Abstract
Olfactory processing starts with the breath and elicits neuronal, metabolic and cortical responses. This process can be investigated centrally via the Olfactory Event-Related Potentials (OERPs) and peripherally via exhaled Volatile Organic Compounds (VOCs). Despite this, the relationship between OERPs (i.e., N1 and Late Positive Component LPC) and exhaled VOCs has not been investigated enough. The aim of this research is to study OERPs and VOCs connection to two different stimuli: phenylethyl alcohol (PEA) and Vaseline Oil (VO). Fifteen healthy subjects performed a perceptual olfactory task with PEA as a smell target stimulus and VO as a neutral stimulus. The results suggest that OERPs and VOCs distributions follow the same amplitude trend and that PEA is highly arousing in both psychophysiological measures. PEA shows ampler and faster N1, a component related to the sensorial aspect of the stimulus. The N1 topographic localization is different between PEA and VO: PEA stimulus evokes greater N1 in the left centroparietal site. LPC, a component elicited by the perceptual characteristic of the stimulus, shows faster latency in the Frontal lobe and decreased amplitude in the Central and Parietal lobe elicited by the PEA smell. Moreover, the delayed time between the onset of N1-LPC and the onset of VOCs seems to be about 3 s. This delay could be identified as the internal metabolic time in which the odorous stimulus, once perceived at the cortical level, is metabolized and subsequently exhaled. Furthermore, the VO stimulus does not allocate the attentive, perceptive and metabolic resource as with PEA.
Keywords: OERP, VOCs, N1, LPC, olfactory perception, olfactory metabolic response
1. Introduction
1.1. Olfactory Function and Breath
The olfactory function, placed in a cortical localization connected to areas dedicated to emotional and mnestic activations (e.g., hippocampus, entorhinal cortex, amygdala), seems to be a borderline function between “perceptions”. It plays a role linked to instinctual emotional responses, evolutionarily less recent both from the phylogenetic and the ontogenetic point of view [1,2]. Moreover, the olfactive function strongly involves and modulates metabolic aspects [3,4], and this peculiarity is more clearly overt if compared to other sensory modalities. Different to olfactory animal models [5,6,7,8], the olfactory whole pathway, linked to human perceptions, has been largely unexplored and needs full investigation because it is a complex sense that has not only a sensorial/perceptive finality but guides internal chemical cues, such as metabolism [3]. Humans can perceive the presence of an odorant according to its concentration (perceptual threshold) [9], with the concentration depending on the single odorant chemical molecule taken into consideration [10,11]. Many brain structures detect olfactory connections. There is an “olfactory map” based on inhibitory and excitatory interactions within and between the two olfactory bulbs [12]. The response of neurons in the bulbs is also subject to modulation through a top down regulation. The precise identification of an odour requires further processing after the physiological stages of the olfactory system. The axons afferent from the olfactory bulb form the olfactory tract and branch out to reach different regions of the frontal lobe, including the olfactory cortex. The neocortex is only reached by some branches from the median dorsal nucleus of the thalamus. It is important to recall that the organization of the olfactory system is unique, because the olfactory system does not have decussation and does not relay to the thalamus [13,14]. This could indicate its extremely ancient origin at the phylogenetic level and could be linked to the more basic physiological aspects (e.g., metabolic/oxidative aspects) [4,15,16]. Furthermore, the sense of smell is mainly modulated by a breath-dependent sensory gate [16,17,18].
1.2. Chemosensory Event Related Potentials
Chemosensory Event Related Potentials (CSERP) and the Olfactory Event Related Potentials (OERP) are the main psychophysiological and electroencephalographic tools used to study the olfactory responses due to chemical stimulus (e.g., odorants) [19]. The registration of CSERP was clinically introduced by Kobal in the early 1970s [20]. CSERPs have become appropriate tools to evaluate the olfactory function, the olfactive sensation and perception [21,22,23]. The characteristic components of CSERPs are N1 and Late Positive Component (LPC). N1 is an early component with negative polarity, reflects the sensory characteristics of the stimulus and depends on the exogenous component. N1 and LPC seem to vary in amplitude and latency in relation to the concentration of odorants (i.e., higher odorant concentration can elicit shorter latency in N1 and ampler amplitude in LPC) [23]. Moreover, in contrast to the other sensory modalities, the effects of selective attention on odours lead to a reduction of the latency instead of an increase of the amplitude in the N1 component.
The LPC is a slow positive component, which follows the N1 and which reflects the perceptive and cognitive characteristics of the stimulus itself and depends on the endogenous components [24].
The LPC component has, instead, the probable function connected to the perceptual-cognitive information processing. We can consider the LPC, evoked by the olfactory stimulation, as a delayed P3 in a cognitive task.
Moreover, it has been shown that aging is accompanied by a decrease in OERP sensitivity, as well as a greater tendency to olfactory adaptation and a slower recovery of threshold sensitivity [20,25]. In any case, the analysis of the olfactory cortical responses must consider some specific methodological biases. The olfactory stimulation usually occurs in a mono-lateralized way (in a single nostril) but this modality can produce cortical lateralization bias, due to both tactile stimulation and odorous stimulation itself. Additionally, some responses to odorous stimuli can activate the trigeminal system, rather than the olfactory system. For example, stimuli such as ammonia, acids or carbon dioxide, activate the trigeminal system and subcortical components linked to nociception [26,27]. As with other sensory modalities, the sense of smell is also susceptible to changes in aging [27], in different cognitive capacity [28], depend on stimulus and is task-related [29,30]; these variations are viewable through the EEG.
1.3. The Volatile Organic Compounds (VOCs)
In a human of healthy condition, 99% of the exhaled breath matrix is composed of a few compounds of the inorganic gasses such us nitrogen, oxygen and carbon dioxide along with vapor aqueous and the inert gases. The residual part consists of a mixture of many molecules: un-volatile organic compounds (e.g., leukotrienes, prostaglandins and serotonin) and by volatile organic compounds (e.g., aldehydes, ketones and benzene derivatives) [31,32]. The VOCs are divided into exogenous and endogenous: the exogenous VOCs are derived from the environment or human habits (e.g., smoker), while the endogenous VOCs are the result of the body’s metabolic processes (e.g., glucose degradation originates acetone; cholesterol biochemical pathway of the mevalonic acid derives isoprene; fat acids peroxidation produces alkanes). Gas exchange at the alveolar-blood capillary membrane of the respiratory tract is a passive diffusion driven by concentration gradients. Following these vital gasses, molecules present in the blood can diffuse passively into the breath. In normal subjects, more than 3400 different VOCs can be detected in the exhaled breath [32]. The VOCs’ profile in exhaled breath reflects the biochemical alterations related to metabolic changes, organ failure, or neuronal dysfunction in disease, which are, at least in part, transmitted via the lung to the alveolar exhaled breath, even at the very onset of disease [32,33,34,35]. In recent studies, VOCs analysis has been applied to neurodegenerative diseases [36,37,38] and cognition [33]. Interestingly, VOC evaluation in a healthy centenarian shows a peculiar pattern in comparison with young and elderly controls [35], as was the case for OERP [27,39,40]. Neuronal metabolism in some diseases induce VOC alteration, as suggested by these studies, and moreover there is a lack of studies on physiological neuronal activity and VOCs release.
1.4. Connection between OERP and VOC
The connection between olfactive perception and metabolic response is not well investigated. Mazzatenta et al. focused on metabolic aspects (i.e., VOCs) and oxidative processes in some neurodegenerative pathologies [34,38,41]. Invitto’s recent studies investigated the OERP variation in Mild Cognitive Impairment [28] and in Obstructive Sleep Apnoea (OSA) syndrome [16], both pathologies that show altered VOCs [34,38]. This aspect is particularly evident in OSA, where the altered breath compromises metabolic and oxidative capacity [16,42,43]. It is certainly known that the respiratory act is the gateway necessary for the activation of the olfactory receptor response [44], but we know nothing about the metabolic activity produced as a function of different stimuli and how this activation is correlated with the sensory response. In the same way, we know that breath can interact with ERP amplitude and EEG rhythms in different ways [45,46,47], but we do not know enough about a parallel investigation into VOCs and OERPs elicited by the same condition (e.g., same stimulus, same cognitive task or same attentional condition).
In the present study, we investigated the trend between OERP and the exhaled VOCs during the PEA sensorial (rose/floral smell) stimulation and the Vaseline Oil (neuter smell) sensorial stimulation [48], focusing in particular on N1 and LPC OERPs components, the main components elicited during an olfactory perceptive task [16,28].
2. Materials and Methods
2.1. Participants
We recruited 15 healthy adult volunteers (10 females; mean age 24.4; standard deviation 4.4). The subjects were university students. All the subjects were non-smokers. The subjects had normal hearing, normal or corrected-to-normal vision and a right manual dominance. All participants signed a written informed consent according to the Helsinki Declaration. The protocol was approved by the local ethical committee (Ethical Committee ‘Vito Fazzi Hospital’ ASL Lecce, Italy—authorization with report number 36-2016)
2.2. OERP Apparatus and OERP Processing
In the present task, we administered olfactory stimuli via an olfactometer [49] interfaced in parallel with the EEG amplifier. This tool allows the administration of scented stimuli triggered on line on EEG recordings [16,28,49,50]. Continuous EEG was recorded (sampled at 500 Hz) during the olfactory task, via the V-AMP-Brain Vision Recorder, using a cap embedded with 16 Ag /AgCl electrodes (Brain Products, Germany), positioned according to the 10–20 system electrode. Impedance was kept below 5 kΩ. Electrodes were referenced online to the FCz [51], and offline referenced as common averaged reference [52]. One electrode was placed at the outer canthus of the left eyebrow and was used to monitor eye movements. The ERPs analysis was obtained using the Brain Vision Analyzer and the time off-line analysis was from 100 pre-stimulus to 600 ms post-stimulus with 100 ms baseline-correction. Thereafter, trials with blinks and eye movements were rejected based on horizontal electro-oculogram with an ICA component analysis. The signal was filtered offline (0.01–50 Hz, 24 dB), and the threshold for artefact rejection was set at > |125|μV [16,28]. Ocular rejection was performed through independent component analysis (ICA). Separate averages were calculated for each odorant segmentation (PEA and Vaseline Oil). Peaks detection was applied according to the latency range of the maximum peak area in Grand Average [16,28].
2.3. VOC Apparatus
During the EEG recordings subjects were, in parallel, recorded with the iAQ-2000 (Applied Sensor, Warren, NJ, USA) equipped with a metal oxide semiconductor (MOS). The e nose has a sensing range of 450–2000 ppm CO2 equivalents and is able to detect a broad range of volatile compounds (both organic and inorganic, e.g., alcohols, aldehydes, aliphatic hydrocarbons, amines, aromatic hydrocarbons, ketones, organic acids, and CO), while correlating directly with the CO2 levels [32,33,34,35].
2.4. Method
The subject was sitting comfortably in the laboratory setting, where there was a constant temperature of 20 degrees centigrade. The subject was sitting in front of a black box (interfaced with the olfactometer) and his task was to try to have an orthonasal breathing and to perceive the odorous stimuli during the task.
The sequence of stimulus presentation S1 (500 ms) and S2 (for 500 ms) was administrated in a pseudorandom way, with a proportion of 50% of the target (i.e., PEA smell). The interstimulus interval was 1 min during which the individual was exposed only to air. The air flow, after collecting the volatile components of substances S1 and S2, was transferred to an exposure open black box, where the subject was required to enter his head. In this paradigm the stimulation was administrated in the central way and in a diffused manner (not in only one nostril), to avoid lateralization bias. The outlet of the tube from which the essences emerged was placed at the centre of the stimulation cave, where the subject had to inhale and exhale. Each experimental session had a duration of about 40 min.
After the Task, the experimenter asked the subject if he had perceived the smell and if he perceived the odour, and if the odour was pleasant, arousing or familiar on a scale from 1 to 5 (i.e., 1 = minimum value; 5 = maximum value) [53].
In order to record OERPs correlated to physiological effect on VOCs emission, we interfaced the olfactometer to the EEG and to an electronic sensor for VOCs parallel recording. This condition allowed us to control olfactory stimulations and to relate them to psychophysiological responses. Consequently, we detected: the olfactory response to record, for each given single step of stimulation, the EEG, the OERPs components and VOCs responses. The experimental settings allowed us to investigate, through OERPs and VOCs, the olfactory response to a neutral stimulus (Vaseline Oil 10 mL added of 20 μL) and to a scented stimulus of a characteristic rose odour (PEA, 2-phenyl ethanol C2H4O2 with a dilution of 20 μL in 10 mL).
3. Statistical Analysis and Results
3.1. Statistical Analysis
VOCs data normalization was made by 1/log10. Data treatment and statistical analysis was performed with Excel, Origin and SPSS software, α was set at 0.05. OERP data analysis was performed with SPSS Software
3.1.1. OERP Analysis
The General Linear Model (GLM), repeated measure, was performed on amplitudes and latencies of N1 and LPC component; as the Within Factor we considered Smell (PEA, Vaseline Oil), and Electrodes (Fp1; Fp2; F3; F4; F7; F8; C3; C4; P7; P8; O1; O2; Fz; Cz; Pz)
3.1.2. N1
The analysis on N1 latency showed significant results on Electrode (F =2.42; p = 0.004; η2 = 0.157) and on Smell × Electrode (F = 2.15; p = 0.011; η2 = 0.142). Post hoc t Test paired comparison highlighted significant differences in terms of an N1 faster latency elicited by PEA stimulation in the Frontal Right localization (F4 PEA smell = 207 ms; vs. F4Neuter Smell = 246.6 ms).
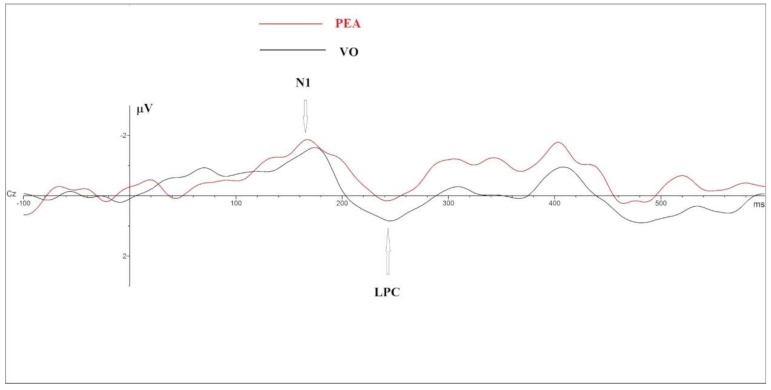
GLM on N1 amplitude highlighted the main significant effect on Smell (F =31.45; p = 0.000; η2 = 0.724) and Electrode (F = 19.9; p = 0.000; η2 = 0.624) and an interaction on Smell × Electrode (F = 4.674; Sign = 0.000; η2 = 0.280). PEA stimulation elicited ampler N1 (PEA = −7.5 µV vs. VO = −5.4 µV); post hoc t Test paired comparison showed significant difference in F4, C3, C4, P7; P8, O1, O2, F8, Cz, Pz in term of ampler N1 in PEA stimulation (see Table 1 and Figure 1 and Figure 2).
Table 1.
Table 1 describes significant results of Post Hoc paired t Test comparison: t Value, p, N1 mean amplitude (in µV) of PEA and VO.
| Electrode | t Value | p | PEA | VO |
|---|---|---|---|---|
| F4 | 3.664 | 0.003 | −3.9 | −2.4 |
| C3 | 3.173 | 0.007 | −6.19 | −4.48 |
| C4 | 3.062 | 0.009 | −6.82 | −4.89 |
| P7 | 4.429 | 0.001 | −12.25 | −9.06 |
| P8 | 5.040 | 0.000 | −13.21 | −9.23 |
| O1 | 4.865 | 0.013 | −14.17 | −11.26 |
| O2 | 3.957 | 0.002 | −14.95 | −11.15 |
| F8 | 2.67 | 0.019 | −8.60 | −5.54 |
| Cz | 2.724 | 0.017 | −3.17 | −2.49 |
| Pz | 4.332 | 0.001 | −10.31 | −8.06 |
Figure 1.
Cz–Grand Average comparison between OERP components elicited during phenylethyl alcohol (PEA) (red line) and Vaseline Oil (black line).
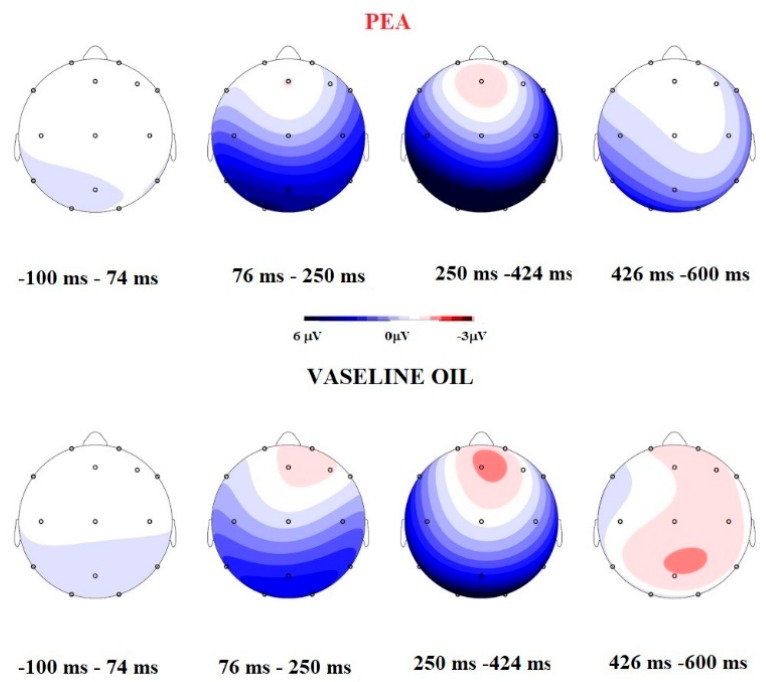
Figure 2.
Comparison between Mapping during four different temporal ranges and between OERP component. The Mapping during PEA stimulation indicates that is present a more negative activation in the temporal range 250–425 ms, and positive components during the Vaseline Oil stimulation. The OERP amplitude comparison shows that PEA elicited ampler N1 and decreased late positive component (LPC).
3.1.3. LPC
The GLM on LPC latency showed a significant effect only in Electrode variable (F = 2.450; p = 0.003; η2 = 0.159) in term of shorter latencies in Frontal topography (see Table 2).
Table 2.
Table 2 points out the descriptive values of Latencies (in ms) for each electrode.
| Electrode | Latency | SEM |
|---|---|---|
| Fp1 | 389.73 | 23.7 |
| Fp2 | 358.13 | 28.36 |
| F3 | 348.67 | 26.18 |
| F4 | 367.63 | 27.55 |
| C3 | 413.6 | 26.42 |
| C4 | 386 | 31.06 |
| P7 | 416 | 28.5 |
| P8 | 404.4 | 30.74 |
| O1 | 424.53 | 25.69 |
| O2 | 424.40 | 24.5 |
| F7 | 384.93 | 25.5 |
| F8 | 348.53 | 34.05 |
| Cz | 426.53 | 23.19 |
| Pz | 447.07 | 18.95 |
| Fz | 338.27 | 20.82 |
GLM on LPC Amplitude showed a significant Interaction between Smell × Electrode (F = 2.075; p = 0.015; η2 =0.147). Post Hoc paired t Test comparison highlighted significant results in Cz (t = 2.44; p = 0.030; VO mean amplitude = 2.702 µV; PEA mean amplitude = 2.095 µV) and in Pz (t = 2.356; p = 0.035; VO mean amplitude = 6.54 µV; PEA mean amplitude = 4.97 µV), in terms of a decreased amplitude elicited by PEA stimulation (see Figure 1).
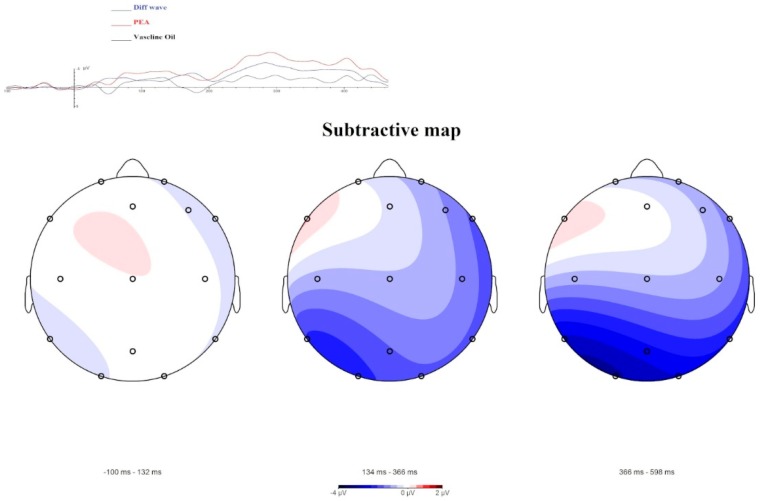
Moreover, the OERP components and Maps obtained (Figure 3), through data comparison (i.e., difference waves between PEA administration and Vaseline Oil administration), highlight greater negativity with a left lateralization.
Figure 3.
A subtractive visualization of the OERP component and of Mapping. The blue line of OERP component shows the difference wave between PEA condition and Vaseline Oil condition. The Mapping representation highlights a negative activity (linked to N1 results) shifted in a delayed temporal range (306–600 ms). This negative amplitude is related to the odorant characteristic of the stimulus.
3.2. VOCs Analysis
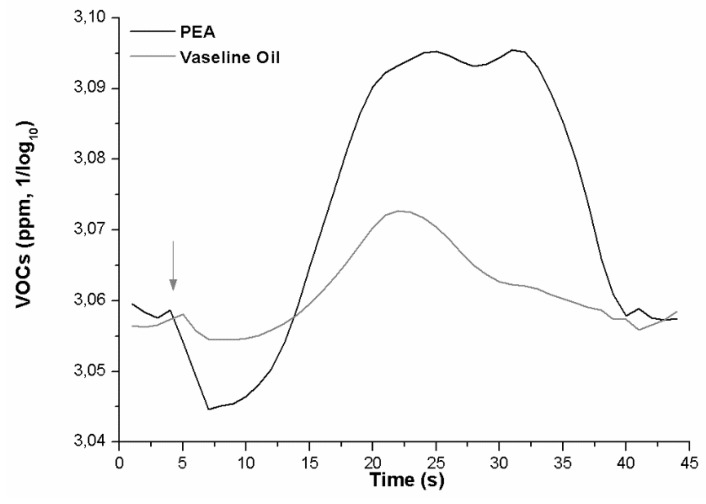
A paired t-test comparison was performed on VOCs Amplitude and Latency. The test returned a significant difference between the VOCs exhaled after PEA administration versus control (t = 5.466; p = 0.012; PEA mean amplitude = 3.08; standard deviation = 0.02; VO mean amplitude = 3.06; standard deviation= 0.006) (Figure 4) in term of greater VOC amplitude elicited by PEA smell.
Figure 4.
Grand average curves of the volatile organic compounds (VOCs) response to PEA (black) and Vaseline oil (grey). The exhaled VOCs metabolic response is about 8 s delayed, by the stimulus administration (arrow), and the recovery occurs after about 20 s.
The analysis of latency highlighted that the onset of the VOCs variation showed dissimilar values (t = 2.917; p = 0.019; PEA onset = 3.56 ms; VO onset = 6.89 ms).
3.3. Behavioral Results
One hundred percent of the subjects reported they perceived the PEA smell during the task. The perceived smell was judged with a low score for the pleasantness (mean = 1.31; standard deviation = 1.01); with low scores for familiarity (mean = 1.8; standard deviation = 1.12) and with a low score for arousal (mean = 1.61; standard deviation = 1.4).
4. Discussion and Conclusions
4.1. OERPs and VOCs can Be Comparable
Recent literature highlights how respiration and metabolic activity can affect ERP components [45,46,47,54] and in particular how the olfactory system is strongly connected to metabolic and respiratory activity [4,41]. However, we are not aware of how our cortical system is related to respiratory metabolic activity. Being in the early stages of this research topic, it seemed particularly useful to work on a single sensorial/anatomic channel that is linked to the olfactory system. Therefore, our investigation is focused on how the olfactory system produces, through the receptor and cortical response, a perceptual variation induced by stimulation and how and when the metabolic system could be affected. Another open question is whether the distributions of the evoked answers (OERP and VOCs) are somehow comparable/superimposable. VOCs and OERPs analysis, through an electrophysiological and psychophysiological approach, can be an interesting starting point to begin an investigation of this evaluation.
The research data show that, in our setting, the N1—a component closely related to the sensory/perceptive characteristics of stimulation [21]—is more sensitive to highlighting differences between the two different stimuli. In particular, our results highlight a significant difference between PEA and Vaseline oil, in the direction of an ampler and faster N1 elicited by PEA stimulation and a decreased LPC in Cz and Pz electrode site. This point can indicate that subjects perceive the PEA smell at a sensorial level and have an allocation of perceptive resource for Vaseline Oil, where the sensorial connotation of the smell is absent. Furthermore, the sites of N1 cortical localization for PEA and Vaseline Oil are different: PEA stimulus evokes greater N1 in the left centroparietal lobe, while Vaseline Oil evokes N1 in an equivalent way, even if less ample, both in the right and left hemisphere. Subtractive Maps indicate that the odour perception (obtained by subtracting the activation acquired with PEA from that with Vaseline Oil), is localized in the left centrotemporal lobe. At the behavioural level the subjects evaluated the PEA odour as having little activating effect, as unfamiliar and unpleasant. The exhaled VOCs show a curve with a trend that starts with a downward deflection and then grows towards increased values. The exhaled VOCs’ amplitudes follow the trend of the OERP results. The results suggest that VOCs are highly sensitive to stimuli and that the PEA stimulus is highly discernible compared to the control. Moreover, the maximum amplitude visible in the grand average of the VOCs is present for around 20 s and thereafter there is a refractory time in which the sensor continues to record the VOCs present in the air, but in decay.
4.2. The Slow Link between Olfactory Perception and VOC Metabolic Response
According to our data, this temporal range present from OERP (a temporal range between baseline time 0 and 600 ms) to VOCs (a temporal range between 600 ms and about 3.5 s) could be the internal metabolic time for which the odorous stimulus, once perceived at the cortical level, is metabolized and subsequently exhaled. In the expiration phase therefore, volatile chemical compounds have their concentration time in the area and decay (temporal range between 2.7 and 40 s). This level of concentration is equivalent to the level of cortical processing of the stimulus. The lateralization showed in the left hemisphere for PEA smell could be considered in this case an attempt to understand and discriminate the stimulation at the conscious level, as the stimulus PEA can be categorized as a floral stimulus or as the smell of a rose. On the other hand, current OERP and VOCs data suggest that the Vaseline Oil stimulus does not allocate the attentive, perceptive and metabolic resource as the PEA does. At the most integrated level, this psychophysiological bridge between cortical activation and metabolic response is a slow pathway because it requires, among other things, the involvement of involuntary and voluntary motor acts, such as respiratory ones.
4.3. Limitation of the Study and Pathway for Future Research
Our future goal will be to investigate, in OERP and VOCs, what happens in the temporal range from a time of 0 to 3 s at the sensory, metabolic and perceptive levels, after sensorial and perpetual olfactive stimulation. Moreover, we aim to evaluate this connection through a survey of the exhaled VOCs analysed through their chemical components and the connection between several different odorants. We think that the perception of a pleasant or unpleasant or neutral substance can prompt different metabolic mechanisms to action and therefore produce different endogenous cascade activations.
A significant limitation of this work included not being able to analyse the VOCs in their main components and in their chemical definitions, elements which therefore did not allow us to thoroughly investigate how central processes can guide peripheral processes but has allowed us exclusively to observe times and overlaps of characteristics of components. In any case, this work is the basis, albeit limited, for developing future research full of possibilities.
Acknowledgments
We thank Giovanni Montagna, Simonetta Capone and Pietro Aleardo Siciliano to support the MI2014A001344.
Author Contributions
Conceptualization, S.I.; methodology, S.I. (OERPs), A.M. (VOCs); validation, S.I. (OERPs), A.M. (VOCs); formal analysis S.I. (OERP and VOCs), A.M. (VOCs); investigation, S.I., A.M.; data curation, S.I., A.M.; writing—original draft preparation, S.I. with the contribute of A.M.; writing—review and editing, S.I.
Funding
This research was co-funded by 5 × 1000 University of Salento, funds for young researcher.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Secundo L., Snitz K., Sobel N. The perceptual logic of smell. Curr. Opin. Neurobiol. 2014;25:107–115. doi: 10.1016/j.conb.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Oelschläger H.H. The dolphin brain—A challenge for synthetic neurobiology. Brain Res. Bull. 2008;75:450–459. doi: 10.1016/j.brainresbull.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 3.Palouzier-Paulignan B., Lacroix M.-C., Aimé P., Baly C., Caillol M., Congar P., Julliard A.K., Tucker K., Fadool D.A. Olfaction Under Metabolic Influences. Chem. Senses. 2012;37:769–797. doi: 10.1093/chemse/bjs059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riera C.E., Tsaousidou E., Halloran J., Follett P., Hahn O., Pereira M.M., Ruud L.E., Alber J., Tharp K., Anderson C.M., et al. The Sense of Smell Impacts Metabolic Health and Obesity. Cell Metab. 2017;26:198–211. doi: 10.1016/j.cmet.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Garrison J.L., Knight Z.A. Linking smell to metabolism and aging. Science. 2017;358:718–719. doi: 10.1126/science.aao5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks K.H., Chuah M.I., King A.E., Vickers J.C. Connectivity of Pathology: The Olfactory System as a Model for Network-Driven Mechanisms of Alzheimer’s Disease Pathogenesis. Front. Neuroinform. 2015;7:234. doi: 10.3389/fnagi.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang M., Crawley J.N. Simple Behavioral Assessment of Mouse Olfaction. Curr. Protoc. Neurosci. 2009;48:8.24.1–8.24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wesson D.W., Levy E., Nixon R.A., Wilson D.A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J. Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedner M., Larsson M., Arnold N., Zucco G.M., Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J. Clin. Exp. Neuropsychol. 2010;32:1062–1067. doi: 10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- 10.Hummel T., Sekinger B., Wolf S.R., Pauli E., Kobal G. ’Sniffin’ sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Khan R.M., Hartley C.A., Sobel N., Sullivan E.V., Gabrieli J.D. Sniffing Longer rather than Stronger to Maintain Olfactory Detection Threshold. Chem. Senses. 2000;25:1–8. doi: 10.1093/chemse/25.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Giessel A.J., Datta S.R. Olfactory maps, circuits and computations. Curr. Opin. Neurobiol. 2014;24:120–132. doi: 10.1016/j.conb.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trimmer C., Mainland J.D. Conn’s Translational Neuroscience. Academic Press; Cambridge, MA, USA: 2016. The Olfactory System. [Google Scholar]
- 14.Doty R.L. Olfaction. Annu. Rev. Psychol. 2001;52:423–452. doi: 10.1146/annurev.psych.52.1.423. [DOI] [PubMed] [Google Scholar]
- 15.Poroyko V.A., Carreras A., Khalyfa A., Khalyfa A.A., Leone V., Peris E., Almendros I., Gileles-Hillel A., Qiao Z., Hubert N., et al. Chronic Sleep Disruption Alters Gut Microbiota, Induces Systemic and Adipose Tissue Inflammation and Insulin Resistance in Mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Invitto S., Calcagnì A., Piraino G., Ciccarese V., Balconi M., De Tommaso M., Toraldo D.M. Obstructive sleep apnea syndrome and olfactory perception: An OERP study. Respir. Physiol. Neurobiol. 2019;259:37–44. doi: 10.1016/j.resp.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Gschwend O., Abraham N.M., Lagier S., Begnaud F., Rodríguez I., Carleton A. Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat. Neurosci. 2015;18:1474–1482. doi: 10.1038/nn.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noguchi D., Sugimoto S., Bannai Y., Okada K.-I. Time characteristics of olfaction in a single breath; Proceedings of the SIGCHI Conference on Human Factors in Computing Systems 2011; Vancouver, BC, Canada. 7–12 May 2011; p. 83. [Google Scholar]
- 19.Lorig T.S. The application of electroencephalographic techniques to the study of human olfaction: A review and tutorial. Int. J. Psychophysiol. 2000;36:91–104. doi: 10.1016/S0167-8760(99)00104-X. [DOI] [PubMed] [Google Scholar]
- 20.Kobal G., Hummel T. Olfactory (chemosensory) event-related potentials. Toxicol. Ind. Heal. 1994;10:587–596. [PubMed] [Google Scholar]
- 21.Gudziol H., Fischer J., Bitter T., Guntinas-Lichius O. Chemosensory event-related brain potentials (CSERP) after strictly monorhinal stimulation. Int. J. Psychophysiol. 2014;93:305–310. doi: 10.1016/j.ijpsycho.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Andrews G., Howie P.M., Dozsa M., Guitar B.E. Stuttering: Speech pattern characteristics under fluency-inducing conditions. J. Hear. Res. 1982;25:208–216. doi: 10.1044/jshr.2502.208. [DOI] [PubMed] [Google Scholar]
- 23.Sojka B., Ferstl R., Pause B.M. Central Processing of Odor Concentration is a Temporal Phenomenon as Revealed by Chemosensory Event-Related Potentials (CSERP) Chem. Senses. 1997;22:9–26. doi: 10.1093/chemse/22.1.9. [DOI] [PubMed] [Google Scholar]
- 24.Pause B.M., Krauel K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int. J. Psychophysiol. 2000;36:105–122. doi: 10.1016/S0167-8760(99)00105-1. [DOI] [PubMed] [Google Scholar]
- 25.Kobal G., Hummel T. Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope Investig. Otolaryngol. 1998;108:1033–1035. doi: 10.1097/00005537-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Geisler M.W., Murphy C. Event-related brain potentials to attended and ignored olfactory and trigeminal stimuli. Int. J. Psychophysiol. 2000;37:309–315. doi: 10.1016/S0167-8760(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 27.Barz S., Pauli E., Kobal G., Hummel T. Chemosensory event-related potentials change with age. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1998;108:208–217. doi: 10.1016/s0168-5597(97)00074-9. [DOI] [PubMed] [Google Scholar]
- 28.Invitto S., Piraino G., Ciccarese V., Carmillo L., Caggiula M., Trianni G., Nicolardi G., Di Nuovo S., Balconi M. Potential Role of OERP as Early Marker of Mild Cognitive Impairment. Front. Neuroinform. 2018;10:272. doi: 10.3389/fnagi.2018.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brauchli P., Rüegg P.B., Etzweiler F., Zeier H. Electrocortical and Autonomic Alteration by Administration of a Pleasant and an Unpleasant Odor. Chem. Senses. 1995;20:505–515. doi: 10.1093/chemse/20.5.505. [DOI] [PubMed] [Google Scholar]
- 30.Lorig T.S., Schwartz G.E. Brain and odor: I. Alteration of human EEG by odor administration. Psychobiology. 1988;16:281–284. [Google Scholar]
- 31.Phillips M., Herrera J., Krishnan S., Zain M., Greenberg J., Cataneo R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999;729:75–88. doi: 10.1016/S0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 32.Mazzatenta A., Pokorski M., Cozzutto S., Barbieri P., Verratti V., Giulio C. Advances in Experimental Medicine and Biology. Springer; Dordrecht, The Netherlands: 2013. Di Non-invasive assessment of exhaled breath pattern in patients with multiple chemical sensibility disorder. [DOI] [PubMed] [Google Scholar]
- 33.Mazzatenta A., Pokorski M., Di Giulio C. Real-Time Breath Analysis in Type 2 Diabetes Patients During Cognitive Effort. Adv. Exp. Med. Biol. 2013;788:247–253. doi: 10.1007/978-94-007-6627-3_35. [DOI] [PubMed] [Google Scholar]
- 34.Mazzatenta A., Di Giulio C., Pokorski M. Pathologies currently identified by exhaled biomarkers. Respir. Physiol. Neurobiol. 2013;187:128–134. doi: 10.1016/j.resp.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Mazzatenta A., Pokorski M., Di Giulio C. Real time analysis of volatile organic compounds (VOCs) in centenarians. Respir. Physiol. Neurobiol. 2015;209:47–51. doi: 10.1016/j.resp.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Ionescu R., Broza Y., Shaltieli H., Sadeh D., Zilberman Y., Feng X., Glass-Marmor L., Lejbkowicz I., Müllen K., Miller A., et al. Detection of Multiple Sclerosis from Exhaled Breath Using Bilayers of Polycyclic Aromatic Hydrocarbons and Single-Wall Carbon Nanotubes. ACS Chem. Neurosci. 2011;2:687–693. doi: 10.1021/cn2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tisch U., Schlesinger I., Ionescu R., Nassar M., Axelrod N., Robertman D., Tessler Y., Azar F., Marmur A., Aharon-Peretz J., et al. Detection of Alzheimer’s and Parkinson’s disease from exhaled breath using nanomaterial-based sensors. Nanomedicine. 2013;8:43–56. doi: 10.2217/nnm.12.105. [DOI] [PubMed] [Google Scholar]
- 38.Mazzatenta A., Pokorski M., Sartucci F., Domenici L., Di Giulio C. Volatile organic compounds (VOCs) fingerprint of Alzheimer’s disease. Respir. Physiol. Neurobiol. 2015;209:81–84. doi: 10.1016/j.resp.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Chopra A., Baur A., Hummel T. Thresholds and chemosensory event-related potentials to malodors before, during, and after puberty: Differences related to sex and age. NeuroImage. 2008;40:1257–1263. doi: 10.1016/j.neuroimage.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Schriever V.A., Góis-Eanes M., Schuster B., Huart C., Hummel T. Olfactory Event-Related Potentials in Infants. J. Pediatr. 2014;165:372–375. doi: 10.1016/j.jpeds.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Mazzatenta A., Pokorski M., Di Tano A., Cacchio M., Di Giulio C. Advances in Experimental Medicine and Biology. Springer; Dordrecht, The Netherlands: 2016. Influence of sensory stimulation on exhaled volatile organic compounds. [DOI] [PubMed] [Google Scholar]
- 42.Bradley T.D., Floras J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 43.Lim D.C., Pack A.I. Obstructive sleep apnea and cognitive impairment: Addressing the blood–brain barrier. Sleep Med. Rev. 2014;18:35–48. doi: 10.1016/j.smrv.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masaoka Y., Harding I.H., Koiwa N., Yoshida M., Harrison B.J., Lorenzetti V., Ida M., Izumizaki M., Pantelis C., Homma I. The neural cascade of olfactory processing: A combined fMRI–EEG study. Respir. Physiol. Neurobiol. 2014;204:71–77. doi: 10.1016/j.resp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Atchley R., Klee D., Memmott T., Goodrich E., Wahbeh H., Oken B. Event-related potential correlates of mindfulness meditation competence. Neuroscience. 2016;320:83–92. doi: 10.1016/j.neuroscience.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menicucci D., Artoni F., Bedini R., Pingitore A., Passera M., Landi A., L’Abbate A., Sebastiani L., Gemignani A. Brain Responses to Emotional Stimuli During Breath Holding and Hypoxia: An Approach Based on the Independent Component Analysis. Brain Topogr. 2014;27:771–785. doi: 10.1007/s10548-013-0349-z. [DOI] [PubMed] [Google Scholar]
- 47.Van Houdt P.J., Ossenblok P.P.W., Van Erp M.G., Schreuder K.E., Krijn R.J.J., Boon P.A.J.M., Cluitmans P.J.M. Automatic breath-to-breath analysis of nocturnal polysomnographic recordings. Med. Biol. Eng. Comput. 2011;49:819–830. doi: 10.1007/s11517-011-0755-x. [DOI] [PubMed] [Google Scholar]
- 48.Miwa T., Tsukatani T., Furukawa M., Costanzo R.M. Detection Thresholds for Phenyl Ethyl Alcohol Using Serial Dilutions in Different Solvents. Chem. Senses. 2003;28:25–32. doi: 10.1093/chemse/28.1.25. [DOI] [PubMed] [Google Scholar]
- 49.Invitto S., Capone S., Montagna G., Siciliano P.A. MI2014A001344 Method and System for Measuring Physiological Parameters of a Subject Undergoing an Olfactory Stimulation. No. 20170127971A1. U.S. Patent. 2017 May 11;
- 50.Invitto S., Capone S., Montagna G., Siciliano P. Convegno Nazionale Sensori. Volume 431. Springer; Cham, Switzerland: 2018. Virtual olfactory device in EEG and olfactory conditioning task: An OERP study. [Google Scholar]
- 51.Nuwer M.R., Lehmann D., Da Silva F.L., Matsuoka S., Sutherling W., Vibert J.-F. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994;91:1–5. doi: 10.1016/0013-4694(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 52.Luck S.J. An Introduction to the Event-Related Potential Technique. MIT press; Cambridge, MA, USA: 2005. [Google Scholar]
- 53.Invitto S., Piraino G., Mignozzi A., Capone S., Montagna G., Siciliano P.A., Mazzatenta A., Rocco G., De Feudis I., Trotta G.F., et al. Smell and Meaning: An OERP Study. Volume 69 Springer; Cham, Switzerland: 2017. [Google Scholar]
- 54.Moore A.W., Gruber T., Derose J., Malinowski P. Regular, brief mindfulness meditation practice improves electrophysiological markers of attentional control. Front. Hum. Neurosci. 2012;6:18. doi: 10.3389/fnhum.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]