Abstract
Inducible cyclization recombinase (Cre) transgenic mouse strains are powerful tools for cell lineage tracing and tissue-specific knockout experiments. However, low efficiency or leaky expression can be important pitfalls. Here, we compared the efficiency and specificity of two commonly used cholangiocyte-specific Cre drivers, the Opn-iCreERT2 and Ck19-CreERT drivers, using a tdTomato reporter strain. We found that Opn-iCreERT2 triggered recombination of the tdTomato reporter in 99.9% of all cholangiocytes while Ck19-CreERT only had 32% recombination efficiency after tamoxifen injection. In the absence of tamoxifen, recombination was also induced in 2% of cholangiocytes for the Opn-iCreERT2 driver and in 13% for the Ck19-CreERT driver. For both drivers, Cre recombination was highly specific for cholangiocytes since recombination was rare in other liver cell types. Toxic liver injury ectopically activated Opn-iCreERT2 but not Ck19-CreERT expression in hepatocytes. However, ectopic recombination in hepatocytes could be avoided by applying a three-day long wash-out period between tamoxifen treatment and toxin injection. Therefore, the Opn-iCreERT2 driver is best suited for the generation of mutant bile ducts, while the Ck19-CreERT driver has near absolute specificity for bile duct cells and is therefore favorable for lineage tracing experiments.
Keywords: Cre, cholangiocytes, bile duct cells, knockout, mouse liver, lineage tracing, Opn, Ck19
1. Introduction
The development of inducible Cre transgenic mouse strains has made it possible to generate cell type-specific knockout and lineage tracing experiments. Such experiments generally require two genetically modified loci. The first locus contains a gene or an artificial transgene that is flanked by two loxP recombination sites, a so-called “floxed” allele. The second locus is a transgene or a knock-in where a cell type-specific promoter drives the expression of the Cre recombinase. Often this transgene encodes a tamoxifen-inducible Cre where Cre is fused to the ligand binding domain of the Estrogen receptor (CreER). In the absence of tamoxifen, CreER is sequestered in the cytoplasm. Upon tamoxifen binding, CreER is released from the cell membrane and translocates into the nucleus where it catalyzes recombination between the two loxP sites. This results in the excision of a DNA segment that is flanked by two equally oriented loxP sites. This inducible CreER-loxP system is broadly used to conditionally knock out a gene of interest in specific cells by combining tissue specific CreER strains with tamoxifen injection at a specific time point. This conditional knock-out strategy thus prevents deletion of floxed segments prematurely or in non-desired cell types.
The CreER-loxP system can also be used for lineage tracing. Here, CreER activation is used to excise a DNA segment that contains a floxed transcriptional STOP cassette (loxP-STOP-loxP cassette) that separates a promoter from a reporter gene. Often used are strains with a loxP-STOP-loxP cassette followed by the coding region of a fluorescent protein inserted into the ubiquitously expressed Rosa26 locus. Upon tamoxifen injection, CreER deletes the STOP cassette and causes expression of the reporter gene in the targeted cells and their progeny.
A large number of mouse strains have been generated that express different versions of Cre in specific cell types. However, Cre lines often do not show 100% efficiency and specificity, two factors that can greatly affect the outcome of a gene knockout experiment. On the one hand, inefficient gene deletion produces mosaic tissues where non-recombined wild-type cells may compensate for the phenotype of mutant cells. On the other hand, recombination in non-target cells caused by unspecific Cre expression may produce misleading phenotypes. Similarly, in lineage tracing experiments, low labeling efficiency can cause underestimation of the contribution of the traced cell type and unspecific reporter activation makes it impossible to know the origin of the labeled cells. Thus, a thorough analysis of the efficiency and specificity of a Cre driver is required to correctly execute knockout and lineage tracing experiments.
A number of different Cre strains have been generated to trigger loxP recombination in cholangiocytes, which are the cells that constitute the bile ducts and play important and specialized roles in development, homeostasis, and regeneration of the liver [1]. These drivers include Ck19-CreERT [2], Hnf1b-CreER [3], Sox9-IRES-CreERT2 [4], and Opn-iCreERT2 [5]. Although all of these transgenes drive CreER in adult cholangiocytes, they display notable differences in their efficiency and specificity which can result in data misinterpretation and lack of reproducibility.
The Hnf1b-CreER driver has 84% recombination efficiency and is highly specific for cholangiocytes, since only 0.0125% of hepatocytes had recombination of a YFP reporter transgene [6,7]. However, use of the Hnf1b-CreER driver in cholangiocyte research has been limited, likely because Hnf1b is expressed in hepatocytes during the early postnatal period [8]. The Sox9-IRES-CreERT2 driver was initially used for lineage tracing of bile duct cells upon liver injury [4], but broad ectopic expression of this driver (and also the Sox9 gene) in hepatocytes upon liver injury resulted in confounding cell tracing results [6,9,10,11]. However, a different Sox9-CreER line generated later showed higher specificity for bile duct cells when used with low doses of tamoxifen [6,9,10,11]. The Ck19-CreERT driver is frequently used and regarded as highly specific towards cholangiocytes; however, this driver suffers from low efficiency in deleting floxed alleles [2,5]. Curiously, another Ck19-Cre driver triggered recombination in cholangiocytes and a subset of hepatocytes [12]. Finally, Opn-iCreERT2 showed high recombination efficiency in cholangiocytes [5]. However, the Opn gene is ectopically expressed in hepatocytes and other liver cells upon chronic injury [13,14,15], raising questions about the specificity of the Opn-iCreERT2 driver.
In this study, we compared the efficiency and specificity of Cre mediated recombination triggered by the Ck19-CreERT and Opn-iCreERT2 drivers. Our results show that both Cre drivers nearly exclusively target cholangiocytes but that recombination in hepatocytes can occasionally occur in either model after tamoxifen injection, although this is a rare event. However, the Opn-iCreERT2 driver showed vastly superior recombination efficiency.
2. Materials and Methods
2.1. Mouse Strains
Opn-iCreERT2-Rosa26-loxP-STOP-loxP-tdTomato mice (Opn-CreER-tdTomato) were generated by crossing Opn-iCreERT2 mice with Rosa26-loxP-STOP-loxP-tdTomato (R26-tdTomato) mice. Opn-iCreERT2 mice were previously described [5]. R26-tdTomato mice were kindly provided by Chris Marine [16]. Ck19-CreERT-Rosa26- loxP-STOP-loxP-tdTomato mice (Ck19-CreER-tdTomato mice) were generated by crossing Ck19-CreERT mice with R26-tdTomato mice. Ck19-CreERT mice were purchased from The Jackson Laboratory (JAX stock #026925; Bar Harbor, Maine) [2]. Male and female mice were used in all experiments. Mice were housed, fed, and treated in accordance with protocols approved by the committee for animal research at KU Leuven. All mouse experiments were approved by the institutional ethical commission at KU Leuven (P146-2017) and were performed in accordance with relevant institutional and national guidelines and regulations.
2.2. Tamoxifen and CCl4 Injections
Tamoxifen (T5648; Sigma, St. Louis, MI, USA) was dissolved in corn oil at a concentration of 10 mg/mL and injected intraperitoneally for five consecutive days at a dose of 80 mg/kg bodyweight into mice aged 6 to 8 weeks. Mice from uninjured groups were sacrificed after either a 3-day or a 3-week washout period following the last tamoxifen injection. Injured groups received an intraperitoneal injection of CCl4 (1 mL/kg bodyweight, in corn oil) after a 3-day or a 3-week washout period. Mice were sacrificed 3 days after CCl4 injection. Mice with liver injury but without tamoxifen washout received CCl4 on day 2 of the 5 days of tamoxifen injection. These mice were sacrificed on day 5, the last day of tamoxifen injection. Vehicle control mice were injected with corn oil on five consecutive days, mice were sacrificed 3 days after corn oil injection.
2.3. Immunostaining and Imaging
Livers were collected and fixed in 4% paraformaldehyde (PFA) in PBS for 48 h at 4 °C and then washed in PBS. Liver lobes were embedded in 4% agarose in PBS and sectioned at 100 μm thickness using a vibratome (model VT 1000S; Leica, Wetzlar, Germany). Liver sections were permeabilized and antigens were retrieved with TRIS-EDTA for 20 min at 95 °C and blocked in 3% Bovine Serum Albumin (BSA) in PBS for 2 h at room temperature. The sections were then incubated in primary antibody solution overnight. The following day, sections were washed and then incubated in secondary antibody solution for 1 h at room temperature. Finally, sections were washed and mounted in mowiol, and analyzed on an FV1200 confocal microscope (Olympus, Tokio, Japan). Images were processed in ImageJ (Version 2.0.0-RC-69/1.52n, open source). Primary antibodies were rat-anti-Ck19 (TROMA III, 1:50; Hybridoma Bank, Iowa City, IA, USA), rabbit-anti-Hnf-4α (ab181604, 1:200; Abcam, Cambridge, UK), goat-anti-Opn (AF143, 1:250; R&D systems, Minneapolis, MN, USA), rabbit-anti-Cd45 (Abcam, ab10558, 1:200) and rabbit-anti-Desmin (RB-9014-P, 1:200; Thermo Fischer Scientific, Waltham, MA, USA). Secondary antibodies were donkey anti-rat-488 (code: 712-225-153, 1:500; Jackson, Bar Harbor, ME, USA), donkey anti-rabbit-647 (A31573, 1:500; Invitrogen, Carlsbad, CA, USA), and donkey anti-goat 488 (Jackson, code: 705-545-147, 1:500) and donkey anti-rabbit-488 (Jackson, code: 711-545-152). DAPI (1:1000; Thermo Fisher Scientific, Waltham, MA, USA) was used to stain nuclei.
2.4. Quantitative Analysis
All quantifications were performed using the Cell Counter plugin in ImageJ software. For both efficiency and specificity experiments, sections from the same mice were analyzed. Heterozygous Opn-CreER-tdTomato mice (n = 30 mice) and heterozygous Ck19-CreER-tdTomato mice (n = 31 mice) were distributed across different treatment groups. Opn-CreER-tdTomato groups were uninjured 3-day after Tamoxifen (n = 7), uninjured 3-week after Tamoxifen (n = 8), CCl4-treated 3-day washout (n = 4), CCl4-treated 3-week washout (n = 3), CCl4-treated no washout (n = 5), and corn oil controls (n = 3). Ck19-CreER-tdTomato groups were uninjured 3-day after Tamoxifen (n = 7), uninjured 3-week after Tamoxifen (n = 8), CCl4-treated 3-day washout (n = 5), CCl4-treated 3-week washout (n = 4), CCl4-treated no washout (n = 5), and corn oil controls (n = 2). Homozygous Opn-CreER-tdTomato mice (n = 3) and homozygous Ck19-CreER-tdTomato mice (n = 4) were checked for bile duct phenotypes.
Hnf-4α+ hepatocytes were quantified by counting 18 pictures from two liver sections per mouse. This amounted to about 5000 Hnf-4α+ cells per section (4697 ± 704). The number of Hnf-4α+,tdTomato+ double positive cells was divided by the total number of Hnf-4α+ cells for each mouse. The resulting fraction was expressed as percentage and used as a measure for R26-tdTomato recombination.
To determine the efficiency of R26-tdTomato recombination in cholangiocytes, per mouse 20 bile duct regions of all sizes were visualized in one liver section and Ck19+ cells were counted. On average 400 (397 ± 69) Ck19+ cells were counted per mouse. Every Ck19+ cell was checked for tdTomato expression. The number of Ck19+,tdTomato+ double positive cells was then divided by the total number of Ck19+ cells, expressed as a percentage, and used as a measure for recombination efficiency.
2.5. Statistical Analysis
Two-way ANOVA with interaction was performed on percentages of Hnf-4α+ cells that were also tdTomato+ to test for differences in specificity between genotypes and between washout types. In the two-way ANOVA for uninjured mice genotypes included Opn-CreER-tdTomato and Ck19-CreER-tdTomato and washout groups included 3-day washout or 3-week washout. In the two-way ANOVA for CCl4-injured mice genotypes included Opn-CreER-tdTomato and Ck19-CreER-tdTomato and washout types included 3-day washout, 3-week washout, or no washout. Tukey’s range test was performed as a post-hoc test to compare all possible pairs of means in both ANOVAs. Two-way ANOVA was performed on percentages of Ck19+ cells that were also tdTomato+ to test for differences in efficiency between genotypes and treatment groups. Genotypes included Opn-CreER-tdTomato and Ck19-CreER-tdTomato, and treatment groups included uninjured or CCl4-treated after either a 3-day or 3-week washout following tamoxifen injections, resulting in a total of 8 groups. Tukey’s range test was performed as a post-hoc test to compare all possible pairs of means. A p-value of 0.05 and lower was considered to be statistically significant for all tests. All statistical tests were performed using R-Studio (Version 1.1.463; R studio Inc., Boston, MA, USA).
3. Results
3.1. Opn-iCreERT2 Drives loxP Site Recombination more Efficiently than Ck19-CreERT
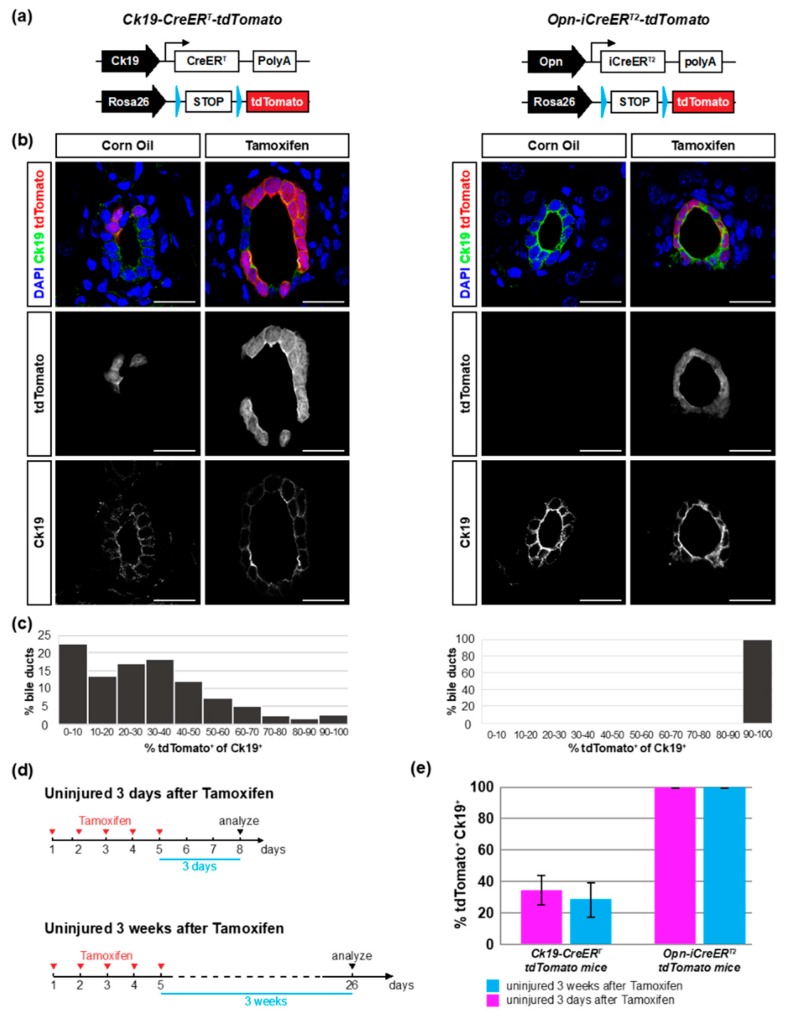
In order to evaluate the efficiency and specificity of Cre-mediated recombination in mice expressing CreER recombinase under the control of the Cytokeratin 19 (Ck19) and Osteopontin (Opn) promoters we crossed the Ck19-CreERT and Opn-iCreERT2 mice with mice containing the Cre-recombination reporter Rosa26-loxP-STOP-loxP-tdTomato (R26-tdTomato). This generated mice with the Ck19-CreERT or Opn-iCreERT2 and the R26-tdTomato transgenes, herewith referred to as Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice respectively (Figure 1a).
Figure 1.
Opn-iCreERT2 drives loxP site recombination more efficiently than Ck19-CreERT. (a) Schematic overview of Cre drivers and recombination alleles of Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice. (b) Immunofluorescent detection of Ck19 and tdTomato in liver sections of Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice after vehicle or tamoxifen injection. (c) Distribution of recombination efficiency in individual bile ducts ranging from 0 to 100 percent. (d) Schematic experimental outline. Mice were injected with tamoxifen on five consecutive days and sacrificed three days or three weeks later. (e) Percentage of tdTomato+ Ck19+ cells quantified in liver sections at different timepoints after tamoxifen injection (n = 7–8 mice per experiment; 20 bile duct regions per mouse). The difference between the two genotypes was significant 3 days (p = 0.001) and 3 weeks (p = 0.001) after tamoxifen injection. Scale bars: 20 μm.
First, we evaluated whether the two Cre drivers induced recombination in the absence of tamoxifen. Liver sections were immunostained for Ck19 to mark all cholangiocytes, and were imaged for Ck19 and tdTomato expression. After corn oil injection (vehicle), Opn-CreER-tdTomato mice showed tdTomato expression in 2% of cholangiocytes (9/388). The 9 tdTomato+ cholangiocytes were found in 7 different bile ducts and mostly appeared as single cells. In Ck19-CreER-tdTomato corn oil injected mice, 13% of cholangiocytes were tdTomato+ (54/418) (Figure 1b). The 54 tdTomato+ cells were found in 8 different bile ducts and appeared as large patches of cells, presumably clones, in large bile ducts. This amounted to 1.80 independent recombination events (clones) per 100 cholangiocytes for Opn-CreERT-tdTomato mice and 1.91 for Ck19-CreERT-tdTomato mice observed at 8 weeks of age. We did not observe tdTomato expression in any other liver cell type in either strain. Thus, while both strains showed some degree of leakiness, Ck19-CreERT had high numbers of recombined cells in the uninduced condition.
Next, we evaluated the recombination efficiency induced by tamoxifen administration. We injected five doses of tamoxifen on consecutive days and analyzed tdTomato expression three days after the last injection. In Ck19-CreER-tdTomato mice, tdTomato expression was highly mosaic and we did not detect any duct that was entirely composed of tdTomato+ cells (Figure 1b, Supplemental Figure S1). Rather, the percentage of tdTomato+ cholangiocytes per bile duct was variable, and in most ducts less than half of the Ck19+ cells expressed tdTomato (Figure 1b,c). Quantification of a large number of bile ducts showed that only 35% of the Ck19+ cholangiocytes (932/2676) expressed tdTomato (Figure 1d,e; Table 1). This number did not increase over time and three weeks after tamoxifen injection still only about 28% of the Ck19+ cells expressed tdTomato (862/3124). In contrast, virtually all bile duct cells expressed tdTomato in Opn-CreER-tdTomato mice (Figure 1b). Quantification showed that tdTomato was detected in 99.96% and 99.94% of the cholangiocytes (2722/2723; 3298/3300) three days and three weeks after tamoxifen injection, respectively (Figure 1d,e; Table 1). These data show that the Opn-iCreERT2 driver efficiently recombines the floxed STOP cassette whereas the Ck19-CreERT driver has limited recombination efficiency when using R26-tdTomato as a reporter.
Table 1.
Quantification of tdTomato+ cholangiocytes.
| Treatment Group | Mice (n) | % tdTomato+ of Ck19+ | ||||
|---|---|---|---|---|---|---|
| Opn-Cre | Ck19-Cre | Opn-Cre | Ck19-Cre | Opn-Cre | Ck19-Cre | |
| Uninjured 3 days after TAM | 7 | 7 | 99.9 ± 0.1 | 34.8 ± 9.1 | ||
| Uninjured 3 weeks after TAM | 8 | 8 | 99.9 ± 0.1 | 27.6 ± 11.7 | ||
| CCl4 3-day washout | 4 | 5 | 100 | 38.9 ± 10.8 | ||
| CCl4 3-week washout | 3 | 4 | 99.9 ± 0.2 | 27.8 ± 5.3 | ||
Table showing the number of mice analyzed per group, the total number of cholangiocytes quantified per group and the amount of tdTomato+ cholangiocytes in absolute values and percentages. Each row represents a different condition. Opn-Cre, Opn-CreER-tdTomato mice; Ck19-Cre, Ck19-CreER-tdTomato mice; TAM, Tamoxifen.
During this analysis, we also observed that heterozygous Ck19-CreER-tdTomato mice had reduced Ck19 expression. This is probably due to the fact that the Ck19-CreERT construct is a knock-in into the Ck19 locus causing loss of function (Figure S2) [2]. Homozygous Ck19-CreER-tdTomato mice lacked Ck19 expression altogether (Figure S2). On the other hand, neither Ck19 nor Opn expression was affected in heterozygous or homozygous Opn-CreER-tdTomato mice consistent with the transgenic nature of the Opn-iCreERT2 construct (Figure S2) [5].
3.2. The Ck19-CreERT and Opn-iCreERT2 Drivers are Highly Specific towards Cholangiocytes
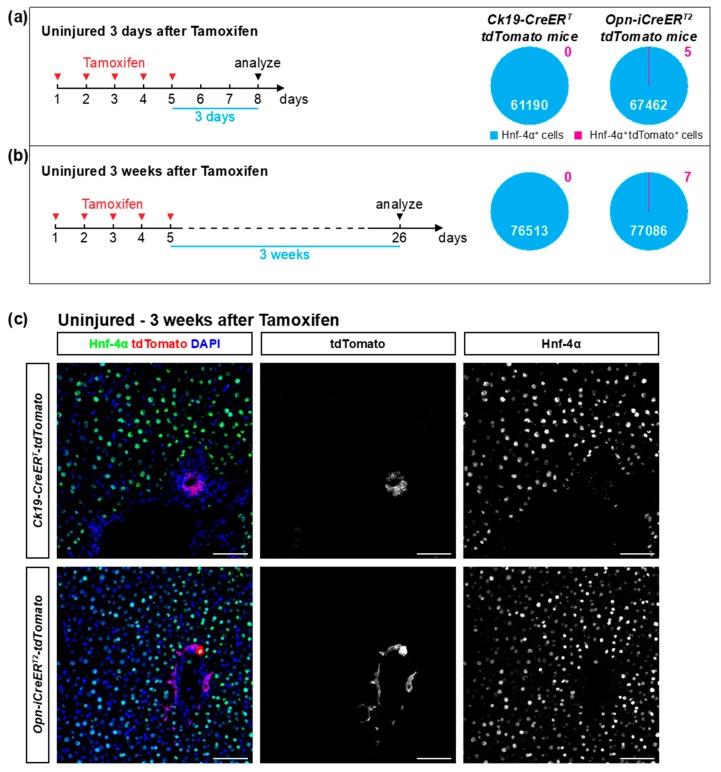
To determine the frequency of recombination in hepatocytes, we stained the Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice for the hepatocyte-specific marker Hnf-4α and determined overlap with tdTomato expression. No tdTomato+ hepatocytes were detected in Ck19-CreER-tdTomato mice three days (0/61190) or three weeks (0/76513) after the last tamoxifen injection (Figure 2; Table 2). Opn-CreER-tdTomato mice had very few tdTomato+ hepatocytes, namely 5 hepatocytes out of 67462 (0.0074%) three days after tamoxifen injection and 7 hepatocytes out of 77086 (0.0091%) three weeks after tamoxifen treatment (Figure 2; Table 2). These tdTomato+ hepatocytes usually appeared as single cells and their location was not restricted to a specific zone. All tdTomato+ Ck19- cells were positive for Hnf-4α, and none of those cells co-stained for the hepatic stellate cell marker Desmin or the immune cell marker Cd45 (Figure S3), confirming earlier reports [17]. We conclude that the Ck19-CreERT driver had absolute specificity in these experiments while the Opn-iCreERT2 driver had extremely low frequency of recombination in cells other than cholangiocytes.
Figure 2.
The Ck19-CreERT and Opn-iCreERT2 drivers are highly specific towards cholangiocytes in non-injured livers. (a,b) Schematic experimental outline. Mice were injected with tamoxifen and sacrificed three days or three weeks later. The percentage of tdTomato+ Hnf-4α+ cells was quantified in liver sections of Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice (n = 7–8 mice per experiment; 18 pictures per mouse). No statistically significant differences were detected between groups. (c) Immunofluorescent detection of Hnf-4α and tdTomato in Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice three weeks after tamoxifen. Scale bars: 50 μm.
Table 2.
Quantification of tdTomato+ hepatocytes.
| Treatment Group | Mice (n) | % tdTomato+ of Hnf-4α+ | ||||
|---|---|---|---|---|---|---|
| Opn-Cre | Ck19-Cre | Opn-Cre | Ck19-Cre | Opn-Cre | Ck19-Cre | |
| uninjured 3 days after TAM | 7 | 7 | 0.0074 ± 0.0126 | 0 | ||
| uninjured 3 weeks after TAM | 8 | 8 | 0.0091 ± 0.0098 | 0 | ||
| CCl4 3-day washout | 4 | 5 | 0 | 0 | ||
| CCl4 3-week washout | 3 | 4 | 0.0226 ± 0.0371 | 0 | ||
| CCl4 no washout † | 5 | 5 | 0.3348 ± 0.3588 | 0.0048 ± 0.0091 | ||
† 9 pictures from 1 liver section were counted per mouse, instead of 18 pictures from 2 sections as in the other groups. Table showing the number of mice analyzed per group, the total number of hepatocytes quantified per group and the amount of tdTomato+ hepatocytes in absolute values and percentages. Each row represents a different condition. Opn-Cre, Opn-CreER-tdTomato mice; Ck19-Cre, Ck19-CreER-tdTomato mice; TAM, Tamoxifen.
3.3. Ectopic Expression of the Cre Drivers after Liver Injury
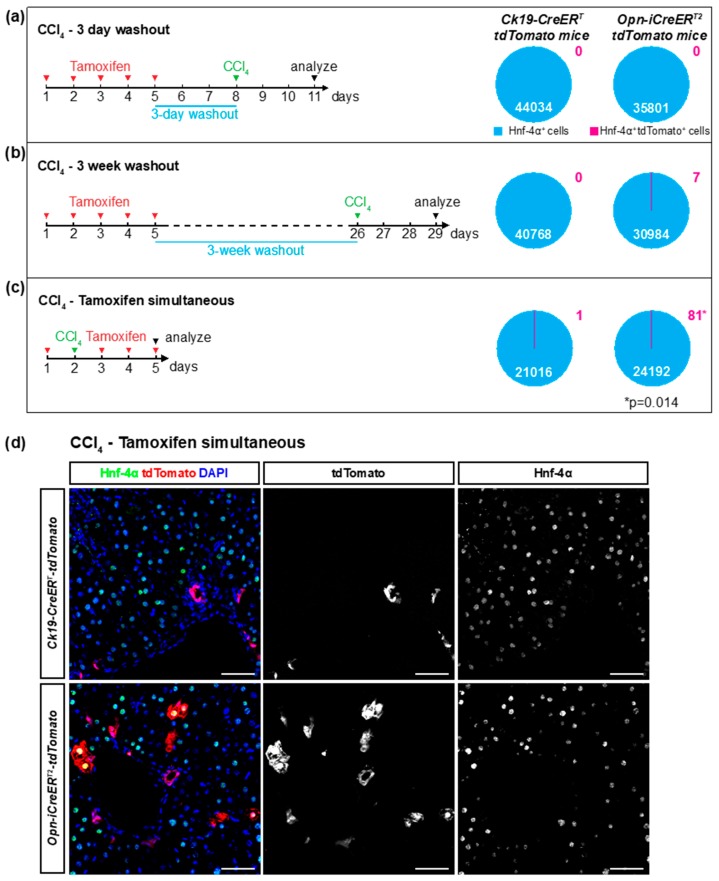
Liver injury can affect gene expression in diverse liver cells. As such, ectopic Opn expression was observed in hepatocytes, HSCs, and immune cells after liver injury [13,15,18,19]. We therefore assessed the specificity of the Ck19-CreERT and the Opn-iCreERT2 drivers after liver injury caused by carbon tetrachloride (CCl4) toxicity. To do this, we injected a single dose of CCl4 into mice either during the tamoxifen treatment, or three days or three weeks after the end of the tamoxifen treatment. We then quantified the number of Hnf-4α+ cells that expressed tdTomato three days after the CCl4 injection (Figure 3a–c). As shown by H&E staining, CCl4 induced necrosis in pericentral hepatocytes (Figure S4). In the Ck19-CreER-tdTomato mice, we found only one of over 100,000 hepatocytes that expressed tdTomato (1/21016; 0/44034; 0/40768 tdTomato+ hepatocytes for the three conditions). This result indicates that CCl4 does not induce ectopic expression of the Ck19-CreERT transgene (Figure 3a–c, Table 2). Indeed, Ck19 staining of CCl4-injected mice showed that Ck19 expression remained restricted to cholangiocytes after liver injury (Figure 3d). Similarly, extremely few tdTomato+ hepatocytes (0/35801; 7/30984) were found in Opn-CreER-tdTomato mice when CCl4 was injected three days or three weeks after tamoxifen administration (Figure 3a,b). However, when CCl4 was injected during tamoxifen treatment, 0.33% of hepatocytes (81/24192) in the Opn-CreER-tdTomato mice expressed tdTomato (Figure 3c–d; Table 2). Thus, elevated ectopic recombination in hepatocytes was observed when the injury was concomitant with the tamoxifen administration. This is consistent with the ectopic expression of the endogenous Opn gene after different types of liver injury [13,19]. No tdTomato expressing HSCs or immune cells were detected in either mouse model, as shown by immunostaining for Desmin and Cd45 respectively (Figure S5). Recombination efficiencies in cholangiocytes were comparable between injured and non-injured conditions (Table 1). Altogether, these data show that Ck19-CreERT and Opn-iCreERT2 retain their high specificity towards cholangiocytes after liver injury. However, CCl4 induces ectopic Opn expression in hepatocytes and therefore a 3-day washout period between tamoxifen and toxin treatment is required to maintain this high level of specificity of the Opn-iCreERT2 driver when using R26-tdTomato as a reporter.
Figure 3.
Ectopic activation of Cre drivers in hepatocytes after liver injury. (a–c) Schematic experimental outline. Mice were injected with CCl4 after a three-day washout (a), a three-week washout (b) or no washout (c) after tamoxifen and sacrificed three days later. The percentage of tdTomato+ Hnf-4α+ cells was quantified in liver sections of Ck19-CreER-tdTomato and Opn-CreER-tdTomato mice (n = 3–5; 18 pictures per mouse). (d) Immunofluorescent detection of Hnf-4α and tdTomato on liver sections of mice treated with tamoxifen and CCl4 without washout period. Scale bars: 50 μm.
4. Discussion
In this study we performed a thorough analysis of the efficiency and specificity by which the Ck19-CreERT and Opn-iCreERT2 drivers trigger recombination of the R26-tdTomato reporter in the liver.
We followed a standardized tamoxifen regimen and compared tdTomato expression in homeostatic livers and livers with acute toxic injury. Our results show that the Ck19-CreERT driver has near absolute specificity for cholangiocytes under normal and injury conditions. However, Ck19-CreERT has relatively low efficiency such that only about 32% of cholangiocytes underwent recombination of the R26-tdTomato reporter. On the other hand, the Opn-iCreERT2 driver showed nearly 100% efficiency in recombining the R26-tdTomato reporter in cholangiocytes. Notably, other studies reported lower levels of recombination with the Opn-iCreERT2 driver, probably because lower amounts of tamoxifen were injected and a less sensitive reporter strain was used (Rosa26-loxP-STOP-loxP-YFP) [5,17]. In any case, our findings indicate that Opn-iCreERT2 drives LoxP site recombination more efficiently than the Ck19-CreERT driver and is therefore more suitable to perform knockout studies in bile ducts.
Despite the fact that Opn-iCreERT2 is a stronger recombination driver than Ck19-CreERT, one should keep in mind that efficiency of creating knockout cells may be lower than 100% in knockout studies. Recombination of one allele is sufficient to produce a positive signal from the R26-tdTomato reporter, yet generation of homozygous mutant cells may require recombination of both alleles. In addition, the sensitivity to Cre recombination for a floxed allele may be lower than for the tdTomato reporter line, which is known to be very sensitive [20]. Determination of the recombination efficiency is therefore important to interpret the results of knockout experiments.
Our study showed Cre activity in the absence of tamoxifen. Recombination occurred in 13% of cholangiocytes in the Ck19-CreERT-tdTomato line and in 2% in the Opn-iCre-ERT2-tdTomato line. Despite its high specificity, the CK19-CreERT might therefore be unsuited for lineage tracing experiments since temporal control over Cre recombination is partially lost. These baseline levels of Cre recombination have to be considered when performing experiments.
Some hepatocytes ectopically activated Opn-iCreERT2 and expressed the tdTomato reporter. This number was minimal, since less than 1 in 10,000 hepatocytes (0.01%) was positive for tdTomato in all conditions. This extremely low level of Cre recombination in hepatocytes may not be sufficient to affect the phenotype of a full knockout in bile ducts. Nevertheless, this low background recombination may make the Opn-iCreERT2 driver unsuited for lineage tracing experiments that relay on absolute specificity for cholangiocytes, yet the driver can be used to generate mutant bile ducts.
The origin of the occasionally observed tdTomato+ hepatocytes is unclear. One possibility is that these hepatocytes were derived from cholangiocytes by trans-differentiation. However, we think that this scenario is improbable because we did not detect signs of a transition from cholangiocytes to hepatocytes, like expression of Opn or Ck19 in tdTomato+ Hnf-4α+ hepatocytes, at any analyzed time point, and because the tdTomato+ hepatocytes were not accumulated around bile ducts as would be expected if they were derived from cholangiocytes. Rather, the tdTomato expression in sparse hepatocytes may be caused by ectopic activation of the promoter driving Cre in hepatocytes. Indeed, expression of Opn is ectopically activated in hepatocytes upon liver injury, indicating that also the Opn-iCreERT2 construct may be ectopically activated [13,19]. However, non-specific recombination driven by the ectopic activation of CreER in hepatocytes could be avoided when liver injury was induced three or more days after the end of the tamoxifen administration. This washout period was thus sufficient to lower the levels of tamoxifen below the threshold required to activate the CreERT2 that was ectopically expressed in hepatocytes [21]. The length of such a washout period will however depend on the tamoxifen regimen and on the injury model applied, and should therefore be re-examined when using a different experimental set-up. In addition, high doses of tamoxifen can cause liver injury and induce the expression of cholangiocyte-specific markers such as Sox9 in hepatocytes [22]. Thus, tamoxifen toxicity may contribute to the ectopic recombination of tdTomato in hepatocytes.
Ck19-CreERT was generated by a knock-in of the CreERT ORF at the start codon of the Ck19 gene. This produced a Ck19 loss-of-function mutation and indeed we observed reduced amounts of Ck19 expression in Ck19-CreERT heterozygous mice. Homozygous Ck19-CreERT mice are viable and appear to have normal bile ducts, although without Ck19 expression [23]. The possibility that reduced expression of Ck19 might impact cholangiocyte function or specific post-injury phenotypes needs to be further evaluated. The Opn-iCreERT2 mice were created by random integration of a bacterial artificial chromosome (BAC) containing the Opn gene with iCreERT2 inserted. The insertion of this construct into the mouse genome did not cause detectable deleterious effects, as homozygous Opn-iCreERT2 mice are viable and did not show altered bile duct morphology or cholangiocyte gene expression. Thus, if desired, both drivers can be bred to homozygosity.
5. Conclusions
Our data indicate that the Opn-iCreERT2 driver is best suited for the generation of mutant bile ducts, while the Ck19-CreERT driver has near absolute specificity for bile duct cells and is therefore favorable for lineage tracing experiments. Importantly however, it has to be considered in this model that Cre recombination is present in the absence of tamoxifen.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/8/4/380/s1, Figure S1: Troma III Ck19 antibody labels all cholangiocytes, Figure S2: Ck19 expression is abrogated in homozygous Ck19-CreER-tdTomato mice, Figure S3: The Ck19-CreERT and Opn-iCreERT2 drivers do not trigger recombination in hepatic stellate cells or immune cells, Figure S4: CCl4 injection causes necrosis of centrilobular hepatocytes, Figure S5: The Ck19-CreERT and Opn-iCreERT2 drivers do not trigger recombination in hepatic stellate cells or immune cells after liver injury.
Author Contributions
B.L. and E.V. conducted experiments and prepared the original draft. L.V.H. conducted experiments. I.M.M. and I.A.L. reviewed the manuscript. L.A.v.G. and F.P.L. provided genetic strains of mice, reviewed the manuscript and provided conceptual advice. G.H. designed and supervised the study, and edited the manuscript.
Funding
E.V. was supported by a doctoral fellowship from the Research Foundation Flanders (FWO). The work of F.P.L. was supported by the D.G. Higher Education and Scientific Research of the French Community of Belgium (ARC 15/20-065), the F.R.S.-FNRS (Belgium: Grants J.0037.17 and J.0105.19), and the Belgian Foundation against Cancer (Grant 2018-078). The work of I.A.L. was supported by a grant from the Fund for Scientific Medical Research (PDR T.1067.14-P) and from unrestricted grant from Gilead, Belgium. The work of L.v.G. and G.H. was supported by FWO (G030616, G095416).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lemaigre F.P. Determining the fate of hepatic cells by lineage tracing: Facts and pitfalls. Hepatology. 2015;61:2100–2103. doi: 10.1002/hep.27659. [DOI] [PubMed] [Google Scholar]
- 2.Means A.L., Xu Y., Zhao A., Ray K.C., Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solar M., Cardalda C., Houbracken I., Martín M., Maestro M.A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Furuyama K., Kawaguchi Y., Akiyama H., Horiguchi M., Kodama S., Kuhara T., Hosokawa S., Elbahrawy A., Soeda T., Koizumi M., et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 5.Español-Suñer R., Carpentier R., Van Hul N., Legry V., Achouri Y., Cordi S., Jacquemin P., Lemaigre F., Leclercq I.A. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Jörs S., Jeliazkova P., Ringelhan M., Thalhammer J., Dürl S., Ferrer J., Sander M., Heikenwalder M., Schmid R.M., Siveke J.T., et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J. Clin. Invest. 2015;125:2445–2457. doi: 10.1172/JCI78585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigo-Torres D., Affò S., Coll M., Morales-Ibanez O., Millán C., Blaya D., Alvarez-Guaita A., Rentero C., Lozano J.J., Maestro M.A., et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367–1377. doi: 10.1002/hep.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lokmane L., Haumaitre C., Garcia-Villalba P., Anselme I., Schneider-Maunoury S., Cereghini S. Crucial role of vHNF1 in vertebrate hepatic specification. Development. 2008;135:2777–2786. doi: 10.1242/dev.023010. [DOI] [PubMed] [Google Scholar]
- 9.Kopp J.L., Dubois C.L., Schaffer A.E., Hao E., Shih H.P., Seymour P.A., Ma J., Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M.A., Ye L., et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766–779. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarlow B.D., Finegold M.J., Grompe M. Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury. Hepatology. 2014;60:278–289. doi: 10.1002/hep.27084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He L., Li Y., Pu W., Huang X., Tian X., Wang Y., Zhang H., Liu Q., Zhang L., Zhao H., et al. Enhancing the precision of genetic lineage tracing using dual recombinases. Nat. Med. 2017;23:1488–1498. doi: 10.1038/nm.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arriazu E., Ge X., Leung T.M., Magdaleno F., Lopategi A., Lu Y., Kitamura N., Urtasun R., Theise N., Antoine D.J., et al. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. 2017;66:1123–1137. doi: 10.1136/gutjnl-2015-310752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Lopategi A., Ge X., Lu Y., Kitamura N., Urtasun R., Leung T.M., Fiel M.I., Nieto N. Osteopontin induces ductular reaction contributing to liver fibrosis. Gut. 2014;63:1805–1818. doi: 10.1136/gutjnl-2013-306373. [DOI] [PubMed] [Google Scholar]
- 15.Kawashima R., Mochida S., Matsui A., You Lu Tu Z.Y., Ishikawa K., Toshima K., Yamanobe F., Inao M., Ikeda H., Ohno A., et al. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: A possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 1999;256:527–531. doi: 10.1006/bbrc.1999.0372. [DOI] [PubMed] [Google Scholar]
- 16.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu X., Español-Suñer R., Mederacke I., Affò S., Manco R., Sempoux C., Lemaigre F.P., Adili A., Yuan D., Weber A., et al. Hepatocellular carcinoma originates from hepatocytes and not from the progenitor/biliary compartment. J. Clin. Invest. 2015;125:3891–3903. doi: 10.1172/JCI77995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorena D., Darby I.A., Gadeau A.P., Leen L.L., Rittling S., Porto L.C., Rosenbaum J., Desmoulière A. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J. Hepatol. 2006;44:383–390. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Sahai A., Malladi P., Melin-Aldana H., Green R.M., Whitington P.F. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G264–G273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Willet S.G., Bankaitis E.D., Xu Y., Wright C.V., Gu G. Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis. 2013;51:436–442. doi: 10.1002/dvg.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinert R.B., Kantz J., Misfeldt A.A., Poffenberger G., Gannon M., Brissova M., Powers A.C. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS ONE. 2012;7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpentier R., Suñer R.E., van Hul N., Kopp J.L., Beaudry J.B., Cordi S., Antoniou A., Raynaud P., Lepreux S., Jacquemin P., et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438.e4. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Guldiken N., Spurny M., Mohammed H.H., Haybaeck J., Pollheimer M.J., Fickert P., Gassler N., Jeon M.K., Trautwein C., et al. Loss of keratin 19 favours the development of cholestatic liver disease through decreased ductular reaction. J. Pathol. 2015;237:343–354. doi: 10.1002/path.4580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.