Abstract
Small guanosine triphosphatases (GTPases) gathered in the Rat sarcoma (Ras) superfamily represent a large family of proteins involved in several key cellular mechanisms. Within the Ras superfamily, the Ras homolog (Rho) family is specialized in the regulation of actin cytoskeleton-based mechanisms. These proteins switch between an active and an inactive state, resulting in subsequent inhibiting or activating downstream signals, leading finally to regulation of actin-based processes. The On/Off status of Rho GTPases implicates two subsets of regulators: GEFs (guanine nucleotide exchange factors), which favor the active GTP (guanosine triphosphate) status of the GTPase and GAPs (GTPase activating proteins), which inhibit the GTPase by enhancing the GTP hydrolysis. In humans, the 20 identified Rho GTPases are regulated by over 70 GAP proteins suggesting a complex, but well-defined, spatio-temporal implication of these GAPs. Among the quite large number of RhoGAPs, we focus on p190RhoGAP, which is known as the main negative regulator of RhoA, but not exclusively. Two isoforms, p190A and p190B, are encoded by ARHGAP35 and ARHGAP5 genes, respectively. We describe here the function of each of these isoforms in physiological processes and sum up findings on their role in pathological conditions such as neurological disorders and cancers.
Keywords: GTPases, GTPase-activating proteins, actin, RhoA, acto-myosin, cancers, neurological diseases
1. Introduction
Small guanosine triphosphatases (GTPases) of the Rat sarcoma (Ras) superfamily are molecular switchers of key signaling pathways. Within the Ras superfamily, members of the Ras homolog (Rho) family are crucial to regulate actin-based processes such as adhesion, migration, or cell division. The alteration of Rho pathways is implicated in various pathological conditions including cancer, cardiovascular diseases, or developmental disorders [1,2,3]. In physiological conditions, these key cellular processes need to be tightly controlled in space and time at the molecular level. Regulation of small GTPases occurs through nucleotide binding and hydrolysis allowing the well-known guanosine diphosphate (GDP)/guanosine triphosphate (GTP) cycle. Thus, the spatiotemporal regulation of this cycle is determinant for the accomplishment of signaling cascades and downstream cellular processes. The main regulators of the GDP/GTP cycle of Rho GTPases are the guanine nucleotide exchange factors (GEFs) and the GTPase-activating proteins (GAPs), allowing, respectively, GTPase activation or inhibition.
The p190RhoGAP paralogs, p190RhoGAP-A (p190A, glucocorticoid receptor DNA-binding factor 1 (GRF-1), or GRLF1, encoded by the ARHGAP35 gene) and p190RhoGAP-B (p190B, encoded by the ARHGAP5 gene), correspond to the two main GAPs regulating the Rho family of small GTPases. Both isoforms are large (≈1500 amino acid long) multidomain proteins with their catalytic RhoGAP domain at the C-terminus. In humans, p190A (Uniprot Q9NRY4) and p190B (Uniprot Q13017) share over 50% sequence identity, and especially 70% for their GAP domain, but only 45% for the middle domain, which is the most divergent domain between both isoforms. The ARHGAP35 gene is conserved in several species like chimpanzee, Rhesus monkey, dog, cow, mouse, rat, chicken, zebrafish, fruit fly, mosquito, and frog, whereas the ARHGAP5 gene is present in mouse, chicken, lizard, and zebrafish. Drosophila shows a single p190RhoGAP (Uniprot Q9VX32), which shares 54% identity with human p190A [4]. Amino acid residues of the human and mouse p190B proteins are approximately 97% identical [5]. Of note, in cellular studies, rat p190A (Uniprot P81128), which is over 98% identical to human p190A, is often used to address p190A function.
Herein, we sum up their best-known cellular roles as regulators of Rho-dependent signaling processes such as cell adhesion, migration, invasion, and cytokinesis, even if Rho-independent implication of p190RhoGAPs emerges in the literature. We also address their important roles in development and in diseases.
2. p190RhoGAP Architecture
The two isoforms of p190RhoGAP share a common domain organization. Each domain has been studied either in a structural and/or functional approach (Figure 1). In addition to Figure 1 showing p190RhoGAP architecture, Figure 2 summarizes p190A and p190B partners.
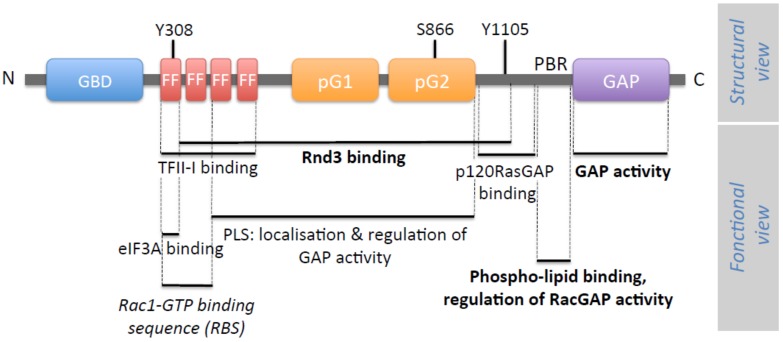
Figure 1.
Structure of p190RhoGAP. The structural organization of p190RhoGAP is represented (structural view) and the corresponding function of each domain is indicated when determined (functional view). From its N-terminus, p190RhoGAP is composed of a guanosine triphosphate (GTP)-binding domain (GBD) (13–249) and four FF domains (FF) (251–533) that can bind TFII-I, eIF3A, or Rac1 proteins. In the middle domain, two pseudoGTPase domains, pG1 (592–767) and pG2 (766–958) have been identified. The protrusion localization sequence (PLS) (380–971) is implicated in the localization and the regulation of the function of p190A. A polybasic region (PBR) (1213–1236), mainly composed of basic amino acids, binds to acidic phospholipids. The GTPase activating proteins (GAP) domain (1259–1513) is located at the C-terminus end of the protein and is responsible for the GAP catalytic function of p190A and p190B. Identified residues involved in the function of p190RhoGAP are indicated on the figure (number of amino acids corresponds to the rat p190A protein sequence). For the functional view, bold indicates data obtained for both p190A and p190B. Italic indicates data obtained only for p190B, whereas basic font indicates data obtained for p190A.
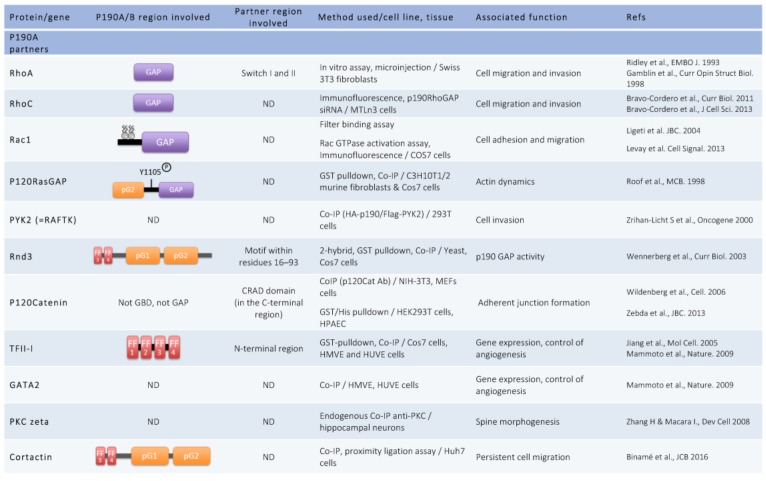
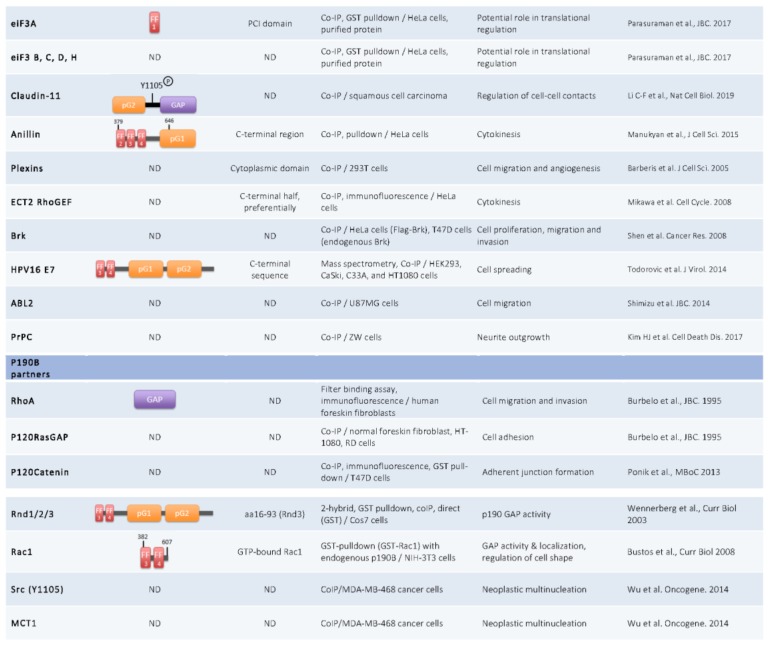
Figure 2.
P190A and p190B partners. Co-IP: Co-immunoprecipitation; ND: Not Determined.
The RhoGAP domain is located at the C-terminal end of p190RhoGAP proteins. Both p190A and B have mainly a catalytic activity towards RhoA [6,7] and p190A represents 60% of the RhoGAP activity in cultured fibroblasts [8]. Arg1283 was identified as the active site allowing efficient catalysis of RhoA-GTP [9]. By regulating RhoA activity, expression of p190RhoGAP in cells alters actin-based stress fibers formation, readout of RhoA function. Work from Dr. Ligeti’s laboratory demonstrates that both p190RhoGAP isoforms can modulate their substrate specificity by switching their GAP activity from RhoA to Rac1 upon interaction between a polybasic region (PBR) located near the GAP domain and phospholipids [10,11] (Figure 1). Like most of the RhoGAP proteins, besides their RhoGAP module, p190A and B contain several domains conferring plasma membrane-binding protein interactions, which modulate their GAP activity as well as provide them additional functions.
The N-terminal part of p190RhoGAPs displays a GTP-binding domain (GBD), which was recently reclassified as a class (ii) pseudo-GTPase domain, meaning a nucleotide-binding catalytically inactive GTPase [4]. Based on crystal structure and on biochemical data, Stiegler and Boggon showed that the N-terminal domain of p190A binds constitutively to GTP/Mg2+ but lacks intrinsic catalytic activity [4]. They further suggested that the role of GTP binding is not to act as a conformational regulator, but rather contributes to stabilizing the structure of the domain. This newly identified domain would so consist of a protein–protein interaction domain. In previous papers, GTP binding was reported to be regulated by a Src-mediated phosphorylation in this domain [12], but the impact of the lack of GTP binding on protein activity is not clear [13,14].
Closed to the GBD, four FF domains have been identified, each consisting of 50 amino acids motifs with two strictly conserved phenylalanines. These FF domains are usually found in nuclear RNA-regulating proteins, making p190RhoGAPs the only cytoplasmic proteins containing FF domains. This region has been implicated in interactions with proteins like the transcription factor TFII-I [15] or the translation preinitiation factor eiF3A [16] (Figure 2). These interactions have been reported to be regulated by phosphorylation. Phosphorylation of Tyr308 by PDGF Receptor within the first FF domain inhibits the interaction with TFII-I [15], but does not affect eiF3A interaction, which, itself, requires phosphorylation of Ser296 [16] (Figure 1 and Figure 2).
The former large “middle domain” defined by the region between the FF and GAP domains contains two newly identified cryptic GTPase-like domains which were termed pG1 and pG2 and classified as class (i) pseudo-GTPase domains described with no nucleotide-binding activity (and therefore no catalytic activity) [17]. pG1 and pG2, encompassing, respectively, residues 592–767 and 766–958 in p190A, constitute two evolutionarily conserved domains, indicating that they may contribute to necessary functions [18]. Intriguingly, based on molecular and cellular studies, we demonstrated that an overlapping region (residues 380–971), including two FF motifs, pG1 and pG2, is necessary to target p190A to actin-based protrusions such as lamellipodia but also to regulate the GAP activity. We further hypothesized that this region, named PLS for protrusion localization sequence, could be involved in an autoinhibitory interaction allowing a closed/inhibited form of the protein [19]. In light of these new structural results, one attractive hypothesis is that one of the pseudo-GTPase domains could bind in cis to the GAP domain to regulate its activity. However, this hypothesis remains to be tested. Within pG2, Ser866 appears as a key residue regulating p190A localization and function. In fact, mutation of this serine into phenylalanine results in a higher GAP activity that alters tumor cell migration [20].
Moreover, in this former middle domain, the regions encompassing residues 382–1007 in p190A/B and residues 382–607 in p190B were shown to interact, respectively, with the atypical Rho family member Rnd3 [21], and the active Rac1 [22], modifying p190RhoGAP activities. Thus, p190RhoGAP function appears tightly regulated by this large middle domain.
Finally, among the other residues important in the regulation and function of p190A, Tyr1105 was identified early as a Src phosphorylation site involved in the association of p190A with the Ras GAP protein, p120RasGAP and, consequently, in its recruitment to the plasma membrane [23,24,25,26] (Figure 2). Although phosphorylation of Y1105 is essential for complex formation, additional phosphorylation of Y1087 in p190A helps to stabilize the interaction [25]. Tyr1105 was also demonstrated to be substrate of other non-receptor tyrosine kinases such as Brk (breast tumor kinase) and ABL2 (v-Abl Abelson murine leukemia viral oncogene homolog 2)/Arg (Abelson-related gene) kinase resulting in the activation of p190RhoGAP [27,28].
3. p190RhoGAP Cellular Functions
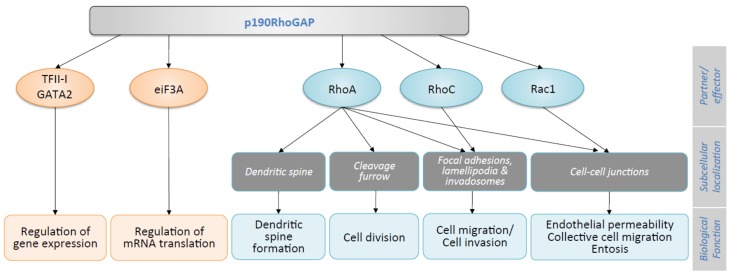
Due to their enzymatic activity consisting of an increased GTP hydrolytic rate and thereby an attenuation of the biological function of small GTPases, GAPs are often labeled as signal terminators. However, thanks to interaction with key partners, GAPs may also function as signal initiators and/or transmitters [29,30]. Function of both p190RhoGAP isoforms is intrinsically linked to RhoA regulation, even if more recently, few roles independently of RhoA were suggested (Figure 3). Of note, most of the data available, summarized in Figure 3, focused on p190A, while only few on p190B.
Figure 3.
Cellular functions of p190A. P190A functions occurring independently of small GTPases are indicated in orange and those implicating the small GTPases are indicated in blue.
3.1. RhoA-Dependent Cell Mechanisms
P190RhoGAPs play their major role by inhibiting the GTPase RhoA and thus its downstream effectors, the Rho-associated coiled-coil-containing protein kinase (ROCK) and the formin mDia. Via ROCK, RhoA controls the phosphorylation of myosin light chain, thus cell contractility, and the cleavage of F-actin by inhibiting cofilin activity. Via mDia, RhoA promotes actin polymerization. Inhibition of RhoA by toxins or dominant negative inhibitors in vitro demonstrated essential functions of this GTPase in stress fiber formation, maintenance of cell–cell contacts, cytokinesis, and cell migration [2,31]. Consequently, p190RhoGAP activity leads to a major decrease in acto-myosin/contractility and thus impacts various RhoA dependent-cell mechanisms. This regulatory function depends on p190RhoGAP subcellular localizations and partner interactions (Figure 2 and Figure 3). Indeed, p190A accumulates in actin-rich structures where a regulation of RhoA is necessary. Localization of the protein in new adhesion sites [32], lamellipodia/ruffles [23], and podosomes/invadopodia [33,34] involved p190RhoGAP in cell migration and invasion. These well-described functions were reviewed elsewhere [19]. Other functions of p190A such as endothelial permeability, collective cell migration, entosis, cell division, and synaptic transmission are described herein. These roles are linked to p190A redistribution at either intercellular junctions [35], cleavage furrow [36], or dendritic spines [37].
3.1.1. Cell–Cell Junctions and Endothelial Permeability
Cadherin engagement to form adherens junctions was previously shown to inhibit RhoA activity via p190A [38]. This occurs through an interaction between p190A and p120-catenin at cell–cell junctions allowing p190A translocation to the membrane where it plays its role of GTPase regulator [35]. A domain in the C-terminus part of p120-catenin, named CRAD (catenin-RhoGAP-association domain) was recently identified as the minimal domain of interaction with p190A (Figure 2). In endothelial cells, depletion of the CRAD of p120-catenin affects p190A translocation to the membrane and is therefore responsible for an increase of RhoA and a decrease of Rac1 signaling, altering endothelial permeability [39].
3.1.2. Cell–Cell Junctions and Collective Cell Migration
The sub-cellular localization of p190A at cell–cell junctions was further described to play a role in collective cell migration of squamous cell carcinoma [40]. Indeed, inhibiting RhoA activity is required to decrease actomyosin activity at cell–cell contacts and favor coordinated cell movement. More recently, this role was demonstrated to be important in the formation of circulating tumor cell clusters. An interaction between claudin-11 and p190A is required to downregulate RhoA at intercellular junctions in order to maintain stable cell–cell contacts during dissemination of these circulating tumor cells [41]. The RhoGAP activity at cell–cell junctions may also implicate p190B. Indeed, in breast cancer cells, RhoA activity at cell–cell contact is regulated by p190B through a complex with p120-catenin (Figure 2). Interestingly, this complex formation is modulated in response to matrix rigidity. This suggests that a cross-talk between cell–cell contact and cell-matrix adhesion is generated by the p190B/p120-catenin complex to spatially regulate RhoA activity in response to changes in tensional homeostasis [42].
3.1.3. Cell–Cell Junctions and Entosis
Throughout its role on attenuation of cell contractility via RhoA down-regulation, p190A was recently implicated in the entotic mechanism. Entosis is described as a non-apoptotic cell death program for cancer cells, where one cell is able to ingest its living neighboring cell. This competition between tumor cells results from a difference in cell contractility between the invading (i.e., loser) and the engulfing (i.e., winner) cell [43]. On one hand, the winner cell exhibits high mechanical deformability, allowing changes in its shape and morphology. On the other hand, the invading cell accumulates actin and myosin at the cell cortex and the resulting mechanical tension drives the cell-in-cell invasion process. In this cellular mechanism, increased cell tension is regulated by Rho/ROCK following establishment of cell–cell adhesion [44,45]. Localization of p190A at cell–cell junctions in the invading cell allows the polarization of RhoA activity and the distribution of tension at the back, which promotes entosis. Accordingly, p190A is required for entosis, and downregulation of its expression reduced entosis [45].
3.1.4. Cell Division
In eukaryotic cells, by its interaction with anillin, p190A localizes at the cleavage furrow of cells allowing a role of this protein during late mitosis [46,47]. Level of endogenous p190A is regulated during cell cycle leading to a decrease of the protein allowing cytokinesis completion. This transient decrease of p190A level during late mitosis is due to degradation of the protein via ubiquitination of the GBD domain [46]. P190A acts antagonistically with the RhoGEF ECT2 at the cleavage furrow where both proteins co-localize and probably interact [48]. A decrease in p190A level consequently regulates RhoA activity at the contractile ring, allowing completion of cytokinesis [36]. However, cells depleted of p190A failed to divide as well, as RhoA GTP induces increased contractility. Thus, p190A, by interacting with anillin, allows a fine regulation of the level of active RhoA necessary for cell division (Figure 2) [47]. However, p190A involvement in mitosis appeared more complex. In addition to its role in cytokinesis, p190A was recently implicated in regulating the mitotic spindle [49]. Interestingly, independently of its GAP activity, p190A is required for bipolar spindle formation in HeLa and RPE cells. This new role of p190A was demonstrated to occur through the regulation of the kinesin Eg5 and the kinase Aurora A activities [49].
3.1.5. Dendritic Spines and Neuronal Morphogenesis
In addition to the roles described above, p190RhoGAP was implicated in the regulation of actin cytoskeletal rearrangements in cells from the central nervous system, with implications in brain functioning [50,51,52,53]. In Drosophila, p190RhoGAP was identified as essential for axon branch stability in body neurons, olfactory learning, and the memory center [53]. In fact, activation of RhoA or inactivation of p190A leads, in both cases, to axon branch retraction. The axon retraction pathway implicates Drok (Drosophila ROCK) and the myosin regulatory light chain [53]. Moreover, outgrowth of cellular extensions and formation of dendritic spines, which constitute actin-rich protrusions on dendrites essential for cognitive functions, are driven by actin cytoskeleton rearrangement and therefore under the control of RhoGTPases which are negatively regulated by p190A. In 2008, Zhang and Macara’s work on dendritic spines implicated the polarity protein PAR-6 and p190A in spine morphogenesis. They showed that, in hippocampal neurons, where p190A and RhoA are downstream effectors of the PKC/PAR6 complex, the catalytic GAP activity of p190A is necessary for spine formation, and depletion of p190A leads to a decrease of spine density [37]. p190A is also implicated in neuronal morphogenesis in the developing brain. Indeed, phosphorylation of p190A by Arg kinase leads to its interaction with p120RasGAP and regulates neuronal morphogenesis in mice postnatal brain. The p190/p120RasGAP complex was demonstrated to induce neuritogenesis in neuroblastoma cells [27]. Finally, in a recent study, p190A has been linked to the cellular prion protein (PrPc), a highly conserved glycosylphosphatidylinositol (GPI)-anchored membrane protein involved in neurite outgrowth (Figure 2). A p190A/PrPc complex, which is impaired in the case of disease-associated mutants of PrPc, may be important to prevent prion-related neurodegeneration [54]. The impact of p190A in neuronal functions was further highlighted by p190A KO mice phenotypes (see paragraph 4) and by its involvement in neuronal diseases (Section 5.1).
3.2. RhoA-Independent Functions
Besides its RhoA-mediated functions, p190A also acts via other small GTPases such as RhoC and Rac1 or, as recently reported, via non-small GTPase pathways (Figure 3).
3.2.1. RhoC/Rac1 Dependent Cell Migration and Invasion
In addition to RhoA, p190A demonstrated GAP activity towards RhoC and Rac1 (Figure 2). RhoC is a Rho family GTPase, which shares a high homology with RhoA [55] and promotes migration and invasion in many cancer cell types [56,57]. The activity of RhoC is regulated by both p190RhoGAP and p190RhoGEF at invadopodia [58] but also at protrusions at the leading edge of cells [59]. The spatial regulation of RhoC modulates protrusion formation by acting on actin dynamics and interfering with either of these proteins affect protrusion formation [60].
P190RhoGAPs display a GAP activity on Rac1 as well [11,61]. Based on in vitro experiments, the ability of p190RhoGAP to switch its GAP activity from RhoA to Rac1 was reported. In fact, due to the presence of some acidic phospholipids in the membrane, the activity of both p190A and p190B is modulated, leading to inhibition of RhoGAP activity and stimulation of RacGAP activity [11]. The switch between RhoGAP and RacGAP activity also depends on post-translational modification like prenylation of the GTPase [61]. The domain of interaction with acidic phospholipids was mapped and named PBR, consisting of a polybasic region composed of 24 amino acids close to the GAP domain in p190A (aa1213–1236) (Figure 1). Phosphorylation of Ser1121 and Thr1226 by PKC in PBR inhibits this interaction regulating thus the switch of GAP activity [62]. Similar to these in vitro experiments, under cellular conditions, p190RhoGAP exhibits RacGAP activity as well. Moreover, the PBR of p190A is necessary for the cellular RacGAP but not for the RhoGAP activity [10].
3.2.2. Gene Expression and Protein Translation
In addition to its classical role of GAP protein acting as a GTPase inhibitor, p190A has been reported to interact with proteins involved in other cell mechanisms such as gene expression or mRNA translation, identifying p190A as a moonlighting protein.
P190A was implicated in gene expression regulation by its interaction with the multifunctional transcription factor TFII-I [63]. Intrigued by the FF domains present in p190A, the group of Pr. Settleman performed a pulldown approach to identify new partners of this domain. They thus identified the serum-responsive transcriptional regulator TFII-I as a specific interactor with the p190A FF domains (Figure 1 and Figure 2), and further demonstrated that these domains sequester TFII-I into the cytoplasm in a phosphorylation-dependent manner in fibroblasts [15]. P190A regulates the nuclear translocation and antagonizes the transcriptional activity of TFII-I. The cytoplasmic retention of TFII-I by p190A was further confirmed in human capillary cells where TFII-I governs vascular endothelial growth factor receptor 2 (VEGFR2) expression [64]. In these cells, p190A also inhibits the nuclear localization of the endothelial transcription factor GATA2. Through the regulation of both transcription factors, TFII-I and GATA2 that act antagonistically on VEGFR2 expression, p190A was showed to control capillary network formation in vitro and retinal angiogenesis in mice [64].
In a similar pulldown approach, p190A was also reported to interact with eiF3A and other translation preinitiation factors (Figure 2). Indeed, five endogenous eIF3 subunits (eiF3A, B, C, D, and H) were identified to co-immunoprecipitate with endogenous p190A, but not p190B, in HeLa cells. This interaction leads to an incomplete and non-functional preinitiation complex. As eIF1A and other important translational preinitiation complex subunits were absent from the p190A complex, the authors hypothesize that p190A might affect the overall rate of mRNA translation by preventing the assembly of complete preinitiation complexes [16]. Given that no previous evidence had suggested a role for p190A GTPase substrates in the rate-limiting initiation step of mRNA translation, this new role of p190A may be independent of its GAP activity, even if this remains to be determined.
These two latter examples suggest that p190A may function as a sequestering molecule through interaction with various proteins in order to regulate key molecular processes such as gene expression and protein synthesis.
4. p190RhoGAP Function during Development
The respective roles of p190A and p190B isoforms in development were studied using Arhgap35 and Arhgap5 knockout in mice. Mice lacking a functional p190A protein die just after birth with several defects in neuronal development. The defects lead to aberrant neuronal morphogenesis and abnormalities in forebrain hemisphere fusion, ventricle shape, optic cup formation, neural tubes closure, and layering of the cerebral cortex [51]. Additional defects were identified in axon guidance and fasciculation [52]. Of note, these animals and mouse embryonic fibroblasts derived from them are not totally knocked out for p190A but instead display a non-functional form of the protein, lacking the GBD domain [51].
Mice lacking p190B also die after birth and exhibit alterations in cell and organism size [65]. As for p190A, p190B-lacking mice exhibit defects in nervous system development suggesting failure in axonogenesis, neuronal differentiation, and neurogenesis [66]. In p190B-deficient mice, a significant reduction in adipogenesis was observed [67] as well as an altered mammary ductal morphogenesis [68]. This latter defect due to p190B deficiency was extensively studied using an inducible system allowing the expression of p190B at different stages of mammary development. p190B overexpression during ductal morphogenesis or during pregnancy alters the terminal end buds (TEB) architecture, leading to disrupted ductal tree, and induces hyperplasic lesions, respectively [69]. As p190B, p190A was involved in mammary gland development but exhibits a distinct role in this process. P190A is implicated in cell adhesion within the TEB and its deletion alters the mammary epithelium differentiation [70].
Altogether, these findings obtained in murine models demonstrated that p190A and p190B play similar but still distinct roles in neuronal and mammary development.
5. p190RhoGAPs in Human Diseases
5.1. p190RhoGAPs in Neuronal Diseases
Due to the implication of p190A in neural development [51,66], few genome-wide and non-bias studies suggested a role of this protein in mental health disorders.
Frontotemporal dementia (FTD) is one of the leading causes of dementia in young patients (<65 years old). Looking for genes potentially implicated in the development of FTD, Mishra and collaborators identified ARHGAP35 as a candidate gene. Indeed, performing gene-based association studies on 3348 clinically identified FTD cases, they reported the novel genetic association of ARHGAP35 with progressive non-fluent aphasia [71]. These data are further consolidated by the fact that ARHGAP35 is strongly expressed in brain tissues, and more specifically, in the anterior cingulate cortex, which is one of the early affected regions in FTD patients [71].
Other studies involved p190A in stress-induced diseases. Stress exposure may lead to neuronal remodeling and structural plasticity. As p190A is localized at dendritic spines, the group of Koleske analyzed the role of p190A in the brain circuit using mice with reduced gene dosage of the protein [72,73]. They determined that p190A is involved in the behavior of mice and cellular response to the prolonged exposure to the major stress hormone, corticosterone [72]. They found that in neural systems, by inhibiting RhoA and subsequently, modulating cellular actomyosin contractility, p190A coordinates behaviorally adaptive outcomes such as mitigating vulnerability to stress hormone exposure, but also to drugs of abuse, as described hereafter. Indeed, underexpression of p190A was correlated with cocaine addiction in mice [73]. p190+/− mice in contrast to WT mice exaggerate locomotor activity and psychomotor sensitivity created by cocaine in link with a structural reorganization in neurons.
In humans, depression and, more precisely, suicide attempts are stress-induced diseases. With the aim to validate several genetic biomarkers associated with suicide attempts in patients with schizophrenia, Li and collaborators confirmed acid phosphatase 1 (ACP1) as a top predictor of suicide attempts [74]. This study also identified ARHGAP35 as a gene whose expression is negatively correlated with APC1, that the authors linked with the transcription repressor of glucocorticoid receptor (NR3C1) to stress response. Altogether, these data suggest that p190A may be involved in phenomena of behavioral vulnerability and resilience throughout life in humans.
Alcohol consumption during pregnancy may lead to severe psychological and organic alterations known as fetal alcohol spectrum (FAS) disorders. Actin cytoskeleton and p190RhoGAP were identified as potential targets of ethanol in astrocytes [75]. Indeed, in in vitro models (HEK293T cells and primary cultures of newborn rat astrocytes), an increase of p190A and p190B activities was detected in response to ethanol chronic exposure. These data provide insights into the molecular mechanism responsible for the neurobehavioral deficiencies induced by alcohol.
In conclusion, p190RhoGAP may act as key factor in cellular response after chronic stress exposure induced by hormones or drugs. Through its role on cytoskeleton remodeling, p190RhoGAP may be linked to the pathogenesis of other stress-related and neurodegenerative diseases.
5.2. p190RhoGAPs in Cancer
Linked to the role of p190RhoGAP in cell cycle progression, cell migration and invasion, many papers described p190A and p190B implication in cancer progression. P190A was first described as a substrate of the oncoprotein c-Src, interacting with p120RasGAP, a key regulator of the Ras oncogene, and subsequently described to contribute to fibroblast transformation [76,77,78,79,80,81]. P190RhoGAP was reported to be involved in several cancer types based on data coming from either patient samples or cell lines (Table 1 and Table 2). Different types of alterations were reported concerning the mRNA or protein expression, the mutational or the phosphorylation status, and/or the GAP activity of p190A and p190B proteins. Most of the studies investigate p190A involvement, whereas only few focus on p190B. Hereinafter, we attempt to summarize data available on p190RhoGAP in the cancer field.
Table 1.
P190RhoGAP’s deregulation in cancers. This table lists data on p190A and 190B obtained from patients. Abbreviations: T, tumoral; NT, non tumoral; nd, not determined; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization.
| Cancer Type | Nb of Tumor/Control | Expression | Detection | Source of Deregulation | Output | References |
|---|---|---|---|---|---|---|
| p190A | ||||||
| Cancer | 3281 T (12 tumor types) | nd | Exome sequencing | Mutations | ARHGAP35 significantly mutated in cancer | Kandoth et al. Nature 2013 |
| 4742 T (21 tumor types) | nd | Exome sequencing | Mutations: 83 missense, 38 nonsense, 16 frameshift and 2 splice site | ARHGAP35 significantly mutated in cancer | Lawrence et al. Nature. 2014 | |
| 191 gliomas + 115 gliomas + 37 ovarian cancers | nd | FISH assay | Locus rearranged/Chromosomal region frequently deleted in solid tumors | Alteration may be responsible of cancerogenesis | Tikoo et al. Gene. 2000 | |
| Osteosarcoma | 247 T | Up | IHC | nd | Poor outcome (larger tumor size, tumor dedifferenciation, high metastatic risk, recurrence-free and overall survival) | Zhao et al. Tumor Biol. 2014 |
| 247 T/428 NT | nd | TaqMan PCR | rs1052667 polymorphism (3′ UTR) | Association with osteosarcoma risk, grade and poor prognosis | Zhao et al. Bio Med Res Int. 2014 | |
| Colorectal cancer | 114 T/NT | Up | qRT-PCR, IHC, WB | nd | Correlation between expression and grade Poor survival outcome |
Li et al. Am Transl Res. 2016 |
| 124 T | nd | PCR-SSCP | c.2834dupA > p.Asn946GlufsX11 | Premature stop/Loss-of-function mutation | Ji Choi et al. Pathol. Oncol. Res. 2017 | |
| Lung adenocarcinoma | 133 T/NT | Up | qRT-PCR, IHC | ↗° phospho-p190A | Poor disease-free survival | Notsuda et al. Int J Oncol. 2013 |
| 660 T/NT | nd | Exome sequencing, RNA seq | Missense & frameshift indel or nonsense mutations | ARHGAP35 alteration in oncogene-negative tumors | Campbell et al. Nature Genetics. 2016 | |
| Hepatocellular carcinoma | 37 T/17 NT | Down | PCR array | nd | Potentially responsible of non-viral hepatocarcinogenesis | Kurokawa et al. J Hepatol. 2003 |
| 44 T | nd | IHC | ↗° Grp78 > FAK > phospho-p190A | Link between Grp78 and tumoral invasion | Su et al. BMC Cancer, 2010 | |
| Hürtle Cell Carcinoma | 41 T/NT | nd | Exome sequencing | p.E1273A | Recurrently mutated in HCC (≈5% samples) | Gopal et al. Cancer cell. 2018 |
| Renal angiomyolipoma | 30 T | nd | Sanger sequencing | p.E1273A | Missense variant with uncertain functional effects | Giannikou et al. PLOS Genetics. 2016 |
| p190B | ||||||
| Hepatocellular carcinoma | 47 T | Up | IHC | Positive correlation between the expression levels of CD147 and p190B | Role in HCC progression by regulation of cell movement through p190B and CD147 | Chen et al. Cancer Cell Int. 2016 |
| Nasopharyngeal carcinoma | 52 T | Up | RT-qPCR, WB | ↗°miR-744 > ↗°p190B | Tumor progression | Fang et al. Oncotarget. 2015 |
| Invasive epithelial ovarian cancer | 18,736 T (10,316 of serous histology) / 26,138 NT | Down | eQTL | nd | G allele of AKAP6 rs927062 correlated with reduced expression of ARHGAP5 | Earp et al. PLoS ONE 2018 |
| Lung cancer | 54 T | Up | IHC, qRT-PCR | Inverse correlation between miR-486-5p and ARHGAP5 expression | Positive correlation of p190B expression and cancer stage and lymph node metastasis | Wang et al. Oncogene. 2014 |
| Colorectal adenomatous polyposis | 221 T / 531 NT | Genome-wide analysis of germline CNV, NGS | Somatic point mutations/Partial duplication | One of 11 promising causative candidate genes | Horpaopan et al. Int. J. Cancer. 2015 | |
| Breast cancer | 120 T / 7 NT | Up | qPCR array | Positive correlation between MCT-1 expression and ARHGAP5 expression in tumors | MCT-1 controls cancer cell dissemination and progression through Src/p190B signaling pathway | Wu et al. Oncogene. 2014 |
Table 2.
Cellular or molecular effects of p190RhoGAP deregulation in cancers. This table lists functional deregulations of p190A or p190B in cancer studied in in vitro and in vivo experiments. Abbreviations: o/e, overexpression; nd, not determined.
| Cancer Type | Cell Lines | Loss or Gain of Function Experiment | Output on p190A Activity | Output on Cell Proliferation | Ouput on Cell Migration/Invasion | Other Output | References |
|---|---|---|---|---|---|---|---|
| p190A | |||||||
| Osteosarcoma | MG63 | Loss (si p190A) | nd | Decrease | nd | Increase of apoptosis | Zhao et al. Tumor Biol. 2014 |
| Breast cancer | MDA-MB-231 | Gain (plasmids p190A) | Increase Brk/p190A interaction, phosphorylation of p190A and p190/p120 complex formation | Increase | Increase | Increase tumorigenicity (mice model) | Shen et al. Cancer Res. 2008 |
| MDA-MB-468, MCF10A | Gain (plasmids) | nd | nd | nd | Induction of caspase- and Rho-dependent apoptosis | Ludwig and Parsons. Genes & Cancer. 2011 | |
| Lung adenocarcinoma | A549, LK87, PC-14, H1975 | Loss (si p190A) | Decrease p190/p120 complex formation, Ras inactivation, Rho activation | Cell cycle arrest | Decrease | Inhibition of Ras pathway | Notsuda et al. Int J Oncol. 2013 |
| Glioma | PDGF/Gtv-a cells | Gain (WT GAP domain) | nd | Inhibition of PDGF-induced proliferation | nd | nd | Wolf et al. Genes Dev. 2003 |
| Renal cancer | RCC, 786-O | Loss (si p190A) | nd | nd | nd | Restore fibronectin matrix assembly | Feijoo-Cuaresma. J Biol Chem. 2008 |
| Melanoma | BLM human melanoma cells | Loss (si p190A) | p120ctn allows p190-RhoA interaction and RhoA activation | nd | E-cadherin melanoma transfectant invasion | nd | Molina-Ortiz et al. J Biol Chem. 2009 |
| LOX melanoma cells | Loss (injection of p190 antibody) | nd | nd | Decrease of matrix degradation | No alteration of cell adhesion | Nakahara et al. J Biol Chem. 1998 | |
| Squamous cell carcinoma | 10 SCC cell lines and a primary HNSCC | Loss (sh p190A) | nd | nd | Dissociation of cancer cells | nd | Li et al. Nat Cell Biol. 2019 |
| Pancreatic cancer | AsPC-1, PANC-1 | Gain (p190-RhoA chimera) | Inhibition of RhoA activity (RhoB and RhoC) | No significant effect | Decrease of cell invasion, metastatic formation | nd | Kusama et al. Cancer Sci. 2006 |
| Human Papillomavirus | HaCat, CaSki, C33A, U2OS, HT1080 | Gain (plasmids p190A-HA) | Interaction MD p190A & CR3 of HPV E7 | nd | nd | Decrease actin stress fiber formation, cell spreading | Todorovic et al. J Virology. 2014 |
| Cancer | Huh7, MDA-MB-231, MEF | Gain (plasmids p190A-HA) | ΔPLS, S886F and Δ865-870 increase RhoGAP activity | nd | Alteration of cell migration directionnality | Defect in cell protrusion localisation and lamellipodia persistence | Binamé et al. J Cell Biol. 2016 |
| MDCK cell lines | Loss (sh p190A) | nd | Loss of CIP | nd | Nuclear translocation of YAP, rescue repression of gene transcription of Hippo pathway | Frank et al. J Cell Biol. 2018 | |
| p190B | |||||||
| Breast cancer | MDA-MB-468 | Loss (si p190B) | Interaction between p190B and MCT1 and also Src | Decrease acytokinetic division and neoplastic multinucleation induced by MCT1 | nd | Inhibition of tumor growth | Wu et al. Oncogene. 2014 |
| MCF7-10A, rats | Gain (o/e p190B) | Overexpression of p190B in some murine mammary tumors | nd | nd | Actin cytoskeleton reorganization, loss of adhesion | Chakravarty et al. Cell Growth Differ. 2000 | |
| Neu mice | p190B haploinsufficiency | Affect Rho signaling pathways | nd | nd | Inhibition of tumor initiation and progression, increase tumor free survival | Heckman-Stoddard et al. Breast Cancer Res. 2009 | |
| Hepatocellular carcinoma | Huh7 | Loss (si p190B) | Increase RhoA activity | nd | Increase cell spreading and cell migration | nd | Gen et al. Cancer Lett. 2009 |
| SMMC-7721, Huh7, HepG2 | Loss (si p190B) | Increase of RhoA activity | nd | Decrease migration induced by CD147 | nd | Chen et al. Cancer Cell Int. 2016 | |
| Nasopharyngeal carcinoma | NPC cell lines | Loss (si p190B) | nd | nd | Abrogates migration and invasion induced by miR-744 | nd | Fang et al. Oncotarget. 2015 |
| Cancer | MDCK cell lines | Loss (sh p190B) | nd | Loss of CIP | nd | Nuclear translocation of YAP, rescue repression of gene transcription of Hippo pathway | Frank et al. J Cell Biol. 2018 |
5.2.1. ARHGAP35 as a Significant Mutated Gene in Cancer
Several large-scale studies mapping somatic variants across thousands of tumors recently identified ARHGAP35, the gene-encoding p190A, as a new major cancer gene [82,83]. In 2013, ARHGAP35 appears among 127 significantly mutated genes from the analysis of 3281 tumors across 12 cancer types, with the highest percentage in uterine corpus endometrial carcinoma [83]. These data were confirmed by Lawrence et al. in 2014. Exome sequencing analysis of almost 5000 samples from 21 tumor types identifies ARHGAP35 as a candidate gene in cancer among 33 new genes. In this study, 2% of whole samples exhibit a mutation on ARHGAP35 gene and this mutational status appears significant for five tumor types: Endometrial tumors (14%), lung squamous cell carcinoma (5%), lung adenocarcinoma (3%), head and neck cancer (3%), and kidney clear cell carcinoma (1%) [82]. The alteration of ARHGAP35 gene in lung cancer was further confirmed by a more recent study dedicated to the identification of new driver genes in lung carcinogenesis. Indeed, ARHGAP35 was highlighted as a new gene significantly altered in lung adenocarcinoma-lacking receptor tyrosine kinase–Ras–Raf pathway or other oncogene alterations [84]. Interestingly, in this study, RASA1-encoding p120RasGAP, a partner of p190A (Figure 2), was also found mutated in squamous cell carcinomas, suggesting that p190A/p120RasGAP pathway may be involved in lung carcinogenesis. Finally, searching for genetic drivers of Hürthle cell carcinoma of the thyroid, whole-exome sequencing also identified recurrent mutations in ARHGAP35, in addition to NRAS, TP53, CDKN1A, and TERT promoter [85]. Altogether, these data demonstrated that ARHGAP35 is a new identified cancer gene. However, investigations should now be performed to correlate ARHGAP35 mutations with clinicopathological features and prognosis of cancer patients.
To better understand p190A function during cancer development and progression, we may attempt to analyze mutation spectrum. Even if few hotspots may be distinguished (i.e., R997* in endometrial tumors [82] or E1273A in thyroid tumors or renal angiomyolipoma [85,86]), ARHGAP35 mutations are observed all along the gene with nonsense mutations and frameshift deletions/insertions supporting a tumor-suppressor role for p190A (tumorportal website (http://www.tumorportal.org/)). At the protein level, as the GAP domain of the protein is localized at its C-terminus, these latter mutations are expected to behave as loss of function mutations, leading to a non-functional protein without GAP activity. This was confirmed by our study of the R997* nonsense mutation in vitro. Ectopically expressed p190A-R977* was unable to bind active RhoA, so to affect stress fiber formation [20]. Besides these mutations, many missense mutations were also reported all along ARHGAP35 sequence. Even if some may have no impact on the protein activity as Y742H or R832Q found in endometrial tumors, we showed that some mutations localized in the pG2 domain (Figure 1) had a strong impact on the protein activity and on cell behavior [20]. Indeed, the point mutation S866F and the in-frame deletion del865-870 when ectopically expressed in tumor cells induce an increase of p190A activity and an alteration of directed tumor cell motility [20]. It is clear that the impact of such mutations remains to be explored in a closer way, but interestingly, these data allow us to hypothesize on a new mode of regulation of p190A, suggesting a potential auto-inhibitory folding of the protein [19]. Thus, even if, at first glance, cancer genome sequencing data support a tumor-suppressor function for p190A, more mechanistic studies about the impact of the cancer-associated mutations are required.
So far, no study has identified mutations in the p190B-encoding gene ARHGAP5 in cancers. However, a genome-wide investigation into copy number variations of 221 colorectal adenomatous polyposis samples revealed recurrent alterations in three genes from the ARHGAP family, among which was ARHGAP5. A partial duplication and several somatic point mutations in this latter gene suggest a role for p190B in colorectal adenoma formation, and classify ARHGAP5 as a candidate gene that may increase the risk of hereditary colorectal tumors [87]. This finding remains to be confirmed with other genetic studies.
5.2.2. ARHGAP35 and ARHGAP5 as Tumor-Suppressor Genes
Without analyzing ARHGAP35 and ARHGAP5 mutational status, several studies reported a decrease of expression of those genes in human tumors.
Hepatitis viruses (HCV and HCB) are major risk factors for hepatocellular carcinoma (HCC), the main primary liver tumor. With the aim to focus on HCC lacking hepatitis virus background, Kurokawa and collaborators performed a PCR array gene profiling and observed a lower expression of p190A mRNA in HCC samples in comparison with non-tumor liver tissues [88]. Similarly, the expression of ARHGAP5, encoding p190B isoform, was found downregulated in invasive epithelial ovarian cancers [89].
Consistent with a tumor-suppressor function, ARHGAP35 gene maps to the chromosomal region 19q13.3, which is also frequently deleted in gliomas, in pancreatic, ovarian, and thyroid tumors [90,91,92]. For gliomas, the tumor-suppressive role of p190A was studied in mice. In a model of oligodendrogliomas using PDGF (platelet-derived growth factor)-expressing retrovirus, co-expression of p190A GAP domain caused a decreased incidence of oligodendrogliomas compared with that observed with PDGF alone [92]. This finding is consistent with the fact that p190A is involved in the differentiation of glial cells [93].
In this context, the function of p190A often occurs through RhoA inactivation. Interestingly, RhoA inhibition by p190A or its C-terminus domain was suggested as a new approach in pancreatic cancer treatment. Indeed, overexpression of p190-RhoA chimera in human pancreatic cancer cells decreases the risk of developing liver metastasis in immune-incompetent mice [94]. As described above, by inactivating RhoA, p190RhoGAP activity leads to a major decrease in acto-myosin/contractility, inhibiting migration and/or invasion of cells. The activation of p190RhoGAP is mainly performed through phosphorylation by non-receptor tyrosine kinases such as Src, Brk, Blk (B lymphocyte kinase), ABL2/Arg kinase, and FAK (focal adhesion kinase), most of the time on Tyr1087 and Tyr1105 (Figure 1). These types of regulation of p190RhoGAP leading to alteration of tumor cell migration and invasion were demonstrated in various cancer cell types. In U87-MG glioma cells, the mediator of axon guidance semaphorin SEMA3F was shown to inhibit in vitro cell migration via the ABL2/Arg kinase-dependent activation of p190A and further inhibition of RhoA [95]. In prostate cancer cells, the microRNA miR-20a, which targets ABL2/Arg, was reported to be overexpressed [96]. In in vitro experiments, inhibition of miR-20a function led to an inhibition of migration and invasion of prostate cancer cells, via an activation of p190A [96]. In patients, this overexpression of miR-20a, which reduces p190A phosphorylation and its activation, correlates with a poor outcome.
Several papers from Teixido’s laboratory focused on the involvement of p190RhoGAP in melanoma progression. They described p190A as a central molecule controlling melanoma cell invasion. Activation of p190A via phosphorylation by the tyrosine kinase Blk inhibits RhoA in melanoma cells [97]. Consequently, this signal decreased cell motility, invasion, and metastasis development in melanoma cellular and murine models [98,99]. They further demonstrated a key role of E-cadherin-based cell–cell junctions in this process via the interaction between p120-catenin and p190A [99].
Another interesting work from Parsons’ laboratory also suggests that p190A may function as a tumor suppressor by regulating apoptosis. Indeed, in breast cancer cell lines, ectopic expression of p190A induced cell death in a RhoA and caspase-dependent manner. Moreover, p190A is able to modulate the apoptotic response to docetaxel (used as chemotherapeutic drug) and increase the sensitivity of breast cancer cell to the drug [100]. More recently, work by Hansen’s laboratory suggests that p190A may alternatively play its tumor-suppressor function by promoting contact inhibition of cell proliferation through its cell–cell junction subcellular localization [101]. Indeed, they demonstrated that absence of p190A (but also p190B) is sufficient to alter contact inhibition of proliferation in epithelial cells cultured in matrigel. This function is dependent on the Hippo pathway, involving the translocation of YAP (yes-associated protein) to the nucleus and the downstream regulation of gene transcription [101].
Thus, altogether these data demonstrate that alterations of p190A arise in tumors from transformed cells of mainly epithelial origin. Thus, p190A through its numerous cellular functions is required to maintain a differentiated state of epithelial cells.
5.2.3. ARHGAP35 and ARHGAP5 as Potential Oncogenes
In contrast with the data summarized above, overexpression and/or overactivity of p190RhoGAP proteins were also reported in a number of studies questioning the status of tumor suppressor of p190A and p190B.
Overexpression of p190A was demonstrated in several cancer types at the protein or mRNA level and was proposed to influence the prognosis of patients. In osteosarcoma, high expression of p190A was correlated with poor outcome and associated with tumor dedifferentiation and increase of tumor size or metastasis risk [102]. Moreover, as a polymorphism in the 3′-UTR of ARHGAP35 gene is associated with tumor size, tumor grade, tumor metastasis, and survival (overall and recurrence-free) of patients, p190A was proposed as an independent prognostic factor influencing survival of patients with osteosarcoma [103]. During colorectal cancer progression, from normal mucosa to liver metastasis through colorectal adenoma and primary carcinoma, p190A expression increased. Overexpression of p190A is thus correlated with poor prognosis in colorectal cancer [104]. In colorectal cancer cellular models, p190A activation together with p120RasGAP were shown to be important effectors of mutant KRAS [105]. In lung adenocarcinoma, p190A mRNA overexpression was associated with a hyperphosphorylation of the protein on Tyr1105 [106]. This hyperactivation of p190A leads to enhanced proliferation, migration, and invasion in EGFR-TKI cell line. This is in line with the poor disease-free survival of patients that harbor tumors with high levels of p190A mRNA [106]. At a glance, these data appear in clear contrast with the non-sense mutations recently found in lung adenocarcinoma [84], but p190A involvement may differ according to the tumor stage and/or mutational status.
As described earlier, p190A was involved in the collective mode of cell migration of squamous cell carcinoma through its role in maintaining cell–cell junctions. P190A is required to decrease actomyosin activity at cell–cell contacts and favor coordinated cell movement [40]. More recently, this role was demonstrated to be important in the formation of circulating tumor cell clusters. Downregulation of RhoA by p190A at intercellular junctions is required to maintain stable cell–cell contact during dissemination of circulating tumor cells [41].
Moreover, in breast cancer, a depletion of p190A leads to a decrease of incidence of bone metastasis in mice model [107]. In vitro and in human breast cancer tissue samples, the non-receptor tyrosine kinase Brk is expressed, but not in benign lesions or in normal breast tissue, and is associated with breast carcinoma cell proliferation [108]. Interaction of Brk with p190A is required to increase Tyr1105 phosphorylation leading to RhoA inactivation and Ras activation in vitro. In breast cancer cells, this will result in an increase in cell migration and invasion but also an increase in tumor growth [28]. Thus, this study revealed that p190A acts downstream of Brk to promote breast tumor proliferation and migration.
Similarly, p190B, which was reported essential for mammary gland development [68], was also suggested to play a role in mammary malignancy. Induction of p190B expression in mammary gland during pregnancy results in hyperplastic lesions in mice [69] and an increased level of p190B was detected in a subset of mutagen-induced mammary tumors [109]. More recently, overexpression of p190B was correlated with MCT1 (multiple copies in T-cell malignancy-1) expression in breast cancer. In vitro, MCT1-Src-p190B interaction gave rise to neoplastic multinucleation of breast cancer cells [110] that was suggested to favor tumorigenicity. This involvement of p190B was also studied in nasopharyngeal carcinoma and lung cancer where, respectively, miR-744 and miR-486-5 expressions correlated with p190B expression resulting in tumor progression and dissemination [111,112]. Several p190B loss-of-function studies in vitro and in vivo confirmed oncogenic role of p190B (Table 2). Depletion of p190B inhibits cell migration, cell invasion, tumor progression, and metastatic dissemination in different cell lines of lung cancer [112], breast cancer [110,113], hepatocellular carcinoma [114], and nasopharyngeal carcinoma [111].
Thus, in the oncogenic context described above, p190A and p190B may be predictive factors for the prognosis of different types of tumors.
5.3. p190A and Other Diseases
Few studies investigate p190A involvement in other diseases. Among them, low p190A activity was observed in idiopathic pulmonary fibrosis (IPF), which is a chronic lung disease characterized by a progressive and irreversible decline in lung function. RhoA activity is increased in IPF fibroblasts and associated with IPF phenotype such as expression of different markers like smooth muscle actin, collagen I, and fibronectin [115]. In glomerulocystic kidney defects, using a small-scale Nethyl-n-nitrosourea mutagenesis screen, Stewart et al. highlighted a point mutation L1396G in p190A leading to an alteration on cilia elongation and its involvement in renal developmental diseases [116].
6. Conclusions and Future Directions
Through Rho-dependent functions, p190RhoGAPs play a critical role in regulating actin cytoskeleton dynamics. Both isoforms p190A and p190B are ubiquitously expressed and co-exist in most cells. They share a common structural organization, often common partners, and similar subcellular localizations, but the interplay between both isoforms at the cellular level remains to be explored in detail. So far, data are in favor of distinct but overlapping functions that may or may not involve regulation of Rho family GTPases. Recent data highlighted new structural domains, i.e., pseudo-GTPases in p190RhoGAPs, and hypothesized a new mode of regulation, i.e., autoinhibitory folding for p190A, opening new paths to explore. Structural, combined with functional, studies would permit us to better understand the molecular mechanisms underlying the function of p190RhoGAPs.
Their involvement in pathologic disorders is important to address to determine whether their signaling may be useful in a therapeutic approach. As described herein, p190RhoGAPs are suggested to play a role mainly in mental health disorders and in tumorigenesis.
Aberrant regulation of Rho GTPase activity and corresponding effector pathways is described to be a hallmark of human tumors. However, the Rho GTPase signaling network is complex. For negative regulators as GAPs, the general trend is toward a reduced expression of GAPs [117]. Nevertheless, the emerging picture for p190RhoGAP is much more complex, probably dependent on the environment and on both tumor type and progression status. Study of cancer-associated mutations by using genome editing would contribute to getting clues about the role of p190RhoGAP in tumorigenesis. This may lead to unexpected functions and regulations of p190RhoGAP.
Abbreviations
| Abl2 | v-Abl Abelson murine leukemia viral oncogene homolog 2 |
| ACP1 | acid phosphatase 1 |
| Arg | Abelson related gene |
| Blk | B lymphocyte kinase |
| Brk | breast tumor kinase |
| CRAD | Catenin-RhoGAP-Association Domain |
| EGFR-TKI | epidermal growth factor receptor tyrosine kinase inhibitor |
| FAK | focal adhesion kinase |
| FAS | fetal alcohol spectrum |
| FTD | Frontotemporal dementia |
| GAP | GTPase activating protein |
| GBD | GTP-binding domain |
| GDP | guanosine diphosphate |
| GEF | guanine nucleotide exchange factor |
| GPI | glycosylphosphatidylinositol |
| GRF-1 | glucocorticoid receptor DNA-binding factor 1 |
| GTP | guanosine triphosphate |
| GTPase | guanosine triphosphate hydrolase |
| HCC | hepatocellular carcinoma |
| IPF | idiopathic pulmonary fibrosis |
| MCT1 | multiple copies in T-cell malignancy-1 |
| PBR | polybasic region |
| PDGF | platelet derived growth factor |
| PLS | protrusion localization sequence |
| PrPc | cellular prion protein |
| Ras | Rat sarcoma |
| ROCK | Rho associated coiled-coil-containing protein kinase |
| Rho | Ras homolog |
| TEB | terminal end buds |
| YAP | yes-associated protein |
Funding
This research was funded by La Fondation pour la Recherche Médicale (grant number DEQ20180839586), by INCa (grant PLBIO2014-182) and by La Ligue contre le Cancer (comité de Gironde). C.H. is supported by a PhD fellowship from the Ministère de l’Enseignement Supérieur et de la Recherche.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Toksoz D., Merdek K.D. The Rho small GTPase: Functions in health and disease. Histol. Histopathol. 2002;17:915–927. doi: 10.14670/HH-17.915. [DOI] [PubMed] [Google Scholar]
- 2.Haga R.B., Ridley A.J. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall A. Rho family GTPases. Biochem. Soc. Trans. 2012;40:1378–1382. doi: 10.1042/BST20120103. [DOI] [PubMed] [Google Scholar]
- 4.Stiegler A.L., Boggon T.J. The N-Terminal GTPase Domain of p190RhoGAP Proteins Is a PseudoGTPase. Structure. 2018;26:1451–1461.e1454. doi: 10.1016/j.str.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbelo P.D., Finegold A.A., Kozak C.A., Yamada Y., Takami H. Cloning, genomic organization and chromosomal assignment of the mouse p190-B gene. Biochim. Biophys. Acta. 1998;1443:203–210. doi: 10.1016/S0167-4781(98)00207-3. [DOI] [PubMed] [Google Scholar]
- 6.Burbelo P.D., Miyamoto S., Utani A., Brill S., Yamada K.M., Hall A., Yamada Y. p190-B, a new member of the Rho GAP family, and Rho are induced to cluster after integrin cross-linking. J. Boil. Chem. 1995;270:30919–30926. doi: 10.1074/jbc.270.52.30919. [DOI] [PubMed] [Google Scholar]
- 7.Ridley A.J., Self A.J., Kasmi F., Paterson H.F., Hall A., Marshall C.J., Ellis C. rho family GTPase activating proteins p190, bcr and rhoGAP show distinct specificities in vitro and in vivo. EMBO J. 1993;12:5151–5160. doi: 10.1002/j.1460-2075.1993.tb06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent S., Settleman J. Inhibition of RhoGAP activity is sufficient for the induction of Rho-mediated actin reorganization. Eur. J. Cell Biol. 1999;78:539–548. doi: 10.1016/S0171-9335(99)80019-3. [DOI] [PubMed] [Google Scholar]
- 9.Li R., Zhang B., Zheng Y. Structural determinants required for the interaction between Rho GTPase and the GTPase-activating domain of p190. J. Boil. Chem. 1997;272:32830–32835. doi: 10.1074/jbc.272.52.32830. [DOI] [PubMed] [Google Scholar]
- 10.Levay M., Bartos B., Ligeti E. p190RhoGAP has cellular RacGAP activity regulated by a polybasic region. Cell. Signal. 2013;25:1388–1394. doi: 10.1016/j.cellsig.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Ligeti E., Dagher M.C., Hernandez S.E., Koleske A.J., Settleman J. Phospholipids can switch the GTPase substrate preference of a GTPase-activating protein. J. Boil. Chem. 2004;279:5055–5058. doi: 10.1074/jbc.C300547200. [DOI] [PubMed] [Google Scholar]
- 12.Roof R.W., Dukes B.D., Chang J.H., Parsons S.J. Phosphorylation of the p190 RhoGAP N-terminal domain by c-Src results in a loss of GTP binding activity. FEBS Lett. 2000;472:117–121. doi: 10.1016/S0014-5793(00)01439-3. [DOI] [PubMed] [Google Scholar]
- 13.Tatsis N., Lannigan D.A., Macara I.G. The function of the p190 Rho GTPase-activating protein is controlled by its N-terminal GTP binding domain. J. Boil. Chem. 1998;273:34631–34638. doi: 10.1074/jbc.273.51.34631. [DOI] [PubMed] [Google Scholar]
- 14.Foster R., Hu K.Q., Shaywitz D.A., Settleman J. p190 RhoGAP, the major RasGAP-associated protein, binds GTP directly. Mol. Cell. Boil. 1994;14:7173–7181. doi: 10.1128/MCB.14.11.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W., Sordella R., Chen G.C., Hakre S., Roy A.L., Settleman J. An FF domain-dependent protein interaction mediates a signaling pathway for growth factor-induced gene expression. Mol. Cell. 2005;17:23–35. doi: 10.1016/j.molcel.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Parasuraman P., Mulligan P., Walker J.A., Li B., Boukhali M., Haas W., Bernards A. Interaction of p190A RhoGAP with eIF3A and Other Translation Preinitiation Factors Suggests a Role in Protein Biosynthesis. J. Boil. Chem. 2017;292:2679–2689. doi: 10.1074/jbc.M116.769216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stiegler A.L., Boggon T.J. p190RhoGAP proteins contain pseudoGTPase domains. Nat. Commun. 2017;8:506. doi: 10.1038/s41467-017-00483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiegler A.L., Boggon T.J. PseudoGTPase domains in p190RhoGAP proteins: A mini-review. Biochem. Soc. Trans. 2018;46:1713–1720. doi: 10.1042/BST20180481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bidaud-Meynard A., Biname F., Lagree V., Moreau V. Regulation of Rho GTPase activity at the leading edge of migrating cells by p190RhoGAP. Small GTPases. 2017;10:99–110. doi: 10.1080/21541248.2017.1280584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biname F., Bidaud-Meynard A., Magnan L., Piquet L., Montibus B., Chabadel A., Saltel F., Lagree V., Moreau V. Cancer-associated mutations in the protrusion-targeting region of p190RhoGAP impact tumor cell migration. J. Cell Boil. 2016;214:859–873. doi: 10.1083/jcb.201601063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wennerberg K., Forget M.A., Ellerbroek S.M., Arthur W.T., Burridge K., Settleman J., Der C.J., Hansen S.H. Rnd proteins function as RhoA antagonists by activating p190 RhoGAP. Curr. Biol. 2003;13:1106–1115. doi: 10.1016/S0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustos R.I., Forget M.A., Settleman J.E., Hansen S.H. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Curr. Biol. 2008;18:1606–1611. doi: 10.1016/j.cub.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGlade J., Brunkhorst B., Anderson D., Mbamalu G., Settleman J., Dedhar S., Rozakis-Adcock M., Chen L.B., Pawson T. The N-terminal region of GAP regulates cytoskeletal structure and cell adhesion. EMBO J. 1993;12:3073–3081. doi: 10.1002/j.1460-2075.1993.tb05976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant S.S., Briggs S., Smithgall T.E., Martin G.A., McCormick F., Chang J.H., Parsons S.J., Jove R. Two SH2 domains of p120 Ras GTPase-activating protein bind synergistically to tyrosine phosphorylated p190 Rho GTPase-activating protein. J. Boil. Chem. 1995;270:17947–17952. doi: 10.1074/jbc.270.30.17947. [DOI] [PubMed] [Google Scholar]
- 25.Hu K.Q., Settleman J. Tandem SH2 binding sites mediate the RasGAP-RhoGAP interaction: A conformational mechanism for SH3 domain regulation. EMBO J. 1997;16:473–483. doi: 10.1093/emboj/16.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roof R.W., Haskell M.D., Dukes B.D., Sherman N., Kinter M., Parsons S.J. Phosphotyrosine (p-Tyr)-dependent and -independent mechanisms of p190 RhoGAP-p120 RasGAP interaction: Tyr 1105 of p190, a substrate for c-Src, is the sole p-Tyr mediator of complex formation. Mol. Cell. Boil. 1998;18:7052–7063. doi: 10.1128/MCB.18.12.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez S.E., Settleman J., Koleske A.J. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr. Biol. 2004;14:691–696. doi: 10.1016/j.cub.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 28.Shen C.H., Chen H.Y., Lin M.S., Li F.Y., Chang C.C., Kuo M.L., Settleman J., Chen R.H. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 29.Csepanyi-Komi R., Pasztor M., Bartos B., Ligeti E. The neglected terminators: Rho family GAPs in neutrophils. Eur. J. Clin. Investig. 2018;48(Suppl. 2) doi: 10.1111/eci.12993. [DOI] [PubMed] [Google Scholar]
- 30.McCormick F. ras GTPase activating protein: Signal transmitter and signal terminator. Cell. 1989;56:5–8. doi: 10.1016/0092-8674(89)90976-8. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor K., Chen M. Dynamic functions of RhoA in tumor cell migration and invasion. Small GTPases. 2013;4:141–147. doi: 10.4161/sgtp.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomar A., Lim S.T., Lim Y., Schlaepfer D.D. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J. Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakahara H., Mueller S.C., Nomizu M., Yamada Y., Yeh Y., Chen W.T. Activation of beta1 integrin signaling stimulates tyrosine phosphorylation of p190RhoGAP and membrane-protrusive activities at invadopodia. J. Boil. Chem. 1998;273:9–12. doi: 10.1074/jbc.273.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Guegan F., Tatin F., Leste-Lasserre T., Drutel G., Genot E., Moreau V. p190B RhoGAP regulates endothelial-cell-associated proteolysis through MT1-MMP and MMP2. J. Cell Sci. 2008;121:2054–2061. doi: 10.1242/jcs.025817. [DOI] [PubMed] [Google Scholar]
- 35.Wildenberg G.A., Dohn M.R., Carnahan R.H., Davis M.A., Lobdell N.A., Settleman J., Reynolds A.B. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 36.Su L., Pertz O., Mikawa M., Hahn K., Parsons S.J. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Exp. Cell Res. 2009;315:1347–1359. doi: 10.1016/j.yexcr.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H., Macara I.G. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev. Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noren N.K., Arthur W.T., Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J. Boil. Chem. 2003;278:13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- 39.Zebda N., Tian Y., Tian X., Gawlak G., Higginbotham K., Reynolds A.B., Birukova A.A., Birukov K.G. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J. Boil. Chem. 2013;288:18290–18299. doi: 10.1074/jbc.M112.432757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidalgo-Carcedo C., Hooper S., Chaudhry S.I., Williamson P., Harrington K., Leitinger B., Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Boil. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C.F., Chen J.Y., Ho Y.H., Hsu W.H., Wu L.C., Lan H.Y., Hsu D.S., Tai S.K., Chang Y.C., Yang M.H. Snail-induced claudin-11 prompts collective migration for tumour progression. Nat. Cell Boil. 2019;21:251–262. doi: 10.1038/s41556-018-0268-z. [DOI] [PubMed] [Google Scholar]
- 42.Ponik S.M., Trier S.M., Wozniak M.A., Eliceiri K.W., Keely P.J. RhoA is down-regulated at cell-cell contacts via p190RhoGAP-B in response to tensional homeostasis. Mol. Boil. Cell. 2013;24:1688–1699. doi: 10.1091/mbc.e12-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fais S., Overholtzer M. Cell-in-cell phenomena in cancer. Nat. Rev. Cancer. 2018;18:758–766. doi: 10.1038/s41568-018-0073-9. [DOI] [PubMed] [Google Scholar]
- 44.Overholtzer M., Mailleux A.A., Mouneimne G., Normand G., Schnitt S.J., King R.W., Cibas E.S., Brugge J.S. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 45.Sun Q., Cibas E.S., Huang H., Hodgson L., Overholtzer M. Induction of entosis by epithelial cadherin expression. Cell Res. 2014;24:1288–1298. doi: 10.1038/cr.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su L., Agati J.M., Parsons S.J. p190RhoGAP is cell cycle regulated and affects cytokinesis. J. Cell Boil. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manukyan A., Ludwig K., Sanchez-Manchinelly S., Parsons S.J., Stukenberg P.T. A complex of p190RhoGAP-A and anillin modulates RhoA-GTP and the cytokinetic furrow in human cells. J. Cell Sci. 2015;128:50–60. doi: 10.1242/jcs.151647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikawa M., Su L., Parsons S.J. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle. 2008;7:2003–2012. doi: 10.4161/cc.7.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manukyan A., Sargsyan L., Parsons S.J., Stukenberg P.T. P190RhoGAP prevents mitotic spindle fragmentation and is required to activate Aurora A kinase at acentriolar poles. Chromosoma. 2018;127:375–386. doi: 10.1007/s00412-018-0670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dupont H., Blancq M. Formation of complexes involving RasGAP and p190 RhoGAP during morphogenetic events of the gastrulation in xenopus. Eur. J. Biochem. 1999;265:530–538. doi: 10.1046/j.1432-1327.1999.00689.x. [DOI] [PubMed] [Google Scholar]
- 51.Brouns M.R., Matheson S.F., Hu K.Q., Delalle I., Caviness V.S., Silver J., Bronson R.T., Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- 52.Brouns M.R., Matheson S.F., Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat. Cell Boil. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- 53.Billuart P., Winter C.G., Maresh A., Zhao X., Luo L. Regulating axon branch stability: The role of p190 RhoGAP in repressing a retraction signaling pathway. Cell. 2001;107:195–207. doi: 10.1016/S0092-8674(01)00522-0. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.J., Choi H.S., Park J.H., Kim M.J., Lee H.G., Petersen R.B., Kim Y.S., Park J.B., Choi E.K. Regulation of RhoA activity by the cellular prion protein. Cell Death Dis. 2017;8:e2668. doi: 10.1038/cddis.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wheeler A.P., Ridley A.J. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp. Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 56.Fingleton B. Molecular targets in metastasis: Lessons from genomic approaches. Cancer Genom. Proteom. 2007;4:211–221. [PubMed] [Google Scholar]
- 57.Vega F.M., Fruhwirth G., Ng T., Ridley A.J. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J. Cell Boil. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bravo-Cordero J.J., Oser M., Chen X., Eddy R., Hodgson L., Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr. Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bravo-Cordero J.J., Sharma V.P., Roh-Johnson M., Chen X., Eddy R., Condeelis J., Hodgson L. Spatial regulation of RhoC activity defines protrusion formation in migrating cells. J. Cell Sci. 2013;126:3356–3369. doi: 10.1242/jcs.123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bravo-Cordero J.J., Hodgson L., Condeelis J.S. Spatial regulation of tumor cell protrusions by RhoC. Cell Adhes. Migr. 2014;8:263–267. doi: 10.4161/cam.28405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ligeti E., Settleman J. Regulation of RhoGAP specificity by phospholipids and prenylation. Methods Enzymol. 2006;406:104–117. doi: 10.1016/S0076-6879(06)06009-5. [DOI] [PubMed] [Google Scholar]
- 62.Levay M., Settleman J., Ligeti E. Regulation of the substrate preference of p190RhoGAP by protein kinase C-mediated phosphorylation of a phospholipid binding site. Biochemistry. 2009;48:8615–8623. doi: 10.1021/bi900667y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy A.L. Biochemistry and biology of the inducible multifunctional transcription factor TFII-I. Gene. 2001;274:1–13. doi: 10.1016/S0378-1119(01)00625-4. [DOI] [PubMed] [Google Scholar]
- 64.Mammoto A., Connor K.M., Mammoto T., Yung C.W., Huh D., Aderman C.M., Mostoslavsky G., Smith L.E., Ingber D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sordella R., Classon M., Hu K.Q., Matheson S.F., Brouns M.R., Fine B., Zhang L., Takami H., Yamada Y., Settleman J. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev. Cell. 2002;2:553–565. doi: 10.1016/S1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 66.Matheson S.F., Hu K.Q., Brouns M.R., Sordella R., VanderHeide J.D., Settleman J. Distinct but overlapping functions for the closely related p190 RhoGAPs in neural development. Dev. Neurosci. 2006;28:538–550. doi: 10.1159/000095116. [DOI] [PubMed] [Google Scholar]
- 67.Sordella R., Jiang W., Chen G.C., Curto M., Settleman J. Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell. 2003;113:147–158. doi: 10.1016/S0092-8674(03)00271-X. [DOI] [PubMed] [Google Scholar]
- 68.Chakravarty G., Hadsell D., Buitrago W., Settleman J., Rosen J.M. p190-B RhoGAP regulates mammary ductal morphogenesis. Mol. Endocrinol. 2003;17:1054–1065. doi: 10.1210/me.2002-0428. [DOI] [PubMed] [Google Scholar]
- 69.Vargo-Gogola T., Heckman B.M., Gunther E.J., Chodosh L.A., Rosen J.M. P190-B Rho GTPase-activating protein overexpression disrupts ductal morphogenesis and induces hyperplastic lesions in the developing mammary gland. Mol. Endocrinol. 2006;20:1391–1405. doi: 10.1210/me.2005-0426. [DOI] [PubMed] [Google Scholar]
- 70.Heckman-Stoddard B.M., Vargo-Gogola T., Herrick M.P., Visbal A.P., Lewis M.T., Settleman J., Rosen J.M. P190A RhoGAP is required for mammary gland development. Dev. Biol. 2011;360:1–10. doi: 10.1016/j.ydbio.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra A., Ferrari R., Heutink P., Hardy J., Pijnenburg Y., Posthuma D., International F.T.D.G.C. Gene-based association studies report genetic links for clinical subtypes of frontotemporal dementia. Brain. 2017;140:1437–1446. doi: 10.1093/brain/awx066. [DOI] [PubMed] [Google Scholar]
- 72.Gourley S.L., Swanson A.M., Koleske A.J. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gourley S.L., Olevska A., Warren M.S., Taylor J.R., Koleske A.J. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J. Neurosci. 2012;32:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J., Yoshikawa A., Meltzer H.Y. Replication of rs300774, a genetic biomarker near ACP1, associated with suicide attempts in patients with schizophrenia: Relation to brain cholesterol biosynthesis. J. Psychiatr. Res. 2017;94:54–61. doi: 10.1016/j.jpsychires.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Selva J., Egea G. Ethanol increases p190RhoGAP activity, leading to actin cytoskeleton rearrangements. J. Neurochem. 2011;119:1306–1316. doi: 10.1111/j.1471-4159.2011.07522.x. [DOI] [PubMed] [Google Scholar]
- 76.Fincham V.J., Chudleigh A., Frame M.C. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. Pt 6J. Cell Sci. 1999;112:947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- 77.Settleman J., Albright C.F., Foster L.C., Weinberg R.A. Association between GTPase activators for Rho and Ras families. Nature. 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 78.Settleman J., Narasimhan V., Foster L.C., Weinberg R.A. Molecular cloning of cDNAs encoding the GAP-associated protein p190: Implications for a signaling pathway from ras to the nucleus. Cell. 1992;69:539–549. doi: 10.1016/0092-8674(92)90454-K. [DOI] [PubMed] [Google Scholar]
- 79.Bouton A.H., Kanner S.B., Vines R.R., Wang H.C., Gibbs J.B., Parsons J.T. Transformation by pp60src or stimulation of cells with epidermal growth factor induces the stable association of tyrosine-phosphorylated cellular proteins with GTPase-activating protein. Mol. Cell. Boil. 1991;11:945–953. doi: 10.1128/MCB.11.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990;343:377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- 81.Wang D.Z., Nur E.K.M.S., Tikoo A., Montague W., Maruta H. The GTPase and Rho GAP domains of p190, a tumor suppressor protein that binds the M(r) 120,000 Ras GAP, independently function as anti-Ras tumor suppressors. Cancer Res. 1997;57:2478–2484. [PubMed] [Google Scholar]
- 82.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kandoth C., McLellan M.D., Vandin F., Ye K., Niu B., Lu C., Xie M., Zhang Q., McMichael J.F., Wyczalkowski M.A., et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell J.D., Alexandrov A., Kim J., Wala J., Berger A.H., Pedamallu C.S., Shukla S.A., Guo G., Brooks A.N., Murray B.A., et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gopal R.K., Kubler K., Calvo S.E., Polak P., Livitz D., Rosebrock D., Sadow P.M., Campbell B., Donovan S.E., Amin S., et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hurthle Cell Carcinoma. Cancer Cell. 2018;34:242–255. doi: 10.1016/j.ccell.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Giannikou K., Malinowska I.A., Pugh T.J., Yan R., Tseng Y.Y., Oh C., Kim J., Tyburczy M.E., Chekaluk Y., Liu Y., et al. Whole Exome Sequencing Identifies TSC1/TSC2 Biallelic Loss as the Primary and Sufficient Driver Event for Renal Angiomyolipoma Development. PLoS Genet. 2016;12:e1006242. doi: 10.1371/journal.pgen.1006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horpaopan S., Spier I., Zink A.M., Altmuller J., Holzapfel S., Laner A., Vogt S., Uhlhaas S., Heilmann S., Stienen D., et al. Genome-wide CNV analysis in 221 unrelated patients and targeted high-throughput sequencing reveal novel causative candidate genes for colorectal adenomatous polyposis. Int. J. Cancer. 2015;136:E578–E589. doi: 10.1002/ijc.29215. [DOI] [PubMed] [Google Scholar]
- 88.Kurokawa Y., Matoba R., Takemasa I., Nakamori S., Tsujie M., Nagano H., Dono K., Umeshita K., Sakon M., Ueno N., et al. Molecular features of non-B, non-C hepatocellular carcinoma: A PCR-array gene expression profiling study. J. Hepatol. 2003;39:1004–1012. doi: 10.1016/S0168-8278(03)00473-2. [DOI] [PubMed] [Google Scholar]
- 89.Earp M., Tyrer J.P., Winham S.J., Lin H.Y., Chornokur G., Dennis J., Aben K.K.H., Anton-Culver H., Antonenkova N., Bandera E.V., et al. Variants in genes encoding small GTPases and association with epithelial ovarian cancer susceptibility. PLoS ONE. 2018;13:e0197561. doi: 10.1371/journal.pone.0197561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tikoo A., Czekay S., Viars C., White S., Heath J.K., Arden K., Maruta H. p190-A, a human tumor suppressor gene, maps to the chromosomal region 19q13.3 that is reportedly deleted in some gliomas. Gene. 2000;257:23–31. doi: 10.1016/S0378-1119(00)00387-5. [DOI] [PubMed] [Google Scholar]
- 91.Zack T.I., Schumacher S.E., Carter S.L., Cherniack A.D., Saksena G., Tabak B., Lawrence M.S., Zhsng C.Z., Wala J., Mermel C.H., et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 2013;45:1134–1140. doi: 10.1038/ng.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf R.M., Draghi N., Liang X., Dai C., Uhrbom L., Eklof C., Westermark B., Holland E.C., Resh M.D. p190RhoGAP can act to inhibit PDGF-induced gliomas in mice: A putative tumor suppressor encoded on human chromosome 19q13.3. Genes Dev. 2003;17:476–487. doi: 10.1101/gad.1040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolf R.M., Wilkes J.J., Chao M.V., Resh M.D. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J. Neurobiol. 2001;49:62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- 94.Kusama T., Mukai M., Endo H., Ishikawa O., Tatsuta M., Nakamura H., Inoue M. Inactivation of Rho GTPases by p190 RhoGAP reduces human pancreatic cancer cell invasion and metastasis. Cancer Sci. 2006;97:848–853. doi: 10.1111/j.1349-7006.2006.00242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimizu A., Mammoto A., Italiano J.E., Jr., Pravda E., Dudley A.C., Ingber D.E., Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J. Boil. Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiang X.F., Zhang Z.W., Liu Q., Sun N., Pan L.L., Shen J., Li T., Yun C., Li H., Shi L.H. miR-20a promotes prostate cancer invasion and migration through targeting ABL2. J. Cell. Biochem. 2014;115:1269–1276. doi: 10.1002/jcb.24778. [DOI] [PubMed] [Google Scholar]
- 97.Bartolome R.A., Diaz-Martinez M., Colo G.P., Arellano-Sanchez N., Torres-Ayuso P., Kleinovink J.W., Merida I., Teixido J. A Blk-p190RhoGAP signaling module downstream of activated Galpha13 functionally opposes CXCL12-stimulated RhoA activation and cell invasion. Cell. Signal. 2014;26:2551–2561. doi: 10.1016/j.cellsig.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 98.Bartolome R.A., Wright N., Molina-Ortiz I., Sanchez-Luque F.J., Teixido J. Activated Gα13 impairs cell invasiveness through p190RhoGAP-mediated inhibition of RhoA activity. Cancer Res. 2008;68:8221–8230. doi: 10.1158/0008-5472.CAN-08-0561. [DOI] [PubMed] [Google Scholar]
- 99.Molina-Ortiz I., Bartolome R.A., Hernandez-Varas P., Colo G.P., Teixido J. Overexpression of E-cadherin on melanoma cells inhibits chemokine-promoted invasion involving p190RhoGAP/p120ctn-dependent inactivation of RhoA. J. Boil. Chem. 2009;284:15147–15157. doi: 10.1074/jbc.M807834200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ludwig K., Parsons S.J. The Tumor Suppressor, p190RhoGAP, Differentially Initiates Apoptosis and Confers Docetaxel Sensitivity to Breast Cancer Cells. Genes Cancer. 2011;2:20–30. doi: 10.1177/1947601911402680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frank S.R., Kollmann C.P., Luong P., Galli G.G., Zou L., Bernards A., Getz G., Calogero R.A., Frodin M., Hansen S.H. p190 RhoGAP promotes contact inhibition in epithelial cells by repressing YAP activity. J. Cell Boil. 2018;217:3183–3201. doi: 10.1083/jcb.201710058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao J., Xu H., He M., Wu Y. Glucocorticoid receptor DNA binding factor 1 expression and osteosarcoma prognosis. Tumour Biol. 2014;35:12449–12458. doi: 10.1007/s13277-014-2563-z. [DOI] [PubMed] [Google Scholar]
- 103.Zhao J., Xu H., He M., Wang Z., Wu Y. Rho GTPase-activating protein 35 rs1052667 polymorphism and osteosarcoma risk and prognosis. Biomed Res. Int. 2014;2014:396947. doi: 10.1155/2014/396947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li L., Li Y.M., Zhou P., Wang X.S., Wang G.Y., Zhao X.H., Cui B.B., Ren Y.L., Dong X.S., Chen Z.Q. Abnormal expression of p190RhoGAP in colorectal cancer patients with poor survival. Am. J. Transl. Res. 2016;8:4405–4414. [PMC free article] [PubMed] [Google Scholar]
- 105.Organ S.L., Hai J., Radulovich N., Marshall C.B., Leung L., Sasazuki T., Shirasawa S., Zhu C.Q., Navab R., Ikura M., et al. p120RasGAP is a mediator of rho pathway activation and tumorigenicity in the DLD1 colorectal cancer cell line. PLoS ONE. 2014;9:e86103. doi: 10.1371/journal.pone.0086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Notsuda H., Sakurada A., Endo C., Okada Y., Horii A., Shima H., Kondo T. p190A RhoGAP is involved in EGFR pathways and promotes proliferation, invasion and migration in lung adenocarcinoma cells. Int. J. Oncol. 2013;43:1569–1577. doi: 10.3892/ijo.2013.2096. [DOI] [PubMed] [Google Scholar]
- 107.Chiu J.H., Wen C.S., Wang J.Y., Hsu C.Y., Tsai Y.F., Hung S.C., Tseng L.M., Shyr Y.M. Role of estrogen receptors and Src signaling in mechanisms of bone metastasis by estrogen receptor positive breast cancers. J. Transl. Med. 2017;15:97. doi: 10.1186/s12967-017-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Harvey A.J., Crompton M.R. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene. 2003;22:5006–5010. doi: 10.1038/sj.onc.1206577. [DOI] [PubMed] [Google Scholar]
- 109.Chakravarty G., Roy D., Gonzales M., Gay J., Contreras A., Rosen J.M. P190-B, a Rho-GTPase-activating protein, is differentially expressed in terminal end buds and breast cancer. Cell Growth Differ. 2000;11:343–354. [PubMed] [Google Scholar]
- 110.Wu M.H., Chen Y.A., Chen H.H., Chang K.W., Chang I.S., Wang L.H., Hsu H.L. MCT-1 expression and PTEN deficiency synergistically promote neoplastic multinucleation through the Src/p190B signaling activation. Oncogene. 2014;33:5109–5120. doi: 10.1038/onc.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fang Y., Zhu X., Wang J., Li N., Li D., Sakib N., Sha Z., Song W. MiR-744 functions as a proto-oncogene in nasopharyngeal carcinoma progression and metastasis via transcriptional control of ARHGAP5. Oncotarget. 2015;6:13164–13175. doi: 10.18632/oncotarget.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang J., Tian X., Han R., Zhang X., Wang X., Shen H., Xue L., Liu Y., Yan X., Shen J., et al. Downregulation of miR-486-5p contributes to tumor progression and metastasis by targeting protumorigenic ARHGAP5 in lung cancer. Oncogene. 2014;33:1181–1189. doi: 10.1038/onc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heckman-Stoddard B.M., Vargo-Gogola T., McHenry P.R., Jiang V., Herrick M.P., Hilsenbeck S.G., Settleman J., Rosen J.M. Haploinsufficiency for p190B RhoGAP inhibits MMTV-Neu tumor progression. Breast Cancer Res. 2009;11:R61. doi: 10.1186/bcr2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen R., Wang S.J., Zhang Y., Hou R., Jiang J.L., Cui H.Y. CD147 promotes cell motility via upregulation of p190-B RhoGAP in hepatocellular carcinoma. Cancer Cell Int. 2016;16:69. doi: 10.1186/s12935-016-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Monaghan-Benson E., Wittchen E.S., Doerschuk C.M., Burridge K. A Rnd3/p190RhoGAP pathway regulates RhoA activity in idiopathic pulmonary fibrosis fibroblasts. Mol. Boil. Cell. 2018;29:2165–2175. doi: 10.1091/mbc.E17-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stewart K., Gaitan Y., Shafer M.E., Aoudjit L., Hu D., Sharma R., Tremblay M., Ishii H., Marcotte M., Stanga D., et al. A Point Mutation in p190A RhoGAP Affects Ciliogenesis and Leads to Glomerulocystic Kidney Defects. PLoS Genet. 2016;12:e1005785. doi: 10.1371/journal.pgen.1005785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Porter A.P., Papaioannou A., Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases. 2016;7:123–138. doi: 10.1080/21541248.2016.1173767. [DOI] [PMC free article] [PubMed] [Google Scholar]