90–120 min [17]. During this period, motor activity sweeps undigested material from the stomach. If the timing of formulation administration coincides with that of the MMC, the GRT of the unit is very short. However, in the presence of food in the stomach, the MMC is interrupted and housekeeper waves are not generated leading to a prolonged GRT [17,47]. Likewise, the gastric emptying rate is also affected by the caloric density and nature of the calories of the ingested food [45]. In general, an increase in the caloric density significantly increases the GRT whereas the nature of calories only have a minor effect in the GRT [48]. In addition, high food viscosity may also increase the GRT [49,50]. Furthermore, the GRT is influenced by posture, and the effect is different for floating and non-floating dosage forms [1]. In the upright position, the floating system floats in the gastric fluid for a prolonged amount of time which can eventually increase the GRT. However, in similar conditions, the non-floating system remains in the lower part of the stomach and the gastric emptying rate is faster as a result of peristaltic contractions [1]. In contrast, in the supine position, the non-floating system has a longer GRT compared to the floating system [51,52].
4.3. Patient-Related Factors
Patient-related factors such as gender, age, illness, and emotional state can influence GRDDS. A recent study reported that gender affected the gastric emptying time and intraluminal pH [53]. The authors demonstrated that females had slower gastric emptying times than males [53]. Hormonal influences could explain the longer GRT in females than in males. Another study showed that males secreted more gastric acid compared to females [54]. Likewise, the age of the patient also affects the GRT. Elderly patients have a longer GRT compared to younger patients [55]. The nature of a patient’s illness may also affect the GRT of the dosage form. For instance, patients with Parkinson’s disease have a prolonged GRT that is frequently accompanied by constipation [56]. Likewise, in diabetic patients, gastric emptying is decreased by 30–50% [57]. The emotional condition of a patient may also influence GRDDS. It was reported that a decrease in gastric emptying rate was observed in patients suffering from depression, whereas an increased rate was observed in patients experiencing anxiety [38,47].
5. Current Pharmaceutical Technologies of GRDDS
In this section, we describe currently used gastroretentive drug delivery approaches. The main mechanism of GRDDS includes floating, sinking, swelling, effervescence, mucoadhesion, and magnetic properties. A brief description for each system is summarized in Table 4.
Table 4.
Summarized mechanisms of the various GRDDS.
| Gastroretentive Approach | Mechanism | References |
|---|---|---|
| Low-density systems/ floating systems | System causes buoyancy in gastric fluid. Density of pellets/tablets is lower than the density of stomach fluid. | [1,17,20,47] |
| High density systems | Uses the density of dosage form as a strategy to produce the retention mechanism. Sinking system remains at the bottom of the stomach, where the density of the dosage form is greater than the gastric fluid. | [1] |
| Expandable systems | Expansion of the dosage form occurs by swelling or unfolding in the stomach. Swelling usually occurs because of diffusion. Unfolding takes place due to mechanical shape memory. | [6,12,61] |
| Bioadhesive systems | A very complex process with several mechanisms, including electrical theory, adsorption, wetting, diffusion, and fracture theories. The interaction between the negatively charged mucosal surface and positively charged polymers might facilitate the bioadhesive process. | [12,45] |
| Raft forming systems | The polymer in presence of mono or di valent cations, absorbs water, swells and forms in situ gel layers, which float above gastric fluid and termed as raft. | [62,63] |
| Super-porous hydrogel systems | Swells up to 100 times due to water update by capillary wetting through numerous pores. | [12,64] |
| Magnetic systems | Consists of the small internal magnet mixed with the drug. Its position inside the stomach is controlled by an extracorporeal magnet. | [16] |
| Ion-exchange resin systems | Drug is loaded into the resin to form the resin loaded drug complex, which can be combined with floating delivery or bioadhesive systems. | [16] |
5.1. Low-Density Systems
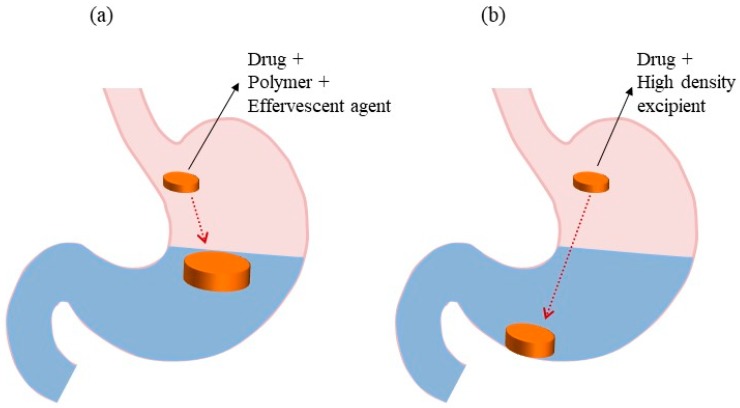
Low-density/floating systems are the most practical and extensively studied gastroretentive dosage forms [1,17,58,59]. The floating system was first introduced by Davis in 1968. In this system, the bulk density of the dosage form is lower than that of the gastric fluid (1.004 g/cm3). This property allows the system to remain buoyant in the stomach for a prolonged period of time while the drug is released at the desired rate from the system during the GRT [1,17,60]. Figure 2a illustrates the concept of low-density systems. These systems are classified into two subtypes based on the mechanism of buoyancy: non-effervescent floating and effervescent floating systems.
Figure 2.
GRDDS based on (a) low-density systems and (b) high-density systems.
5.1.1. Non-Effervescent Floating Systems
In non-effervescent systems, highly swellable cellulose derivatives or gel-forming polymers are used [5]. The formulation technique of non-effervescent systems involves mixing the drug with a gel-forming polymer. Various non-effervescent systems include the hydrodynamically balanced system (HBS), single- and double-layer floating tablets, and microballoons/hollow microspheres.
The HBS system was first designed by Sheth and Tossounian in 1984 [65]. It is a single unit dosage form composed of one or more gel-forming hydrophilic polymers. HPMC, hydroxy propyl cellulose (HPC), hydroxyethylcellulose, sodium carboxymethylcellulose, carrageenan, agar, and alginic acid are some of the polymers that are used to design the HBS system [17,66]. In this system, the drug is mixed with the polymer and filled in the gelatin capsule.
The floating tablet can be designed by uniform mixing of the drug and gel-forming hydrophilic polymer, which hydrates and swells upon contact with the gastric fluid and maintains the bulk density of the tablet at <1 g/cm3 [17,67]. Thus, the low-density systems float on the gastric fluid and prolong the GRT. The commonly used hydrophilic polymers in floating tablet include HPMC, polyethylene oxide, HPC, and cellulose acetate phthalate. Wei et al. [34] studied the bilayer floating tablet containing an immediate-release layer and sustained-release layer of the drug. The immediate-release layer contained a disintegrating agent, which aided the prompt release of drug, whereas the sustained release layer contained a hydrophilic polymer to control the drug release rate and also provided the tablet buoyancy. Drug-loaded microballoons/hollow microspheres are formulated by simple solvent evaporation or solvent diffusion techniques [68], and are multiple-unit floating systems. Polycarbonate, cellulose acetate, calcium alginate, Eudragit S, agar, and low-methoxylated pectin are commonly used polymers to design microballons. Various formulation variables such as the amount of polymer, ratio of plasticizer and polymer, and solvent can affect the floating behavior and drug release of these kinds of dosage forms [69]. One drawback of the HBS is that this system, being a matrix formulation, consists of a blend of drug and low-density polymers. The release kinetics of the drug cannot be changed without changing the floating properties of the dosage form and vice versa.
5.1.2. Effervescent Floating Systems
Effervescent floating systems include a gas-generating agent and volatile liquids. This approach has been applied for single- and multiple-unit systems. In the gas-generating floating system, effervescent agents such as sodium bicarbonate, calcium carbonate, tartaric acid, and citric acid are used in combination with hydrophilic polymers [9,70]. When this system comes into contact with gastric fluid, CO2 is liberated due to the reaction of the effervescent agent with gastric fluid. The liberated CO2 gas is entrapped in the hydrocolloid matrix, which provides the tablet buoyancy and influences the drug release properties [71]. In volatile liquid systems, volatile liquids such as ether and cyclopentane are introduced into an inflatable chamber, which volatilize at body temperature allowing inflation of the chamber in the stomach [38]. Hydrophilic polymers are often used to control the drug release rate in this system.
Effervescent floating systems can be categorized into single- and double-layer effervescent floating tablets and multiple-unit effervescent floating systems [14,17]. Single-layer effervescent tablets are formulated by intimately mixing effervescent agent, polymer, drug, and excipients. However, in bilayer effervescent floating tablets, one layer comprises the drug, polymer, and CO2 gas-generating agent, whereas the other layer constitutes an immediate-release drug and excipients without CO2 and polymer. In a recent study, sodium bicarbonate in HPMC matrix formulation was used to improve the GRT by increasing the hydration volume of dosage form and increasing the surface area of drug diffusion [14]. In addition, an increase in the amount of sodium bicarbonate decreased the drug release rate from the matrix, which could be due to obstruction of the diffusion path by CO2 gas bubbles [14]. Another study also utilized this approach to evaluate the in vitro and in vivo behaviors of ciprofloxacin hydrochloride effervescent floating tablets [72]. The optimized formulation was selected based on the GRT in humans (i.e., 5.50 ± 0.77 h). Multiple-unit effervescent floating systems consist of sustained-release pills as seeds surrounded by double layers. The inner layer contains effervescent agents such as sodium bicarbonate, calcium carbonate, and tartaric acid whereas the outer layer consists of polymers with swelling properties [17]. A low density system may be associated with problems such as sticking together or being obstructed in the GIT, which could produce gastric irritation. This system requires high fluid levels in the stomach to float and work effectively. Therefore, drugs with irritant effects on the gastric mucosa are not suitable candidates for low-density systems [1,17,20,47].
5.2. High-Density Systems
High-density systems have a density greater than that of gastric fluid (Figure 2b). Commonly used excipients of these systems include barium sulfate, zinc oxide, iron powder, and titanium dioxide [17]. In 1930, Hoelzel first discovered the effects of dosage form density on the GRT of several animal species. The densities of the tested dosage forms ranged from 0.9 to 10.5 g/cm3. The author concluded that high-density materials had slower GRTs than light-density materials. Thereafter, the impact of dosage form density on GRT has been studied. Garg and Gupta [51] reported that small high-density pellets are able to resist gastric peristaltic movements due to their retention in the antrum rugae or folds, increasing the gastrointestinal tract time from 5.8 to 25 h. Even though this system has the potential to improve the GRT, it is difficult to design high-density pellets containing high-dose drugs. Moreover, only a few clinical studies on high-density pellet formulations have been reported in the literature; as a result, the clinical significance of these systems is still questionable [73]. Therefore, future directions need to be focused on animal studies to investigate the clinical significance of such dosage forms.
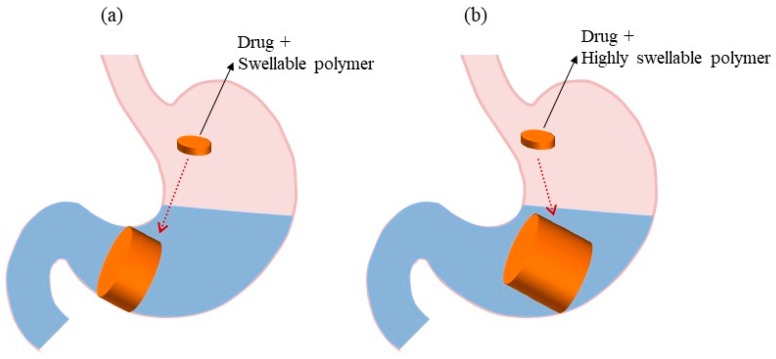
5.3. Expandable Systems
Expandable drug delivery systems are designed to have a longer GRT through an increase in their volume or shape (Figure 3a). Initially, they were used for veterinary purposes and, subsequently, their applications were extended to humans [51]. Three general configurations need to be considered for the proper functioning of the system: small size for easy oral intake, expanded form in the stomach to prevent passage through the pyloric sphincter, and size reduction of the system after complete drug release to enable evacuation [1,6,12]. This system is also termed as a “plug type system” because it has the ability to block the pyloric sphincter. Expansion of the system occurs by two methods, swelling and unfolding, which allow for volume and shape modification, respectively [6,18]. The main mechanism for swelling and drug release from the system is diffusion. These systems utilize hydrophilic polymers (e.g., HPMC, polyethylene oxide, and carbopol) that can absorb water from the gastric fluids and increase the volume of the system. Likewise, in unfolding systems, the polymer and drug are in a folded/compressed state inside the gelatin capsule. When they come into contact with the gastric fluid, gelatin is dissolved and releases the mechanically preferred expanded configuration. Different geometrical forms of biodegradable polymer can be prepared and compressed within a capsule [12]. It is crucial to select a suitable biodegradable polymer with an appropriate molecular weight, viscosity grade, and swelling properties to maintain the sustained release profile of the dosage form [12,74]. Various novel polymers have the ability to swell promptly in contact with the GI fluid. Sivaneswari et al. developed and characterized a novel expandable GRDDS of levetiracetam based on an unfolding mechanism. In their study, the drug was loaded onto a polymeric patch made of HPMC, carbopol 934 P, and xanthum gum, which was designed to adhere to the gastric mucosa, where the drug was released in a sustained manner. Chen et al. [61] formulated GRDDS tablets of Losartan using an equivalent ratio of hydroxyethylcellulose and chitosan, and sodium bicarbonate was added as a gas-generating agent. The optimized formulation showed a sustained release profile of more than 16 h with good swelling and floating behavior. Their results suggested that the addition of sodium bicarbonate could improve the floating ability of the dosage form but might reduce the swelling ability of the low viscosity grade of chitosan. Therefore, the optimum ratio of the polymer and gas-generating agent is necessary to obtain the preferred GRDDS. However, expandable systems have a few limitations such as difficulty in storing easily hydrolysable, biodegradable polymers; being difficult to manufacture and may not be cost-effective; difficulty in maintaining the structural integrity; and may cause bowel obstruction, intestinal adhesion, and gastropathy [6,12,61].
Figure 3.
GRDDS based on (a) expandable systems and (b) superporous hydrogel systems.
5.4. Superporous Hydrogel Systems
In 1998, the superporous hydrogel was presented as a different category of water-absorbent polymer system. This system has gained popularity in the controlled-release formulation due to its high mechanical strength and elastic properties [75]. It has a pore size greater than 100 μm, and as a result, it swells rapidly to an equilibrium size due to water uptake by capillary wetting through numerous pores. Figure 3b depicts the schematic concept of the superporous hydrogel system. The conventional hydrogel system is a slow process and takes several hours to reach equilibrium; thus, the dosage form can be easily evacuated from the stomach. On the contrary, the superporous hydrogel systems swell up to 100 times or more, and gain enough mechanical strength to withstand pressure by gastric contraction, thereby increasing the GRT. Highly swellable polymers, such as croscarmellose sodium and sodium alginate are used in these systems [12,64]. However, these systems can be highly sensitive to pH, and swelling can be reversible due to changes in pH and poor mechanical strength of the structure [12,64].
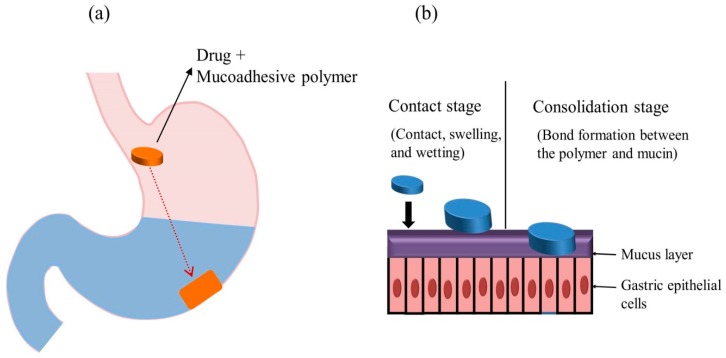
5.5. Bioadhesive/Mucoadhesive Systems
The mucoadhesive/bioadhesive system was first introduced by Park and Robinson in 1984 [76]. It was designed to adhere to the gastric epithelial cell surface and prolong the GRT of drug compounds [8,17]. Figure 4a illustrates the concept of this system. In this approach, drugs are incorporated in a mucoadhesive agent, which can be either natural or synthetic polymers. Bonding established between the polymer and mucosal surface facilitates the mucoadhesion process [77], which generally involves two steps: the contact stage and the consolidation stage (Figure 4b) [78]. The mechanism of mucoadhesion is highly complex and is not fully understood; however, different theories have been postulated, as summarized in Table 5. Various gastrointestinal mucoadhesive dosage forms such as beads, microspheres, films, capsules, and tablets have been prepared and reported in the literature [79]. Commonly used mucoadhesive polymers include carbopol, chitosan, sodium alginate, HPMC, polyethylene glycol, and poly(acrylic acid) [12,17]. Mucoadhesive polymers assist in binding drug substances to the mucosal surfaces and prolonging the drug residence time at the application site. An ideal mucoadhesive polymer is inert, non-irritating, nontoxic, adheres to the mucosal surface, and possesses site specificity; and interacts with the mucin through electrostatic, disulfide, hydrogen, and hydrophobic bonding. The mucoadhesive properties and interaction strength of the polymer depend on the molecular weight, structure, flexibility of the polymeric chains, hydrogen bonding capacity, cross-linking density, charge, concentration, or hydration degree of the polymer [80].
Figure 4.
Mucoadhesive GRDDS (a) general representation of mucoadhesive systems and (b) mechanism of mucoadhesive systems.
Table 5.
| Theories | Mechanisms of Mucoadhesion |
|---|---|
| Wettability | Bioadhesive polymers penetrate and develop intimate contact with the mucous layers. |
| Diffusion | Physical entanglement of mucin strands and flexible polymer chains. Influenced by molecular weight, cross-linking density, chain flexibility, and expansion capacity of both networks. |
| Adsorption | Bioadhesion is due to primary forces (ionic, covalent, and metallic) and secondary forces (van der Waals, hydrophobic and hydrogen bonds) between surfaces. |
| Electronic | Attractive electrostatic forces between the glycoprotein mucin network and the bioadhesive material. |
| Fracture | Detachment force needed to separate the mucus and polymer reflects the force of the adhesive binding. |
Various studies have focused on the combination of floating and mucoadhesive properties in order to improve the GRT of the dosage form by forming mucoadhesive floating drug delivery systems (MFDDS) [61,80,82,83,84]. Liu et al. developed a hollow-bioadhesive microsphere of psoralen using glycerol monooleate as a bioadhesive polymer. The prepared microspheres showed strong mucoadhesive properties with good buoyancy both in vitro and in vivo. Moreover, the pharmacokinetic analysis of the drug in the microsphere showed the prolonged elimination half-life time and reduction in elimination rate. Recently, a novel gastroretentive oil entrapped alginate beads containing resperidone was prepared by using ionotropic emulsion gelation technique. The alginate beads were coated with the cross-linked alginate sterculia gum gel membrane. The dosage form demonstrated good floating and ex vivo mucoadhesion behavior and sustained drug release characteristics [84]. Generally, different types of ex vivo mucosal tissues are used to evaluate the mucoadhesive properties (total work of mucoadhesion, force of system detachment, percentage of mucoadhesion) of the dosage form [85,86,87]. However, mucosal tissues taken from laboratory animals can be difficult to obtain and are not considered good from an ethical point of view. In such cases, synthetic hydrogels that can mimic biological mucosa could be a good synthetic substitute for animal tissues. The hydrophilic structure of the hydrogels renders them capable of imbibing large amounts of water in their three-dimensional networks and resembling the properties of biological tissues [88]. In this system, specific targeting might be difficult because composition of the mucus is different according to the region of the mucous membrane. In addition, the constant turnover of the mucus, and high stomach hydration might decrease the bioadhesion of polymers. Moreover, there is high risk of adhesion to the esophagus which may lead to collateral lesions [12,45].
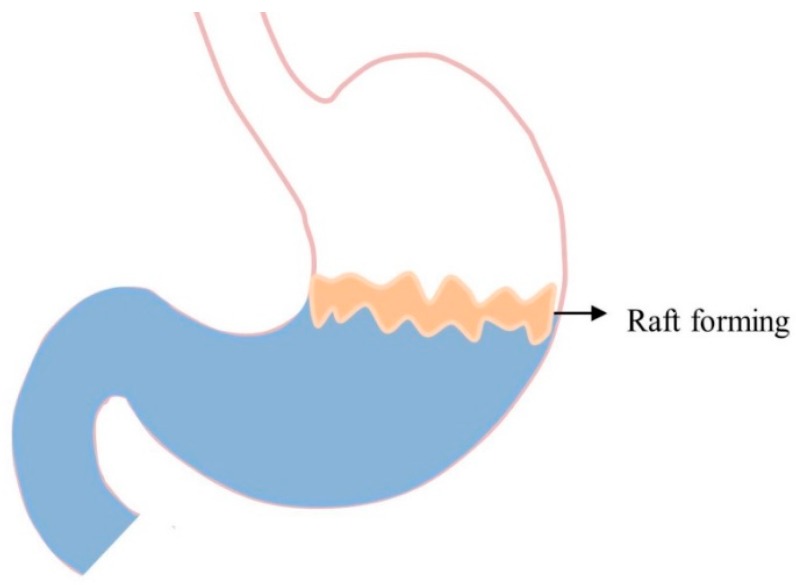
5.6. Raft-Forming Systems
Raft-forming systems are another type of GRDDS, formulated with effervescent excipients and gel forming polymers in order to achieve the sustained drug delivery. Figure 5 illustrates the concept of these systems, which mainly focuses on achieving localized effects because floating rafts act as blockades between esophagus and stomach. Thus, they can be used for the effective management of gastric esophageal reflux disease. When raft-forming systems come into contact with gastric fluid, they swell and form a viscous cohesive gel leading to the formation of a continuous layer termed as rafts [17,62]. Fabregas et al. [89] explained the antacid raft-forming floating system. The authors used sodium alginate as a gel-forming polymer, and sodium bicarbonate and acid neutralizer as gas-generating agents. Thus, CO2 gas is generated that lowers the bulk density of the system, and as a result, the raft floats on the gastric fluid. Nabarawi et al. [62] developed a controlled release floating raft system of mebeverine hydrochloride, and evaluated different excipients for their floating behavior and in vitro controlled-release. It forms a viscous and cohesive gel when it swells and entraps CO2 bubbles produced by the reaction of carbonates and gastric fluid [12]. The formed raft can remain intact in the stomach for several hours, promoting the sustained release of the drug. Such rafts are particularly useful for delivering antacid drugs such as aluminum hydroxide, calcium carbonate, and simethicone [90]. However, the mechanical strength of the systems is weak and can be easily disrupted by the MMC [62,63].
Figure 5.
GRDDS based on raft-forming systems.
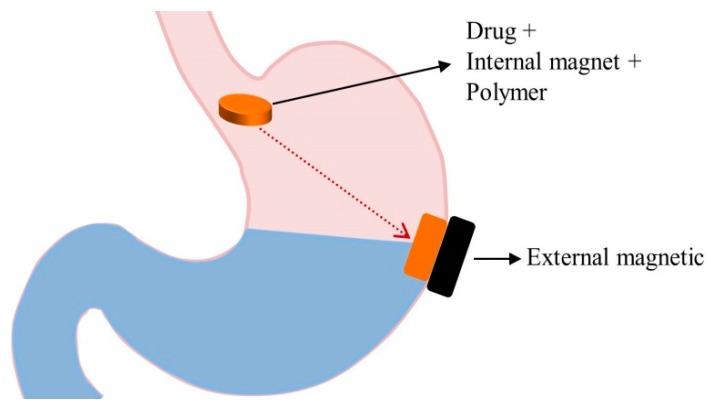
5.7. Magnetic Systems
In magnetic systems, a dosage form consists of active pharmaceutical ingredient, excipients and also a small amount of internal magnet. An extracorporeal magnet is placed over the stomach to control the position of the dosage form containing internal magnet as presented in Figure 6 [17]. The position and the magnetic field intensity of the extracorporeal magnet can affect the GRT [16]. Previous studies have reported that the GRT and bioavailability are improved by magnetic tablets [3,91]. Groning et al. performed a study in human volunteers using magnetic acyclovir tablets with and without an external magnetic field. The authors observed that the GRT and plasma drug concentration were increased in the presence of an extracorporeal magnet. Ito et al. formulated bioadhesive granules containing ultra-fine ferrite and performed in vivo experiment in rabbits [92]. They found that an external magnetic field intensity of 1700 G retained all granules in the stomach for more than 2 h. However, specific positioning of the magnet might be difficult and results in low patient compliance [16]. Only a few studies have been conducted on magnetic systems and their clinical significance has yet to be explored. Therefore, future research studies on these systems need to focus more on their clinical significance.
Figure 6.
GRDDS based on magnetic systems.
5.8. Ion-Exchange Resin Systems
The ion-exchange resin system consists of the water insoluble cross-linked polymer (resin) that can be either cationic or anionic. In general, it is designed to release the drug in a controlled manner. The suitable resins can be chosen according to the drug properties. In case of GRDDS, drugs should be released in the stomach and hence this system is applicable to cationic drugs. Therefore, cationic resin can be selected. A specific amount of resin is poured on a known drug concentration and mixed homogeneously for a certain period. The drug ions from the solution get adsorbed onto the resin matrix and displace cations from the resin. Such loaded drug resin complexes are called resinates. When the resinates come into contact with the hydrogen ions in the acidic environment of the stomach, hydrogen ions are exchanged with the drug ions present in the resinates matrix. As a consequence, the drug ions are released into the gastric fluid while the resin particles are eliminated through the large intestine [93]. The release rate of the drug from resins depends on inherent properties of the resins such as the particle size, cross-linking density, type of ionogenic group. Moreover, it also depends on the nature of the drugs, ionic environment and test solution [93]. When an ion exchange resin is highly cross-linked, the drug loading efficiency gets decreased [94,95]. The degree of drug resin complexation can be calculated by dry weight resin capacity measurement methods. It is determined by weighing a dry resin, rewetting it in drug solution, and displacing completely from the resin. The displaced ions can be assayed giving the degree of drug resin complexation. Even though this system alone may not be suitable to increase the GRT, the ion exchange resin can be combined with floating delivery systems or bioadhesive systems to prolong the GRT [96]. Some of its limitations may be difficulty in estimating the amount of bound resin with drug, and safety issues concerning its ingestion.
6. Evaluation Parameters of GRDDS
6.1. In Vitro Evaluation Parameters
In vitro assessments of GRDDS can be used to predict the in vivo performance. The routine evaluation methods of gastroretentive tablets include measurement of tablet tensile strength, weight variation, friability, drug content, content uniformity, and in vitro drug release. Floating behaviors such as floating lag time and total floating duration have been used for the assessment of floating behavior of low-density systems. Furthermore, floating force is also used to measure the floating capacity of the floating tablet. In addition, swelling rate, water uptake capacity, and gel strength of the polymeric dosage form can be evaluated using dissolution medium and tested for at least 8 h to ensure the floating mechanism, drug release, and gel strength. Table 6 summarizes various in vitro evaluation parameters of different GRDDS.
Table 6.
In vitro evaluation parameters of various GRDDS.
| GRDDS | Evaluation | Comment | References |
|---|---|---|---|
| Low-density system, raft-forming system | Floating Lag Time (FLT), total floating time (TFT), floating strength | The test is carried out in a simulated gastric fluid (SGF) at 37 °C. The time between introduction of dosage form and its buoyancy on the SGF (FLT) and the time during which the dosage form remains buoyant (TFT) were measured. The floating strength is measured using specifically designed basket holder connected with analytical balance. The reduction of weight on the analytical balance over time determines the floating strength. | [9,17,19,31,97,98] |
| Superporous hydrogel system, expandable system | Swelling studies | The test is carried out by placing the weighed amount of dosage form into the swelling medium (0.01 N HCl) and weight, diameter, and length of swollen samples are measured at predetermined time point. | [61,99] |
| Raft-forming and Mucoadhesion systems | Viscosity and Rheology | Viscosity of polymer affects the consistency of the dosage form upon contact with the gastric fluid. Brookfield/Ostwald’s viscometer and texture analyzer are commonly used. | [100] |
| Expandable system | In vitro unfolding study | The test is carried out by placing the folded dosage form into the dissolution medium and examining its unfolding behavior in different time interval. | [101] |
| Ion-exchange resin system | Particle size, ion exchange capacity, moisture content | Particle size analysis is carried out using a sieve shaker, laser diffraction, and coulter counter analyzer. The ion exchange capacity depends upon the functional group available for crosslinking. Moisture content can be measured with Karl Fischer. | [96,102,103,104] |
| Applicable for all GRDDS | In vitro drug release | The test is carried out in SGF at a predefined time interval (generally 0 to 12 h) using USP type-II apparatus at 50 rpm and maintained at 37 °C. | [9,27,72,80] |
| Gel strength | The high gel strength is desirable for better mechanical integrity. | [9,105] | |
| Drug-excipient interaction study | It can be studied by using FT-IR spectroscopy, Differential scanning calorimetry, and High Performance Liquid Chromatography. | [17,106] |
6.2. In Vivo Evaluation Parameters
In order to provide the evidence of in vivo efficacy of GRDDS, a well-designated in vivo study in an animal model or humans is required. In vivo studies provide information about the GRT and bioavailability of the drug. Selection of a suitable animal model is the first requirement for a successful in vivo study. For example, in small animals such as mouse, rat, guinea pig, and rabbit, there might be an issue of animal handling especially for large dosage forms [18,107]. As a result, measurements of the GRT and bioavailability are still difficult. Table 6 summarizes the in vivo evaluation parameters of different GRDDS.
Various diagnostic imaging techniques including gamma scintigraphy, radiology, gastroscopy, ultrasonography, and magnetic resonance imaging (MRI) can be applied for in vivo evaluations of GRDDS [6,18,36,108]. Gamma scintigraphy studies have been conducted to determine the location and extent of GRDDS and their transit through the GIT. In this technique, small amounts of stable isotope are added to the dosage form during its preparation [18]. Then, this isotope is converted into γ-emitting material by irradiating the dosage form in a neutron source. Gamma rays are released and captured as an image after processing by a computer. This method can also be used for the identification of dissolution and disintegration properties of the dosage form. A good safety profile and relatively low doses of radiation are the major advantages of the technique [6,18].
Likewise, the radiology/X-ray technique is used for the preclinical evaluation of GRT, disintegration rate, dimensions of the dosage form, and esophageal transit of GRDDS [18,36]. In this technique, a radio-opaque material such as barium sulphate is incorporated with the dosage form, and radiographs taken after ingestion of the dosage form help in locating the dosage forms at various periodic time intervals. Its major advantages compared to γ-scintigraphy are simplicity and cost. Even though this technique has been successfully used in human volunteers, dogs, and rabbits, safety issues still need to be considered because repetitive exposure to x-rays may lead to various health hazards.
Gastroscopy is a type of per-oral endoscopy used for the diagnosis and monitoring of GRDDS [18]. This technique composed of optical fibers and a video camera to determine the location of the dosage form. This method is applicable for all types of GRDDS; however, it is less convenient and might require minor or complete anesthesia to assess gastric retention of GRDDS. Similarly, ultrasonography is an alternative technique used in GRDDS. Ultrasonic waves are generated that enable the imaging of some abdominal organs and determine the intragastric location of the hydrogels, solvent penetration into the gel, and interactions between the dosage form and gastric mucosa during peristalsis [6]. MRI is another technique for determining the in vivo gastric retention of GRDDS. This technique uses magnetic fields and radiowaves to view the complete anatomical structure as well as location of the ingested dosage form [6,36]. The compounds with super paramagnetic properties (e.g., ferrous oxide) are incorporated for visualization purposes. Steingoetter et al. used this technique to report the in vivo gastric retention of gadolinium chelates floating tablets containing Fe3O4 as a super paramagnetic agent and succeeded in analyzing intra-gastric tablet position and residence time in human volunteers.
7. Future Perspectives of GRDDS
The GRT of the conventional dosage form is one of the main challenges in the pharmaceutical industry, especially for drugs that are absorbed from the upper part of the intestine. Developing GRDDS will help to overcome the drawbacks associated with conventional dosage form, although further work is needed on its shortcomings. To date, many studies have been performed on GRDDS utilizing the single system approach such as floating, expandable, and mucoadhesive systems.
Even though various GRDDS technologies have been extensively explored to achieve successful gastroretentive systems, most have their own limitations (Table 4). The variation in GRT, especially in the fed and fasted states, is still one of the main challenges faced by many formulation scientists. No single approach might be the best for resolving the problems. Therefore, it is desirable to explore suitable GRDDS that can overcome the limitations of a single approach. Using combination approaches such as expandable and effervescent floating systems, mucoadhesive and floating systems, swellable and floating systems, and mucoadhesive and high-density system may be useful strategies for minimizing the variability of GRT. Moreover, dual-working systems are less affected by the physiological condition of the stomach such as the fasting and fed states and these systems can ensure delayed gastric emptying. Therefore, future works on GRDDS should be focused on combinations of different mechanisms in order to prolong gastric retention of dosage forms even in the fasted state.
It is essential to assess gastroretentive dosage forms on a case-by-case basis because the physiochemical nature of drug and excipients, types and composition of polymers, drug dose, and manufacturability may depend on product specification [19]. Another important aspect for improving GRDDS is to understand the effects of formulation and process variables on the critical quality attributes of GRDDS. The critical quality attributes of GRDDS include floating behavior, floating force, gel strength, mucoadhesive strength, mucoadhesive time, in vitro drug release, swelling capacity, porosity of hydrogel, tablet tensile strength, and friability. From formulation viewpoints, understanding polymer behavior and its role in formulation is crucial for the rational development of the gastroretentive dosage form. Furthermore, selection of an appropriate concentration of polymer is equally important for designing such dosage forms. In this regard, the quality by design (QbD) approach can be a useful tool for investigating the influence of formulation and process variables on the critical quality attributes of GRDDS. With implementation of the QbD approach in pharmaceutical fields, there has been a significant transformation in the understanding and control of the manufacturing process, which notably minimizes the risk of product failure [9].
Some gastroretentive approaches such as magnetic systems have not been extensively studied. The clinical studies of these systems have not yet been reported in detail. Therefore, future works on magnetic systems need to be focused on clinical candidates to specify their practical applications in humans. Moreover, incorporating magnetic systems into the superporous hydrogel system can help extracorporeal magnets precisely locate the ingested dosage form since it swells and occupies larger volume. The advancement of technologies offers efficient measurement tools that can help to predict and correlate the gastric emptying time and passage of drug into the GIT. For example radiology and scintigraphy can be used for the in vivo evaluation of gastric emptying of dosage forms from the stomach [17]. Moreover, magnetic marker monitoring techniques can also be utilized to capture images of dosage forms in the stomach [109].
8. Conclusions
GRDDS have great potential to improve the therapeutic efficacy of drugs with narrow absorption windows, high solubility at acidic pH, and instability at alkaline pH. A thorough understanding of the anatomy and physiological state of the stomach, investigations into the impact of formulation and process variables on dosage form quality is a prerequisite for the successful design of GRDDS. Even though various GRDDS such as bio/mucoadhesive, magnetic, low-, and high-density systems have been reported in the literature, their clinical significance still needs to be studied. From the pharmaceutical aspect, future directions of GRDDS may need to focus on a combination approach of GRDDS to achieve better product quality. Moreover, a QbD approach can be used to better understand the effects of formulation and process variable on product performance.
Author Contributions
Conceptualization, S.H.J and P.T.; Methodology, P.T., J.T.; Formal Analysis, P.T.; Data Curation, P.T; Resources, S.H.J.; Writing-Original Draft Preparation, P.T, R.M, J.T.; Writing-Review & Editing, S.H.J, P.T., J.T., R.M.; Supervision, S.H.J.; Funding Acquisition, S.J.H.
Funding
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (MSIT) (NRF-2018R1A5A2023127) and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2018R1D1A1B07045154).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lopes C.M., Bettencourt C., Rossi A., Buttini F., Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016;510:144–158. doi: 10.1016/j.ijpharm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Rouge N., Buri P., Doelker E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996;136:117–139. doi: 10.1016/0378-5173(96)85200-8. [DOI] [Google Scholar]
- 3.Fujimori J., Machida Y., Tanaka S., Nagai T. Effect of magnetically controlled gastric residence of sustained release tablets on bioavailability of acetaminophen. Int. J. Pharm. 1995;119:47–55. doi: 10.1016/0378-5173(94)00368-F. [DOI] [Google Scholar]
- 4.Hwang K.-M., Cho C.-H., Tung N.-T., Kim J.-Y., Rhee Y.-S., Park E.-S. Release kinetics of highly porous floating tablets containing cilostazol. Eur. J. Pharm. Biopharm. 2017;115:39–51. doi: 10.1016/j.ejpb.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Kim S., Hwang K.-M., Park Y.S., Nguyen T.-T., Park E.-S. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int. J. Pharm. 2018;550:160–169. doi: 10.1016/j.ijpharm.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Klausner E.A., Lavy E., Friedman M., Hoffman A. Expandable gastroretentive dosage forms. J. Control. Release. 2003;90:143–162. doi: 10.1016/S0168-3659(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar D., Nandi G., Changder A., Hudati P., Sarkar S., Ghosh L.K. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int. J. Biol. Macromol. 2017;96:137–148. doi: 10.1016/j.ijbiomac.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Sarparanta M.P., Bimbo L.M., Mäkilä E.M., Salonen J.J., Laaksonen P.H., Helariutta A.K., Linder M.B., Hirvonen J.T., Laaksonen T.J., Santos H.A. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials. 2012;33:3353–3362. doi: 10.1016/j.biomaterials.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 9.Thapa P., Jeong S. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics. 2018;10:161. doi: 10.3390/pharmaceutics10030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavanpatil M.D., Jain P., Chaudhari S., Shear R., Vavia P.R. Novel sustained release, swellable and bioadhesive gastroretentive drug delivery system for ofloxacin. Int. J. Pharm. 2006;316:86–92. doi: 10.1016/j.ijpharm.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Sawicki W. Pharmacokinetics of verapamil and norverapamil from controlled release floating pellets in humans. Eur. J. Pharm. Biopharm. 2002;53:29–35. doi: 10.1016/S0939-6411(01)00189-8. [DOI] [PubMed] [Google Scholar]
- 12.Bardonnet P., Faivre V., Pugh W., Piffaretti J., Falson F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release. 2006;111:1–18. doi: 10.1016/j.jconrel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 13.El-Zahaby S.A., Kassem A.A., El-Kamel A.H. Design and evaluation of gastroretentive levofloxacin floating mini-tablets-in-capsule system for eradication of Helicobacter pylori. Saudi Pharm. J. 2014;22:570–579. doi: 10.1016/j.jsps.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiménez-Martínez I., Quirino-Barreda T., Villafuerte-Robles L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008;362:37–43. doi: 10.1016/j.ijpharm.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S., Mangla B., Kamboj S., Rana V. A QbD approach for the fabrication of immediate and prolong buoyant cinnarizine tablet using polyacrylamide-g-corn fibre gum. Int. J. Biol. Macromol. 2018;117:350–361. doi: 10.1016/j.ijbiomac.2018.05.178. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi R., Kulkarni G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Durg Deliv. 2016;23:378–394. doi: 10.3109/10717544.2014.936535. [DOI] [PubMed] [Google Scholar]
- 17.Prajapati V.D., Jani G.K., Khutliwala T.A., Zala B.S. Raft forming system—An upcoming approach of gastroretentive drug delivery system. J. Control. Release. 2013;168:151–165. doi: 10.1016/j.jconrel.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Mandal U.K., Chatterjee B., Senjoti F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharm. Sci. 2016;11:575–584. doi: 10.1016/j.ajps.2016.04.007. [DOI] [Google Scholar]
- 19.Prinderre P., Sauzet C., Fuxen C. Advances in gastro retentive drug-delivery systems. Expert Opin. Drug Deliv. 2011;8:1189–1203. doi: 10.1517/17425247.2011.592828. [DOI] [PubMed] [Google Scholar]
- 20.Hwang S.-J., Park H., Park K. Gastric retentive drug-delivery systems. Crit. Rev. Ther. Drug. 1998;15 doi: 10.1615/CritRevTherDrugCarrierSyst.v15.i3.20. [DOI] [PubMed] [Google Scholar]
- 21.Cvijic S., Ibric S., Parojcic J., Djuris J. An in vitro—In silico approach for the formulation and characterization of ranitidine gastroretentive delivery systems. J. Drug Deliv. Sci. Technol. 2018;45:1–10. doi: 10.1016/j.jddst.2018.02.013. [DOI] [Google Scholar]
- 22.Hooda A., Nanda A., Jain M., Kumar V., Rathee P. Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software. Int. J. Biol. Macromol. 2012;51:691–700. doi: 10.1016/j.ijbiomac.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Odeku O., Aderogba A., Ajala T., Akin-Anjani O., Okunlola A. Formulation of floating metronidazole microspheres using cassava starch (Manihot esculenta) as polymer. J. Pharm. Investig. 2017;47:445–451. doi: 10.1007/s40005-017-0319-7. [DOI] [Google Scholar]
- 24.Diós P., Nagy S., Pál S., Pernecker T., Kocsis B., Budán F., Horváth I., Szigeti K., Bölcskei K., Máthé D., et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015;96:196–206. doi: 10.1016/j.ejpb.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Jain S.K., Jangdey M.S. Lectin conjugated gastroretentive multiparticulate delivery system of clarithromycin for the effective treatment of Helicobacter pylori. Mol. Pharm. 2008;6:295–304. doi: 10.1021/mp800193n. [DOI] [PubMed] [Google Scholar]
- 26.Hardikar S., Bhosale A. Formulation and evaluation of gastro retentive tablets of clarithromycin prepared by using novel polymer blend. Bull. Fac. Pharm. Cairo Univ. 2018;56:147–157. doi: 10.1016/j.bfopcu.2018.07.001. [DOI] [Google Scholar]
- 27.Inukai K., Takiyama K., Noguchi S., Iwao Y., Itai S. Effect of gel formation on the dissolution behavior of clarithromycin tablets. Int. J. Pharm. 2017;521:33–39. doi: 10.1016/j.ijpharm.2017.01.065. [DOI] [PubMed] [Google Scholar]
- 28.Mostafavi A., Emami J., Varshosaz J., Davies N.M., Rezazadeh M. Development of a prolonged-release gastroretentive tablet formulation of ciprofloxacin hydrochloride: Pharmacokinetic characterization in healthy human volunteers. Int. J. Pharm. 2011;409:128–136. doi: 10.1016/j.ijpharm.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Fu J., Yin H., Yu X., Xie C., Jiang H., Jin Y., Sheng F. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems. Int. J. Pharm. 2018;549:370–379. doi: 10.1016/j.ijpharm.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Kagan L., Lapidot N., Afargan M., Kirmayer D., Moor E., Mardor Y., Friedman M., Hoffman A. Gastroretentive Accordion Pill: Enhancement of riboflavin bioavailability in humans. J. Control. Release. 2006;113:208–215. doi: 10.1016/j.jconrel.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Qin C., Wu M., Xu S., Wang X., Shi W., Dong Y., Yang L., He W., Han X., Yin L. Design and optimization of gastro-floating sustained-release tablet of pregabalin: In vitro and in vivo evaluation. Int. J. Pharm. 2018;545:37–44. doi: 10.1016/j.ijpharm.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Ngwuluka N.C., Choonara Y.E., Kumar P., du Toit L.C., Modi G., Pillay V. An optimized gastroretentive nanosystem for the delivery of levodopa. Int. J. Pharm. 2015;494:49–65. doi: 10.1016/j.ijpharm.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Oh T.-O., Kim J.-Y., Ha J.-M., Chi S.-C., Rhee Y.-S., Park C.-W., Park E.-S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013;83:460–467. doi: 10.1016/j.ejpb.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 34.He W., Li Y., Zhang R., Wu Z., Yin L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014;476:223–231. doi: 10.1016/j.ijpharm.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 35.Patil S., Talele G.S. Gastroretentive mucoadhesive tablet of lafutidine for controlled release and enhanced bioavailability. Durg Deliv. 2015;22:312–319. doi: 10.3109/10717544.2013.877099. [DOI] [PubMed] [Google Scholar]
- 36.Pawar V.K., Kansal S., Garg G., Awasthi R., Singodia D., Kulkarni G.T. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems. Durg Deliv. 2011;18:97–110. doi: 10.3109/10717544.2010.520354. [DOI] [PubMed] [Google Scholar]
- 37.Kotreka U., Adeyeye M.C. Gastroretentive floating drug-delivery systems: A critical review. Crit. Rev. Ther. Drug. 2011;28 doi: 10.1615/CritRevTherDrugCarrierSyst.v28.i1.20. [DOI] [PubMed] [Google Scholar]
- 38.Talukder R., Fassihi R. Gastroretentive delivery systems: A mini review. Drug Dev. Ind. Pharm. 2004;30:1019–1028. doi: 10.1081/DDC-200040239. [DOI] [PubMed] [Google Scholar]
- 39.Garg S., Sharma S. Gastroretentive drug delivery systems. Expert opin. Drug Deliv. 2006;3:217–233. doi: 10.1517/17425247.3.2.217. [DOI] [PubMed] [Google Scholar]
- 40.Salessiotis N. Measurement of the diameter of the pylorus in man: Part I. Experimental project for clinical application. Am. J. Surg. 1972;124:331–333. doi: 10.1016/0002-9610(72)90036-0. [DOI] [PubMed] [Google Scholar]
- 41.Timmermans J., Moes A.J. How well do floating dosage forms float? Int. J. Pharm. 1990;62:207–216. doi: 10.1016/0378-5173(90)90234-U. [DOI] [Google Scholar]
- 42.Chauhan M.S., Kumar A., Pathak K. Osmotically regulated floating asymmetric membrane capsule for controlled site-specific delivery of ranitidine hydrochloride: Optimization by central composite design. AAPS PharmSciTech. 2012;13:1492–1501. doi: 10.1208/s12249-012-9870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali J., Arora S., Ahuja A., Babbar A.K., Sharma R.K., Khar R.K., Baboota S. Formulation and development of hydrodynamically balanced system for metformin: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2007;67:196–201. doi: 10.1016/j.ejpb.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Clarke G., Newton J., Short M. Gastrointestinal transit of pellets of differing size and density. Int. J. Pharm. 1993;100:81–92. doi: 10.1016/0378-5173(93)90078-T. [DOI] [Google Scholar]
- 45.Streubel A., Siepmann J., Bodmeier R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006;6:501–508. doi: 10.1016/j.coph.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Arora S., Ali J., Ahuja A., Khar R.K., Baboota S. Floating drug delivery systems: A review. AAPS PharmSciTech. 2005;6:E372–E390. doi: 10.1208/pt060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaha S., Patel J., Pundarikakshudu K., Patel N. An overview of a gastro-retentive floating drug delivery system. Asian J. Pharm. Sci. 2009;4:65–80. [Google Scholar]
- 48.Calbet J.A., MacLean D.A. Role of caloric content on gastric emptying in humans. Pt 2J. Physiol. 1997;498:553–559. doi: 10.1113/jphysiol.1997.sp021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juvonen K.R., Purhonen A.-K., Salmenkallio-Marttila M., Lahteenmaki L., Laaksonen D.E., Herzig K.-H., Uusitupa M.I., Poutanen K.S., Karhunen L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009;139:461–466. doi: 10.3945/jn.108.099945. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y., Hsu W.H., Hollis J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE. 2013;8:e67482. doi: 10.1371/journal.pone.0067482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garg R., Gupta G. Progress in controlled gastroretentive delivery systems. Trop. J. Pharm. Res. 2008;7:1055–1066. doi: 10.4314/tjpr.v7i3.14691. [DOI] [Google Scholar]
- 52.Nguyen N.Q., Debreceni T.L., Burgstad C.M., Wishart J.M., Bellon M., Rayner C.K., Wittert G.A., Horowitz M. Effects of posture and meal volume on gastric emptying, intestinal transit, oral glucose tolerance, blood pressure and gastrointestinal symptoms after Roux-en-Y gastric bypass. Obes. Surg. 2015;25:1392–1400. doi: 10.1007/s11695-014-1531-4. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y.T., Mohammed S.D., Farmer A.D., Wang D., Zarate N., Hobson A.R., Hellström P.M., Semler J.R., Kuo B., Rao S.S. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharmacol. Ther. 2015;42:761–772. doi: 10.1111/apt.13329. [DOI] [PubMed] [Google Scholar]
- 54.Feldman M., Barnett C. Fasting gastric pH and its relationship to true hypochlorhydria in humans. Dig. Dis. Sci. 1991;36:866–869. doi: 10.1007/BF01297133. [DOI] [PubMed] [Google Scholar]
- 55.Mojaverian P., Vlasses P.H., Kellner P.E., Rocci M.L. Effects of gender, posture, and age on gastric residence time of an indigestible solid: Pharmaceutical considerations. Pharm. Res. 1988;5:639–644. doi: 10.1023/A:1015922903843. [DOI] [PubMed] [Google Scholar]
- 56.Krygowska-Wajs A., Cheshire W.P., Wszolek Z.K., Hubalewska-Dydejczyk A., Jasinska-Myga B., Farrer M.J., Moskala M., Sowa-Staszczak A. Evaluation of gastric emptying in familial and sporadic Parkinson disease. Parkinsonism Relat. D. 2009;15:692–696. doi: 10.1016/j.parkreldis.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Triantafyllou K., Kalantzis C., Papadopoulos A., Apostolopoulos P., Rokkas T., Kalantzis N., Ladas S. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig. Liver Dis. 2007;39:575–580. doi: 10.1016/j.dld.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 58.N Abduljabbar H., M Badr-Eldin S., M Aldawsari H. Gastroretentive ranitidine hydrochloride tablets with combined floating and bioadhesive properties: Factorial design analysis, in vitro evaluation and in vivo abdominal X-ray imaging. Curr. Drug Deliv. 2015;12:578–590. doi: 10.2174/1567201812666150608101720. [DOI] [PubMed] [Google Scholar]
- 59.Choi B., Park H., Hwang S., Park J. Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 2002;239:81–91. doi: 10.1016/S0378-5173(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 60.Rossi A., Conti C., Colombo G., Castrati L., Scarpignato C., Barata P., Sandri G., Caramella C., Bettini R., Buttini F. Floating modular drug delivery systems with buoyancy independent of release mechanisms to sustain amoxicillin and clarithromycin intra-gastric concentrations. Drug Dev. Ind. Pharm. 2016;42:332–339. doi: 10.3109/03639045.2015.1054397. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y.-C., Ho H.-O., Lee T.-Y., Sheu M.-T. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int. J. Pharm. 2013;441:162–169. doi: 10.1016/j.ijpharm.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Abouelatta S.M., Aboelwafa A.A., El-Gazayerly O.N. Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug. Durg Deliv. 2018;25:1161–1174. doi: 10.1080/10717544.2018.1474969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Youssef N.A.H.A., Kassem A.A., El-Massik M.A.E., Boraie N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015;486:297–305. doi: 10.1016/j.ijpharm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Bhalla S., Nagpal M. Comparison of various generations of superporous hydrogels based on chitosan-acrylamide and in vitro drug release. ISRN Pharm. 2013;2013:624841. doi: 10.1155/2013/624841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheth P., Tossounian J. The hydrodynamically balanced system (HBS™): A novel drug delivery system for oral use. Drug Dev. Ind. Pharm. 1984;10:313–339. doi: 10.3109/03639048409064653. [DOI] [Google Scholar]
- 66.Reddy L.H.V., Murthy R. Floating dosage systems in drug delivery. Crit. Rev. Ther. Drug. 2002;19:553–585. doi: 10.1615/CritRevTherDrugCarrierSyst.v19.i6.20. [DOI] [PubMed] [Google Scholar]
- 67.Hilton A., Deasy P. In vitro and in vivo evaluation of an oral sustained-release floating dosage form of amoxycillin trihydrate. Int. J. Pharm. 1992;86:79–88. doi: 10.1016/0378-5173(92)90033-X. [DOI] [Google Scholar]
- 68.Michaels A. Drug Delivery Device with Self Actuated Mechanism for Retaining Device in Selected Area. 3,786,813. U.S. Patent. 1974 Jan 22;
- 69.Kawashima Y., Niwa T., Takeuchi H., Hino T., Itoh Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J. Pharm. Sci. 1992;81:135–140. doi: 10.1002/jps.2600810207. [DOI] [PubMed] [Google Scholar]
- 70.Rahim S.A., Carter P., Elkordy A.A. Influence of calcium carbonate and sodium carbonate gassing agents on pentoxifylline floating tablets properties. Powder Technol. 2017;322:65–74. doi: 10.1016/j.powtec.2017.09.001. [DOI] [Google Scholar]
- 71.Baumgartner S., Kristl J., Vrečer F., Vodopivec P., Zorko B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000;195:125–135. doi: 10.1016/S0378-5173(99)00378-6. [DOI] [PubMed] [Google Scholar]
- 72.Tadros M.I. Controlled-release effervescent floating matrix tablets of ciprofloxacin hydrochloride: Development, optimization and in vitro–in vivo evaluation in healthy human volunteers. Eur. J. Pharm. Biopharm. 2010;74:332–339. doi: 10.1016/j.ejpb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Moes A. Business Briefing: Pharmatech. Business Briefings Ltd.; Singapore: 2003. Gastric retention systems for oral drug delivery; pp. 157–159. [Google Scholar]
- 74.Sivaneswari S., Karthikeyan E., Chandana P. Novel expandable gastro retentive system by unfolding mechanism of levetiracetam using simple lattice design–Formulation optimization and in vitro evaluation. Bull. Fac. Pharm. Cairo Univ. 2017;55:63–72. doi: 10.1016/j.bfopcu.2017.02.003. [DOI] [Google Scholar]
- 75.Omidian H., Rocca J.G., Park K. Advances in superporous hydrogels. J. Control. Release. 2005;102:3–12. doi: 10.1016/j.jconrel.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 76.Park K., Robinson J.R. Bioadhesive polymers as platforms for oral-controlled drug delivery: Method to study bioadhesion. Int. J. Pharm. 1984;19:107–127. doi: 10.1016/0378-5173(84)90154-6. [DOI] [Google Scholar]
- 77.Wang J., Tauchi Y., Deguchi Y., Morimoto K., Tabata Y., Ikada Y. Positively charged gelatin microspheres as gastric mucoadhesive drug delivery system for eradication of H. pylori. Durg Deliv. 2000;7:237–243. doi: 10.1080/107175400455173. [DOI] [PubMed] [Google Scholar]
- 78.Smart J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 79.Patil H., Tiwari R.V., Repka M.A. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. J. Drug Deliv. Sci. Technol. 2016;31:65–71. doi: 10.1016/j.jddst.2015.12.002. [DOI] [Google Scholar]
- 80.Shtenberg Y., Goldfeder M., Prinz H., Shainsky J., Ghantous Y., El-Naaj I.A., Schroeder A., Bianco-Peled H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018;111:62–69. doi: 10.1016/j.ijbiomac.2017.12.137. [DOI] [PubMed] [Google Scholar]
- 81.Andrews G.P., Laverty T.P., Jones D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009;71:505–518. doi: 10.1016/j.ejpb.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 82.Dey S.K., De P.K., De A., Ojha S., De R., Mukhopadhyay A.K., Samanta A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016;89:622–631. doi: 10.1016/j.ijbiomac.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 83.Darandale S.S., Vavia P.R. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. Acta Pharm. Sin. B. 2012;2:509–517. doi: 10.1016/j.apsb.2012.05.004. [DOI] [Google Scholar]
- 84.Bera H., Kandukuri S.G., Nayak A.K., Boddupalli S. Alginate-sterculia gum gel-coated oil-entrapped alginate beads for gastroretentive risperidone delivery. Carbohydr. Polym. 2015;120:74–84. doi: 10.1016/j.carbpol.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Nappinnai M., Sivaneswari S. Formulation optimization and characterization of gastroretentive cefpodoxime proxetil mucoadhesive microspheres using 32 factorial design. J. Pharm. Res. 2013;7:304–309. doi: 10.1016/j.jopr.2013.04.014. [DOI] [Google Scholar]
- 86.Pund S., Joshi A., Vasu K., Nivsarkar M., Shishoo C. Gastroretentive delivery of rifampicin: In vitro mucoadhesion and in vivo gamma scintigraphy. Int. J. Pharm. 2011;411:106–112. doi: 10.1016/j.ijpharm.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 87.Wang L., Wu Y., Li J., Qiao H., Di L. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. Int. J. Biol. Macromol. 2018;120:529–536. doi: 10.1016/j.ijbiomac.2018.08.127. [DOI] [PubMed] [Google Scholar]
- 88.Khutoryanskiy V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011;11:748–764. doi: 10.1002/mabi.201000388. [DOI] [PubMed] [Google Scholar]
- 89.Fabregas J., Claramunt J., Cucala J., Pous R., Siles A. “In-Vitro” Testing of an Antacid Formulation with Prolonged Gastric Residence Time (Almagate Flot-Coat®) Drug Dev. Ind. Pharm. 1994;20:1199–1212. doi: 10.3109/03639049409038361. [DOI] [Google Scholar]
- 90.Hampson F.C., Jolliffe I.G., Bakhtyari A., Taylor G., Sykes J., Johnstone L.M., Dettmar P.W. Alginate–antacid combinations: Raft formation and gastric retention studies. Drug Dev. Ind. Pharm. 2010;36:614–623. doi: 10.3109/03639040903388290. [DOI] [PubMed] [Google Scholar]
- 91.Murphy C.S., Pillay V., Choonara Y.E., du Toit L.C. Gastroretentive drug delivery systems: Current developments in novel system design and evaluation. Curr. Drug Deliv. 2009;6:451–460. doi: 10.2174/156720109789941687. [DOI] [PubMed] [Google Scholar]
- 92.Ito R., Machida Y., Sannan T., Nagai T. Magnetic granules: A novel system for specific drug delivery to esophageal mucosa in oral administration. Int. J. Pharm. 1990;61:109–117. doi: 10.1016/0378-5173(90)90049-A. [DOI] [Google Scholar]
- 93.Gaur P.K., Mishra S., Bhardwaj S., Puri D., Kumar S.S. Ion Exchange Resins in Gastroretentive Drug Delivery: Characteristics, Selection, Formulation and Applications. J. Pharm. Sci. Pharmacol. 2014;1:304–312. doi: 10.1166/jpsp.2014.1037. [DOI] [Google Scholar]
- 94.Jeong S.H., Park K. Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix. Int. J. Pharm. 2008;361:26–32. doi: 10.1016/j.ijpharm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Jeong S.H., Berhane N.H., Haghighi K., Park K. Drug Release Properties of Polymer Coated Ion-Exchange Resin Complexes: Experimental and Theoretical Evaluation. J. Pharm. Sci. 2007;96:618–632. doi: 10.1002/jps.20677. [DOI] [PubMed] [Google Scholar]
- 96.Anand V., Kandarapu R., Garg S. Ion-exchange resins: Carrying drug delivery forward. Drug Discov. Today. 2001;6:905–914. doi: 10.1016/S1359-6446(01)01922-5. [DOI] [PubMed] [Google Scholar]
- 97.Eisenächer F., Garbacz G., Mäder K. Physiological relevant in vitro evaluation of polymer coats for gastroretentive floating tablets. Eur. J. Pharm. Biopharm. 2014;88:778–786. doi: 10.1016/j.ejpb.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 98.Strübing S., Abboud T., Contri R.V., Metz H., Mäder K. New insights on poly(vinyl acetate)-based coated floating tablets: Characterisation of hydration and CO2 generation by benchtop MRI and its relation to drug release and floating strength. Eur. J. Pharm. Biopharm. 2008;69:708–717. doi: 10.1016/j.ejpb.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 99.Chen J., Blevins W.E., Park H., Park K. Gastric retention properties of superporous hydrogel composites. J. Control. Release. 2000;64:39–51. doi: 10.1016/S0168-3659(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 100.Kashyap N., Viswanad B., Sharma G., Bhardwaj V., Ramarao P., Kumar M.R. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials. 2007;28:2051–2060. doi: 10.1016/j.biomaterials.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Verma S., Nagpal K., Singh S., Mishra D. Unfolding type gastroretentive film of Cinnarizine based on ethyl cellulose and hydroxypropylmethyl cellulose. Int. J. Biol. Macromol. 2014;64:347–352. doi: 10.1016/j.ijbiomac.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 102.Jeong S.H., Park K. Development of sustained release fast-disintegrating tablets using various polymer-coated ion-exchange resin complexes. Int. J. Pharm. 2008;353:195–204. doi: 10.1016/j.ijpharm.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 103.Torres D., Boado L., Blanco D., Vila-Jato J.L. Comparison between aqueous and non-aqueous solvent evaporation methods for microencapsulation of drug–resin complexes. Int. J. Pharm. 1998;173:171–182. doi: 10.1016/S0378-5173(98)00224-5. [DOI] [Google Scholar]
- 104.Farag Y., Nairn J.G. Rate of release of organic carboxylic acids from ion-exchange resins. J. Pharm. Sci. 1988;77:872–875. doi: 10.1002/jps.2600771012. [DOI] [PubMed] [Google Scholar]
- 105.El-said I.A., Aboelwafa A.A., Khalil R.M., ElGazayerly O.N. Baclofen novel gastroretentive extended release gellan gum superporous hydrogel hybrid system: In vitro and in vivo evaluation. Durg Deliv. 2016;23:101–112. doi: 10.3109/10717544.2014.905654. [DOI] [PubMed] [Google Scholar]
- 106.Chandrashekar G., Udupa N. Biodegradable Injectable Implant Systems for Long Term Drug Delivery Using Poly (Lactic-co-glycolic) Acid Copolymers. J. Pharm. Pharmacol. 1996;48:669–674. doi: 10.1111/j.2042-7158.1996.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 107.Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 108.Badve S.S., Sher P., Korde A., Pawar A.P. Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur. J. Pharm. Biopharm. 2007;65:85–93. doi: 10.1016/j.ejpb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 109.Soni R.P., Patel A.V., Patel R.B., Patel M., Patel K., Patel N. Gastroretentive drug delivery systems: A review. Int. J. Pharm. World Res. 2011;2:1–24. [Google Scholar]