Abstract
Simple Summary
Mycotoxicosis in poultry has been seriously damaging the poultry production in Pakistan, resulting in economic losses to the country. The present study may act as a preliminary step for exploring the effect of indigenously characterized potential probiotic lactobacilli on aflatoxin production by Aspergillus flavus. The present study explored anti-fungal Lactobacillus strains. Further investigations revealed their in vitro aflatoxin binding and anti-aflatoxigenic capabilities. These findings demonstrated L. gallinarum PL 149 to be an effective binder of aflatoxin B1 which may be used as a biocontrol agent against A. flavus and aflatoxin B1 production. It may be further employed for aflatoxin binding in poultry gut after in vivo evaluations.
Abstract
Aflatoxin contamination in human food and animal feed is a threat to public safety. Aflatoxin B1 (AFB1) can be especially damaging to poultry production and consequently economic development of Pakistan. The present study assessed the in vitro binding of AFB1 by indigenously characterized probiotic lactobacilli. Six isolates (Lactobacillus gallinarum PDP 10, Lactobacillus reuetri FYP 38, Lactobacillus fermentum PDP 24, Lactobacillus gallinarum PL 53, Lactobacillus paracasei PL 120, and Lactobacillus gallinarum PL 149) were tested for activity against toxigenic Aspergillus flavus W-7.1 (AFB1 producer) by well diffusion assay. Only three isolates (PL 53, PL 120, and PL 149) had activity against A. flavus W-7.1. The ameliorative effect of these probiotic isolates on AFB1 production was determined by co-culturing fungus with lactobacilli for 12 days, followed by aflatoxin quantification by high-performance liquid chromatography. In vitro AFB1 binding capacities of lactobacilli were determined by their incubation with a standard amount of AFB1 in phosphate buffer saline at 37 °C for 2 h. AFB1 binding capacities of isolates ranged from 28–65%. Four isolates (PDP 10, PDP 24, PL 120, and PL 149) also ceased aflatoxin production completely, whereas PL 53 showed 55% reduction in AFB1 production as compared to control. The present study demonstrated Lactobacillus gallinarum PL 149 to be an effective candidate AFB1 binding agent against Aspergillus flavus. These findings further support the binding ability of lactic acid bacteria for dietary contaminants.
Keywords: Aflatoxin B1, Lactobacillus, anti-fungal, Aspergillus flavus, in vitro, poultry
1. Introduction
Poultry is one of the major sectors playing a role in the enhanced economic activity of Pakistan but still it faces a lot of problems, including mycotoxicosis. Mycotoxins are toxic secondary metabolites of fungal origin, which can cause various diseases and death in animals and humans. Ergot alkaloids, fumonisins, patulin, aflatoxin, citrinin, trichothecenes, ochratoxin A, and zearalenone are all examples of some different mycotoxins. Aflatoxins, produced by Aspergillus parasiticus, Aspergillus flavus, and Aspergillus nomius, are of great importance because of their biological and biochemical effects on living systems [1]. Aflatoxin-producing molds are globally and can flourish on a variety of food and feed commodities during production, processing, storage, and transportation procedures [1,2,3]. These molds can infect crops, especially in hot and humid conditions, resulting in economic loss and adverse effects on consumers’ health.
Aflatoxin is a potent carcinogen, mutagen, contains hepatotoxic and immunosuppressant effects and inhibit several metabolic systems resulting in liver and kidney damage [1,4]. Aflatoxin and citrinin cause increased fragility of the vascular system and produce hemorrhages in body tissues. Among aflatoxins, aflatoxin B1 is the most potent, and it is categorized among class 1 human carcinogens. Different factors including pH, temperature, water activity, available nutrients, and competitive inhibition by other microorganisms can affect aflatoxin production in feed [3]. Appropriate harvesting and storage conditions of crops and feed play important roles in aflatoxin reduction.
Various methods have been employed for the removal or inactivation of aflatoxins, including physical, biological, and chemical methods. Chemical treatments may include roasting, ammoniation, and other solvent extraction techniques. Many aflatoxin binders, like activated carbon and various mineral clays, are commercially available and act as sequestering agents and tightly bind aflatoxin; the resulting binding complex is then excreted from the animal’s body [5]. These toxin binders can restore the nutritional value of the feed, but these chemical methods are unsafe, unhealthy, and expensive [6]. Toxin removal by microorganisms is a promising and economical method for decontaminating raw materials and food [7]. Numerous investigations have reported the inhibitory effects of microbes including actinomycetes, yeast, mold, and bacteria on mold growth and aflatoxin production [3]. Thus, beneficial microorganisms may serve as an alternative therapy for mycotoxicosis.
Anti-mutagenic lactic acid bacteria can remove mutagens from food by physical means [8]. Toxin binding by bacteria occurs through cell wall components, namely polysaccharides or polypeptides. Many researchers have studied this binding mechanism, but the exact mechanism of binding is still unknown [9].
Researchers are paying more attention towards preventing the absorption of aflatoxins in the gastrointestinal tracts of users by the aid of probiotic bacterial supplements in food and feed [10]. According to the World Health Organization (WHO), probiotics are defined as live microorganisms which when administered in adequate amounts exert healthy effects to host [11]. Lactobacillus, Bifidobacterium, Enterococcus, Saccharomyces, and Bacillus may serve as probiotics.
Lactobacilli can efficiently remove aflatoxins from contaminated broth. The toxin removal mechanism involves sequestration by binding the toxin to the cell wall instead of metabolic degradation [12]. The present study may act as a preliminary step for studying the effect of indigenously characterized potential probiotic lactobacilli on aflatoxin production by Aspergillus flavus, so that lactobacilli can be used as biocontrol agents. The present study also assessed the in vitro AFB1 binding capacity of Lactobacillus spp., so that these probiotic strains can be employed as toxin binders in place of chemicals in animal feed and thereby the harmful effects of chemical toxin binders can be avoided.
2. Materials and Methods
2.1. Identification of Isolates
Previously characterized probiotic lactobacilli (n = 6) of poultry and fermented food origin [13] and toxigenic Aspergillus flavus W-7.1 were procured from the Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, as listed in Table 1. Lactobacilli were revived using De Man, Rogosa, and Sharpe (MRS) agar and identified as describe previously [14]. Fungal strain was cultured on Sabouraud Dextrose Agar (SDA) medium incubated at 37 °C for 5–6 days. Culture and microscopic characters were observed for identification as described previously [15].
Table 1.
Antifungal activity of cell free supernatants of lactobacilli.
| Isolates | GenBank Accession # | Zones of Inhibition (mm) | |
|---|---|---|---|
| pH 4 | pH 7 | ||
| L. gallinarum PDP 10 | MF980924 | NZ | NZ |
| L. reuteri PDP 24 | MF980925 | NZ | NZ |
| L. fermentum FYP 38 | MF980923 | NZ | NZ |
| L. gallinarum PL 53 | MK182967 | 13 | 12 |
| L. paracasei PL 120 | MK182968 | 16 | 14 |
| L. gallinarum PL 149 | MK182969 | 17 | 15 |
NZ: No zone of inhibition.
2.2. Antifungal Activity of Lactobacilli
Antifungal activity of lactobacilli (n = 6) was determined by well diffusion assay as described elsewhere [16]. Briefly, SDA medium seeded with fungal spores (107 spores/mL) was poured into sterile Petri dishes and allowed to solidify. Wells were punctured in the medium which were then sealed with sterile molten agar. Cell free supernatant (100 μL) of each lactobacilli strain was added into the respective wells. After 3–4 days incubation at 28 °C aerobically, the diameter of zones of inhibition (mm) was measured.
2.3. Effect of Lactobacilli on Aflatoxin Production
The effect of lactobacilli on aflatoxin production by Aspergillus flavus was observed by inoculating 1 mL bacterial suspension (1 McFarland) in yeast extract sucrose broth (YESB) supplemented with a standard amount of fungal spores (107 spores/mL), followed by incubation at 28 °C and 100 rpm for 10 days. YESB media supplemented with known fungal spores and plain YESB media without any inoculation were also incubated as positive and negative controls, respectively. After incubation, medium containing lactobacilli and fungus was filtered through Whatman filter paper no 1 and aflatoxin B1 quantity in filtrate was measured by high-performance liquid chromatography (HPLC) and compared with controls [6]. Aflatoxin B1 was detected by HPLC and quantified using the following formulae:
| (1) |
| (2) |
2.4. Aflatoxin B1 Extraction
For toxin extraction, a previously established protocol was used with modifications [17]. Briefly, broth culture of Aspergillus flavus was autoclaved at 121 °C and 15 psi and then homogenized using homogenizer. Twenty-five grams of homogenate was treated with chloroform (90 mL), methanol (10 mL), NaCl (5 g), and distilled water (10 mL) and incubated at 37 °C with continuous shaking (150–160 rpm) for 30 min. Filtration was carried out using Whatman filter paper #4 and filtrate was concentrated in a water bath at 50 °C. Concentrate was ground to fine powder and reconstituted in 3 mL chloroform volume and stored at 4 °C.
2.5. Toxin Binding Assay
Standard aflatoxin B1 solution was prepared by the method described elsewhere [18]. Prepared standard aflatoxin solution was then added to sterile phosphate buffer saline (PBS) containing lactobacilli culture (1 McFarland). After 2 h of incubation, cells with bound toxin were separated by centrifugation at 10,000 rpm for 5 min and unbound aflatoxin in supernatant was quantified by HPLC.
2.6. High Performance Liquid Chromatography (HPLC)
Aflatoxins were quantified by Agilent HPLC system, 1100 series (Agilent, Santa Clara, CA, USA) as described previously [19]. A mixture of acetonitrile, water, and methanol was used as mobile phase at a flow rate of 1 mL per minute. Mobile phase was firstly purified using a filtration assembly and then sonicated for 10 min at 20 °C in order to avoid gas bubbles. Next, 20 µL samples were injected using a micro-syringe. After 15 min, ultra violet (UV) absorbance was recorded at 254 nm. Sample peaks were analyzed and compared with standard UV absorption data of secondary metabolites at various retention times. Limit of detection (LOD) and limit of quantification (LOQ) of standard aflatoxin were 0.01 ng/mL–100 µg/mL and 0.1 ng/mL–100 µg/mL, respectively.
2.7. Statistical Analysis
Mitigation of aflatoxin production and toxin binding capacity of lactobacilli was compared by one-way ANOVA (analysis of variance) followed by Turkey’s multiple comparison test using Graph pad prism 5.0 software (GraphPad Software, San Diego, CA, USA).
3. Results
A total of six potential probiotic lactobacilli, including Lactobacillus gallinarum PDP 10, Lactobacillus reuteri PDP 24, Lactobacillus fermentum FYP 38, Lactobacillus gallinarum PL 53, Lactobacillus paracasei PL 120, and Lactobacillus gallinarum PL 149, were procured from the Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan. All isolates were Gram-positive rods and catalase negative.
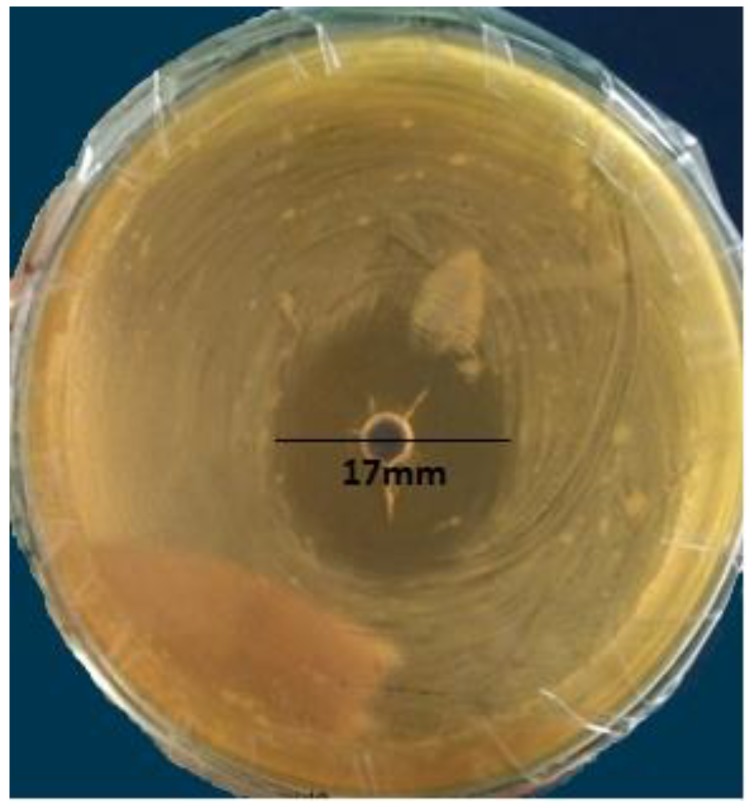
Only three isolates (PL 53, PL 120, and PL 149) had antifungal activity observed by well diffusion assay, as illustrated in Table 1 and Figure 1.
Figure 1.
Activity of cell free supernatant of Lactobacillus gallinarum PL 149 against Aspergillus flavus.
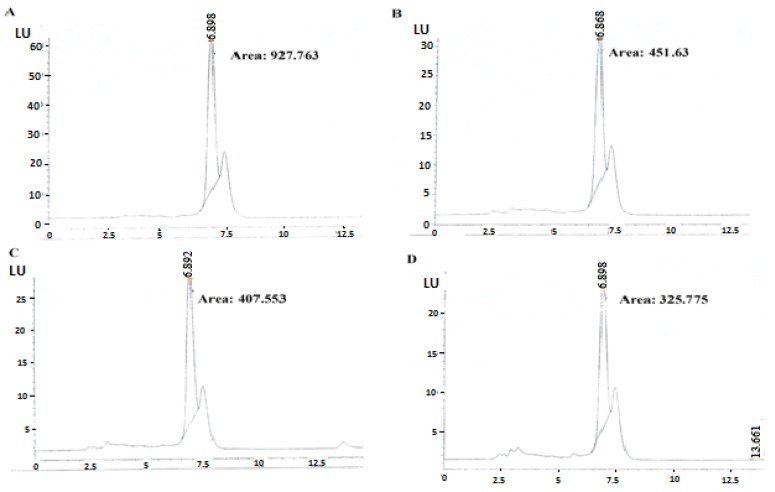
Four isolates (PDP 10, PDP 24, PL 120, and PL 149) showed 100% removal of AFB1, PL 53 caused 55.2% reduction, while FYP 38 showed an enhancing effect on aflatoxin B1 production, as described in Table 2. All isolates showed a varied degree of toxin binding capacities, as described in Table 3 and Figure 2. PL 149 was the most effective binder of aflatoxin B1, with 65% capacity.
Table 2.
Effect of lactobacilli on aflatoxin B1 production.
| Isolates | Peak Areas | Quantity of AFB1 (ng/mL) | % Age Reduction |
|---|---|---|---|
| Standard | 120.205 | 100 | - |
| Control | 0.58439 | 0.4 | - |
| L. gallinarum PDP 10 | ND | ND | 100% |
| L. fermentum FYP 38 | 0.815847 | 0.6 | −39.6% |
| L. reuteri PDP 24 | ND | ND | 100% |
| L. gallinarum PL 53 | 0.26124 | 0.2 | 55.2% |
| L. paracasei PL 120 | ND | ND | 100% |
| L. gallinarum PL 149 | ND | ND | 100% |
AFB1: Aflatoxin B1; ND: Not detected.
Table 3.
Aflatoxin B1 binding capacity of probiotic lactobacilli.
| Isolates | Peak Areas | Quantity of AFB1 Bound (ng/mL) | % Age Reduction (Binding Capacity) |
|---|---|---|---|
| Standard | 108.246 | 100 | - |
| Control | 927.763 | 857 | - |
| L. gallinarum PDP 10 | 451.63 | 417.2 | 51.3% |
| L. fermentum FYP 38 | 407.553 | 376.5 | 56% |
| L. reuteri PDP 24 | 909.624 | 840 | 2% |
| L. gallinarum PL 53 | 546.523 | 504.8 | 42% |
| L. paracasei PL 120 | 676.472 | 624.9 | 28% |
| L. gallinarum PL 149 | 326.775 | 301.8 | 65% |
AFB1: Aflatoxin B1.
Figure 2.
High-performance liquid chromatography chromatograms of aflatoxin B1 present in control and suspension after treatment with lactobacilli: (a) Control; (b) PDP 10; (c) FYP 38; (d) PL 149.
4. Discussion
Aflatoxins represent a group of fungal secondary metabolites that are of great health and economic importance. In developing countries, greater than five billion people are at risk of chronic exposure to aflatoxins, which are capable of causing liver cancer [4]. Consequently, there is an increasing demand for novel preventive and controlling strategies for aflatoxin contaminations in food and feed. Recent studies have revealed the aflatoxin binding ability of lactobacilli. Many bacteria have been reported as aflatoxin binders, including Flavobacterium aurantiacum, L. plantarum, L. pentosus, and L. beveris [20,21,22]. Likewise, Lactobacillus casei psuedoplantarum 371, obtained from silage inoculum, inhibited aflatoxin B1 and G1 synthesis by Aspergillus flavus subsp. parasiticus NRRL 2999 in liquid medium [23]. In a previous study, a mixture of lactobacilli was found to reduce mold growth, germination of spores, and production of aflatoxins by Aspergillus flavus subsp. parasiticus [24]. A large number of such studies have been reported worldwide, but few related studies have been reported in Pakistan.
The present study can act as a preliminary step in a multistep study to investigate the anti-fungal, anti-aflatoxigenic, and in vitro AFB1 binding capacities of previously characterized indigenous phytase-solubilizing probiotic lactobacilli spp. of poultry and fermented food origin [13] against toxigenic Aspergillus flavus. This study identified three probiotic lactobacilli isolates (Lactobacillus gallinarum PL 53, Lactobacillus paracasei PL 120, and Lactobacillus gallinarum PL 149) as antifungal agents. Such inhibitory effects may be a result of lactic acid production or physical interaction of lactobacilli with mold. Similar inhibitory effects of L. acidophilus ATCC 4495 and L. brevis were also demonstrated previously against Aspergillus flavus and Aspergillus parasiticus, respectively [25,26].
Four isolates (PDP 10, PDP 24, PL 120, PL 149) in the present study ceased aflatoxin production completely, whereas PL 53 showed 55% reduction. On the other hand, FYP 38 showed an enhancing effect on aflatoxin B1 production. These variable results may depict different bacterial cell wall structures. Many other investigators have reported similar results, in which various lactic acid-producing bacteria, including Lactobacillus, were capable of inhibiting aflatoxin production, whereas some lactic acid bacteria, like Lactococcus lactis, stimulated aflatoxin biosynthesis [27]. Cell wall polysaccharides and peptidoglycans have been considered bacterial tools for mycotoxin binding [28]. Extracellular metabolites of Lactobacillus casei KC 324 has been reported to mitigate mold growth and aflatoxin production of Aspergillus flavus ATCC 15517 [29]. Commercial silage was once reported to contain inhibitory lactobacilli against aflatoxin B1 and G1 production [30]. L. plantarum ATCC 4008, L. plantarum 12006, Lactobacillus plantarum 299V, L. paracasei subsp. paracasei LMG 13552, and L. rhamnosus VT1 reduced aflatoxin production by 85–92% to 96.3–98.3% [31], whereas in the present study a 100% reduction in AFB1 production by L. gallinarum PDP 10 and PL 149, L. reuteri PDP 24, and L. paracasei PL 120 was observed. It may also be a result of very low aflatoxin production in control conditions as well. Yeast can also act as an effective biocontrol agent against aflatoxins. S. boulardii and S. cerevisiae individually reduced aflatoxin production by 72.8% and 65.8%, respectively, while their combinations reduced aflatoxin production from 71.1% to 96.1%. Supplementation of peanut grains with combinations of S. boulardii plus L. delbrueckii, S. boulardii and S. cerevisiae, L. delbrueckii and S. cerevisiae showed reduction by 96.1%, 66.7%, and 71.1%, respectively [32]. Lactobacillus fermentum PTCC 1744 and Bifidobacterium bifidum PTCC 1644 were also reported to reduce aflatoxin production by more than 99% in comparison with controls [6], although this report is contradictory to the present research which revealed the enhancing effect of Lactobacillus fermentum on AFB1 production by A. flavus.
In the present study, Lactobacillus gallinarum PDP 10, Lactobacillus fermentum FYP 38, Lactobacillus reuteri PDP 24, Lactobacillus gallinarum PL 53, Lactobacillus paracasei PL 120, and Lactobacillus gallinarum PL 149 showed aflatoxin binding capacities of 51.3%, 56%, 2%, 42%, 28%, and 65%, respectively. These results were quite similar with that of Fazeli et al. [33]. In a previous study, the aflatoxin B1 binding capacities of Lactobacillus and Bifidobacterium strains were assessed, which were found to range from 5.8% to 31.3% [12]. On the other hand, the present study reported up to 65% AFB1 binding abilities of probiotic lactobacilli. A previous study reported that Lactobacillus casei had a 20% AFB1 binding capacity [34], which is less than that of L. paracasei PL 120 (28%), whereas Lactobacillus delbrueckii subsp. lactis was reported to have the maximum antifungal (67.43% reduction) and anti-aflatoxigenic (94.33% reduction) activity against A. flavus [35]. Another previous study reported 43.9–64.2% aflatoxin degrading ability of lactobacilli strains [36]. Past investigations revealed similar responses of non-viable and viable cells of Enterococcus faecium strains, whose binding abilities were insignificant statistically. Hence, it was hypothesized that AFB1 detoxification of Enterococcus faecium is a result of aflatoxin binding to bacterial cell wall; a similar mechanism has been also described by other relevant studies [37]. An in vivo experiment revealed the neutralizing capability of Lactobacillus casei Shirota on AFB1 toxicity on the intestine and body weight of host via binding processes [38]. Thus, lactic acid bacteria have been declared as good candidates to prevent aflatoxicosis in farm animals and poultry [9].
5. Conclusions
The present study reported the anti-fungal, anti-aflatoxigenic, and AFB1 binding capacity of six indigenously characterized probiotic strains. It was concluded that L. gallinarum PL 149 may inhibit the AFB1 production by A. flavus and also bind AFB1. L. gallinarum PL 149 may be employed for aflatoxin binding in poultry gut after in vivo evaluations.
Author Contributions
Conceptualization: M.N. and A.A.A.; methodology: M.N., A.A.A., N.A., and S.S. (Shagufta Saeed); software: M.N.; validation: M.N., A.A.A., M.R.Y., S.S. (Shagufta Saeed), and S.S. (Saba Sana); formal analysis: M.N., N.A., M.R.Y., A.M., and S.S. (Saba Sana); investigation: N.A., A.M., and S.S. (Shagufta Saeed); resources: M.N. and A.A.A.; data curation: N.A. and A.M.; writing—original draft preparation: A.M. and N.A.; writing—review and editing: A.M., N.A., S.S. (Saba Sana), M.R.Y., and M.N.; visualization: M.N., A.A.A., S.S. (Shagufta Saeed), and N.A.; supervision: M.N., A.A.A., S.S. (Shagufta Saeed), and S.S. (Saba Sana); project administration: M.N. and A.A.A.; funding acquisition: M.N. and A.A.A.
Funding
This project was partially supported through Higher Education Commission (HEC) project No. 4333/NRPU/R & D/HEC/14/278 and NRPU Project # 4148.
Conflicts of Interest
The authors declare no conflict of interest. The funding agency had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Naseem M.N., Saleemi M.K., Abbas R.Z., Khan A., Khatoon A., Gul S.T., Imran M., Sindhu Z.U.D., Sultan A. Hematological and serum biochemical effects of aflatoxin B1 intoxication in broilers experimentally infected with fowl adenovirus-4 (FAdV-4) Pak. Vet. J. 2018;38:209–213. doi: 10.29261/pakvetj/2018.028. [DOI] [Google Scholar]
- 2.Elsanhoty R.M., Salam S.A., Ramadan M.F., Badr F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. doi: 10.1016/j.foodcont.2014.03.002. [DOI] [Google Scholar]
- 3.Ellis W.O., Smith J.P., Simpson B.K., Oldham J.H., Scott P.M. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit. Rev. Food. Sci. Nutr. 1991;30:403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- 4.Williams J.H., Phillips T.D., Jolly P.E., Stiles J.K., Jolly C.M., Aggarwal D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 5.Diaz D.E., Hagler W.M., Hopkins B.A., Whitlow L.W. Aflatoxin binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia. 2003;156:223–226. doi: 10.1023/A:1023388321713. [DOI] [PubMed] [Google Scholar]
- 6.Ghazvini R.D., Kouhsari E., Zibafar E., Hashemi S.J., Amini A., Niknejad F. Antifungal activity and aflatoxin degradation of bifidobacterium bifidum and lactobacillus fermentum against toxigenic aspergillus parasiticus. Open. Microbiol. J. 2016;10:197. doi: 10.2174/1874285801610010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoghi A., Khosravi-Darani K., Sohrabvandi S. Surface binding of toxins and heavy metals by probiotics. Mini. Rev. Med. Chem. 2014;14:84–98. doi: 10.2174/1389557513666131211105554. [DOI] [PubMed] [Google Scholar]
- 8.Corthier G. The health benefits of probiotics. Danone Nutr. 2004;29:1–18. [Google Scholar]
- 9.Pizzolitto R.P., Bueno D.J., Armando M.R., Cavaglieri L., Dalcero A.M., Salvano M.A. Aflatoxins-Biochemistry and Molecular Biology. IntechOpen; London, UK: 2011. Binding of aflatoxin B1 to lactic acid bacteria and Saccharomyces cerevisiae in vitro: A useful model to determine the most efficient microorganism. [Google Scholar]
- 10.Manubolu M., Goodla L., Pathakoti K., Malmlöf K. Enzymes in Human and Animal Nutrition. Academic Press; Cambridge, MA, USA: 2018. Enzymes as direct decontaminating agents—mycotoxins; pp. 313–330. [Google Scholar]
- 11.Morelli L., Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012;46:S1–S2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 12.Peltonen K.D., El-Nezami H.S., Salminen S.J., Ahokas J.T. Binding of aflatoxin B1 by probiotic bacteria. J. Sci. Food Agric. 2000;80:1942–1945. doi: 10.1002/1097-0010(200010)80:13<1942::AID-JSFA741>3.0.CO;2-7. [DOI] [Google Scholar]
- 13.Arif A., Nawaz M., Rabbani M., Iqbal S., Mustafa A., Yousuf M.R., Muhammad K. Screening, characterization and physicochemical optimization of phosphorus solubilization activity of potential probiotic Lactobacillus spp. Pak. Vet. J. 2018;38:316–320. doi: 10.29261/pakvetj/2018.061. [DOI] [Google Scholar]
- 14.Saleem N., Nawaz M., Ghafoor A., Javeed A., Mustafa A., Yousuf M.R., Khan I. Phenotypic and molecular analysis of antibiotic resistance in Lactobacilli of poultry origin from Lahore, Pakistan. Pak. Vet. J. 2018;38:341–346. doi: 10.29261/pakvetj/2018.084. [DOI] [Google Scholar]
- 15.AL-Ruwaili M., Alkhalaileh N.I., Herzallah S.M., Rawashdeh A., Fataftah A., Holley R. Reduction of aflatoxin B1 residues in meat and organs of broiler chickens by lactic acid bacteria. Pak. Vet. J. 2018;38:325–328. doi: 10.29261/pakvetj/2018.064. [DOI] [Google Scholar]
- 16.Hernández D., Cardell E., Zárate V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: Initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J. Appl. Microbiol. 2005;99:77–84. doi: 10.1111/j.1365-2672.2005.02576.x. [DOI] [PubMed] [Google Scholar]
- 17.Alberts J.F., Engelbrecht Y., Steyn P.S., Holzapfel W.H., van Zyl W.H. Biological degradation of aflatoxin B1 by Rhodococcus erythropolis cultures. Int. J. Food Microbiol. 2006;109:121–126. doi: 10.1016/j.ijfoodmicro.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Zinedine A., Faid M., Benlemlih M. In vitro reduction of aflatoxin B1 by strains of lactic acid bacteria isolated from Moroccan sourdough bread. Int. J. Agric. Biol. 2005;7:67–70. [Google Scholar]
- 19.Yalcin N.F., Avci T., Isik M.K., Oguz H. In vitro activity of toxin binders on aflatoxin B1 in poultry gastrointestinal medium. Pak. Vet. J. 2018;38:61–65. doi: 10.29261/pakvetj/2018.012. [DOI] [Google Scholar]
- 20.Huang L., Duan C., Zhao Y., Gao L., Niu C., Xu J., Li S. Reduction of aflatoxin B1 toxicity by Lactobacillus plantarum C88: A potential probiotic strain isolated from Chinese traditional fermented food “Tofu”. PLoS ONE. 2017;12:1. doi: 10.1371/journal.pone.0170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smiley R., Draughon F. Preliminary evidence that degradation of aflatoxin B1 by Flavobacterium aurantiacum is enzymatic. J. Food. Prot. 2000;63:415–418. doi: 10.4315/0362-028X-63.3.415. [DOI] [PubMed] [Google Scholar]
- 22.Hamidi A., Mirnejad R., Yahaghi E., Behnod V., Mirhosseini A., Amani S., Sattari S., Darian E.K. The aflatoxin B1 isolating potential of two lactic acid bacteria. Asian Pac. J. Trop. Biomed. 2013;3:732–736. doi: 10.1016/S2221-1691(13)60147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gourama H., Bullerman L.B. Anti-aflatoxigenic activity of Lactobacillus casei pseudoplantarum. Int. J. Food. Microbiol. 1997;34:131–143. doi: 10.1016/S0168-1605(96)01176-2. [DOI] [PubMed] [Google Scholar]
- 24.Gourama H., Bullerman L. Aspergillus flavus and Aspergillus parasiticus: Aflatoxigenic fungi of concern in foods and feeds: A Review. J. Food Prot. 1995;58:1395–1404. doi: 10.4315/0362-028X-58.12.1395. [DOI] [PubMed] [Google Scholar]
- 25.Onilude A., Fagade O., Bello M., Fadahunsi I. Inhibition of aflatoxin-producing aspergilli by lactic acid bacteria isolates from indigenously fermented cereal gruels. Afr. J. Biotechnol. 2005;4:1404–1408. [Google Scholar]
- 26.Ghonaimy G., Yonis A., Abolela M. Inhibition of Aspergillus flavus and A. Parasiticus fungal growth and its aflatoxins [B1, B2, G1 and G2] production by Lactobacillus acidophillus. J. Egypt. Soc. Toxicol. 2007;37:53–60. [Google Scholar]
- 27.Gourama H., Bullerman L.B. Antimycotic and antiaflatoxigenic effect of lactic acid bacteria: A review. J. Food. Prot. 1995;58:1275–1280. doi: 10.4315/0362-028X-58.11.1275. [DOI] [PubMed] [Google Scholar]
- 28.Hosono A. Desmutagenic property of cell walls of Streptococcus faecalis on the mutagenicities induced by amino acid pyrolyzates. Milchwissenschaft. 1988;43:168–170. [Google Scholar]
- 29.Chang I., Kim J.-D. Inhibition of aflatoxin production of Aspergillus flavus by Lactobacillus casei. Mycobiology. 2007;35:76–81. doi: 10.4489/MYCO.2007.35.2.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gourama H., Bullerman L.B. Inhibition of growth and aflatoxin production of Aspergillus flavus by Lactobacillus species. J. Food. Prot. 1995;58:1249–1256. doi: 10.4315/0362-028X-58.11.1249. [DOI] [PubMed] [Google Scholar]
- 31.Gomah N.H., Ragab W., Bullerman L. Inhibition of fungal growth and aflatoxin b1 production by some Lactobacillus strains. Assiut. J. Agric. Sci. 2009;40:27–36. [Google Scholar]
- 32.Da Silva J.F., Peluzio J.M., Prado G., Madeira J.E., Silva M.O., de Morais P.B., Rosa C.A., Pimenta R.S., Nicoli J.R. Use of probiotics to control aflatoxin production in peanut grains. Sci. World J. 2015;2015:959138. doi: 10.1155/2015/959138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fazeli M.R., Hajimohammadali M., Moshkani A., Samadi N., Jamalifar H., Khoshayand M.R., Vaghari E., Pouragahi S. Aflatoxin B1 binding capacity of autochthonous strains of lactic acid bacteria. J. Food. Prot. 2009;72:189–192. doi: 10.4315/0362-028X-72.1.189. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Mendoza A., Garcia H., Steele J. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B 1. Food. Chem. Toxicol. 2009;47:1064–1068. doi: 10.1016/j.fct.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 35.Prathivadi Bayankaram P., Sellamuthu P.S. Antifungal and anti-aflatoxigenic effect of probiotics against Aspergillus flavus and Aspergillus parasiticus. Toxin. Rev. 2016;35:10–15. doi: 10.1080/15569543.2016.1178147. [DOI] [Google Scholar]
- 36.Roger T., Léopold T.N. Effect of selected lactic acid bacteria on growth of Aspergillus flavus and aflatoxin B1 production in kutukutu. J. Microbiol. Res. 2015;5:84–94. doi: 10.5923/j.microbiology.20150503.02. [DOI] [Google Scholar]
- 37.El-Nezami H., Kankaanpaa P., Salminen S., Ahokas J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998;36:321–326. doi: 10.1016/S0278-6915(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 38.Liew W.-P.-P., Nurul-Adilah Z., Than L.T.L., Mohd-Redzwan S. The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front. Microbiol. 2018;9:1503. doi: 10.3389/fmicb.2018.01503. [DOI] [PMC free article] [PubMed] [Google Scholar]