Abstract
Background: Exercise is one of the best nonpharmacologic therapies to treat hypertension. The blood pressure (BP) response to exercise is heritable. Yet, the genetic basis for the antihypertensive effects of exercise remains elusive. Methods: To assemble a prioritized gene signature, we performed a systematic review with a series of Boolean searches in PubMed (including Medline) from earliest coverage. The inclusion criteria were human genes in major BP regulatory pathways reported to be associated with: (1) the BP response to exercise; (2) hypertension in genome-wide association studies (GWAS); (3) the BP response to pharmacotherapy; (4a) physical activity and/or obesity in GWAS; and (4b) BP, physical activity, and/or obesity in non-GWAS. Included GWAS reports disclosed the statistically significant thresholds used for multiple testing. Results: The search yielded 1422 reports. Of these, 57 trials qualified from which we extracted 11 genes under criteria 1, 18 genes under criteria 2, 28 genes under criteria 3, 27 genes under criteria 4a, and 29 genes under criteria 4b. We also included 41 genes identified from our previous work. Conclusions: Deep-sequencing the exons of this systematically assembled signature of genes represents a cost and time efficient approach to investigate the genomic basis for the antihypertensive effects of exercise.
Keywords: antihypertensive therapy, healthy lifestyle, physical exercise
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, claiming 31% (17.5 million) of deaths globally [1,2]. Hypertension is the most common, costly, and preventable CVD risk factor [1]. The American College of Cardiology (ACC)/American Heart Association (AHA) recently redefined hypertension to a lower blood pressure (BP) threshold of 130 mmHg for systolic BP (SBP) or 80 mmHg for diastolic BP (DBP) [3]. This change has been met with some opposition. Nonetheless, the lower BP thresholds now classify 50% of adults in the United States with hypertension [1], underscoring the importance of hypertension as a public health problem.
The authors of the AHA/ACC report rated exercise as one of the best nonpharmacologic therapies to treat hypertension because aerobic exercise training lowers BP 5–8 mmHg among adults with hypertension [3]. The magnitude of these BP reductions rival those that result from taking antihypertensive medication [4,5], may lower the risk of CVD risk by 4–22% and stroke by 6–41% [6,7,8], and reduce the resting BP of some adults with hypertension into normal ranges [9,10]. Accordingly, professional organizations from around the world recommend adults with hypertension participate in 30–60 min/day of aerobic exercise, such as walking or jogging, on most days of the week [5,11].
Despite the well-documented antihypertensive benefits of exercise, many adults with hypertension do not exercise to lower their BP [12,13]. Reasons for this non-adherence are varied; however, one reason relevant to the topic of this systematic review is that there is significant inter-individual variability in the BP response to exercise partially attributed to genetic predispositions that led some to believe exercise does not work as antihypertensive therapy [14,15]. Indeed, investigators from the HEalth, RIsk Factors Exercise TrAining and GEnetics or HERITAGE Family Study involving over 700 subjects established the BP response to aerobic exercise is heritable (h = 0.13–0.42) [14,16,17,18,19]. Nearly 20 years ago this discovery prompted our laboratory group [20,21,22,23,24] and others [16,17,18,19,25] to conduct candidate genes association studies to identify genetic variants that account for a clinically meaningful proportion of the variability in the BP response to exercise. However, these efforts have met with little success [17,25,26].
More recently, we exploited advances in genomic technology that emerged since our discovery phase candidate gene association studies as well as other strategies to bolster our statistical power to detect genetic variants associated with the BP response to exercise in a replication cohort of subjects with hypertension whose characteristics resembled those from our earlier studies. In this series of studies, we deep-sequenced the exons of genes using the Illumina TruSeq Custom Amplicon kit (Catalog# FC-130-1001, Illumina, San Diego, CA, USA) on a prioritized signature of 41 BP and exercise genes that contained genes identified from our prior work in addition to those obtained from a systematic review of the literature of genes reported to be associated with hypertension or the BP response to exercise or pharmacotherapy [27,28,29]. After adjustment for multiple testing, despite the small sample size, we found variants in 61% of the 41 genes in the prioritized panel associated with the BP response to exercise. We attributed the high proportion of the significant BP-genotype associations that we found to a focused inquiry of variants with a gene signature obtained from a systematic review of the literature that reduced the search space within the genome; and the use of high throughput exon sequencing to concentrate on functional gene regions and standardized BP and exercise protocols that were well controlled and closely supervised.
The purpose of this systematic review is to update and expand our original systematic review to assemble a prioritized signature of BP and exercise genes whose exons can then be deep-sequenced among a larger, more ethnically and gender diverse sample of adults that were reported in the qualifying studies to have hypertension to better inform the genomic basis for the antihypertensive effects of exercise. The long-term goal of this work is to develop personalized exercise prescriptions based upon genetic predispositions and other clinical characteristics to optimize the BP benefits of exercise.
2. Systematic Review Methods
This systematic review followed the specifications of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [30,31]. A series of comprehensive Boolean searches were run in PubMed (including Medline). The first search replicated the original search done by Bruneau et al. [25] in the four databases of Medline, Biosis, Scopus, and Web of Science and included contiguous dates. The additional three searches were run from earliest coverage to 11 May 2017 to locate trials that met our predetermined inclusion criteria. These a priori criteria were human genes in major BP regulatory pathways reported to be associated with: (1) the BP response to exercise; (2) hypertension in genome-wide association studies (GWAS); (3) the BP response to pharmacotherapy; (4a) physical activity and/or obesity, a major risk factor for hypertension as 80% of adults with hypertension are overweight to obese [32] in GWAS; and (4b) BP, physical activity, and/or obesity in non-GWAS from relevant reference searches of the authors’ files. We also included the genes from the prioritized signature of BP and exercise genes from our previous work [27,28,29].
The major regulatory BP pathways that a qualifying gene could be in were the renin angiotensin aldosterone system, endothelial nitric oxide synthase pathway, and/or the sympathetic nervous system, and/or pathways involved with vascular function and structure, fluid and electrolyte control, inflammation, insulin sensitivity, lipid metabolism, and obesity. Of note, many of the GWAS we initially located as indicating they involved genes associated with hypertension upon closer scrutiny involved genes associated with resting BP rather than hypertension per se so that they were excluded. In all GWAS for the gene to be included on the panel, the reported associations with the BP response to exercise must have met preestablished statistically significant thresholds for multiple testing which is commonly set at p = 5 × 10−8. The full search strategy for each of the four a priori criteria are depicted in Table 1, Table 2, Table 3 and Table 4.
Table 1.
Criteria 1: Genes (n = 11) in major blood pressure regulatory pathways associated with the blood pressure response to exercise. Two searches were conducted on 05/11/2017 and a total of 321 records were identified.
| Search 1: | (2006/01:2017/06 [edat]) AND (“mean arterial” OR “blood pressure”[mesh] OR “blood pressure” OR “blood pressures” OR “arterial pressure” OR “arterial pressures” OR hypertension OR hypotension OR normotension OR hypertensive OR hypotensive OR normotensive OR “systolic pressure” OR “diastolic pressure” OR “pulse pressure” OR “venous pressure” OR “pressure monitor” OR hypotension OR “pre hypertension” OR “bp response” OR “bp decrease” OR “bp reduction” OR “bp monitor” OR “bp monitors” OR “bp measurement”) AND (“exercise”[mesh] OR exercise OR exercises OR running[mesh] OR “bicycle” OR “bicycles” OR “bicycling” OR walking[mesh] OR treadmill* OR “weight lifting” OR “weight training” OR “weight bearing” OR “resistance training” OR “strength training” OR “endurance training” OR “speed training” OR “training duration” OR “training frequency” OR “training intensity” OR “aerobic endurance”) AND (“randomized controlled trial”[pt] OR “nonrandomized controlled” OR “nonrandomized control” OR controlled clinical trial[pt] OR “randomized controlled trial”[publication type] OR random allocation[mh] OR clinical trial[pt] OR “comparative study” OR “comparative studies” OR clinical trials[mh] OR “clinical trial”[tw] OR “latin square”[tw] OR random*[tw] OR research design[mh:noexp] OR “comparative study”[publication type] OR “evaluation studies”[publication type] OR “prospective studies”[mh] OR “cross-over studies”[mh] OR “control”[tw] OR “controlled”[tw]) AND (“gene” OR “genes” OR “genotype” OR “genotypes” OR “snp” OR polymorphism* OR “DNA” OR “minor allele” OR “minor alleles” OR “single nucleotide polymorphism” OR “single nucleotides polymorphisms” OR genetic*) NOT (“DASH”[tiab] OR “cancer” OR “neoplasms” OR “review”[pt] OR “fibromyalgia” OR “alzheimers” OR “alzheimer” OR “pregnant” OR “pregnancy” OR “obesity/drug therapy”[mesh] OR “diet therapy”[mesh] OR “diet therapy”[subheading] OR “caffeine” OR “eating change” OR “activities of daily living” OR “dehydration” OR “dehydrate” OR “dehydrated” OR “dietary salt” OR “epilepsy” OR “influenza” OR “flu” OR “pneumonia” OR “septicemia” OR “hiv” OR “Acquired Immunodeficiency Syndrome” OR “meningitis” OR “substance abuse” OR “alcoholism” OR “drug abuse” OR “Cross-Sectional Studies”[MeSH Terms] OR “Prospective Studies”[MeSH Terms] OR “epidemiology”[Subheading]). |
| Search 2: | (“mean arterial” OR “blood pressure”[mesh] OR “blood pressure” OR “blood pressures” OR “arterial pressure” OR “arterial pressures” OR hypertension OR hypotension OR normotension OR hypertensive OR hypotensive OR normotensive OR “systolic pressure” OR “diastolic pressure” OR “pulse pressure” OR “venous pressure” OR “pressure monitor” OR hypotension OR “pre hypertension” OR “bp response” OR “bp decrease” OR “bp reduction” OR “bp monitor” OR “bp monitors” OR “bp measurement”) AND (“ambulatory blood pressure” OR “exercise”[mesh] OR exercise[ti] OR exercises OR running[mesh] OR running[ti] OR “bicycle” OR “bicycles” OR “bicycling” OR walking[mesh] OR walking[ti] OR treadmill* OR “weight lifting” OR “weight training” OR “weight bearing” OR “resistance training” OR “strength training” OR “endurance training” OR “speed training” OR “training duration” OR “training frequency” OR “training intensity” OR “aerobic endurance” OR “aerobic training”) AND (gwa[ti] OR gwas[ti] OR genome[ti] OR “gene”[ti] OR “genes”[ti] OR “genotype”[ti] OR “genotypes”[ti] OR “genotyping”[ti] OR “snp”[ti] OR “snps”[ti] OR polymorphism*[ti] OR “DNA”[ti] OR allele[ti] OR alleles[ti] OR “minor allele” OR “minor alleles” OR “single nucleotide polymorphism” OR “single nucleotides polymorphisms” OR genetic*[ti] OR “trait locus”[ti] OR “loci”[ti] OR “Genetic Predisposition to Disease”[MeSH] OR “Genotype”[MeSH] OR “Gene Frequency”[MeSH] OR “Polymorphism, Single Nucleotide/genetics”[MESH] OR “Polymorphism, Single Nucleotide”[MAJR] OR “Genetic Loci”[Mesh] OR “Genetic Association Studies”[Mesh] OR “Genetic Variation”[Mesh]) NOT (“DASH”[tiab] OR “cancer” OR “neoplasms” OR “review”[pt] OR “fibromyalgia” OR “alzheimers” OR “alzheimer” OR “pregnant” OR “pregnancy” OR “obesity/drug therapy”[mesh] OR “diet therapy”[mesh] OR “diet therapy”[subheading] OR “caffeine” OR “eating change” OR “activities of daily living” OR “dehydration” OR “dehydrate” OR “dehydrated” OR “dietary salt” OR “epilepsy” OR “influenza” OR “flu” OR “pneumonia” OR “septicemia” OR “hiv” OR “Acquired Immunodeficiency Syndrome” OR “meningitis” OR “substance abuse” OR “alcoholism” OR “drug abuse” OR “Cross-Sectional Studies”[MeSH Terms] OR “Prospective Studies”[MeSH Terms] OR “epidemiology”[Subheading]). |
Table 2.
Criteria 2: Genes (n = 18) in major blood pressure regulatory pathways associated with hypertension in genome wide association studies. The search was conducted on 05/11/2017 and a total of 188 records were identified.
| (“mean arterial” OR “blood pressure”[mesh] OR “blood pressure” OR “blood pressures” OR “arterial pressure” OR “arterial pressures” OR hypertension OR hypotension OR normotension OR hypertensive OR hypotensive OR normotensive OR “systolic pressure” OR “diastolic pressure” OR “pulse pressure” OR “venous pressure” OR “pressure monitor” OR hypotension OR “pre hypertension” OR “bp response” OR “bp decrease” OR “bp reduction” OR “bp monitor” OR “bp monitors” OR “bp measurement” OR “Blood Pressure/genetics”[MeSH]) AND (“resting blood pressure” OR “resting BP” OR “exercise”[mesh] OR exercise[ti] OR exercises OR running[mesh] OR running[ti] OR “bicycle” OR “bicycles” OR “bicycling” OR walking OR walking[mesh] OR walking[ti] OR treadmill* OR “weight lifting” OR “weight training” OR “weight bearing” OR “resistance training” OR “strength training” OR “endurance training” OR “speed training” OR “training duration” OR “training frequency” OR “training intensity” OR “aerobic endurance” OR “aerobic training” OR “physical activity” OR “motor activity”[mesh] OR Overweight OR “Overweight”[Mesh] OR BMI OR “body mass” OR “Body Mass Index”[MeSH] OR “Waist Circumference”[MeSH] OR obesity OR “Obesity”[Mesh] OR obese) AND (gwa OR gwas OR “Genetic Association Studies”[Mesh] OR genomewide OR “genome wide” OR “genomewide association” OR “genome wide association” OR “genome-wide interaction”) NOT (“review”[pt] OR “Cross-Sectional Studies”[MeSH Terms] OR Comment[pt] OR Editorial[pt] OR Letter[pt] OR “Case Reports”[pt] OR “case control”[ti] OR “case report”[ti] OR “case study”[ti] OR “case series”[ti] OR “Case-Control Studies”[Mesh] OR “Follow-Up Studies”[Mesh] OR “observational study”[ti] OR “prospective cohort”[ti] OR “cohort studies” [Mesh:NoExp] OR “cohort study”[ti] OR “Longitudinal Studies” [Mesh:NoExp] OR “Follow-Up Studies”[mesh] OR “Retrospective Studies”[mesh] OR “non-randomized”[ti] OR “follow up study”[ti] OR “Cross-Sectional Studies”[MeSH Terms] OR “Prospective Studies”[MeSH Terms] OR “epidemiology”[Subheading] OR “pulmonary hypertension” OR “pulmonary arterial hypertension” OR “heart transplant” OR “heart failure” OR “cystic fibrosis” OR “cancer” OR “neoplasms” OR “fibromyalgia” OR “alzheimers” OR “alzheimer” OR “pregnant” OR “pregnancy” OR “obesity/drug therapy”[mesh] OR “diet therapy”[mesh] OR “diet therapy”[subheading] OR “DASH”[tiab] OR meal[ti] OR “nutritional intervention” OR “dietary intervention” OR “nutritional counseling” OR “dietary counseling” OR “caffeine” OR “eating change” OR “activities of daily living” OR “dehydration” OR “dehydrate” OR “dehydrated” OR “dietary salt” OR sodium OR “epilepsy” OR “influenza” OR “flu” OR “pneumonia” OR “septicemia” OR arthritis OR “hiv” OR “Acquired Immunodeficiency Syndrome” OR “meningitis” OR “substance abuse” OR “alcoholism” OR “drug abuse” OR “spinal cord”[ti] OR “Sleep”[Majr] OR “Sleep Apnea Syndromes”[Majr] OR sleep[ti] OR contraceptive*[ti] OR (animals[mesh] NOT humans[mesh]) OR rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR pig[ti] OR pigs[ti] OR dog[ti] OR dogs[ti] OR canine[ti] OR cow[ti] OR cows[ti] OR bovine[ti]). |
Table 3.
Criteria 3. Genes (n = 28) in major blood pressure regulatory pathways associated with the blood pressure response to pharmacotherapy. The search was conducted on 05/11/2017 and a total of 711 records were identified.
| (“mean arterial” OR “blood pressure”[mesh] OR “blood pressure” OR “blood pressures” OR “arterial pressure” OR “arterial pressures” OR hypertension OR hypotension OR normotension OR hypertensive OR hypotensive OR normotensive OR “systolic pressure” OR “diastolic pressure” OR “pulse pressure” OR “venous pressure” OR “pressure monitor” OR hypotension OR “pre hypertension” OR “bp response” OR “bp decrease” OR “bp reduction” OR “bp monitor” OR “bp monitors” OR “bp measurement” OR “Blood Pressure/genetics”[Mesh]) AND (“Antihypertensive Agents”[Mesh] OR “Antihypertensive Agents” [Pharmacological Action] OR “anti-hypertensive agent” OR “antihypertensive agent” OR “anti hypertensive agent” OR “anti-hypertensive agents” OR “antihypertensive agents” OR “anti hypertensive agents” OR “anti-hypertensive drug” OR “antihypertensive drug” OR “anti hypertensive drug” OR “anti-hypertensive drugs” OR “antihypertensive drugs” OR “anti hypertensive drugs” OR “anti-hypertensive medication” OR “antihypertensive medication” OR “anti hypertensive medication” OR “anti-hypertensive medications” OR “antihypertensive medications” OR “anti hypertensive medications” OR “anti-hypertensives” OR antihypertensives OR “anti hypertensives” OR diuretic OR diuretics OR “Diuretics”[Mesh] OR acebutolol OR aliskiren OR Ambrisentan OR amlodipine OR atenolol OR “azilsartan medoxomil” OR benazepril OR betaxolol OR bisoprolol OR bosentan OR “candesartan cilexetil” OR captopril OR carteolol OR carvedilol OR chlorthalidone OR clonidine OR cilazapril OR clevidipine OR deserpidine OR diazoxide OR diltiazem OR doxazosin OR enalapril OR enalaprilat OR “eprosartan mesylate” OR hydrochlorothiazide OR felodipine OR fenoldopam OR fosinopril OR guanabenz OR guanadrel OR guanethidine OR guanfacine OR hydralazine OR irbesartan OR isradipine OR labetalol OR lisinopril OR “losartan potassium” OR macitentan OR mecamylamine OR methyldopa OR metoprolol OR metyrosine OR mibefradil OR minoxidil OR moexipril OR moxonidine OR nadolol OR nebivolol OR nicardipine OR nifedipine OR nisoldipine OR nitroprusside OR “olmesartan medoxomil” OR omapatrilat OR penbutolol OR perindopril OR phentolamine OR pindolol OR prazosin OR propranolol OR quinapril OR ramipril OR rescinnamine OR reserpine OR sildenafil OR “sodium nitroprusside” OR tadalafil OR telmisartan OR terazosin OR timolol OR trandolapril OR treprostinil OR trimethaphan OR valsartan OR verapamil OR diuretic OR diuretics OR thiazide OR “adrenergic beta-antagonist” OR “adrenergic beta-antagonists” OR “adrenergic alpha-antagonist” OR “adrenergic alpha-antagonists” OR “Angiotensin-Converting Enzyme Inhibitors”[Mesh] OR “ace inhibitor” OR “ace inhibitors” OR “angiotensin-converting enzyme inhibitor” OR “angiotensin-converting enzyme inhibitors” OR “angiotensin II Receptor Blockers” OR “angiotensin II Receptor Blockers” OR “Angiotensin II Type 2 Receptor Blockers”[Mesh] OR “calcium channel blocker” OR “calcium channel blockers” OR “ganglionic blocker” OR “ganglionic blockers” OR “vasodilator agent” OR “vasodilator agents” OR “Vasodilator Agents”[Mesh] OR nitrates OR “Nitrates”[Mesh] OR nitrites OR “Nitrites”[Mesh]) AND (gwa[ti] OR gwas[ti] OR genome[ti] OR “gene”[ti] OR “genes”[ti] OR “genotype”[ti] OR “genotypes”[ti] OR “genotyping”[ti] OR “snp”[ti] OR “snps”[ti] OR polymorphism*[ti] OR “DNA”[ti] OR allele[ti] OR alleles[ti] OR “minor allele” OR “minor alleles” OR “single nucleotide polymorphism” OR “single nucleotides polymorphisms” OR genetic*[ti] OR “trait locus”[ti] OR “loci”[ti] OR “Genetic Predisposition to Disease”[MeSH] OR “Genotype”[MeSH] OR “Gene Frequency”[MeSH] OR “Polymorphism, Single Nucleotide/genetics”[MESH] OR “Polymorphism, Single Nucleotide”[MAJR] OR “Genetic Loci”[Mesh] OR “Genetic Association Studies”[Mesh] OR “Genetic Variation”[Mesh] OR “Blood Pressure/genetics”[Mesh]) NOT (“review”[pt] OR “Cross-Sectional Studies”[MeSH Terms] OR Comment[pt] OR Editorial[pt] OR Letter[pt] OR “Case Reports”[pt] OR “case control”[ti] OR “case report”[ti] OR “case study”[ti] OR “case series”[ti] OR “Case-Control Studies”[Mesh] OR “Follow-Up Studies”[Mesh] OR “observational study”[ti] OR “prospective cohort”[ti] OR “cohort studies” [Mesh:NoExp] OR “cohort study”[ti] OR “Longitudinal Studies” [Mesh:NoExp] OR “Follow-Up Studies”[mesh] OR “Retrospective Studies”[mesh] OR “non-randomized”[ti] OR “follow up study”[ti] OR “Cross-Sectional Studies”[MeSH Terms] OR “Prospective Studies”[MeSH Terms] OR “epidemiology”[Subheading] OR “pulmonary hypertension” OR “pulmonary arterial hypertension” OR “heart transplant” OR “heart failure” OR “cystic fibrosis” OR “cancer” OR “neoplasms” OR “fibromyalgia” OR “alzheimers” OR “alzheimer” OR “pregnant” OR “pregnancy” OR “obesity/drug therapy”[mesh] OR “diet therapy”[mesh] OR “diet therapy”[subheading] OR “DASH”[tiab] OR meal[ti] OR “nutritional intervention” OR “dietary intervention” OR “nutritional counseling” OR “dietary counseling” OR “caffeine” OR “eating change” OR “activities of daily living” OR “dehydration” OR “dehydrate” OR “dehydrated” OR “dietary salt” OR sodium OR “epilepsy” OR “influenza” OR “flu” OR “pneumonia” OR “septicemia” OR arthritis OR “hiv” OR “Acquired Immunodeficiency Syndrome” OR “meningitis” OR “substance abuse” OR “alcoholism” OR “drug abuse” OR “spinal cord”[ti] OR “Sleep”[Majr] OR “Sleep Apnea Syndromes”[Majr] OR sleep[ti] OR contraceptive*[ti] OR (animals[mesh] NOT humans[mesh]) OR rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR pig[ti] OR pigs[ti] OR dog[ti] OR dogs[ti] OR canine[ti] OR cow[ti] OR cows[ti] OR bovine[ti]). |
Table 4.
Criteria 4a: Genes (n = 27) in major blood pressure regulatory pathways associated with physical activity and/or obesity in genome wide association studies. The search was conducted on 05/11/2017 and a total of 188 records were identified.
| (“mean arterial” OR “blood pressure”[mesh] OR “blood pressure” OR “blood pressures” OR “arterial pressure” OR “arterial pressures” OR hypertension OR hypotension OR normotension OR hypertensive OR hypotensive OR normotensive OR “systolic pressure” OR “diastolic pressure” OR “pulse pressure” OR “venous pressure” OR “pressure monitor” OR hypotension OR “pre hypertension” OR “bp response” OR “bp decrease” OR “bp reduction” OR “bp monitor” OR “bp monitors” OR “bp measurement” OR “Blood Pressure/genetics”[MeSH]) AND (“resting blood pressure” OR “resting BP” OR “exercise”[mesh] OR exercise[ti] OR exercises OR running[mesh] OR running[ti] OR “bicycle” OR “bicycles” OR “bicycling” OR walking OR walking[mesh] OR walking[ti] OR treadmill* OR “weight lifting” OR “weight training” OR “weight bearing” OR “resistance training” OR “strength training” OR “endurance training” OR “speed training” OR “training duration” OR “training frequency” OR “training intensity” OR “aerobic endurance” OR “aerobic training” OR “physical activity” OR “motor activity”[mesh] OR Overweight OR “Overweight”[Mesh] OR BMI OR “body mass” OR “Body Mass Index”[MeSH] OR “Waist Circumference”[MeSH] OR obesity OR “Obesity”[Mesh] OR obese) AND (gwa OR gwas OR “Genetic Association Studies”[Mesh] OR genomewide OR “genome wide” OR “genomewide association” OR “genome wide association” OR “genome-wide interaction”) NOT (“review”[pt] OR “Cross-Sectional Studies”[MeSH Terms] OR Comment[pt] OR Editorial[pt] OR Letter[pt] OR “Case Reports”[pt] OR “case control”[ti] OR “case report”[ti] OR “case study”[ti] OR “case series”[ti] OR “Case-Control Studies”[Mesh] OR “Follow-Up Studies”[Mesh] OR “observational study”[ti] OR “prospective cohort”[ti] OR “cohort studies” [Mesh:NoExp] OR “cohort study”[ti] OR “Longitudinal Studies” [Mesh:NoExp] OR “Follow-Up Studies”[mesh] OR “Retrospective Studies”[mesh] OR “non-randomized”[ti] OR “follow up study”[ti] OR “Cross-Sectional Studies”[MeSH Terms] OR “Prospective Studies”[MeSH Terms] OR “epidemiology”[Subheading] OR “pulmonary hypertension” OR “pulmonary arterial hypertension” OR “heart transplant” OR “heart failure” OR “cystic fibrosis” OR “cancer” OR “neoplasms” OR “fibromyalgia” OR “alzheimers” OR “alzheimer” OR “pregnant” OR “pregnancy” OR “obesity/drug therapy”[mesh] OR “diet therapy”[mesh] OR “diet therapy”[subheading] OR “DASH”[tiab] OR meal[ti] OR “nutritional intervention” OR “dietary intervention” OR “nutritional counseling” OR “dietary counseling” OR “caffeine” OR “eating change” OR “activities of daily living” OR “dehydration” OR “dehydrate” OR “dehydrated” OR “dietary salt” OR sodium OR “epilepsy” OR “influenza” OR “flu” OR “pneumonia” OR “septicemia” OR arthritis OR “hiv” OR “Acquired Immunodeficiency Syndrome” OR “meningitis” OR “substance abuse” OR “alcoholism” OR “drug abuse” OR “spinal cord”[ti] OR “Sleep”[Majr] OR “Sleep Apnea Syndromes”[Majr] OR sleep[ti] OR contraceptive*[ti] OR (animals[mesh] NOT humans[mesh]) OR rat[ti] OR rats[ti] OR mouse[ti] OR mice[ti] OR pig[ti] OR pigs[ti] OR dog[ti] OR dogs[ti] OR canine[ti] OR cow[ti] OR cows[ti] OR bovine[ti]). |
Potential reports were screened by PP for title and abstract, and by PP and LSP for full text review to determine if they qualified. In addition, the authors performed manual searches of reference lists from relevant reports in their files for possible inclusion. Any questions regarding qualification were resolved by discussion by PP and LSP.
3. Results
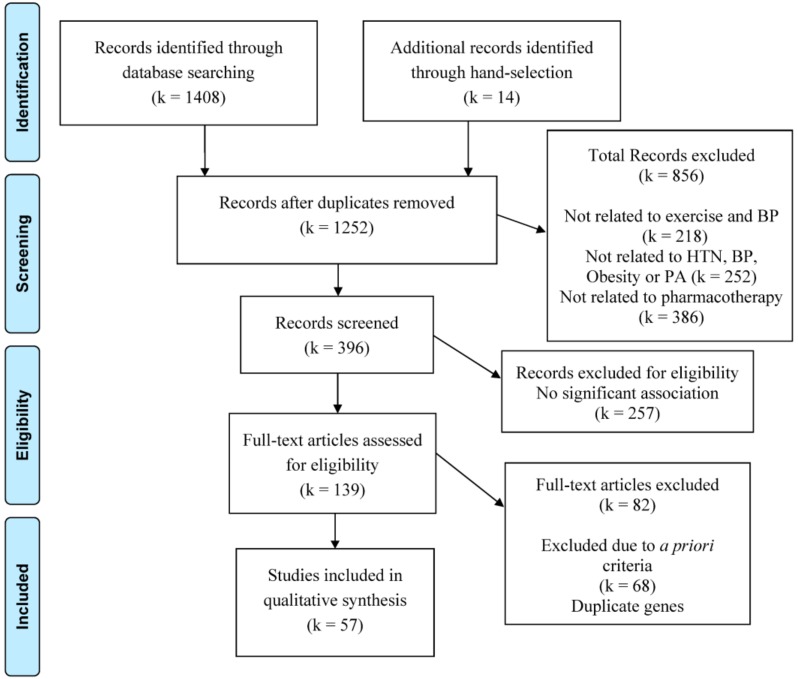
The search yielded 1408 reports plus 14 records obtained from manual searches of the authors’ files. Of these, 57 trials qualified. Please see Figure 1 for the flowchart detailing the systematic search of potential reports and selection process for the reports that qualified. Of the qualifying trials containing human genes in major BP regulatory pathways, we extracted 11 genes associated with the BP response to exercise under criteria 1 [33,34,35,36,37,38,39,40]; 18 genes associated with hypertension in GWAS under criteria 2 [41,42,43,44,45,46,47,48]; 28 genes associated with the BP response to pharmacotherapy under criteria 3 [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]; 27 genes associated with physical activity and/or obesity in GWAS under criteria 4a [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]; and 29 genes associated with BP, physical activity, and/or obesity in non-GWAS under criteria 4b [86,87,88] displayed in Supplemental Digital Content (SDC) 1 Table S1. In addition, we included the 41 genes from our preliminary work that were reported to be associated with hypertension or the BP response to exercise or pharmacotherapy [27,28,29].
Figure 1.
The systematic search trial selection process. BP: blood pressure; HTN: hypertension; PA: physical activity.
See SDC 1 Table S1 for the complete signature of 154 prioritized BP and exercise genes by our predetermined inclusion criteria.
4. Discussion
The AHA/ACC rated exercise as one of the best lifestyle therapeutic approaches to prevent and treat hypertension because aerobic exercise training lowers BP 5-8 mmHg among adults with hypertension [3]. Nearly 20 years ago, the HERITAGE study investigators established that the BP response to exercise was heritable. This finding set off investigator-initiated studies examining the genetic basis for the antihypertensive effects of exercise using a candidate gene approach, as this was the technology available at that time. Despite the best intentions and efforts of these research teams, these candidate gene studies were met with little success [17,25,26]. The failure of the candidate gene approach is best illustrated by a systematic review we recently completed on the influence of the angiotensin converting enzyme (ACE) insertion/deletion polymorphism rs4340 on human endurance exercise performance and cardiovascular health [89]. ACE rs4340 is the most widely investigated genetic variant in the exercise genomic literature. Despite the extensive volume of literature on ACE rs4340, we concluded that due to disparate findings no definitive conclusions could be made regarding the role of ACE rs4340 on endurance exercise performance or the BP response to exercise.
More recently, using newer genomic technology that emerged since our discovery phase candidate gene association studies, in a replication cohort we deep-sequenced the exons of genes contained on a prioritized signature of 41 genes identified from our earlier work combined with those from a systematic review of the literature of genes reported to be associated with hypertension or the BP response to exercise or pharmacotherapy using the Illumina TruSeq Custom Amplicon kit [27,28,29]. Even after adjustment for multiple testing, we found that 61% of the genes in the prioritized panel associated with the BP response to exercise. Clinical features such as resting BP, age, gender, and cardiometabolic biomarkers explained 66%–92% of the variation in the BP response to exercise. Yet, the genetic variants that emerged from these analyses explained 2%–15% of the variance in the BP response to exercise, a magnitude that is larger than typically reported in exercise genomic studies [25,90].
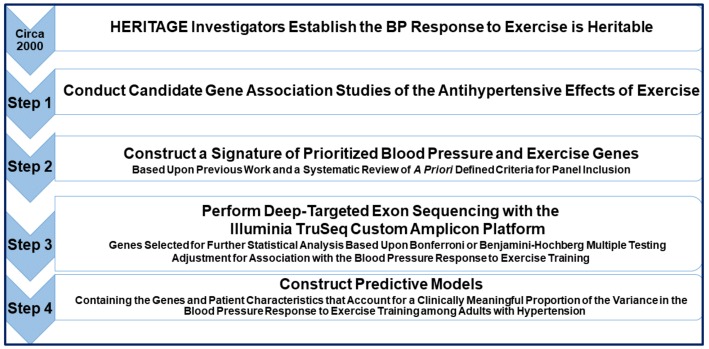
We attributed the high proportion of significant BP-genotype associations that we found to the following methodological strategies we instituted [27,28,29]: a randomized control repeated measure design with subjects who were their own control; a focused inquiry of variants with a prioritized panel of genes obtained from a systematic review of the literature that reduced the search space within the genome; high throughput exon sequencing on functional gene regions; standardized protocols that included a closely monitored, well-controlled exercise exposure; and adjustment for multiple testing based on genetic variants exhibiting variability in the number of minor alleles and with unique genotypic values. This series of studies are proof of concept that a focused genomic inquiry on functional portions of genes based upon a systematic review of the literature is a time and cost-efficient way to investigate the genomic basis for the antihypertensive effects of exercise. See Figure 2 for a conceptual overview of this systematic review approach to investigate the genomics of the antihypertensive effects of exercise. However, future investigator-initiated studies remain to be done expanding upon this approach among a large ethnically and gender diverse sample of adults with hypertension to continue to gain information about the genomic basis for the antihypertensive effects of exercise with the long-term goal of fine-tuning exercise prescriptions to optimize the BP benefits of exercise.
Figure 2.
A Systematic review approach to assemble a targeted gene panel to study the genomics of the antihypertensive effects of exercise. HERITAGE: HEalth, RIsk Factors Exercise TrAining and Genetics; BP: blood pressure.
We acknowledge a limitation of our approach is that there are more sophisticated system analyses and bioinformatic approaches now being used with GWAS, human disease, and pathway data sets such as ENCODE and NHGRI GWAS that integrate omic high throughput technology of the genome, transcriptome, proteome, and epigenome to elucidate the genomic basis for hypertension (See SDC 1 Table 1 criteria 4a). In fact, we used such data sets in our most recent work to derive regulatory elements for the polymorphisms that passed multiple testing threshold for significant associations with the BP to exercise [27,28,29]. Another limitation is that genes have transcription-factor and post-transcriptional regulators so that RNA expression levels could also be used to find signatures associated with the antihypertensive effects of exercise as opposed to BP and exercise genes per se. Genes have epistatic and pleiotropic effects further complicating the identification of genetic variants that explain a clinically meaningful proportion of the BP response to exercise. Presently no data set repositories exist for genes that have been reported to associated with the BP response to exercise that have passed preestablished thresholds for multiple testing. Considering the importance of hypertension as a public health problem and the critical role exercise has in the treatment of hypertension, we posit that the methods we have presented in this systematic review represent a time and cost-efficient approach to construct targeted gene signatures whose exons can be deep-sequenced to gain insight into the genomic basis for the antihypertensive effects of exercise. Furthermore, these methods could easily be adapted to design prioritized signatures of the transcriptome, proteome, and epigenome as they regulate the BP response to exercise.
Acknowledgments
The Jackson Laboratory’s Scientific Services (Genome Technologies) for performing the deep-targeting exon sequencing and preparation of the variant calling files. L.S.P. contributed to study design conceptualization, data collection, statistical analyses, results interpretation, manuscript writing and reviewing, and acquisition of funding; Elizabeth D. Schifano contributed to statistical analyses, results interpretation, and manuscript writing and reviewing; Garrett I. Ash contributed to study design conceptualization, data collection, statistical analyses, results interpretation, manuscript reviewing, and acquisition of funding; Gregory A. Panza contributed to data collection, statistical analyses, results interpretation, and manuscript writing and reviewing; Lauren ML Corso contributed to data collection and manuscript writing and reviewing; Ming-Hui Chen contributed to statistical analyses, results interpretation, and manuscript reviewing; Ved Deshpande contributed to statistical analyses, results interpretation, and manuscript reviewing; Amanda Zaleski contributed to data collection, manuscript writing and reviewing, and acquisition of funding; Burak Cilhoroz contributed to statistical analyses, results interpretation, and manuscript writing and reviewing; Paulo Farinatti contributed to data collection, manuscript writing and reviewing, and acquisition of funding; B.A.T. contributed to data collection, manuscript writing and reviewing, and acquisition of funding; Rachel J. O’Neill contributed to results interpretation and manuscript writing and reviewing; and Paul D. Thompson contributed to data collection and manuscript writing and reviewing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/10/4/295/s1, Supplemental Digital Content 1. Table S1. The Prioritized Panel of 154 Blood Pressure Genes from a Systematic Review of the Literature by the Four A Priori Criteria Combined with Our Preliminary Work.
Funding
The University of Connecticut (UConn) Research Foundation; UConn Institute for Health, Intervention, and Policy; Connecticut Institute for Clinical and Translational Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Fisher N.D.L., Curfman G. Hypertension-A Public Health Challenge of Global Proportions. JAMA. 2018;320:1757–1759. doi: 10.1001/jama.2018.16760. [DOI] [PubMed] [Google Scholar]
- 3.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Jr., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Naci H., Salcher-Konrad M., Dias S., Blum M.R., Anova S., Nunan D., Ioannidis J. How Does Exercise Treatment Compare with Antihypertensive Medications? A Network Meta-Analysis of 391 RCTs Assessing Exercise and Medication Effects on Systolic Blood Pressure. Br. J. Sports Med. 2018 doi: 10.1136/bjsports-2018-099921. [DOI] [PubMed] [Google Scholar]
- 5.Pescatello L.S. Exercise Measures Up to Medication as Antihypertensive Therapy: Its Value has Long been Underestimated. Br. J. Sports Med. 2018 doi: 10.1136/bjsports-2018-100359. [DOI] [PubMed] [Google Scholar]
- 6.Chobanian A.V., Bakris G.L., Black H.R., Cushman W.C., Green L.A., Izzo J.L., Jr., Jones D.W., Materson B.J., Oparil S., Wright J.T., Jr., et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Law M.R., Morris J.K., Wald N.J. Use of Blood Pressure Lowering Drugs in the Prevention of Cardiovascular Disease: Meta-Analysis of 147 Randomised Trials in the Context of Expectations from Prospective Epidemiological Studies. BMJ. 2009;338:b1665. doi: 10.1136/bmj.b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelton P.K., He J., Appel L.J., Cutler J.A., Havas S., Kotchen T.A., Roccella E.J., Stout R., Vallbona C., Winston M.C., et al. Primary Prevention of Hypertension: Clinical and Public Health Advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 9.Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Department of Health and Human Services; Washington, DC, USA: 2018. [Google Scholar]
- 10.Powell K.E., King A.C., Buchner D.M., Campbell W.W., DiPietro L., Erickson K.I., Hillman C.H., Jakicic J.M., Janz K.F., Katzmarzyk P.T., et al. The Scientific Foundation for the Physical Activity Guidelines for Americans, 2nd Edition. J. Phys. Act. Health. 2018;16:1–11. doi: 10.1123/jpah.2018-0618. [DOI] [PubMed] [Google Scholar]
- 11.Pescatello L.S., MacDonald H.V., Ash G.I., Lamberti L.M., Farquhar W.B., Arena R., Johnson B.T. Assessing the Existing Professional Exercise Recommendations for Hypertension: A Review and Recommendations for Future Research Priorities. Mayo Clin. Proc. 2015;90:801–812. doi: 10.1016/j.mayocp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Andrade F.C.D. Diagnostic Status and Age at Diagnosis of Hypertension on Adherence to Lifestyle Recommendations. Prev. Med. Rep. 2018;13:52–56. doi: 10.1016/j.pmedr.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu L., Cohen A.J., Mukamal K.J. Prevalence and Predictors of Resistance and Aerobic Exercise among Hypertensive Adults in the United States. J. Hum. Hypertens. 2015;29:394–395. doi: 10.1038/jhh.2014.104. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard C., Rankinen T. Individual Differences in Response to Regular Physical Activity. Med. Sci. Sports Exerc. 2001;33:S446–S451. doi: 10.1097/00005768-200106001-00013. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard C., Blair S.N., Church T.S., Earnest C.P., Hagberg J.M., Hakkinen K., Jenkins N.T., Karavirta L., Kraus W.E., Leon A.S., et al. Adverse Metabolic Response to Regular Exercise: Is it a Rare Or Common Occurrence? PLoS ONE. 2012;7:e37887. doi: 10.1371/journal.pone.0037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankinen T., Gagnon J., Perusse L., Chagnon Y.C., Rice T., Leon A.S., Skinner J.S., Wilmore J.H., Rao D.C., Bouchard C. AGT M235T and ACE ID Polymorphisms and Exercise Blood Pressure in the HERITAGE Family Study. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H368–H374. doi: 10.1152/ajpheart.2000.279.1.H368. [DOI] [PubMed] [Google Scholar]
- 17.Rankinen T., Roth S.M., Bray M.S., Loos R., Perusse L., Wolfarth B., Hagberg J.M., Bouchard C. Advances in Exercise, Fitness, and Performance Genomics. Med. Sci. Sports Exerc. 2010;42:835–846. doi: 10.1249/MSS.0b013e3181d86cec. [DOI] [PubMed] [Google Scholar]
- 18.Rankinen T., Rice T., Perusse L., Chagnon Y.C., Gagnon J., Leon A.S., Skinner J.S., Wilmore J.H., Rao D.C., Bouchard C. NOS3 Glu298Asp Genotype and Blood Pressure Response to Endurance Training: The HERITAGE Family Study. Hypertension. 2000;36:885–889. doi: 10.1161/01.HYP.36.5.885. [DOI] [PubMed] [Google Scholar]
- 19.Rankinen T., Church T., Rice T., Markward N., Leon A.S., Rao D.C., Skinner J.S., Blair S.N., Bouchard C. Effect of Endothelin 1 Genotype on Blood Pressure is Dependent on Physical Activity Or Fitness Levels. Hypertension. 2007;50:1120–1125. doi: 10.1161/HYPERTENSIONAHA.107.093609. [DOI] [PubMed] [Google Scholar]
- 20.Augeri A.L., Tsongalis G.J., van Heest J.L., Maresh C.M., Thompson P.D., Pescatello L.S. The Endothelial Nitric Oxide Synthase -786 T>C Polymorphism and the Exercise-Induced Blood Pressure and Nitric Oxide Responses among Men with Elevated Blood Pressure. Atherosclerosis. 2009;204:e28–e34. doi: 10.1016/j.atherosclerosis.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Ash G.I., Eicher J.D., Pescatello L.S. The Promises and Challenges of the use of Genomics in the Prescription of Exercise for Hypertension: The 2013 Update. Curr. Hypertens. Rev. 2013;9:130–147. doi: 10.2174/15734021113099990010. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard B.E., Tsongalis G.J., Guidry M.A., LaBelle L.A., Poulin M., Taylor A.L., Maresh C.M., Devaney J., Thompson P.D., Pescatello L.S. RAAS Polymorphisms Alter the Acute Blood Pressure Response to Aerobic Exercise among Men with Hypertension. Eur. J. Appl. Physiol. 2006;97:26–33. doi: 10.1007/s00421-006-0142-8. [DOI] [PubMed] [Google Scholar]
- 23.Pescatello L.S., Blanchard B.E., Tsongalis G.J., Maresh C.M., O’Connell A., Thompson P.D. The α-Adducin Gly460Trp Polymorphism and the Antihypertensive Effects of Exercise among Men with High Blood Pressure. Clin. Sci. (Lond.) 2007;113:251–258. doi: 10.1042/CS20060345. [DOI] [PubMed] [Google Scholar]
- 24.Pescatello L.S., Blanchard B.E., van Heest J.L., Maresh C.M., Gordish-Dressman H., Thompson P.D. The Metabolic Syndrome and the Immediate Antihypertensive Effects of Aerobic Exercise: A Randomized Control Design. BMC Cardiovasc. Disord. 2008;8:12. doi: 10.1186/1471-2261-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruneau M.L., Jr., Johnson B.T., Huedo-Medina T.B., Larson K.A., Ash G.I., Pescatello L.S. The Blood Pressure Response to Acute and Chronic Aerobic Exercise: A Meta-Analysis of Candidate Gene Association Studies. J. Sci. Med. Sport. 2016;19:424–431. doi: 10.1016/j.jsams.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Bouchard C. Overcoming Barriers to Progress in Exercise Genomics. Exerc. Sport Sci. Rev. 2011;39:212–217. doi: 10.1097/JES.0b013e31822643f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cilhoroz B.T., Schifano E.D., Panza G.A., Ash G.I., Corso L., Chen M.H., Deshpande V., Zaleski A., Farinatti P., Santos L.P., et al. FURIN Variant Associations with Postexercise Hypotension are Intensity and Race Dependent. Physiol. Rep. 2019;7:e13952. doi: 10.14814/phy2.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pescatello L.S., Shifano E.D., Ash G.I., Panza G.A., Lamberti L., Chen M., Deshpande V., Zaleski A., Farinatti P., Taylor B.A., et al. Deep-Targeted Exon Sequencing Reveals Renal Polymorphisms Associate with Postexercise Hypotension among African Americans. Physiol. Rep. 2016;4:e12992. doi: 10.14814/phy2.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pescatello L.S., Schifano E.D., Ash G.I., Panza G.A., Corso L.M.L., Chen M.H., Deshpande V., Zaleski A., Cilhoroz B., Farinatti P., et al. Deep-Targeted Sequencing of Endothelial Nitric Oxide Synthase Gene Exons Uncovers Exercise Intensity and Ethnicity-Dependent Associations with Post-Exercise Hypotension. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D., Liberati A., Tetzlaff J., Altman D.G. PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavie C.J., de Schutter A., Parto P., Jahangir E., Kokkinos P., Ortega F.B., Arena R., Milani R.V. Obesity and Prevalence of Cardiovascular Diseases and Prognosis-the Obesity Paradox Updated. Prog. Cardiovasc. Dis. 2016;58:537–547. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Deschamps C.L., Connors K.E., Klein M.S., Johnsen V.L., Shearer J., Vogel H.J., Devaney J.M., Gordish-Dressman H., Many G.M., Barfield W. The ACTN3 R577X Polymorphism is Associated with Cardiometabolic Fitness in Healthy Young Adults. PLoS ONE. 2015;10:e0130644. doi: 10.1371/journal.pone.0130644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franks P.W., Bhattacharyya S., Luan J., Montague C., Brennand J., Challis B., Brage S., Ekelund U., Middelberg R.P., O’Rahilly S., et al. Association between Physical Activity and Blood Pressure is Modified by Variants in the G-Protein Coupled Receptor 10. Hypertension. 2004;43:224–228. doi: 10.1161/01.HYP.0000109319.63240.08. [DOI] [PubMed] [Google Scholar]
- 35.Heradien M., Revera M., van der Merwe L., Goosen A., Corfield V.A., Brink P.A., Mayosi B.M., Moolman-Smook J.C. Abnormal Blood Pressure Response to Exercise Occurs More Frequently in Hypertrophic Cardiomyopathy Patients with the R92W Troponin T Mutation than in those with Myosin Mutations. Heart Rhythm. 2009;6:S18–S24. doi: 10.1016/j.hrthm.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayewardene A.F., Mavros Y., Gwinn T., Hancock D.P., Rooney K.B. Associations between CD36 Gene Polymorphisms and Metabolic Response to a Short-Term Endurance-Training Program in a Young-Adult Population. Appl. Physiol. Nutr. Metab. 2015;41:157–167. doi: 10.1139/apnm-2015-0430. [DOI] [PubMed] [Google Scholar]
- 37.Masuki S., Mori M., Tabara Y., Miki T., Sakurai A., Morikawa M., Miyagawa K., Higuchi K., Nose H., Shinshu University Genetic Research Consortium Vasopressin V1a Receptor Polymorphism and Interval Walking Training Effects in Middle-Aged and Older People. Hypertension. 2010;55:747–754. doi: 10.1161/HYPERTENSIONAHA.109.147728. [DOI] [PubMed] [Google Scholar]
- 38.Nunes R.A.B., Barroso L.P., da Costa Pereira A., Krieger J.E., Mansur A.J. Gender-Related Associations of Genetic Polymorphisms of α-Adrenergic Receptors, Endothelial Nitric Oxide Synthase and Bradykinin B2 Receptor with Treadmill Exercise Test Responses. Open Heart. 2014;1:e000132. doi: 10.1136/openhrt-2014-000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera M.A., Echegaray M., Rankinen T., Pérusse L., Rice T., Gagnon J., Leon A.S., Skinner J.S., Wilmore J.H., Rao D. Angiogenin Gene-Race Interaction for Resting and Exercise BP Phenotypes: The HERITAGE Family Study. J. Appl. Physiol. 2001;90:1232–1238. doi: 10.1152/jappl.2001.90.4.1232. [DOI] [PubMed] [Google Scholar]
- 40.Shiwaku K., Nogi A., Anuurad E., Kitajima K., Enkhmaa B., Shimono K., Yamane Y. Difficulty in Losing Weight by Behavioral Intervention for Women with Trp64Arg Polymorphism of the β 3-Adrenergic Receptor Gene. Int. J. Obes. 2003;27:1028. doi: 10.1038/sj.ijo.0802375. [DOI] [PubMed] [Google Scholar]
- 41.Fujimaki T., Oguri M., Horibe H., Kato K., Matsuoka R., Abe S., Tokoro F., Arai M., Noda T., Watanabe S. Association of a Transcription Factor 21 Gene Polymorphism with Hypertension. Biomed. Rep. 2015;3:118–122. doi: 10.3892/br.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huan T., Esko T., Peters M.J., Pilling L.C., Schramm K., Schurmann C., Chen B.H., Liu C., Joehanes R., Johnson A.D. A Meta-Analysis of Gene Expression Signatures of Blood Pressure and Hypertension. PLoS Genet. 2015;11:e1005035. doi: 10.1371/journal.pgen.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu X., Wang L., Lin X., Huang J., Charles Gu C., He M., Shen H., He J., Zhu J., Li H. Genome-Wide Association Study in Chinese Identifies Novel Loci for Blood Pressure and Hypertension. Hum. Mol. Genet. 2014;24:865–874. doi: 10.1093/hmg/ddu478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murakata Y., Fujimaki T., Yamada Y. Association of a Butyrophilin, Subfamily 2, Member A1 Gene Polymorphism with Hypertension. Biomed. Rep. 2014;2:818–822. doi: 10.3892/br.2014.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ong K.L., Li M., Tso A.W., Xu A., Cherny S.S., Sham P.C., Tse H.F., Lam T.H., Cheung B.M., Lam K.S., et al. Association of Genetic Variants in the Adiponectin Gene with Adiponectin Level and Hypertension in Hong Kong Chinese. Eur. J. Endocrinol. 2010;163:251–257. doi: 10.1530/EJE-10-0251. [DOI] [PubMed] [Google Scholar]
- 46.Rong S., Zhou X., Wang Z., Wang X., Wang Y., Xue C., Li B. Glutathione S-Transferase M1 and T1 Polymorphisms and Hypertension Risk: An Updated Meta-Analysis. J. Hum. Hypertens. 2018:1. doi: 10.1038/s41371-018-0133-3. [DOI] [PubMed] [Google Scholar]
- 47.Zhang K., Rao F., Miramontes-Gonzalez J.P., Hightower C.M., Vaught B., Chen Y., Greenwood T.A., Schork A.J., Wang L., Mahata M. Neuropeptide Y (NPY): Genetic Variation in the Human Promoter Alters Glucocorticoid Signaling, Yielding Increased NPY Secretion and Stress Responses. J. Am. Coll. Cardiol. 2012;60:1678–1689. doi: 10.1016/j.jacc.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang W., Wang H., Guan X., Niu Q., Li W. Variant rs2237892 of KCNQ1 is Potentially Associated with Hypertension and Macrovascular Complications in Type 2 Diabetes Mellitus in a Chinese Han Population. Genom. Proteom. Bioinform. 2015;13:364–370. doi: 10.1016/j.gpb.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y., Liu D., Zhang P., Zhong J., Zhang C., Wu S., Zhang Y., Liu G., He M., Jin L. Impact of ACE2 Gene Polymorphism on Antihypertensive Efficacy of ACE Inhibitors. J. Hum. Hypertens. 2016;30:766. doi: 10.1038/jhh.2016.24. [DOI] [PubMed] [Google Scholar]
- 50.Bruck H., Schwerdtfeger T., Toliat M., Leineweber K., Heusch G., Philipp T., Nürnberg P., Brodde O. Presynaptic α-2C Adrenoceptor-mediated Control of Noradrenaline Release in Humans: Genotype-or Age-Dependent? Clin. Pharmacol. Ther. 2007;82:525–530. doi: 10.1038/sj.clpt.6100181. [DOI] [PubMed] [Google Scholar]
- 51.Chen G., Jiang S., Mao G., Zhang S., Hong X., Tang G., Li Z., Liu X., Zhang Y., Xing H. CYP2C9 Ile359Leu Polymorphism, Plasma Irbesartan Concentration and Acute Blood Pressure Reductions in Response to Irbesartan Treatment in Chinese Hypertensive Patients. Methods Find. Exp. Clin. Pharmacol. 2006;28:19–24. doi: 10.1358/mf.2006.28.1.962773. [DOI] [PubMed] [Google Scholar]
- 52.Chen W., Shu Y., Li Q., Xu L., Roederer M.W., Fan L., Wu L., He F., Luo J., Tan Z. Polymorphism of ORM1 is Associated with the Pharmacokinetics of Telmisartan. PLoS ONE. 2013;8:e70341. doi: 10.1371/journal.pone.0070341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anthony E.G., Richard E., Lipkowitz M.S., Kelley S.T., Alcaraz J.E., Shaffer R.A., Bhatnagar V. Association of Phosphodiesterase 4 Polymorphism (rs702553) with Blood Pressure in the African American Study of Kidney Disease and Hypertension Genomics Study. Pharm. Genom. 2013;23:442–444. doi: 10.1097/FPC.0b013e3283636840. [DOI] [PubMed] [Google Scholar]
- 54.Donner K.M., Hiltunen T.P., Hannila-Handelberg T., Suonsyrjä T., Kontula K. STK39 Variation Predicts the Ambulatory Blood Pressure Response to Losartan in Hypertensive Men. Hypertens. Res. 2012;35:107. doi: 10.1038/hr.2011.166. [DOI] [PubMed] [Google Scholar]
- 55.Duarte J.D., Turner S.T., Tran B., Chapman A.B., Bailey K.R., Gong Y., Gums J.G., Langaee T.Y., Beitelshees A.L., Cooper-Dehoff R.M. Association of Chromosome 12 Locus with Antihypertensive Response to Hydrochlorothiazide may Involve Differential YEATS4 Expression. Pharm. J. 2013;13:257. doi: 10.1038/tpj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frau F., Zaninello R., Salvi E., Ortu M.F., Braga D., Velayutham D., Argiolas G., Fresu G., Troffa C., Bulla E. Genome-Wide Association Study Identifies CAMKID Variants Involved in Blood Pressure Response to Losartan: The SOPHIA Study. Pharmacogenomics. 2014;15:1643–1652. doi: 10.2217/pgs.14.119. [DOI] [PubMed] [Google Scholar]
- 57.Duan R., Cui W., Wang H. Association of the Antihypertensive Response of Iptakalim with KCNJ11 (Kir6. 2 Gene) Polymorphisms in Chinese Han Hypertensive Patients. Acta Pharmacol. Sin. 2011;32:1078. doi: 10.1038/aps.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhatnagar V., O’connor D.T., Brophy V.H., Schork N.J., Richard E., Salem R.M., Nievergelt C.M., Bakris G.L., Middleton J.P., Norris K.C. G-Protein-Coupled Receptor Kinase 4 Polymorphisms and Blood Pressure Response to Metoprolol among African Americans: Sex-Specificity and Interactions. Am. J. Hypertens. 2009;22:332–338. doi: 10.1038/ajh.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamide K., Asayama K., Katsuya T., Ohkubo T., Hirose T., Inoue R., Metoki H., Kikuya M., Obara T., Hanada H. Genome-Wide Response to Antihypertensive Medication using Home Blood Pressure Measurements: A Pilot Study Nested within the HOMED-BP Study. Pharmacogenomics. 2013;14:1709–1721. doi: 10.2217/pgs.13.161. [DOI] [PubMed] [Google Scholar]
- 60.Kamide K., Yang J., Matayoshi T., Takiuchi S., Horio T., Yoshii M., Miwa Y., Yasuda H., Yoshihara F., Nakamura S. Genetic Polymorphisms of L-Type Calcium Channel α1C and α1D Subunit Genes are Associated with Sensitivity to the Antihypertensive Effects of L-Type Dihydropyridine Calcium-Channel Blockers. Circ. J. 2009;73:732–740. doi: 10.1253/circj.CJ-08-0761. [DOI] [PubMed] [Google Scholar]
- 61.Konoshita T., Kato N., Fuchs S., Mizuno S., Aoyama C., Motomura M., Makino Y., Wakahara S., Inoki I., Miyamori I., et al. Genetic Variant of the Renin-Angiotensin System and Diabetes Influences Blood Pressure Response to Angiotensin Receptor Blockers. Diabetes Care. 2009;32:1485–1490. doi: 10.2337/dc09-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He F., Luo J., Luo Z., Fan L., He Y., Zhu D., Gao J., Deng S., Wang Y., Qian Y. The KCNH2 Genetic Polymorphism (1956, C> T) is a Novel Biomarker that is Associated with CCB and α, β-ADR Blocker Response in EH Patients in China. PLoS ONE. 2013;8:e61317. doi: 10.1371/journal.pone.0061317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He F., Luo J., Zhang Z., Luo Z., Fan L., He Y., Wen J., Zhu D., Gao J., Wang Y. The RGS2 (-391, C> G) Genetic Variation Correlates to Antihypertensive Drug Responses in Chinese Patients with Essential Hypertension. PLoS ONE. 2015;10:e0121483. doi: 10.1371/journal.pone.0121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong X., Xing H., Yu Y., Wen Y., Zhang Y., Zhang S., Tang G., Xu X. Genetic Polymorphisms of the Urea Transporter Gene are Associated with Antihypertensive Response to Nifedipine GITS. Methods Find. Exp. Clin. Pharmacol. 2007;29:3–10. doi: 10.1358/mf.2007.29.1.1063490. [DOI] [PubMed] [Google Scholar]
- 65.Guo R., Chen L., Li L., Guo X., Sun J., Xiong X., Cheng Z., Li Y., Chen X. Association of GSTM1 Null Polymorphism with Isosorbide-5-Mononitrate Cardiovascular Response and Involvement of CGRP in Healthy Chinese Male Volunteers. Pharm. Genom. 2011;21:142–151. doi: 10.1097/FPC.0b013e328343ea0a. [DOI] [PubMed] [Google Scholar]
- 66.Geshi E., Kimura T., Yoshimura M., Suzuki H., Koba S., Sakai T., Saito T., Koga A., Muramatsu M., Katagiri T. A Single Nucleotide Polymorphism in the Carboxylesterase Gene is Associated with the Responsiveness to Imidapril Medication and the Promoter Activity. Hypertens. Res. 2005;28:719. doi: 10.1291/hypres.28.719. [DOI] [PubMed] [Google Scholar]
- 67.Jia J., Men C., Tang K., Zhan Y. Apelin Polymorphism Predicts Blood Pressure Response to Losartan in Older Chinese Women with Essential Hypertension. Genet. Mol. Res. 2015;14:6561–6568. doi: 10.4238/2015.June.12.10. [DOI] [PubMed] [Google Scholar]
- 68.Lanzani C., Citterio L., Glorioso N., Manunta P., Tripodi G., Salvi E., Carpini S.D., Ferrandi M., Messaggio E., Staessen J.A., et al. Adducin- and Ouabain-Related Gene Variants Predict the Antihypertensive Activity of Rostafuroxin, Part 2: Clinical Studies. Sci. Transl. Med. 2010;2:59ra87. doi: 10.1126/scitranslmed.3001814. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y., Zhang M., Niu T., Xu X., Zhu G., Huo Y., Chen C., Wang X., Xing H., Peng S. D919G Polymorphism of Methionine Synthase Gene is Associated with Blood Pressure Response to Benazepril in Chinese Hypertensive Patients. J. Hum. Genet. 2004;49:296. doi: 10.1007/s10038-004-0149-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y., Hong X., Xing H., Li J., Yong H., Xu X. E112D Polymorphism in the Prolylcarboxypeptidase Gene is Associated with Blood Pressure Response to Benazepril in Chinese Hypertensive Patients. Chin. Med. J. 2009;122:2461–2465. [PubMed] [Google Scholar]
- 71.Andreassen O.A., Djurovic S., Thompson W.K., Schork A.J., Kendler K.S., O’Donovan M.C., Rujescu D., Werge T., van de Bunt M., Morris A.P. Improved Detection of Common Variants Associated with Schizophrenia by Leveraging Pleiotropy with Cardiovascular-Disease Risk Factors. Am. J. Hum. Genet. 2013;92:197–209. doi: 10.1016/j.ajhg.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flaquer A., Baumbach C., Kriebel J., Meitinger T., Peters A., Waldenberger M., Grallert H., Strauch K. Mitochondrial Genetic Variants Identified to be Associated with BMI in Adults. PLoS ONE. 2014;9:e105116. doi: 10.1371/journal.pone.0105116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flores-Alfaro E., Fernández-Tilapa G., Salazar-Martínez E., Cruz M., Illades-Aguiar B., Parra-Rojas I. Common Variants in the CRP Gene are Associated with Serum C-Reactive Protein Levels and Body Mass Index in Healthy Individuals in Mexico. Genet. Mol. Res. 2012;11:2258. doi: 10.4238/2012.May.14.5. [DOI] [PubMed] [Google Scholar]
- 74.Hotta K., Kitamoto A., Kitamoto T., Mizusawa S., Teranishi H., Matsuo T., Nakata Y., Hyogo H., Ochi H., Nakamura T., et al. Genetic Variations in the CYP17A1 and NT5C2 Genes are Associated with a Reduction in Visceral and Subcutaneous Fat Areas in Japanese Women. J. Hum. Genet. 2012;57:46–51. doi: 10.1038/jhg.2011.127. [DOI] [PubMed] [Google Scholar]
- 75.Hager J., Dina C., Francke S., Dubois S., Houari M., Vatin V., Vaillant E., Lorentz N., Basdevant A., Clement K. A Genome-Wide Scan for Human Obesity Genes Reveals a Major Susceptibility Locus on Chromosome 10. Nat. Genet. 1998;20:304. doi: 10.1038/3123. [DOI] [PubMed] [Google Scholar]
- 76.Kaess B.M., Barnes T.A., Stark K., Charchar F.J., Waterworth D., Song K., Wang W.Y., Vollenweider P., Waeber G., Mooser V. FGF21 Signalling Pathway and Metabolic traits–genetic Association Analysis. Eur. J. Hum. Genet. 2010;18:1344. doi: 10.1038/ejhg.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kong X., Zhang X., Xing X., Zhang B., Hong J., Yang W. The Association of Type 2 Diabetes Loci Identified in Genome-Wide Association Studies with Metabolic Syndrome and its Components in a Chinese Population with Type 2 Diabetes. PLoS ONE. 2015;10:e0143607. doi: 10.1371/journal.pone.0143607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim Y.K., Kim Y., Hwang M.Y., Shimokawa K., Won S., Kato N., Tabara Y., Yokota M., Han B., Lee J.H. Identification of a Genetic Variant at 2q12. 1 Associated with Blood Pressure in East-Asians by Genome-Wide Scan Including Gene-Environment Interactions. BMC Med. Genet. 2014;15:65. doi: 10.1186/1471-2350-15-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muiya N.P., Wakil S., Al-Najai M., Tahir A.I., Baz B., Andres E., Al-Boudari O., Al-Tassan N., Al-Shahid M., Meyer B.F. A Study of the Role of GATA2 Gene Polymorphism in Coronary Artery Disease Risk Traits. Gene. 2014;544:152–158. doi: 10.1016/j.gene.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 80.Sull J.W., Yang S.J., Kim S., Jee S.H. The ABCG2 Polymorphism rs2725220 is Associated with Hyperuricemia in the Korean Population. Genom. Inform. 2014;12:231–235. doi: 10.5808/GI.2014.12.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Teixeira A.A., Quinto B.M.R., Dalboni M.A., Rodrigues C.J.D.O., Batista M.C. Association of IL-6 Polymorphism-174G/C and Metabolic Syndrome in Hypertensive Patients. BioMed Res. Int. 2015;2015:927589. doi: 10.1155/2015/927589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M., Li J., Yeung V., Zee B., Yu R., Ho S., Waye M. Four Pairs of gene–gene Interactions Associated with Increased Risk for Type 2 Diabetes (CDKN2BAS–KCNJ11), Obesity (SLC2A9–IGF2BP2, FTO–APOA5), and Hypertension (MC4R–IGF2BP2) in Chinese Women. Meta Gene. 2014;2:384–391. doi: 10.1016/j.mgene.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y., Bos S.D., Harbo H.F., Thompson W.K., Schork A.J., Bettella F., Witoelar A., Lie B.A., Li W., McEvoy L.K. Genetic Overlap between Multiple Sclerosis and several Cardiovascular Disease Risk Factors. Mult. Scler. J. 2016;22:1783–1793. doi: 10.1177/1352458516635873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei F., Cai C., Yu P., Lv J., Ling C., Shi W., Jiao H., Chang B., Yang F., Tian Y. Quantitative Candidate Gene Association Studies of Metabolic Traits in Han Chinese Type 2 Diabetes Patients. Genet. Mol. Res. 2015;14:15471–15481. doi: 10.4238/2015.November.30.25. [DOI] [PubMed] [Google Scholar]
- 85.Zeller T., Wild P., Szymczak S., Rotival M., Schillert A., Castagne R., Maouche S., Germain M., Lackner K., Rossmann H., et al. Genetics and Beyond--the Transcriptome of Human Monocytes and Disease Susceptibility. PLoS ONE. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akintunde A., Nondi J., Gogo K., Jones E.S., Rayner B.L., Hackam D.G., Spence J.D. Physiological Phenotyping for Personalized Therapy of Uncontrolled Hypertension in Africa. Am. J. Hypertens. 2017;30:923–930. doi: 10.1093/ajh/hpx066. [DOI] [PubMed] [Google Scholar]
- 87.Ehret G.B., Ferreira T., Chasman D.I., Jackson A.U., Schmidt E.M., Johnson T., Thorleifsson G., Luan J., Donnelly L.A., Kanoni S., et al. The Genetics of Blood Pressure Regulation and its Target Organs from Association Studies in 342,415 Individuals. Nat. Genet. 2016;48:1171–1184. doi: 10.1038/ng.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wahl S., Drong A., Lehne B., Loh M., Scott W.R., Kunze S., Tsai P., Ried J.S., Zhang W., Yang Y. Epigenome-Wide Association Study of Body Mass Index, and the Adverse Outcomes of Adiposity. Nature. 2017;541:81. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pescatello L.S., Corso L.M.L., Santos L.P., Livingston J., Taylor B.A. Chapter 21 Angiotensin Converting Enzyme and the Genomics of Endurance Performance. In: Lightfoot J.T., Hubal M.J., Roth S.M., editors. Routledge Handbook of Sport and Exercise Systems Genetics. 1st ed. Routledge Taylor and France Group; London, UK: 2019. [Google Scholar]
- 90.Pescatello L.S., Devaney J.M., Hubal M.J., Thompson P.D., Hoffman E.P. Highlights from the Functional Single Nucleotide Polymorphisms Associated with Human Muscle Size and Strength Or FAMuSS Study. Biomed. Res. Int. 2013;2013:643575. doi: 10.1155/2013/643575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.