Highlights
-
•
Acute exercise is an immune system adjuvant that improves defense activity and metabolic health.
-
•
Data support a clear inverse relationship between moderate exercise training and illness risk.
-
•
Exercise training has an anti-inflammatory influence mediated through multiple pathways.
-
•
Illness risk is increased in athletes during periods of intensified training and competition.
-
•
Increased carbohydrate and polyphenol intake is an effective nutritional strategy for immune support.
-
•
Habitual exercise improves immune regulation, delaying the onset of age-related dysfunction.
-
•
Advances in mass spectrometry technology will provide new insights on exercise–immune responses.
Keywords: Aging, Exercise, Immunology, Infection, Inflammation, Mass Spectrometry, Nutrition
Abstract
This review summarizes research discoveries within 4 areas of exercise immunology that have received the most attention from investigators: (1) acute and chronic effects of exercise on the immune system, (2) clinical benefits of the exercise–immune relationship, (3) nutritional influences on the immune response to exercise, and (4) the effect of exercise on immunosenescence. These scientific discoveries can be organized into distinctive time periods: 1900–1979, which focused on exercise-induced changes in basic immune cell counts and function; 1980–1989, during which seminal papers were published with evidence that heavy exertion was associated with transient immune dysfunction, elevated inflammatory biomarkers, and increased risk of upper respiratory tract infections; 1990–2009, when additional focus areas were added to the field of exercise immunology including the interactive effect of nutrition, effects on the aging immune system, and inflammatory cytokines; and 2010 to the present, when technological advances in mass spectrometry allowed system biology approaches (i.e., metabolomics, proteomics, lipidomics, and microbiome characterization) to be applied to exercise immunology studies. The future of exercise immunology will take advantage of these technologies to provide new insights on the interactions between exercise, nutrition, and immune function, with application down to the personalized level. Additionally, these methodologies will improve mechanistic understanding of how exercise-induced immune perturbations reduce the risk of common chronic diseases.
1. Introduction to exercise immunology
Although exercise immunology is considered a relatively new area of scientific endeavor with 90% of papers published after 1990,1 some of the earliest studies were published well over a century ago. For example, in 1902, Larrabee2 provided evidence that changes in white blood cell differential counts in Boston marathon runners paralleled those seen in certain diseased conditions. He also observed that “the exertion had gone far beyond physiological limits and our results certainly show that where this is the case we may get a considerable leukocytosis of the inflammatory type.”2
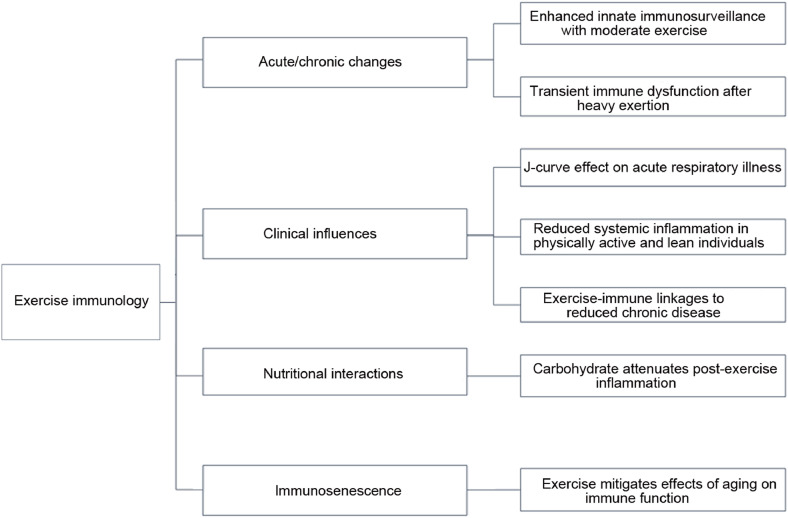
The immune system is very responsive to exercise, with the extent and duration reflecting the degree of physiological stress imposed by the workload. This review paper summarizes the research discoveries within 4 areas of exercise immunology that have received the most consideration: acute and chronic effects of exercise on the immune system, clinical benefits of this exercise–immune relationship, nutritional influences on the immune response to exercise, and the exercise effect on immunosenescence (Fig. 1).3, 4, 5, 6, 7
Fig. 1.
Key research areas and basic findings in exercise immunology.
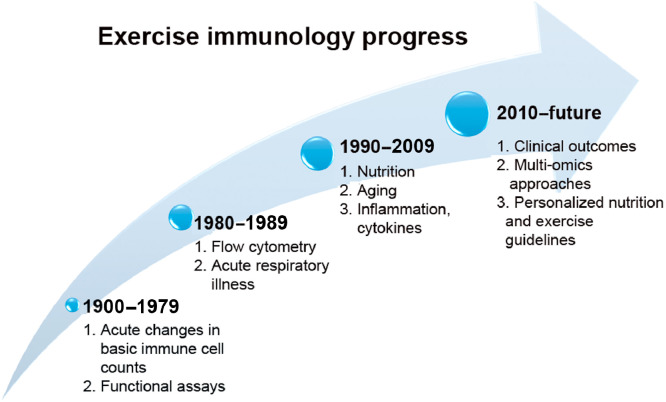
These scientific discoveries can be organized into distinctive time periods (Fig. 2). The earliest exercise immunology studies (1900–1979) focused on exercise-induced changes in basic immune cell counts and function.5 The human immunodeficiency virus was identified as the cause of the AIDS in 1984. One of the markers for AIDS diagnosis was the CD4 antigen on helper T cells that required a flow cytometer for detection. Many medical universities acquired flow cytometers in the 1980s, and these instruments became available to exercise investigators, initiating the modern era of exercise immunology research. Another impetus was the publication of a brief review in a special issue of the Journal of the American Medical Association for the 1984 Olympic Games in Los Angeles.8 This review concluded there was “no clear experimental or clinical evidence that exercise will alter the frequency or severity of human infections… Further studies will be needed before it can be concluded that exercise affects the host response to infection in any clinically meaningful way.”8 This conclusion was consistent with the existing evidence at that time and at the same time provided a framework for future investigations. During the same time period (1980–1989), seminal papers were published with evidence that heavy exertion was associated with transient immune dysfunction, elevated inflammatory biomarkers, and an increased risk of upper respiratory tract infections (URTIs).9, 10, 11, 12, 13, 14, 15, 16, 17, 18 For example, acute bouts of intense and prolonged exercise were linked by several early exercise immunology pioneer investigators to suppressed salivary immunoglobulin A (IgA) output, decreased natural killer cell (NK) lytic activity, reduced T- and B-cell function, and a 2- to 6-fold increased URTI risk during the 1–2 week postrace time period.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 In 1989, the International Society of Exercise Immunology was founded, leading to biannual conferences and the highly successful Exercise Immunology Review journal (www.isei.dk).5
Fig. 2.
Exercise immunology research can be organized into 4 distinctive periods.
During the time period from 1990 to 2009, additional focus areas were added to the field of exercise immunology, including the interactive effect of nutrition,7, 19,20 effects on the aging immune system,21, 22, 23 and influences on inflammatory cytokines.24, 25, 26, 27 With advances in mass spectrometry and genetic testing technology since 2010, increasing attention is being focused on metabolomics, proteomics, lipidomics, gut microbiome characterization, and genomic approaches to exercise immunology, and how this information can be used to provide personalized exercise and nutrition guidelines.28, 29, 30, 31, 32, 33 Additionally, acute and chronic exercise-induced immune changes are now being described as important mechanistic pathways for elucidating reduced cancer and heart disease risk among the physically active.34, 35, 36
2. Acute and chronic effects of exercise on the immune system
The acute immune response to exercise depends on the intensity and duration of effort. For the purposes of this review, moderate and vigorous exercises are differentiated using an intensity threshold of 60% of the oxygen update and heart rate reserve, and a duration threshold of 60 min. Exercise immunology investigators had an early focus on the large perturbations of basic leukocyte subsets associated with the physiological stress of athletic endeavor.2,9, 10, 11, 12, 13, 14,27 Increasing attention is being directed to the enhanced immunosurveillance of distinct immune cell subtypes during exercise bouts of less than 60 min that have potential prevention and therapeutic value.37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48
2.1. Enhanced immunosurveillance with acute exercise bouts of less than 60 min
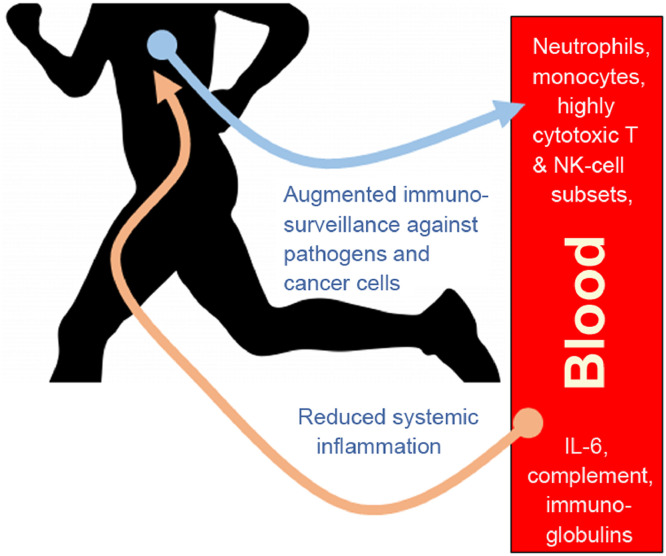
During moderate- and vigorous-intensity aerobic exercise bouts of less than 60 min duration, the antipathogen activity of tissue macrophages occurs in parallel with an enhanced recirculation of immunoglobulins, anti-inflammatory cytokines, neutrophils, NK cells, cytotoxic T cells, and immature B cells, all of which play critical roles in immune defense activity and metabolic health (Fig. 3).37, 38, 39, 40,44, 45, 46, 47 Acute exercise bouts preferentially mobilize NK cells and CD8+ T lymphocytes that exhibit high cytotoxicity and tissue migrating potential.38, 46,48 Stress hormones, which can suppress immune cell function, and proinflammatory cytokines, indicative of intense metabolic activity, do not reach high levels during short duration, moderate exercise bouts.40 Over time, these transient, exercise-induced increases in selective lymphocyte subsets enhance immunosurveillance and lower inflammation, and may be of particular clinical value for obese and diseased individuals.41, 42, 43
Fig. 3.
Acute exercise stimulates the interchange of innate immune system cells and components between lymphoid tissues and the blood compartment. Although transient, a summation effect occurs over time, with improved immunosurveillance against pathogens and cancer cells and decreased systemic inflammation.
In general, acute exercise is now viewed as an important immune system adjuvant to stimulate the ongoing exchange of leukocytes between the circulation and tissues.37 An ancillary benefit is that acute exercise may serve as a simple strategy to enrich the blood compartment of highly cytotoxic T-cell and NK cell subsets that can be harvested for clinical use.38,44, 45, 46 Metabolically, moderate exercise induces small, acute elevations in IL-6 that exert direct anti-inflammatory effects, improving glucose and lipid metabolism over time.49, 50 Another benefit may include an enhanced antibody-specific response when vaccinations are preceded by an acute exercise bout, but more research is needed with better study designs to control for potential confounding influences.51
2.2. Transient immune dysfunction after heavy exertion
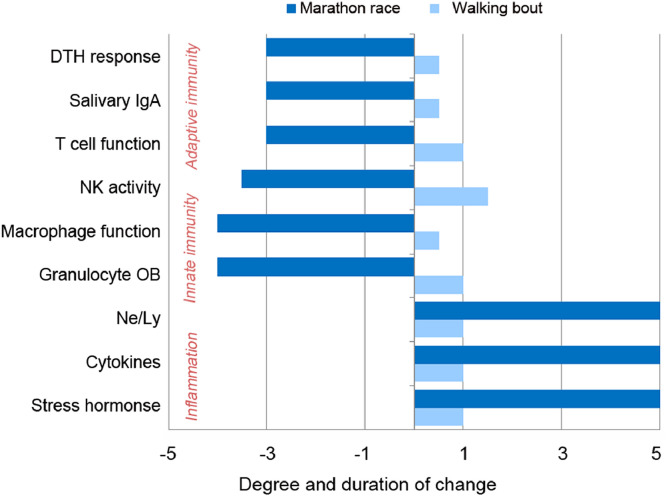
The measurement of immune responses to prolonged and intensive exercise by athletes continues to receive high attention. Taken together, the best evidence supports that high exercise training workloads, competition events, and the associated physiological, metabolic, and psychological stress are linked to immune dysfunction, inflammation, oxidative stress, and muscle damage.9, 10, 11, 12, 13, 14,24, 27,52, 53, 54 NK cell and neutrophil function, various measures of T- and B-cell function, salivary IgA output, skin delayed-type hypersensitivity response, major histocompatibility complex II expression in macrophages, and other biomarkers of immune function are altered for several hours to days during recovery from prolonged and intensive endurance exercise.52, 53, 54, 55, 56, 57, 58 The contrast in the magnitude of immune responses between a 30- to 45-min walking bout and 42.2-km marathon race is summarized in Fig. 4.3, Fig. 4,27, 40,52, 53, 54, 55, 56, 57 These immune changes occur in several compartments of the immune system and body including the skin, upper respiratory tract mucosal tissue, lung, blood, muscle, and peritoneal cavity. Although some investigators have challenged the clinical significance and linkage between heavy exertion and transient immune dysfunction,58 the majority of investigators in the field of exercise immunology have supported the viewpoint that the immune system reflects the magnitude of physiological stress experienced by the exerciser.3, 4, 5,27, 54,56, 57
Fig. 4.
The contrast in acute immune responses to heavy exertion (e.g., a marathon race) and a 30- to 45-min walking bout. DTH = delayed-type hypersensitivity; IgA = immunoglobulin A; Ne/Ly = neutrophil/lymphocyte ratio; NK = natural killer; OB = oxidative burst.
Recent improvements in mass spectrometry technology and bioinformatics support have improved the capacity to use a systems biology approach when measuring the complex interactions between exercise stress and immune function within the human athlete.29, 30, 31, 32, 33,59, 60, 61, 62, 63 Metabolomics, proteomics, and lipidomics have revealed that metabolism and immunity are inextricably interwoven and has led to a new area of research endeavor termed immunometabolism.33, 64 In a typical study with human athletes exercising intensely for more than 2 h, significant increases in at least 300 identified metabolites can be measured as body glycogen stores are depleted and an extensive increase occurs in numerous and varied lipid super-pathway metabolites, including oxidized derivatives called oxylipins.32,60, 61, 62 Exercise-induced muscle tissue injury and inflammation elicit a strong innate immune response involving granulocytes, monocytes, and macrophages. Immune-specific proteins are produced to regulate the innate immune response, with oxylipins involved in initiating, mediating, and resolving this process.29, 30,60, 63 Most of the expressed immune-related proteins including lysozyme C, neutrophil elastase and defensin 1, proteins S100-A8/A12, cathelicidin antimicrobial peptide, α-actinin-1, and profilin-1 are involved with pathogen defense and immune cell chemotaxis and locomotion. Other proteins including serum amyloid A-4, myeloperoxidase, complements C4B and C7, plasma protease C1 inhibitor, α-2-HS-glycoprotein, and α-1-acid glycoprotein 2 increase chronically during recovery and are involved in the inflammatory acute phase response.30
This profound, exercise-induced perturbation in metabolites, lipid mediators, and proteins more than likely has a direct influence on immune function, decreasing the capacity of immune cells to increase oxygen consumption rates after activation.31 In response to an acute immunologic challenge such as exercise stress, cells of the immune system must be able to engage in growth and proliferation to generate effector cells that produce specific molecules such as cytokines and the proteins listed in the previous paragraph.64 Immune activation is associated with oxygen and biosynthetic demands, and immune cells must engage in metabolic reprogramming to generate sufficient energy to fuel these demands. Although more research is needed, preliminary data support that immune cell metabolic capacity is decreased during recovery from physiologically demanding bouts of intensive exercise, resulting in transient immune dysfunction.31, 33 Immunonutrition support, especially increased intake of carbohydrate and polyphenols, has been shown to counter these exercise-induced decrements in immune cell metabolic capacity.31, 33
2.3. Illness risk and high exercise workloads
The potential linkage between prolonged, intensive exercise and increased risk for illness has been an active area of research since the 1980s.3,65, 66, 67, 68, 69 Early epidemiologic studies indicated that athletes engaging in marathon and ultramarathon race events and/or very heavy training were at increased risk of URTI.17, 67 (Table 1). For example, in a large group of 2311 endurance runners, nearly 13.0% reported illness during the week after the Los Angeles Marathon race compared with 2.2% of control runners (odds ratio (OR) = 5.9; 95% confidence interval (CI): 1.9–18.8).17 Forty percent of the runners reported at least 1 illness episode during the 2-month winter period before the marathon race, and those running more than 96 km/week vs. less than 32 km/week doubled their odds for illness. A 1-year retrospective study of 852 German athletes showed that URTI risk was highest in endurance athletes who also reported significant stress and sleep deprivation.70 These seminal studies indicated that illness risk may be increased when an athlete participates in competitive events, goes through repeated cycles of unusually heavy exertion, or experiences other stressors to the immune system including lack of sleep and mental stress. The direct connection between exercise-induced immune changes and infection risk has not yet been established, and will require long-term studies with large cohorts. More research is needed to more clearly demonstrate the linkage between heavy exertion, illness symptoms, and pathogen-based illnesses, and the relative importance of associated factors such as travel, pathogen exposure, exercise-induced immune perturbations, sleep disruption, mental stress, and nutrition support.3, 4
Table 1.
Research on the relationship between vigorous exercise and illness.
| Investigator | Study population | Research design | Key finding |
|---|---|---|---|
| Peters and Bateman67 | 141 ultramarathon runners and 124 controls (aged 18–65 years) | Participants reported 2-week recall of illness symptoms after 56-km race | Illness incidence 2× higher in runners after race vs. controls (33% vs. 15%). |
| Nieman et al.21 | 1828 marathon runners and 134 runner controls (aged 36.9 ± 0.2 years) | Participants reported illness symptoms 2 months before and 1 week after March 42.2-km race | Illness incidence 6× higher in runners who finished race vs. controls (13% vs. 2%). Runners training ≥97 km/week vs. <32 km/week at higher URTl risk. |
| Heath et al.69 | 530 runners (aged 39.4 years) | Participants reported training log and illness symptoms every month for 1 year | Running >485 miles/year (780 km/year) increased risk of illness. |
| Konig et al.70 | 852 German athletes (aged 23.6 ± 9.5 years) | Participants retrospectively reported illness episodes over past 12 months | Illness incidence 2× higher in endurance sports (OR = 2.2); 2× higher with stress (OR = 2.0); and nearly 2× with sleep deprivation (OR = 1.7). |
| Spence et al.71 | 20 elite triathletes/cyclists, 30 recreational triathletes/cyclists, 20 sedentary controls (aged 18–34 years) | Participants followed for 5 months in summer/autumn; reported daily illness symptoms | Illness incidence 4× higher in elite athletes and 2× greater in controls vs. recreational athletes. Higher number of illness days in elite athletes (311 days) and control (137 days) and recreational (92 days). |
| Gleeson et al.72 | 75 endurance trained university students (aged 18–35 years) | Participants followed for 4 months in winter; reported weekly illness symptoms | Greater illness incidence in high and medium vs. low training groups (2.4 ± 2.6 episodes and 2.6 ± 2.2 episodes vs. 1.0 ± 1.7 episodes). |
| Rama et al.73 | 19 elite swimmers vs. 11 nonathlete controls (aged 17.6 ± 1.0 years) | Participants followed for 7 months in winter; reported daily illness symptoms | 67% of illness episodes occurred during high volume training in swimmers vs. no illness in control at same time points. |
| Hellard et al.74 | 28 elite swimmers (aged 16–30 years) | Participants followed for 4 years; monitored weekly for illness | Illness increased 1.08× (95%CI: 1.01–1.16) every 10% increase in resistance training and 1.10× (95%CI: 1.01–1.19) for every 10% increase in high-load training. |
| Svendsen et al.75 | 42 elite cross-country skiers (aged 24 ± 4 years) | Participants followed for 8 years; reported illness symptoms daily for 10 days after the Tour de Ski race | Illness incidence was 3× higher in skiers who raced the Tour de Ski vs. non-competing skiers (48% vs. 16%). |
| Raysmith and Drew77 | 33 international track and field athletes | Participants reported illness symptoms during 6 months preceding competition for 5 years | Illness incidence was 23%; one-half of illnesses occurred 2 months before competition. Better performing athletes had a lower incidence of illness. |
| Drew et al.78 | 132 elite athletes preparing for the Olympics | 3 months before competition, participants reported illness symptoms during a 1-month time period | Illness symptoms in 100% athletes (46% upper respiratory). Risk factors were female sex, low energy availability. |
| Prien et al.,79 Timpka et al.80 | 1551 elite athletes preceding World Championship competition | Participants retrospectively reported illness symptoms during 4 weeks preceding competition | Illness incidence ranged from 5% to 13%. |
| Engebretsen et al.,81, 82 Palmer-Green and Elliott,83 Soligard et al.84, 85 | 27,245 elite athletes during an international Olympic competition | Medical staff reported illness symptoms during competition event (<4 weeks) | Illness incidence ranged from 5% to 18%; Risk factor was female sex. |
| Mountjoy et al.,86, 87 Prien et al.79 | 5293 elite aquatics athletes during the international World Championships | Medical staff reported illness symptoms during competition event (<4 weeks) | Illness incidence ranged from 7% to 13%. |
| Alonso et al.,88, 89 Timpka et al.80 | 3305 elite track and field athletes during the international World Championships | Medical staff reported illness symptoms during competition event (<4 weeks) | Illness incidence ranged from 2% to 7%; 10× greater illness incidence in endurance events vs. speed/power events. |
Abbreviations: CI = confidence interval; OR = odds ratio; URTI = upper respiratory tract infections.
As illness data from additional studies mounted,71, 72, 73, 74, 75, 76, 77 several athletic organizations including the International Olympic Committee (IOC) and the International Association of Athletics Federation (IAAF) initiated acute illness surveillance systems to delineate the extent of the problem and underlying risk factors.65,78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 The stated goal was to improve illness prevention and treatment procedures.65, 80 The IOC has also focused on the inappropriate management of both internal (e.g., psychological responses) and external loads (e.g., training and competition workloads). Load management is a key strategy, according to the IOC, to decrease illness incidence and associated downturns in exercise performance, interruptions in training, missed competitive events, and risk of serious medical complications. The wealth of acute illness epidemiologic data collected during international competition events has revealed that 2%–18% of elite athletes experience illness episodes, with higher proportions for females and those engaging in endurance events (Table 1).78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89 At least one-half of the acute illness bouts involve the respiratory tract, with other affected systems including the digestive tract, skin tissues, and the genitourinary tract.65 Significant illness risk factors include female gender, high levels of depression or anxiety, engaging in unusually intensive training periods with large fluctuations, international travel across several time zones, participation in competitive events especially during the winter, lack of sleep, and low diet energy intake.65,68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92 The decrease in exercise performance after an URTI can last 2–4 days, and runners who unwisely start an endurance race with systemic URTI symptoms are 2–3 times less likely to complete the race.65, 92,93 Paralympic athletes have unique preexisting medical conditions that predispose them to an increased risk of illness, and the incidence rate of illness is high in the Summer (10.0–13.2 episodes per 1000 athlete-days) and Winter (18.7 episodes per 1000 athlete-days) Paralympic Games.94
Athletes must train hard for competition and are interested in strategies to keep their immune systems robust and illness rates low despite the physiologic stress experienced. The ultimate objective is to achieve performance goals with little interruption from illness and fatigue from training-induced subclinical immune dysfunction. Several training, hygienic, nutritional, and psychological strategies are recommended, and these require the coordinated involvement of the medical staff, coaches, and athletes.4, 6,65, 95 The medical staff should develop and implement an illness prevention program, with a focus on full preventative precautions for high-risk individuals such as female endurance athletes. Adjustments to the guidelines can be applied based on how each individual athlete responds. Here is a summary of the most important guidelines provided from consensus statements:4, 6,65, 95
2.3.1. Training and competition load management
-
a.
Develop a detailed, individualized training and competition plan that also provides for sufficient recovery using sleep, nutrition, hydration, and psychological strategies.
-
b.
Use small increments when changing the training load (typically less than 10% weekly).
-
c.
Develop a competition event calendar that is based on the health of the athlete.
-
d.
Monitor for early signs and symptoms of over-reaching, overtraining, and illness.
-
e.
Avoid intensive training when ill or experiencing the early signs and symptoms of illness (which can make the illness more severe and prolonged).
-
f.
Participate in ongoing illness surveillance systems by sport agencies.
2.3.2. Hygienic, lifestyle, nutritional, and behavioral strategies
-
a.
Minimize pathogen exposure by avoiding close contact with infected individuals in crowded, enclosed spaces, and not sharing drinking or eating implements. Avoid exercise sessions in poorly ventilated clubs and gymnasium facilities. The medical staff should isolate infected athletes.
-
b.
Limit hand-to-face contact (i.e., self-inoculation) and wash hands regularly and effectively. The medical staff should educate the athletes to minimize pathogen spread to others (e.g., sneezing and coughing into the crook of the elbow).
-
c.
Follow other hygienic practices to limit all types of infections including safe sex and the use of condoms, wearing open footwear when using public facilities to limit skin infections, using insect repellents, and covering the arms and legs with clothing at dawn or dusk.
-
d.
Maintain vaccines needed for home and foreign travel, with a focus on annual influenza vaccination.
-
e.
Follow strategies that facilitate regular, high-quality sleep.
-
f.
Avoid excessive alcohol intake.
-
g.
Consume a well-balanced diet with sufficient energy to maintain a healthy weight, with a focus on grains, fruits, and vegetables to provide sufficient carbohydrate and polyphenols that reduce exercise-induced inflammation and improve viral protection.
2.3.3. Psychological load management
-
a.
Follow stress management techniques that decrease the extraneous load of life hassles and stresses.
-
b.
Develop coping strategies that minimize the internalized impact of negative life events and emotions.
-
c.
Periodically monitor psychological stresses using available instruments.
3. Clinical influences of immune responses to chronic exercise
Each bout of moderate physical activity promotes improved but transient immunosurveillance and, when repeated on a regular basis, confers multiple health benefits including decreased illness incidence and dampened systemic inflammation.95
3.1. J-curve relationship between exercise and URTIs
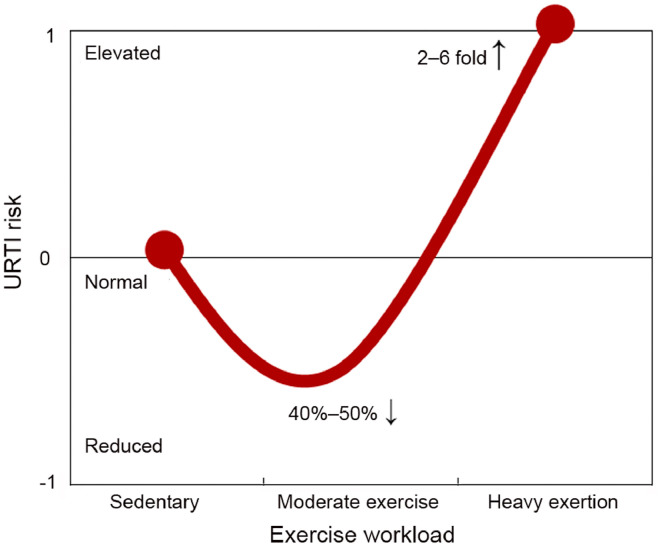
Table 2 summarizes published evidence from randomized clinical trials and epidemiologic studies on the inverse relationship between moderate exercise training and URTI incidence. The randomized clinical trials (8 weeks to 1 year in length) are consistent in demonstrating that study participants assigned to moderate exercise programs experience reduced URTI incidence and duration.21,96, 97, 98, 99, 100 The magnitude of reduction in URTI symptom days with near-daily moderate exercise in these randomized clinical trials (typically 40%–50%) exceeds levels reported for most medications and supplements, and bolsters public health guidelines urging individuals to be physically active on a regular basis. The protective effect of moderate activity on illness incidence contrasts with the increased illness risk linked with prolonged and intensive exercise, as summarized in the J-curve model (Fig. 5).95 The IOC consensus group provided support for the J-curve model, but cautioned that the right side of the model may not apply to elite athletes on the highest level, where high training loads are not consistently associated with an increased risk of illness.65
Table 2.
Research on the relationship between moderate exercise and illness.
| Investigator | Study population | Research design | Key finding |
|---|---|---|---|
| Randomized controlled trials | |||
| Nieman et al.96 | 36 mildly obese sedentary women (aged 34.4 ± 1.1 years) | Randomized to 15 weeks of moderate intensity (45 min/day × 5 days/week) walking program or observational control | Fewer days with illness symptoms reported in walkers vs. controls (5.1 ± 1.2 days vs. 10.8 ± 2.3 days). |
| Nieman et al.21 | 32 sedentary women (aged 73.4 ± 1.2 years); 12 highly conditioned women (aged 72.5 ± 1.8 years) | Sedentary women randomized to a 12-week moderate intensity (30- to 40-min/day × 5 days/week) walking program or stretching (45 min/day × 5 days/week) in fall season | Illness incidence 8% in highly conditioned, 21% in walkers, and 50% in controls. |
| Nieman et al.97 | 91 obese women (aged 45.6 ± 1.1 years) | Randomized to a 12-week moderate intensity (45 min/day × 5 days/week) walking program or stretching 45 min/day × 4 days/week | Fewer days with illness symptoms reported in walkers vs. controls (5.6 ± 0.9 days vs. 9.4 ± 1.1 days). |
| Chubak et al.98 | 115 postmenopausal women (aged 60.7 ± 6.9 years) | Randomized to 1 year of moderate intensity exercise (45 min/day × 5 days/week) or stretching control (45 min/day × 1 day/week) | Illness incidence 30% in exercise vs. 48% in controls. Three-fold decreased risk of illness in exercise group vs. control in final 3 months. |
| Barrett et al.99, 100 | 373 male and female older adults (aged 59.3 ± 6.6 years (2012); 49.9 ± 11.8 years (2018)) | Randomized to 8 week moderate-intensity sustained exercise (group sessions; home practice) or observational control | Pooled datasets: proportional reductions of incidence, days-of-illness, and global severity were 14%, 23%, and 31% for exercise compared with controls. |
| Epidemiologic studies | |||
| Mathews et al.101 | 547 male and female adults (aged 48.0 ± 12.4 years) | Participants followed for 1 year; interviewed for physical activity and illness symptoms every 90 days | 29% decreased illness risk in upper vs. lower quartile of activity. |
| Fondell et al.102 | 1509 male and female adults (aged 20–60 years) | Participants followed for 4 months; baseline questionnaire on physical activity; illness symptoms assessed every 3 weeks | 18% decreased illness risk in high vs. low physical activity. |
| Nieman et al.103 | 1002 male and female adults (aged 18–85 years) | Participants followed for 12 weeks in winter and autumn seasons; baseline questionnaire on physical fitness levels; daily illness symptoms checklist | 46% decrease in total day with illness in high vs. low physical fitness tertile. 43% decrease in those who reported ≥5 days/week aerobic activity vs. <1 day/week. |
| Zhou et al.104 | 1413 male and female adults (aged 38.9 ± 9.0 years) | Participants retrospectively reported frequency of illness and physical activity over the past year | 26% decreased illness risk in high vs. low physical activity. |
Fig. 5.
J-curve model of the relationship between the exercise workload continuum and risk for upper respiratory tract infection (URTI). Other factors such as travel, pathogen exposure, sleep disruption, mental stress, and dietary patterns may influence this relationship. This figure was adapted from Nieman.95
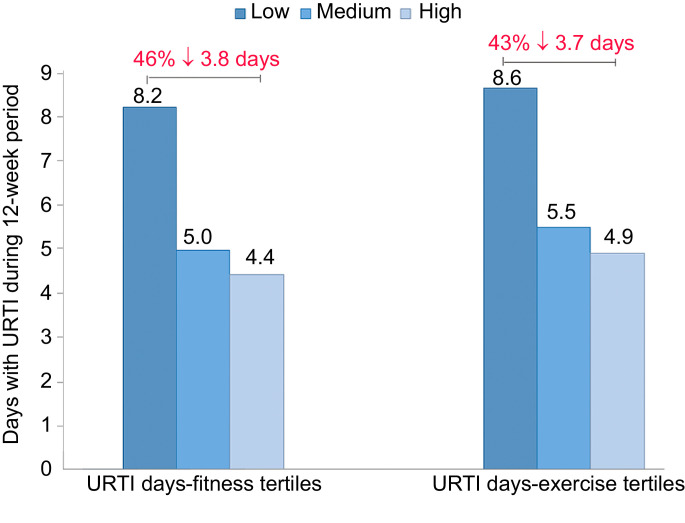
Retrospective and prospective epidemiologic studies have measured illness incidence in large groups of individuals engaging in self-selected and varied physical activity workloads (Table 2).101, 102, 103, 104 Collectively, the epidemiologic studies summarized in Table 2 consistently show reduced URTI rates (weighted mean, 28%) in high vs. low physical activity and fitness groups. Fig. 6 summarizes the results from a group of 1002 adults (aged 18–85 years; 60% female and 40% male) studied for 12 weeks (one-half during the winter, one-half during the fall), with monitoring of URTI symptoms and severity using the validated Wisconsin Upper Respiratory Symptom Survey.103, 105 The number of days with URTI was 43% lower in subjects engaging in an average of 5 or more days per week of aerobic exercise (20 min bouts or longer) compared with those who were largely sedentary (≤1 day/week), and 46% lower when comparing subjects in the highest vs. lowest tertile for perceived physical fitness. This relationship persisted, even after adjustment for confounders such as age, education level, marital status, gender, body mass index (BMI), and perceived mental stress.
Fig. 6.
The upper tertiles of fitness and exercise frequency are associated with reduced numbers of days with upper respiratory tract infections (URTI). Data from Nieman et al.103
Physical activity may lower rates of infection for other types of viral and bacterial diseases, but more data are needed. Several epidemiologic studies suggest that regular physical activity is associated with decreased mortality and incidence rates for influenza and pneumonia.106, 107, 108, 109 These findings are in accordance with rodent-based studies demonstrating a positive link between chronic exercise and improved host responses to influenza and pneumonia infection.110, 111, 112, 113 These data must be carefully balanced with published reports of increased infectious disease severity when vigorous exercise was engaged in during active influenza or other viral infections.114, 115, 116 There is also increasing support for improved antibody responses to influenza immunization in elderly adults who engage in regular exercise training regimens.117, 118, 119
3.2. Reduced systemic inflammation in physically active and lean individuals
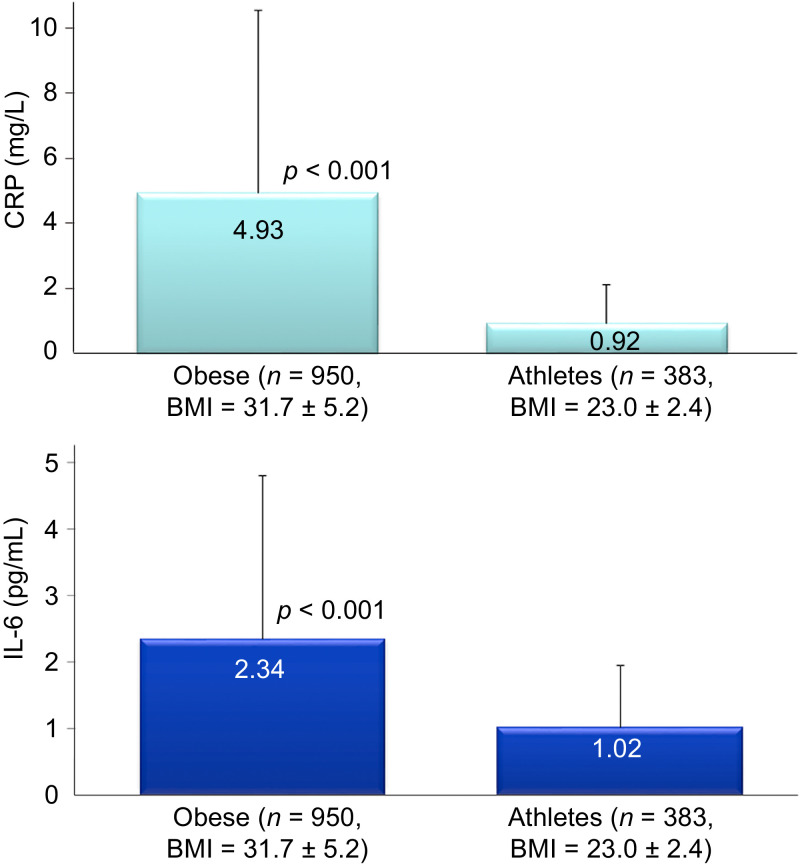
Each exercise bout causes transient increases in total white blood cells, granulocyte-related proteins, and a variety of plasma cytokines including interleukin-6 (IL-6), IL-8, IL-10, IL-18, IL-1 receptor antagonist (IL-1ra), granulocyte colony stimulating factor, and monocyte chemoattractant protein 1.30, 40,52 The magnitude of change in these inflammation-related biomarkers depends on the overall exercise workload. Acute phase proteins including C-reactive protein (CRP) are also increased after heavy exertion, but increases are delayed in comparison with most cytokines.30, 52 Despite regular increases in these inflammation biomarkers during each intense exercise bout, physically fit individuals have lower resting levels in contrast with those who are overweight and unfit. Fig. 7 compares serum CRP (4.4-fold difference) and plasma IL-6 (1.3-fold) in large groups of obese individuals (n = 950; mean BMI = 31.7 kg/m2) and endurance athletes (n = 383; mean BMI = 23.0 kg/m2) studied over the course of the past 2 decades in the author's laboratory. There is increasing evidence that regular exercise training has an overall anti-inflammatory influence mediated through multiple pathways including improved control of inflammatory signaling pathways, release of muscle myokines that stimulate production of IL-1ra and IL-10 (perhaps by blood mononuclear immune cells), a decrease in dysfunctional adipose tissue and improved oxygenation, enhanced innate immune function, and an improved balance of oxylipins.7, 33,50, 55,120
Fig. 7.
C-reactive protein (CRP) and interleukin-6 (IL-6) values for obese and athletic groups (data expressed as mean ± SD). Data are from ongoing studies in the first author's lab during the past 2 decades. BMI = body mass index.
The persistent increase in inflammation biomarkers is defined as chronic or systemic inflammation, and is linked with multiple disorders and diseases including obesity, arthritis, atherosclerosis and cardiovascular disease, chronic kidney disease, liver disease, metabolic syndrome, insulin resistance and type 2 diabetes mellitus, sarcopenia, arthritis, bone resorption and osteoporosis, chronic obstructive pulmonary disease, dementia, depression, and various types of cancers.121, 122, 123, 124, 125, 126, 127 Obesity induces a constant state of low-grade inflammation characterized by activation and infiltration of proinflammatory immune cells such as macrophages and granulocytes, and a dysregulated production of acute-phase proteins, reactive oxygen species, metalloproteinases, oxylipins, adipokines, and proinflammatory cytokines.124, 128 Many of the inflammation biomarkers increased transiently after intense and prolonged exercise bouts are chronically expressed at lower levels in obese individuals (resting state).
Epidemiologic studies consistently show reduced white blood cell count, CRP, IL-6, IL-18, tumor necrosis factor alpha, and other inflammatory biomarkers in adults with higher levels of physical activity and fitness, even after adjustment for potential confounders such as BMI.129, 130, 131 For example, in a study of 1002 community-dwelling adults (aged 18–85 years), a general linear model analysis showed that BMI had the strongest effect on CRP followed by gender (greater in females), exercise frequency, age, and smoking status.129 Another study of 1293 middle-aged Danes showed that cardiorespiratory fitness was inversely associated with CRP, IL-6, and IL-18, and was only partly explained by lower levels of abdominal obesity.130
Most randomized, controlled trials, however, have failed to demonstrate that inflammation is decreased by a clinically significant level with exercise training in the absence of weight loss.132, 133, 134, 135, 136 There are several potential explanations for these null findings when compared with epidemiologic studies, including the small reported changes in aerobic fitness and activity levels, the short duration of the intervention trials, and issues with compliance. In general, moderate exercise training is unlikely to lower chronic inflammation at the individual level unless the exercise workload is increased to more than 300 min per week and significant weight loss is experienced.
3.3. Exercise–immune linkages to reduced chronic disease
Do exercise-induced perturbations in immunity help to explain altered risks of cancer, heart disease, type 2 diabetes, arthritis, nonalcoholic fatty liver disease, and other chronic conditions? Research in this area is still emergent, but there is increasing evidence that the circulation surge in cells of the innate immune system with each exercise bout and the anti-inflammatory and antioxidant effect of exercise training have a summation effect over time in modulating tumorigenesis, atherosclerosis, and other disease processes.36,137, 138, 139
Obesity, the metabolic syndrome, and most common chronic diseases such as atherosclerosis, specific types of cancer, and type 2 diabetes are characterized in part by high inflammation, oxidative stress, and immune dysfunction.137 Exercise training counters these elements of the disease process, stimulating many cellular and molecular changes throughout body tissues that promote anti-inflammatory and antioxidant responses, and augment immunosurveillance. For example, IL-1β is a proinflammatory cytokine that is involved in disease pathogenesis, and the release of IL-6 from the exercising muscle induces high levels of plasma IL-1ra during recovery that competitively inhibits IL-1β signaling.137 Exercise training also downregulates Toll-like receptor 4 expression, a key transmembrane receptor that is activated by numerous ligands including oxidized low-density lipoproteins and involved in obesity-induced insulin resistance and type 2 diabetes, and atherosclerosis.137
Inflammation involves several types of immune cells, including macrophages and neutrophils, and is an important mediator of oxidative stress. Reactive oxygen species (ROS) or reactive nitrogen species (RNS), are double-edged molecules. ROS/RNS can function as important inflammatory effectors in supporting immune system clearance of pathogens and repair of damaged muscle tissue, or they can amplify chronic inflammation (e.g., during obesity) and induce tissue damage. Oxinflammation is a term used to describe the complex interactions between oxidative stress and inflammation.138 Exercise training decreases oxidative stress by augmenting antioxidant defenses consisting of enzymes such as catalase, superoxide dismutase, and glutathione peroxidase, and nonenzymatic antioxidants including glutathione.137, 138
Exercise training has immunomodulating effects that may alter the cross-talk between the immune system and tumorigenesis. For example, exercise may increase intra-tumoral cytotoxic T-cell infiltration and reduce regulatory T-cell infiltration, enhance the recirculation and function of tumor-specific NK cells, and decrease inflammatory influences that support cancer cell growth.139
In general, exercise promotes the recirculation of key immune cells and mediates an anti-inflammatory and antioxidant state through multiple mechanisms. Although many information gaps exist, these exercise-induced effects may help to counter the development of chronic metabolic diseases and are likely multiplied when body fat mass is reduced.
3.4. Exercise, gut immune function, and the microbiome
The gastrointestinal tract is colonized by trillions of micro-organisms that include a gene set 150 times larger than that of the human genome.140 The most abundant bacterial phyla are the Firmicutes (∼60%) and Bacteriodetes (∼20%), with low proportions of Actinobacteria, Proteobacteria, and Verrucomicrobia. One-third of the adult gut microbiota is similar between most individuals, but diversity is associated with a healthier status. The gut bacteria composition and diversity is influenced by a variety of factors, including dietary and exercise habits, age, gender, genetics, ethnicity, antibiotics, health, and disease.
The gut microbiota influences human health and immune function, in part through the fermentation of indigestible food components in the large intestine. The microbiome and derived metabolites including short chain fatty acids and biotransformed bile acids have been shown to influence immune function both within the gut and systematically.141 Although research in this area is emerging, recent studies indicate that exercise and physical fitness diversifies the gut microbiota, enhancing the number of benign microbial communities.142, 143 The underlying mechanisms are still being explored, with no clear consensus, in part owing to confounding from diet, exercise workload and intensity, and body composition. More human research is needed to establish whether the positive linkage between long-term exercise training and a diverse microbiome translates to improved immune function in physically fit individuals and athletes.144, 145
4. Nutritional interactions on exercise-induced immune changes
Several comprehensive reviews have been published on the value of nutritional support as countermeasures to exercise-induced immune dysfunction, inflammation, and oxidative stress.4, 6,7, 33 The most effective nutritional strategies for athletes, especially when evaluated from a multiomics perspective, include increased intake of carbohydrates and polyphenols.
4.1. Carbohydrates attenuate postexercise inflammation
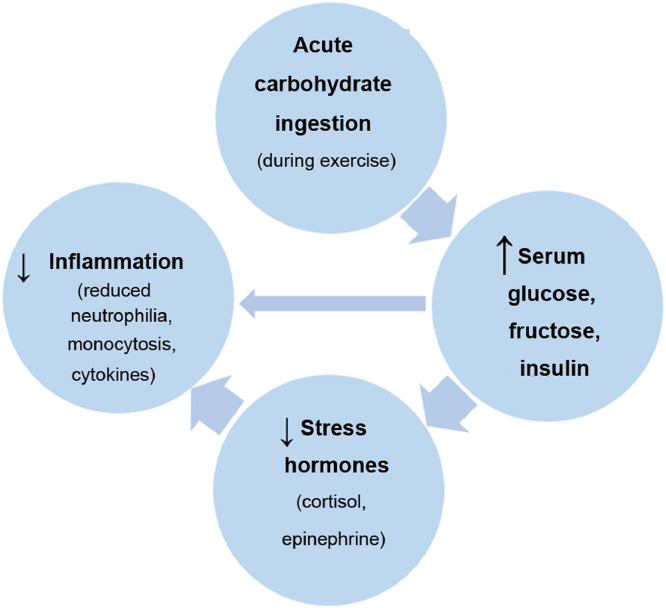
During the 1990s, several studies reported that carbohydrate ingestion (30–60 g/h) during prolonged endurance exercise (90 min and longer) was linked with lower postexercise plasma stress hormone levels and inflammation.20,146, 147, 148, 149, 150 These results have been confirmed by many subsequent studies (Table 3).31,151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165 A consistent finding is that carbohydrate intake during prolonged and intense exercise, whether from 6%–8% beverages or sugar-dense fruits such as bananas (with water), is associated with higher plasma glucose and insulin levels; lower plasma stress hormones (epinephrine and cortisol), adrenocorticotropic hormone, and growth hormone; diminished fatty acid mobilization and oxidation; and reduced inflammation as measured by a variety of biomarkers including skeletal muscle IL-6 and IL-8 messenger ribonucleic acid (mRNA), blood neutrophil and monocyte cell counts, cytokines such as IL-6, IL-1ra, and IL-10, and granulocyte phagocytosis (Table 3 and Fig. 8). The effect of carbohydrate ingestion in attenuating postexercise inflammation is strong (about 30%–40%), especially when contrasted with water-only intake in overnight fasted athletes.7, 27,31, 32, 33,164, 166
Table 3.
Research showing the effect of carbohydrates on inflammation and immune biomarkers after >90 min of endurance exercise.
| Investigator | Study population | Exercise protocol | Carbohydrate intervention | Postexercise immune response | |
|---|---|---|---|---|---|
| Nieman et al.,146 Nehlsen-Cannarella et al.,20 Henson et al.147 |
30 male and female marathon runners (aged 41.5 ± 2.0 years) randomized to CHO or placebo | 2.5-h run at 75%–80%VO2max | 6% CHO or placebo beverage consumed before, during, and after exercise | CHO ↑ glucose; ↓ cortisol; ↓ total leukocytes, neutrophils, monocytes, and lymphocytes; ↓ IL-6, IL-1ra vs. placebo group. Placebo ↑ T cells and NK cells immediately after running; ↓ 3-h recovery vs. the CHO group. | |
| Nieman et al.,148 Nieman et al.,149 Henson et al.150 |
10 male and female triathletes (aged 34.0 ± 2.1 years) in cross-over design | 2.5-h cycle and run at 75%VO2max | 6% CHO or placebo beverage consumed before, during, and after exercise | CHO ↑ glucose, insulin; ↓ cortisol, growth hormone; ↓ neutrophils, monocytes, lymphocytes; ↓ granulocyte, monocyte phagocytosis and oxidative burst activity; ↓ neutrophil/lymphocyte ratio; ↓ NK cell cytotoxicity; ↓ IL-6, IL-1ra vs. placebo trial. | |
| Henson et al.151 | 15 Olympic female rowers (aged 22.4 ± 0.5 years) in a cross-over design | 2-h rowing session | 6% CHO or placebo beverage consumed before, during, and after exercise | CHO ↑ glucose; ↓ total leukocytes, neutrophils, and monocytes; ↓ phagocytosis; ↓ IL-1ra vs. placebo trial. | |
| Nieman et al.152 | 16 marathon runners (aged 50.1 ± 1.5 years) in a cross-over design | 3-h run at 70%VO2max | 6% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose, insulin; ↓ cortisol; ↓ total leukocytes, monocytes, lymphocytes, and granulocytes; ↓ plasma IL-6, IL-10, IL-1ra; ↓ skeletal muscle IL-6, IL-8 mRNA vs. placebo trial. | |
| Bishop et al.153 | 9 trained male cyclists (aged 25.0 ± 2.0 years) in a cross-over design | 2-h cycle at 75%VO2max | 6.4% CHO or placebo beverage consumed before, during, and after exercise | CHO ↑ glucose; ↓ cortisol; ↓ neutrophils vs. placebo trial. | |
| Keller et al.154 | 8 untrained men (aged 24.0 ± 1.0 years) in a cross-over design | 3-h cycle at 60% maximal workload | 6% CHO or placebo beverage consumed during exercise | CHO ↑ glucose; ↓ free fatty acids; ↓ plasma IL-6, ↓ adipose tissue IL-6 mRNA vs. placebo trial. | |
| Febbraio et al.155 | 7 men (aged 22.1 ± 3.8 years) in cross-over design | 2-h cycle at 65%VO2max | 6.4% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose; ↓ free fatty acids; ↓ IL-6 vs. placebo trial. | |
| Henson et al.156 | 48 male and female marathon runners (aged 42.5 ± 2.4 years) randomized to CHO or placebo | 42-km marathon | 6% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose, insulin; ↓ cortisol; ↓ total leukocytes, neutrophils, and monocytes vs. placebo group. | |
| Davison and Gleeson157 | 6 moderately trained men (aged 25.0 ± 2.0 years) in a cross-over design | 2.5-h cycle at 60%VO2max | 6% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose; ↓ cortisol; ↓ ACTH; ↓ total leukocytes, neutrophils; ↑ bacterial-stimulated neutrophil degranulation vs. placebo trial. | |
| Lancaster et al.158 | 7 moderately trained men (aged 25.0 ± 1.0 years) in a cross-over design | 2.5-h cycle at 65%VO2max | 6.4%, 12.8% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose; ↓ cortisol; ↓ growth hormone; ↓ total leukocytes, neutrophils, monocytes; ↓ CD4+ T cell IFN-γ and CD8+ T cell IFN-γ lymphocytes. No significant difference between CHO concentrations. | |
| Li and Gleeson159 | 9 men (aged 28.7 ± 1.6 years) in a cross-over design | 90-min cycle at 60%VO2max | 10% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose; ↓ cortisol, epinephrine, ACTH, growth hormone; ↓ total leukocytes, monocytes, lymphocytes; ↓ IL-6 vs. placebo trial. | |
| Nieman et al.160 | 15 trained male cyclists (aged 29.2 ± 6.0 years) in a cross-over design | 2.5-h cycle at 75%VO2max | 6% CHO or placebo beverage consumed before, during, and after exercise | CHO ↑ glucose, insulin; ↓ cortisol, epinephrine; ↓ total leukocytes, neutrophils; ↓ IL-6, IL-10, IL-1ra vs. placebo trial. | |
| Nieman et al.161 | 12 trained male cyclists (aged 21.0 ± 1.0 years) in a cross-over design | 2-h cycle at 75%VO2max | 6% CHO or placebo beverage consumed before and during exercise | CHO ↑ glucose, insulin; ↓ cortisol; ↓ total leukocytes, neutrophils, and monocytes vs. placebo trial. | |
| Scharhag et al.162 | 14 trained male cyclists/triathletes (aged 25.0 ± 5.0 years) in a cross-over design | 4-h cycle at 70% anaerobic threshold | 6%, 12% CHO, or placebo beverage consumed before and during exercise | 6% and 12% CHO ↑ glucose; ↓ cortisol; ↓ total leukocytes, neutrophils, and monocytes vs. placebo trial. 12% CHO ↓ CRP and NK cells vs. placebo trial. | |
| Nieman et al.163 | 14 trained male cyclists (aged 37.0 ± 7.1 years) in a cross-over design | 75-km time trial | 6% CHO beverage or matched CHO banana consumed before and during exercise | No difference in immune and inflammation measures (e.g., IL-6, granulocyte and monocyte phagocytosis) between banana and CHO beverage; higher FRAP and plasma dopamine with banana. | |
| Nieman et al.164 | 20 trained male cyclists (aged 39.2 ± 1.9 years) in a cross-over design | 75-km time trial | Banana, pear, or water consumed before and during exercise | Banana and pear ↑ glucose, RER; ↓ cortisol, IL-10; ↓ neutrophil/ lymphocyte ratio; ↑ antioxidant capacity (sulfated phenolics, FRAP), ↓ fatty acid mobilization and oxidation metabolites vs. water trial. ↑ in fruit-specific metabolites. | |
| Shanely et al.165 | 20 trained male cyclists (aged 48.5 ± 2.3 years) in a cross-over design | 75-km time trial | 6% CHO beverage or matched CHO watermelon consumed before and during exercise | No difference in inflammation measures (e.g., cytokines and immune cell counts) between watermelon and CHO beverage; watermelon ↑ antioxidant capacity (FRAP, ORAC); ↑ citrulline, arginine, nitrate vs. CHO beverage. | |
| Nieman et al.31 | 20 trained male and female cyclists (aged 39.1 ± 2.4 years) in a cross-over design | 75-km time trial | 6% CHO beverage, 2 types of banana, or water consumed before and during exercise | Bananas and CHO beverage ↑ glucose, fructose; ↓ cortisol; ↓ IL-6, IL-10, IL-1ra; ↓ total leukocytes; ↓ 9+13 HODES; ↓ fatty acid mobilization and oxidation metabolites vs. water trial. Both banana trials ↓ COX2 mRNA expression; ↑ amino acid and xenobiotics metabolites. | |
Abbreviations: ACTH = adrenocorticotropic hormone; CHO = carbohydrate; COX2 = cyclo-oxygenase 2; CRP = C-reactive protein; FRAP = ferric reducing ability of plasma; HODES = hydroxyoctadecadienoic acid; IFN-γ = interferon gamma; IL-1ra = interleukin-1 receptor antagonist; mRNA = messenger ribonucleic acid; NK = natural killer; ORAC = oxygen radical absorbance capacity; RER = respiratory exchange ratio; VO2max = maximal oxygen uptake.
Fig. 8.
Carbohydrate ingestion before and during exercise attenuates postexercise inflammation.
4.2. Polyphenols counter exercise-induced immune changes
Fruits contain a mixture of sugars and a wide variety of biologically active polyphenols. Polyphenols, in particular flavonoids, have attracted much attention owing to their bioactivity and related health benefits, and new evidence using metabolomics supports their value as potential countermeasures to exercise-induced immune changes.6, 7,31, 33
Many of the earlier studies reported few discernable immune-related influences of increased polyphenol intake for athletes, but research design deficiencies portrayed a misunderstanding of polyphenol bioavailability and metabolism, and the appropriate outcome measures. Polyphenol absorption, disposition, metabolism, and excretion is complex and requires both untargeted and targeted metabolomics procedures to measure small molecule shifts in humans after increased intake.167 A high proportion of ingested polyphenols from fruits, vegetables, and other plant foods pass through the small intestine unabsorbed and reach the colon, where bacterial degradation produces smaller phenolics that can reabsorbed into the circulation after undergoing phase 2 conjugation in the liver.33 The biotransformed, gut-derived phenolics circulate throughout the body, exerting a variety of bioactive effects that are important to athletes including anti-inflammatory, antiviral, antioxidative, and immune cell signaling effects.167, 168, 169, 170, 171, 172
Several studies using metabolomics and ex vivo cell cultures comparing ingestion of bananas with intake of water only or a 6% sugar beverage during prolonged and intensive cycling have shown large-fold increases in at least 18 banana-related metabolites.31, 163,164 Banana flesh contains many unique molecules including serotonin, dopamine, phenolics, and xenobiotics. Soon after ingestion, plasma levels of metabolites derived from banana flesh molecules increase, and may confer anti-inflammatory effects by countering cyclooxygenase-2 (COX-2) mRNA expression the morning after heavy exertion.31
In general, evolving data support the intake of fruits such as dates, raisins, and bananas by athletes during training to provide the sugars and polyphenols that exert anti-inflammatory influences that may enhance metabolic recovery. Future studies using system-wide approaches such as metabolomics, lipidomics, and proteomics will improve scientific understanding regarding the complex and multilevel interactions between exercise, nutrition, and the immune and metabolic systems.
5. Exercise influences immunosenescence
Immunosenescence is defined as immune dysregulation with aging and is related to an increased susceptibility to infections, autoimmune diseases, neoplasias, metabolic diseases, osteoporosis, and neurologic disorders. Recent evidence supports that immunity can be remodeled during the aging process as a result of interactions with the environment and lifestyle and is instrumental in shaping immune status in later life.173, 174, 175 Immune system interactions with pathogens, the host microbiome, nutritional and exercise influences, mental stress, and many other extrinsic factors are considered as crucial modulators of the immunosenescence process.
Early cross-sectional studies compared immune function in highly conditioned and sedentary elderly men and women.31, 176 One study contrasted immune function in 30 sedentary elderly women and 12 age-matched, highly conditioned elderly women who were active in state and national senior game and road race endurance events.31 The highly conditioned elderly women had significantly higher levels of NK cells and T-lymphocyte function and reduced illness rates compared with the 30 sedentary elderly women. Another study compared immune function in 17 elderly runners who had trained for about 17 years and 19 elderly controls, and reported significantly higher T lymphocyte function in the elderly runners.176
These studies stimulated many additional studies on the effects of exercise training on immunosenescence. Data support that habitual exercise is capable of regulating the immune system and delaying the onset of immunosenescence, and has been associated with the following:31, 175,177, 178, 179
-
•
Enhanced vaccination responses,
-
•
Lower numbers of exhausted/senescent T cells,
-
•
Increased T-cell proliferative capacity,
-
•
Lower circulatory levels of inflammatory cytokines (i.e., decreased “inflamm-aging”),
-
•
Increased neutrophil phagocytic activity,
-
•
Lowered inflammatory response to bacterial challenge,
-
•
Greater NK cell cytotoxic activity, and
-
•
Longer leukocyte telomere lengths.
6. Conclusion
This review summarized research discoveries within 4 areas of exercise immunology: acute and chronic effects of exercise on the immune system, clinical benefits of the exercise–immune relationship, nutritional influences on the immune response to exercise, and the exercise effect on immunosenescence. The immune system is very responsive to exercise, with the extent and duration reflecting the degree of physiological stress imposed by the workload. Key exercise immunology discoveries since 1980 include the following.
-
•
Acute exercise (moderate-to-vigorous intensity, less than 60 min) is now viewed as an important immune system adjuvant to stimulate the ongoing exchange of distinct and highly active immune cell subtypes between the circulation and tissues. In particular, each exercise bout improves the antipathogen activity of tissue macrophages in parallel with an enhanced recirculation of immunoglobulins, anti-inflammatory cytokines, neutrophils, NK cells, cytotoxic T cells, and immature B cells. With near daily exercise, these acute changes operate through a summation effect to enhance immune defense activity and metabolic health.
-
•
In contrast, high exercise training workloads, competition events, and the associated physiological, metabolic, and psychological stress are linked with transient immune perturbations, inflammation, oxidative stress, muscle damage, and increased illness risk. Metabolomics, proteomics, and lipidomics have revealed that metabolism and immunity are inextricably interwoven, providing new insights on how intense and prolonged exercise can cause transient immune dysfunction by decreasing immune cell metabolic capacity.
-
•
Illness risk may be increased when an athlete competes, goes through repeated cycles of unusually heavy exertion, and experiences other stressors to the immune system. The wealth of acute illness epidemiologic data collected during international competition events has revealed that 2%–18% of elite athletes experience illness episodes, with higher proportions for females and those engaging in endurance events. Other illness risk factors include high levels of depression or anxiety, participation in unusually intensive training periods with large fluctuations, international travel across several time zones, participation in competitive events especially during the winter, lack of sleep, and low diet energy intake.
-
•
The IOC has also focused on load management of both internal (e.g., psychological responses) and external factors (e.g., training and competition workloads), and lifestyle strategies (e.g., hygiene, nutritional support, vaccination, regular sleep) to reduce illness incidence and associated downturns in exercise performance, interruptions in training, missed competitive events, and risk of serious medical complications.
-
•
Randomized clinical trials and epidemiologic studies consistently support the inverse relationship between moderate exercise training and incidence of URTI. These data led to the development of the J-curve model that links URTI risk with the exercise workload continuum. Several epidemiologic studies also suggest that regular physical activity is associated with decreased mortality and incidence rates for influenza and pneumonia.
-
•
Regular exercise training has an overall anti-inflammatory influence mediated through multiple pathways. Epidemiologic studies consistently show decreased levels of inflammatory biomarkers in adults with higher levels of physical activity and fitness, even after adjustment for potential confounders such as BMI.
-
•
There is increasing evidence that the circulation surge in cells of the innate immune system with each exercise bout and the anti-inflammatory and antioxidant effect of exercise training have a summation effect over time in modulating tumorigenesis, atherosclerosis, and other disease processes.
-
•
Recent studies indicate that exercise and physical fitness diversifies the gut microbiota, but more human research is needed to determine potential linkages to immune function in physically fit individuals and athletes.
-
•
The most effective nutritional strategies for athletes, especially when evaluated from a multiomics perspective, include increased intake of carbohydrates and polyphenols. A consistent finding is that carbohydrate intake during prolonged and intense exercise, whether from 6%–8% beverages or sugar-dense fruits such as bananas is associated with reduced stress hormones, diminished blood levels of neutrophils and monocytes, and dampened inflammation. Gut-derived phenolics circulate throughout the body after increased polyphenol intake, exerting a variety of bioactive effects that are important to athletes including anti-inflammatory, antiviral, antioxidative, and immune cell signaling effects.
-
•
Immunosenescence is defined as immune dysregulation with aging. Emergent data support that habitual exercise is capable of improving regulation of the immune system and delaying the onset of immunosenescence.
The future of exercise immunology will take advantage of advances in mass spectrometry and genetic testing technology, with increased utilization of metabolomics, proteomics, lipidomics, microbiome characterization, and genomics. Use of these system-wide approaches will provide new insights on the interactions between exercise, nutrition, and immune function, with application down to the personalized level. Additionally, these methodologies will improve mechanistic understanding of how exercise-induced immune changes reduce risk for common chronic diseases.
Acknowledgments
Authors' contributions
DCN and LMW conducted the literature review and wrote the manuscript. Both authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
Both authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Van Dijk J.G., Matson K.D. Ecological immunology through the lens of exercise immunology: new perspective on the links between physical activity and immune function and disease susceptibility in wild animals. Integr Comp Biol. 2016;56:290–303. doi: 10.1093/icb/icw045. [DOI] [PubMed] [Google Scholar]
- 2.Larrabee R.C. Leukocytosis after violent exercise. J Med Res (NS) 1902;7:76–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Walsh N.P., Gleeson M., Shephard R.J., Gleeson M., Woods J.A., Bishop N.C. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 4.Walsh N.P., Gleeson M., Pyne D.B., Nieman D.C., Dhabhar F.S., Shephard R.J. Position statement. Part two: maintaining immune health. Exerc Immunol Rev. 2011;17:64–103. [PubMed] [Google Scholar]
- 5.Shephard R.J. Development of the discipline of exercise immunology. Exerc Immunol Rev. 2010;16:194–222. [PubMed] [Google Scholar]
- 6.Bermon S., Castell L.M., Calder P.C., Bishop N.C., Blomstrand E., Mooren F.C. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 7.Nieman D.C., Mitmesser S.H. Potential impact of nutrition on immune system recovery from heavy exertion: a metabolomics perspective. Nutrients. 2017;9 doi: 10.3390/nu9050513. pii: E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon H.B. The immunology of exercise: a brief review. JAMA. 1984;252:2735–2738. [PubMed] [Google Scholar]
- 9.Mackinnon L.T. Changes in some cellular immune parameters following exercise training. Med Sci Sports Exerc. 1986;18:596–597. [PubMed] [Google Scholar]
- 10.Mackinnon L.T., Chick T.W., van As A., Tomasi T.B. The effect of exercise on secretory and natural immunity. Adv Exp Med Biol. 1987;216A:869–876. doi: 10.1007/978-1-4684-5344-7_102. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman-Goetz L., Keir R., Thorne R., Houston M.E., Young C. Chronic exercise stress in mice depresses splenic T lymphocyte mitogenesis in vitro. Clin Exp Immunol. 1986;66:551–557. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman-Goetz L., Thorne R.J., Houston M.E. Splenic immune responses following treadmill exercise in mice. Can J Physiol Pharmacol. 1988;66:1415–1419. doi: 10.1139/y88-230. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen B.K., Tvede N., Hansen F.R., Andersen V., Bendix T., Bendixen G. Modulation of natural killer cell activity in peripheral blood by physical exercise. Scand J Immunol. 1988;27:673–678. doi: 10.1111/j.1365-3083.1988.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 14.Tvede N., Pedersen B.K., Hansen F.R., Bendix T., Christensen L.D., Galbo H. Effect of physical exercise on blood mononuclear cell subpopulations and in vitro proliferative responses. Scand J Immunol. 1989;29:383–389. doi: 10.1111/j.1365-3083.1989.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 15.Nieman D.C., Tan S.A., Lee J.W., Berk L.S. Complement and immunoglobulin levels in athletes and sedentary controls. Int J Sports Med. 1989;10:124–128. doi: 10.1055/s-2007-1024887. [DOI] [PubMed] [Google Scholar]
- 16.Nieman D.C., Johanssen L.M., Lee J.W. Infectious episodes in runners before and after a roadrace. J Sports Med Phys Fitness. 1989;29:289–296. [PubMed] [Google Scholar]
- 17.Nieman D.C., Johanssen L.M., Lee J.W., Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- 18.Cannon J.G., Kluger M.J. Endogenous pyrogen activity in human plasma after exercise. Science. 1983;220:617–619. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- 19.Peters E.M., Goetzsche J.M., Grobbelaar B., Noakes T.D. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr. 1993;57:170–174. doi: 10.1093/ajcn/57.2.170. [DOI] [PubMed] [Google Scholar]
- 20.Nehlsen-Cannarella S.L., Fagoaga O.R., Nieman D.C., Henson D.A., Butterworth D.E., Schmitt R.L. Carbohydrate and the cytokine response to 2.5 h of running. J Appl Physiol (1985) 1997;82:1662–1667. doi: 10.1152/jappl.1997.82.5.1662. [DOI] [PubMed] [Google Scholar]
- 21.Nieman D.C., Henson D.A., Gusewitch G., Warren B.J., Dotson R.C., Butterworth D.E. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Nieman D.C., Henson D.A. Role of endurance exercise in immune senescence. Med Sci Sports Exerc. 1994;26:172–181. doi: 10.1249/00005768-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Shinkai S., Konishi M., Shephard R.J. Aging, exercise, training, and the immune system. Exerc Immunol Rev. 1997;3:68–95. [PubMed] [Google Scholar]
- 24.Northoff H., Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int J Sports Med. 1991;12(Suppl. 1):S9–15. doi: 10.1055/s-2007-1024743. [DOI] [PubMed] [Google Scholar]
- 25.Cannon J.G., Meydani S.N., Fielding R.A., Fiatarone M.A., Meydani M., Farhangmehr M. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am J Physiol. 1991;260:R1235–R1240. doi: 10.1152/ajpregu.1991.260.6.R1235. [DOI] [PubMed] [Google Scholar]
- 26.Rohde T., MacLean D.A., Richter E.A., Kiens B., Pedersen B.K. Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL-6. Am J Physiol. 1997;273:E85–E91. doi: 10.1152/ajpendo.1997.273.1.E85. [DOI] [PubMed] [Google Scholar]
- 27.Nieman D.C. Immune response to heavy exertion. J Appl Physiol (1985) 1997;82:1385–1394. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- 28.Colbey C., Cox A.J., Pyne D.B., Zhang P., Cripps A.W., West N.P. Upper respiratory symptoms, gut health and mucosal immunity in athletes. Sports Med. 2018;48(Suppl. 1):S65–S77. doi: 10.1007/s40279-017-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markworth J.F., Vella L., Lingard B.S., Tull D.L., Rupasinghe T.W., Sinclair A.J. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1281–R1296. doi: 10.1152/ajpregu.00128.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieman D.C., Groen A.J., Pugachev A., Vacca G. Detection of functional overreaching in endurance athletes using proteomics. Proteomes. 2018;6 doi: 10.3390/proteomes6030033. pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieman D.C., Gillitt N.D., Sha W., Esposito D., Ramamoorthy S. Metabolic recovery from heavy exertion following banana compared to sugar beverage or water only ingestion: a randomized, crossover trial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieman D.C., Gillitt N.D., Sha W. Identification of a targeted metabolomics panel for measuring metabolic perturbation in response to heavy exertion. Metabolomics. 2018;14:147. doi: 10.1007/s11306-018-1444-7. [DOI] [PubMed] [Google Scholar]
- 33.Nieman D.C., Lila M.A., Gillitt N.D. Immunometabolism: a multi-omics approach to interpreting the influence of exercise and diet on the immune system. Ann Rev Food Sci Tech. 2019;10:341–363. doi: 10.1146/annurev-food-032818-121316. [DOI] [PubMed] [Google Scholar]
- 34.Koelwyn G.J., Wennerberg E., Demaria S., Jones L.W. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology (Williston Park) 2015;29:908–920. [PMC free article] [PubMed] [Google Scholar]
- 35.Bigley A.B., Spielmann G., LaVoy E.C., Simpson R.J. Can exercise-related improvements in immunity influence cancer prevention and prognosis in the elderly? Maturitas. 2013;76:51–56. doi: 10.1016/j.maturitas.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Antunes B.M., Cayres S.U., Lira F.S., Fernandes R.A. Arterial thickness and immunometabolism: the mediating role of chronic exercise. Curr Cardiol Rev. 2016;12:47–51. doi: 10.2174/1573403X12666160126115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams G.R., Zaldivar F.P., Nance D.M., Kodesh E., Radom-Aizik S., Cooper D.M. Exercise and leukocyte interchange among central circulation, lung, spleen, and muscle. Brain Behav Immun. 2011;25:658–666. doi: 10.1016/j.bbi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bigley A.B., Rezvani K., Chew C., Sekine T., Pistillo M., Crucian B. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160–171. doi: 10.1016/j.bbi.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Gupta P., Bigley A.B., Markofski M., Laughlin M., LaVoy E.C. Autologous serum collected 1 h post-exercise enhances natural killer cell cytotoxicity. Brain Behav Immun. 2018;71:81–92. doi: 10.1016/j.bbi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Nieman D.C., Henson D.A., Austin M.D., Brown V.A. Immune response to a 30-minute walk. Med Sci Sports Exerc. 2005;37:57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- 41.Viana J.L., Kosmadakis G.C., Watson E.L., Bevington A., Feehally J., Bishop N.C. Evidence for anti-inflammatory effects of exercise in CKD. J Am Soc Nephrol. 2014;25:2121–2130. doi: 10.1681/ASN.2013070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans E.S., Hackney A.C., McMurray R.G., Randell S.H., Muss H.B., Deal A.M. Impact of acute intermittent exercise on natural killer cells in breast cancer survivors. Integr Cancer Ther. 2015;14:436–445. doi: 10.1177/1534735415580681. [DOI] [PubMed] [Google Scholar]
- 43.Ferrandi P.J., Fico B.G., Whitehurst M., Zourdos M.C., Bao F., Dodge K.M. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and interleukin-6 in obese and normal-weight individuals. Life Sci. 2018;202:161–166. doi: 10.1016/j.lfs.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Simpson R.J., Kunz H., Agha N., Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Simpson R.J., Bigley A.B., Agha N., Hanley P.J., Bollard C.M. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc Sport Sci Rev. 2017;45:163–172. doi: 10.1249/JES.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaVoy E.C., Bollard C.M., Hanley P.J., Blaney J.W., O'Connor D.P., Bosch J.A. A single bout of dynamic exercise enhances the expansion of MAGE-A4 and PRAME-specific cytotoxic T-cells from healthy adults. Exerc Immunol Rev. 2015;21:144–153. [PubMed] [Google Scholar]
- 47.Turner J.E., Spielmann G., Wadley A.J., Aldred S., Simpson R.J., Campbell J.P. Exercise-induced B cell mobilization: preliminary evidence for an influx of immature cells into the bloodstream. Physiol Behav. 2016;164:376–382. doi: 10.1016/j.physbeh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Campbell J.P., Riddell N.E., Burns V.E., Turner M., van Zanten J.J., Drayson M.T. Acute exercise mobilizes CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Karstoft K., Pedersen B.K. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146–150. doi: 10.1038/icb.2015.101. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen B.K. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47:600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- 51.Grande A.J., Reid H., Thomas E.E., Nunan D., Foster C. Exercise prior to influenza vaccination for limiting influenza incidence and its related complications in adults. Cochrane Database Syst Rev. 2016;22 doi: 10.1002/14651858.CD011857.pub2. CD011857.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peake J.M., Della Gatta P., Suzuki K., Nieman D.C. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. [PubMed] [Google Scholar]
- 53.Peake J.M., Neubauer O., Della Gatta P.A., Nosaka K. Muscle damage and inflammation during recovery from exercise. J Appl Physiol (1985) 2017;122:559–570. doi: 10.1152/japplphysiol.00971.2016. [DOI] [PubMed] [Google Scholar]
- 54.Peake J.M., Neubauer O., Walsh N.P., Simpson R.J. Recovery of the immune system after exercise. J Appl Physiol (1985) 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 55.Simpson R.J., Kunz H., Agha N., Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen B.K., Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- 57.Siedlik J.A., Benedict S.H., Landes E.J., Weir J.P., Vardiman J.P., Gallagher P.M. Acute bouts of exercise induce a suppressive effect on lymphocyte proliferation in human subjects: a meta-analysis. Brain Behav Immun. 2016;56:343–351. doi: 10.1016/j.bbi.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Campbell J.P., Turner J.E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balfoussia E., Skenderi K., Tsironi M., Anagnostopoulos A.K., Parthimos N., Vougas K. A proteomic study of plasma protein changes under extreme physical stress. J Proteomics. 2014;98:1–14. doi: 10.1016/j.jprot.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Markworth J.F., Maddipati K.R., Cameron-Smith D. Emerging roles of pro-resolving lipid mediators in immunological and adaptive responses to exercise-induced muscle injury. Exerc Immunol Rev. 2016;22:110–134. [PubMed] [Google Scholar]
- 61.Nieman D.C., Shanely R.A., Gillitt N.D., Pappan K.L., Lila M.A. Serum metabolic signatures induced by a three-day intensified exercise period persist after 14 h of recovery in runners. J Proteome Res. 2013;12:4577–4584. doi: 10.1021/pr400717j. [DOI] [PubMed] [Google Scholar]
- 62.Nieman D.C., Shanely R.A., Luo B., Meaney M.P., Dew D.A., Pappan K.L. Metabolomics approach to assessing plasma 13- and 9-hydroxy-octadecadienoic acid and linoleic acid metabolite responses to 75-km cycling. Am J Physiol Regul Integr Comp Physiol. 2014;307:R68–R74. doi: 10.1152/ajpregu.00092.2014. [DOI] [PubMed] [Google Scholar]
- 63.Whitham M., Parker B.L., Friedrichsen M., Hingst J.R., Hjorth M., Hughes W.E. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27:237–251. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Hotamisligil G.S. Foundations of immunometabolism and implications for metabolic health and disease. Immunity. 2017;47:406–420. doi: 10.1016/j.immuni.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwellnus M., Soligard T., Alonso J.M., Bahr R., Clarsen B., Dijkstra H.P. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br J Sports Med. 2016;50:1043–1052. doi: 10.1136/bjsports-2016-096572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meeusen R., Duclos M., Foster C., Fry A., Gleeson M., Nieman D. European College of Sport Science; American College of Sports Medicine. Prevention, diagnosis, and treatment of the overtraining syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine. Med Sci Sports Exerc. 2013;45:186–205. doi: 10.1249/MSS.0b013e318279a10a. [DOI] [PubMed] [Google Scholar]
- 67.Peters E.M., Bateman E.D. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- 68.Nieman D.C. Exercise immunology: practical applications. Int J Sports Med. 1997;18(Suppl. 1):S91–100. doi: 10.1055/s-2007-972705. [DOI] [PubMed] [Google Scholar]
- 69.Heath G.W., Ford E.S., Craven T.E., Macera C.A., Jackson K.L., Pate R.R. Exercise and the incidence of upper respiratory tract infections. Med Sci Sports Exerc. 1991;23:152–157. [PubMed] [Google Scholar]
- 70.Konig D., Grathwohl D., Weinstock C., Northoff H., Berg A. Upper respiratory tract infection in athletes: influence of lifestyle, type of sport, training effort, and immunostimulant intake. Exerc Immunol Rev. 2000;6:102–120. [PubMed] [Google Scholar]
- 71.Spence L., Brown W.J., Pyne D.B., Nissen M.D., Sloots T.P., McCormack J.G. Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med Sci Sports Exerc. 2007;39:577–586. doi: 10.1249/mss.0b013e31802e851a. [DOI] [PubMed] [Google Scholar]
- 72.Gleeson M., Bishop N., Oliveira M., Tauler P. Influence of training load on upper respiratory tract infection incidence and antigen-stimulated cytokine production. Scand J Med Sci Sports. 2013;23:451–457. doi: 10.1111/j.1600-0838.2011.01422.x. [DOI] [PubMed] [Google Scholar]
- 73.Rama L., Teixeira A.M., Matos A., Borges G., Henriques A., Gleeson M. Changes in natural killer cell subpopulations over a winter training season in elite swimmers. Eur J Appl Physiol. 2013;113:859–868. doi: 10.1007/s00421-012-2490-x. [DOI] [PubMed] [Google Scholar]
- 74.Hellard P., Avalos M., Guimaraes F., Toussaint J.F., Pyne D.B. Training-related risk of common illnesses in elite swimmers over a 4-yr period. Med Sci Sports Exerc. 2015;47:698–707. doi: 10.1249/MSS.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 75.Svendsen I.S., Gleeson M., Haugen T.A., Tønnessen E. Effect of an intense period of competition on race performance and self-reported illness in elite cross-country skiers. Scand J Med Sci Sports. 2015;25:846–853. doi: 10.1111/sms.12452. [DOI] [PubMed] [Google Scholar]
- 76.Svendsen I.S., Taylor I.M., Tønnessen E., Bahr R., Gleeson M. Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br J Sports Med. 2016;50:809–815. doi: 10.1136/bjsports-2015-095398. [DOI] [PubMed] [Google Scholar]
- 77.Raysmith B.P., Drew M.K. Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: a 5-year prospective study. J Sci Med Sport. 2016;19:778–783. doi: 10.1016/j.jsams.2015.12.515. [DOI] [PubMed] [Google Scholar]
- 78.Drew M., Vlahovich N., Hughes D., Appaneal R., Burke L.M., Lundy B. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br J Sports Med. 2018;52:47–53. doi: 10.1136/bjsports-2017-098208. [DOI] [PubMed] [Google Scholar]
- 79.Prien A., Mountjoy M., Miller J., Boyd K., van den Hoogenband C., Gerrard D. Injury and illness in aquatic sport: how high is the risk? A comparison of results from three FINA World Championships. Br J Sports Med. 2017;51:277–282. doi: 10.1136/bjsports-2016-096075. [DOI] [PubMed] [Google Scholar]
- 80.Timpka T., Jacobsson J., Bargoria V., Périard J.D., Racinais S., Ronsen O. Preparticipation predictors for championship injury and illness: cohort study at the Beijing 2015 International Association of Athletics Federations World Championships. Br J Sports Med. 2017;51:271–276. doi: 10.1136/bjsports-2016-096580. [DOI] [PubMed] [Google Scholar]
- 81.Engebretsen L., Steffen K., Alonso J.M., Aubry M., Dvorak J., Junge A. Sports injuries and illnesses during the Winter Olympic Games 2010. Br J Sports Med. 2010;44:772–780. doi: 10.1136/bjsm.2010.076992. [DOI] [PubMed] [Google Scholar]
- 82.Engebretsen L., Soligard T., Steffen K., Alonso J.M., Aubry M., Budgett R. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br J Sports Med. 2013;47:407–414. doi: 10.1136/bjsports-2013-092380. [DOI] [PubMed] [Google Scholar]
- 83.Palmer-Green D., Elliott N. Sports injury and illness epidemiology: Great Britain Olympic Team (TeamGB) surveillance during the Sochi 2014 Winter Olympic Games. Br J Sports Med. 2015;49:25–29. doi: 10.1136/bjsports-2014-094206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soligard T., Steffen K., Palmer-Green D., Aubry M., Grant M.E., Meeuwisse W. Sports injuries and illnesses in the Sochi 2014 Olympic Winter Games. Br J Sports Med. 2015;49:441–447. doi: 10.1136/bjsports-2014-094538. [DOI] [PubMed] [Google Scholar]
- 85.Soligard T., Steffen K., Palmer D., Alonso J.M., Bahr R., Lopes A.D. Sports injury and illness incidence in the Rio de Janeiro 2016 Olympic Summer Games: a prospective study of 11274 athletes from 207 countries. Br J Sports Med. 2017;51:1265–1271. doi: 10.1136/bjsports-2017-097956. [DOI] [PubMed] [Google Scholar]
- 86.Mountjoy M., Junge A., Alonso J.M., Engebretsen L., Dragan I., Gerrard D. Sports injuries and illnesses in the 2009 FINA World Championships (Aquatics) Br J Sports Med. 2010;44:522–527. doi: 10.1136/bjsm.2010.071720. [DOI] [PubMed] [Google Scholar]
- 87.Mountjoy M., Junge A., Benjamen S., Boyd K., Diop M., Gerrard D. Competing with injuries: injuries prior to and during the 15th FINA World Championships 2013 (aquatics) Br J Sports Med. 2015;49:37–43. doi: 10.1136/bjsports-2014-093991. [DOI] [PubMed] [Google Scholar]
- 88.Alonso J.M., Tscholl P.M., Engebretsen L., Mountjoy M., Dvorak J., Junge A. Occurrence of injuries and illnesses during the 2009 IAAF World Athletics Championships. Br J Sports Med. 2010;44:1100–1105. doi: 10.1136/bjsm.2010.078030. [DOI] [PubMed] [Google Scholar]
- 89.Alonso J.M., Edouard P., Fischetto G., Adams B., Depiesse F., Mountjoy M. Determination of future prevention strategies in elite track and field: analysis of Daegu 2011 IAAF Championships injuries and illnesses surveillance. Br J Sports Med. 2012;46:505–514. doi: 10.1136/bjsports-2012-091008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wentz L.M., Ward M.D., Potter C., Oliver S.J., Jackson S., Izard R.M. Increased risk of upper respiratory infection in military recruits who report sleeping less than 6 hour per night. Mil Med. 2018;183:e699–e704. doi: 10.1093/milmed/usy090. [DOI] [PubMed] [Google Scholar]
- 91.Drew M.K., Vlahovich N., Hughes D., Appaneal R., Peterson K., Burke L. A multifactorial evaluation of illness risk factors in athletes preparing for the Summer Olympic Games. J Sci Med Sport. 2017;20:745–750. doi: 10.1016/j.jsams.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Van Tonder A., Schwellnus M., Swanevelder S., Jordaan E., Derman W., Janse van Rensburg D.C. A prospective cohort study of 7031 distance runners shows that 1 in 13 report systemic symptoms of an acute illness in the 8-12 day period before a race, increasing their risk of not finishing the race 1.9 times for those runners who started the race: SAFER study IV. Br J Sports Med. 2016;50:939–945. doi: 10.1136/bjsports-2016-096190. [DOI] [PubMed] [Google Scholar]
- 93.Gordon L., Schwellnus M., Swanevelder S., Jordaan E., Derman W. Recent acute prerace systemic illness in runners increases the risk of not finishing the race: SAFER study V. Br J Sports Med. 2017;51:1295–1300. doi: 10.1136/bjsports-2016-096964. [DOI] [PubMed] [Google Scholar]
- 94.Janse Van Rensburg D.C., Schwellnus M., Derman W., Webborn N. Illness among Paralympic athletes: epidemiology, risk markers, and preventative strategies. Phys Med Rehabil Clin N Am. 2018;29:185–203. doi: 10.1016/j.pmr.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Nieman D.C. Is infection risk linked to exercise workload? Med Sci Sports Exerc. 2000;32(Suppl. 7):S406–S411. doi: 10.1097/00005768-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 96.Nieman D.C., Nehlsen-Cannarella S.L., Markoff P.A., Balk-Lamberton A.J., Yang H., Chritton D.B. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 97.Nieman D.C., Nehlsen-Cannarella S.L., Henson D.A., Koch A.J., Butterworth D.E., Fagoaga O.R. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 98.Chubak J., McTiernan A., Sorensen B., Wener M.H., Yasui Y., Velasquez M. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119:937–942. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 99.Barrett B., Hayney M.S., Muller D., Rakel D., Ward A., Obasi C.N. Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial. Ann Fam Med. 2012;10:337–346. doi: 10.1370/afm.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barrett B., Hayney M.S., Muller D., Rakel D., Brown R., Zgierska A.E. Meditation or exercise for preventing acute respiratory infection (MEPARI-2): a randomized controlled trial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matthews C.E., Ockene I.S., Freedson P.S., Rosal M.C., Merriam P.A., Hebert J.R. Moderate to vigorous physical activity and risk of upper-respiratory tract infection. Med Sci Sports Exerc. 2002;34:1242–1248. doi: 10.1097/00005768-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Fondell E., Lagerros Y.T., Sundberg C.J., Lekander M., Bälter O., Rothman K.J. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc. 2011;43:272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 103.Nieman D.C., Henson D.A., Austin M.D., Sha W. Upper respiratory tract infection is reduced in physically fit and active adults. Br J Sports Med. 2011;45:987–992. doi: 10.1136/bjsm.2010.077875. [DOI] [PubMed] [Google Scholar]
- 104.Zhou G., Liu H., He M., Yue M., Gong P., Wu F. Smoking, leisure-time exercise and frequency of self-reported common cold among the general population in northeastern China: a cross-sectional study. BMC Public Health. 2018;18:294. doi: 10.1186/s12889-018-5203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barrett B., Brown R., Mundt M., Safdar N., Dye L., Maberry R. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol. 2005;58:609–617. doi: 10.1016/j.jclinepi.2004.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams P.T. Reduced total and cause-specific mortality from walking and running in diabetes. Med Sci Sports Exerc. 2014;46:933–939. doi: 10.1249/MSS.0000000000000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Williams P.T. Dose-response relationship between exercise and respiratory disease mortality. Med Sci Sports Exerc. 2014;46:711–717. doi: 10.1249/MSS.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu S., Ma C., Yang Z., Yang P., Chu Y., Zhang H. Hygiene behaviors associated with influenza-like illness among adults in Beijing, China: a large, population-based survey. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wong C.M., Lai H.K., Ou C.Q., Ho S.Y., Chan K.P., Thach T.Q. Is exercise protective against influenza-associated mortality? PLoS One. 2008;3:e2108. doi: 10.1371/journal.pone.0002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sim Y.J., Yu S., Yoon K.J., Loiacono C.M., Kohut M.L. Chronic exercise reduces illness severity, decreases viral load, and results in greater anti-inflammatory effects than acute exercise during influenza infection. J Infect Dis. 2009;200:1434–1442. doi: 10.1086/606014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warren K.J., Olson M.M., Thompson N.J., Cahill M.L., Wyatt T.A., Yoon K.J. Exercise improves host response to influenza viral infection in obese and non-obese mice through different mechanisms. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stravinskas Durigon T., MacKenzie B., Carneiro Oliveira-Junior M., Santos-Dias A., De Angelis K., Malfitano C. Aerobic exercise protects from Pseudomonas aeruginosa-induced pneumonia in elderly mice. J Innate Immun. 2018;10:279–290. doi: 10.1159/000488953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olivo C.R., Miyaji E.N., Oliveira M.L., Almeida F.M., Lourenço J.D., Abreu R.M. Aerobic exercise attenuates pulmonary inflammation induced by Streptococcus pneumoniae. J Appl Physiol (1985) 2014;117:998–1007. doi: 10.1152/japplphysiol.00290.2014. [DOI] [PubMed] [Google Scholar]
- 114.Parker S., Brukner P., Rosier M. Chronic fatigue syndrome and the athlete. Sport Med Train Rehab. 1996;6:269–278. [Google Scholar]
- 115.Folsom R.W., Littlefield-Chabaud M.A., French D.D., Pourciau S.S., Mistric L., Horohov D.W. Exercise alters the immune response to equine influenza virus and increases susceptibility to infection. Equine Vet J. 2001;33:664–669. doi: 10.2746/042516401776249417. [DOI] [PubMed] [Google Scholar]
- 116.Roberts J.A. Viral illnesses and sports performance. Sports Med. 1986;3:298–303. doi: 10.2165/00007256-198603040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kohut M.L., Arntson B.A., Lee W., Rozeboom K., Yoon K.J., Cunnick J.E. Moderate exercise improves antibody response to influenza immunization in older adults. Vaccine. 2004;22:2298–2306. doi: 10.1016/j.vaccine.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 118.de Araújo A.L., Silva L.C., Fernandes J.R., Matias Mde S., Boas L.S., Machado C.M. Elderly men with moderate and intense training lifestyle present sustained higher antibody responses to influenza vaccine. Age (Dordr) 2015;37:105. doi: 10.1007/s11357-015-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kohut M.L., Cooper M.M., Nickolaus M.S., Russell D.R., Cunnick J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 120.Ringseis R., Eder K., Mooren F.C., Krüger K. Metabolic signals and innate immune activation in obesity and exercise. Exerc Immunol Rev. 2015;21:58–68. [PubMed] [Google Scholar]
- 121.Beavers K.M., Beavers D.P., Newman J.J., Anderson A.M., Loeser R.F., Nicklas B.J. Effects of total and regional fat loss on plasma CRP and IL-6 in overweight and obese, older adults with knee osteoarthritis. Osteoarthritis Cartilage. 2015;23:249–256. doi: 10.1016/j.joca.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rahmati M., Mobasheri A., Mozafari M. Inflammatory mediators in osteoarthritis: a critical review of the state-of-the-art, current prospects, and future challenges. Bone. 2016;85:81–90. doi: 10.1016/j.bone.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 123.Antunes B.M., Cayres S.U., Lira F.S., Fernandes R.A. Arterial thickness and immunometabolism: the mediating role of chronic exercise. Curr Cardiol Rev. 2016;12:47–51. doi: 10.2174/1573403X12666160126115317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Apostolopoulos V., de Courten M.P., Stojanovska L., Blatch G.L., Tangalakis K., de Courten B. The complex immunological and inflammatory network of adipose tissue in obesity. Mol Nutr Food Res. 2016;60:43–57. doi: 10.1002/mnfr.201500272. [DOI] [PubMed] [Google Scholar]
- 125.Kiecolt-Glaser J.K., Derry H.M., Fagundes C.P. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172:1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Akchurin O.M., Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 127.Rajendran P., Chen Y.F., Chen Y.F., Chung L.C., Tamilselvi S., Shen C.Y. The multifaceted link between inflammation and human diseases. J Cell Physiol. 2018;233:6458–6471. doi: 10.1002/jcp.26479. [DOI] [PubMed] [Google Scholar]
- 128.Guzik T.J., Skiba D.S., Touyz R.M., Harrison D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shanely R.A., Nieman D.C., Henson D.A., Jin F., Knab A.M., Sha W. Inflammation and oxidative stress are lower in physically fit and active adults. Scand J Med Sci Sports. 2013;23:215–223. doi: 10.1111/j.1600-0838.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- 130.Wedell-Neergaard A.S., Krogh-Madsen R., Petersen G.L., Hansen A.M., Pedersen B.K., Lund R. Cardiorespiratory fitness and the metabolic syndrome: roles of inflammation and abdominal obesity. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Parsons T.J., Sartini C., Welsh P., Sattar N., Ash S., Lennon L.T. Physical activity, sedentary behavior, and inflammatory and hemostatic markers in men. Med Sci Sports Exerc. 2017;49:459–465. doi: 10.1249/MSS.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Church T.S., Earnest C.P., Thompson A.M., Priest E.L., Rodarte R.Q., Saunders T. Exercise without weight loss does not reduce C-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42:708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arsenault B.J., Côté M., Cartier A., Lemieux I., Després J.P., Ross R. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese post-menopausal women with elevated blood pressure. Atherosclerosis. 2009;207:530–533. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.You T., Arsenis N.C., Disanzo B.L., Lamonte M.J. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43:243–256. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 135.Beavers K.M., Brinkley T.E., Nicklas B.J. Effect of exercise training on chronic inflammation. Clin Chim Acta. 2010;411:785–793. doi: 10.1016/j.cca.2010.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fedewa M.V., Hathaway E.D., Ward-Ritacco C.L. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomized and non-randomized controlled trials. Br J Sports Med. 2017;51:670–676. doi: 10.1136/bjsports-2016-095999. [DOI] [PubMed] [Google Scholar]
- 137.Lancaster G.I., Febbraio M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014;35:262–269. doi: 10.1016/j.it.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 138.Valacchi G., Virgili F., Cervellati C., Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Front Physiol. 2018;9:858. doi: 10.3389/fphys.2018.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Koelwyn G.J., Wennerberg E., Demaria S., Jones L.W. Exercise in regulation of inflammation-immune axis function in cancer initiation and progression. Oncology (Williston Park) 2015;29:908–922. [PMC free article] [PubMed] [Google Scholar]
- 140.Codella R., Luzi L., Terruzzi I. Exercise has the guts: how physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig Liver Dis. 2018;50:331–341. doi: 10.1016/j.dld.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 141.Rizzetto L., Fava F., Tuohy K.M., Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun. 2018;92:12–34. doi: 10.1016/j.jaut.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 142.Estaki M., Pither J., Baumeister P., Little J.P., Gill S.K., Ghosh S. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barton W., Penney N.C., Cronin O., Garcia-Perez I., Molloy M.G., Holmes E. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625–633. doi: 10.1136/gutjnl-2016-313627. [DOI] [PubMed] [Google Scholar]
- 144.Cook M.D., Allen J.M., Pence B.D., Wallig M.A., Gaskins H.R., White B.A. Exercise and gut immune function: evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol. 2016;94:158–163. doi: 10.1038/icb.2015.108. [DOI] [PubMed] [Google Scholar]
- 145.Bermon S., Petriz B., Kajėnienė A., Prestes J., Castell L., Franco O.L. The microbiota: an exercise immunology perspective. Exerc Immunol Rev. 2015;21:70–79. [PubMed] [Google Scholar]
- 146.Nieman D.C., Fagoaga O.R., Butterworth D.E., Warren B.J., Utter A., Davis J.M. Carbohydrate supplementation affects blood granulocyte and monocyte trafficking but not function after 2.5 h or running. Am J Clin Nutr. 1997;66:153–159. doi: 10.1093/ajcn/66.1.153. [DOI] [PubMed] [Google Scholar]
- 147.Henson D.A., Nieman D.C., Parker J.C., Rainwater M.K., Butterworth D.E., Warren B.J. Carbohydrate supplementation and the lymphocyte proliferative response to long endurance running. Int J Sports Med. 1998;19:574–580. doi: 10.1055/s-2007-971962. [DOI] [PubMed] [Google Scholar]
- 148.Nieman D.C., Nehlsen-Cannarella S.L., Fagoaga O.R., Henson D.A., Utter A., Davis J.M. Effects of mode and carbohydrate on the granulocyte and monocyte response to intensive, prolonged exercise. J Appl Physiol (1985) 1998;84:1252–1259. doi: 10.1152/jappl.1998.84.4.1252. [DOI] [PubMed] [Google Scholar]
- 149.Nieman D.C., Nehlsen-Cannarella S.L., Fagoaga O.R., Henson D.A., Utter A., Davis J.M. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med Sci Sports Exerc. 1998;30:671–678. doi: 10.1097/00005768-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 150.Henson D.A., Nieman D.C., Blodgett A.D., Butterworth D.E., Utter A., Davis J.M. Influence of exercise mode and carbohydrate on the immune response to prolonged exercise. Int J Sport Nutr. 1999;9:213–228. doi: 10.1123/ijsn.9.2.213. [DOI] [PubMed] [Google Scholar]
- 151.Henson D.A., Nieman D.C., Nehlsen-Cannarella S.L., Fagoaga O.R., Shannon M., Bolton M.R. Influence of carbohydrate on cytokine and phagocytic responses to 2 h of rowing. Med Sci Sports Exerc. 2000;32:1384–1389. doi: 10.1097/00005768-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 152.Nieman D.C., Davis J.M., Henson D.A., Walberg-Rankin J., Shute M., Dumke C.L. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985) 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- 153.Bishop N.C., Walsh N., Scanlon G.A. Effect of prolonged exercise and carbohydrate on total neutrophil elastase content. Med Sci Sports Exerc. 2003;35:1326–1332. doi: 10.1249/01.MSS.0000078927.08049.A8. [DOI] [PubMed] [Google Scholar]
- 154.Keller C., Keller P., Marshal S., Pedersen B.K. IL-6 gene expression in human adipose tissue in response to exercise−effect of carbohydrate ingestion. J Physiol. 2003;550:927–931. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Febbraio M.A., Steensberg A., Keller C., Starkie R.L., Nielsen H.B., Krustrup P. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol. 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Henson D.A., Nieman D.C., Pistilli E.E., Schilling B., Colacino A., Utter A.C. Influence of carbohydrate and age on lymphocyte function following a marathon. Int J Sport Nutr Exerc Metab. 2004;14:308–322. doi: 10.1123/ijsnem.14.3.308. [DOI] [PubMed] [Google Scholar]
- 157.Davison G., Gleeson M. Influence of acute vitamin C and/or carbohydrate ingestion on hormonal, cytokine, and immune responses to prolonged exercise. Int J Sport Nutr Exerc Metab. 2005;15:465–479. doi: 10.1123/ijsnem.15.5.465. [DOI] [PubMed] [Google Scholar]
- 158.Lancaster G.I., Khan Q., Drysdale P.T., Wallace F., Jeukendrup A.E., Drayson M.T. Effect of prolonged exercise and carbohydrate ingestion on type 1 and type 2 T lymphocyte distribution and intracellular cytokine production in humans. J Appl Physiol (1985) 2005;98:565–571. doi: 10.1152/japplphysiol.00754.2004. [DOI] [PubMed] [Google Scholar]
- 159.Li T.L., Gleeson M. The effects of carbohydrate supplementation during the second of two prolonged cycling bouts on immunoendocrine responses. Eur J Appl Physiol. 2005;95:391–399. doi: 10.1007/s00421-005-0024-5. [DOI] [PubMed] [Google Scholar]
- 160.Nieman D.C., Davis J.M., Henson D.A., Gross S.J., Dumke C.L., Utter A.C. Muscle cytokine mRNA changes after 2.5 h of cycling: influence of carbohydrate. Med Sci Sports Exerc. 2005;37:1283–1290. doi: 10.1249/01.mss.0000175054.99588.b1. [DOI] [PubMed] [Google Scholar]
- 161.Nieman D.C., Henson D.A., Gojanovich G., Davis J.M., Murphy E.A., Mayer E.P. Influence of carbohydrate on immune function following 2 h cycling. Res Sports Med. 2006;14:225–237. doi: 10.1080/15438620600854793. [DOI] [PubMed] [Google Scholar]
- 162.Scharhag J., Meyer T., Auracher M., Gabriel H.H., Kindermann W. Effects of graded carbohydrate supplementation on the immune response in cycling. Med Sci Sports Exerc. 2006;38:286–292. doi: 10.1249/01.mss.0000191437.69493.d4. [DOI] [PubMed] [Google Scholar]
- 163.Nieman D.C., Gillitt N.D., Henson D.A., Sha W., Shanely R.A., Knab A.M. Bananas as an energy source during exercise: a metabolomics approach. PLoS One. 2012;7:e37479. doi: 10.1371/journal.pone.0037479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nieman D.C., Gillitt N.D., Sha W., Meaney M.P., John C., Pappan K.L. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J Proteome Res. 2015;14:5367–5377. doi: 10.1021/acs.jproteome.5b00909. [DOI] [PubMed] [Google Scholar]
- 165.Shanely R.A., Nieman D.C., Perkins-Veazie P., Henson D.A., Meaney M.P., Knab A.M. Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant capacity. Nutrients. 2016;8:E518. doi: 10.3390/nu8080518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nieman D.C. Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc Immunol Rev. 1998;4:64–76. [PubMed] [Google Scholar]
- 167.Kay C.D., Pereira-Caro G., Ludwig I.A., Clifford M.N., Crozier A. Anthocyanins and flavanones are more bioavailable than previously perceived: a review of recent evidence. Annu Rev Food Sci Technol. 2017;8:155–180. doi: 10.1146/annurev-food-030216-025636. [DOI] [PubMed] [Google Scholar]
- 168.Ahmed M., Henson D.A., Sanderson M.C., Nieman D.C., Gillitt N.D., Lila M.A. The protective effects of a polyphenol-enriched protein powder on exercise-induced susceptibility to virus infection. Phytother Res. 2014;28:1829–1836. doi: 10.1002/ptr.5208. [DOI] [PubMed] [Google Scholar]
- 169.Amin H.P., Czank C., Raheem S., Zhang Q., Botting N.P., Cassidy A. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res. 2015;59:1095–1106. doi: 10.1002/mnfr.201400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Edwards M., Czank C., Woodward G.M., Cassidy A., Kay C.D. Phenolic metabolites of anthocyanins modulate mechanisms of endothelial function. J Agric Food Chem. 2015;63:2423–2431. doi: 10.1021/jf5041993. [DOI] [PubMed] [Google Scholar]
- 171.Di Gesso J.L., Kerr J.S., Zhang Q., Raheem S., Yalamanchili S.K., O'Hagan D. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol Nutr Food Res. 2015;59:1143–1154. doi: 10.1002/mnfr.201400799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Warner E.F., Smith M.J., Zhang Q., Raheem K.S., O'Hagan D., O'Connell M.A. Signatures of anthocyanin metabolites identified in humans inhibit biomarkers of vascular inflammation in human endothelial cells. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201700053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Müller L., Pawelec G. Aging and immunity-impact of behavioral intervention. Brain Behav Immun. 2014;39:8–22. doi: 10.1016/j.bbi.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 174.Pascoe A.R., Fiatarone Singh M.A., Edwards K.M. The effects of exercise on vaccination responses: a review of chronic and acute exercise interventions in humans. Brain Behav Immun. 2014;39:33–41. doi: 10.1016/j.bbi.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 175.Simpson R.J., Lowder T.W., Spielmann G., Bigley A.B., LaVoy E.C., Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11:404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 176.Shinkai S., Kohno H., Kimura K., Komura T., Asai H., Inai R. Physical activity and immune senescence in men. Med Sci Sports Exerc. 1995;27:1516–1526. [PubMed] [Google Scholar]
- 177.Nieman D.C., Henson D.A. Role of endurance exercise in immune senescence. Med Sci Sports Exerc. 1994;26:172–181. doi: 10.1249/00005768-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 178.Turner J.E., Brum P.C. Does regular exercise counter T cell immunosenescence reducing the risk of developing cancer and promoting successful treatment of malignancies. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/4234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Simpson R.J., Kunz H., Agha N., Graff R. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]