Highlights
-
•
Tactile feedback through smaller type II afferents is critical for balance.
-
•
Proprioceptive feedback through larger type I afferents is important for balance.
-
•
Peripheral neuropathy often lead to chronic foot sole tactile desensitization.
-
•
CNS adapt to type II dysfunction by relying on more type I afferents.
Keywords: Balance, Biomechanics, Neuroplasticity, Peripheral neuropathy, Postural control, Rehabilitation
Abstract
Peripheral neuropathy (PN) is a multifarious disorder that is caused by damage to the peripheral nerves. Although the symptoms of PN vary with the etiology, most cases are characterized by impaired tactile and proprioceptive sensation that progresses in a distal to proximal manner. Balance also tends to deteriorate as the disorder becomes more severe, and those afflicted are substantially more likely to fall while walking compared with those who are healthy. Most patients with PN walk more cautiously and with greater stride variability than age-matched controls, but the majority of their falls occur when they must react to a perturbation such as a slippery or uneven surface. The purpose of this study was to first describe the role of somatosensory feedback in the control of posture and then discuss how that relationship is typically affected by the most common types of PN. A comprehensive review of the scientific literature was conducted using MEDLINE, and the relevant information was synthesized. The evidence indicates that the proprioceptive feedback that is conveyed primarily through larger type I afferents is important for postural control. However, the evidence indicates that the tactile feedback communicated through smaller type II afferents is particularly critical to the maintenance of balance. Many forms of PN often lead to chronic tactile desensitization in the soles of the feet and, although the central nervous system seems to adapt to this smaller type II afferent dysfunction by relying on more larger type I afferent reflex loops, the result is still decreased stability. We propose a model that is intended both to help explain the relationship between stability and the smaller type II afferent and the larger type I afferent feedback that may be impaired by PN and to assist in the development of pertinent rehabilitative interventions.
1. Introduction
Peripheral neuropathy (PN) is a complex disorder that arises from damage to ≥1 peripheral nerves, and it is estimated to affect as much as 2.4% of the adult population and 8%–10% of those over the age of 55.1 The majority of cases of PN are secondary to a preexisting illness, the most common of which is diabetes mellitus,2 but as many as 30% of cases are idiopathic.3 In short, researchers have identified >100 types of PN, and the term, therefore, describes a highly diverse set of diseases that are characterized by a wide variety of etiologies and pathologies.4 However, many of the most common types of PN, including diabetic PN (DPN), frequently result in specific functional impairment, which is a loss of balance that greatly enhances the risk of falling.5, 6, 7, 8, 9, 10, 11, 12, 13 That is, although PN is a heterogeneous set of diseases that lead to many different forms of clinical presentation, the scope of this review is limited to the majority of types of the disorder that often result in functional impairments to balance. Indeed, the purposes of the review are to describe how and why balance is typically impaired with PN and to propose a conceptual model that may assist in the development of rehabilitative interventions for those with decreased postural stability that is caused by PN. To help unveil the nature of these impairments in individuals with PN, it is necessary to first elucidate the effect of PN on peripheral nerve function and the risk of falling and to then summarize postural control and how it is typically impacted by the disorder.
2. PN and nerve conduction velocity
The defining characteristic of PN is damage to the axons and/or myelin of peripheral nerves in a manner that typically results in abnormal conduction velocities and amplitudes.14 Although α-motoneuron dysfunction frequently occurs, as is indicated by symptoms like muscle atrophy and strength loss,4, 14 chronic damage to the sensory nervous system occurs in >85% of documented cases of PN.15 It is therefore common for those with PN to experience both positive and negative sensory symptoms with the disease. Positive symptoms include the presence of sensations such as burning, tingling, and exaggerated pain responses (e.g., allodynia and hyperalgesia), whereas negative symptoms include the loss of tactile sensation, proprioception, and temperature sensitivity.14, 16 The nerve damage associated with most types of PN typically progresses in a distal to proximal manner,4 such as from the foot sole to the ankle to the leg, which helps to explain why positive symptoms are regularly worse after long periods of weightbearing activity and negative symptoms are often described as numbness or “feet feel dead”.17 Still, the clinical presentation of PN is often highly inconsistent, which is why nerve conduction velocity (NCV) is the leading assessment of sensory nerve impairment used in clinics4, 18 and epidemiologic studies.19, 20, 21, 22, 23, 24
The standard sensory NCV test assesses the velocity and amplitude of action potentials in the sural, or short saphenous, nerve, which innervates the skin along the posterior aspect of the lower legs, ankles, and feet.25 Given that nearly 30% of people with diabetes over the age of 40 have impaired sensation in their feet and hands,26 it is not surprising that a majority of the studies that have used sural NCV to monitor the progression of PN have done so in those with DPN. For example, Claus et al.20 demonstrated that sural NCV diminished approximately 0.5 m/s each year in those with DPN, and Jarmuzewska and Ghidoni21 reported that sural NCV decreased an average of 3.9 m/s every 10 years in patients diagnosed with type II diabetes.21 Decreased sural NCV has also been linked with impaired glycemic control,24 abnormal sensations,22 and decreased quality of life23 in this population. In light of these and other important pertinent studies that used sensory NCV, it is important to remember that PN is a disease unto itself that has many different causes and is associated with pathologic processes in various combinations of sensory nerve fibers. However, most cases of PN do involve pathology in the smaller sensory fibers like the types II, III, and unmyelinated 4 that transmit cutaneous sensations like touch, sharp pain, and temperature.27, 28 By contrast, sensory NCV is a measure that is limited to large diameter nerves, and at least 1 study29 has demonstrated that PN can result in significant degeneration in sural nerve fiber density without a decrease in sural NCV. Therefore, small fiber involvement is at least to some degree independent of large fiber involvement with most cases of PN. Sensory NCV, which is considered the gold standard diagnostic technique, may not adequately assess the degeneration of the smaller diameter nerves that often occurs at the earliest stages of the disorder.30
3. PN and the risk of falling
Balance may be described as the dynamics of body posture to prevent falling,31 and the risk of falling, in turn, can be predicted by one's ability to control postural sway and center of pressure (COP) while standing.32 A particular concern with many of the most common types of PN is that balance tends to deteriorate as the disease becomes more severe.8, 9,11, 12 The resulting deficits in sensory feedback lead to well-documented increases in postural sway while standing,5, 10,33 including exaggerated COP outcomes such as 95% area of COP and velocity of COP movement.34 Furthermore, many individuals with PN tend to perform more poorly on tests like the 6-Minute Walk and Timed Up-and-Go tests, which are tests of functional mobility that highly correlate with standing balance and are used clinically to predict the risk of falling.10, 35
The majority of falls in those with PN occur while they are walking,7 and individuals with PN are 15 times more likely to experience an injury while walking than age-matched participants with intact sensation.5 The predictive factors that are associated with an increased risk of falling in the elderly are the relative measures of dynamic stability in walking, including variability of stride-to-stride, step lengths, and step widths.36 Although these increased measures of variability associated with PN are due to slow walking speeds and are not directly related to sensory loss, it is possible that years of loss of peripheral sensation and fear of falling cause those individuals to self-select slower walking speeds.7 Those afflicted with the most common types of PN often do walk cautiously, as is indicated by their significantly decreased speed,7,37, 38, 39 step lengths,39 ankle moments, ankle powers, and ground reaction forces.40 Similar walking alterations have also been observed in healthy individuals with experimentally decreased plantar cutaneous sensation (e.g., using ice immersion), but individuals with PN exhibit persistent variability on those measures.41 However, it is important to emphasize that most patients with PN can generate relatively normal and stable locomotory behavior, and the majority of the falls they experience occur when they need to quickly react to perturbations such as irregular surfaces or unexpected objects.6, 7,13 That is, the ability to detect postural changes and make corrections to COP after a perturbation is diminished because it depends on a complex response involving cutaneous and proprioceptive sensory receptors, as well as both small and large sensory fibers, which may be impaired in those with PN. Those difficulties in responding to perturbations, along with the observed differences between individuals with pathologically versus experimentally reduced sensation, suggest that the most common causes of PN impair not just specific cutaneous receptors or sensory fibers, but all peripheral sensory systems.42 In light of that information, we first provide an overview of the relationship between postural control and somatosensation and then describe how that relationship is typically impaired in those with PN.
4. Overview of postural control
Postural control may be defined as the act of achieving, maintaining, or restoring a state of balance during any posture or activity,43 and it depends on a combination of both passive and active mechanical controls.31, 44 Passive control refers to the stiffness and kinematic proprieties associated with the pertinent anatomical structures (e.g., bones and other components of the joints), as well as the effect that gravity exerts on them, whereas active control describes the nervous regulation of skeletal muscle in a manner that requires energy expenditure.45 Passive control helps to explain phenomena like the consistency of postural control observed across many different types of tasks and the decline in postural stability that may occur with muscle fatigue in the lower extremities. However, active control is responsible for sway detection and postural correction,45, 46 and it is critical to our ability to stabilize and maintain balance while standing and walking.47, 48
Active postural control depends on a complex interaction between the joints, skeletal muscles, and both the peripheral nervous system and the central nervous system (CNS). The functional role of the nervous system in active control may be subdivided into 4 components: stimulation collection via sensory receptors, afferent signaling via sensory neurons, CNS control of information processing and decision making in the CNS, and efferent signaling to skeletal muscles via α-motoneurons. The latter 2 are the sole components used in feedforward control, which is accomplished using internal preprogrammed models that are based on anticipation.49 By contrast, feedback control involves modification of ongoing movement using the information that is gathered by sensory receptors and transmitted to the CNS by sensory neurons. Consequently, feedback control allows for a higher degree of accuracy because it is based on error detection and correction, but it is also necessarily slower than feedforward control. Optimal postural control depends on a combination of both feedforward and feedback processes.50
The mechanics of postural control during standing are often described using an inverted pendulum model, and the goal of control is to maintain the COP, the weighted average of all pressures over the area that is contacting the ground, about the base of support.31 Simply put, we naturally sway as we stand, and our stability depends on our ability to sense, control, and correct those movements. Postural control during walking is, of course, quite different because the goal is to actually move outside the base of support and yet maintain stability from one stride to the next. Two popular theories of postural control of gait are passive dynamic walking (PDW) and a central pattern generator. PDW develops from a simple mechanistic model in which gait is a natural repetitive motion that is generated by gravity and inertia.51 Under the frame of PDW theory, segmental inertia and joint stiffness account for most of the control for walking, and the role of the nervous system is to provide more guidance than overt control.47 By contrast, the central pattern generator theory depends heavily on the nervous system and feedforward control, because walking is considered a rhythmic movement that is preprogrammed at the upper level of the spinal cord.52 According to this theory, sensory feedback is important to the control of posture during the stance phase of walking while just 1 foot is on the ground, but it is less important during the swing phase.42 In fact, studies in quadrupeds suggest that locomotion can occur in the absence of afferent inputs53, 54 or even a cerebrum. Nevertheless, bipedal gait is consistently less stable than that in quadrupeds, and it is generally presumed to require some level of feedback control,55 particularly during perturbations. Similarly, active nervous control and sensory feedback are also required within the PDW model to optimize lateral balance in the gait45 and to correct errors.56 To summarize, the evidence indicates that sensory feedback is required to respond to perturbations and maintain posture while standing and walking.
5. Postural control and somatosensation
The sensory receptors and afferent neurons that are most critical to providing information about the difference between current posture and upright position are those associated with the visual, vestibular, and somatosensory systems.57 All 3 of those sensory systems contribute to the maintenance of balance at all times,57 but they are weighted differently according to the specific task.58, 59, 60, 61 For example, the somatosensory and visual systems provide sufficient sensory information to maintain balance during quiet standing with eyes open and feet shoulder width apart,62 whereas the vestibular system is more significantly involved while balancing on an unstable platform.63 That said, the somatosensory system is of particular interest to this review because it provides the most accurate information to assist postural control,57 and it is the sensory system that is most often impaired by PN.14, 27,28
Somatosensation refers to feedback from the body surface and its interaction with the external environment, and it includes the proprioceptive and tactile subdivisions. The tactile subdivision pertains mostly to cutaneous sensations such as touch, pressure, and vibration, while the proprioceptive includes muscle spindles and Golgi tendon organs that contribute to the detection of joint position and joint motion.64, 65 More specifically, Golgi tendon organs monitor muscle loading and their information is conveyed through type Ib sensory neurons, whereas muscle spindles provide feedback about both dynamic and static muscle length through large type Ia and II sensory neurons, respectively. By comparison, smaller diameter sensory neurons are responsible for all tactile sensations, including some information about touch that uses the type III neurons that are particularly susceptible to PN.27, 28 The 4 main tactile receptors in the skin include Merkel's cells, Pacinian corpuscles, Meissner's corpuscles, and Ruffini endings.66, 67 Meissner's and Pacinian corpuscles are rapidly adapting receptors that are responsible for vibrotactile sensation, whereas Merkel's cells and Ruffini endings adapt slowly and are responsible for touch and pressure sensitivity. Because slowly adapting receptors better retain their sensitivity throughout continuous stimulation, Merkel's cells and Ruffini endings likely provide more important tactile feedback for postural control during slow movements and quiet standing. 68, 69
In general, somatosensory information is thought to influence static stability in standing and dynamic stability primarily by affecting the activities of lower leg muscles like the tibialis anterior and soleus, as well as by mediating gait patterns at the ankle, knee, and hip joints.70 We discuss these items in the following text: the specific roles that proprioceptive and tactile sensations play in feedback control of posture and how those relationships may be affected by PN. However, it is worth first noting that many of the studies that have investigated the effect of somatosensory dysfunction on postural control during standing and walking have not done so with the elderly population, who are more likely to have decreased postural stability with degenerative neurologic disorders.6, 71 Instead, many of those studies have used healthy adults whose somatosensation was decreased using soft or moving supporting surfaces, ischemic injections, mechanical vibratory stimuli, or inflated blood pressure cuffs at the ankle or thigh.72, 73, 74, 75, 76
6. Role of ankle proprioception and stretch reflex postural control
Much of the research on the proprioceptive influence of postural control has focused on the ankle proprioceptors and pertinent stretch reflexes because the ankle–foot complex is the only part of the body that contacts the ground and it is the site in which most postural sway occurs.77 In addition to the influence of proprioception, ankle joint ligaments and the surrounding muscles could also contribute to ankle joint stability.44, 78,79 The 3 most likely contributors to stability reduction and enhanced sway at the ankle joints are a decrease in muscular strength of the ankle evertors, an increase in ligamentous laxity, and proprioceptive deficits resulting from a disruption in the integrity of the receptors.80, 81, 82, 83 Because the stiffness of muscles and ligaments around the ankle joint alone cannot achieve joint stabilization,78 ankle proprioception is believed to be a critical determinant of functional joint stability,78, 84 which, in turn, may influence postural stability in standing and walking. Indeed, studies by Fu and Hui-Chan85 and Jerosch and Prymka,86 who conducted joint reposition tests after ankle injury, demonstrated a high correlation between joint stability and ankle proprioception. In addition, Lee and Lin87 reported that 12 weeks of biomechanical ankle platform system training improved joint and postural stability in conjunction with enhancements in ankle proprioception.
In light of the correlations discussed, very few studies have directly investigated the importance of ankle proprioception in postural control owing, in part, to the difficulty of experimentally inducing temporary dysfunction in the pertinent receptors without affecting other sensory receptors. In 1 such study by Hertel et al.,88 the investigators anesthetized portions of the ankle and then analyzed postural sway under both static and dynamic conditions. The results indicated that the anesthesia treatment did adversely affect joint proprioception, but the reduction of joint sensory input did not affect postural sway. In a similar study, De Carlo and Talbot89 examined dynamic stability using a multiaxial platform, and they also reported no difference between anesthetized and unanesthetized ankles. One could argue that the observations of both of those studies were limited by the fact that the injections they used produced uneven or incomplete decreased in ankle proprioception. Most types of PN are not like acute desensitization because those afflicted with progressive forms of the disease for a longer period adapt to ankle joint proprioception through neuroplasticity. A more recent study did study patients with DPN with confirmed lower leg proprioceptive dysfunction, and those investigators reported no differences in balance-correcting responses between patients and healthy controls.90 However, that study did not provide any information regarding participants’ foot sole cutaneous sensation, which is known to be an important component of postural control.91 It is possible that some compensation from other sensory divisions (e.g., foot sole cutaneous sensation) masked the importance of proprioception in the studies described, and proprioceptive feedback continues to be considered an important component of postural control.9, 77,92 Clearly, the precise role that ankle and lower leg proprioceptors play in the control of balance during standing and walking has yet to be fully elucidated. 35
Another important consideration with the relationship between ankle proprioceptors and postural control is the stretch reflex and the information it can provide about the connection between large afferent fibers (LAF), the CNS, and α-motoneuron stimulation of skeletal muscle. Interneurons within the spinal cord elicited this reflexive stimulation of muscle contraction in response to feedback from the muscle spindles.93 Proprioceptive feedback also travels up to the cerebellum that, in turn, can modify the sensitivity and excitation of the spinal interneurons in a manner that helps to control muscle tension to maintain posture and locomotion.94, 95 The sensory feedback provided by spindles and their contribution to the stretch reflex arc is divided into primary and secondary components. Primary spindle fibers convey feedback about the velocity of muscle length changes using large-diameter type Iα sensory neurons, whereas secondary fibers provide information about static muscle length using smaller type II neurons.
Among the more important muscles for postural control are those that dorsiflex (tibialis anterior) and plantar flex (gastrocnemius) the ankle, including the soleus muscles that are critical agonists during both standing and the push-off phase of gait.96 The soleus stretch reflex is necessary to both inhibit plantar flexion during the swing phase of locomotion and provide excitation during the stance phase, and it is thought to help correct balance when responding to perturbations and unexpected stretching of the plantar flexors.93, 95,97 When stretching the soleus in a seated position (i.e., with unloaded soleus), the resulting stretch reflex produces 2 bursts of afferent activity with different latencies. The burst with the shorter latency has an onset of approximately 40 ms and is attributed to the excitation of the primary spindle fibers and type Iα sensory afferents,97, 98 which is why it has been described as the LAF reflex loop. The other burst has a latency of about 70 ms and is associated with the type II afferents that originate from secondary spindle endings;99 therefore, it is often called the small afferent fiber (SAF) reflex loop. Both stretch reflex loops and types of sensory afferents are thought to contribute to postural control during standing and walking,93, 100,101 but the SAF and reflex loop are thought to be more important.66, 99,100, 102
The Hoffman reflex (H-reflex) is a reflective skeletal muscle contraction that occurs in response to an electrical stimulation of the sensory afferents that are associated with the spindles. Although H-reflex and stretch reflex are not identical, the H-reflex is a common tool used to estimate the function of the stretch reflex because they are both dependent on the same afferent neurons and α-motoneurons, as well as the interneurons that connect them.103, 104 As compared with the stretch reflex, the latency of the H-reflex indicates the efficiency of the synaptic transmission between the afferents and α-motoneurons, and the amplitude of the H-reflex reflects the excitation level of the α-motoneurons. Also like the stretch reflex, the CNS alters the latency and amplitude of the H-reflex when the brain modifies the sensitivity and threshold of excitability of the spinal interneurons.105, 106 One of the advantages of the H-reflex is that it is less influenced by joint motions and the activities of other peripheral sensory receptors; consequently, it is often used to investigate central adaptive neuroplasticity during interventional studies.107 The ratio between the amplitude of the H-reflex (H-wave) and the amplitude of the depolarization in the α-motoneuron that is distal to the electrical stimulation (M-wave) is also commonly used as an index for estimating the level of reflex excitability of the motor pool.106, 108
Capaday and Stein105 investigated the influence of posture on the H-reflex in the soleus, and they reported variances between standing and walking that indicated differences in CNS control. While standing with relatively small leg muscle activity, body sway results in relatively larger H-wave amplitudes and intense stretch reflexes to counteract the sway and maintain stability. By contrast, the amplitudes of the H-reflex are generally smaller during walking, but they do vary between the swing and stance phases. Walking requires more compliance and less rigid control of the ankle than standing,109 and the smaller amplitudes of the H-reflex during walking are partly due to the relaxation of the soleus throughout the swing phase. The stronger modulation of the H-reflex during walking is not simply a passive effect of the α-motoneuron excitation level, it indicates that sensory feedback modifies the CNS control at the mean time.
7. Role of foot sole sensation in postural control
Because at least 1 foot is always in contact with the ground during standing and walking, the cutaneous tactile receptors in the soles of the feet provide constant feedback about the surface characteristics of the terrain and whether it becomes slippery, unstable, irregular, and so on. Additionally, foot sole sensation (FSS) is important to postural control because it helps to inform the CNS as to how the body mass and the COP are moving relative to the base(s) of support. Plantar cutaneous feedback is also a logical place to investigate the enhanced risk of falls that occur with PN because the loss of FSS is often one of the earliest and most obvious clinical signs of the disease.91, 106,110
Numerous investigators have reported that the feedback from the cutaneous receptors in the soles helps to regulate postural sway,41, 65,91, 106,111, 112, 113, 114 but Nardone et al.66, 67, 68 have conducted some of the key studies. These investigators examined body sway area during quiet stance in patients with either Charcot–Marie–Tooth (CMT) type 1A, CMT type 2, or DPN. CMT type 1A is a neurologic disease that impairs the function of type Ia and larger diameter type II sensory neurons, whereas CMT type 2 and DPN both cause additional impairment to the smaller type IIβ neurons. The investigators reported that the patients with CMT type 1 were able to stand upright normally, but those with DPN or CMT type 2 had decreased postural stability. These observations indicate that tactile sensory feedback is critical to postural control during standing,66, 68 especially feedback about touch and pressure that is detected by Merkel's cells and Ruffini endings and then conveyed through smaller diameter type II neurons. 67
Another important measure that is used to help understand the role of FSS in the control of posture, and how that relationship may be affected by PN, is the distribution of force over the foot sole, or plantar pressure distribution. Numerous studies have demonstrated that plantar pressure distribution is altered in healthy individuals with experimentally reduced FSS,11, 41,110, 111, 112,115, 116 as well as in patients with PN.91, 106,115, 117 Those alterations have typically consisted of shifts in COP away from the toes and toward the midfoot,41, 110,111, 115 but shifts away from specific regions of insensitivity have also been described.91, 112 Still, it is important to note that not all pertinent studies have produced similar results. For example, some more recent investigations reported that targeted decreases in FSS using anesthetic injections failed to affect plantar pressure distribution,118 and, perhaps more important, did not impair dynamic stability.119, 120 Although the inconsistent observations across these studies may be explained by differences in experimental methods and the extent to which sensation was decreased,34, 116,118 it is clear that more investigation is required to fully understand how changes in FSS affect the plantar pressure distribution and the basic characteristics of gait. Nevertheless, studies involving both patients with PN10, 121 and healthy individuals with experimentally decreased sensation41 have demonstrated that reductions in FSS do lead to slower and more cautious patterns of walking. It is also relevant that Perry et al.69 have shown that FSS is important to the maintenance of posture when perturbation evokes compensatory stepping.
8. Sensory reweighting and PN
An intact somatosensory system is thought to provide the most accurate information to assist postural control,57 but it has been established that alternative sources of sensory information can be used to compensate for those who have been impaired by disease or destabilizing environments.122, 123, 124 Regarding postural control, sensory reweighting occurs when the CNS uses one type of sensory stimulus that is coupled to the control of balance (upweighted) to compensate for another weakened stimulus.125, 126 Somatosensory reweighting can occur acutely, such as while walking blindfolded or with experimentally decreased somatosensation, or it may be prolonged by neuroplastic changes to the CNS in response to chronic impairments occurring with diseases such as PN. Although the exact nature of the neuroplastic adaptations is not yet clear, studies have demonstrated that they occur in the spine,127 supraspinal areas,128, 129 and cerebellum.37 What is clear is that there are differences between acute and chronic sensory reweighting; consequently, we should be careful when comparing postural responses to acutely versus chronically decreased somatosensation because they may involve different compensatory strategies. For example, the distinctions between the tactile and proprioceptive systems that are evident in healthy individuals with experimentally decreased somatosensation are not present in those with the chronic sensory adaptations that are caused by the most common types of PN.34
One measure that can help to elucidate the impact of somatosensory reweighting on postural control in many of those with PN versus healthy individuals with experimentally reduced sensation is the H-index. The H-index is a variation of the H-reflex, and it provides a normalized time course between the onset of the M-wave and the onset of the H-wave relative to an individual's height:130
The H-index represents the entire arc of the type I LAF reflex loop, including the synapses of the spinal cord that integrate peripheral sensory information and are affected by chronic reweighting.130, 131 The H-index has been shown to correlate with other measures of balance, and it is considered to be both a helpful tool for diagnosing neurologic impairments132 and a reliable measure for individuals with PN.117
Although both the LAF reflex loop and the smaller type II afferent (SAF) reflex loop are thought to be important to the control of posture,93, 100,101 the latter is generally considered to play a more significant role.66, 99,102 Furthermore, the decrease in FSS that often occurs with the more common types of PN is associated with impairment to the SAF reflex loop and is thought to diminish postural control.11, 66,67 What is less well-known is how the decrease in FSS and chronic sensory adaptations that may occur with chronic forms of PN affect the relationship between SAF and LAF reflex loops in the control of posture. We have recently investigated this relationship106 by comparing postural control and the H-index in patients with the plantar cutaneous sensation that was impaired by chronic PN versus age-matched controls. The results indicated that the individuals with PN had a decreased H-index, greater postural sway, and impaired functional mobility. There was also a significant correlation between the H-index and postural sway in those with PN, but not in the controls that exhibited normal FSS. These observations indicate that the LAF reflex loop moderates postural control for those with impaired plantar cutaneous sensation. That is, balance control may depend more on LAF reflex loops in those with PN, and sensory reweighting may allow their LAF loop and proprioception to compensate for their smaller fiber degeneration and impaired cutaneous sensation. For example, Dixit et al.133 recently provided indirect evidence of sensory reweighting in individuals with DPN. After 8 weeks of aerobic exercise training, participants had improved control of the COP movement while quietly standing on a foam surface with their eyes closed, which indicates the proprioceptive adaptation occurred without visual feedback.
9. Conceptual model based on this literature
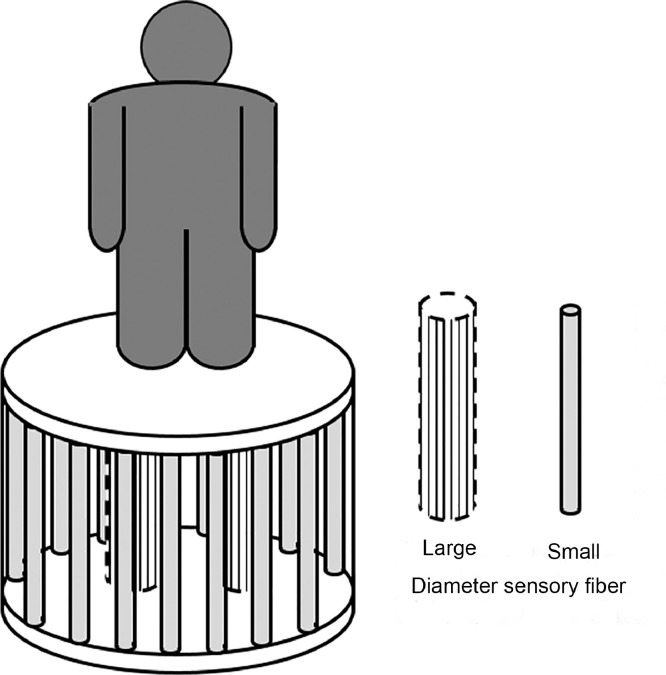
To encapsulate the common effect of chronic PN on the postural control that is discussed above, we propose the following conceptual model to describe the relationship between stability and the SAF and LAF feedback that is often impaired by the disease (Fig. 1). Imagine that an individual is standing on a platform that is supported by both LAFs near the center and SAFs around the perimeter. This conceptual model shows the relationship between the functions of LAFs and SAFs in the development of PN. Impaired LAF function will not threaten the balance of the system, as long as the SAFs remain healthy and function normally, because the balance of the system is mainly supported by the columns in the perimeter (the SAFs). In contrast, the system will be less stable if the SAFs (pillars at the perimeter) are impaired, but the stability decrease may not be clinically evident if the LAFs (support columns in the center of the platform) are healthy. However, the decrease in stability would become apparent if the system with SAF impairment is challenged by an external or internal perturbation, because the LAFs provide a much smaller base of support. Such a conceptual model can help us to explain why individuals with PN-induced impairments in SAF function and tactile sensation, compared with people with only LAF impairments, are far more likely to become unstable and fall when they encounter perturbations.
Fig. 1.
Current understanding of the functions of large afferent fibers (LAF) and small afferent fibers (SAFs) in relation to postural control. SAFs plays an important role in the feedback process for postural control, whereas LAFs become more important with impaired SAFs.
10. Conclusion
To quickly review some of the highlights discussed herein, many types of PN typically include degeneration and dysfunction in the distal sensory neurons,4, 15 especially those that transmit tactile sensations like touch.10, 27,28, 116 As the disease progresses and becomes more severe, balance deteriorates8, 9, 10, 11, 12 and the risk of falling and sustaining an injury while walking increases substantially.5 Individuals with these forms of PN walk more cautiously and with greater stride variability than those with intact somatosensation,7,37, 38, 39 and most of their falls occur as a result of their impaired ability to react to perturbations such as slippery surfaces and unexpected obstacles.6, 7,13 One of the most important determinants of postural control is the cutaneous tactile feedback that is transmitted by SAFs,66, 67, 68 particularly that at the soles of the feet. The decreased FSS that typically occurs with PN17, 114,134 leads to cautious walking10, 41,121 and has been shown to inhibit the recovery of balance after perturbations.69 Finally, the evidence indicates that patients with PN may compensate for their impaired FSS through a greater coupling of postural control to proprioceptive feedback and the LAF reflexive loop.106
PN is a complex disorder that is often difficult to control, and most medical treatments are focused on decreasing pain rather than decreasing the increased risk of falling that frequently accompanies the most common types of the condition. Our recent observations106 suggest that improved LAF reflex function might enhance postural control in those who have impaired SAF function. Some intervention studies have already shown that exercise can improve the function of the LAF reflex loop in athletes and elderly adults.135, 136 Studies have also demonstrated that routine exercise can help individuals with PN to improve strength and balance,137, 138, 139 reaction time and the risk of falling,140 and FSS and functional gait.141 Future pertinent studies should continue to investigate the CNS adaptations that affect postural control, and they should continue to explore how exercise affects those adaptations and how it improves balance in this clinical population.
Acknowledgments
Authors’ contributions
LL initiated the concept and designed the basic structure of the review paper; SZ contributed to the main structure of the manuscript and provided most of the references; JD drafted the manuscript. All 3 authors contributed to the editing and finalization of the manuscript. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Martyn CN, Hughes RA. Epidemiology of peripheral neuropathy. J Neurol Neurosurg Psychiatry. 1997;62:310–318. doi: 10.1136/jnnp.62.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–318. doi: 10.3122/jabfm.17.5.309. [DOI] [PubMed] [Google Scholar]
- 3.Smith AG, Singleton JR. Idiopathic neuropathy, prediabetes and the metabolic syndrome. J Neurol Sci. 2006;242:9–14. doi: 10.1016/j.jns.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders and Stroke. Peripheral neuropathy fact sheet. 2012 https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Peripheral-Neuropathy-Fact-Sheet Available at: [accessed 13.05.2018] [Google Scholar]
- 5.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 6.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med. 2007;86:125–132. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 7.Dingwell JB, Cavanagh PR. Increased variability of continuous overground walking in neuropathic patients is only indirectly related to sensory loss. Gait Posture. 2001;14:1–10. doi: 10.1016/s0966-6362(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 8.Geurts AC, Mulder TW, Nienhuis B, Mars P, Rijken RA. Postural organization in patients with hereditary motor and sensory neuropathy. Arch Phys Med Rehabil. 1992;73:569–572. [PubMed] [Google Scholar]
- 9.Kavounoudias A, Roll R, Roll JP. The plantar sole is a “dynamometric map” for human balance control. Neuroreport. 1998;9:3247–3252. doi: 10.1097/00001756-199810050-00021. [DOI] [PubMed] [Google Scholar]
- 10.Manor B, Li L. Characteristics of functional gait among people with and without peripheral neuropathy. Gait Posture. 2009;30:253–256. doi: 10.1016/j.gaitpost.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- 12.Rogers MW, Wardman DL, Lord SR, Fitzpatrick RC. Passive tactile sensory input improves stability during standing. Exp Brain Res. 2001;136:514–522. doi: 10.1007/s002210000615. [DOI] [PubMed] [Google Scholar]
- 13.Stolze H, Klebe S, Zechlin C, Baecker C, Friege L, Deuschl G. Falls in frequent neurological diseases. J Neurol. 2004;251:79–84. doi: 10.1007/s00415-004-0276-8. [DOI] [PubMed] [Google Scholar]
- 14.Azhary H, Farooq MU, Bhanushali M, Majid A, Kassab MY. Peripheral neuropathy: differential diagnosis and management. Am Fam Physician. 2010;81:887–892. [PubMed] [Google Scholar]
- 15.Padua L, Schenone A, Aprile I, Benedetti L, Caliandro P, Tonali P. Quality of life and disability assessment in neuropathy: a multicentre study. J Peripher Nerv Syst. 2005;10:3–10. doi: 10.1111/j.1085-9489.2005.10103.x. [DOI] [PubMed] [Google Scholar]
- 16.Apfel SC, Asbury AK, Bril V, Bruns TM, Campbell JN, Chalk CH. Positive neuropathic sensory symptoms as endpoints in diabetic neuropathy trials. J Neurol Sci. 2001;189:3–5. doi: 10.1016/s0022-510x(01)00584-6. [DOI] [PubMed] [Google Scholar]
- 17.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 18.Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50:1767–1773. doi: 10.1046/j.1532-5415.2002.50503.x. [DOI] [PubMed] [Google Scholar]
- 19.Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977;40:1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claus D, Mustafa C, Vogel W, Herz M, Neundörfer B. Assessment of diabetic neuropathy: definition of normal and discrimination of abnormal nerve function. Muscle Nerve. 1993;16:757–768. doi: 10.1002/mus.880160711. [DOI] [PubMed] [Google Scholar]
- 21.Jarmuzewska EA, Ghidoni A. Study of the onset and progression of peripheral neuropathy and hypertension in NIDDM. Minerva Med. 2000;91:1–5. [PubMed] [Google Scholar]
- 22.Løseth S, Lindal S, Stålberg E, Mellgren SI. Intraepidermal nerve fibre density, quantitative sensory testing and nerve conduction studies in a patient material with symptoms and signs of sensory polyneuropathy. Eur J Neurol. 2006;13:105–111. doi: 10.1111/j.1468-1331.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 23.Padua L, Saponara C, Ghirlanda G, Padua R, Aprile I, Caliandro P. Lower limb nerve impairment in diabetic patients: multiperspective assessment. Eur J Neurol. 2002;9:69–73. doi: 10.1046/j.1468-1331.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- 24.Tkac I, Bril V. Glycemic control is related to the electrophysiologic severity of diabetic peripheral sensorimotor polyneuropathy. Diabetes Care. 1998;21:1749–1752. doi: 10.2337/diacare.21.10.1749. [DOI] [PubMed] [Google Scholar]
- 25.Umphred DA, Lazaro RT, Roller M, Burton G. Neurological rehabilitation-E-book. Elsevier Health Sciences; Edinburgh: 2013. [Google Scholar]
- 26.Centers for Disease Control and Prevention Nation diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Available at: [accessed 13.05.2018]
- 27.Baron R. Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Rev Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 28.Talbot S, Couture R. Emerging role of microglial kinin B1 receptor in diabetic pain neuropathy. Exp Neurol. 2012;234:373–381. doi: 10.1016/j.expneurol.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM. Painful sensory neuropathy prospective evaluation using skin biopsy. Neurology. 1999;53:1641–1647. doi: 10.1212/wnl.53.8.1641. [DOI] [PubMed] [Google Scholar]
- 30.Lacomis D, Giuliani MJ, Steen V, Powell HC. Small fiber neuropathy and vasculitis. Arthritis Rheumatol. 1997;40:1173–1177. doi: 10.1002/art.1780400624. [DOI] [PubMed] [Google Scholar]
- 31.Winter DA. Biomechanics of normal and pathological gait: implications for understanding human locomotor control. J Mot Behav. 1989;21:337–355. doi: 10.1080/00222895.1989.10735488. [DOI] [PubMed] [Google Scholar]
- 32.Sheldon JH. The effect of age on the control of sway. Gerontol Clin. 1963;5:129–138. doi: 10.1159/000244784. [DOI] [PubMed] [Google Scholar]
- 33.Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. doi: 10.2337/diacare.17.12.1411. [DOI] [PubMed] [Google Scholar]
- 34.Kars HJ, Hijmans JM, Geertzen JH, Zijlstra W. The effect of reduced somatosensation on standing balance: a systematic review. J Diabetes Sci Technol. 2009;3:931–943. doi: 10.1177/193229680900300441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manor B, Doherty A, Li L. The reliability of physical performance measures in peripheral neuropathy. Gait Posture. 2008;28:343–346. doi: 10.1016/j.gaitpost.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 37.Manor B, Newton E, Abduljalil A, Novak V. The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy. Diabetes Care. 2012;35:1907–1912. doi: 10.2337/dc11-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menz HB, Lord SR, Fitzpatrick RC. A tactile stimulus applied to the leg improves postural stability in young, old and neuropathic subjects. Neurosci Lett. 2006;406:23–26. doi: 10.1016/j.neulet.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004;52:1532–1537. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- 40.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 41.Eils E, Behrens S, Mers O, Thorwesten L, Völker K, Rosenbaum D. Reduced plantar sensation causes a cautious walking pattern. Gait Posture. 2004;20:54–60. doi: 10.1016/S0966-6362(03)00095-X. [DOI] [PubMed] [Google Scholar]
- 42.Sinkjær T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollock AS, Durward BR, Rowe PJ, Paul JP. What is balance? Clin Rehabil. 2000;14:402–406. doi: 10.1191/0269215500cr342oa. [DOI] [PubMed] [Google Scholar]
- 44.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- 45.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 46.Qu X, Nussbaum MA. Evaluation of the roles of passive and active control of balance using a balance control model. J Biomech. 2009;42:1850–1855. doi: 10.1016/j.jbiomech.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Garcia M, Ruina A, Coleman M, Chatterjee A. Some results in passive-dynamic walking. In: Pfeiffer F, editor. Proceedings of the Euromech 375: Biology and Technology of Walking. München. Technical University of Munich; March 23–25, 1998. pp. 268–275. [Google Scholar]
- 48.Morasso PG, Schieppati M. Can muscle stiffness alone stabilize upright standing. J Neurophysiol. 1999;82:1622–1626. doi: 10.1152/jn.1999.82.3.1622. [DOI] [PubMed] [Google Scholar]
- 49.Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 50.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 51.McGeer T. Passive dynamic walking. Int J Robotics Res. 1990;9:62–82. [Google Scholar]
- 52.Duysens J, Van de Crommert HW. Neural control of locomotion; part 1: the central pattern generator from cats to humans. Gait Posture. 1998;7:131–141. doi: 10.1016/s0966-6362(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 53.Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- 54.Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. J Neurophysiol. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- 55.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo AD. The relative roles of feedforward and feedback in the control of rhythmic movements. Motor Control. 2002;6:129–145. doi: 10.1123/mcj.6.2.129. [DOI] [PubMed] [Google Scholar]
- 57.Manchester D, Woollacott M, Zederbauer-Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–M127. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- 58.Diener HC, Dichgans J. On the role of vestibular, visual and somatosensory information for dynamic postural control in humans. Prog Brain Res. 1988;76:253–262. doi: 10.1016/s0079-6123(08)64512-4. [DOI] [PubMed] [Google Scholar]
- 59.Mergner T, Rosemeier T. Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions—a conceptual model. Brain Res Rev. 1998;28:118–135. doi: 10.1016/s0165-0173(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 60.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res. 1995;105:101–110. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- 61.Simoneau GG, Ulbrecht JS, Derr JA, Cavanagh PR. Role of somatosensory input in the control of human posture. Gait Posture. 1995;3:115–122. [Google Scholar]
- 62.Timmann D, Belting C, Schwarz M, Diener HC. Influence of visual and somatosensory input on leg EMG responses in dynamic posturography in normals. Electroencephalogr Clin Neurophysiol. 1994;93:7–14. doi: 10.1016/0168-5597(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 63.Lacour M, Borel L. Vestibular control of posture and gait. Arch Ital Biol. 1993;131:81–104. [PubMed] [Google Scholar]
- 64.Hijmans JM, Geertzen JH, Dijkstra PU, Postema K. A systematic review of the effects of shoes and other ankle or foot appliances on balance in older people and people with peripheral nervous system disorders. Gait Posture. 2007;25:316–323. doi: 10.1016/j.gaitpost.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 65.McKeon PO, Hertel J. Diminished plantar cutaneous sensation and postural control. Percept Mot Skills. 2007;104:56–66. doi: 10.2466/pms.104.1.56-66. [DOI] [PubMed] [Google Scholar]
- 66.Nardone A, Galante M, Pareyson D, Schieppati M. Balance control in sensory neuron disease. Clin Neurophysiol. 2007;118:538–550. doi: 10.1016/j.clinph.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Nardone A, Grasso M, Schieppati M. Balance control in peripheral neuropathy: are patients equally unstable under static and dynamic conditions? Gait Posture. 2006;23:364–373. doi: 10.1016/j.gaitpost.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Nardone A, Tarantola J, Miscio G, Pisano F, Schenone A, Schieppati M. Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: evidence from neuropathy. Exp Brain Res. 2000;135:155–162. doi: 10.1007/s002210000513. [DOI] [PubMed] [Google Scholar]
- 69.Perry SD, McIlroy WE, Maki BE. The role of plantar cutaneous mechanoreceptors in the control of compensatory stepping reactions evoked by unpredictable, multi-directional perturbation. Brain Res. 2000;877:401–406. doi: 10.1016/s0006-8993(00)02712-8. [DOI] [PubMed] [Google Scholar]
- 70.Erickson MA, Oliver T, Baldini T, Bach J. Biomechanical assessment of conventional unit rod fixation versus a unit rod pedicle screw construct: a human cadaver study. Spine. 2004;29:1314–1319. doi: 10.1097/01.brs.0000127182.36142.95. [DOI] [PubMed] [Google Scholar]
- 71.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson's disease and Huntington's disease. Mov Disord. 1998;13:428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 72.Anacker SL, Di Fabio RP. Influence of sensory inputs on standing balance in community-dwelling elders with a recent history of falling. Phys Ther. 1992;72:575–581. doi: 10.1093/ptj/72.8.575. [DOI] [PubMed] [Google Scholar]
- 73.Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res. 1990;82:167–177. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- 74.Lord SR, Clark RD, Webster IW. Postural stability and associated physiological factors in a population of aged persons. J Gerontol. 1991;46:M69–M76. doi: 10.1093/geronj/46.3.m69. [DOI] [PubMed] [Google Scholar]
- 75.Mauritz KH, Dietz V. Characteristics of postural instability induced by ischemic blocking of leg afferents. Exp Brain Res. 1980;38:117–119. doi: 10.1007/BF00237939. [DOI] [PubMed] [Google Scholar]
- 76.Teasdale N, Stelmach GE, Breunig A. Postural sway characteristics of the elderly under normal and altered visual and support surface conditions. J Gerontol. 1991;46:B238–B244. doi: 10.1093/geronj/46.6.b238. [DOI] [PubMed] [Google Scholar]
- 77.Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morasso PG, Sanguineti V. Ankle muscle stiffness alone cannot stabilize balance during quiet standing. J Neurophysiol. 2002;88:2157–2162. doi: 10.1152/jn.2002.88.4.2157. [DOI] [PubMed] [Google Scholar]
- 79.Winter DA, Patla AE, Rietdyk S, Ishac MG. Ankle muscle stiffness in the control of balance during quiet standing. J Neurophysiol. 2001;85:2630–2633. doi: 10.1152/jn.2001.85.6.2630. [DOI] [PubMed] [Google Scholar]
- 80.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47:669–677. [PubMed] [Google Scholar]
- 81.Garn SN, Newton RA. Kinesthetic awareness in subjects with multiple ankle sprains. Phys Ther. 1988;68:1667–1671. doi: 10.1093/ptj/68.11.1667. [DOI] [PubMed] [Google Scholar]
- 82.Lentell GL, Katzman LL, Walters MR. The relationship between muscle function and ankle stability. J Orthop Sports Phys Ther. 1990;11:605–611. doi: 10.2519/jospt.1990.11.12.605. [DOI] [PubMed] [Google Scholar]
- 83.Tropp H. Pronator muscle weakness in functional instability of the ankle joint. Int J Sports Med. 1986;7:291–294. doi: 10.1055/s-2008-1025777. [DOI] [PubMed] [Google Scholar]
- 84.Irrgang JJ, Whitney SL, Cox ED. Balance and proprioceptive training for rehabilitation of the lower extremity. J Sport Rehabil. 1994;3:68–83. [Google Scholar]
- 85.Fu AS, Hui-Chan CW. Ankle joint proprioception and postural control in basketball players with bilateral ankle sprains. Am J Sports Med. 2005;33:1174–1182. doi: 10.1177/0363546504271976. [DOI] [PubMed] [Google Scholar]
- 86.Jerosch J, Prymka M. Proprioception and joint stability. Knee Surg Sports Traumatol Arthrosc. 1996;4:171–179. doi: 10.1007/BF01577413. [DOI] [PubMed] [Google Scholar]
- 87.Lee AJ, Lin WH. Twelve-week biomechanical ankle platform system training on postural stability and ankle proprioception in subjects with unilateral functional ankle instability. Clin Biomech. 2008;23:1065–1072. doi: 10.1016/j.clinbiomech.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Hertel JN, Guskiewicz KM, Kahler DM, Perrin DH. Effect of lateral ankle joint anesthesia on center of balance, postural sway, and joint position sense. J Sport Rehabil. 1996;5:111–119. [Google Scholar]
- 89.De Carlo MS, Talbot RW. Evaluation of ankle joint proprioception following injection of the anterior talofibular ligament. J Orthop Sports Phys Ther. 1986;8:70–76. doi: 10.2519/jospt.1986.8.2.70. [DOI] [PubMed] [Google Scholar]
- 90.Bloem B, Allum JH, Carpenter M, Verschuuren JJ, Honegger F. Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss. Exp Brain Res. 2002;142:91–107. doi: 10.1007/s00221-001-0926-3. [DOI] [PubMed] [Google Scholar]
- 91.Zhang S, Li L. The differential effects of foot sole sensory on plantar pressure distribution between balance and gait. Gait Posture. 2013;37:532–535. doi: 10.1016/j.gaitpost.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 92.Simmons RW, Richardson C, Pozos R. Postural stability of diabetic patients with and without cutaneous sensory deficit in the foot. Diabetes Res Clin Pract. 1997;36:153–160. doi: 10.1016/s0168-8227(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 93.Clarac F, Cattaert D, Le Ray D. Central control components of a “simple” stretch reflex. Trends Neurosci. 2000;23:199–208. doi: 10.1016/s0166-2236(99)01535-0. [DOI] [PubMed] [Google Scholar]
- 94.Hoffer JA, Andreassen S. Regulation of soleus muscle stiffness in premammillary cats: intrinsic and reflex components. J Neurophysiol. 1981;45:267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- 95.Sinkjær T, Andersen JB, Larsen BI. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- 96.Di Giulio I, Maganaris CN, Baltzopoulos V, Loram ID. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol. 2009;587:2399–2416. doi: 10.1113/jphysiol.2009.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matthews PB. The human stretch reflex and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- 98.Taylor J, Stein RB, Murphy PR. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985;53:341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]
- 99.Schieppati M, Nardone A. Medium–latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J Physiol. 1997;503:691–698. doi: 10.1111/j.1469-7793.1997.691bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjær T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Prog Brain Res. 1999;123:461–472. doi: 10.1016/s0079-6123(08)62882-4. [DOI] [PubMed] [Google Scholar]
- 102.Mazzaro N, Grey MJ, do Nascimento OF, Sinkjær T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res. 2006;173:713–723. doi: 10.1007/s00221-006-0451-5. [DOI] [PubMed] [Google Scholar]
- 103.Akazawa K, Aldridge JW, Steeves JD, Stein RB. Modulation of stretch reflexes during locomotion in the mesencephalic cat. J Physiol. 1982;329:553–567. doi: 10.1113/jphysiol.1982.sp014319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burke D. Critical examination of the case for or against fusimotor involvement in disorders of muscle tone. Adv Neurol. 1983;39:133–150. [PubMed] [Google Scholar]
- 105.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang S, Manor B, Li L. H-index is important for postural control for people with impaired foot sole sensation. PloS One. 2015;10 doi: 10.1371/journal.pone.0121847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]
- 108.Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- 109.Houk JC. An assessment of stretch reflex function. Prog Brain Res. 1976;44:303–314. doi: 10.1016/S0079-6123(08)60741-4. [DOI] [PubMed] [Google Scholar]
- 110.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23:606–611. doi: 10.2337/diacare.23.5.606. [DOI] [PubMed] [Google Scholar]
- 111.Chen H, Nigg BM, Hulliger M, De Koning J. Influence of sensory input on plantar pressure distribution. Clin Biomech. 1995;10:271–274. doi: 10.1016/0268-0033(95)99806-d. [DOI] [PubMed] [Google Scholar]
- 112.Eils E, Nolte S, Tewes M, Thorwesten L, Völker K, Rosenbaum D. Modified pressure distribution patterns in walking following reduction of plantar sensation. J Biomech. 2002;35:1307–1313. doi: 10.1016/s0021-9290(02)00168-9. [DOI] [PubMed] [Google Scholar]
- 113.Nurse MA, Nigg BM. The effect of changes in foot sensation on plantar pressure and muscle activity. Clin Biomech. 2001;16:719–727. doi: 10.1016/s0268-0033(01)00090-0. [DOI] [PubMed] [Google Scholar]
- 114.Wu G, Chiang JH. The significance of somatosensory stimulations to the human foot in the control of postural reflexes. Exp Brain Res. 1997;114:163–169. doi: 10.1007/pl00005616. [DOI] [PubMed] [Google Scholar]
- 115.Caselli A, Pham H, Giurini JM, Armstrong DG, Veves A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care. 2002;25:1066–1071. doi: 10.2337/diacare.25.6.1066. [DOI] [PubMed] [Google Scholar]
- 116.Hong SL, Manor B, Li L. Stance and sensory feedback influence on postural dynamics. Neurosci Lett. 2007;423:104–108. doi: 10.1016/j.neulet.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 117.Zhang S, Holmes M, Li L. Reliability of nerve function assessments for people with peripheral neuropathy. Int J Neurosci. 2015;125:201–207. doi: 10.3109/00207454.2014.920332. [DOI] [PubMed] [Google Scholar]
- 118.Höhne A, Stark C, Brüggemann GP. Plantar pressure distribution in gait is not affected by targeted reduced plantar cutaneous sensation. Clin Biomech. 2009;24 doi: 10.1016/j.clinbiomech.2009.01.001. 308–3. [DOI] [PubMed] [Google Scholar]
- 119.England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait Posture. 2007;25:172–178. doi: 10.1016/j.gaitpost.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Höhne A, Stark C, Brüggemann GP, Arampatzis A. Effects of reduced plantar cutaneous afferent feedback on locomotor adjustments in dynamic stability during perturbed walking. J Biomech. 2011;44:2194–2200. doi: 10.1016/j.jbiomech.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 121.Tsai YJ, Lin SI. Older adults adopted more cautious gait patterns when walking in socks than barefoot. Gait Posture. 2013;37:88–92. doi: 10.1016/j.gaitpost.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 122.Curthoys IS, Halmagyi GM. Vestibular compensation: a review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J Vestib Res. 1995;5:67–107. [PubMed] [Google Scholar]
- 123.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nashner LM. Adaptation of human movement to altered environments. Trends Neurosci. 1982;5:358–361. [Google Scholar]
- 125.Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Brain Res Cogn Brain Res. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 126.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 127.Bove M, Trompetto C, Abbruzzese G, Schieppati M. The posture-related interaction between Ia-afferent and descending input on the spinal reflex excitability in humans. Neurosci Lett. 2006;397:301–306. doi: 10.1016/j.neulet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 128.Lalonde R, Strazielle C. Brain regions and genes affecting postural control. Prog Neurobiol. 2007;81:45–60. doi: 10.1016/j.pneurobio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 129.Visser JE, Bloem BR. Role of the basal ganglia in balance control. Neural Plast. 2005;12:161–174. doi: 10.1155/NP.2005.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- 131.Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008;171:1–2. doi: 10.1016/j.jneumeth.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 132.Aiello I, Rosati G, Serra G, Manca M. The diagnostic value of H-index in S1 root compression. J Neurol Neurosurg Psychiatry. 1981;44:171–172. doi: 10.1136/jnnp.44.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dixit S, Maiya A, Shastry BA, Guddattu V. Analysis of postural control during quiet standing in a population with diabetic peripheral neuropathy undergoing moderate intensity aerobic exercise training: a single blind, randomized controlled trial. Am J Phys Med Rehabil. 2016;95:516–524. doi: 10.1097/PHM.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 134.Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998;15:508–514. doi: 10.1002/(SICI)1096-9136(199806)15:6<508::AID-DIA613>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 135.Gruber M, Taube W, Gollhofer A, Beck S, Amtage F, Schubert M. Training-specific adaptations of H-and stretch reflexes in human soleus muscle. J Mot Behav. 2007;39:68–78. doi: 10.3200/JMBR.39.1.68-78. [DOI] [PubMed] [Google Scholar]
- 136.Guan H, Koceja DM. Effects of long-term Tai Chi practice on balance and H-reflex characteristics. Am J Chin Med. 2011;39:251–260. doi: 10.1142/S0192415X11008798. [DOI] [PubMed] [Google Scholar]
- 137.Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li L, Hondzinski JM. Select exercise modalities may reverse movement dysfunction because of peripheral neuropathy. Exerc Sport Sci Rev. 2012;40:133–137. doi: 10.1097/JES.0b013e31825f7483. [DOI] [PubMed] [Google Scholar]
- 139.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 140.Morrison S, Colberg SR, Mariano M, Parson HK, Vinik AI. Balance training reduces falls risk in older individuals with type 2 diabetes. Diabetes Care. 2010;33:748–750. doi: 10.2337/dc09-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li L, Manor B. Long term Tai Chi exercise improves physical performance among people with peripheral neuropathy. Am J Chin Med. 2010;38:449–459. doi: 10.1142/S0192415X1000797X. [DOI] [PubMed] [Google Scholar]