Abstract
Levodopa (LD) is the most effective drug in the treatment of Parkinson’s disease (PD). However, although it represents the “gold standard” of PD therapy, LD can cause side effects, including gastrointestinal and cardiovascular symptoms as well as transient elevated liver enzyme levels. Moreover, LD therapy leads to LD-induced dyskinesia (LID), a disabling motor complication that represents a major challenge for the clinical neurologist. Due to the many limitations associated with LD therapeutic use, other dopaminergic and non-dopaminergic drugs are being developed to optimize the treatment response. This review focuses on recent investigations about non-dopaminergic central nervous system (CNS) receptor ligands that have been identified to have therapeutic potential for the treatment of motor and non-motor symptoms of PD. In a different way, such agents may contribute to extending LD response and/or ameliorate LD-induced side effects.
Keywords: Parkinson’s disease, levodopa therapy, levodopa-induced side effects, dopaminergic drugs, non-dopaminergic receptor ligands
1. Introduction
Parkinson’s disease (PD), also known as idiopathic paralysis agitans, is one of the most frequent chronic neurodegenerative diseases worldwide. Although its etiology has not been determined so far, the main pathological characteristic is the decrease of the dopamine (DA) level due to the degeneration of the dopaminergic neurons in the substantia nigra pars compacta [1,2]. This leads to motor (i.e., postural instability, dyskinesias, tremor, and rigidity) and non-motor (i.e., depression, cognitive impairment, pain, hallucinations) symptoms [3,4,5,6,7,8,9,10,11,12,13]. Another pathologically severe aspect is the abnormal formation of protein aggregates inside nerve cells (Lewy bodies), whose primary structural component is the presynaptic neuronal protein α-synuclein. For this reason, PD is classified as synucleopathy. Unfortunately, effective inhibition of progression or the cure for PD is not yet available, while all the available therapies only provide relief for symptoms.
Dopaminergic medications are currently the most effective treatment for both motor and non-motor symptoms, though they are not devoid of limitations and frequently produce undesired side effects. The standard treatment of PD patients consists in the administration of DA) in the form of levodopa (LD), a catecholamine produced by the intraneuronal tyrosine hydroxylation [14,15,16,17,18,19]. Its combination with a peripheral DOPA decarboxylase inhibitor (i.e., carbidopa) increases LD availability in the central nervous system (CNS) and ameliorates the therapeutic profile of LD, prolonging its efficacy [20,21,22]. An increase in the efficacy of dopaminergic therapy is also obtained by the simultaneous blockade of the DA metabolism with monoaminooxidase B (MAO-B) and/or catechol-O-methyl transferase (COMT) inhibitors [23,24]. Although LD represents the “gold standard” of PD therapy [25], unfortunately, orally administered LD can cause side effects, including gastrointestinal and cardiovascular symptoms as well as transient elevated liver enzyme levels. Moreover, LD therapy leads to LD-induced dyskinesia (LID) [26], a disabling motor complication that represents a major challenge for the clinical neurologist [27]. Indeed, LID negatively affects the quality of life [28,29,30] and constitutes a serious obstacle to the management of PD imposing a limit and a reduction of LD dosage, thus restricting treatment efficacy [27].
Numerous therapies are currently being developed to treat the motor and non-motor complications of PD and LID [31]. (See Appendix A for the PubChem CIDs (or Reaxys IDs) of the compounds reported in the review).
Mostly a customized combination of DA agonists and LD formulations is performed. The striatal D1 and D2 receptors are the common binding sites of DA ligands for PD treatment, but lately D3 and D4 subtypes have also become potential targets.
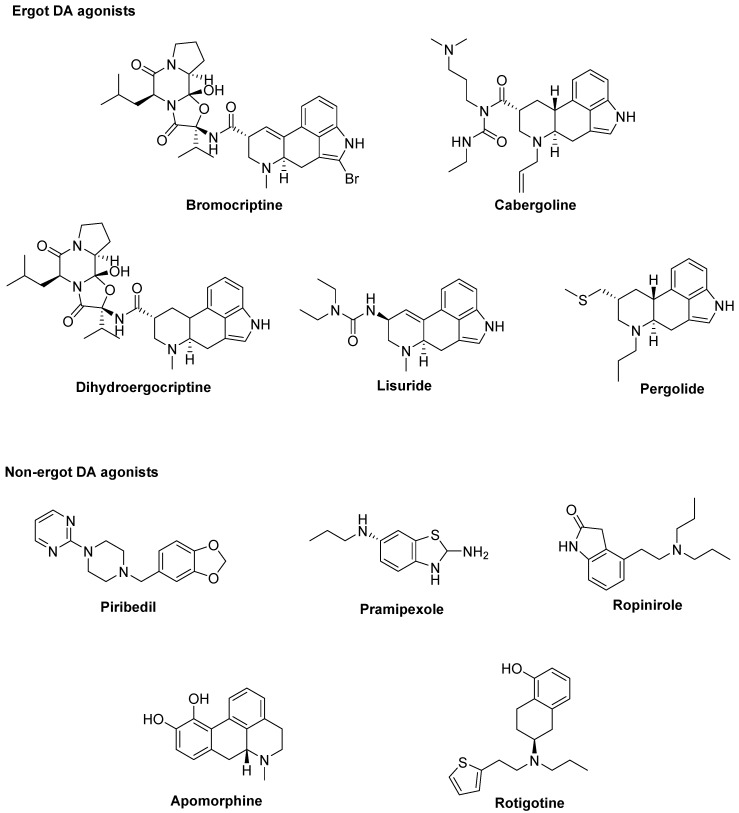
At the best of our knowledge, ten DA agonists are so far available for this disease. They can be listed in ergot DA agonists, including Bromocriptine, Cabergoline, Dihydroergocriptine, Lisuride, and Pergolide and non-ergot DA agonists, including Piribedil, Pramipexole, Ropinirole, Apomorphine, and Rotigotine [32] (Figure 1).
Figure 1.
Dopamine (DA) agonists available for Parkinson’s disease (PD) treatment.
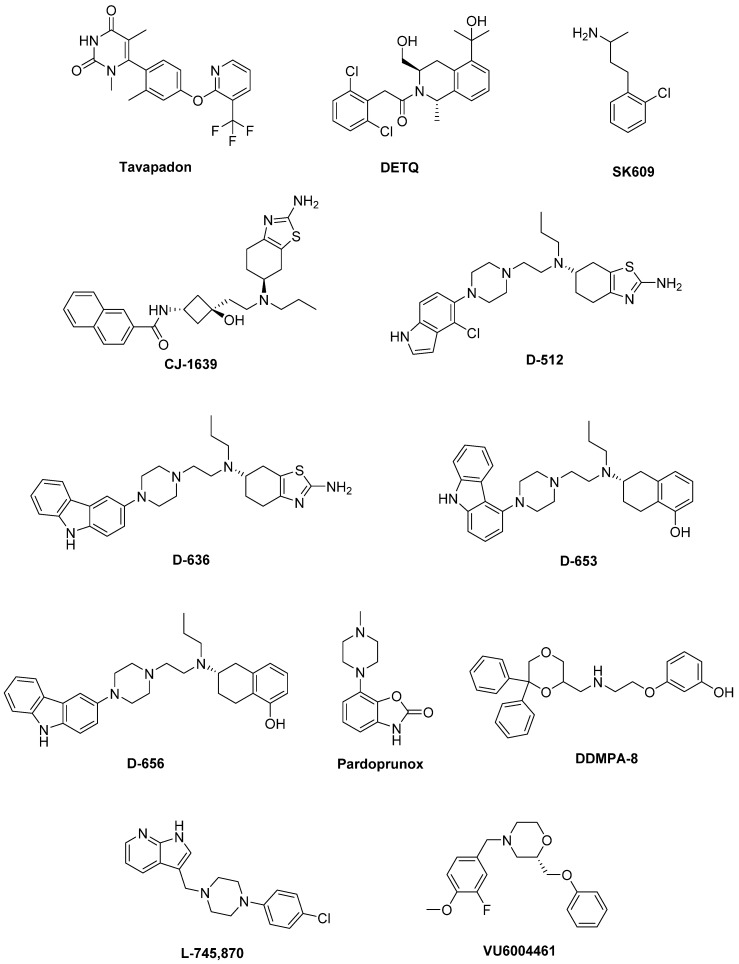
Unluckily, DA agonists are not devoid of significant side effects such as hallucinations, hypotension, nausea, vomiting, pathological gambling, compulsive shopping and hypersexuality [33,34]. As a therapeutic example, symptoms of early stage PD may be controlled by the treatment with Pramipexole [35], but after a while a combination with LD is needed to optimize the management of PD symptoms [36]. Thus, DA agonists are typically used either to reduce the dosage of LD or to delay its use (LD sparing) [37], although it has been discussed that dyskinesia evolvement is due to disease persistence rather than protracted LD use [38]. Recently, research on dopaminergic targets has produced some new interesting candidates (Figure 2).
Figure 2.
Emerging dopaminergic ligands as new levodopa (LD) adjuvant candidates.
Among these, Tavapadon (or PF-06649751) is a novel, highly selective D1/D5 agonist. A recent paper, reporting about Phase I PD studies, candidates Tavapadon as a novel therapeutic agent for PD with an initial safety, tolerability, and pharmacokinetic profile as well as potential for efficacy. The same report asserts that Phase II clinical trials have been initiated to deeper investigate the potential safety and efficacy of Tavapadon with the aim to determine the dose that can produce relief of symptoms while reducing dependence on LD and, in the meanwhile, avoiding the problems associated with long-term LD administration [39].
Preclinical and clinical studies have indicated the potential utility of D1 agonists for the treatment of neuropsychiatric disorders. However, these agents are not devoid of limitations. For instance, it has been demonstrated that LID results from increased D1 receptor-mediated transmission at the level of the direct pathway. Moreover, unlike positive allosteric modulators (PAMs), orthosteric D1 receptor agonists produce receptor desensitization and an inverted U-shaped dose-response curve [40]. The development of the D1 PAM DETQ has been reported as a different approach to D1 receptor activation [41]. Being able to amplify the effects of released endogenous DA in situ, DETQ gives a more physiological response. Its CNS pharmacology strictly reminds that of D1 agonists, but also shows remarkable differences (i.e., it does not induce stereotypy or desensitization) [41]. The reported behavioral and neurochemical test results suggest a therapeutic utility in neuropsychiatric disorders such as PD [42,43].
It has also been hypothesized that DA receptors in the striatum can form heteromeric complexes. Such an heteromerization leads to changes in the functional and pharmacological properties of receptors compared to their monomeric subtypes [44,45]. It has been observed a correlation between the expression of D1–D3 receptor heteromers and the development of LID [46]. Furthermore, D3 receptor stimulation can potentiate the D1 receptor signaling pathway [46,47]. Thus, future D3 antagonists or partial agonists able to selectively modulate the activity of striatal D1–D3 receptor heteromers could be very promising in LID control [48]. Treatment with LD also induces an ectopic expression of D3 receptors in the DA depleted dorsal striatum, which is associated with dyskinesia [40,46,49]. D3 receptor involvement in dyskinesia has further been proved by PET studies in humans showing an elevated D3 receptor binding in patients with dyskinesia [50]. It has been reported that D3 receptor agonists may produce neuroprotective effects by directly scavenging free radicals, improving the activity of free radical scavenging enzymes, stabilizing the mitochondrial membrane, directly inhibiting neuronal apoptosis. Moreover, being D3 receptors primarily localized in the midbrain limbic system, which is unrelated to motor function, selective D3 receptor agonists may have suitable anti-PD activity without significant extrapyramidal side effects [51,52,53,54,55].
The D3 receptor subtype has also been shown to exhibit biased signaling and desensitization pattern in response to certain agonists, DA included. Such an evidence could significantly contribute to the development of motor and hyperkinetic symptoms in PD and LID, respectively. On the contrary, the closely related D2 receptors have not demonstrated these D3 characteristics [40,56]. Thus, it has been demonstrated that the selective D3 agonist SK609, which does not induce desensitization of D3 receptors in vivo [57,58], was able to decrease locomotor activity [59,60]. Moreover, it has also been observed a dose dependent efficacy of SK609 in improving motor deficits in PD and ameliorating abnormal involuntary movements (AIMs) in LID using the hemiparkinsonian unilateral lesioned rodent PD model. A combination of SK609 and a low dose of LD induced a motor symptomatic relief without producing AIMs [61].
CJ-1639 is actually one of the most potent and selective D3 full agonist reported to date that may become one of the newer anti-PD drugs [62,63].
The novel ‘multifunctional’ D2/D3 high-affinity compound D-512, endowed with receptor agonist activity together with antioxidant and other neuroprotective features has recently been developed [64,65]. Compared with Ropinirole, it showed greater peak-dose efficacy and a longer lasting action, thus deserving consideration for clinical investigation.
The novel carbazole-based multifunctional D2/D3 receptor ligands D-636, D-653, and D-656, endowed with high binding affinity and full agonist activity at both receptors [66], have been proved to be highly efficacious in a PD rat model indicating their potential in relieving motor dysfunction in PD. They also exhibited neuroprotective property in an in vitro cellular model of PD. Furthermore, D-636 and D-653 demonstrated potent modulator effect on aggregation and toxicity of α-synuclein protein in vitro. Thus, it has been postulated that multifunctional drugs like D-636, D-653, and D-656 have the potential to alleviate motor dysfunction in PD patients, as well as to modify the disease progression.
Pardoprunox (SLV-308), a D2/D3 receptor partial agonist and 5-HT1A receptor full agonist, reached Phase III clinical trials for the treatment of PD. Compared with other dopaminergic agents, it displayed lower propensity to elicit side effects like dyskinesia [67].
Since ligands endowed with such a multitarget profile might be effective in PD pharmacotherapy, novel multitarget compounds based on the N-((6,6-diphenyl-1,4-dioxan-2-yl)methyl)-2-phenoxyethan-1-amine (DDMPA) scaffold were studied. Interestingly, the 3-hydroxy derivative, here named for the first time DDMPA-8, behaved as a partial agonist at D2 and as a potent full agonist at D3 and D4 subtypes. In addition to its potent 5-HT1A receptor agonism, that might be helpful in reducing dyskinetic side effects associated with the dopaminergic stimulation, such a dopaminergic profile makes DDMPA-8 a potential multitarget compound for the treatment of PD. In perspective, its evaluation in PD animal models would shed light on its therapeutic potential [68].
D4 receptors are present within the basal ganglia that represent a key area involved in parkinsonism and, in particular, in dyskinesia [69,70]. During the last years, a renewed interest has emerged around D4 receptors as potential therapeutic target for the treatment of PD, in which D4 antagonists can attenuate LID [71,72,73,74]. It has been observed that associating the potent D4 antagonist L-745,870 to LD significantly ameliorates the dyskinesia scenario in LID models. Such a result was quite remarkable since this compound has also demonstrated to be well tolerated in clinical trials. Thus, it could have a rapid development as a new tool for LID treatment. Unfortunately, disappointing results were obtained in the rotarod performance test when co-administered with LD. In fact, L-745,870 reduced the overall LD antiparkinsonian benefit in this model opening only a narrow therapeutic window to its use for the treatment of LIDs [75,76].
The effect of the novel selective D4 antagonist, VU6004461 [77], endowed with high blood–brain barrier penetrability has also been investigated. The clear antidyskinetic effect of both L-745,870 and VU6004461 points to the D4 as a possible future target for the treatment of LID [78]. At present more work is needed, but the use of D4 antagonists for the treatment of LIDs in PD remains a very promising area of research and the development of more highly optimized ligands is still an acceptable challenge [73].
All the results obtained so far are not enough and the rising of the aged population imposes new strategies in PD that may help to manage known limitations of current therapies. Some of the alternative strategies investigated as potential treatment of LID in PD involve non-dopaminergic receptors. To help researchers in such a challenge, this review focuses on recent investigations about non-dopaminergic CNS receptor ligands that have been identified to have therapeutic potential for the treatment of motor and non-motor symptoms of PD. Such agents in different way may contribute to extend LD response and/or ameliorate LD-induced side effects.
2. Serotonin Receptors
The serotonin (5-HT) system has been demonstrated to play a crucial role in the pathogenesis of LID in animal models of PD [79]. Indeed, after advanced dopaminergic cell loss, remaining serotonin neurons can convert exogenous LD to DA and mediate its vesicular storage and release [38,80]. The non-physiological DA release from these neurons might cause DA receptor overstimulation, leading to generation of dyskinesia [81]. Consequently, modulation of 5-HT system has emerged as a promising strategy for LID management. Several studies have shown a reduction of LID induced by targeting different 5-HT receptor (5-HTR) subtypes. 5-HT1AR (dorsal raphe nucleus and striatum), 5-HT1BR (striatopallidal pathways), and 5-HT2AR (substantia nigra pars reticulata and internal segment of the globus pallidus) can modulate DA, GABA, and glutamate release within the basal ganglia to improve motor symptoms of PD and to reduce dyskinesia [82]. 5-HT1AR and 5-HT1BR agonists, as well as 5-HT2AR and 5-HT3R antagonists have demonstrated a potential as antidyskinetic agents, while 5-HT4 agonists can increase LD-stimulated DA release in CNS.
2.1. 5-HT1ARs
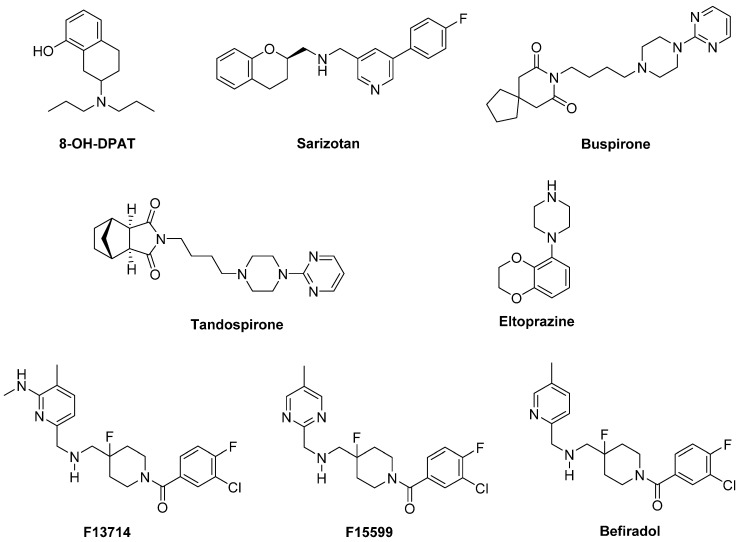
5-HT1AR is the most studied of the 5-HT family. Indeed, several preclinical and clinical studies demonstrated that 5-HT1AR stimulation (auto- and heteroreceptors) [49,83,84] may reduce dyskinesia through the decrease of DA release [79]. Moreover, 5-HT1AR activation may also weaken glutamatergic transmission ameliorating motor symptoms [83].
In a preclinical study, the highly selective 5-HT1A full agonist 8-OH-DPAT and its (R)-(+) eutomer reduced LID, but also worsened motor function in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model [85] (Figure 3).
Figure 3.
5-HT1AR agonists.
Sarizotan, another 5-HT1AR full agonist, also endowed with partial D2-like agonist/antagonist profile [86], showed better results as an antidyskinetic agent in a 6-hydroxydopamine (6-OHDA)-lesioned rat model of PD [87]. When evaluated in a Phase II clinical study, at low dose it ameliorated some PD symptoms [88]. However, it failed in attenuating LID compared with placebo in two Phase III studies [89], limiting its therapeutic potential.
The partial 5-HT1AR agonists Buspirone and Tandospirone have been shown to possess antidyskinetic properties in humans, but also negatively impacted on parkinsonian symptoms [90,91]. Currently, a Phase III clinical trial is investigating the antidyskinetic potential of Buspirone, which also behaves as a D2-like receptor antagonist [92], on LID (NCT02617017), while a Phase I clinical trial is exploring its potential in combination with the non-selective NMDA antagonist Amantadine (NCT02589340).
Another 5-HT1AR partial agonist able to reduce LID in combination with LD in preclinical studies is Eltoprazine [93]. Unlike Buspirone and Tandospirone, this compound also behaves as a 5-HT1BR agonist and a 5-HT2CR antagonist [94]. In both rodent and monkey models, it abolished LD-mediated motor improvements, suggesting that it may have a narrow therapeutic window [93]. However, the loss of LD efficacy proved to be mitigated by co-administration with 5-hydroxy-tryptophan [95]. When tested in a clinical Phase I/IIa study, Eltoprazine attenuated LID without affecting the antiparkinsonian action of LD [96]. To further validate its efficacy, another Phase II trial is currently ongoing to assess the duration of Eltoprazine’s efficacy in LID management and its effects on motor function (NCT02439125). Preclinical studies have also highlighted the potential efficacy of combining eltoprazine with other compounds able to attenuate LID, such as Amantadine and the selective adenosine A2A receptor antagonist Preladenant [93,97,98].
The 5-HT1A agonists so far evaluated in clinical trials have shown off-target effects and only partial agonist efficacy at 5-HT1AR. In this contest, the new highly selective 5-HT1AR biased agonists F13714, F15599, and Befiradol (also known as F13640 or NLX112) were recently demonstrated to exhibit exceptionally potent antidyskinetic activity in animal models of PD, while minimally interfering with LD antiparkinsonian effects [99,100,101]. Biased 5-HT1A agonists are selective ligands that act in specific brain regions and preferentially target different 5-HT1AR subpopulations [102]. While F13714 and Befiradol preferentially bind presynaptic 5-HT1A autoreceptors, F15599 activates postsynaptic 5-HT1ARs [100,103,104]. Befiradol has recently been shown to possess a distinctive in vitro G-protein activation profile in rat brain cell membranes which differs from those of F13714 or F15599 [105]. In particular, it preferentially activate Gαo proteins over other G-protein subtypes. This compound is currently undergoing clinical development as an antidyskinetic agent (www.parkinsons.org.uk/news/investing-new-treatment-dyskinesia).
2.2. 5-HT1BRs
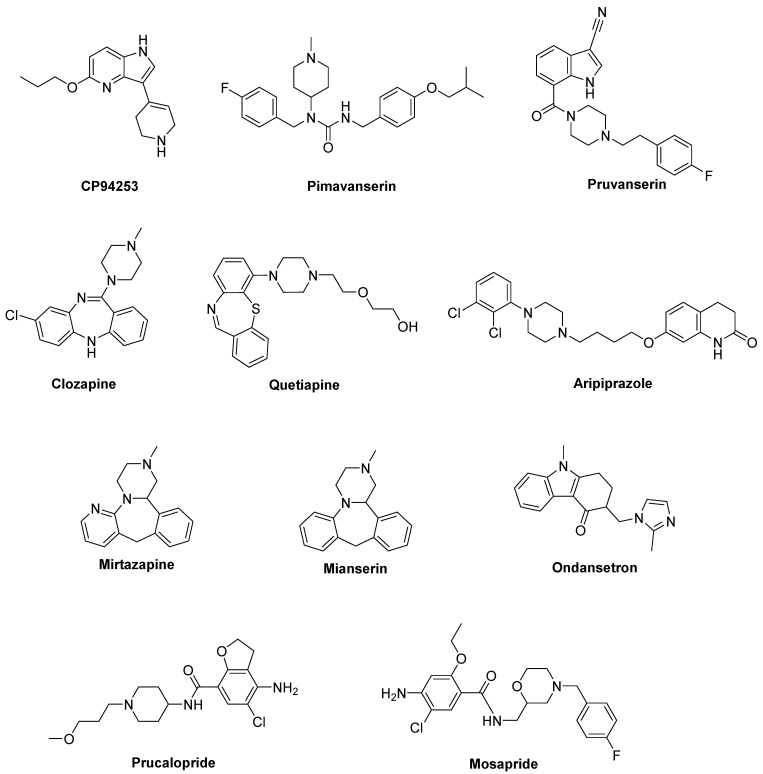
Although studies with compounds selectively targeting 5-HT1BR are limited, the selective 5-HT1BR agonist CP94253 demonstrated to attenuate LID in a 6-OHDA-lesioned rat model [81,106] (Figure 4). Interestingly, CP94253 also reduced dyskinesia induced by D1 receptor agonists at low doses [106]. However, a definitive role of 5-HT1BR in LID is difficult to be defined due to very limited clinical studies. Instead, the combination of 5-HT1BR and 5-HT1AR agonism is more often studied both by monotherapies with mixed actions (e.g., the 5-HT1AR/5-HT1BR agonist Eltoprazine) or by combined therapies [81]. More recently, the co-administration of the 5-HT1B agonist CP94253 with the 5-HT1A agonist 8-OH-DPAT and the metabotropic glutamate 5 receptor (mGlu5R) antagonist MTEP elicited a great synergistic antidyskinetic effect without impairment of the antiparkinsonian effects [107].
Figure 4.
5-HT1BR, 5-HT2AR, 5-HT3R, and 5-HT4R ligands.
2.3. 5-HT2ARs
Among 5-HT2R subtypes, a potential role in PD and LID has been suggested for 5-HT2AR. Pimavanserin (ACP-103), a potent 5-HT2AR and less potent 5-HT2CR inverse agonist [108], demonstrated to attenuate the expression of LID in cynomolgus monkeys without reducing LD efficacy [109] (Figure 4). This compound has been approved in the United States for the treatment of dopamimetic-induced psychosis in PD patients [110]. However, clinical studies examining this compound against LID have not been reported so far. Evidence in support of Pimavanserin for the management of psychosis in PD patients comes from a Phase III placebo-controlled trial, showing that it was well tolerated and didn’t worse motor function [111].
The highly selective 5-HT2A receptor antagonist Pruvanserin (EMD-281,014, LY-2,442,347) demonstrated to reduce the severity of LID and psychosis in a primate PD model, without affecting LD anti-parkinsonian activity [112]. On the contrary, it failed to reduce LD-induced AIMs in 6-OHDA-lesioned rat model, highlighting differences between rodent and primate models of PD [113].
Other evidences of 5-HT2AR involvement in LID derive from studies with antipsychotic compounds that are not selective towards such a subtype. For example, in both 6-OHDA-lesioned rat and MPTP-lesioned marmoset models, the atypical antipsychotic Clozapine reduced LID psychosis-like behaviors [114,115]. When tested in humans, Clozapine successfully reduced both duration and severity of LID symptoms without affecting LD efficacy [116,117]. However, the observation that Clozapine also displays affinity for other receptors, including 5-HT2C, D2 and D4, makes it difficult to evaluate the direct involvement of 5-HT2AR.
Quetiapine, another atypical antipsychotic agent targeting 5-HT2AR in addition to other systems including adrenergic, muscarinic, histaminergic, and dopaminergic receptors [118,119], effectively reduced LID without interfering with LD efficacy in 6-OHDA-lesioned rat and MPTP-lesioned macaque models [120]. Nevertheless, the results of clinical trials with this compound seem to be conflicting. Indeed, while a clinical study reported that low doses of Quetiapine did not significantly attenuate LID [121], another study found that it reduced LID with worsening of few motor symptoms [117].
The antipsychotic Aripiprazole is endowed with a multitarget profile, showing antagonism at 5-HT2AR and partial agonism at both 5-HT1AR and D2 [122]. In clinical studies it was able to attenuate hallucinations associated with PD, but also reduced LD efficacy in some patients [123]. Moreover, at a very low dose, it provided long-term LID relief [124].
Finally, both Mirtazapine and its analogue Mianserin, antagonists at noradrenergic receptors and 5-HT2R/5-HT3R [125], demonstrated to reduce LID in NHP models [126,127]. However, Mianserin also reduced LD efficacy, limiting its clinical use. In clinical trials mirtazapine was reported to reduce LID without worsening PD symptoms, particularly in patients that were non-responsive to Amantadine [128]. Further clinical studies are in progress.
2.4. 5-HT3Rs
Recently it has been proposed that stimulation of the receptor channel 5-HT3R might affect DA release in striatum. The 5-HT3 antagonist Ondansetron decreased AIMs scores in 6-OHDA-lesioned rat model of PD, suggesting its efficacy in LID. However, it had no effects on motor coordination in rotarod behavioral test [129] (Figure 4).
2.5. 5-HT4Rs
In a recent study, the 5-HT4 agonist Prucalopride, evaluated in a 6-OHDA-lesioned rat model of PD, selectively enhanced LD-stimulated DA release in the substantia nigra pars reticulata and prefrontal cortex (Figure 4). The enterokinetic properties of 5-HT4R agonists suggested their potential use against LD-induced fluctuations in patients with PD [130]. Moreover, since 5-HT4 agonists displayed anxiolytic/antidepressant properties in a mouse corticosterone model [131], Prucalopride may represent an alternative approach to the treatment of anxiety and/or depression in LD-treated patients with PD [132].
The mixed 5-HT3R antagonist/5-HT4R agonist Mosapride proved to be effective in promoting the lower gastrointestinal tract motility and in ameliorating constipation in PD patients [133].
3. Glutamate Receptors
In rodent models of LID, high extracellular levels of glutamate were observed in the striatum and substantia nigra pars reticulata. Molecular imaging studies suggested that similar neurochemical changes of this system are evident in PD patients [134]. Therefore, glutamate receptors represent attractive targets for the treatment of LID. While the first efforts were addressed to antagonize the ionotropic glutamate receptors (iGluRs) N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtypes [135], more recently, metabotropic glutamate receptors (mGluRs) have also been considered as potential targets for PD and LID treatment [136,137].
3.1. iGluRs
Several studies performed in animal models of LID and in post-mortem basal ganglia tissues from dyskinetic PD patients have revealed modifications in the expression and state of phosphorylation of iGluRs, in particular NMDA and AMPA receptors [134]. Therefore, these receptor systems are considered of major importance to the pathophysiology of LID.
3.1.1. NMDA Receptors
Alterations in NMDA receptor trafficking and distribution in the postsynaptic neurons appear to be associated with the extent of DA denervation as well as with the development of LID. However, the exact mechanisms regulating NMDA receptor subcellular trafficking and function in PD and LID are not fully elucidated yet [138,139]. Among the subunits forming the NMDA receptor, GluN2B subunit has attracted considerable interest. Indeed, from radioligand binding studies, performed both in NHP models of LID and dyskinetic PD patients, increased binding densities at GluN2B-containing NMDA receptors in the putamen were observed [140,141]. Furthermore, increased levels of GluN2B phosphorylation have been found in 6-OHDA-lesioned rats after chronic LD treatment [142].
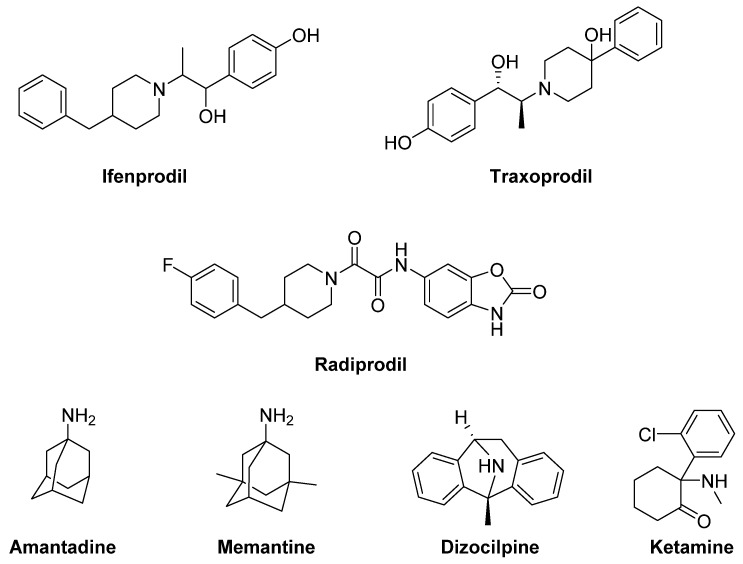
The GluN2B-selective antagonists Ifenprodil and Traxoprodil (CP-101606) were reported to ameliorate parkinsonian symptoms and to reduce LID in rat and NHP models [143,144,145,146,147,148] (Figure 5). However, their use has been discouraged in NHP owing to the development of severe side effects, including amnesia and dissociation [143,145,149,150].
Figure 5.
N-methyl-D-aspartate (NMDA) receptor ligands.
In general, divergent results have been obtained following treatment with GluN2B-selective antagonists in animal models of LID, ranging from improvement to no effect, and even to a worsening of AIMs [134].
Radiprodil, another GluN2B-selective antagonist, in combination with the selective A2A receptor antagonist Tozadenant, significantly improved motor activity both in 6-OHDA-lesioned rats and MPTP-lesioned NHP models, suggesting that the use of such a combination could lead to motor improvement to PD patients, without inducing the motor complications induced by LD therapy [151,152].
Promising results for the treatment of LID were also obtained with the weak non-competitive NMDA receptor antagonists Amantadine and Memantine. Amantadine, historically used as an antiviral agent, showed moderate but significant antidyskinetic efficacy in various clinical trials performed in the last two decades [153,154,155]. For this reason, it is the only drug with established antidyskinetic activity available in the market [156]. Amantadine treatment proved to reduce the duration of LID and to improve motor disability in PD [157] without major complications [154]. However, there are contrasting results concerning its long-term efficacy [158,159]. A Phase II clinical trial is currently ongoing to study the impact of Amantadine in preventing LID in early PD (NCT01538329). Other Phase II clinical trials are currently underway to evaluate the efficacy of Amantadine, in combination with other classes of drugs (e.g., Buspirone or Eltoprazine, see the section “Serotonin receptors”), in reducing LID in preclinical or clinical trials. Moreover, a recent study has revealed that the combination of a sub-effective dose of Amantadine and the nitric oxide synthase inhibitor 7-Nitroindazole potentiated the effect of reducing LD-induced AIMs in 6-OHDA-lesioned rats when compared to the effect of the drugs alone. This strategy may provide therapeutic benefits to PD patients at lower and thus more tolerable doses [160]. Memantine has also been investigated for its antidyskinetic potential in PD patients, but the results were conflicting. Although Memantine treatment was associated with lower LID scores and reduced daytime duration of dyskinesia, no significant effects on dyskinesia severity were found [161,162,163].
Other non-competitive NMDA receptor antagonists, including Neu-120 (structure not disclosed), Dizocilpine (MK-810) and Ketamine, displayed potential antidyskinetic effects.
Neu-120 is produced by Neurim Pharmaceuticals for the treatment of drug-induced dyskinesias. This compound, that also inhibits MAO-B and GSK-3β, has been subjected to a Phase I/II clinical trial to determine its safety, tolerability, pharmacokinetic and pharmacodynamic profiles in reducing LID in patients with advanced-phase idiopathic PD (NCT00607451). The study has been completed, but the results are not available yet.
Dizocilpine also reduced LD-induced AIMs in a rat model of LID, but only at concentrations that worsen parkinsonism [164]. However, when this compound was co-administered with the opioid glycopeptide Lactomorphin (see Figure 17), its pro-parkinsonian activity was suppressed, while a strong antidyskinetic effect remained [165].
Finally, the dissociative anesthetic Ketamine, administered at low sub-anesthetic doses, displayed a long-term effect in reducing LID in a preclinical 6-OHDA-lesioned rat model [166]. This result was confirmed by a clinical trial, in which intravenous infusion of low doses of Ketamine induced a long-lasting therapeutic benefit to reduce LID and depression in PD patients [167].
3.1.2. AMPA Receptors
Analogously to NMDA receptors, synaptic localization and phosphorylation of AMPA receptors proved to be altered in animal models of LID and in PD patients [168,169,170,171]. Moreover, in MPTP-lesioned monkeys and 6-OHDA-lesioned rats, the pharmacological blockade of AMPA receptors decreased LIDs and enhanced the antiparkinsonian effect of LD [172,173,174]. Conversely, AMPA receptor agonists triggered dyskinesias [174]. In the light of these findings, treatments with selective AMPA receptor antagonists alone or in combination with selective NMDA receptor antagonists showed beneficial effect in reducing dyskinesia [172].
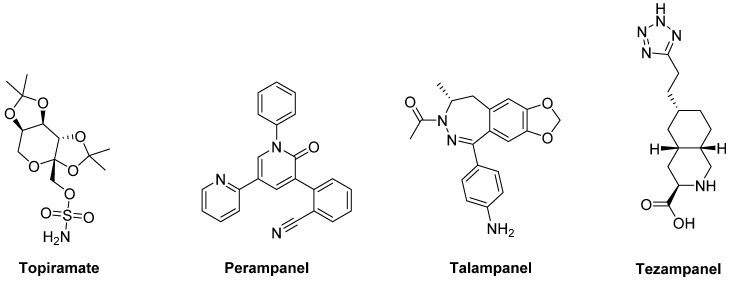
The anticonvulsants Topiramate and Perampanel are the only AMPA receptor antagonists which have reached clinical trials (Figure 6). Topiramate is a negative modulator of AMPA receptors [175] and a PAM of GABAA receptors [176]. It has been reported to improve LID in MPTP-lesioned NHPs [177]. Moreover, in combination with the non-competitive NMDA receptor antagonist Amantadine, Topiramate elicited a synergistic antidyskinetic effect in both rodent and marmoset models of LID at low doses [178]. Despite these positive preclinical experiences, clinical trials have provided conflicting results. In a double-blind trial involving patients with idiopathic PD, Topiramate worsened dyskinesia and was poorly tolerated [179]. No results are so far available for other two Phase II clinical trials evaluating the efficacy of the combination of Amantadine and Topiramate versus Amantadine alone in PD patients with or without dyskinesia (NCT00794313, NCT01789047). Conflicting results were also found in clinical trials with the non-competitive antagonist Perampanel [180,181,182].
Figure 6.
amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor ligands.
In preclinical studies, Talampanel (LY-300164, GYKI 537773), another non-competitive AMPA receptor antagonist, increased the anti-parkinsonian benefits of LD in MPTP-treated monkeys [174], while the competitive AMPA antagonist Tezampanel (LY-293558) was able to reduce wearing-off of LD-induced motor responses in 6-OHDA-lesioned rats [183].
3.2. mGluRs
mGluRs modulate intracellular signaling pathways without blocking the main action of glutamate on excitatory synaptic transmission. For this reason, they may be considered drug targets more convenient than iGluRs. Evidence demonstrated that mGluRs regulate pathophysiologically crucial events related to PD and LID [184,185].
3.2.1. mGlu2/3Rs
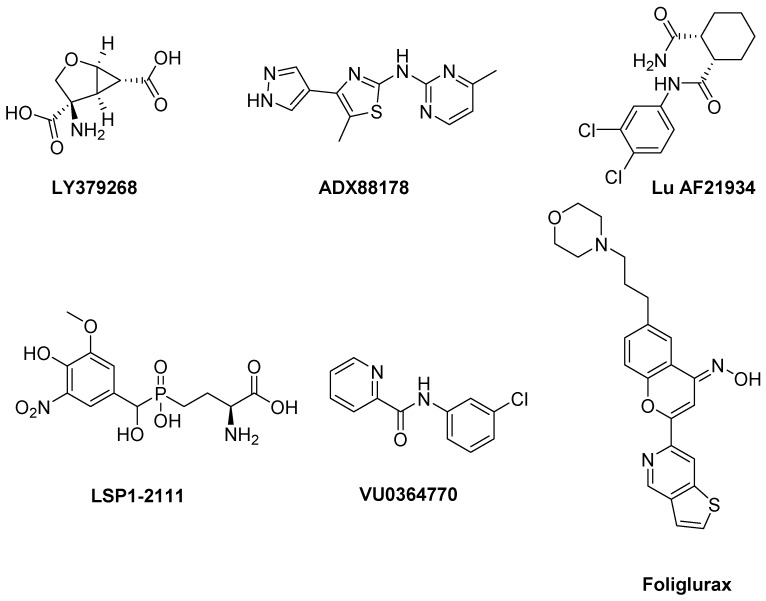
mGlu2R and mGlu3R agonists have been proposed to be used in the treatment of PD and LID [134]. In a reserpine-treated model of PD, the mGlu2/3R agonist LY379268 was able to reduce akinesia [186] (Figure 7). However, this result was in contrast with that obtained in the 6-OHDA-lesioned model of PD, in which LY379268 failed to modify the bias towards ipsiversive rotations [186]. It should be considered that discrepancies between results from different PD models may depend on different degrees of DA depletion. LY379268 also failed to produce any amelioration of AIM scores in a rat model of LID [149]. Because of these contrasting effects, the use of mGlu2R and mGlu3R agonists for the treatment of PD and LID may be challenging.
Figure 7.
mGlu2/3R and mGlu4R ligands.
3.2.2. mGlu4Rs
Activation of mGlu4R using PAMs or orthosteric agonists induces antiparkinsonian effects in animal models of PD [187]. The mGlu4R PAMs ADX88178 [188] and Lu AF21934 [189] potentiated the effect of LD without increasing LID in a 6-OHDA-lesioned rat model (Figure 7). The above mentioned PAMs and the orthosteric agonist LSP1-2111 [190], when co-administered with LD, showed LD sparing effect. This makes mGlu4R agonists potentially useful to allow LD to maintain the same benefit on PD motor symptoms al lower doses. Such an effect would indirectly improve LID. In a more recent study the observation that the stimulation of mGlu4R with the orthosteric agonist LSP1-2111 lacked antidyskinetic and LD-sparing activities while the PAM VU0364770 decreased LID in 6-OHDA-lesioned rats demonstrated that an mGlu4R PAM might be an antidyskinetic agent better than an orthosteric agonist [191].
Recently, novel potent and selective mGlu4R PAMs with improved pharmacokinetic profiles after oral administration have been discovered. Among them, Foliglurax (PXT002331) fully reversed hypokinetic deficits in 6-OHDA-lesioned rats when co-administered with sub-threshold doses of LD [192] and proved to alleviate the motor symptoms of PD and the motor complications induced by LD in primates [193]. Foliglurax is currently being evaluated in Phase IIa trial (NCT03162874) in PD patients affected by LID and wearing-off fluctuations. These results support mGlu4R as a novel and promising therapeutic target for PD and LID.
3.2.3. mGlu5Rs
Several preclinical and clinical studies demonstrated the involvement of mGlu5R in PD and LID. In particular, negative allosteric modulators (NAMs) have proven high efficacy to reverse motor deficits and inhibit LID in both 6-OHDA-lesioned rat and MPTP-lesioned NHP models of PD [184,185].
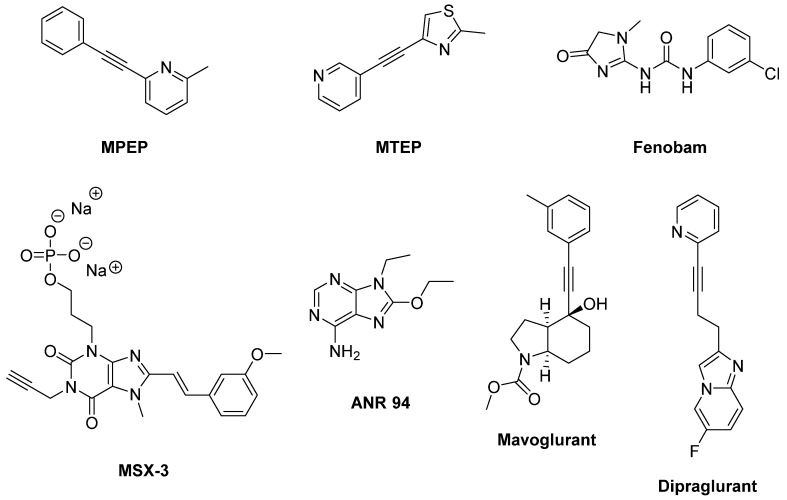
The NAMs MPEP and MTEP attenuated the effects of LD in inducing involuntary movements in the 6-OHDA-lesioned rat model of LID (Figure 8) [194,195]. Accordingly, MTEP potently inhibited AIMs triggered by the D1 receptor agonist SKF38393 [107]. MPEP and MTEP also reduced the intensity of LID after acute administration in NHP models of PD [196]. Moreover, MPEP proved to be efficacious after chronic administration without affecting LD efficacy [197]. These results were in line with a previous study, in which the mGlu5R NAM Fenobam reduced LID in both 6-OHDA-lesioned rats and MPTP-lesioned monkeys [198]. Moreover, a combination of Fenobam and Amantadine at sub-threshold doses reduced LID without worsening PD [199], while a combination of MPEP and the adenosine A2A antagonists MSX-3 and ANR 94 synergistically increased LD-induced turning [200].
Figure 8.
mGlu5R ligands.
Accordingly, in Phase II clinical studies (NCT00582673, NCT00888004, and NCT00986414) the mGlu5R NAM Mavoglurant (AFQ056) demonstrated antidyskinetic efficacy without worsening PD motor symptoms [137,201,202]. The most common adverse events were reported to be dizziness, hallucinations, diarrhea, and insomnia. Unfortunately, Mavoglurant failed to replicate the previous outcome in two subsequent Phase II clinical studies (NCT01491529, NCT01385592), leading to discontinue clinical trials of this compound for the treatment of LID [203]. In another clinical study (NCT01092065), administration of Mavoglurant in patients treated with high doses of LD avoided a worsening of dyskinesia. However, this study was limited by the reduced number of patients, the short treatment duration and the conflicting clinician-rated measures [204].
Among the mGlu5R NAMs, Dipraglurant (ADX48621) showed the most encouraging clinical results. Indeed, in a recent Phase II clinical trial it effectively reduced LID severity including a reduction of dystonia severity and chorea (two major LID components) with no evidence of worsening parkinsonism. Moreover, Dipraglurant demonstrated good safety and tolerability [205], deserving to be further investigated in a larger number of patients to confirm its efficacy in the treatment of LID.
3.2.4. mGlu7Rs and mGlu8Rs
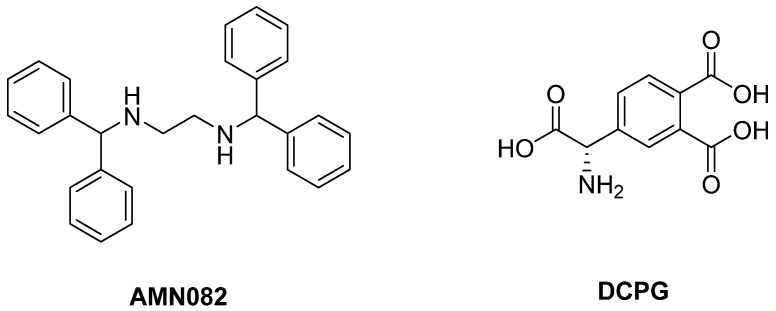
The role played by mGlu7R and mGlu8R in PD and LID need to be elucidated as highly potent and selective ligands have become available only recently and have not been fully pharmacologically characterized yet. Only AMN082, a selective mGlu7R allosteric agonist [206], was shown to have some modest antiparkinsonian effects in reserpine-induced akinesia [207] as well as in haloperidol-induced akinesia animal models [208] (Figure 9).
Figure 9.
mGlu7R and mGlu8R ligands.
The mGlu8R agonist DCPG failed to have antiparkinsonian effect in rodent models of PD [207]. However, other studies reported that this compound elicited a reduction of haloperidol-induced catalepsy and reserpine-induced akinesia but only when Haloperidol or Reserpine are administered for a prolonged period of time [209] in 6-OHDA-lesioned rats. This evidence highlighted the need for further studies to understand the mechanisms underlying the antiparkinsonian effects of DCPG.
4. Noradrenergic Receptors
The noradrenergic system plays an important role in the pathophysiology of PD. Noradrenergic neurons in the locus coeruleus [210] undergo degeneration in PD and may even anticipate the death of DA neurons [211,212,213]. They appear to play a protective role by establishing the extent of nigral degeneration induced by both neurotoxic damage and pathological events underlying PD [212,214,215]. Therefore, the indirect activation of adrenergic pathways by blocking presynaptic α2 adrenergic autoreceptors should prevent the nigrostriatal DA degeneration and subsequent motor deficits in PD.
Moreover, being the noradrenergic system implicated in autonomic function, targeting α2 or β adrenergic receptors (α2-Ars or β-Ars, respectively) appears to have potential to improve symptomatic orthostatic hypertension in PD.
4.1. α2-Ars
Stimulation of α2-Ars overexpressed in striatal GABAergic neurons activates direct basal ganglia pathway and is involved in the generation of LID, justifying the investigation of α2-AR antagonists as antidyskinetic agents [216].
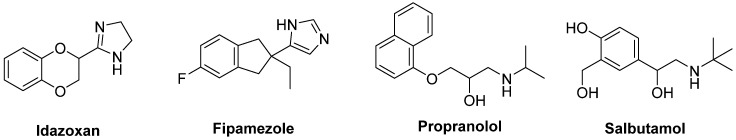
The non-selective α2-AR antagonist Idazoxan was effective in alleviating the expression of AIMs in 6-OHDA-lesioned rats [217] (Figure 10). In a randomized, placebo-controlled pilot study, Idazoxan improved the severity of LIDs without affecting the antiparkinsonian effect of LD [218], but increasing the frequency of cardiovascular side effects.
Figure 10.
Noradrenergic receptor ligands.
Fipamezole, a more recently developed α2-AR antagonist, has also been shown to extend both the duration and quality of LD action in MPTP-lesioned NHP [219]. A clinical trial with ten PD patients has demonstrated good tolerability and sound antidyskinetic effect [220]. In a Phase II double-blind, placebo-controlled study in US and Indian PD patients Fipamezole did not show any significant antidyskinetic effect. However, the analysis of US subjects revealed that it reduced LIDs in a dose-dependent manner with an acceptable profile of adverse effects [221]. Other clinical trials with Fipamezole have been performed but the results have not been published yet (NCT01149811, NCT01140841, NCT00040209).
4.2. β-ARs
Pharmacological and neuroanatomical evidences support a role for β-ARs as potential therapeutic targets against LID. Indeed, both β1- and β2-ARs are expressed in the striatum [222] and are integral in PD patients [223].
The β2-AR antagonist Propranolol has been reported to reduce LID without affecting LD’s efficacy in several experimental and clinical studies (Figure 10). However, it failed to reduce dyskinesia produced by the D1 receptor agonist SKF81297 or the D2 receptor agonist Quinpirole. Antidyskinetic properties of Propranolol appear to be mediated via attenuation of LD-induced extra-physiological efflux of DA [224]. β blockers might be preferred first-line agents in PD patients who has co-morbid hypertension. Moreover, they are associated with a lower risk of constipation, which is one of the most frequent non-motor symptoms of PD [225]. However, in patients with asthma or chronic obstructive pulmonary disease, β blockers should not be used owing to the risk of bronchospasm [226].
Evidences also support the use of β2-AR agonists in PD therapy. Indeed, from molecular and immunological studies adrenergic stimulation has been suggested to decrease both α-synuclein deposition and release of neurotoxic molecules. In small clinical trials the β2-AR agonist Salbutamol in combination with LD improved parkinsonian symptoms in patients with fluctuating PD. Nevertheless, large randomized controlled trials are lacking [227].
5. Adenosine Receptors
Adenosine is a neuromodulator that regulates responses to DA and other neurotransmitters in areas of the brain that are responsible for motor function as well as learning and memory [228]. While the monotherapy with adenosine receptor antagonists reaches limited efficacy in the treatment of PD, their use as coadjuvants to LD appears to be a promising strategy.
5.1. A1 Receptors
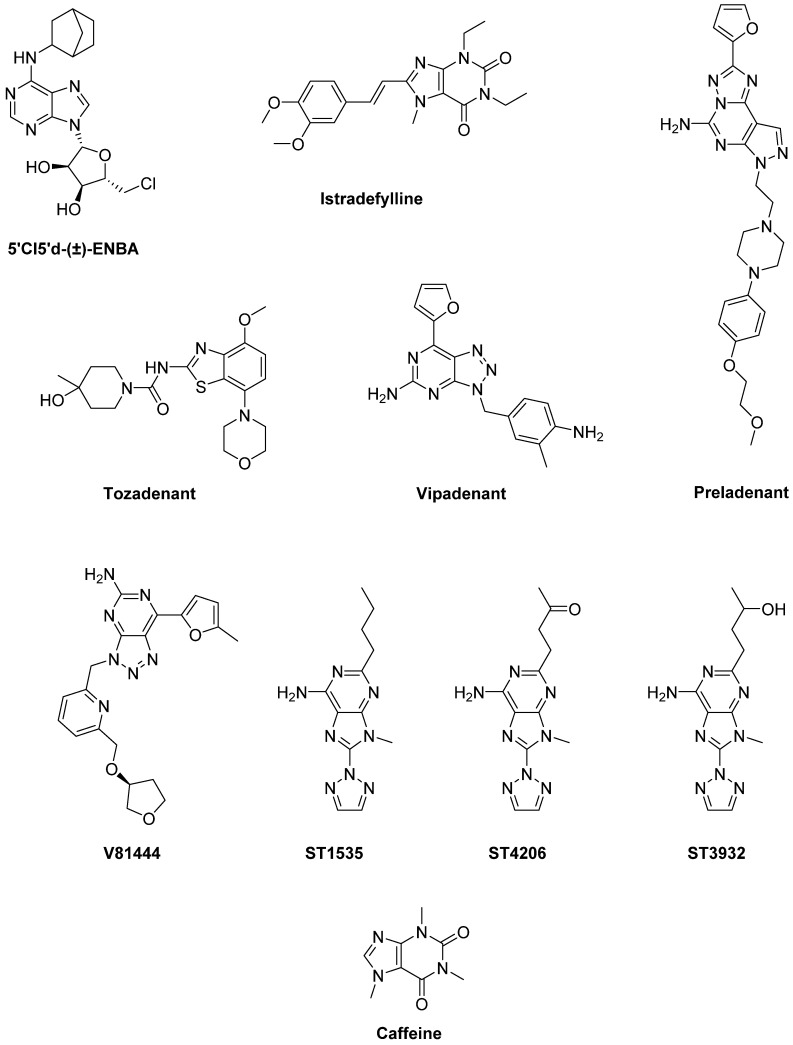
Adenosine has been reported to antagonize D1 receptor-mediated transmission through the stimulation of A1 receptors [229,230], which are widely expressed in the substantia nigra pars reticulate [231]. The selective adenosine A1 receptor agonist 5′Cl5′d-(±)-ENBA, administered in combination with LD, reduced the development of AIMs, indicating the potential efficacy of A1 agonists for the treatment of LID and hyperkinetic disorders [232] (Figure 11).
Figure 11.
Adenosine receptor ligands.
5.2. A2A Receptors
A2A receptors are highly expressed and co-localized with D2 and D3 receptors on striatopallidal output neurons in the striatum. Activation of A2A receptors causes the hetero-dimerization with D2 receptors and inhibits indirect basal ganglia pathway from striatum to thalamus [233,234]. As demonstrated by preclinical and clinical studies, A2A receptor antagonists are able to improve motor dysfunctions of PD while reducing side effects such as dyskinesia [235].
Istradefylline (KW6002), one of the first selective A2A antagonists tested in clinics, received marketing approval in Japan in 2013 for the treatment of PD [236] (Figure 11). In preclinical studies, administration of Istradefylline to 6-OHDA-lesioned rats, previously exposed to LD and exhibiting AIMs with each LD intake, did not elicit AIMs. Moreover, Istradefylline didn’t increase AIMs when administered with LD. However, it didn’t enhance the antiparkinsonian action of LD, assessed by the rotarod performance [237]. In the MPTP-lesioned marmoset, administration of Istradefylline reversed parkinsonism similarly to LD, without eliciting dyskinesia [238] and increasing motor activity [239]. When Istradefylline was administered to MPTP-lesioned marmosets previously treated with LD, it enhanced the antiparkinsonian action of the D2 agonist Quinpirole and, at lesser extent, of the D1 agonist SKF-80,723 [240]. The combination of Istradefylline and a low dose of LD caused a reduction of LID after chronic treatment, while maintaining the antiparkinsonian effect [241]. In the MPTP-lesioned macaque, Istradefylline monotherapy reduced parkinsonism [242]. After exposure to LD, it reversed parkinsonian disability without eliciting dyskinesia. Finally, when administered with sub-active dose of LD, Istradefylline did not enhance the antiparkinsonian action and dyskinesia, but specifically alleviated bradykinesia [243,244]. Taken together, these data support the clinical use of Istradefylline as co-adjuvant in PD therapy to manage various LD-induced complications. Istradefylline has been found to improve LD-related motor complications in many clinical trials. Some Phase II and III studies showed significant OFF time reduction in PD patients [245,246,247,248]. In contrast, in a large study it failed to demonstrate significant OFF time reduction [249]. Nevertheless, a meta-analysis of all randomized trials concluded that Istradefylline is clinically useful for increasing ON time and reducing OFF time in PD patients with motor fluctuations [250], as supported by a subsequent Phase III trial performed in patients with advanced PD [251]. The findings of an analysis of a post-marketing surveillance study are comparable with previous pre-approval clinical trials in Japan, demonstrating safety and effectiveness of Istradefylline in LD-treated PD patients with the wearing-off phenomenon [252]. Overall, from most of clinical data reported to date, Istradefylline demonstrated to be a well-tolerated and easy to use drug which shows efficacy in advanced PD patients without significantly increasing dyskinesia. This compound might represent a valid adjuvant in LD and other dopaminergic drug therapy to maximize their efficacy and minimize motor fluctuations [253]. Other clinical trials are currently ongoing to confirm the efficacy of Istradefylline in moderate to advanced PD patients (NCT01968031, NCT02610231).
Preladenant (SCH-420,814/MK-3814), another selective competitive A2A receptor antagonist, reversed parkinsonian disability without eliciting dyskinesia in rodent and primate models of PD [254,255]. When added to a low dose of LD, it enhanced its antiparkinsonian effect, without increasing LID [254]. In two Phase II trials evaluating Preladenant in combination with LD in PD patients for 12 or 36 weeks, a significant OFF time reduction was shown [256,257]. On the contrary, in other Phase III and Phase II trials, Preladenant failed to elicit the same effect probably owing to inappropriate study design and execution [258,259,260].
The A2A antagonist Tozadenant (SYN115) could also alleviate motor fluctuation. Indeed, in a Phase IIa study it elicited faster tapping speed before and during LD infusion compared to placebo [261] and, in a Phase IIb trial it was effective in reducing OFF time [262]. A Phase III study, assessing safety and efficacy of Tozadenant to treat end of dose wearing-off in PD patients using LD is currently ongoing (NCT02453386).
Vipadenant (V2006, BIIB014) is also a selective A2A antagonist which has reached clinical trials for the treatment of PD. This compound reduced OFF time duration and extended ON time in PD patients, without troublesome dyskinesia. However, 41% of Vipadenant-treated patients experienced adverse effects [263].
Other A2A antagonists that have progressed to Phase I clinical trials include V81444, PBF-509 (structure not disclosed), ST1535 and its metabolites ST4206 and ST3932. V81444 is an A2A antagonist currently under development. In a Phase I study it showed rapid absorption when orally administered, a half-life compatible with twice daily dosing, and minimal urinary excretion [264]. Moreover, a Phase Ib/II study is ongoing (NCT01634568). PBF509 potentiated the activity of LD in reversing parkinsonian motor impairments and inhibited LID in 6-OHDA-lesioned rats [265]. In a Phase I clinical trial (NCT01691924) it showed safety, tolerability and feasibility. ST1535 enhanced LD-induced rotational behavior in 6-OHDA-lesioned rats [266,267] and potentiated the antiparkinsonian action of a sub-active dose of LD in MPTP-lesioned marmosets [268]. A Phase I clinical trial demonstrated that ST1535 was well tolerated [269]. Its metabolites ST4206 and ST3932 showed a similar pharmacological activity and may be considered potentially therapeutic alternatives to ST1535.
5.3. A1/A2A Receptors
The non-specific adenosine receptor antagonist Caffeine has shown antiparkinsonian and neuroprotective effects in animal models of PD [270,271,272] (Figure 11). A clinical trial demonstrated that Caffeine could reduce the probability of developing dyskinesia [273]. However, in another randomized trial no significant changes in motor features were observed [274]. A Phase III trial to evaluate the efficacy of Caffeine in PD is currently ongoing (NCT01738178).
6. Histamine Receptors
Histamine H2 and H3 receptors are highly expressed in basal ganglia and might be involved in motor activity, thus representing another potential target for the treatment of LID in PD patients [275].
6.1. H2 Receptors
H2 receptors are mainly distributed in basal ganglia, particularly in the striatum. In mouse models the activation of cholinergic interneurons in LID has been demonstrated to be inhibited by blocking H2 histaminergic transmission, providing a strong rationale to reduce LID in PD patients by targeting such receptors [276].
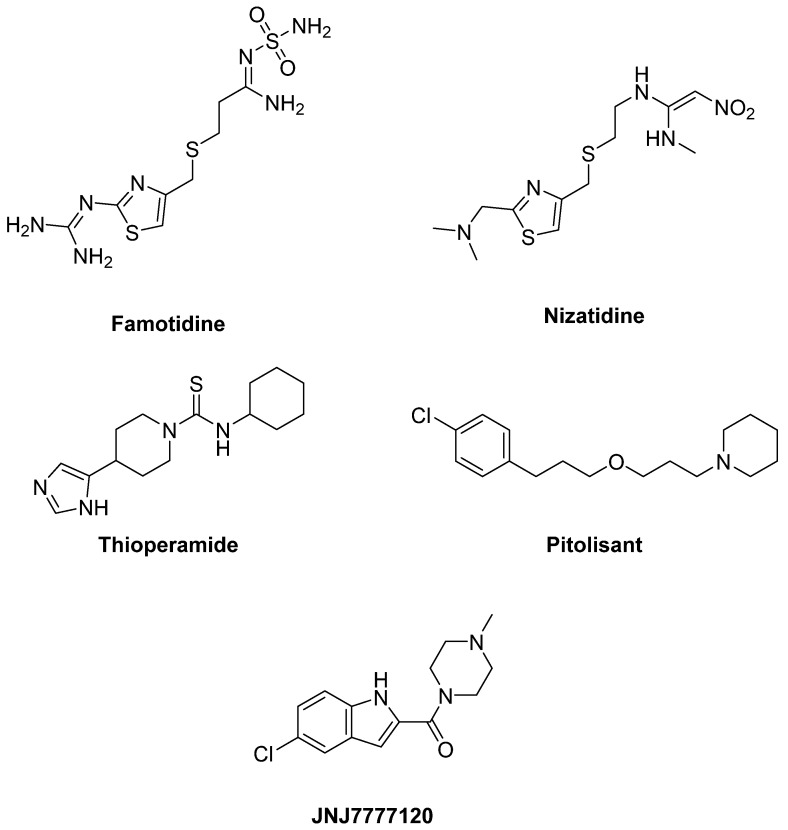
The selective H2 antagonist Famotidine enhanced the antiparkinsonian effects of LD and reduced LID in two mouse models [276] and a primate model of PD [277] (Figure 12). However, a Phase II trial evaluating Famotidine failed to demonstrate efficacy in reducing dyskinesia severity, although this trial used relatively low doses [278].
Figure 12.
Histamine receptor ligands.
In PD patient, Nizatidine, another selective H2 antagonist, demonstrated to be efficacious in ameliorating gastroparesis and slow transit constipation [279,280].
6.2. H3 Receptors
The observation that the H3 antagonist Thioperamide potentiated DA agonist-induced locomotor activation suggested a potential benefit of H3 antagonists on motor control in PD patients [281]. Thioperamide was also demonstrated to counteract memory and sleep impairment in a 6-OHDA-lesioned mouse model of PD [282] (Figure 12).
Pitolisant, the only H3 inverse agonist approved for the treatment of narcolepsy with and without catalepsy, is in Phase III clinical trials for the treatment of excessive daytime sleepiness in PD patients (NCT01036139, NCT01066442, NCT00642928).
6.3. H4 Receptors
The activity of microglia, which is regulated by H4 receptors, seems to play a key role in the pathogenesis of PD. Accordingly mRNA expression of H4 receptors proved to be increased in the basal ganglia of PD patients [283]. In rotenone-induced PD rat model the specific H4 antagonist JNJ7777120 blocked the microglial activation, reduced apomorphine-induced rotational behavior, prevented decreases in striatal DA levels, providing the first evidence of the efficacy of an H4 antagonist in PD [284] (Figure 12).
7. Cholinergic Receptors
It is well known that DA action is contrasted by acetylcholine (ACh) in striatum and unbalanced signaling between these neurotransmitter systems could alter basal ganglia activity and motor function, as it occurs in PD and LID. Numerous studies show that in PD nigrostriatal damage with severe DA depletion causes abnormal increase in cholinergic interneurons activity that, via strategically positioned nicotinic and muscarinic ACh receptors, promote striatal signaling to attenuate normal movements. Recently, new technology and pharmacological agents have facilitated understanding the role of ACh transmission in PD and LID, thus offering new therapeutic strategies in movement disorders [285,286,287].
7.1. Muscarinic Receptors
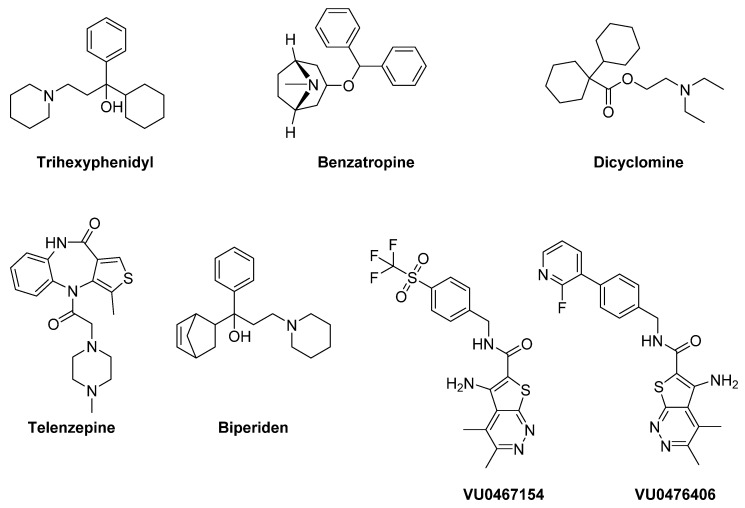
Muscarinic cholinergic antagonists have been considered in the treatment of PD for decades [288]. They are effective in preventing acute dyskinesias, especially in young patients. However, poor subtype selectivity and the occurrence of severe side-effects (confusion, hallucination, dry mouth, memory disturbance, urinary retention) have limited their use [289,290]. Trihexyphenidyl (benzhexol) has been considered one of the most representative compounds within this class. It inhibits the excitability of cholinergic neurons by blocking striatal M1 receptors. Although it was taken into account for the treatment of PD since 1949, only quite recently it was approved by the FDA for the treatment of parkinsonian tremor, dyskinetic movements and spastic contractions [291] (Figure 13). When the above-mentioned forms of parkinsonism are treated with LD, Trihexyphenidyl is often used as an adjuvant therapy [291].
Figure 13.
Muscarinic receptor ligands.
The M1 receptor antagonist Benzatropine also demonstrated to be efficacious for PD tremors [292]. Moreover, the non-subtype selective muscarinic antagonist Dicyclomine proved to enhance LD’s antiparkinsonian effects and to significantly attenuate LID in Pitx3-deficient aphakia mice [293].
M1 muscarinic receptors may also play a role in the modulation of PD non-motor deficits. Indeed, the preferential M1 antagonist Telenzepine demonstrated to improve anxiety-like behavior and social memory recognition in 6-OHDA-lesioned mice, suggesting that dysfunction of the striatal cholinergic system affects emotional and cognitive deficits in mice with reduced DA levels [294].
Biperiden is another muscarinic receptor antagonist with high affinity for the M1 subtype used in the treatment of PD and neuroleptic-induced extrapyramidal motor side effects. Recently, it has also been demonstrated to behave as a weak inhibitor of acetylcholinesterase [295].
Recent studies report that the M4 PAMs VU0467154 and VU0476406 significantly attenuated dyskinetic behaviors in mouse and primate models of LID in PD. These results suggest that activation of M4 muscarinic receptors, facilitating long-term depotentiation in D1 medium spiny projection neurons, might represent a novel pharmacological strategy to alleviate LID in PD patients [296].
7.2. Nicotinic Agonists
Activation of nicotinic receptors expressed on dopaminergic neurons indirectly affects DA release [297,298]. Moreover, it can also indirectly modulate GABA, serotonin and glutamate release, since nicotinic receptors are also localized on GABAergic, serotoninergic and glutamatergic interneurons [299,300]. Preclinical evidence demonstrated that nicotinic receptor ligands reduced LID by up to 60% in different PD animal models [285,287]. However, clinical studies on the involvement of the nicotinic system in LIDs are only emerging [301]. Interestingly, both nicotinic receptor agonists and antagonists similarly demonstrated to reduce LIDs in PD animal models. This can be due to the fact that prolonged exposure to agonist can lead to nicotinic receptor desensitization, ultimately reducing neurotransmitter release [297,302,303,304,305]. Therefore, nicotinic agonists and antagonists induce a similar functional blockade [306].
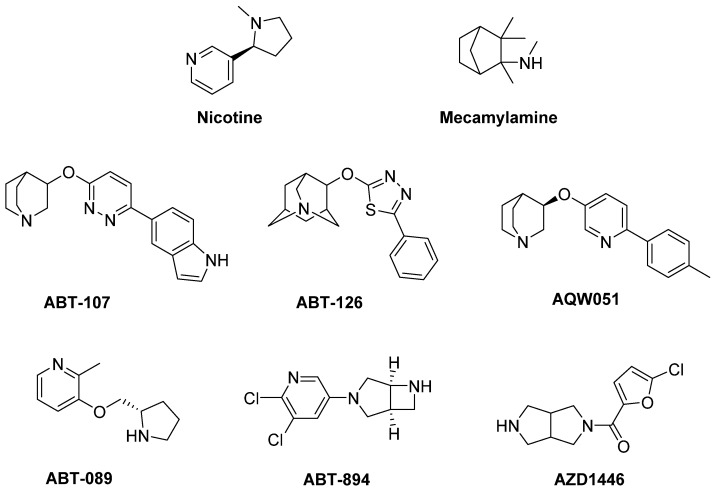
In addition to providing neuroprotection, Nicotine also demonstrated to protect against LID in different models of PD [306,307,308,309,310,311,312,313,314,315,316,317] (Figure 14).
Figure 14.
Nicotinic receptor ligands.
Interestingly, similar effects were observed with the non-selective nicotinic receptor antagonist Mecamylamine [306,310]. In clinical trials Nicotine showed antidyskinetic effect in PD patients after oral treatment [318,319]. Results of a small Phase II trial have never been published (NCT00957918).
Preclinical studies have shown that the nicotinic receptor subtypes α7 and β2* (the asterisk indicates the possible presence of other subunits in the receptor complex) are mainly implicated in mediating both neuroprotective and antidyskinetic effects, suggesting that nicotinic subtype selective drugs may be beneficial therapeutic agents for LID management [300,308,320]. The α7 nicotinic receptor agonists ABT-107 and ABT-126 significantly reduced LID in PD monkeys without developing tolerance or worsening parkinsonism. ABT-126 was also effective in monkeys with both severe and moderate nigrostriatal damages, suggesting its ability to reduce dyskinesias in early- and later-stage PD [321,322].
Analogously, the selective α7 nicotinic receptor partial agonist AQW051, studied in MPTP-lesioned monkeys, reduced LID and extended LD antiparkinsonian response [323]. However, it failed to reduce dyskinesia or parkinsonian severity in idiopathic PD patients [324]. Several β2* nicotinic receptor subtype agonists, including ABT-089, ABT-894, and AZD1446, also demonstrated to significantly reduce LID in most dyskinetic animals without worsening parkinsonism and developing tolerance [325,326]. The extent of LID reduction didn’t increase by co-administration of α7 and β2* nicotinic receptor subtype agonists with respect to the drugs administered alone, suggesting that they act through a common mechanism of action [321]. Overall, the use of compounds selectively targeting β2* or α7 subtype appears to be a good therapeutic approach to alleviate LID. Thus, both classes of drugs may be promising antidyskinetic agents to be tested in clinical trials.
Since attenuated dopaminergic neurodegeneration and motor dysfunction have been observed in hemiparkinsonian α5-KO mice, nicotinic receptors containing the α5 subunit represent potential novel targets in the treatment of PD [327].
8. GABA Receptors
Considering that alterations in GABAergic neurotransmission may contribute to some of the axial symptoms of PD [328], GABA modulation has been proposed as a new strategy for PD treatment [329].
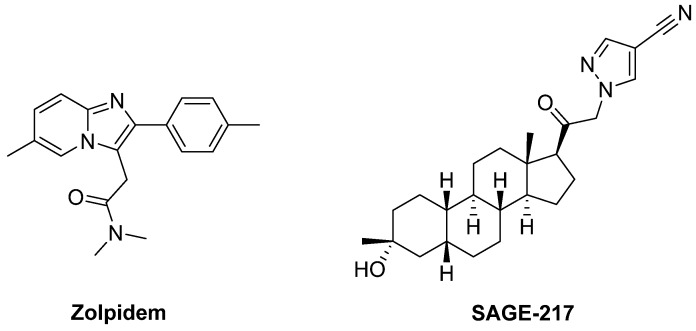
The GABAA receptor agonist Zolpidem, a PAM with selective affinity for receptors expressing the α1 subunit, improved motor impairments in unilateral 6-OHDA-lesioned rats, suggesting that targeting Zolpidem-sensitive GABAA receptors may be a novel approach to treat motor symptoms in PD [330] (Figure 15).
Figure 15.
GABA receptor ligands.
SAGE-217, another GABAA receptor PAM, is an orally bioavailable steroidal derivative which has reached a Phase II trial for the treatment of PD as monotherapy or in combination with LD. The results are not available yet (NCT03000569).
9. Neurokinin Receptors
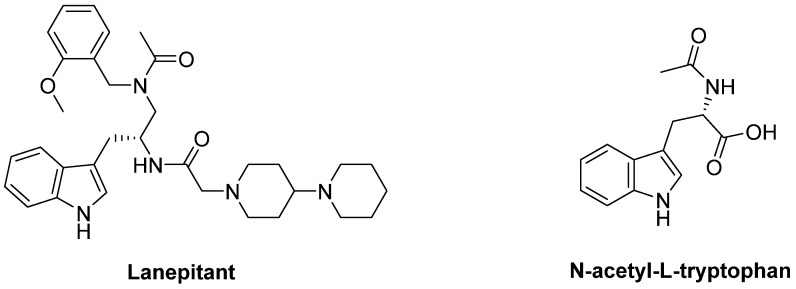
The abnormal stimulation of DA receptors, associated with LID and AIMs, induces up-regulation of FosB expression in dynorphin containing striatal cells where substance P (SP) is co-localized. LD treatment proved to increase SP in the substantia nigra. SP receptor antagonists has been suggested to reduce LID by blocking neurokinin 1 (NK1) receptors. Indeed, in 6-OHDA-lesioned rats the NK1 antagonists Lanepitant (LY303870) and N-acetyl-L-tryptophan demonstrated to ameliorate LID without affecting the therapeutic effect of LD and conserving motor function [331,332] (Figure 16).
Figure 16.
Neurokinin receptor ligands.
10. Opioid Receptors
Opioid receptors, especially δ receptor subtype, and the endogenous opioid peptides enkephalin and dynorphin are expressed in basal ganglia and cortex, where the opioid system modulates the activity of spiny projection neurons in motor disorders such as PD [333,334]. The level of opioid peptides demonstrated to be increased in the striatum, thalamus and anterior cingulate cortex [225] in PD animal models and PD patients exhibiting dyskinesia. Therefore, selective agonists and antagonists of opioid receptors have been used to contrast akinesia and LID in PD [335]. Moreover, due to the well known involvement of opioid in pain, several studies have investigated their potential for the treatment of pain in PD [288].
Tapentadol, a µ opioid receptor agonist with a serotonin/noradrenaline reuptake inhibitor activity, efficaciously reduced pain and was well tolerated in PD patients [336] (Figure 17).
Figure 17.
Opioid receptor ligands.
The µ opioid receptor antagonists might also be involved in PD therapy. Indeed, in MPTP-lesioned NHP Cyprodine and ADL5510 (structure not available) reduced LID without affecting the antiparkinsonian effects of LD [337,338].
A similar effect was induced by the selective δ antagonist Naltrindole, which demonstrated to alleviate LID in MPTP-lesioned marmoset and 6-OHDA-lesioned rats [337,339], while the δ agonist SNC-80 increased locomotor activity in PD animal models [340,341,342]. On the contrary, the selective κ receptor agonist U50,488 reduced LID in rat and monkey models of PD, although it contrasted the anti-parkinsonian effects of LD [343].
Analogously, the κ agonist and µ antagonist Nalbuphine alleviated LID in an NHP model of PD and decreased the levels of specific dyskinetic molecular markers [344].
Contrasting results were obtained with the non-selective opioid antagonist Naloxone, which reduced LID in 6-OHDA-lesioned rats [114,345], while the same effect was not observed in NHP and PD patients [346,347].
Particularly interesting are the results obtained with DPI-289 a δ Agonist/µ Antagonist (DAMA), which provided anti-parkinsonian action in rodent and NHP models of PD both alone or in combination with LD, without increasing dyskinesia, thus representing an LD-sparing strategy for clinical development [348].
The glycosylated derivative of the opioid peptide Leu-enkephalin Lactomorphin (MMP-2200) [H2N-Tyr-D-Thr-Gly-Phe-Leu-Ser-(O-β-d-lactose)-CONH2] behaved as a mixed δ/μ opioid receptor agonist [349]. This compound showed a modest antiparkinsonian activity, but reduced dyskinesia induced by D2-like receptor agonists [165]. A study evaluating Lactomorphin in combination with the NMDA receptor antagonist MK-801 is reported in the section “glutamate receptors”.
11. Sigma-1 (σ1) Receptors
σ1 Receptor is a type of non-opioid receptor [350] that is down-regulated in the brains of early stage PD patients [351,352]. Recently, the pharmacological stimulation of such a receptor has shown improvement of LID and neurorestorative and protective properties in experimental PD models [352,353].
σ1 Receptor ligands, such as the antagonist BMY-14802 [354], have been reported to be potentially useful for the treatment of LID [355] (Figure 18).
Figure 18.
σ1 receptor ligands.
The σ1 receptor agonist Dextromethorphan caused a reduction of dyskinesia by about 30–40%, without affecting the beneficial effect of LD [356]. This compound, that also behaves as a non-competitive NMDA receptor antagonist, as well as a serotonin and norepinephrine reuptake inhibitor is rapidly metabolized by hepatic cytochrome P450 CYP2D6. A Phase IIa clinical trial (NCT01767129) provided preliminary evidence of the efficacy, safety, and tolerability of Dextromethorphan in combination with the potent CYP2D6 inhibitor Quinidine for the treatment of LID in PD patients [357]. However, further studies with a longer treatment duration are needed to validate these early findings.
Pridopidine, a small molecule under development for the treatment of Huntington’s disease [358], produced a significant decrease in LID maintaining the antiparkinsonian benefit of LD in MPTP-lesioned macaques. Although such an effect was associated with full σ1 occupancy, such a mechanism alone is unlikely responsible for the antidyskinetic efficacy of Pridopidine which may be associated with the involvement of non-σ receptors [358].
12. Conclusions
Two hundred years ago James Parkinson described in his work “An essay on the shaking palsy” the characteristic of a CNS chronic degenerative disease lately named with his name (PD) [359]. Despite great progresses over the last 200 years, the therapeutic treatment of this disease, which has become the second most diffused neurodegenerative pathology over the world, still remains an unfulfilled dream and a challenge that scientists have to face. The currently available therapies have demonstrated limited efficacy for the following reasons:
-
-
the causes of such a pathology are mostly unknown;
-
-
the dopaminergic system and other receptors, as well as several enzymatic targets not discussed in this review, mutually affect each other and are deeply altered over the course of the disease (Figure 19);
-
-
the same drug used in PD therapy or for the treatment of co-morbidities may aggravate the progression of different disease symptoms;
-
-
the symptoms are individual and fluctuating during the day;
-
-
frequently divergent results come from the experimental models used in the evaluation of drug candidates.
Figure 19.
Pathophysiology of levodopa-induced dyskinesia (Reprinted with permission from Springer Nature: Springer CNS Drugs Pharmacological Strategies for the Management of Levodopa-Induced Dyskinesia in Patients with Parkinson’s Disease, Schaeffer, E.; Pilotto, A.; Berg, D., 2014, doi: 10.1007/s40263-014-0205-z. [360]).  Physiological activation,
Physiological activation, 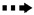 pathological alterations in LD-induced dyskinesia (LID),
pathological alterations in LD-induced dyskinesia (LID),  pathological alterations in LID,
pathological alterations in LID,  modulation, ++ increased activation, -- decreased activation. A2A adenosine receptor, α2a and α2ab noradrenergic receptors, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, Cb1 cannabinoid receptor, D1 and D2 dopaminergic receptors, DA dopamine, DA-autoR dopamine autoreceptor, DAT dopamine transporter, GABA γ-aminobutyric acid, Glu glutamate, H3 histamine receptor, LID levodopa-induced dyskinesia, mGluR metabotropic glutamate receptor, NA noradrenaline, nAchR nicotinic acetylcholine receptors, NMDA N-methyl-D-aspartate, OP opioid receptor.
modulation, ++ increased activation, -- decreased activation. A2A adenosine receptor, α2a and α2ab noradrenergic receptors, AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, Cb1 cannabinoid receptor, D1 and D2 dopaminergic receptors, DA dopamine, DA-autoR dopamine autoreceptor, DAT dopamine transporter, GABA γ-aminobutyric acid, Glu glutamate, H3 histamine receptor, LID levodopa-induced dyskinesia, mGluR metabotropic glutamate receptor, NA noradrenaline, nAchR nicotinic acetylcholine receptors, NMDA N-methyl-D-aspartate, OP opioid receptor.
However, the severity of the disease and its increasing diffusion due to the rising of the aged population prompt to the research of new therapeutic tools both administered alone and/or as LD adjuvant. From this point of view, interesting perspectives are given by the discovery of new ligands targeting different receptor systems, which are discussed in this review.
Moreover, based on the evolution of the traditional concept “one molecule-one target” to the newer “one molecule-one disease” that represents a trend of the modern medicinal chemistry, another helpful “stick of the LD old age” may be represented by multitarget ligands, synergistically able to restore dysfunctions of different system.
Considering the numerous possibilities existing in the field of target-based drug discovery, efficacious therapeutic tools might be hopefully available to PD patients in the future.
Acknowledgments
This work was supported by grant from the University of Camerino (Fondo di Ateneo per la Ricerca 2018).
Appendix A
PubChem CIDs (or Reaxys IDs) of the compounds reported in the review
| Compound | PubChem CID (Reaxys ID) |
| 5’Cl5’d-(±)-ENBA | 15599147 |
| 8-OH-DPAT | 1220 |
| ABT-089 | 178052 |
| ABT-107 | 11151363 |
| ABT-126 | 24987875 |
| ABT-894 | 10131048 |
| ADX88178 | 46836872 |
| Amantadine | 2130 |
| AMN082 | 11698390 |
| ANR 94 | 11805896 |
| Apomorphine | 6005 |
| AQW051 | 50914822 |
| Aripiprazole | 60795 |
| AZD1446 | 24795080 |
| Befiradol | 9865384 |
| Benzatropine | 1201549 |
| Biperiden | 2381 |
| BMY-14802 | 108046 |
| Bromocriptine | 31101 |
| Buspirone | 2477 |
| Cabergoline | 54746 |
| Caffeine | 2519 |
| CJ-1639 | 53475319 |
| Clozapine | 135398737 |
| CP94253 | 4029677 |
| Cyprodine | 24758534 |
| D-512 | (26962985) |
| D-636 | (33944059) |
| D-653 | (33944076) |
| D-656 | (33944078) |
| DCPG | 16062593 |
| DDMPA-8 | (33958274) |
| DETQ | 117720272 |
| Dextromethorphan | 5360696 |
| Dicyclomine | 3042 |
| Dihydroergocriptine | 114948 |
| Dipraglurant | 44557636 |
| Dizocilpine | 180081 |
| DPI-289 | (12841869) |
| Eltoprazine | 65853 |
| F13714 | (8361393) |
| F15599 | 11741361 |
| Famotidine | 5702160 |
| Fenobam | 135659063 |
| Fipamezole | 213041 |
| Foliglurax | 135565465 |
| Idazoxan | 54459 |
| Ifenprodil | 3689 |
| Istradefylline | 5311037 |
| JNJ7777120 | 4908365 |
| Ketamine | 3821 |
| L-745,870 | 5311200 |
| Lactomorphin | Not Available |
| Lanepitant | 3086681 |
| Lisuride | 28864 |
| LSP1-2111 | 46898088 |
| Lu AF21934 | 66553157 |
| LY379268 | 10197984 |
| Mavoglurant | 9926832 |
| Mecamylamine | 4032 |
| Memantine | 4054 |
| Mianserin | 4184 |
| Mirtazapine | 4205 |
| Mosapride | 119584 |
| MPEP | 3025961 |
| MSX-3 | 10256041 |
| MTEP | 9794218 |
| N-acetyl-L-tryptophan | 700653 |
| Nalbuphine | 5311304 |
| Naloxone | 5284596 |
| Naltrindole | 5497186 |
| Nicotine | 89594 |
| Nizatidine | 3033637 |
| Ondansetron | 4595 |
| Perampanel | 9924495 |
| Pergolide | 47811 |
| Pimavanserin | 10071196 |
| Piribedil | 4850 |
| Pitolisant | 9948102 |
| Pramipexole | 119570 |
| Preladenant | 10117987 |
| Pridopidine | 9795739 |
| Propranolol | 4946 |
| Prucalopride | 3052762 |
| Pruvanserin | 6433122 |
| Quetiapine | 5002 |
| Radiprodil | 10200813 |
| Ropinirole | 5095 |
| Rotigotine | 59227 |
| SAGE-217 | 86294073 |
| Salbutamol | 2083 |
| Sarizotan | 6918388 |
| SK609 | 6486733 |
| SLV-308 | 6918524 |
| SNC-80 | 123924 |
| ST1535 | 9860294 |
| ST3932 | (20692973) |
| ST4206 | 46912314 |
| Talampanel | 164509 |
| Tandospirone | 91273 |
| Tapentadol | 9838022 |
| Tavapadon | 86764100 |
| Telenzepine | 5387 |
| Tezampanel | 127894 |
| Thioperamide | 3035905 |
| Topiramate | 5284627 |
| Tozadenant | 11618368 |
| Traxoprodil | 219101 |
| Trihexyphenidyl | 5572 |
| U50,488 | 3036289 |
| V81444 | 44537963 |
| Vipadenant | 21874557 |
| VU0364770 | 836002 |
| VU0467154 | 73774630 |
| VU0476406 | (23873237) |
| VU6004461 | (29581513) |
| Zolpidem | 5732 |
Funding
This research received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Apostolova I., Taleb D.S., Lipp A., Galazky I., Kupitz D., Lange C., Makowski M.R., Brenner W., Amthauer H., Plotkin M., et al. Utility of Follow-up Dopamine Transporter Spect with 123i-Fp-Cit in the Diagnostic Workup of Patients with Clinically Uncertain Parkinsonian Syndrome. Clin. Nucl. Med. 2017;42:589–594. doi: 10.1097/RLU.0000000000001696. [DOI] [PubMed] [Google Scholar]
- 2.Nido G.S., Dolle C., Flones I., Tuppen H.A., Alves G., Tysnes O.B., Haugarvoll K., Tzoulis C. Ultradeep Mapping of Neuronal Mitochondrial Deletions in Parkinson’s Disease. Neurobiol. Aging. 2018;63:120–127. doi: 10.1016/j.neurobiolaging.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Agosti V., Vitale C., Avella D., Rucco R., Santangelo G., Sorrentino P., Varriale P., Sorrentino G. Effects of Global Postural Reeducation on Gait Kinematics in Parkinsonian Patients: A Pilot Randomized Three-Dimensional Motion Analysis Study. Neurol. Sci. 2016;37:515–522. doi: 10.1007/s10072-015-2433-5. [DOI] [PubMed] [Google Scholar]
- 4.Falaki A., Huang X., Lewis M.M., Latash M.L. Dopaminergic Modulation of Multi-Muscle Synergies in Postural Tasks Performed by Patients with Parkinson’s Disease. J. Electromyogr. Kinesiol. 2017;33:20–26. doi: 10.1016/j.jelekin.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kataoka H., Okada Y., Kiriyama T., Kita Y., Nakamura J., Morioka S., Shomoto K., Ueno S. Can Postural Instability Respond to Galvanic Vestibular Stimulation in Patients with Parkinson’s Disease? J. Mov. Disord. 2016;9:40–43. doi: 10.14802/jmd.15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozinga S.J., Koop M.M., Linder S.M., Machado A.G., Dey T., Alberts J.L. Three-Dimensional Evaluation of Postural Stability in Parkinson’s Disease with Mobile Technology. NeuroRehabilitation. 2017;41:211–218. doi: 10.3233/NRE-171473. [DOI] [PubMed] [Google Scholar]
- 7.Picconi B., Hernandez L.F., Obeso J.A., Calabresi P. Motor Complications in Parkinson’s Disease: Striatal Molecular and Electrophysiological Mechanisms of Dyskinesias. Mov. Disord. 2018;33:867–876. doi: 10.1002/mds.27261. [DOI] [PubMed] [Google Scholar]
- 8.Silva K.G., De Freitas T.B., Dona F., Gananca F.F., Ferraz H.B., Torriani-Pasin C., Pompeu J.E. Effects of Virtual Rehabilitation Versus Conventional Physical Therapy on Postural Control, Gait, and Cognition of Patients with Parkinson’s Disease: Study Protocol for a Randomized Controlled Feasibility Trial. Pilot Feasibility Stud. 2017;3:68. doi: 10.1186/s40814-017-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilczyński J., Habik N. The Effect of L-Dopa on Postural Stability in Parkinson’s Disease Patients. Appl. Sci. 2019;9:409. doi: 10.3390/app9030409. [DOI] [Google Scholar]
- 10.Steib S., Klamroth S., Gassner H., Pasluosta C., Eskofier B., Winkler J., Klucken J., Pfeifer K. Perturbation During Treadmill Training Improves Dynamic Balance and Gait in Parkinson’s Disease: A Single-Blind Randomized Controlled Pilot Trial. Neurorehabil. Neural Repair. 2017;31:758–768. doi: 10.1177/1545968317721976. [DOI] [PubMed] [Google Scholar]
- 11.Wilczynski J., Pedrycz A., Mucha D., Ambrozy T., Mucha D. Body Posture, Postural Stability, and Metabolic Age in Patients with Parkinson’s Disease. BioMed Res. Int. 2017;2017:3975417. doi: 10.1155/2017/3975417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant M.S., Hou J.G., Collins R.L., Protas E.J. Contribution of Axial Motor Impairment to Physical Inactivity in Parkinson Disease. Am. J. Phys. Med. Rehabil. 2016;95:348–354. doi: 10.1097/PHM.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caetano M.J.D., Lord S.R., Allen N.E., Brodie M.A., Song J., Paul S.S., Canning C.G., Menant J.C. Stepping Reaction Time and Gait Adaptability Are Significantly Impaired in People with Parkinson’s Disease: Implications for Fall Risk. Parkinsonism Relat. Disord. 2018;47:32–38. doi: 10.1016/j.parkreldis.2017.11.340. [DOI] [PubMed] [Google Scholar]
- 14.Beuter A., Hernandez R., Rigal R., Modolo J., Blanchet P.J. Postural Sway and Effect of Levodopa in Early Parkinson’s Disease. Can. J. Neurol. Sci. 2008;35:65–68. doi: 10.1017/S0317167100007575. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet C.T., Delval A., Szaffarczyk S., Defebvre L. Levodopa Has Primarily Negative Influences on Postural Control in Patients with Parkinson’s Disease. Behav. Brain Res. 2017;331:67–75. doi: 10.1016/j.bbr.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri N.B., Bernardi G. The ‘Magic’ of L-Dopa: Why Is It the Gold Standard Parkinson’s Disease Therapy? Trends Pharmacol. Sci. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Olanow C.W., Agid Y., Mizuno Y., Albanese A., Bonucelli U., Damier P., De Yebenes J., Gershanik O., Guttman M., Grandas F., et al. Levodopa in the Treatment of Parkinson’s Disease: Current Controversies. Mov. Disord. 2004;19:997–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- 18.Poewe W., Antonini A., Zijlmans J.C., Burkhard P.R., Vingerhoets F. Levodopa in the Treatment of Parkinson’s Disease: An Old Drug Still Going Strong. Clin. Interv. Aging. 2010;5:229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith E.S., Hardy G.A., Schallert T., Lee H.J. The Impact of L-Dopa on Attentional Impairments in a Rat Model of Parkinson’s Disease. Neuroscience. 2016;337:295–305. doi: 10.1016/j.neuroscience.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 20.Cossu G., Ricchi V., Pilleri M., Mancini F., Murgia D., Ricchieri G., Mereu A., Melis M., Antonini A. Levodopa-Carbidopa Intrajejunal Gel in Advanced Parkinson Disease with “on” Freezing of Gait. Neurol. Sci. 2015;36:1683–1686. doi: 10.1007/s10072-015-2234-x. [DOI] [PubMed] [Google Scholar]
- 21.Standaert D.G., Rodriguez R.L., Slevin J.T., Lobatz M., Eaton S., Chatamra K., Facheris M.F., Hall C., Sail K., Jalundhwala Y.J., et al. Effect of Levodopa-Carbidopa Intestinal Gel on Non-Motor Symptoms in Patients with Advanced Parkinson’s Disease. Mov. Disord. Clin. Pract. 2017;4:829–837. doi: 10.1002/mdc3.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zibetti M., Merola A., Artusi C.A., Rizzi L., Angrisano S., Reggio D., De Angelis C., Rizzone M., Lopiano L. Levodopa/Carbidopa Intestinal Gel Infusion in Advanced Parkinson’s Disease: A 7-Year Experience. Eur. J. Neurol. 2014;21:312–318. doi: 10.1111/ene.12309. [DOI] [PubMed] [Google Scholar]
- 23.Kocer B., Guven H., Comoglu S.S. Homocysteine Levels in Parkinson’s Disease: Is Entacapone Effective? BioMed Res. Int. 2016;2016:7563705. doi: 10.1155/2016/7563705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott L.J. Opicapone: A Review in Parkinson’s Disease. Drugs. 2016;76:1293–1300. doi: 10.1007/s40265-016-0623-y. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira J.J., Katzenschlager R., Bloem B.R., Bonuccelli U., Burn D., Deuschl G., Dietrichs E., Fabbrini G., Friedman A., Kanovsky P., et al. Summary of the Recommendations of the EFNS/MDS-ES Review on Therapeutic Management of Parkinson’s Disease. Eur. J. Neurol. 2013;20:5–15. doi: 10.1111/j.1468-1331.2012.03866.x. [DOI] [PubMed] [Google Scholar]
- 26.Cenci M.A. Molecular Mechanisms of L-Dopa-Induced Dyskinesia. In: Steiner H., Tseng K.Y., editors. Handbook of Behavioral Neuroscience. Vol. 24. Elsevier; Amsterdam, The Netherlands: 2016. pp. 857–871. [Google Scholar]
- 27.Cerri S., Siani F., Blandini F. Investigational Drugs in Phase I and Phase II for Levodopa-Induced Dyskinesias. Expert Opin. Investig. Drugs. 2017;26:777–791. doi: 10.1080/13543784.2017.1333598. [DOI] [PubMed] [Google Scholar]
- 28.Curtze C., Nutt J.G., Carlson-Kuhta P., Mancini M., Horak F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease. Mov. Disord. 2015;30:1361–1370. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hechtner M.C., Vogt T., Zöllner Y., Schröder S., Sauer J.B., Binder H., Singer S., Mikolajczyk R. Quality of Life in Parkinson’s Disease Patients with Motor Fluctuations and Dyskinesias in Five European Countries. Parkinsonism Relat. Disord. 2014;20:969–974. doi: 10.1016/j.parkreldis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Lloret S., Negre-Pages L., Damier P., Delval A., Derkinderen P., Destee A., Meissner W.G., Tison F., Rascol O. L-DOPA-Induced Dyskinesias, Motor Fluctuations and Health-Related Quality of Life: The Copark Survey. Eur. J. Neurol. 2017;24:1532–1538. doi: 10.1111/ene.13466. [DOI] [PubMed] [Google Scholar]
- 31.Dragasevic-Miskovic N., Petrovic I., Stankovic I., Kostic V.S. Chemical Management of Levodopa-Induced Dyskinesia in Parkinson’s Disease Patients. Expert Opin. Pharmacother. 2019;20:219–230. doi: 10.1080/14656566.2018.1543407. [DOI] [PubMed] [Google Scholar]
- 32.Oertel W., Schulz J.B. Current and Experimental Treatments of Parkinson Disease: A Guide for Neuroscientists. J. Neurochem. 2016;139(Suppl. 1):325–337. doi: 10.1111/jnc.13750. [DOI] [PubMed] [Google Scholar]
- 33.Dong J., Cui Y., Li S., Le W. Current Pharmaceutical Treatments and Alternative Therapies of Parkinson’s Disease. Curr. Neuropharmacol. 2016;14:339–355. doi: 10.2174/1570159X14666151120123025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore T.J., Glenmullen J., Mattison D.R. Reports of Pathological Gambling, Hypersexuality, and Compulsive Shopping Associated with Dopamine Receptor Agonist Drugsimpulse Control Disorders and Dopamine Agonistsimpulse Control Disorders and Dopamine Agonists. JAMA Intern. Med. 2014;174:1930–1933. doi: 10.1001/jamainternmed.2014.5262. [DOI] [PubMed] [Google Scholar]
- 35.Connolly B.S., Lang A.E. Pharmacological Treatment of Parkinson Disease: A Review. JAMA. 2014;311:1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 36.Noyes K., Dick A.W., Holloway R.G. Pramipexole and Levodopa in Early Parkinson’s Disease. Pharmacoeconomics. 2005;23:1257–1270. doi: 10.2165/00019053-200523120-00009. [DOI] [PubMed] [Google Scholar]
- 37.Thanvi B., Lo N., Robinson T. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Clinical Features, Pathogenesis, Prevention and Treatment. Postgrad. Med. J. 2007;83:384–388. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espay A.J., Morgante F., Merola A., Fasano A., Marsili L., Fox S.H., Bezard E., Picconi B., Calabresi P., Lang A.E. Levodopa-Induced Dyskinesia in Parkinson Disease: Current and Evolving Concepts. Ann. Neurol. 2018;84:797–811. doi: 10.1002/ana.25364. [DOI] [PubMed] [Google Scholar]
- 39.Sohur U.S., Gray D.L., Duvvuri S., Zhang Y., Thayer K., Feng G. Phase 1 Parkinson’s Disease Studies Show the Dopamine D1/D5 Agonist PF-06649751 Is Safe and Well Tolerated. Neurol. Ther. 2018;7:307–319. doi: 10.1007/s40120-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guigoni C., Aubert I., Li Q., Gurevich V.V., Benovic J.L., Ferry S., Mach U., Stark H., Leriche L., Håkansson K., et al. Pathogenesis of Levodopa-Induced Dyskinesia: Focus on D1 and D3 Dopamine Receptors. Parkinsonism Relat. Disord. 2005;11:S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Svensson K.A., Heinz B.A., Schaus J.M., Beck J.P., Hao J., Krushinski J.H., Reinhard M.R., Cohen M.P., Hellman S.L., Getman B.G., et al. An Allosteric Potentiator of the Dopamine D1 Receptor Increases Locomotor Activity in Human D1 Knock-in Mice without Causing Stereotypy or Tachyphylaxis. J. Pharmacol. Exp. Ther. 2017;360:117–128. doi: 10.1124/jpet.116.236372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruns R.F., Mitchell S.N., Wafford K.A., Harper A.J., Shanks E.A., Carter G., O’Neill M.J., Murray T.K., Eastwood B.J., Schaus J.M., et al. Preclinical Profile of a Dopamine D1 Potentiator Suggests Therapeutic Utility in Neurological and Psychiatric Disorders. Neuropharmacology. 2018;128:351–365. doi: 10.1016/j.neuropharm.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Meltzer H.Y., Rajagopal L., Matrisciano F., Hao J., Svensson K.A., Huang M. The Allosteric Dopamine D1 Receptor Potentiator, Detq, Ameliorates Subchronic Phencyclidine-Induced Object Recognition Memory Deficits and Enhances Cortical Acetylcholine Efflux in Male Humanized D1 Receptor Knock-in Mice. Behav. Brain Res. 2019;361:139–150. doi: 10.1016/j.bbr.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Fiorentini C., Busi C., Gorruso E., Gotti C., Spano P., Missale C. Reciprocal Regulation of Dopamine D1 and D3 Receptor Function and Trafficking by Heterodimerization. Mol. Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- 45.Marcellino D., Ferré S., Casadó V., Cortés A., Le Foll B., Mazzola C., Drago F., Saur O., Stark H., Soriano A., et al. Identification of Dopamine D1–D3 Receptor Heteromers. Indications for a Role of Synergistic D1-D3 Receptor Interactions in the Striatum. J. Biol. Chem. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solis O., Garcia-Montes J.R., Gonzalez-Granillo A., Xu M., Moratalla R. Dopamine D3 Receptor Modulates L-Dopa-Induced Dyskinesia by Targeting D1 Receptor-Mediated Striatal Signaling. Cereb. Cortex. 2017;27:435–446. doi: 10.1093/cercor/bhv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiorentini C., Savoia P., Bono F., Tallarico P., Missale C. The D3 Dopamine Receptor: From Structural Interactions to Function. Eur. Neuropsychopharmacol. 2015;25:1462–1469. doi: 10.1016/j.euroneuro.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Solis O., Moratalla R. Dopamine Receptors: Homomeric and Heteromeric Complexes in L-DOPA-Induced Dyskinesia. J. Neural Transm. 2018;125:1187–1194. doi: 10.1007/s00702-018-1852-x. [DOI] [PubMed] [Google Scholar]
- 49.Bézard E., Ferry S., Mach U., Stark H., Leriche L., Boraud T., Gross C., Sokoloff P. Attenuation of Levodopa-Induced Dyskinesia by Normalizing Dopamine D3 Receptor Function. Nat. Med. 2003;9:762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- 50.Payer D.E., Guttman M., Kish S.J., Tong J., Adams J.R., Rusjan P., Houle S., Furukawa Y., Wilson A.A., Boileau I. D3 Dopamine Receptor-Preferring [11C]PHNO PET Imaging in Parkinson Patients with Dyskinesia. Neurology. 2016;86:224–230. doi: 10.1212/WNL.0000000000002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghosh B., Antonio T., Zhen J., Kharkar P., Reith M.E.A., Dutta A.K. Development of (S)-N6-(2-(4-(Isoquinolin-1-yl)Piperazin-1-yl)Ethyl)-N6-Propyl-4,5,6,7-Tetrahydrobenzo[d]-Thiazole-2,6-Diamine and Its Analogue as a D3 Receptor Preferring Agonist: Potent in Vivo Activity in Parkinson’s Disease Animal Models. J. Med. Chem. 2010;53:1023–1037. doi: 10.1021/jm901184n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiller C., Kling R.C., Heinemann F.W., Meyer K., Hübner H., Gmeiner P. Functionally Selective Dopamine D2/D3 Receptor Agonists Comprising an Enyne Moiety. J. Med. Chem. 2013;56:5130–5141. doi: 10.1021/jm400520c. [DOI] [PubMed] [Google Scholar]
- 53.Tschammer N., Elsner J., Goetz A., Ehrlich K., Schuster S., Ruberg M., Kühhorn J., Thompson D., Whistler J., Hübner H., et al. Highly Potent 5-Aminotetrahydropyrazolopyridines: Enantioselective Dopamine D3 Receptor Binding, Functional Selectivity, and Analysis of Receptor−Ligand Interactions. J. Med. Chem. 2011;54:2477–2491. doi: 10.1021/jm101639t. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H., Tong R., Bai L., Shi J., Ouyang L. Emerging Targets and New Small Molecule Therapies in Parkinson’s Disease Treatment. Bioorg. Med. Chem. 2016;24:1419–1430. doi: 10.1016/j.bmc.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y., Lu X., Yang C.-y., Huang Z., Fu W., Hou T., Zhang J. Computational Modeling toward Understanding Agonist Binding on Dopamine 3. J. Chem. Inf. Model. 2010;50:1633–1643. doi: 10.1021/ci1002119. [DOI] [PubMed] [Google Scholar]
- 56.Gil-Mast S., Kortagere S., Kota K., Kuzhikandathil E.V. An Amino Acid Residue in the Second Extracellular Loop Determines the Agonist-Dependent Tolerance Property of the Human D3 Dopamine Receptor. ACS Chem. Neurosci. 2013;4:940–951. doi: 10.1021/cn3002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kortagere S., Kuzhikandahil E.V. Novel D3 Dopamine Receptor Agonists to Treat Dyskinesia in Parkinson’s Disease. WO2012021629 e. U.S. Patent. 16 February 2012
- 58.Westrich L., Gil-Mast S., Kortagere S., Kuzhikandathil E.V. Development of Tolerance in D3 Dopamine Receptor Signaling Is Accompanied by Distinct Changes in Receptor Conformation. Biochem. Pharmacol. 2010;79:897–907. doi: 10.1016/j.bcp.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Cote S.R., Kuzhikandathil E.V. In vitro and In vivo Characterization of the Agonist-Dependent D3 Dopamine Receptor Tolerance Property. Neuropharmacology. 2014;79:359–367. doi: 10.1016/j.neuropharm.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Xu W., Wang X., Tocker A.M., Huang P., Reith M.E., Liu-Chen L.Y., Smith A.B., 3rd, Kortagere S. Functional Characterization of a Novel Series of Biased Signaling Dopamine D3 Receptor Agonists. ACS Chem. Neurosci. 2017;8:486–500. doi: 10.1021/acschemneuro.6b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simms S.L., Huettner D.P., Kortagere S. In Vivo Characterization of a Novel Dopamine D3 Receptor Agonist to Treat Motor Symptoms of Parkinson’s Disease. Neuropharmacology. 2016;100:106–115. doi: 10.1016/j.neuropharm.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Chen J., Collins G.T., Levant B., Woods J., Deschamps J.R., Wang S. Cj-1639: A Potent and Highly Selective Dopamine D3 Receptor Full Agonist. ACS Med. Chem. Lett. 2011;2:620–625. doi: 10.1021/ml200100t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das B., Modi G., Dutta A. Dopamine D3 Agonists in the Treatment of Parkinson’s Disease. Curr. Top. Med. Chem. 2015;15:908–926. doi: 10.2174/156802661510150328223428. [DOI] [PubMed] [Google Scholar]
- 64.Lindenbach D., Das B., Conti M.M., Meadows S.M., Dutta A.K., Bishop C. D-512, a Novel Dopamine D2/3 Receptor Agonist, Demonstrates Greater Anti-Parkinsonian Efficacy Than Ropinirole in Parkinsonian Rats. Br. J. Pharmacol. 2017;174:3058–3071. doi: 10.1111/bph.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santra S., Xu L., Shah M., Johnson M., Dutta A. D-512 and D-440 as Novel Multifunctional Dopamine Agonists: Characterization of Neuroprotection Properties and Evaluation of in Vivo Efficacy in a Parkinson’s Disease Animal Model. ACS Chem. Neurosci. 2013;4:1382–1392. doi: 10.1021/cn400106n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elmabruk A., Das B., Yedlapudi D., Xu L., Antonio T., Reith M.E.A., Dutta A.K. Design, Synthesis, and Pharmacological Characterization of Carbazole Based Dopamine Agonists as Potential Symptomatic and Neuroprotective Therapeutic Agents for Parkinson’s Disease. ACS Chem. Neurosci. 2019;10:396–411. doi: 10.1021/acschemneuro.8b00291. [DOI] [PubMed] [Google Scholar]
- 67.Glennon J.C., Van Scharrenburg G., Ronken E., Hesselink M.B., Reinders J.H., Van Der Neut M., Long S.K., Feenstra R.W., McCreary A.C. In Vitro Characterization of SLV308 (7-[4-Methyl-1-Piperazinyl]-2(3h)-Benzoxazolone, Monohydrochloride): A Novel Partial Dopamine D2 and D3 Receptor Agonist and Serotonin 5-HT1A Receptor Agonist. Synapse. 2006;60:599–608. doi: 10.1002/syn.20330. [DOI] [PubMed] [Google Scholar]
- 68.Del Bello F., Ambrosini D., Bonifazi A., Newman A.H., Keck T.M., Giannella M., Giorgioni G., Piergentili A., Cappellacci L., Cilia A., et al. Multitarget 1,4-Dioxane Compounds Combining Favorable D2-Like and 5-HT1A Receptor Interactions with Potential for the Treatment of Parkinson’s Disease or Schizophrenia. ACS Chem. Neurosci. 2019 doi: 10.1021/acschemneuro.8b00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeLong M.R. Primate Models of Movement Disorders of Basal Ganglia Origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-V. [DOI] [PubMed] [Google Scholar]
- 70.Huot P., Johnston T.H., Koprich J.B., Fox S.H., Brotchie J.M. The Pharmacology of L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Pharmacol. Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 71.Del Bello F., Bonifazi A., Giorgioni G., Cifani C., Micioni Di Bonaventura M.V., Petrelli R., Piergentili A., Fontana S., Mammoli V., Yano H., et al. 1-[3-(4-Butylpiperidin-1-yl)Propyl]-1,2,3,4-Tetrahydroquinolin-2-One (77-LH-28-1) as a Model for the Rational Design of a Novel Class of Brain Penetrant Ligands with High Affinity and Selectivity for Dopamine D4 Receptor. J. Med. Chem. 2018;61:3712–3725. doi: 10.1021/acs.jmedchem.8b00265. [DOI] [PubMed] [Google Scholar]
- 72.DeLong M.R., Wichmann T. Circuits and Circuit Disorders of the Basal Ganglia. Arch. Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 73.Lindsley C.W., Hopkins C.R. Return of D4 Dopamine Receptor Antagonists in Drug Discovery. J. Med. Chem. 2017;60:7233–7243. doi: 10.1021/acs.jmedchem.7b00151. [DOI] [PubMed] [Google Scholar]
- 74.Wichmann T., DeLong M.R. Functional Neuroanatomy of the Basal Ganglia in Parkinson’s Disease. Adv. Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- 75.Huot P., Johnston T.H., Koprich J.B., Aman A., Fox S.H., Brotchie J.M. L-745,870 Reduces L-DOPA-Induced Dyskinesia in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Lesioned Macaque Model of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2012;342:576–585. doi: 10.1124/jpet.112.195693. [DOI] [PubMed] [Google Scholar]
- 76.Huot P., Johnston T.H., Koprich J.B., Espinosa M.C., Reyes M.G., Fox S.H., Brotchie J.M. L-745,870 Reduces the Expression of Abnormal Involuntary Movements in the 6-OHDA-Lesioned Rat. Behav. Pharmacol. 2015;26:101–108. doi: 10.1097/FBP.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 77.Witt J.O., McCollum A.L., Hurtado M.A., Huseman E.D., Jeffries D.E., Temple K.J., Plumley H.C., Blobaum A.L., Lindsley C.W., Hopkins C.R. Synthesis and Characterization of a Series of Chiral Alkoxymethyl Morpholine Analogs as Dopamine Receptor 4 (D4R) Antagonists. Bioorg. Med. Chem. Lett. 2016;26:2481–2488. doi: 10.1016/j.bmcl.2016.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sebastianutto I., Maslava N., Hopkins C.R., Cenci M.A. Validation of an Improved Scale for Rating L-Dopa-Induced Dyskinesia in the Mouse and Effects of Specific Dopamine Receptor Antagonists. Neurobiol. Dis. 2016;96:156–170. doi: 10.1016/j.nbd.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Lanza K., Bishop C. Serotonergic Targets for the Treatment of L-DOPA-Induced Dyskinesia. J. Neural Transm. 2018;125:1203–1216. doi: 10.1007/s00702-017-1837-1. [DOI] [PubMed] [Google Scholar]
- 80.Carta M., Tronci E. Serotonin System Implication in L-Dopa-Induced Dyskinesia: From Animal Models to Clinical Investigations. Front. Neurol. 2014;5:78. doi: 10.3389/fneur.2014.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carta M., Carlsson T., Kirik D., Bjorklund A. Dopamine Released from 5-HT Terminals Is the Cause of L-DOPA-Induced Dyskinesia in Parkinsonian Rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- 82.Nicholson S.L., Brotchie J.M. 5-Hydroxytryptamine (5-HT, Serotonin) and Parkinson’s Disease—Opportunities for Novel Therapeutics to Reduce the Problems of Levodopa Therapy. Eur. J. Neurol. 2002;9(Suppl. 3):1–6. doi: 10.1046/j.1468-1331.9.s3.1.x. [DOI] [PubMed] [Google Scholar]
- 83.Dupre K.B., Ostock C.Y., Eskow Jaunarajs K.L., Button T., Savage L.M., Wolf W., Bishop C. Local Modulation of Striatal Glutamate Efflux by Serotonin 1A Receptor Stimulation in Dyskinetic, Hemiparkinsonian Rats. Exp. Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dupre K.B., Ostock C.Y., George J.A., Eskow Jaunarajs K.L., Hueston C.M., Bishop C. Effects of 5-HT1A Receptor Stimulation on D1 Receptor Agonist-Induced Striatonigral Activity and Dyskinesia in Hemiparkinsonian Rats. ACS Chem. Neurosci. 2013;4:747–760. doi: 10.1021/cn300234z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iravani M.M., Tayarani-Binazir K., Chu W.B., Jackson M.J., Jenner P. In 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Primates, the Selective 5-Hydroxytryptamine 1a Agonist (R)-(+)-8-Ohdpat Inhibits Levodopa-Induced Dyskinesia but Only with\ Increased Motor Disability. J. Pharmacol. Exp. Ther. 2006;319:1225–1234. doi: 10.1124/jpet.106.110429. [DOI] [PubMed] [Google Scholar]
- 86.Bartoszyk G.D., Van Amsterdam C., Greiner H.E., Rautenberg W., Russ H., Seyfried C.A. Sarizotan, a Serotonin 5-HT1A Receptor Agonist and Dopamine Receptor Ligand. 1. Neurochemical Profile. J. Neural Transm. 2004;111:113–126. doi: 10.1007/s00702-003-0094-7. [DOI] [PubMed] [Google Scholar]
- 87.Bibbiani F., Oh J.D., Chase T.N. Serotonin 5-HT1A Agonist Improves Motor Complications in Rodent and Primate Parkinsonian Models. Neurology. 2001;57:1829–1834. doi: 10.1212/WNL.57.10.1829. [DOI] [PubMed] [Google Scholar]
- 88.Goetz C.G., Damier P., Hicking C., Laska E., Muller T., Olanow C.W., Rascol O., Russ H. Sarizotan as a Treatment for Dyskinesias in Parkinson’s Disease: A Double-Blind Placebo-Controlled Trial. Mov. Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- 89.Goetz C.G., Laska E., Hicking C., Damier P., Müller T., Nutt J., Warren Olanow C., Rascol O., Russ H. Placebo Influences on Dyskinesia in Parkinson’s Disease. Mov. Disord. 2008;23:700–707. doi: 10.1002/mds.21897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kannari K., Kurahashi K., Tomiyama M., Maeda T., Arai A., Baba M., Suda T., Matsunaga M. Tandospirone Citrate, a Selective 5-HT1A Agonist, Alleviates L-DOPA-Induced Dyskinesia in Patients with Parkinson’s Disease. No to shinkei = Brain and nerve. 2002;54:133–137. [PubMed] [Google Scholar]
- 91.Ludwig C.L., Weinberger D.R., Bruno G., Gillespie M., Bakker K., LeWitt P.A., Chase T.N. Buspirone, Parkinson’s Disease, and the Locus Ceruleus. Clin. Neuropharmacol. 1986;9:373–378. doi: 10.1097/00002826-198608000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Politis M., Wu K., Loane C., Brooks D.J., Kiferle L., Turkheimer F.E., Bain P., Molloy S., Piccini P. Serotonergic Mechanisms Responsible for Levodopa-Induced Dyskinesias in Parkinson’s Disease Patients. J. Clin. Invest. 2014;124:1340–1349. doi: 10.1172/JCI71640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bezard E., Tronci E., Pioli E.Y., Li Q., Porras G., Bjorklund A., Carta M. Study of the Antidyskinetic Effect of Eltoprazine in Animal Models of Levodopa-Induced Dyskinesia. Mov. Disord. 2013;28:1088–1096. doi: 10.1002/mds.25366. [DOI] [PubMed] [Google Scholar]
- 94.Schipper J., Tulp M.T.M., Sijbesma H. Neurochemical Profile of Eltoprazine. Drug Metabol. Drug Interact. 1990;8:85–114. doi: 10.1515/DMDI.1990.8.1-2.85. [DOI] [PubMed] [Google Scholar]
- 95.Tronci E., Fidalgo C., Stancampiano R., Carta M. Effect of Selective and Non-Selective Serotonin Receptor Activation on L-DOPA-Induced Therapeutic Efficacy and Dyskinesia in Parkinsonian Rats. Behav. Brain Res. 2015;292:300–304. doi: 10.1016/j.bbr.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 96.Svenningsson P., Rosenblad C., Af Edholm Arvidsson K., Wictorin K., Keywood C., Shankar B., Lowe D.A., Bjorklund A., Widner H. Eltoprazine Counteracts L-Dopa-Induced Dyskinesias in Parkinson’s Disease: A Dose-Finding Study. Brain. 2015;138:963–973. doi: 10.1093/brain/awu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ko W.K.D., Li Q., Cheng L.Y., Morelli M., Carta M., Bezard E. A Preclinical Study on the Combined Effects of Repeated Eltoprazine and Preladenant Treatment for Alleviating L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Eur. J. Pharmacol. 2017;813:10–16. doi: 10.1016/j.ejphar.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 98.Pinna A., Ko W.K., Costa G., Tronci E., Fidalgo C., Simola N., Li Q., Tabrizi M.A., Bezard E., Carta M., et al. Antidyskinetic Effect of A2A and 5HT1A/1B Receptor Ligands in Two Animal Models of Parkinson’s Disease. Mov. Disord. 2016;31:501–511. doi: 10.1002/mds.26475. [DOI] [PubMed] [Google Scholar]
- 99.Huot P., Johnston T.H., Fox S.H., Newman-Tancredi A., Brotchie J.M. The Highly-Selective 5-HT(1A) Agonist F15599 Reduces L-DOPA-Induced Dyskinesia without Compromising Anti-Parkinsonian Benefits in the MPTP-Lesioned Macaque. Neuropharmacology. 2015;97:306–311. doi: 10.1016/j.neuropharm.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 100.Iderberg H., McCreary A.C., Varney M.A., Cenci M.A., Newman-Tancredi A. Activity of Serotonin 5-HT(1A) Receptor ’Biased Agonists’ in Rat Models of Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Neuropharmacology. 2015;93:52–67. doi: 10.1016/j.neuropharm.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 101.Iderberg H., McCreary A.C., Varney M.A., Kleven M.S., Koek W., Bardin L., Depoortere R., Cenci M.A., Newman-Tancredi A. NLX-112, a Novel 5-HT1A Receptor Agonist for the Treatment of L-DOPA-Induced Dyskinesia: Behavioral and Neurochemical Profile in Rat. Exp. Neurol. 2015;271:335–350. doi: 10.1016/j.expneurol.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 102.Chilmonczyk Z., Bojarski A.J., Pilc A., Sylte I. Functional Selectivity and Antidepressant Activity of Serotonin 1A Receptor Ligands. Int. J. Mol. Sci. 2015;16:18474–18506. doi: 10.3390/ijms160818474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Llado-Pelfort L., Assie M.B., Newman-Tancredi A., Artigas F., Celada P. Preferential in Vivo Action of F15599, a Novel 5-HT(1A) Receptor Agonist, at Postsynaptic 5-HT(1A) Receptors. Br. J. Pharmacol. 2010;160:1929–1940. doi: 10.1111/j.1476-5381.2010.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Newman-Tancredi A., Martel J.C., Assie M.B., Buritova J., Lauressergues E., Cosi C., Heusler P., Bruins Slot L., Colpaert F.C., Vacher B., et al. Signal Transduction and Functional Selectivity of F15599, a Preferential Post-Synaptic 5-HT1A Receptor Agonist. Br. J. Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Newman-Tancredi A., Varney M.A., McCreary A.C. Effects of the Serotonin 5-HT1A Receptor Biased Agonists, F13714 and F15599, on Striatal Neurotransmitter Levels Following L-DOPA Administration in Hemi-Parkinsonian Rats. Neurochem. Res. 2018;43:1035–1046. doi: 10.1007/s11064-018-2514-y. [DOI] [PubMed] [Google Scholar]
- 106.Eskow Jaunarajs K.L., Dupre K.B., Steiniger A., Klioueva A., Moore A., Kelly C., Bishop C. Serotonin 1B Receptor Stimulation Reduces D1 Receptor Agonist-Induced Dyskinesia. Neuroreport. 2009;20:1265–1269. doi: 10.1097/WNR.0b013e3283300fd7. [DOI] [PubMed] [Google Scholar]
- 107.Iderberg H., Rylander D., Bimpisidis Z., Cenci M.A. Modulating mGluR5 and 5-HT1A/1B Receptors to Treat L-Dopa-Induced Dyskinesia: Effects of Combined Treatment and Possible Mechanisms of Action. Exp. Neurol. 2013;250:116–124. doi: 10.1016/j.expneurol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 108.Roberts C. ACP-103, a 5-HT2A Receptor Inverse Agonist. Curr. Opin. Investig. Drugs. 2006;7:653–660. [PubMed] [Google Scholar]
- 109.Vanover K.E., Betz A.J., Weber S.M., Bibbiani F., Kielaite A., Weiner D.M., Davis R.E., Chase T.N., Salamone J.D. A 5-HT2A Receptor Inverse Agonist, ACP-103, Reduces Tremor in a Rat Model and Levodopa-Induced Dyskinesias in a Monkey Model. Pharmacol. Biochem. Behav. 2008;90:540–544. doi: 10.1016/j.pbb.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stahl S.M. Mechanism of Action of Pimavanserin in Parkinson’s Disease Psychosis: Targeting Serotonin 5HT2A and 5HT2C Receptors. CNS Spectr. 2016;21:271–275. doi: 10.1017/S1092852916000407. [DOI] [PubMed] [Google Scholar]
- 111.Cummings J., Isaacson S., Mills R., Williams H., Chi-Burris K., Corbett A., Dhall R., Ballard C. Pimavanserin for Patients with Parkinson’s Disease Psychosis: A Randomised, Placebo-Controlled Phase 3 Trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 112.Hamadjida A., Nuara S.G., Bedard D., Gaudette F., Beaudry F., Gourdon J.C., Huot P. The Highly Selective 5-HT2A Antagonist EMD-281,014 Reduces Dyskinesia and Psychosis in the L-Dopa-Treated Parkinsonian Marmoset. Neuropharmacology. 2018;139:61–67. doi: 10.1016/j.neuropharm.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 113.Frouni I., Kwan C., Bedard D., Belliveau S., Bourgeois-Cayer E., Gaudette F., Beaudry F., Hamadjida A., Huot P. Effect of the Selective 5-HT2A Receptor Antagonist EMD-281,014 on L-DOPA-Induced Abnormal Involuntary Movements in the 6-OHDA-Lesioned Rat. Exp. Brain Res. 2019;237:29–36. doi: 10.1007/s00221-018-5390-4. [DOI] [PubMed] [Google Scholar]
- 114.Lundblad M., Andersson M., Winkler C., Kirik D., Wierup N., Cenci M.A. Pharmacological Validation of Behavioural Measures of Akinesia and Dyskinesia in a Rat Model of Parkinson’s Disease. Eur. J. Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 115.Visanji N.P., Gomez-Ramirez J., Johnston T.H., Pires D., Voon V., Brotchie J.M., Fox S.H. Pharmacological Characterization of Psychosis-Like Behavior in the MPTP-Lesioned Nonhuman Primate Model of Parkinson’s Disease. Mov. Disord. 2006;21:1879–1891. doi: 10.1002/mds.21073. [DOI] [PubMed] [Google Scholar]
- 116.Durif F., Debilly B., Galitzky M., Morand D., Viallet F., Borg M., Thobois S., Broussolle E., Rascol O. Clozapine Improves Dyskinesias in Parkinson Disease. Neurology. 2004;62:381–388. doi: 10.1212/01.WNL.0000110317.52453.6C. [DOI] [PubMed] [Google Scholar]
- 117.Morgante L., Epifanio A., Spina E., Zappia M., Di Rosa A.E., Marconi R., Basile G., Di Raimondo G., La Spina P., Quattrone A. Quetiapine and Clozapine in Parkinsonian Patients with Dopaminergic Psychosis. Clin. Neuropharmacol. 2004;27:153–156. doi: 10.1097/01.wnf.0000136891.17006.ec. [DOI] [PubMed] [Google Scholar]
- 118.DeVane C.L., Nemeroff C.B. Clinical Pharmacokinetics of Quetiapine: An Atypical Antipsychotic. Clin. Pharmacokinet. 2001;40:509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 119.Gefvert O., Bergstrom M., Langstrom B., Lundberg T., Lindstrom L., Yates R. Time Course of Central Nervous Dopamine-D2 and 5-HT2 Receptor Blockade and Plasma Drug Concentrations after Discontinuation of Quetiapine (Seroquel) in Patients with Schizophrenia. Psychopharmacology. 1998;135:119–126. doi: 10.1007/s002130050492. [DOI] [PubMed] [Google Scholar]
- 120.Oh J.D., Bibbiani F., Chase T.N. Quetiapine Attenuates Levodopa-Induced Motor Complications in Rodent and Primate Parkinsonian Models. Exp. Neurol. 2002;177:557–564. doi: 10.1006/exnr.2002.8009. [DOI] [PubMed] [Google Scholar]
- 121.Katzenschlager R., Manson A.J., Evans A., Watt H., Lees A.J. Low Dose Quetiapine for Drug Induced Dyskinesias in Parkinson’s Disease: A Double Blind Cross over Study. J. Neurol. Neurosurg. Psychiatry. 2004;75:295–297. [PMC free article] [PubMed] [Google Scholar]
- 122.Grunder G., Kungel M., Ebrecht M., Gorocs T., Modell S. Aripiprazole: Pharmacodynamics of a Dopamine Partial Agonist for the Treatment of Schizophrenia. Pharmacopsychiatry. 2006;39(Suppl. 1):S21–25. doi: 10.1055/s-2006-931485. [DOI] [PubMed] [Google Scholar]
- 123.Friedman J.H., Berman R.M., Goetz C.G., Factor S.A., Ondo W.G., Wojcieszek J., Carson W.H., Marcus R.N. Open-Label Flexible-Dose Pilot Study to Evaluate the Safety and Tolerability of Aripiprazole in Patients with Psychosis Associated with Parkinson’s Disease. Mov. Disord. 2006;21:2078–2081. doi: 10.1002/mds.21091. [DOI] [PubMed] [Google Scholar]
- 124.Meco G., Stirpe P., Edito F., Purcaro C., Valente M., Bernardi S., Vanacore N. Aripiprazole in L-Dopa-Induced Dyskinesias: A One-Year Open-Label Pilot Study. J. Neural Transm. 2009;116:881–884. doi: 10.1007/s00702-009-0231-z. [DOI] [PubMed] [Google Scholar]
- 125.Anttila S.A.K., Leinonen E.V.J. A Review of the Pharmacological and Clinical Profile of Mirtazapine. CNS Drug Rev. 2001;7:249–264. doi: 10.1111/j.1527-3458.2001.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamadjida A., Nuara S.G., Gourdon J.C., Huot P. The Effect of Mianserin on the Severity of Psychosis and Dyskinesia in the Parkinsonian Marmoset. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;81:367–371. doi: 10.1016/j.pnpbp.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 127.Hamadjida A., Nuara S.G., Veyres N., Frouni I., Kwan C., Sid-Otmane L., Harraka M.J., Gourdon J.C., Huot P. The Effect of Mirtazapine on Dopaminergic Psychosis and Dyskinesia in the Parkinsonian Marmoset. Psychopharmacology. 2017;234:905–911. doi: 10.1007/s00213-017-4530-z. [DOI] [PubMed] [Google Scholar]
- 128.Meco G., Fabrizio E., Di Rezze S., Alessandri A., Pratesi L. Mirtazapine in L-Dopa-Induced Dyskinesias. Clin. Neuropharmacol. 2003;26:179–181. doi: 10.1097/00002826-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 129.Aboulghasemi N., Hadipour Jahromy M., Ghasemi A. Anti-Dyskinetic Efficacy of 5-Ht3 Receptor Antagonist in the Hemi-Parkinsonian Rat Model. IBRO Rep. 2019;6:40–44. doi: 10.1016/j.ibror.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Asai H., Udaka F., Hirano M., Minami T., Oda M., Kubori T., Nishinaka K., Kameyama M., Ueno S. Increased Gastric Motility During 5-HT4 Agonist Therapy Reduces Response Fluctuations in Parkinson’s Disease. Parkinsonism Relat. Disord. 2005;11:499–502. doi: 10.1016/j.parkreldis.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 131.Mendez-David I., David D.J., Darcet F., Wu M.V., Kerdine-Romer S., Gardier A.M., Hen R. Rapid Anxiolytic Effects of a 5-HT(4) Receptor Agonist Are Mediated by a Neurogenesis-Independent Mechanism. Neuropsychopharmacology. 2014;39:1366–1378. doi: 10.1038/npp.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Navailles S., Di Giovanni G., De Deurwaerdere P. The 5-HT4 Agonist Prucalopride Stimulates L-DOPA-Induced Dopamine Release in Restricted Brain Regions of the Hemiparkinsonian Rat in Vivo. CNS Neurosci. Ther. 2015;21:745–747. doi: 10.1111/cns.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y., Liu Z., Li F., Kong F. Therapeutic Effect of Mosapride Citrate on Gastrointestinal Tract Function Disorder of Patients with Parkinson’s Syndrome. J. Jilin Univ. Med. Ed. 2017;43:125–129. [Google Scholar]
- 134.Sgambato-Faure V., Cenci M.A. Glutamatergic Mechanisms in the Dyskinesias Induced by Pharmacological Dopamine Replacement and Deep Brain Stimulation for the Treatment of Parkinson’s Disease. Prog. Neurobiol. 2012;96:69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 135.Chase T.N., Oh J.D., Konitsiotis S. Antiparkinsonian and Antidyskinetic Activity of Drugs Targeting Central Glutamatergic Mechanisms. J. Neurol. 2000;247(Suppl. 2):II36–42. doi: 10.1007/PL00007759. [DOI] [PubMed] [Google Scholar]
- 136.Cenci M.A., Ohlin K.E., Odin P. Current Options and Future Possibilities for the Treatment of Dyskinesia and Motor Fluctuations in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets. 2011;10:670–684. doi: 10.2174/187152711797247885. [DOI] [PubMed] [Google Scholar]
- 137.Rascol O., Fox S., Gasparini F., Kenney C., Di Paolo T., Gomez-Mancilla B. Use of Metabotropic Glutamate 5-Receptor Antagonists for Treatment of Levodopa-Induced Dyskinesias. Parkinsonism Relat. Disord. 2014;20:947–956. doi: 10.1016/j.parkreldis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 138.Mellone M., Stanic J., Hernandez L.F., Iglesias E., Zianni E., Longhi A., Prigent A., Picconi B., Calabresi P., Hirsch E.C., et al. NMDA Receptor Glun2a/Glun2b Subunit Ratio as Synaptic Trait of Levodopa-Induced Dyskinesias: From Experimental Models to Patients. Front. Cell. Neurosci. 2015;9:245. doi: 10.3389/fncel.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paille V., Picconi B., Bagetta V., Ghiglieri V., Sgobio C., Di Filippo M., Viscomi M.T., Giampa C., Fusco F.R., Gardoni F., et al. Distinct Levels of Dopamine Denervation Differentially Alter Striatal Synaptic Plasticity and NMDA Receptor Subunit Composition. J. Neurosci. 2010;30:14182–14193. doi: 10.1523/JNEUROSCI.2149-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Calon F., Morissette M., Ghribi O., Goulet M., Grondin R., Blanchet P.J., Bedard P.J., Di Paolo T. Alteration of Glutamate Receptors in the Striatum of Dyskinetic 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Monkeys Following Dopamine Agonist Treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:127–138. doi: 10.1016/S0278-5846(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 141.Calon F., Rajput A.H., Hornykiewicz O., Bédard P.J., Di Paolo T. Levodopa-Induced Motor Complications Are Associated with Alterations of Glutamate Receptors in Parkinson’s Disease. Neurobiol. Dis. 2003;14:404–416. doi: 10.1016/j.nbd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 142.Chase T.N., Oh J.D. Striatal Dopamine- and Glutamate-Mediated Dysregulation in Experimental Parkinsonism. Trends Neurosci. 2000;23:S86–S91. doi: 10.1016/S1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- 143.Hadj Tahar A., Gregoire L., Darre A., Belanger N., Meltzer L., Bedard P.J. Effect of a Selective Glutamate Antagonist on L-Dopa-Induced Dyskinesias in Drug-Naive Parkinsonian Monkeys. Neurobiol. Dis. 2004;15:171–176. doi: 10.1016/j.nbd.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 144.Morissette M., Dridi M., Calon F., Tahar A.H., Meltzer L.T., Bédard P.J., Di Paolo T. Prevention of Dyskinesia by an NMDA Receptor Antagonist in MPTP Monkeys: Effect on Adenosine A2A Receptors. Synapse. 2006;60:239–250. doi: 10.1002/syn.20295. [DOI] [PubMed] [Google Scholar]
- 145.Nash J.E., Fox S.H., Henry B., Hill M.P., Peggs D., McGuire S., Maneuf Y., Hille C., Brotchie J.M., Crossman A.R. Antiparkinsonian Actions of Ifenprodil in the MPTP-Lesioned Marmoset Model of Parkinson’s Disease. Exp. Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- 146.Nash J.E., Ravenscroft P., McGuire S., Crossman A.R., Menniti F.S., Brotchie J.M. The NR2B-Selective NMDA Receptor Antagonist CP-101,606 Exacerbates L-DOPA-Induced Dyskinesia and Provides Mild Potentiation of Anti-Parkinsonian Effects of L-DOPA in the MPTP-Lesioned Marmoset Model of Parkinson’s Disease. Exp. Neurol. 2004;188:471–479. doi: 10.1016/j.expneurol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 147.Nutt J.G., Gunzler S.A., Kirchhoff T., Hogarth P., Weaver J.L., Krams M., Jamerson B., Menniti F.S., Landen J.W. Effects of a NR2B Selective NMDA Glutamate Antagonist, CP-101,606, on Dyskinesia and Parkinsonism. Mov. Disord. 2008;23:1860–1866. doi: 10.1002/mds.22169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Igarashi M., Habata T., Akita H., Noda K., Ogata M., Saji M. The NR2B Antagonist, Ifenprodil, Corrects the L-Dopa-Induced Deficit of Bilateral Movement and Reduces C-Fos Expression in the Subthalamic Nucleus of Hemiparkinsonian Rats. Neurosci. Res. 2015;96:45–53. doi: 10.1016/j.neures.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 149.Rylander D., Recchia A., Mela F., Dekundy A., Danysz W., Cenci M.A. Pharmacological Modulation of Glutamate Transmission in a Rat Model of L-DOPA-Induced Dyskinesia: Effects on Motor Behavior and Striatal Nuclear Signaling. J. Pharmacol. Exp. Ther. 2009;330:227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wessell R.H., Ahmed S.M., Menniti F.S., Dunbar G.L., Chase T.N., Oh J.D. NR2B Selective NMDA Receptor Antagonist CP-101,606 Prevents Levodopa-Induced Motor Response Alterations in Hemi-Parkinsonian Rats. Neuropharmacology. 2004;47:184–194. doi: 10.1016/j.neuropharm.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 151.Michel A., Downey P., Van Damme X., De Wolf C., Schwarting R., Scheller D. Behavioural Assessment of the A2a/NR2B Combination in the Unilateral 6-OHDA-Lesioned Rat Model: A New Method to Examine the Therapeutic Potential of Non-Dopaminergic Drugs. PLoS ONE. 2015;10:e0135949. doi: 10.1371/journal.pone.0135949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Michel A., Nicolas J.-M., Rose S., Jackson M., Colman P., Briône W., Sciberras D., Muglia P., Scheller D.K., Citron M., et al. Antiparkinsonian Effects of the "Radiprodil and Tozadenant" Combination in MPTP-Treated Marmosets. PLoS ONE. 2017;12:e0182887. doi: 10.1371/journal.pone.0182887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Snow B.J., Macdonald L., McAuley D., Wallis W. The Effect of Amantadine on Levodopa-Induced Dyskinesias in Parkinson’s Disease: A Double-Blind, Placebo-Controlled Study. Clin. Neuropharmacol. 2000;23:82–85. doi: 10.1097/00002826-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 154.Wolf E., Seppi K., Katzenschlager R., Hochschorner G., Ransmayr G., Schwingenschuh P., Ott E., Kloiber I., Haubenberger D., Auff E., et al. Long-Term Antidyskinetic Efficacy of Amantadine in Parkinson’s Disease. Mov. Disord. 2010;25:1357–1363. doi: 10.1002/mds.23034. [DOI] [PubMed] [Google Scholar]
- 155.Sawada H., Oeda T., Kuno S., Nomoto M., Yamamoto K., Yamamoto M., Hisanaga K., Kawamura T. Amantadine for Dyskinesias in Parkinson’s Disease: A Randomized Controlled Trial. PLoS ONE. 2010;5:e15298. doi: 10.1371/journal.pone.0015298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perez-Lloret S., Rascol O. Efficacy and Safety of Amantadine for the Treatment of L-DOPA-Induced Dyskinesia. J. Neural Transm. 2018;125:1237–1250. doi: 10.1007/s00702-018-1869-1. [DOI] [PubMed] [Google Scholar]
- 157.Da Silva-Junior F.P., Braga-Neto P., Sueli Monte F., de Bruin V.M. Amantadine Reduces the Duration of Levodopa-Induced Dyskinesia: A Randomized, Double-Blind, Placebo-Controlled Study. Parkinsonism Relat. Disord. 2005;11:449–452. doi: 10.1016/j.parkreldis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 158.Ory-Magne F., Corvol J.C., Azulay J.P., Bonnet A.M., Brefel-Courbon C., Damier P., Dellapina E., Destee A., Durif F., Galitzky M., et al. Withdrawing Amantadine in Dyskinetic Patients with Parkinson Disease: The Amandysk Trial. Neurology. 2014;82:300–307. doi: 10.1212/WNL.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 159.Thomas A., Iacono D., Luciano A.L., Armellino K., Di Iorio A., Onofrj M. Duration of Amantadine Benefit on Dyskinesia of Severe Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry. 2004;75:141–143. [PMC free article] [PubMed] [Google Scholar]
- 160.Bortolanza M., Bariotto-Dos-Santos K.D., Dos-Santos-Pereira M., da-Silva C.A., Del-Bel E. Antidyskinetic Effect of 7-Nitroindazole and Sodium Nitroprusside Associated with Amantadine in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2016;30:88–100. doi: 10.1007/s12640-016-9618-4. [DOI] [PubMed] [Google Scholar]
- 161.Merello M., Nouzeilles M.I., Cammarota A., Leiguarda R. Effect of Memantine (NMDA Antagonist) on Parkinson’s Disease: A Double-Blind Crossover Randomized Study. Clin. Neuropharmacol. 1999;22:273–276. [PubMed] [Google Scholar]
- 162.Moreau C., Delval A., Tiffreau V., Defebvre L., Dujardin K., Duhamel A., Petyt G., Hossein-Foucher C., Blum D., Sablonniere B., et al. Memantine for Axial Signs in Parkinson’s Disease: A Randomised, Double-Blind, Placebo-Controlled Pilot Study. J. Neurol. Neurosurg. Psychiatry. 2013;84:552–555. doi: 10.1136/jnnp-2012-303182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wictorin K., Widner H. Memantine and Reduced Time with Dyskinesia in Parkinson’s Disease. Acta Neurol. Scand. 2016;133:355–360. doi: 10.1111/ane.12468. [DOI] [PubMed] [Google Scholar]
- 164.Paquette M.A., Anderson A.M., Lewis J.R., Meshul C.K., Johnson S.W., Paul Berger S. MK-801 Inhibits L-DOPA-Induced Abnormal Involuntary Movements Only at Doses That Worsen Parkinsonism. Neuropharmacology. 2010;58:1002–1008. doi: 10.1016/j.neuropharm.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Flores A.J., Bartlett M.J., Root B.K., Parent K.L., Heien M.L., Porreca F., Polt R., Sherman S.J., Falk T. The Combination of the Opioid Glycopeptide MMP-2200 and a NMDA Receptor Antagonist Reduced L-Dopa-Induced Dyskinesia and MMP-2200 by Itself Reduced Dopamine Receptor 2-Like Agonist-Induced Dyskinesia. Neuropharmacology. 2018;141:260–271. doi: 10.1016/j.neuropharm.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bartlett M.J., Joseph R.M., LePoidevin L.M., Parent K.L., Laude N.D., Lazarus L.B., Heien M.L., Estevez M., Sherman S.J., Falk T. Long-Term Effect of Sub-Anesthetic Ketamine in Reducing L-DOPA-Induced Dyskinesias in a Preclinical Model. Neurosci. Lett. 2016;612:121–125. doi: 10.1016/j.neulet.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sherman S.J., Estevez M., Magill A.B., Falk T. Case Reports Showing a Long-Term Effect of Subanesthetic Ketamine Infusion in Reducing L-Dopa-Induced Dyskinesias. Case Rep. Neurol. 2016;8:53–58. doi: 10.1159/000444278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Santini E., Sgambato-Faure V., Li Q., Savasta M., Dovero S., Fisone G., Bezard E. Distinct Changes in cAMP and Extracellular Signal-Regulated Protein Kinase Signalling in L-DOPA-Induced Dyskinesia. PLoS ONE. 2010;5:e12322. doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Santini E., Valjent E., Usiello A., Carta M., Borgkvist A., Girault J.A., Herve D., Greengard P., Fisone G. Critical Involvement of cAMP/DARPP-32 and Extracellular Signal-Regulated Protein Kinase Signaling in L-DOPA-Induced Dyskinesia. J. Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ba M., Kong M., Yang H., Ma G., Lu G., Chen S., Liu Z. Changes in Subcellular Distribution and Phosphorylation of GluR1 in Lesioned Striatum of 6-Hydroxydopamine-Lesioned and L-Dopa-Treated Rats. Neurochem. Res. 2006;31:1337–1347. doi: 10.1007/s11064-006-9177-9. [DOI] [PubMed] [Google Scholar]
- 171.Errico F., Bonito-Oliva A., Bagetta V., Vitucci D., Romano R., Zianni E., Napolitano F., Marinucci S., Di Luca M., Calabresi P., et al. Higher Free D-Aspartate and N-Methyl-D-Aspartate Levels Prevent Striatal Depotentiation and Anticipate L-DOPA-Induced Dyskinesia. Exp. Neurol. 2011;232:240–250. doi: 10.1016/j.expneurol.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 172.Bibbiani F., Oh J.D., Kielaite A., Collins M.A., Smith C., Chase T.N. Combined Blockade of AMPA and NMDA Glutamate Receptors Reduces Levodopa-Induced Motor Complications in Animal Models of PD. Exp. Neurol. 2005;196:422–429. doi: 10.1016/j.expneurol.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 173.Kobylecki C., Cenci M.A., Crossman A.R., Ravenscroft P. Calcium-Permeable AMPA Receptors Are Involved in the Induction and Expression of L-Dopa-Induced Dyskinesia in Parkinson’s Disease. J. Neurochem. 2010;114:499–511. doi: 10.1111/j.1471-4159.2010.06776.x. [DOI] [PubMed] [Google Scholar]
- 174.Konitsiotis S., Blanchet P.J., Verhagen L., Lamers E., Chase T.N. AMPA Receptor Blockade Improves Levodopa-Induced Dyskinesia in MPTP Monkeys. Neurology. 2000;54:1589–1595. doi: 10.1212/WNL.54.8.1589. [DOI] [PubMed] [Google Scholar]
- 175.Vijayakumar D., Jankovic J. Drug-Induced Dyskinesia, Part 1: Treatment of Levodopa-Induced Dyskinesia. Drugs. 2016;76:759–777. doi: 10.1007/s40265-016-0566-3. [DOI] [PubMed] [Google Scholar]
- 176.White H.S., Brown S.D., Woodhead J.H., Skeen G.A., Wolf H.H. Topiramate Modulates GABA-Evoked Currents in Murine Cortical Neurons by a Nonbenzodiazepine Mechanism. Epilepsia. 2000;41(Suppl. 1):S17–20. doi: 10.1111/j.1528-1157.2000.tb02165.x. [DOI] [PubMed] [Google Scholar]
- 177.Silverdale M.A., Nicholson S.L., Crossman A.R., Brotchie J.M. Topiramate Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Marmoset Model of Parkinson’s Disease. Mov. Disord. 2005;20:403–409. doi: 10.1002/mds.20345. [DOI] [PubMed] [Google Scholar]
- 178.Kobylecki C., Hill M.P., Crossman A.R., Ravenscroft P. Synergistic Antidyskinetic Effects of Topiramate and Amantadine in Animal Models of Parkinson’s Disease. Mov. Disord. 2011;26:2354–2363. doi: 10.1002/mds.23867. [DOI] [PubMed] [Google Scholar]
- 179.Kobylecki C., Burn D.J., Kass-Iliyya L., Kellett M.W., Crossman A.R., Silverdale M.A. Randomized Clinical Trial of Topiramate for Levodopa-Induced Dyskinesia in Parkinson’s Disease. Parkinsonism Relat. Disord. 2014;20:452–455. doi: 10.1016/j.parkreldis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 180.Eggert K., Squillacote D., Barone P., Dodel R., Katzenschlager R., Emre M., Lees A.J., Rascol O., Poewe W., Tolosa E., et al. Safety and Efficacy of Perampanel in Advanced Parkinson’s Disease: A Randomized, Placebo-Controlled Study. Mov. Disord. 2010;25:896–905. doi: 10.1002/mds.22974. [DOI] [PubMed] [Google Scholar]
- 181.Lees A., Fahn S., Eggert K.M., Jankovic J., Lang A., Micheli F., Maral Mouradian M., Oertel W.H., Olanow C.W., Poewe W., et al. Perampanel, an AMPA Antagonist, Found to Have No Benefit in Reducing “Off” Time in Parkinson’s Disease. Mov. Disord. 2012;27:284–288. doi: 10.1002/mds.23983. [DOI] [PubMed] [Google Scholar]
- 182.Rascol O., Barone P., Behari M., Emre M., Giladi N., Olanow C.W., Ruzicka E., Bibbiani F., Squillacote D., Patten A., et al. Perampanel in Parkinson Disease Fluctuations: A Double-Blind Randomized Trial with Placebo and Entacapone. Clin. Neuropharmacol. 2012;35:15–20. doi: 10.1097/WNF.0b013e318241520b. [DOI] [PubMed] [Google Scholar]
- 183.Marin C., Jimenez A., Bonastre M., Vila M., Agid Y., Hirsch E.C., Tolosa E. Ly293558, an AMPA Glutamate Receptor Antagonist, Prevents and Reverses Levodopa-Induced Motor Alterations in Parkinsonian Rats. Synapse. 2001;42:40–47. doi: 10.1002/syn.1097. [DOI] [PubMed] [Google Scholar]
- 184.Litim N., Morissette M., Di Paolo T. Metabotropic Glutamate Receptors as Therapeutic Targets in Parkinson’s Disease: An Update from the Last 5 Years of Research. Neuropharmacology. 2017;115:166–179. doi: 10.1016/j.neuropharm.2016.03.036. [DOI] [PubMed] [Google Scholar]
- 185.Sebastianutto I., Cenci M.A. mGlu Receptors in the Treatment of Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Curr. Opin. Pharm. 2018;38:81–89. doi: 10.1016/j.coph.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 186.Murray T.K., Messenger M.J., Ward M.A., Woodhouse S., Osborne D.J., Duty S., O’Neill M.J. Evaluation of the mGluR2/3 Agonist LY379268 in Rodent Models of Parkinson’s Disease. Pharmacol. Biochem. Behav. 2002;73:455–466. doi: 10.1016/S0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 187.Amalric M., Lopez S., Goudet C., Fisone G., Battaglia G., Nicoletti F., Pin J.P., Acher F.C. Group III and Subtype 4 Metabotropic Glutamate Receptor Agonists: Discovery and Pathophysiological Applications in Parkinson’s Disease. Neuropharmacology. 2013;66:53–64. doi: 10.1016/j.neuropharm.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 188.Le Poul E., Bolea C., Girard F., Poli S., Charvin D., Campo B., Bortoli J., Bessif A., Luo B., Koser A.J., et al. A Potent and Selective Metabotropic Glutamate Receptor 4 Positive Allosteric Modulator Improves Movement in Rodent Models of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2012;343:167–177. doi: 10.1124/jpet.112.196063. [DOI] [PubMed] [Google Scholar]
- 189.Bennouar K.E., Uberti M.A., Melon C., Bacolod M.D., Jimenez H.N., Cajina M., Kerkerian-Le Goff L., Doller D., Gubellini P. Synergy between L-DOPA and a Novel Positive Allosteric Modulator of Metabotropic Glutamate Receptor 4: Implications for Parkinson’s Disease Treatment and Dyskinesia. Neuropharmacology. 2013;66:158–169. doi: 10.1016/j.neuropharm.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 190.Lopez S., Bonito-Oliva A., Pallottino S., Acher F., Fisone G. Activation of Metabotropic Glutamate 4 Receptors Decreases L-DOPA-Induced Dyskinesia in a Mouse Model of Parkinson’s Disease. J. Parkinsons Dis. 2011;1:339–346. doi: 10.3233/JPD-2011-11066. [DOI] [PubMed] [Google Scholar]
- 191.Iderberg H., Maslava N., Thompson A.D., Bubser M., Niswender C.M., Hopkins C.R., Lindsley C.W., Conn P.J., Jones C.K., Cenci M.A. Pharmacological Stimulation of Metabotropic Glutamate Receptor Type 4 in a Rat Model of Parkinson’s Disease and L-DOPA-Induced Dyskinesia: Comparison between a Positive Allosteric Modulator and an Orthosteric Agonist. Neuropharmacology. 2015;95:121–129. doi: 10.1016/j.neuropharm.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Charvin D., Pomel V., Ortiz M., Frauli M., Scheffler S., Steinberg E., Baron L., Deshons L., Rudigier R., Thiarc D., et al. Discovery, Structure-Activity Relationship, and Antiparkinsonian Effect of a Potent and Brain-Penetrant Chemical Series of Positive Allosteric Modulators of Metabotropic Glutamate Receptor 4. J. Med. Chem. 2017;60:8515–8537. doi: 10.1021/acs.jmedchem.7b00991. [DOI] [PubMed] [Google Scholar]
- 193.Charvin D., Di Paolo T., Bezard E., Gregoire L., Takano A., Duvey G., Pioli E., Halldin C., Medori R., Conquet F. An Mglu4-Positive Allosteric Modulator Alleviates Parkinsonism in Primates. Mov. Disord. 2018;33:1619–1631. doi: 10.1002/mds.27462. [DOI] [PubMed] [Google Scholar]
- 194.Marin C., Bonastre M., Aguilar E., Jimenez A. The Metabotropic Glutamate Receptor Antagonist 2-Methyl-6-(Phenylethynyl) Pyridine Decreases Striatal VGlut2 Expression in Association with an Attenuation of L-DOPA-Induced Dyskinesias. Synapse. 2011;65:1080–1086. doi: 10.1002/syn.20941. [DOI] [PubMed] [Google Scholar]
- 195.Mela F., Marti M., Dekundy A., Danysz W., Morari M., Cenci M.A. Antagonism of Metabotropic Glutamate Receptor Type 5 Attenuates L-Dopa-Induced Dyskinesia and Its Molecular and Neurochemical Correlates in a Rat Model of Parkinson’s Disease. J. Neurochem. 2007;101:483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 196.Morin N., Gregoire L., Gomez-Mancilla B., Gasparini F., Di Paolo T. Effect of the Metabotropic Glutamate Receptor Type 5 Antagonists MPEP and MTEP in Parkinsonian Monkeys. Neuropharmacology. 2010;58:981–986. doi: 10.1016/j.neuropharm.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 197.Morin N., Morissette M., Gregoire L., Gomez-Mancilla B., Gasparini F., Di Paolo T. Chronic Treatment with MPEP, an mGlu5 Receptor Antagonist, Normalizes Basal Ganglia Glutamate Neurotransmission in L-DOPA-Treated Parkinsonian Monkeys. Neuropharmacology. 2013;73:216–231. doi: 10.1016/j.neuropharm.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 198.Rylander D., Iderberg H., Li Q., Dekundy A., Zhang J., Li H., Baishen R., Danysz W., Bezard E., Cenci M.A. A mGluR5 Antagonist under Clinical Development Improves L-DOPA-Induced Dyskinesia in Parkinsonian Rats and Monkeys. Neurobiol. Dis. 2010;39:352–361. doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 199.Ko W.K., Pioli E., Li Q., McGuire S., Dufour A., Sherer T.B., Bezard E., Facheris M.F. Combined Fenobam and Amantadine Treatment Promotes Robust Antidyskinetic Effects in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Lesioned Primate Model of Parkinson’s Disease. Mov. Disord. 2014;29:772–779. doi: 10.1002/mds.25859. [DOI] [PubMed] [Google Scholar]
- 200.Fuzzati-Armentero M.T., Cerri S., Levandis G., Ambrosi G., Montepeloso E., Antoninetti G., Blandini F., Baqi Y., Muller C.E., Volpini R., et al. Dual Target Strategy: Combining Distinct Non-Dopaminergic Treatments Reduces Neuronal Cell Loss and Synergistically Modulates L-DOPA-Induced Rotational Behavior in a Rodent Model of Parkinson’s Disease. J. Neurochem. 2015;134:740–747. doi: 10.1111/jnc.13162. [DOI] [PubMed] [Google Scholar]
- 201.Berg D., Godau J., Trenkwalder C., Eggert K., Csoti I., Storch A., Huber H., Morelli-Canelo M., Stamelou M., Ries V., et al. AFQ056 Treatment of Levodopa-Induced Dyskinesias: Results of 2 Randomized Controlled Trials. Mov. Disord. 2011;26:1243–1250. doi: 10.1002/mds.23616. [DOI] [PubMed] [Google Scholar]
- 202.Stocchi F., Rascol O., Destee A., Hattori N., Hauser R.A., Lang A.E., Poewe W., Stacy M., Tolosa E., Gao H., et al. AFQ056 in Parkinson Patients with Levodopa-Induced Dyskinesia: 13-Week, Randomized, Dose-Finding Study. Mov. Disord. 2013;28:1838–1846. doi: 10.1002/mds.25561. [DOI] [PubMed] [Google Scholar]
- 203.Trenkwalder C., Stocchi F., Poewe W., Dronamraju N., Kenney C., Shah A., von Raison F., Graf A. Mavoglurant in Parkinson’s Patients with L-Dopa-Induced Dyskinesias: Two Randomized Phase 2 Studies. Mov. Disord. 2016;31:1054–1058. doi: 10.1002/mds.26585. [DOI] [PubMed] [Google Scholar]
- 204.Kumar R., Hauser R.A., Mostillo J., Dronamraju N., Graf A., Merschhemke M., Kenney C. Mavoglurant (AFQ056) in Combination with Increased Levodopa Dosages in Parkinson’s Disease Patients. Int. J. Neurosci. 2016;126:20–24. doi: 10.3109/00207454.2013.841685. [DOI] [PubMed] [Google Scholar]
- 205.Tison F., Keywood C., Wakefield M., Durif F., Corvol J.C., Eggert K., Lew M., Isaacson S., Bezard E., Poli S.M., et al. A Phase 2a Trial of the Novel mGluR5-Negative Allosteric Modulator Dipraglurant for Levodopa-Induced Dyskinesia in Parkinson’s Disease. Mov. Disord. 2016;31:1373–1380. doi: 10.1002/mds.26659. [DOI] [PubMed] [Google Scholar]
- 206.Mitsukawa K., Yamamoto R., Ofner S., Nozulak J., Pescott O., Lukic S., Stoehr N., Mombereau C., Kuhn R., McAllister K.H., et al. A Selective Metabotropic Glutamate Receptor 7 Agonist: Activation of Receptor Signaling Via an Allosteric Site Modulates Stress Parameters in Vivo. Proc. Natl. Acad. Sci. USA. 2005;102:18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Broadstock M., Austin P.J., Betts M.J., Duty S. Antiparkinsonian Potential of Targeting Group III Metabotropic Glutamate Receptor Subtypes in the Rodent Substantia Nigra Pars Reticulata. Br. J. Pharmacol. 2012;165:1034–1045. doi: 10.1111/j.1476-5381.2011.01515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Konieczny J., Lenda T. Contribution of the mGluR7 Receptor to Antiparkinsonian-Like Effects in Rats: A Behavioral Study with the Selective Agonist AMN082. Pharmacol. Rep. 2013;65:1194–1203. doi: 10.1016/S1734-1140(13)71477-4. [DOI] [PubMed] [Google Scholar]
- 209.Johnson K.A., Jones C.K., Tantawy M.N., Bubser M., Marvanova M., Ansari M.S., Baldwin R.M., Conn P.J., Niswender C.M. The Metabotropic Glutamate Receptor 8 Agonist (S)-3,4-DCPG Reverses Motor Deficits in Prolonged but Not Acute Models of Parkinson’s Disease. Neuropharmacology. 2013;66:187–195. doi: 10.1016/j.neuropharm.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Foote S.L., Bloom F.E., Aston-Jones G. Nucleus Locus Ceruleus: New Evidence of Anatomical and Physiological Specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 211.Zarow C., Lyness S.A., Mortimer J.A., Chui H.C. Neuronal Loss Is Greater in the Locus Coeruleus Than Nucleus Basalis and Substantia Nigra in Alzheimer and Parkinson Diseases. Arch. Neurol. 2003;60:337–341. doi: 10.1001/archneur.60.3.337. [DOI] [PubMed] [Google Scholar]
- 212.Fornai F., di Poggio A.B., Pellegrini A., Ruggieri S., Paparelli A. Noradrenaline in Parkinson’s Disease: From Disease Progression to Current Therapeutics. Curr. Med. Chem. 2007;14:2330–2334. doi: 10.2174/092986707781745550. [DOI] [PubMed] [Google Scholar]
- 213.McMillan P.J., White S.S., Franklin A., Greenup J.L., Leverenz J.B., Raskind M.A., Szot P. Differential Response of the Central Noradrenergic Nervous System to the Loss of Locus Coeruleus Neurons in Parkinson’s Disease and Alzheimer’s Disease. Brain Res. 2011;1373:240–252. doi: 10.1016/j.brainres.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mavridis M., Degryse A.D., Lategan A.J., Marien M.R., Colpaert F.C. Effects of Locus Coeruleus Lesions on Parkinsonian Signs, Striatal Dopamine and Substantia Nigra Cell Loss after 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine in Monkeys: A Possible Role for the Locus Coeruleus in the Progression of Parkinson’s Disease. Neuroscience. 1991;41:507–523. doi: 10.1016/0306-4522(91)90345-O. [DOI] [PubMed] [Google Scholar]
- 215.Rommelfanger K.S., Edwards G.L., Freeman K.G., Liles L.C., Miller G.W., Weinshenker D. Norepinephrine Loss Produces More Profound Motor Deficits Than MPTP Treatment in Mice. Proc. Natl. Acad. Sci. USA. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Savola J.M., Hill M., Engstrom M., Merivuori H., Wurster S., McGuire S.G., Fox S.H., Crossman A.R., Brotchie J.M. Fipamezole (JP-1730) Is a Potent Alpha2 Adrenergic Receptor Antagonist That Reduces Levodopa-Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Mov. Disord. 2003;18:872–883. doi: 10.1002/mds.10464. [DOI] [PubMed] [Google Scholar]
- 217.Barnum C.J., Bhide N., Lindenbach D., Surrena M.A., Goldenberg A.A., Tignor S., Klioueva A., Walters H., Bishop C. Effects of Noradrenergic Denervation on L-DOPA-Induced Dyskinesia and Its Treatment by A- and B-Adrenergic Receptor Antagonists in Hemiparkinsonian Rats. Pharmacol. Biochem. Behav. 2012;100:607–615. doi: 10.1016/j.pbb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rascol O., Arnulf I., Peyro-Saint Paul H., Brefel-Courbon C., Vidailhet M., Thalamas C., Bonnet A.M., Descombes S., Bejjani B., Fabre N., et al. Idazoxan, an Alpha-2 Antagonist, and L-DOPA-Induced Dyskinesias in Patients with Parkinson’s Disease. Mov. Disord. 2001;16:708–713. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- 219.Johnston T.H., Fox S.H., Piggott M.J., Savola J.M., Brotchie J.M. The Alpha(2) Adrenergic Antagonist Fipamezole Improves Quality of Levodopa Action in Parkinsonian Primates. Mov. Disord. 2010;25:2084–2093. doi: 10.1002/mds.23172. [DOI] [PubMed] [Google Scholar]
- 220.Dimitrova T., Bara-Jimenez W., Savola J., Encarnacion E., Mouradian M., Chase T. Alpha-2 Adrenergic Antagonist Effects in Parkinson’s Disease. Lippincott Williams & Wilkins 530 Walnut St; Philadelphia, PA, USA: p. A540. Neurology, 2011. [Google Scholar]
- 221.Lewitt P.A., Hauser R.A., Lu M., Nicholas A.P., Weiner W., Coppard N., Leinonen M., Savola J.M. Randomized Clinical Trial of Fipamezole for Dyskinesia in Parkinson Disease (Fjord Study) Neurology. 2012;79:163–169. doi: 10.1212/WNL.0b013e31825f0451. [DOI] [PubMed] [Google Scholar]
- 222.Rainbow T.C., Parsons B., Wolfe B.B. Quantitative Autoradiography of Beta 1- and Beta 2-Adrenergic Receptors in Rat Brain. Proc. Natl. Acad. Sci. USA. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Waeber C., Rigo M., Chinaglia G., Probst A., Palacios J.M. Beta-Adrenergic Receptor Subtypes in the Basal Ganglia of Patients with Huntington’s Chorea and Parkinson’s Disease. Synapse. 1991;8:270–280. doi: 10.1002/syn.890080405. [DOI] [PubMed] [Google Scholar]
- 224.Bhide N., Lindenbach D., Barnum C.J., George J.A., Surrena M.A., Bishop C. Effects of the Beta-Adrenergic Receptor Antagonist Propranolol on Dyskinesia and L-DOPA-Induced Striatal Da Efflux in the Hemi-Parkinsonian Rat. J. Neurochem. 2015;134:222–232. doi: 10.1111/jnc.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Pagano G., Tan E.E., Haider J.M., Bautista A., Tagliati M. Constipation Is Reduced by Beta-Blockers and Increased by Dopaminergic Medications in Parkinson’s Disease. Parkinsonism Relat. Disord. 2015;21:120–125. doi: 10.1016/j.parkreldis.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 226.Julius A., Longfellow K. Movement Disorders: A Brief Guide in Medication Management. Med. Clin. N. Am. 2016;100:733–761. doi: 10.1016/j.mcna.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 227.Magistrelli L., Comi C. Beta2-Adrenoceptor Agonists in Parkinson’s Disease and Other Synucleinopathies. J. Neuroimmune Pharmacol. 2019 doi: 10.1007/s11481-018-09831-0. [DOI] [PubMed] [Google Scholar]
- 228.Latini S., Pedata F. Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J. Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 229.Ferré S. Adenosine-Dopamine Interactions in the Ventral Striatum. Implications for the Treatment of Schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- 230.Ferré S., Popoli P., Giménez-Llort L., Rimondini R., Müller C.E., Strömberg I., Ögren S.O., Fuxe K. Adenosine/Dopamine Interaction: Implications for the Treatment of Parkinson’s Disease. Parkinsonism Relat. Disord. 2001;7:235–241. doi: 10.1016/S1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- 231.Fastbom J., Pazos A., Palacios J.M. The Distribution of Adenosine A1 Receptors and 5’-Nucleotidase in the Brain of Some Commonly Used Experimental Animals. Neuroscience. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- 232.Mango D., Bonito-Oliva A., Ledonne A., Cappellacci L., Petrelli R., Nistico R., Berretta N., Fisone G., Mercuri N.B. Adenosine A1 Receptor Stimulation Reduces D1 Receptor-Mediated Gabaergic Transmission from Striato-Nigral Terminals and Attenuates L-Dopa-Induced Dyskinesia in Dopamine-Denervated Mice. Exp. Neurol. 2014;261:733–743. doi: 10.1016/j.expneurol.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 233.Ferre S., Quiroz C., Woods A.S., Cunha R., Popoli P., Ciruela F., Lluis C., Franco R., Azdad K., Schiffmann S.N. An Update on Adenosine A2A-Dopamine D2 Receptor Interactions: Implications for the Function of G Protein-Coupled Receptors. Curr. Pharm. Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pollack A.E., Fink J.S. Adenosine Antagonists Potentiate D2 Dopamine-Dependent Activation of Fos in the Striatopallidal Pathway. Neuroscience. 1995;68:721–728. doi: 10.1016/0306-4522(95)00168-I. [DOI] [PubMed] [Google Scholar]
- 235.Zheng J., Zhang X., Zhen X. Development of Adenosine A2A Receptor Antagonists for the Treatment of Parkinson’s Disease: A Recent Update and Challenge. ACS Chem. Neurosci. 2019;10:783–791. doi: 10.1021/acschemneuro.8b00313. [DOI] [PubMed] [Google Scholar]
- 236.Approval for Manufacturing and Marketing of Nouriast Tablets 20 Mg, a Novel Antiparkinsonian Agent, Kyowa Hakko Kirin Co Ltd Press Release. [(accessed on 9 April 2019)];2019 Mar 25; Available online: https://www.kyowa-kirin.com/news_releases/2013/e20130325_04.html.
- 237.Lundblad M., Vaudano E., Cenci M.A. Cellular and Behavioural Effects of the Adenosine A2a Receptor Antagonist KW-6002 in a Rat Model of L-Dopa-Induced Dyskinesia. J. Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 238.Kanda T., Jackson M.J., Smith L.A., Pearce R.K., Nakamura J., Kase H., Kuwana Y., Jenner P. Adenosine A2A Antagonist: A Novel Antiparkinsonian Agent That Does Not Provoke Dyskinesia in Parkinsonian Monkeys. Ann. Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- 239.Kanda T., Tashiro T., Kuwana Y., Jenner P. Adenosine A2A Receptors Modify Motor Function in MPTP-Treated Common Marmosets. Neuroreport. 1998;9:2857–2860. doi: 10.1097/00001756-199808240-00032. [DOI] [PubMed] [Google Scholar]
- 240.Kanda T., Jackson M.J., Smith L.A., Pearce R.K., Nakamura J., Kase H., Kuwana Y., Jenner P. Combined Use of the Adenosine A(2A) Antagonist KW-6002 with L-DOPA or with Selective D1 or D2 Dopamine Agonists Increases Antiparkinsonian Activity but Not Dyskinesia in MPTP-Treated Monkeys. Exp. Neurol. 2000;162:321–327. doi: 10.1006/exnr.2000.7350. [DOI] [PubMed] [Google Scholar]
- 241.Uchida S., Tashiro T., Kawai-Uchida M., Mori A., Jenner P., Kanda T. Adenosine a(2)a-Receptor Antagonist Istradefylline Enhances the Motor Response of L-DOPA without Worsening Dyskinesia in MPTP-Treated Common Marmosets. J. Pharmacol. Sci. 2014;124:480–485. doi: 10.1254/jphs.13250FP. [DOI] [PubMed] [Google Scholar]
- 242.Bibbiani F., Oh J.D., Petzer J.P., Castagnoli N., Jr., Chen J.F., Schwarzschild M.A., Chase T.N. A2A Antagonist Prevents Dopamine Agonist-Induced Motor Complications in Animal Models of Parkinson’s Disease. Exp. Neurol. 2003;184:285–294. doi: 10.1016/S0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 243.Grondin R., Bedard P.J., Hadj Tahar A., Gregoire L., Mori A., Kase H. Antiparkinsonian Effect of a New Selective Adenosine A2A Receptor Antagonist in MPTP-Treated Monkeys. Neurology. 1999;52:1673–1677. doi: 10.1212/WNL.52.8.1673. [DOI] [PubMed] [Google Scholar]
- 244.Ko W.K.D., Camus S.M., Li Q., Yang J., McGuire S., Pioli E.Y., Bezard E. An Evaluation of Istradefylline Treatment on Parkinsonian Motor and Cognitive Deficits in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)-Treated Macaque Models. Neuropharmacology. 2016;110:48–58. doi: 10.1016/j.neuropharm.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 245.Hauser R.A., Hubble J.P., Truong D.D. Randomized Trial of the Adenosine A(2A) Receptor Antagonist Istradefylline in Advanced PD. Neurology. 2003;61:297–303. doi: 10.1212/01.WNL.0000081227.84197.0B. [DOI] [PubMed] [Google Scholar]
- 246.Hauser R.A., Shulman L.M., Trugman J.M., Roberts J.W., Mori A., Ballerini R., Sussman N.M. Study of Istradefylline in Patients with Parkinson’s Disease on Levodopa with Motor Fluctuations. Mov. Disord. 2008;23:2177–2185. doi: 10.1002/mds.22095. [DOI] [PubMed] [Google Scholar]
- 247.Stacy M., Silver D., Mendis T., Sutton J., Mori A., Chaikin P., Sussman N.M. A 12-Week, Placebo-Controlled Study (6002-US-006) of Istradefylline in Parkinson Disease. Neurology. 2008;70:2233–2240. doi: 10.1212/01.wnl.0000313834.22171.17. [DOI] [PubMed] [Google Scholar]
- 248.LeWitt P.A., Guttman M., Tetrud J.W., Tuite P.J., Mori A., Chaikin P., Sussman N.M., Group U.S.S. Adenosine A2A Receptor Antagonist Istradefylline (KW-6002) Reduces “Off” Time in Parkinson’s Disease: A Double-Blind, Randomized, Multicenter Clinical Trial (6002-US-005) Ann. Neurol. 2008;63:295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- 249.Pourcher E., Fernandez H.H., Stacy M., Mori A., Ballerini R., Chaikin P. Istradefylline for Parkinson’s Disease Patients Experiencing Motor Fluctuations: Results of the KW-6002-US-018 Study. Parkinsonism Relat. Disord. 2012;18:178–184. doi: 10.1016/j.parkreldis.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 250.Zhu C., Wang G., Li J., Chen L., Wang C., Wang Y., Lin P., Ran H. Adenosine A2A Receptor Antagonist Istradefylline 20 Versus 40 Mg/Day as Augmentation for Parkinson’s Disease: A Meta-Analysis. Neurol. Res. 2014;36:1028–1034. doi: 10.1179/1743132814Y.0000000375. [DOI] [PubMed] [Google Scholar]
- 251.Kondo T., Mizuno Y. A Long-Term Study of Istradefylline Safety and Efficacy in Patients with Parkinson Disease. Clin. Neuropharmacol. 2015;38:41–46. doi: 10.1097/WNF.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 252.Takahashi M., Fujita M., Asai N., Saki M., Mori A. Safety and Effectiveness of Istradefylline in Patients with Parkinson’s Disease: Interim Analysis of a Post-Marketing Surveillance Study in Japan. Expert Opin. Pharmacother. 2018;19:1635–1642. doi: 10.1080/14656566.2018.1518433. [DOI] [PubMed] [Google Scholar]
- 253.Torti M., Vacca L., Stocchi F. Istradefylline for the Treatment of Parkinson’s Disease: Is It a Promising Strategy? Expert Opin. Pharmacother. 2018;19:1821–1828. doi: 10.1080/14656566.2018.1524876. [DOI] [PubMed] [Google Scholar]
- 254.Hodgson R.A., Bedard P.J., Varty G.B., Kazdoba T.M., Di Paolo T., Grzelak M.E., Pond A.J., Hadjtahar A., Belanger N., Gregoire L., et al. Preladenant, a Selective A(2A) Receptor Antagonist, Is Active in Primate Models of Movement Disorders. Exp. Neurol. 2010;225:384–390. doi: 10.1016/j.expneurol.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 255.Hodgson R.A., Bertorelli R., Varty G.B., Lachowicz J.E., Forlani A., Fredduzzi S., Cohen-Williams M.E., Higgins G.A., Impagnatiello F., Nicolussi E., et al. Characterization of the Potent and Highly Selective A2A Receptor Antagonists Preladenant and SCH 412348 [7-[2-[4-2,4-Difluorophenyl]-1-Piperazinyl]Ethyl]-2-(2-Furanyl)-7h-Pyrazolo[4,3-E][1,2,4]Triazolo[1,5-C]Pyrimidin-5-Amine] in Rodent Models of Movement Disorders and Depression. J. Pharmacol. Exp. Ther. 2009;330:294–303. doi: 10.1124/jpet.108.149617. [DOI] [PubMed] [Google Scholar]
- 256.Factor S.A., Wolski K., Togasaki D.M., Huyck S., Cantillon M., Ho T.W., Hauser R.A., Pourcher E. Long-Term Safety and Efficacy of Preladenant in Subjects with Fluctuating Parkinson’s Disease. Mov. Disord. 2013;28:817–820. doi: 10.1002/mds.25395. [DOI] [PubMed] [Google Scholar]
- 257.Hauser R.A., Cantillon M., Pourcher E., Micheli F., Mok V., Onofrj M., Huyck S., Wolski K. Preladenant in Patients with Parkinson’s Disease and Motor Fluctuations: A Phase 2, Double-Blind, Randomised Trial. Lacet. Neurol. 2011;10:221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 258.Hattori N., Kikuchi M., Adachi N., Hewitt D., Huyck S., Saito T. Adjunctive Preladenant: A Placebo-Controlled, Dose-Finding Study in Japanese Patients with Parkinson’s Disease. Parkinsonism Relat. Disord. 2016;32:73–79. doi: 10.1016/j.parkreldis.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 259.Hauser R., Stocchi F., Rascol O., Huyck S., Ha X., Capece R., Wolski K., Ho T., Sklar P., Lines C., et al. Phase-3 Clinical Trials of Adjunctive Therapy with Preladenant, an Adenosine 2a Antagonist, in Patients with Parkinson’s Disease (P7.087) Neurology. 2014;82:P7.087. [Google Scholar]
- 260.Hauser R.A., Stocchi F., Rascol O., Huyck S.B., Capece R., Ho T.W., Sklar P., Lines C., Michelson D., Hewitt D. Preladenant as an Adjunctive Therapy with Levodopa in Parkinson Disease: Two Randomized Clinical Trials and Lessons Learned. JAMA neurol. 2015;72:1491–1500. doi: 10.1001/jamaneurol.2015.2268. [DOI] [PubMed] [Google Scholar]
- 261.Black K.J., Koller J.M., Campbell M.C., Gusnard D.A., Bandak S.I. Quantification of Indirect Pathway Inhibition by the Adenosine A2a Antagonist SYN115 in Parkinson Disease. J. Neurosci. 2010;30:16284–16292. doi: 10.1523/JNEUROSCI.2590-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hauser R.A., Olanow C.W., Kieburtz K.D., Pourcher E., Docu-Axelerad A., Lew M., Kozyolkin O., Neale A., Resburg C., Meya U., et al. Tozadenant (SYN115) in Patients with Parkinson’s Disease Who Have Motor Fluctuations on Levodopa: A Phase 2b, Double-Blind, Randomised Trial. Lacet. Neurol. 2014;13:767–776. doi: 10.1016/S1474-4422(14)70148-6. [DOI] [PubMed] [Google Scholar]
- 263.Papapetropoulos S., Borgohain R., Kellet M., Giladi N., Tomic D., Coppell A. The Adenosine A2A Receptor Antagonist BIIB014 Is Effective in Improving on-Time in Parkinson’s Disease (PD) Patients with Motor Fluctuations. Mov. Disord. 2010;25:S305. [Google Scholar]
- 264.Pawsey S., Donaldson K., Warrington S., Tranter E. A Phase I Single and Repeated Dose Pharmacokinetic Study of Oral V81444, a Selective Non-Xanthine Adenosine A2A Receptor Antagonist. J. Neurol. Sci. 2013;333:e135. doi: 10.1016/j.jns.2013.07.450. [DOI] [Google Scholar]
- 265.Núñez F., Taura J., Camacho J., López-Cano M., Fernández-Dueñas V., Castro N., Castro J., Ciruela F. PBF509, an Adenosine A(2A) Receptor Antagonist with Efficacy in Rodent Models of Movement Disorders. Front. Pharmacol. 2018;9:1200. doi: 10.3389/fphar.2018.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tronci E., Simola N., Borsini F., Schintu N., Frau L., Carminati P., Morelli M. Characterization of the Antiparkinsonian Effects of the New Adenosine A2A Receptor Antagonist ST1535: Acute and Subchronic Studies in Rats. Eur. J. Pharmacol. 2007;566:94–102. doi: 10.1016/j.ejphar.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 267.Rose S., Ramsay Croft N., Jenner P. The Novel Adenosine A2a Antagonist ST1535 Potentiates the Effects of a Threshold Dose of L-Dopa in Unilaterally 6-OHDA-Lesioned Rats. Brain Res. 2007;1133:110–114. doi: 10.1016/j.brainres.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 268.Rose S., Jackson M.J., Smith L.A., Stockwell K., Johnson L., Carminati P., Jenner P. The Novel Adenosine A2a Receptor Antagonist ST1535 Potentiates the Effects of a Threshold Dose of L-DOPA in MPTP Treated Common Marmosets. Eur. J. Pharmacol. 2006;546:82–87. doi: 10.1016/j.ejphar.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 269.Pinna A. Adenosine A2A Receptor Antagonists in Parkinson’s Disease: Progress in Clinical Trials from the Newly Approved Istradefylline to Drugs in Early Development and Those Already Discontinued. CNS Drugs. 2014;28:455–474. doi: 10.1007/s40263-014-0161-7. [DOI] [PubMed] [Google Scholar]
- 270.Bagga P., Chugani A.N., Patel A.B. Neuroprotective Effects of Caffeine in MPTP Model of Parkinson’s Disease: A (13)C NMR Study. Neurochem. Int. 2016;92:25–34. doi: 10.1016/j.neuint.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 271.Rivera-Oliver M., Diaz-Rios M. Using Caffeine and Other Adenosine Receptor Antagonists and Agonists as Therapeutic Tools against Neurodegenerative Diseases: A Review. Life Sci. 2014;101:1–9. doi: 10.1016/j.lfs.2014.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Roshan M.H., Tambo A., Pace N.P. Potential Role of Caffeine in the Treatment of Parkinson’s Disease. Open Neurol. J. 2016;10:42–58. doi: 10.2174/1874205X01610010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wills A.-M.A., Eberly S., Tennis M., Lang A.E., Messing S., Togasaki D., Tanner C.M., Kamp C., Chen J.-F., Oakes D., et al. Caffeine Consumption and Risk of Dyskinesia in Calm-PD. Mov. Disord. 2013;28:380–383. doi: 10.1002/mds.25319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Postuma R.B., Lang A.E., Munhoz R.P., Charland K., Pelletier A., Moscovich M., Filla L., Zanatta D., Rios Romenets S., Altman R., et al. Caffeine for Treatment of Parkinson Disease: A Randomized Controlled Trial. Neurology. 2012;79:651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pilleri M., Antonini A. Therapeutic Strategies to Prevent and Manage Dyskinesias in Parkinson’s Disease. Expert Opin. Drug Saf. 2015;14:281–294. doi: 10.1517/14740338.2015.988137. [DOI] [PubMed] [Google Scholar]
- 276.Lim S.A.O., Xia R., Ding Y., Won L., Ray W.J., Hitchcock S.A., McGehee D.S., Kang U.J. Enhanced Histamine H2 Excitation of Striatal Cholinergic Interneurons in L-DOPA-Induced Dyskinesia. Neurobiol. Dis. 2015;76:67–76. doi: 10.1016/j.nbd.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Johnston T.H., van der Meij A., Brotchie J.M., Fox S.H. Effect of Histamine H2 Receptor Antagonism on Levodopa-Induced Dyskinesia in the MPTP-Macaque Model of Parkinson’s Disease. Mov. Disord. 2010;25:1379–1390. doi: 10.1002/mds.23069. [DOI] [PubMed] [Google Scholar]
- 278.Mestre T.A., Shah B.B., Connolly B.S., de Aquino C., Al Dhakeel A., Walsh R., Ghate T., Lui J.P., Fox S.H. Famotidine, a Histamine H2 Receptor Antagonist, Does Not Reduce Levodopa-Induced Dyskinesia in Parkinson’s Disease: A Proof-of-Concept Study. Mov. Disord. Clin. Pract. 2014;1:219–224. doi: 10.1002/mdc3.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Doi H., Sakakibara R., Sato M., Hirai S., Masaka T., Kishi M., Tsuyusaki Y., Tateno A., Tateno F., Takahashi O., et al. Nizatidine Ameliorates Gastroparesis in Parkinson’s Disease: A Pilot Study. Mov. Disord. 2014;29:562–566. doi: 10.1002/mds.25777. [DOI] [PubMed] [Google Scholar]
- 280.Sakakibara R., Doi H., Sato M., Hirai S., Masaka T., Kishi M., Tsuyusaki Y., Tateno A., Tateno F., Aiba Y., et al. Nizatidine Ameliorates Slow Transit Constipation in Parkinson’s Disease. J. Am. Geriatr. Soc. 2015;63:399–401. doi: 10.1111/jgs.13279. [DOI] [PubMed] [Google Scholar]
- 281.Ferrada C., Ferre S., Casado V., Cortes A., Justinova Z., Barnes C., Canela E.I., Goldberg S.R., Leurs R., Lluis C., et al. Interactions between Histamine H3 and Dopamine D2 Receptors and the Implications for Striatal Function. Neuropharmacology. 2008;55:190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Masini D., Lopes-Aguiar C., Bonito-Oliva A., Papadia D., Andersson R., Fisahn A., Fisone G. The Histamine H3 Receptor Antagonist Thioperamide Rescues Circadian Rhythm and Memory Function in Experimental Parkinsonism. Transl. Psychiatry. 2017;7:e1088. doi: 10.1038/tp.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shan L., Bossers K., Luchetti S., Balesar R., Lethbridge N., Chazot P.L., Bao A.M., Swaab D.F. Alterations in the Histaminergic System in the Substantia Nigra and Striatum of Parkinson’s Patients: A Postmortem Study. Neurobiol. Aging. 2012;33:1488.e1–1488.e13. doi: 10.1016/j.neurobiolaging.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 284.Zhou P., Homberg J.R., Fang Q., Wang J., Li W., Meng X., Shen J., Luan Y., Liao P., Swaab D.F., et al. Histamine-4 Receptor Antagonist JNJ7777120 Inhibits Pro-Inflammatory Microglia and Prevents the Progression of Parkinson-Like Pathology and Behaviour in a Rat Model. Brain. Behav. Immun. 2019;76:61–73. doi: 10.1016/j.bbi.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 285.Bordia T., Perez X.A. Cholinergic Control of Striatal Neurons to Modulate L-Dopa-Induced Dyskinesias. Eur. J. Neurosci. 2019;49:859–868. doi: 10.1111/ejn.14048. [DOI] [PubMed] [Google Scholar]
- 286.Conti M.M., Chambers N., Bishop C. A New Outlook on Cholinergic Interneurons in Parkinson’s Disease and L-DOPA-Induced Dyskinesia. Neurosci. Biobehav. Rev. 2018;92:67–82. doi: 10.1016/j.neubiorev.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 287.Perez X.A., Bordia T., Quik M. The Striatal Cholinergic System in L-Dopa-Induced Dyskinesias. J. Neural Transm. 2018;125:1251–1262. doi: 10.1007/s00702-018-1845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Freitas M.E., Fox S.H. Nondopaminergic Treatments for Parkinson’s Disease: Current and Future Prospects. Neurodegener. Dis. Manag. 2016;6:249–268. doi: 10.2217/nmt-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhang H., Bai L., He J., Zhong L., Duan X., Ouyang L., Zhu Y., Wang T., Zhang Y., Shi J. Recent Advances in Discovery and Development of Natural Products As source for Anti-Parkinson’s Disease Lead Compounds. Eur. J. Med. Chem. 2017;141:257–272. doi: 10.1016/j.ejmech.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 290.Montastruc J.L., Llau M.E., Rascol O., Senard J.M. Drug-Induced Parkinsonism: A Review. Fund. Clin. Pharmacol. 1994;8:293–306. doi: 10.1111/j.1472-8206.1994.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 291.Brocks D.R. Anticholinergic Drugs Used in Parkinson’s Disease: An Overlooked Class of Drugs from a Pharmacokinetic Perspective. J. Pharm. Pharm. Sci. 1999;2:39–46. [PubMed] [Google Scholar]
- 292.Fox S.H., Katzenschlager R., Lim S.Y., Ravina B., Seppi K., Coelho M., Poewe W., Rascol O., Goetz C.G., Sampaio C. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the Motor Symptoms of Parkinson’s Disease. Mov. Disord. 2011;26(Suppl. 3):S2–41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 293.Ding Y., Won L., Britt J.P., Lim S.A., McGehee D.S., Kang U.J. Enhanced Striatal Cholinergic Neuronal Activity Mediates L-DOPA-Induced Dyskinesia in Parkinsonian Mice. Proc. Natl. Acad. Sci. USA. 2011;108:840–845. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ztaou S., Lhost J., Watabe I., Torromino G., Amalric M. Striatal Cholinergic Interneurons Regulate Cognitive and Affective Dysfunction in Partially Dopamine-Depleted Mice. Eur. J. Neurosci. 2018;48:2988–3004. doi: 10.1111/ejn.14153. [DOI] [PubMed] [Google Scholar]
- 295.Kostelnik A., Cegan A., Pohanka M. Anti-Parkinson Drug Biperiden Inhibits Enzyme Acetylcholinesterase. BioMed Res. Int. 2017;2017:2532764. doi: 10.1155/2017/2532764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shen W., Plotkin J.L., Francardo V., Ko W.K., Xie Z., Li Q., Fieblinger T., Wess J., Neubig R.R., Lindsley C.W., et al. M4 Muscarinic Receptor Signaling Ameliorates Striatal Plasticity Deficits in Models of L-DOPA-Induced Dyskinesia. Neuron. 2015;88:762–773. doi: 10.1016/j.neuron.2015.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Exley R., Clements M.A., Hartung H., McIntosh J.M., Franklin M., Bermudez I., Cragg S.J. Striatal Dopamine Transmission Is Reduced after Chronic Nicotine with a Decrease in Alpha6-Nicotinic Receptor Control in Nucleus Accumbens. Eur. J. Neurosci. 2013;38:3036–3043. doi: 10.1111/ejn.12298. [DOI] [PubMed] [Google Scholar]
- 298.Kosillo P., Zhang Y.-F., Threlfell S., Cragg S.J. Cortical Control of Striatal Dopamine Transmission Via Striatal Cholinergic Interneurons. Cereb. Cortex. 2016;26:4160–4169. doi: 10.1093/cercor/bhw252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Luo R., Janssen M.J., Partridge J.G., Vicini S. Direct and GABA-Mediated Indirect Effects of Nicotinic Ach Receptor Agonists on Striatal Neurones. J. Physiol. 2013;591:203–217. doi: 10.1113/jphysiol.2012.241786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Quik M., Zhang D., McGregor M., Bordia T. Alpha7 Nicotinic Receptors as Therapeutic Targets for Parkinson’s Disease. Biochem. Pharmacol. 2015;97:399–407. doi: 10.1016/j.bcp.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Brumberg J., Küsters S., Al-Momani E., Marotta G., Cosgrove K.P., van Dyck C.H., Herrmann K., Homola G.A., Pezzoli G., Buck A.K., et al. Cholinergic Activity and Levodopa-Induced Dyskinesia: A Multitracer Molecular Imaging Study. Ann. Clin. Transl. Neurol. 2017;4:632–639. doi: 10.1002/acn3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Dajas-Bailador F., Wonnacott S. Nicotinic Acetylcholine Receptors and the Regulation of Neuronal Signalling. Trends Pharmacol. Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 303.Garcia-Montes J.R., Boronat-Garcia A., Lopez-Colome A.M., Bargas J., Guerra-Crespo M., Drucker-Colin R. Is Nicotine Protective against Parkinson’s Disease? An Experimental Analysis. CNS Neurol. Disord. Drug Targets. 2012;11:897–906. doi: 10.2174/1871527311201070897. [DOI] [PubMed] [Google Scholar]
- 304.Perez X.A., Ly J., McIntosh J.M., Quik M. Long-Term Nicotine Exposure Depresses Dopamine Release in Nonhuman Primate Nucleus Accumbens. J. Pharmacol. Exp. Ther. 2012;342:335–344. doi: 10.1124/jpet.112.194084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Perez X.A., McIntosh J.M., Quik M. Long-Term Nicotine Treatment Down-Regulates Alpha6beta2* Nicotinic Receptor Expression and Function in Nucleus Accumbens. J. Neurochem. 2013;127:762–771. doi: 10.1111/jnc.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Bordia T., Campos C., McIntosh J.M., Quik M. Nicotinic Receptor-Mediated Reduction in L-DOPA-Induced Dyskinesias May Occur Via Desensitization. J. Pharmacol. Exp. Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bordia T., Campos C., Huang L., Quik M. Continuous and Intermittent Nicotine Treatment Reduces L-3,4-Dihydroxyphenylalanine (L-DOPA)-Induced Dyskinesias in a Rat Model of Parkinson’s Disease. J. Pharmacol. Exp. Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- 308.Bordia T., McGregor M., McIntosh J.M., Drenan R.M., Quik M. Evidence for a Role for α6(∗) Nachrs in L-Dopa-Induced Dyskinesias Using Parkinsonian α6(∗) nAChR Gain-of-Function Mice. Neuroscience. 2015;295:187–197. doi: 10.1016/j.neuroscience.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bordia T., McIntosh J.M., Quik M. The Nicotine-Mediated Decline in L-Dopa-Induced Dyskinesias Is Associated with a Decrease in Striatal Dopamine Release. J. Neurochem. 2013;125:291–302. doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Bordia T., Zhang D., Perez X.A., Quik M. Striatal Cholinergic Interneurons and D2 Receptor-Expressing Gabaergic Medium Spiny Neurons Regulate Tardive Dyskinesia. Exp. Neurol. 2016;286:32–39. doi: 10.1016/j.expneurol.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Huang L.Z., Campos C., Ly J., Ivy Carroll F., Quik M. Nicotinic Receptor Agonists Decrease L-Dopa-Induced Dyskinesias Most Effectively in Partially Lesioned Parkinsonian Rats. Neuropharmacology. 2011;60:861–868. doi: 10.1016/j.neuropharm.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Huang L.Z., Grady S.R., Quik M. Nicotine Reduces L-DOPA-Induced Dyskinesias by Acting at Beta2* Nicotinic Receptors. J. Pharmacol. Exp. Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Quik M., Campos C., Bordia T., Strachan J.P., Zhang J., McIntosh J.M., Letchworth S., Jordan K. Alpha4beta2 Nicotinic Receptors Play a Role in the nAChR-Mediated Decline in L-Dopa-Induced Dyskinesias in Parkinsonian Rats. Neuropharmacology. 2013;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Quik M., Campos C., Grady S.R. Multiple Cns Nicotinic Receptors Mediate L-Dopa-Induced Dyskinesias: Studies with Parkinsonian Nicotinic Receptor Knockout Mice. Biochem. Pharmacol. 2013;86:1153–1162. doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Quik M., Mallela A., Ly J., Zhang D. Nicotine Reduces Established Levodopa-Induced Dyskinesias in a Monkey Model of Parkinson’s Disease. Mov. Disord. 2013;28:1398–1406. doi: 10.1002/mds.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Quik M., Park K.M., Hrachova M., Mallela A., Huang L.Z., McIntosh J.M., Grady S.R. Role for Alpha6 Nicotinic Receptors in L-Dopa-Induced Dyskinesias in Parkinsonian Mice. Neuropharmacology. 2012;63:450–459. doi: 10.1016/j.neuropharm.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Quik M., Zhang D., Perez X.A., Bordia T. Role for the Nicotinic Cholinergic System in Movement Disorders; Therapeutic Implications. Pharmacol. Ther. 2014;144:50–59. doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Barreto G.E., Iarkov A., Moran V.E. Beneficial Effects of Nicotine, Cotinine and Its Metabolites as Potential Agents for Parkinson’s Disease. Front. Aging Neurosci. 2014;6:340. doi: 10.3389/fnagi.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Quik M., Bordia T., Zhang D., Perez X.A. Nicotine and Nicotinic Receptor Drugs: Potential for Parkinson’s Disease and Drug-Induced Movement Disorders. Int. Rev. Neurobiol. 2015;124:247–271. doi: 10.1016/bs.irn.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 320.Leino S., Koski S.K., Rannanpää S., Salminen O. Effects of Antidyskinetic Nicotine Treatment on Dopamine Release in Dorsal and Ventral Striatum. Neurosci. Lett. 2018;672:40–45. doi: 10.1016/j.neulet.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 321.Zhang D., McGregor M., Bordia T., Perez X.A., McIntosh J.M., Decker M.W., Quik M. A7 Nicotinic Receptor Agonists Reduce Levodopa-Induced Dyskinesias with Severe Nigrostriatal Damage. Mov. Disord. 2015;30:1901–1911. doi: 10.1002/mds.26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Zhang D., McGregor M., Decker M.W., Quik M. The Alpha7 Nicotinic Receptor Agonist ABT-107 Decreases L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys. J. Pharmacol. Exp. Ther. 2014;351:25–32. doi: 10.1124/jpet.114.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Di Paolo T., Grégoire L., Feuerbach D., Elbast W., Weiss M., Gomez-Mancilla B. AQW051, a Novel and Selective Nicotinic Acetylcholine Receptor α7 Partial Agonist, Reduces L-Dopa-Induced Dyskinesias and Extends the Duration of L-Dopa Effects in Parkinsonian Monkeys. Parkinsonism Relat. Disord. 2014;20:1119–1123. doi: 10.1016/j.parkreldis.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 324.Trenkwalder C., Berg D., Rascol O., Eggert K., Ceballos-Baumann A., Corvol J.-C., Storch A., Zhang L., Azulay J.-P., Broussolle E., et al. A Placebo-Controlled Trial of AQW051 in Patients with Moderate to Severe Levodopa-Induced Dyskinesia. Mov. Disord. 2016;31:1049–1054. doi: 10.1002/mds.26569. [DOI] [PubMed] [Google Scholar]
- 325.Mather J., Burdette C., Posener A. In Potential of Azd1446, a Novel Nicotinic Agonist, for the Treatment of L-DOPA-Induced Dyskinesia in Parkinson’s Disease, Society for Neuroscience Abstr, 2014; p L1
- 326.Zhang D., Bordia T., McGregor M., McIntosh J.M., Decker M.W., Quik M. ABT-089 and ABT-894 Reduce Levodopa-Induced Dyskinesias in a Monkey Model of Parkinson’s Disease. Mov. Disord. 2014;29:508–517. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Leino S., Koski S.K., Hanninen R., Tapanainen T., Rannanpaa S., Salminen O. Attenuated Dopaminergic Neurodegeneration and Motor Dysfunction in Hemiparkinsonian Mice Lacking the Alpha5 Nicotinic Acetylcholine Receptor Subunit. Neuropharmacology. 2018;138:371–380. doi: 10.1016/j.neuropharm.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 328.O’Gorman Tuura R.L., Baumann C.R., Baumann-Vogel H. Beyond Dopamine: GABA, Glutamate, and the Axial Symptoms of Parkinson Disease. Front. Neurol. 2018;9:806. doi: 10.3389/fneur.2018.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Błasczyk J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016;10:269. doi: 10.3389/fnins.2016.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Assini R., Abercrombie E.D. Zolpidem Ameliorates Motor Impairments in the Unilaterally 6-Hydroxydopamine-Lesioned Rat. Eur. J. Neurosci. 2018;48:1896–1905. doi: 10.1111/ejn.14075. [DOI] [PubMed] [Google Scholar]
- 331.Yang X., Zhao H., Shi H., Wang X., Zhang S., Zhang Z., Zu J., Zhang W., Shen X., Cui G., et al. Intranigral Administration of Substance P Receptor Antagonist Attenuated Levodopa-Induced Dyskinesia in a Rat Model of Parkinson’s Disease. Exp. Neurol. 2015;271:168–174. doi: 10.1016/j.expneurol.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 332.Thornton E., Hassall M.M., Corrigan F., Vink R. The Nk1 Receptor Antagonist N-Acetyl-L-Tryptophan Reduces Dyskinesia in a Hemi-Parkinsonian Rodent Model. Parkinsonism Relat. Disord. 2014;20:508–513. doi: 10.1016/j.parkreldis.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 333.Mabrouk O.S. Delta Opioid Pharmacology in Parkinson’s Disease. In: Jutkiewicz E.M., editor. Delta Opioid Receptor Pharmacology and Therapeutic Applications. Springer International Publishing; Cham, Switzerland: 2018. [DOI] [Google Scholar]
- 334.Huang J.-Z., Ren Y., Xu Y., Chen T., Xia T.C., Li Z.-R., Zhao J.-N., Hua F., Sheng S.-Y., Xia Y. The Delta-Opioid Receptor and Parkinson’s Disease. CNS Neurosci. Ther. 2018;24:1089–1099. doi: 10.1111/cns.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sgroi S., Tonini R. Opioidergic Modulation of Striatal Circuits, Implications in Parkinson’s Disease and Levodopa Induced Dyskinesia. Front. Neurol. 2018;9:524. doi: 10.3389/fneur.2018.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Freo U., Furnari M., Ori C. Effects of Tapentadol on Pain, Motor Symptoms and Cognitive Functions in Parkinson’s Disease. J. Pain Res. 2018;11:1849–1856. doi: 10.2147/JPR.S164939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Henry B., Fox S.H., Crossman A.R., Brotchie J.M. Mu- and Delta-Opioid Receptor Antagonists Reduce Levodopa-Induced Dyskinesia in the MPTP-Lesioned Primate Model of Parkinson’s Disease. Exp. Neurol. 2001;171:139–146. doi: 10.1006/exnr.2001.7727. [DOI] [PubMed] [Google Scholar]
- 338.Koprich J.B., Fox S.H., Johnston T.H., Goodman A., Le Bourdonnec B., Dolle R.E., DeHaven R.N., DeHaven-Hudkins D.L., Little P.J., Brotchie J.M. The Selective Mu-Opioid Receptor Antagonist Adl5510 Reduces Levodopa-Induced Dyskinesia without Affecting Antiparkinsonian Action in MPTP-Lesioned Macaque Model of Parkinson’s Disease. Mov. Disord. 2011;26:1225–1233. doi: 10.1002/mds.23631. [DOI] [PubMed] [Google Scholar]
- 339.Billet F., Costentin J., Dourmap N. Influence of Corticostriatal Δ-Opioid Receptors on Abnormal Involuntary Movements Induced by L-DOPA in Hemiparkinsonian Rats. Exp. Neurol. 2012;236:339–350. doi: 10.1016/j.expneurol.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 340.Hudzik T.J., Howell A., Payza K., Cross A.J. Antiparkinson Potential of Delta-Opioid Receptor Agonists. Eur. J. Pharmacol. 2000;396:101–107. doi: 10.1016/S0014-2999(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 341.Nozaki C., Le Bourdonnec B., Reiss D., Windh R.T., Little P.J., Dolle R.E., Kieffer B.L., Gaveriaux-Ruff C. Delta-Opioid Mechanisms for Adl5747 and ADL5859 Effects in Mice: Analgesia, Locomotion, and Receptor Internalization. J. Pharmacol. Exp. Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Spina L., Longoni R., Mulas A., Chang K.J., Di Chiara G. Dopamine-Dependent Behavioural Stimulation by Non-Peptide Delta Opioids BW373U86 and SNC 80: 1. Locomotion, Rearing and Stereotypies in Intact Rats. Behav. Pharmacol. 1998;9:1–8. [PubMed] [Google Scholar]
- 343.Cox H., Togasaki D.M., Chen L., Langston J.W., Di Monte D.A., Quik M. The Selective Kappa-Opioid Receptor Agonist U50,488 Reduces L-Dopa-Induced Dyskinesias but Worsens Parkinsonism in MPTP-Treated Primates. Exp. Neurol. 2007;205:101–107. doi: 10.1016/j.expneurol.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Potts L.F., Park E.S., Woo J.M., Dyavar Shetty B.L., Singh A., Braithwaite S.P., Voronkov M., Papa S.M., Mouradian M.M. Dual Κ-Agonist/Μ-Antagonist Opioid Receptor Modulation Reduces Levodopa-Induced Dyskinesia and Corrects Dysregulated Striatal Changes in the Nonhuman Primate Model of Parkinson Disease. Ann. Neurol. 2015;77:930–941. doi: 10.1002/ana.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Klintenberg R., Svenningsson P., Gunne L., Andren P.E. Naloxone Reduces Levodopa-Induced Dyskinesias and Apomorphine-Induced Rotations in Primate Models of Parkinsonism. J. Neural Transm. 2002;109:1295–1307. doi: 10.1007/s00702-002-0715-6. [DOI] [PubMed] [Google Scholar]
- 346.Fox S., Silverdale M., Kellett M., Davies R., Steiger M., Fletcher N., Crossman A., Brotchie J. Non-Subtype-Selective Opioid Receptor Antagonism in Treatment of Levodopa-Induced Motor Complications in Parkinson’s Disease. Mov. Disord. 2004;19:554–560. doi: 10.1002/mds.10693. [DOI] [PubMed] [Google Scholar]
- 347.Gomez-Mancilla B., Bedard P.J. Effect of Nondopaminergic Drugs on L-Dopa-Induced Dyskinesias in MPTP-Treated Monkeys. Clin. Neuropharmacol. 1993;16:418–427. doi: 10.1097/00002826-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 348.Johnston T.H., Versi E., Howson P.A., Ravenscroft P., Fox S.H., Hill M.P., Reidenberg B.E., Corey R., Brotchie J.M. DPI-289, a Novel Mixed Delta Opioid Agonist / Mu Opioid Antagonist (DAMA), Has L-DOPA-Sparing Potential in Parkinson’s Disease. Neuropharmacology. 2018;131:116–127. doi: 10.1016/j.neuropharm.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 349.Lowery J.J., Raymond T.J., Giuvelis D., Bidlack J.M., Polt R., Bilsky E.J. In Vivo Characterization of MMP-2200, a Mixed Delta/Mu Opioid Agonist, in Mice. J. Pharmacol. Exp. Ther. 2011;336:767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Su T.P., London E.D., Jaffe J.H. Steroid Binding at Sigma Receptors Suggests a Link between Endocrine, Nervous, and Immune Systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 351.Konitsiotis S. Novel Pharmacological Strategies for Motor Complications in Parkinson’s Disease. Expert Opin. Investig. Drugs. 2005;14:377–392. doi: 10.1517/13543784.14.4.377. [DOI] [PubMed] [Google Scholar]
- 352.Mishina M., Ishiwata K., Ishii K., Kitamura S., Kimura Y., Kawamura K., Oda K., Sasaki T., Sakayori O., Hamamoto M., et al. Function of Sigma1 Receptors in Parkinson’s Disease. Acta Neurol. Scand. 2005;112:103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 353.Francardo V., Bez F., Wieloch T., Nissbrandt H., Ruscher K., Cenci M.A. Pharmacological Stimulation of Sigma-1 Receptors Has Neurorestorative Effects in Experimental Parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- 354.Paquette M.A., Foley K., Brudney E.G., Meshul C.K., Johnson S.W., Berger S.P. The Sigma-1 Antagonist Bmy-14802 Inhibits L-DOPA-Induced Abnormal Involuntary Movements by a Way-100635-Sensitive Mechanism. Psychopharmacology. 2009;204:743–754. doi: 10.1007/s00213-009-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tsai S.Y., Pokrass M.J., Klauer N.R., De Credico N.E., Su T.P. Sigma-1 Receptor Chaperones in Neurodegenerative and Psychiatric Disorders. Expert Opin. Ther. Targets. 2014;18:1461–1476. doi: 10.1517/14728222.2014.972939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Verhagen-Metman L., Blanchet P.J., van den Munckhof P., Del Dotto P., Natté R., Chase T.N. A Trial of Dextromethorphan in Parkinsonian Patients with Motor Response Complications. Mov. Disord. 1998;13:414–417. doi: 10.1002/mds.870130307. [DOI] [PubMed] [Google Scholar]
- 357.Fox S.H., Metman L.V., Nutt J.G., Brodsky M., Factor S.A., Lang A.E., Pope L.E., Knowles N., Siffert J. Trial of Dextromethorphan/Quinidine to Treat Levodopa-Induced Dyskinesia in Parkinson’s Disease. Mov. Disord. 2017;32:893–903. doi: 10.1002/mds.26976. [DOI] [PubMed] [Google Scholar]
- 358.Johnston T.H., Geva M., Steiner L., Orbach A., Papapetropoulos S., Savola J.M., Reynolds I.J., Ravenscroft P., Hill M., Fox S.H., et al. Pridopidine, a Clinic-Ready Compound, Reduces 3,4-Dihydroxyphenylalanine-Induced Dyskinesia in Parkinsonian Macaques. Mov. Disord. 2018 doi: 10.1002/mds.27565. [DOI] [PubMed] [Google Scholar]
- 359.Parkinson J. An Essay on the Shaking Palsy. 1817. J. Neuropsychiatry. Clin. Neurosci. 2002;14:223–236. doi: 10.1176/jnp.14.2.223. discussion 222. [DOI] [PubMed] [Google Scholar]
- 360.Schaeffer E., Pilotto A., Berg D. Pharmacological Strategies for the Management of Levodopa-Induced Dyskinesia in Patients with Parkinson’s Disease. CNS Drugs. 2014;28:1155–1184. doi: 10.1007/s40263-014-0205-z. [DOI] [PubMed] [Google Scholar]