Abstract
As key post-transcriptional regulators, microRNAs (miRNAs) play an indispensable role in skeletal muscle development. Our previous study suggested that miR-34b-5p and IGFBP2 could have a potential role in skeletal muscle growth. Our goal in this study is to explore the function and regulatory mechanism of miR-34b-5p and IGFBP2 in myogenesis. In this study, the dual-luciferase reporter assay and Western blot analysis showed that IGFBP2 is a direct target of miR-34b-5p. Flow cytometric analysis and EdU assay showed that miR-34b-5p could repress the cell cycle progression of myoblasts, and miR-34b-5p could promote the formation of myotubes by promoting the expression of MyHC. On the contrary, the overexpression of IGFBP2 significantly facilitated the proliferation of myoblasts and hampered the formation of myotubes. Together, our results indicate that miR-34b-5p could mediate the proliferation and differentiation of myoblasts by targeting IGFBP2.
Keywords: miR-34b-5p, IGFBP2, skeletal muscle, myoblast, myogenesis
1. Introduction
MicroRNAs (miRNAs) are small non-coding RNAs which normally can bind to the 3′UTR (3′-Untranslated Region, 3′UTR) of mRNA and function as endogenous translational repressors [1]. Recent studies have proved that miRNAs widely participate in a variety of biological processes such as innate immune responses, cell proliferation and apoptosis, colon, lung, and breast carcinogenesis, and lipid metabolism [2,3,4,5]. In our previous study, we found that the miR-34b-5p was differentially expressed between breast muscle tissues of Recessive White Rock (WRR) and Xinghua Chickens (XH) [6]. Combining this miRNA-Seq data with our previous RNA-Seq data [7], we predicted that the differentially expressed gene IGFBP2 could be the target gene of miR-34b-5p. Thus, we speculated that miR-34b-5p and IGFBP2 could play a potential role in broiler growth.
MiR-34b-5p is a member of the miR-34 family (miR-34a, miR-34b and miR-34c) and can be processed to its precursor miRNA (miR-34b) with a mature sequence of 23 nucleotides. The miR-34 family is a well-known cancer suppressor, which can be induced by p53 and participates in many cancer biological processes [8,9,10]. In chicken, it has been reported that miR-34b-5p can promote the proliferation of ALV-J-infected cells and ALV-J replication by targeting MDA5 [11]. Besides cancer, miR-34 was differentially expressed miRNA during the development of skeletal muscle in Nile tilapia [12]. All members of the miR-34 family could be involved in and affect osteoblast differentiation during embryonic development and bone-mass accrual after birth in mice [13,14]. miR-34a can also promote the proliferation of human pulmonary artery smooth muscle cells [15]. In addition, miR-34a and miR-34c could inhibit the proliferation of smooth muscle cells [16,17]. miR-34c was also reported to repress the proliferation of muscle satellite cells while promoting the differentiation process [18]. All these studies indicate that miR-34 not only can mediate the physiological process of cancer, but also can participate in the regulation of muscle growth.
Insulin-like growth factor binding protein 2 (IGFBP2) is a member of the IGFBPs (IGFBP1-6) family. These six homologous proteins are famous for their high-affinity binding to IGF1 and IGF2 [19]. IGFBP2 can participate in many different kinds of cancer as a potential biomarker [20]. Previous research has found that IGFBP2 was abundantly expressed in the eyes, brain, skeletal muscle, and heart of chickens during the embryonic period [21], and highly expressed in embryonic tissues in mammals [22]. The DNA polymorphism of IGFBP2 was significantly correlated with growth, carcass, meat quality, and reproductive traits of pigs [23,24]. However, in addition to the above studies, few reports have been made about IGFBP2 in poultry skeletal muscle development.
In this study, we explored the functional role and regulation of miR-34b-5p and IGFBP2 in chicken skeletal muscle development. We confirmed that miR-34b-5p could suppress the cell cycle progression of myoblast and accelerate the formation of myotubes by directly repressing the expression of IGFBP2.
2. Materials and Methods
2.1. Animals and Cells
The tissues including heart, liver, spleen, lung, kidney, breast muscle, and leg muscle were collected from female Xinghua chickens at 7 weeks of age. The chicken primary myoblasts (CPM) were isolated from leg muscle tissues of Yuhe chickens in at an embryo age of 11 (Zhuhai Yuhe Company Ltd, Zhuhai, China), following the method that we previously reported [25]. All experimental animals were operated on in accordance with approved guidelines of the Animal Care and Use Committee of the South China Agricultural University (Approval number: SCAU#0014). Please provide the ethic code.
2.2. Cell Culture and Transfection
DF-1 cell lines were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 0.5% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA). CPM cells were cultured in growth medium (GM, Roswell Park Memorial Institute (RPMI)-1640 medium with 20% FBS and 0.5% penicillin/streptomycin) and induced to differentiate in differentiation medium (DM, FBS reduced to 5%).
All the RNA oligonucleotides used in this study, including miR-34b-5p mimics, miR-34b-5p inhibitor, and si-gga-IGFBP2 (small interfering RNA (siRNA) used for the knockdown of IGFBP2), were obtained from RiboBio (Guangzhou, China) and the sequences are showed in Table 1. The transfection concentrations of these three oligonucleotides were 10 nM, 100 nM, and 100 nM. The oligonucleotides were transfected using Lipofectamine 3000 reagent (Invitrogen, USA) according to the manufacturer’s protocol with at least three replications.
Table 1.
Oligonucleotide sequences used in this study.
| Fragment Name | Sequence (5′ to 3′) |
|---|---|
| miR-34b-5p mimic | CAGGCAGUGUAGUUAGCUGAUUG |
| miR-34b-5p inhibitor | CUUUCUGCUUUCUUCUCUGCCUG |
| si-gga-IGFBP2 | GGGAGTGTCTCTCTTTCTT |
2.3. Plasmid Construction
Primers used in this study were designed using Premier Primer 5.0 software (Premier Biosoft International, Palo Alto, CA, USA), and synthesized by Sangon Biotech (Shanghai, China).
PmirGLO dual-luciferase reporter vector: The 3′UTR fragment of IGFBP2 (NCBI (National Center for Biotechnology Information) Reference Sequence: NM_205359.1) containing the miR-34b-5p binding site was cloned into the pmirGLO vector through the PMDTM-18T cloning vector, along with the mutation vector (Takara, Ostu, Japan). The primers were named IGFBP2-WT and IGFBP2-MT and the sequences are listed in Table 2.
Table 2.
Primers used in this study.
| Primer Name | Primer Sequences (5′ to 3′) | Size (bp) |
|---|---|---|
| IGFBP2-WT | F:ACCATTTCCCTCTTCCTCC | 295 |
| R:ACCAAGCATTCAGCTCCAC | ||
| IGFBP2-MT | F:CGAGACCAAAGACTGTAAATTGTGAGTCTTGTGTCCTGCC | 2987 |
| R:GGCAGGACACAAGACTCACAATTTACAGTCTTTGGTCTCG | ||
| IGFBP2 | F:AGCGGCAGATGGGCAAAGT | 184 |
| R:GGGGATGTGGAGGGAGTAGAGG | ||
| β-actin | F:TCATTGTGCTAGGTGCCA | 160 |
| R:TCATTGTGCTAGGTGCCA | ||
| MyoD | F: GCTACTACACGGAATCACCAAAT | 200 |
| R: CTGGGCTCCACTGTCACTCA | ||
| MyoG | F: CGGAGGCTGAAGAAGGTGAA | 320 |
| R: CGGTCCTCTGCCTGGTCAT | ||
| MyHC | F: CTCCTCACGCTTTGGTAA | 213 |
| R: TGATAGTCGTATGGGTTGGT |
The IGFBP2 overexpression vector: The full coding sequence (CDS) of IGFBP2 was synthesized by Sangon Biotech (Shanghai, China) and cloned into the pcDNA3.1 expression vector.
2.4. Dual-Luciferase Reporter Assay
The pmirGLO dual-luciferase reporter vector (200 ng) containing a wild-type or mutant IGFBP2-3′UTR fragment was co-transfected with miR-34b-5p mimic or NC (Negative Control) duplexes (100 nM) into DF-1 cells in a 96-well plate with six independent repeats. After 48 h of transfection, Firefly and Renilla luciferase activities were measured in a Fluorescence/Multi-Detection Microplate Reader (BioTek, Winooski, VT, USA) using a Dual-GLO Luciferase Assay System Kit (Promega, Madison, WI, USA).
2.5. RNA Isolation, Complementary DNA (cDNA) Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from skeletal muscle tissues or cells using RNAiso Plus (Takara, Ostu, Japan). cDNA synthesis for mRNA was carried out using a PrimeScript RT Reagent Kit with gDNA Eraser (Perfect Real Time) (Takara, Japan). The reverse transcription reaction for miRNA was performed with ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan) using specific Bulge-loop miRNA qRT-PCR Primer for miR-34b-5p and U6 designed by RiboBio (Guangzhou, China). qRT-PCR reactions were carried out in a QuantStudio 5 Real-Time PCR Systems (Thermo Fisher, Waltham, MA, USA) with iTaq Universal SYBR Green Supermix Kit (Toyobo, Japan). The comparative 2−ΔΔCT method [26] was used for qRT-PCR data analysis. The primers used for qRT-PCR are also listed in Table 2.
2.6. EdU (5-Ethynyl-2′-Deoxyuridine) Assay
After 2 h of incubation with 50 μM 5-ethynyl-2′-deoxyuridine (EdU; RiboBio, China), the CPM cells were fixed and stained with a C10310 EdU Apollo In Vitro Imaging Kit (RiboBio, China). We used a Leica DMi8 fluorescent microscope to capture the EdU-stained cells with three randomly selected fields. The proliferation rate was calculated by the ratio of the number of EdU-stained cells to the number of Hoechst 33342-stained cells.
2.7. Flow Cytometric Analysis
After 48 h of transfection, the CPM cells cultured in 12-well plates were collected and fixed in 70% ethanol at −20 °C overnight. The flow cytometric analysis was carried on a BD AccuriC6 flow cytometer (BD Biosciences, San Jose, CA, USA) with a Cell Cycle Analysis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The data were processed using the FlowJo7.6 software (Treestar Incorporated, Ashland, OR, USA).
2.8. Western Blotting
Radio-immunoprecipitation assay (RIPA) buffer and phenylmenthanesulfonyl fluoride (PMSF) protease inhibitor were used here to extract the CPM cellular proteins. The Western blot assays were carried out as previously reported [27]. The primary antibodies used in this study were as follows: IGFBP2 rabbit polyclonal antibody (11065-3-AP; proteintech, Chicago, IL, USA; 1:300), MyHC mouse monoclonal antibody (B103; DHSB, Lowa City, IA, USA; 1:500), and β-Tubulin monoclonal antibody (A01030; Abbkine, California, CA, USA; 1:5000). HRP conjugated goat anti-rabbit IgG (A21020; Abbkine, USA; 1:10000) and HRP conjugated goat anti-mouse IgG (A21010; Abbkine, USA; 1:10000) were used as secondary antibodies.
2.9. Immunofluorescence
After 48 h of transfection, CPM cells cultured in 12-well plates were first treated with 4% formaldehyde for 20 min and then permeabilized by 0.1% Triton X-100. Next, after being blocked for 30 min with goat serum, the cells were then incubated overnight with anti-MyHC (B103; DHSB, USA; 1:50). The cells were treated with ProteinFind goat anti-mouse IgG (H+L) FITC conjugate (HS221; Transgen, China; 1:200) or goat anti-mouse IgG (H+L)-Dylight 594 (BS10027; Bioworld, USA; 1:200) for 1 h. The cell nuclei were stained with DAPI (Beyotime, Shanghai, China) for 5 min and then images were captured with a Leica DMi8 fluorescent microscope (Leica, Wetzlar, Germary). We calculated the percentage of the total image area covered by myotubes as the total myotube area by using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.10. Statistical Analysis
All experimental results are presented as the mean ± S.E.M, with at least three independent replications. The statistically significant difference between groups was tested by independent sample t-test. We considered P < 0.05 to be statistically significant. * P < 0.05; ** P < 0.01; *** P < 0.001.
3. Results
3.1. miR-34b-5p Represses the Cell Cycle Progression of Myoblasts
In order to reveal the function of miR-34b-5p in myoblasts, we transfected the miR-34b-5p mimic and inhibitor into CPM cells to assess its effect on cell cycle progression (Figure 1A). The EdU assay found that the overexpression of miR-34b-5p could hamper the proliferation of CPM cells and the opposite results could be found when we inhibited the expression of miR-34b-5p (Figure 1B,C). In addition, the cells in G0/G1 phase were significantly increased after being transfected with miR-34b-5p mimic, while the number of cells in S phase were lower than that in the control group (Figure 1D,F). The cell cycle changes transfected with miR-34b-5p inhibitor showed the opposite trend (Figure 1E,F). Collectively, these results demonstrate that miR-34b-5p could repress the cell cycle progression of myoblasts.
Figure 1.
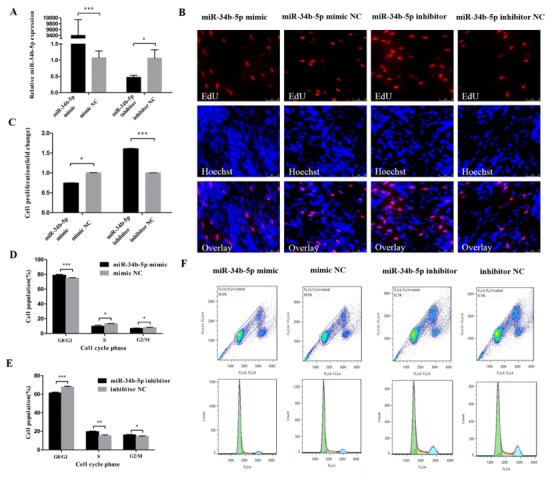
miR-34b-5p represses the cell cycle progression of myoblasts. (A) The transfection efficiency of miR-34b-5p after overexpression and inhibition of miR-34b-5p. (B) EdU staining after the transfection of miR-34b-5p mimic and inhibitor in chicken primary myoblasts (CPM) cells. (C) The proliferation rate of CPM cells transfected with miR-34b-5p mimic and inhibitor. (D,E) The statistical results of cell cycle analysis after overexpression and inhibition of miR-34b-5p in myoblast. (F) Flow cytometry raw data of cell cycle analysis in myoblasts after overexpression or inhibition of miR-34b-5p. Results are shown as mean ± S.E.M. and the data are representative of at least three independent assays. Independent sample t-test was used to analyze the statistical differences between groups. (* P < 0.05; ** P < 0.01, *** P < 0.001). Please provide a sharper figure.
3.2. miR-34b-5p Promotes the Formation of Myotubes
miR-34b-5p showed a relatively high expression in kidney, breast muscle, and leg muscle of 7 week old Xinghua chickens (Figure 2A). CPM cells at around 90% confluency were induced to undergo differentiation by changing the growth media (GM) to differentiation media (DM), as previously described. GM represents myoblasts in the proliferative phase, while DM1–DM6 represent the myoblasts that were successfully induced to differentiate from day 1 to day 6 (at different time points). Results showed that miR-34b-5p expression increased abruptly at middle (DM3) and late (DM5) differentiation stages (Figure 2B). Therefore, we transfected miR-34b-5p mimic into CPM cells and found that the overexpression of miR-34b-5p remarkably increased the expression of MyoD (myogenic differentiation), MyoG (myogenin), and MyHC (myosin heavy chain) (three important marker genes during myogenesis [28,29,30,31,32]), whereas the inhibition of miR-34b-5p had an opposite effect on these genes (Figure 2E,F). The Western blot analysis showed that miR-34b-5p could increase the expression of MyHC (Figure 2G,H). Also, the MyHC staining results showed that the number and the area of myotubes were greatly increased after miR-34b-5p mimic transfection, while the knockdown of miR-34b-5p could inhibit the formation of myotubes (Figure 2C,D). These results suggest that miR-34b-5p promotes myoblast differentiation.
Figure 2.
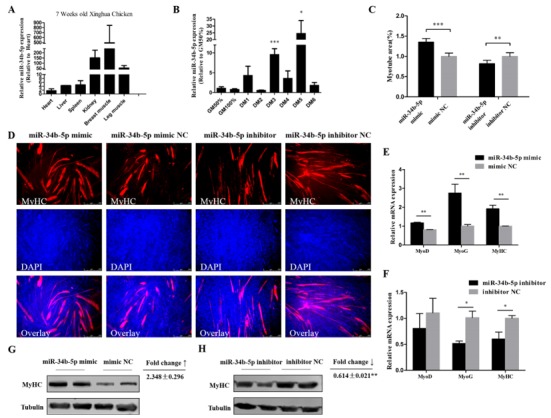
miR-34b-5p promotes the formation of myotubes. (A) The relative expression of miR-34b-5p in six tissues of 7 week old Xinghua chickens. (B) The relative expression of miR-34b-5p during CPM differentiation. GM represents myoblasts in the proliferative phase, while DM1–DM6 represent myoblasts that were successfully induced to differentiate from day 1 to day 6. (C) Myotube area (%) of CPM cells 72 h after overexpression and inhibition of miR-34b-5p. (D) MyHC staining of CPM cells 72 h after transfection of miR-34b-5p mimic and inhibitor. (E,F) MicroRNA (mRNA) level of MyoD, MyoG, and MyHC after transfection with miR-34b-5p mimic or inhibitor in CPM cells. (G,H) The protein expression of MyHC was determined by Western blotting in CPM cells after transfection with miR-34b-5p mimic and inhibitor. Results are shown as mean ± S.E.M. and the data are representative of at least three independent assays. Independent sample t-test was used to analyze the statistical differences between groups. (* P < 0.05; ** P < 0.01, *** P < 0.001).
3.3. IGFBP2 is a Direct Target of miR-34b-5p
The miR-34b-5p binding site in the 3′UTR region of IGFBP2 was successfully mutated (Figure 3A,B). A dual-luciferase reporter assay carried out in DF-1 cells showed that miR-34b-5p could reduce the luciferase activity of pmirGLO-IGFBP2-WT compared with the NC group (Figure 3E). The mRNA and protein level of IGFBP2 showed that miR-34b-5p could directly inhibit the expression of IGFBP2 (Figure 3C,D).
Figure 3.
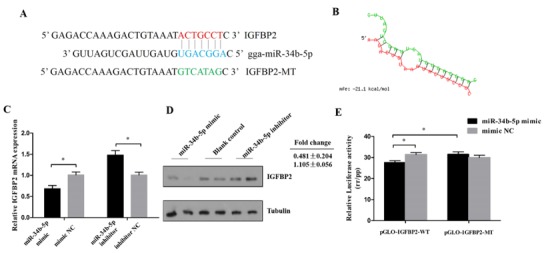
IGFBP2 is a direct target of miR-34b-5p. (A) The miR-34b-5p binding site in the IGFBP2 mRNA 3′UTR. The seed sequence and mutant sequence in miR-34b-5p were highlighted in blue and green, respectively. (B) The potential miR-34b-5p targeting site in IGFBP2 mRNA 3′UTR was analyzed by RNAhybrid software. (C,D) The mRNA and protein level of IGFBP2 in CPM cells after being transfected with miR-34b-5p mimic or inhibitor. (E) The dual-luciferase reporter assay was performed by co-transfecting a wild-type or mutant IGFBP2 3′UTR with miR-34b-5p mimic or mimic NC in DF-1 cells. Results are shown as mean ± S.E.M. and the data are representative of at least three independent assays. Independent sample t-test was used to analyze the statistical differences between groups. (* P < 0.05).
3.4. IGFBP2 Facilitates the Cell Cycle Progression of Myoblasts
IGFBP2 had a relatively high expression in kidney, breast muscle, and leg muscle of 7 week old Xinghua chickens (Figure 4A), similar to the miR-34b-5p expression (see Figure 2A). The overexpression plasmid (pcDNA3.1-IGFBP2) and si-IGFBP2 efficiency were detected by qRT-PCR and Western blot analysis. These results confirm that IGFBP2 was successfully overexpressed and knocked-down in CPM cells (Figure 4B,C). The EdU assay showed that the number of cells in the proliferative phase was significantly increased due to the overexpression of IGFBP2, and decreased after IGFBP2 knockdown (Figure 4D,E). The number of cells in G0/G1 phase were less than that in the control group after IGFBP2 overexpression, and the contrary trend could be found after IGFBP2 knockdown (Figure 4F–H).
Figure 4.
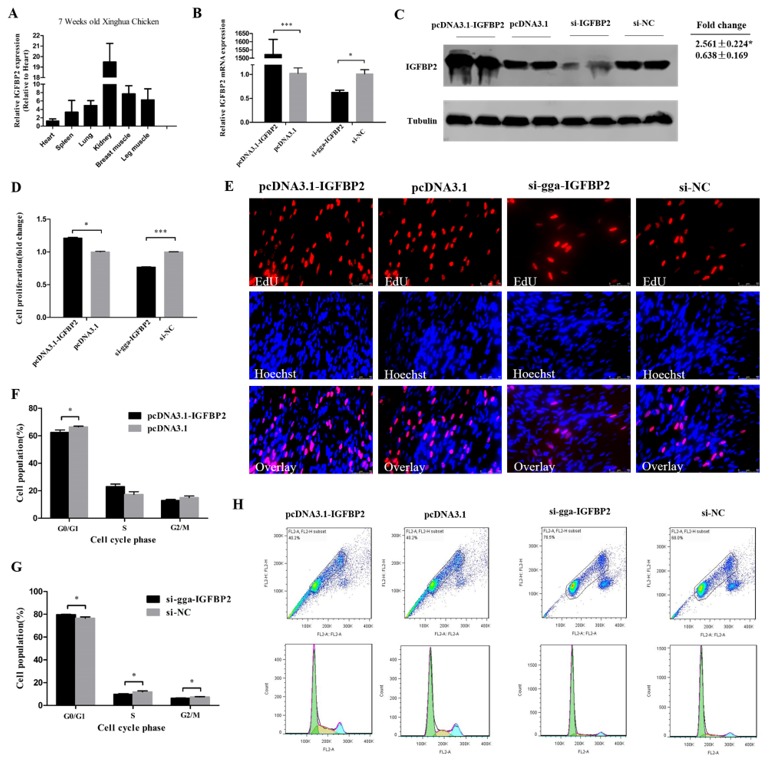
IGFBP2 facilitates the cell cycle progression of myoblasts. (A) The relative mRNA expression of IGFBP2 in six different tissues of 7 week old Xinghua chickens. (B,C) The mRNA and protein level of IGFBP2 after IGFBP2 overexpression and knockdown. (D,E) The proliferation rate and EdU assay of CPM cells after overexpression and inhibition of IGFBP2. (F–H) Flow cytometry raw data and statistical results of cell cycle analysis after IGFBP2 overexpression or knockdown in myoblasts. Results are shown as mean ± S.E.M. and the data are representative of at least three independent assays. Independent sample t-test was used to analyze the statistical differences between groups. (* P < 0.05; *** P < 0.001).
3.5. IGFBP2 is an Inhibitor of Myotube Formation
qRT-PCR results indicate that IGFBP2 could have a potential role during myoblast differentiation, since it showed a relatively high expression level during myoblast differentiation, especially at DM2 and DM4 (Figure 5A). Meanwhile, the mRNA expression of MyoD and MyHC was downregulated by IGFBP2 overexpression (Figure 5B), and upregulated after IGFBP2 inhibition (Figure 5C). The protein level of MyHC was inhibited by IGFBP2 and upregulated after IGFBP2 knockdown (Figure 5F,G). Immunofluorescence staining showed that the formation of myotubes could be hampered by IGFBP2 overexpression; conversely, IGFBP2 knockdown could promote the formation of myotubes (Figure 5D,E). These results suggest that IGFBP2 could serve as an inhibitor of myotube formation.
Figure 5.
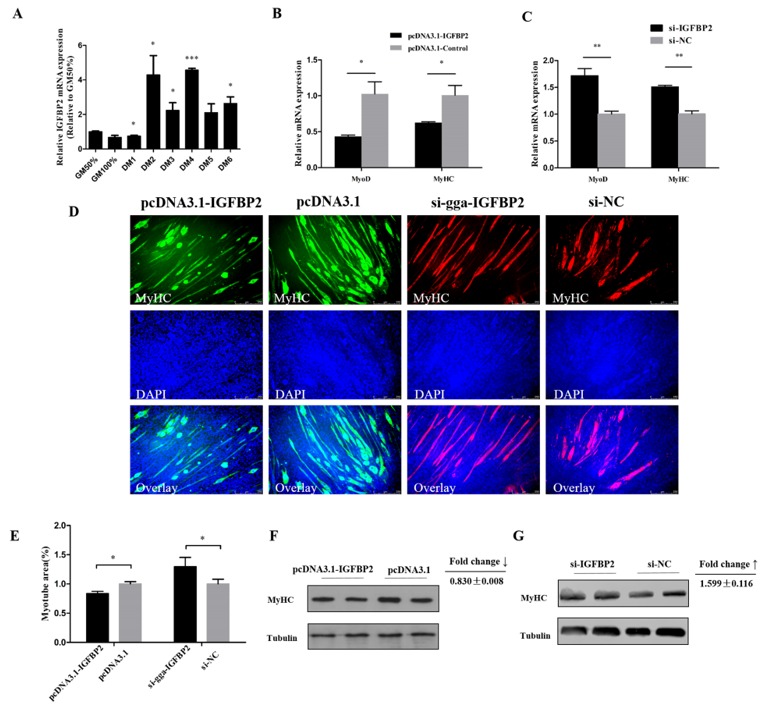
IGFBP2 suppresses myotube formation. (A) The relative expression of IGFBP2 during CPM differentiation. (B,C) The mRNA level of MyoD and MyHC after overexpression or knockdown of IGFBP2 in CPM cells. (D,E) MyHC staining and the myotube area (%) of CPM cells 72 h after overexpression and knockdown of IGFBP2. (F,G) The protein level of MyHC after overexpression and inhibition of IGFBP2. Results are shown as mean ± S.E.M. and the data are representative of at least three independent assays. Independent sample t-test was used to analyze the statistical differences between groups. (* P < 0.05; ** P < 0.01, *** P < 0.001).
4. Discussion
Myoblast proliferation, differentiation, and fusion into multinucleated myotubes occur to form myofibers, and the formation of myofibers is one of the processes of skeletal muscle development that only happens in the embryonic stage [28,29]. Besides some muscle-specific miRNAs (such as miR-206, miR-1, and miR-133), many non-muscle-specific miRNAs are also required for the differentiation of myoblasts. This is the reason why we chose chicken primary myoblasts to investigate the function of miR-34b-5p and IGFBP2.
It has been reported that miR-34 can target many genes involved in cell cycle progression, such as CCND1, CCNE2, CDK4, CDK6, E2F3, MAP2K1, MAP3K9, MYC, and so on [30], and our present study with gga-miR-34b-5p shared the same binding sites with hsa-miR-34b-5p. This means that gga-miR-34b-5p could possibly mediate the cell cycle progression through these genes. In our study, we found that miR-34b-5p overexpression increased the proportion of cells in G0/G1 phase and reduced the proportion of cells in S phase, suggesting that it can suppress cells from G0/G1 to S phase in CPM. It was reported that miR-34b could repress muscle cell proliferation and promote myotube formation by targeting NUCL in mouse myoblast C2C12 cells [31], which proved that our results are true and reliable. Previous studies have found that miR-34b-5p could induce G1/S shift by targeting MDA5 in DF-1 cells, while in our study, we found that IGFBP2 could facilitate the cell cycle progression of myoblasts. This difference may be explained by a study that showed that miR-34b-5p had a totally different effect when compared between DF-1 and myoblast cells [11]. Our results in Figure 2A indicate that miR-34b-5p could also play a potential regulatory role in postnatal myofiber hypertrophy. MyoD and MyoG are important marker genes during myogenesis [32,33,34]. The major difference between muscle fiber types is related to their myosin complement [35]. So, myosin heavy chain (MyHC) staining would be an appropriate way to describe myoblast differentiation. Here, we confirmed that miR-34b-5p could accelerate the formation of myotubes by promoting the mRNA level of MyoD, MyoG, and MyHC as well as the protein level of MyHC.
The role of IGFBP2 has been studied in angiogenesis [36] and many types of cancers [20], but not in chicken skeletal muscle development. In the present study, we proved that IGFBP2 had a relatively high expression in the breast muscle and leg muscle of 7 week old Xinghua chickens (Figure 4A) and it could participate in the proliferation and differentiation of myoblasts. We used chicken primary myoblast cells to better understand the how IGFBP2 regulates skeletal muscle development. Previous studies have found that the DNA polymorphism of IGFBP2 is associated with chicken growth, body composition, and carcass traits [37,38]. In this study, we found that IGFBP2 could facilitate the cell cycle progression of myoblasts by promoting the transition of myoblasts from G0/G1 phase to S phase, and could suppress the formation of myotubes by inhibiting MyoD and MyHC. It is also reported that c-Myc and ZEB1 could be repressors of myoblast differentiation [39,40], but we found that IGFBP2 could repress the expression of these two genes (data not shown), indicating that IGFBP2 might play a more complex regulatory role in the differentiation of myoblasts than we expected.
5. Conclusions
In conclusion, we found that miR-34b-5p could repress the proliferation and promote the differentiation of myoblasts by targeting IGFBP2 (Figure 6).
Figure 6.
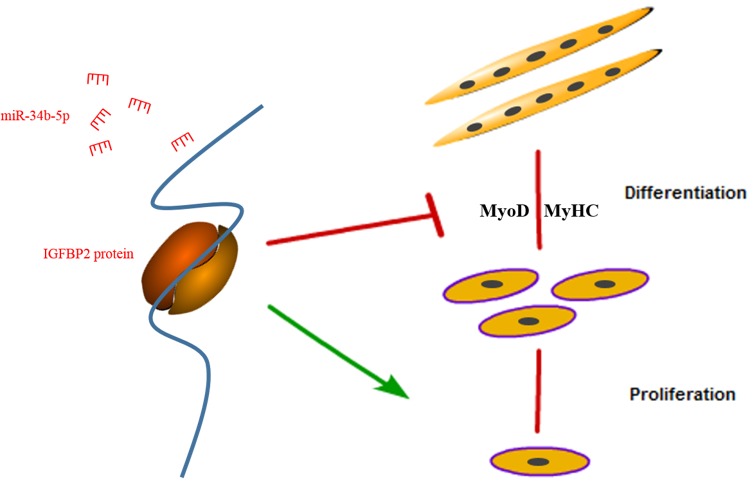
A schematic model of an IGFBP2-mediated regulatory network during myoblast proliferation and differentiation.
Author Contributions
Z.W. and X.Z. designed the study, carried out all experiments, analyzed data, and wrote the paper. Z.L. participated in the design of the experiment and data analysis. B.A. and Y.C. helped with performing some of the manuscripts’ experiments, useful discussion, and language correction. Q.N. participated in the design, manuscript writing, and final approval of the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by the grants from Science and Technology Planning Project of Guangzhou City (201604020007; 201504010017) and the Ten Thousand Talents Program of China (W03020593).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Saunders M.A., Liang H., Li W. Human polymorphism at microRNAs and microRNA target sites. Proc. Natl. Acad. Sci. USA. 2007;104:3300–3305. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullen B.R. MicroRNAs as mediators of viral evasion of the immune system. Nat. Immunol. 2013;14:205–210. doi: 10.1038/ni.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey R., Ahmed R.P.H. MicroRNAs Inducing Proliferation of Quiescent Adult Cardiomyocytes. Cardiovasc. Regener. Med. 2015;2:e519. doi: 10.14800/crm.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taft R.J., Pang K.C., Mercer T.R., Dinger M., Mattick J.S. Non-coding RNAs: Regulators of disease. J. Pathol. 2010;220:126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- 5.Yu J., Peng J., Luan Z., Zheng F., Su W. MicroRNAs as a Novel Tool in the Diagnosis of Liver Lipid Dysregulation and Fatty Liver Disease. Molecules. 2019;24:230. doi: 10.3390/molecules24020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouyang H., He X., Li G., Xu H., Jia X., Nie Q., Zhang X. Deep Sequencing Analysis of miRNA Expression in Breast Muscle of Fast-Growing and Slow-Growing Broilers. Int. J. Mol. Sci. 2015;16:16242–16262. doi: 10.3390/ijms160716242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jebessa E., Ouyang H., Abdalla B.A., Li Z., Abdullahi A.Y., Liu Q., Nie Q., Zhang X. Characterization of miRNA and their target gene during chicken embryo skeletal muscle development. Oncotarget. 2018;9:17309–17324. doi: 10.18632/oncotarget.22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He L., He X., Lim L.P., De Stanchina E., Xuan Z., Liang Y., Xue W., Zender L., Magnus J., Ridzon D., et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1116–1130. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bommer G.T., Gerin I., Feng Y., Kaczorowski A.J., Kuick R., Love R.E., Zhai Y., Giordano T.J., Qin Z.S., Moore B.B., et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Luo Q., Xu H., Zheng M., Abdalla B.A., Feng M., Cai B., Zhang X., Nie Q., Zhang X. MiR-34b-5p Suppresses Melanoma Differentiation-Associated Gene 5 (MDA5) Signaling Pathway to Promote Avian Leukosis Virus Subgroup J (ALV-J)-Infected Cells Proliferaction and ALV-J Replication. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C.W., Li Y.H., Hu S.Y., Chi J.R., Lin G.H., Lin C.C., Gong H.Y., Chen J.Y., Chen R.H., Chang S.J., et al. Differential expression patterns of growth-related microRNAs in the skeletal muscle of Nile tilapia (Oreochromis niloticus) J. Anim. Sci. 2012;90:4266–4279. doi: 10.2527/jas.2012-5142. [DOI] [PubMed] [Google Scholar]
- 13.Bae Y., Yang T., Zeng H., Campeau P.M., Chen Y., Bertin T., Dawson B.C., Munivez E., Tao J., Lee B.H. miRNA-34c regulates Notch signaling during bone development. Hum. Mol. Genet. 2012;21:2991–3000. doi: 10.1093/hmg/dds129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J., Shi Y., Zheng L., Zhou B., Inose H., Wang J., Guo X.E., Grosschedl R., Karsenty G. miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J. Cell Biol. 2012;197:509–521. doi: 10.1083/jcb.201201057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P., Xu J., Hou Z., Wang F., Song Y., Wang J., Zhu H., Jin H. miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif. 2016;49:484–493. doi: 10.1111/cpr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q., Yang F., Guo M., Wen G., Zhang C., Le Anh L., Zhu J., Xiao Q., Zhang L. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J. Mol. Cell. Cardiol. 2015;89:75–86. doi: 10.1016/j.yjmcc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Choe N., Kwon J., Kim Y.S., Eom G.H., Ahn Y.K., Baik Y.H., Park H., Kook H. The microRNA miR-34c inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by targeting stem cell factor. Cell Signal. 2015;27:1056–1065. doi: 10.1016/j.cellsig.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Hou L., Xu J., Li H., Ou J., Jiao Y., Hu C., Wang C. MiR-34c represses muscle development by forming a regulatory loop with Notch1. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-09688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxter R.C. Insulin-like growth factor (IGF)-binding proteins: Interactions with IGFs and intrinsic bioactivities. Am. J. Physiol. Endocrinol. Metab. 2000;278:E967–E976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 20.Pickard A., McCance D.J. IGF-binding protein 2-oncogene or tumor suppressor? Front. Endocrinol. 2015;6 doi: 10.3389/fendo.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoen T.J., Mazuruk K., Waldbillig R.J., Potts J., Beebe D.C., Chader G.J., Rodriguez I.R. Cloning and characterization of a chick embryo cDNA and gene for IGF-binding protein-2. J. Mol. Endocrinol. 1995;15:49–59. doi: 10.1677/jme.0.0150049. [DOI] [PubMed] [Google Scholar]
- 22.Gerrard D.E., Okamura C.S., Grant A.L. Expression and location of IGF binding proteins-2, -4, and -5 in developing fetal tissues. J. Anim. Sci. 1999;77:1431–1441. doi: 10.2527/1999.7761431x. [DOI] [PubMed] [Google Scholar]
- 23.Sironen A.I., Uimari P., Serenius T., Mote B., Rothschild M., Vilkki J. Effect of polymorphisms in candidate genes on reproduction traits in Finnish pig populations. J. Anim. Sci. 2010;88:821–827. doi: 10.2527/jas.2009-2426. [DOI] [PubMed] [Google Scholar]
- 24.Prasongsook S., Choi I., Bates R.O., Raney N.E., Ernst C.W., Tumwasorn S. Association of Insulin-like growth factor binding protein 2 genotypes with growth, carcass and meat quality traits in pigs. J. Anim. Sci. Technol. 2015;57:31. doi: 10.1186/s40781-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z., Ouyang H., Chen X., Yu J., Abdallat B.A., Chen BNie Q. Gga-miR-205a Affecting Myoblast Proliferation and Differentiation by Targeting CDH11. Front. Genet. 2018;9 doi: 10.3389/fgene.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Bass J.J., Wilkinson D.J., Rankin D., Phillips B.E., Szewczyk N.J., Smith KAtherton P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports. 2017;27:4–25. doi: 10.1111/sms.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen E.H., Grote E., Mohler W., Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Luo W., Nie Q., Zhang X. MicroRNAs Involved in Skeletal Muscle Differentiation. J. Genet. Genom. 2013;40:107–116. doi: 10.1016/j.jgg.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Rokavec M., Li H., Jiang L., Hermeking H. The p53/miR-34 axis in development and disease. J. Mol. Cell Biol. 2014;6:214–230. doi: 10.1093/jmcb/mju003. [DOI] [PubMed] [Google Scholar]
- 31.Tang Z., Qiu H., Luo L., Liu N., Zhong J., Kang K., Gou D. miR-34b Modulates Skeletal Muscle Cell Proliferation and Differentiation. J. Cell. Biochem. 2017;118:4285–4295. doi: 10.1002/jcb.26079. [DOI] [PubMed] [Google Scholar]
- 32.Venuti J.M., Morris J.H., Vivian J.L., Olson E.N., Klein W.H. Myogenin is required for late but not early aspects of myogenesis during mouse development. J. Cell Biol. 1995;128:563–576. doi: 10.1083/jcb.128.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudnicki M.A., Schnegelsberg P.N., Stead R.H., Braun T., Arnold H.H., Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-V. [DOI] [PubMed] [Google Scholar]
- 34.Zammit P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017;72:19–32. doi: 10.1016/j.semcdb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Pette D., Staron R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Azar W.J., Zivkovic S., Werther G.A., Russo V.C. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33:578–588. doi: 10.1038/onc.2012.630. [DOI] [PubMed] [Google Scholar]
- 37.Lei M.M., Nie Q.H., Peng X., Zhang D.X., Zhang X.Q. Single nucleotide polymorphisms of the chicken insulin-like factor binding protein 2 gene associated with chicken growth and carcass traits. Poultry Sci. 2005;84:1191–1198. doi: 10.1093/ps/84.8.1191. [DOI] [PubMed] [Google Scholar]
- 38.Li Z.H., Li H., Zhang H., Wang S.Z., Wang Q.G., Wang Y.X. Identification of a single nucleotide polymorphism of the insulin-like growth factor binding protein 2 gene and its association with growth and body composition traits in the chicken. J. Anim. Sci. 2006;84:2902–2906. doi: 10.2527/jas.2006-144. [DOI] [PubMed] [Google Scholar]
- 39.Li G., Luo W., Abdalla B.A., Ouyang H., Yu J., Hu F., Nie Q., Zhang X. miRNA-223 upregulated by MYOD inhibits myoblast proliferation by repressing IGF2 and facilitates myoblast differentiation by inhibiting ZEB1. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo W., Chen J., Li L., Ren X., Cheng T., Lu S., Lawal R.A., Nie Q., Zhang X., Hanotte O. c-Myc inhibits myoblast differentiation and promotes myoblast proliferation and muscle fibre hypertrophy by regulating the expression of its target genes, miRNAs and lincRNAs. Cell Death Differ. 2018 doi: 10.1038/s41418-018-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


