Abstract
Patient: Female, 5
Final Diagnosis: Enterovirus infection
Symptoms: Weakness in all 4 limbs
Medication: —
Clinical Procedure: Nerve decompression • neurolysis and nerve transfer
Specialty: Neurosurgery
Objective:
Rare disease
Background:
Acute flaccid myelitis is an emerging polio-like illness mostly affecting young children, characterized by rapid onset of extremity weakness and paralysis in 1 or more limbs. Certain viruses, including enteroviruses such as EV-68, EV-71, poliovirus, and West Nile virus, can cause this disorder. The largest known outbreak of EVD68 in the United States was in the summer of 2014, causing severe respiratory illness and acute flaccid myelitis, mainly in young children. Furthermore, the US Centers for Disease Control and Prevention noted an increase in the number of patients with clinical symptoms of acute flaccid myelitis in 2018, and 134 confirmed cases by December 2018 were reported in the USA.
Case Report:
The patient in our present study was a 5-year-old female who had significant weakness and paralysis in all 4 extremities due to acute flaccid myelitis. EV-D68 had caused this disorder in this patient in August 2014. Conservative management had not helped her condition. Specific areas of concern were both shoulders and biceps, and the femoral and peroneal nerves in both sides. Of these, the right shoulder function was the worst, at less than grade 3. The patient also had marked atrophy and weakness of the right quadricep muscles. The patient underwent surgical treatment and had steady improvements in all 4 extremity functional movements.
Conclusions:
We demonstrated that decompression, neurolysis, and nerve transfer surgical procedures can be used successfully to correct the paralyzed upper and lower extremity movements in acute flaccid myelitis patients.
MeSH Keywords: Decompression, Enterovirus Infections, Motor Activity, Nerve Transfer, Quadriplegia
Background
Acute flaccid myelitis (AFM) is an emerging polio-like illness mostly affecting young children, characterized by rapid onset of extremity weakness and paralysis in 1 or more limbs [1]. Certain viruses, including non-polio enteroviruses [2–6] such as EV-68, EV-71, poliovirus, and West Nile virus, are known to cause AFM. Several studies have reported on patients with AFM since its recent worldwide outbreak [2,3,5,7–22].
The US Centers for Disease Control and Prevention (CDC) noted an increase in the number of patients with clinical symptoms of AFM in August 2018 [1]. There had been 134 confirmed cases of AFM by December 2018 in the USA (December 6, 2018 news report in Nature). These viral infections also cause similar limb weakness and paralysis such as transverse myelitis and Guillain-Barre syndrome [23]. Guillain-Barre syndrome is an immune-mediated condition affecting peripheral nerves and nerve roots. Both motor and sensory functions are affected in Guillain-Barre syndrome affected patients, whereas there is only a minimal or no sensory loss in AFM patients [8]. There was no sensory loss in the patient in our study.
There is no specific treatment for patients with AFM. Neurologists recommend physical or occupational therapy for arm and leg functional movements. CDC and the experts in this field are working together to understand the long-term outcomes in AFM affected patients. Saltzman et al. [24] recently reported functional improvements after nerve transfer in 2 AFM patients. Here, we report the functional improvement of upper and lower extremities, after decompression and neurolysis and nerve transfer in a pediatric patient with acute flaccid myelitis.
Case Report
The patient in our case study was a 5-year-old female who had significant weakness and paralysis in all 4 extremities due to AFM. Conservative management did not help the patient, and there were ongoing weakness and instability of all 4 extremities. Specific areas of concern were both shoulders, biceps, and the femoral and peroneal nerves in both sides. Of these, the right shoulder function was the worst, at less than grade 3. The patient also had marked atrophy and weakness of the right quadricep muscles. An emerging and increased pathogenic enteroviral infection, possibly EV-D68 (non-polio enteroviruses) had caused this illness in this patient in August 2014. There was a temporal association of AFM with a nationwide outbreak of EV-D68 in the summer 2014. This was about 16 months before the patient presented to our clinic, and she had received only conservative treatment with physical therapy since that time. The patient underwent surgical treatment at our hospital and had stable improvements in all 4 extremity functional movements.
History of the patient’s illness and her hospital visit
The patient’s mother reported that the child had a mild cold on August 28, 2014. Then, the patient had severe headaches, fatigue, tremor in her hands, and woke up the next day with double vision and tongue deviated to the left. She was brought to the emergency department. Later, she was unable to move her right arm and her head fell to the right side, she could not walk, support her head, or sit up on her own. The patient was then taken to another hospital for further examinations and treatments.
Nerve conduction velocity and electromyography reports
Indications were cranial nerve deficits, extremity weakness, facial droop on the left upper and lower extremities, lethargy, and fever. Nerve conduction velocity and electromyography studies reported the following findings. 1) No evidence for diffuse peripheral neuropathy disorder affecting the motor or sensory nerves in the 4 extremities. 2) There was evidence of significant neurogenic axon degeneration of the right median nerve. There was also evidence of reduced amplitude of all motor responses from nerve conduction when recording distally in the extremities. 3) There was an active sign of denervation and axon degeneration in C5–C8 innervated muscles in the right upper extremity and in the L3–L4 innervated muscles in the right lower extremity. 4) Sensory functions were normal.
Nerve conduction velocity and electromyography study reports suggested an unusual central and proximal peripheral demyelinating disorder that was multifocal in its distribution. There was also evidence of focal axon degeneration at variable spinal levels that may involve anterior horn cell degeneration and was compatible with an acute motor neuron disorder that was multifocal.
Spine cervical magnetic resonance imaging
Findings
Nonspecific signal abnormality and mild swelling of the cervical spinal cord were detected on spine cervical magnetic resonance imaging.
Indications for surgery
Specific areas of concern were both shoulders, biceps, and the femoral and peroneal nerves in both sides. Of these, the right shoulder function was the worst, at less than grade 3, yet with some deltoid function that could support surgical reinnervation. The patient also had marked atrophy and weakness of the right quadricep muscles. Conservative management had not helped and there was ongoing weakness and instability of all 4 extremities.
Based on the findings of the upper trunk neurolysis, the lead author and the operating surgeon (RKN) conferred at this point with the child’s parent/guardian, who wished then to proceed with nerve transfer to the non-conducting elements of the right axillary nerve branch to the deltoid muscle. Conducting elements of that nerve were left intact.
Surgical procedures performed on the patient
Surgical procedures performed on the patient included: external and internal neurolysis of right and left anterior and posterior divisions of brachial plexus, and suprascapular nerve; right and left anterior scalene nerve release; external and internal neurolysis of right axillary and radial nerve; external and internal neurolysis of right and left femoral, deep and superficial peroneal nerves.
The patient was brought to the operating room and underwent general anesthesia. The bilateral necks and the right axilla and arm were prepped and draped in the usual sterile fashion. An incision was created in the right neck, and the brachial plexus was exposed in the usual fashion. Scarring was noted over the entire upper trunk and components. The suprascapular nerve was tested, then decompressed, externally and internally neurolysed.
Similarly, the posterior division of the upper trunk was externally and internally neurolysed. The anterior division of the upper trunk was externally and internally neurolysed. The anterior scalene muscle was partially released to relieve any compression over the upper trunk. Electrical testing confirmed improved conduction through all elements except the posterior division, which remained poor in conductivity although with some improvement. The skin was closed in 2 layers after antibiotic irrigation and hemostasis.
A new incision was created in the right posterior axillary fold. Contracture of the latissimus dorsi muscle was released. Dissection revealed the axillary nerve at the superomedial border of the triceps tendon. The deltoid elements were externally neurolysed, then separated with intraneural dissection and further subdivided into component fascicle groups. Each group was electrically tested and those with conducting nerve fibers were excluded from further dissection. Those that did not conduct were severed to await a triceps branch of the radial nerve for nerve transfer. The radial nerve was then neurolysed in the roof of the axilla. The axial vessels were retracted to access the nerve and its triceps branches. One branch was selected through internal neurolysis, measured for length, and severed as a donor for the previously chosen axillary group fascicles. The radial nerve fibers and the axillary nerve fibers were sutured together in a circumferential fashion using 9-0 epineurial stitches in strict microsurgical fashion and under high magnification. The axillary wound was closed in 2 layers after antibiotic irrigation and hemostasis.
An incision was created in the left neck and the brachial plexus was exposed in the usual fashion. Scarring was noted over the entire upper trunk and components. The suprascapular nerve was tested, then decompressed, externally and internally neurolysed. Similarly, the posterior division of the upper trunk was externally and internally neurolysed. The anterior division of the upper trunk was also externally and internally neurolysed. The anterior scalene muscle was partially released to relieve any compression over the upper trunk. Electrical testing confirmed improved the conduction through all elements. The skin was closed in 2 layers after antibiotic irrigation and hemostasis.
The drapes were taken down and the lower extremities were prepped and draped in the usual sterile fashion. A vertical incision was made in the right groin inferior to the palpated inguinal ligament and medial to the palpable femoral arterial pulse. The distal branches of the femoral nerve were isolated and dissected. Several branches were noted to arise from the distal trunk of the nerve. This complex underwent electrical testing then external and internal neurolysis, resulting in improved measured electrical conduction. The skin was closed in 2 layers after antibiotic irrigation and hemostasis.
An incision was created in the right knee inferior to the fibular neck. The peroneal nerve was dissected free and the superficial and deep branches were isolated. The deep branch underwent external neurolysis and internal neurolysis, followed by the superficial branch which also underwent external neurolysis and internal neurolysis. Electrical testing proved improvement in conduction of both branches. The skin was closed in 2 layers after antibiotic irrigation and hemostasis.
An incision was then created in the left knee inferior to the fibular neck. The peroneal nerve was dissected free and the superficial and deep branches were isolated. The deep branch underwent external and internal neurolysis, followed by the superficial branch which also underwent external and internal neurolysis. Electrical testing proved improvement in conduction of both branches. The skin was closed in 2 layers after antibiotic irrigation and hemostasis.
All wounds were covered with dry dressings. No complications occurred and no specimens were sent. The patient was awake, alert and extubated in stable condition following surgery.
Shoulder movements were assessed pre-operatively and post-operatively by evaluating video recordings of standardized movements according to the modified Mallet classification [25]. Ankle eversion, inversion and dorsiflexion, toe extension, flexion and plantar flexion were clinically evaluated by the lead author (RKN) [26] using the Medical Research Council (MRC) scale for muscle strength pre-operatively and 18 months after surgery.
Pre-operative evaluation of upper and lower extremities
The patient had severe atrophy and paralysis of the right shoulder. There were ongoing atrophy and paralysis of the right shoulder with MRC scale grade 2 function remaining, indicating useful muscle mass for a nerve transfer and neurolysis. There was weakness also in the entire left upper extremity with grade 3–3+ function. There were antigravity ankle movements throughout, but there was a partial steppage gait in the patient’s right leg. Patient’s left leg also had generalized weakness but had become her dominate leg. The patient reported that she could not walk up the stairs bilaterally and therefore would place her right leg on the step and use momentum to bring her left leg to the same step and repeated. The patient had no pain and was not on medication.
Post-operative functional improvements
There were stable improvements in all areas of the left and right upper extremities, except for right shoulder abduction (Table 1, Figure 1). The patient’s total Mallet score for right shoulder functions improved significantly from 19 to 23 (of 30) (P<0.03). Left shoulder and hand movements were close to normal. Patient’s walking speed has increased, and she was able to lift her right foot better when walking after 4 weeks of peroneal nerve decompression, micro-neurolysis and neuroplasty surgery. Functional recovery was remarkable in foot anti-gravity and walking gait after 18 months of surgery. Overall significant clinical improvement to MRC grade 4+ was achieved. There were no complaints with her left leg (Table 2, Figure 2).
Table 1.
Improvements in the right upper extremity modified Mallet functions.
| Mallet functions | Pre-op | Post-op |
|---|---|---|
| Shoulder abduction | 2 | 2 |
| External rotation | 3 | 4 |
| Hand to mouth | 4 | 5 |
| Hand to neck | 2 | 3 |
| Hand to spine | 4 | 4 |
| Supination | 4 | 5 |
| Total Mallet score | 19 | 23 |
| Mean Mallet score | 3.2 | 3.8 |
| STD | 1.0 | 1.2 |
| P value | <0.03 |
Figure 1.
Photographs and video stills of the patient performing modified Mallet functional movements. Upper panel: pre-operative pictures (A) abduction, (B) hand to spine, (C) hand to mouth, (D) supination. Lower panel: post-operative pictures (E) abduction, (F) external rotation, (G) hand to spine, (H) hand to neck, (I) hand to mouth, and (J) supination.
Table 2.
Improvements in the lower extremity functions.
| Functions | Pre-op | Post-op |
|---|---|---|
| Toe extension | 3 | 4+ |
| Toe flexion | 4 | 4+ |
| Plantar flexion | 4+ | 5 |
| Ankle eversion | 3 | 4+ |
| Ankle Dorsiflexion | 3 | 4+ |
| Ankle inversion | 4+ | 4+ |
| Mean foot function | 3.5 | 4+ |
Figure 2.
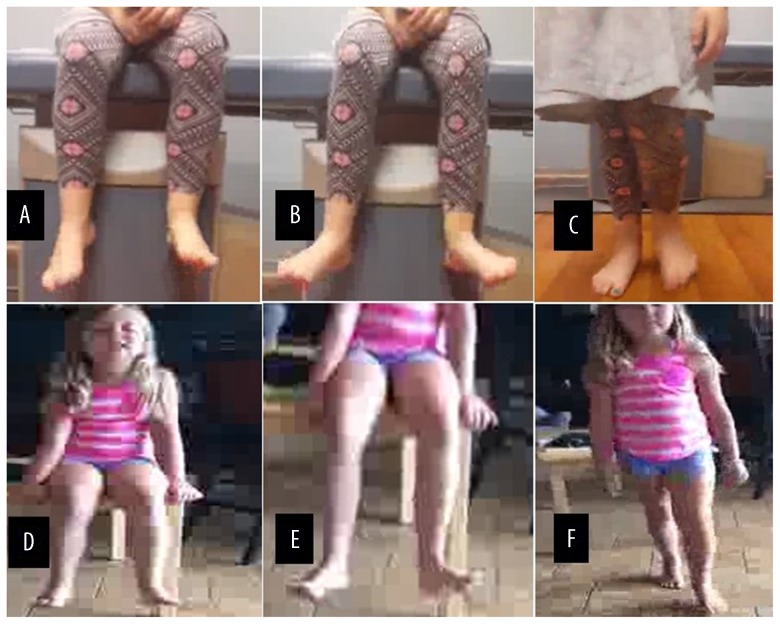
Video stills of the patient performing foot movements before and after peroneal nerve decompression and microneurolysis. Upper panel: (A, B) the patient was unable to dorsiflex the ankle, and (C) with steppage gait before surgery. Lower panel: (D, E) significant improvement in ankle dorsiflexion toe extension, and eversion, and (F) the patient was able to walk without slapping or tripping of the foot after peroneal nerve decompression and microneurolysis.
Discussion
Immunosuppressive therapy is ineffective for these patients. CDC and experts in this field are working together to develop an enteroviral vaccine. Martin et al. [21] found 3 of 12 children develop permanent extremity functional impairment in their study on outcomes of Colorado children with AFM. AFM symptoms are typically described as asymmetric motor weakness mostly affecting the upper limbs [5]. However, the patient in our case study had paralysis in both upper and lower limbs. It was still asymmetric, considering her right side was severely damaged in comparison to her left extremities.
Neurologists recommend physical or occupational therapy for upper and lower extremity movements. Nerve and muscle transfer procedures have been historically used for poliomyelitis associated paralysis [5]. Recently, nerve transfer was shown to improve muscle strength and range of motion in 2 pediatric AFM patients, who sustained upper extremity paralysis [24]. Similarly, another study [27] found that surgery was suitable in EV-D68-induced dysphagia patient; this patient had motor dysfunction but preserved sensory function.
We and other investigators have previously shown that decompression and neurolysis and nerve transfer have significantly improved both upper and lower extremity functional movements in brachial plexus injury (BPI), winging scapula, and foot drop patients [28–32]. We have used these surgical techniques successfully for the patient in this study report.
Conclusions
We demonstrate that decompression, neurolysis, and nerve transfer surgical procedures can be used successfully to correct the paralyzed upper and lower extremity movements in AFM patients.
Acknowledgments
We thank the patient and family who participated in this study.
References:
- 1.McKay SL, Lee AD, Lopez AS, et al. Increase in acute flaccid myelitis – United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:1273–75. doi: 10.15585/mmwr.mm6745e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliabadi N, Messacar K, Pastula DM, et al. Enterovirus D68 infection in children with acute flaccid myelitis, Colorado, USA, 2014. Emerg Infect Dis. 2016;22:1387–94. doi: 10.3201/eid2208.151949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messacar K, Abzug MJ, Dominguez SR. 2014 outbreak of enterovirus D68 in North America. J Med Virol. 2016;88:739–45. doi: 10.1002/jmv.24410. [DOI] [PubMed] [Google Scholar]
- 4.Messacar K, Abzug MJ, Dominguez SR. The emergence of enterovirus-D68. Microbiol Spectr. 2016;4(3) doi: 10.1128/microbiolspec.EI10-0018-2016. [DOI] [PubMed] [Google Scholar]
- 5.Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: A clinical review of US cases 2012–2015. Ann Neurol. 2016;80:326–38. doi: 10.1002/ana.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suresh S, Forgie S, Robinson J. Non-polio Enterovirus detection with acute flaccid paralysis: A systematic review. J Med Virol. 2018;90:3–7. doi: 10.1002/jmv.24933. [DOI] [PubMed] [Google Scholar]
- 7.Maloney JA, Mirsky DM, Messacar K, et al. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. Am J Neuroradiol. 2015;36:245–50. doi: 10.3174/ajnr.A4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millichap JG. Acute flaccid myelitis outbreak. Pediatr Neurol Briefs. 2015;29:96. doi: 10.15844/pedneurbriefs-29-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pabbaraju K, Wong S, Drews SJ, et al. Full genome analysis of enterovirus D-68 strains circulating in Alberta, Canada. J Med Virol. 2016;88:1194–203. doi: 10.1002/jmv.24444. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. 2015;20:21062. doi: 10.2807/1560-7917.es2015.20.10.21062. [DOI] [PubMed] [Google Scholar]
- 11.Schuster JE, Newland JG. Management of the 2014 enterovirus 68 outbreak at a pediatric tertiary care center. Clin Ther. 2015;37:2411–18. doi: 10.1016/j.clinthera.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Tan Y, Hassan F, Schuster JE, et al. Molecular evolution and intraclade recombination of enterovirus D68 during the 2014 outbreak in the United States. J Virol. 2016;90:1997–2007. doi: 10.1128/JVI.02418-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Haren K, Ayscue P, Waubant E, et al. Acute flaccid myelitis of unknown etiology in California, 2012–2015. JAMA. 2015;314:2663–71. doi: 10.1001/jama.2015.17275. [DOI] [PubMed] [Google Scholar]
- 14.Nelson GR, Bonkowsky JL, Doll E, et al. Recognition and management of acute flaccid myelitis in children. Pediatr Neurol. 2016;55:17–21. doi: 10.1016/j.pediatrneurol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August–December 2014: Results of nationwide surveillance. Clin Infect Dis. 2016;63:737–45. doi: 10.1093/cid/ciw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubner J, Kruse B, Christen HJ, et al. Acute flaccid myelitis in German children in 2016 – the return of polio? Dtsch Arztebl Int. 2017;114:551–57. doi: 10.3238/arztebl.2017.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helfferich J, Kingma EM, Meiners LC, et al. [Acute flaccid myelitis after a respiratory tract infection; first Dutch case related to enterovirus type D68 infection] Ned Tijdschr Geneeskd. 2017;161:D1566. [in Dutch] [PubMed] [Google Scholar]
- 18.Bonwitt J, Poel A, DeBolt C, et al. Acute flaccid myelitis among children – Washington, September–November 2016. MMWR Morb Mortal Wkly Rep. 2017;66:826–29. doi: 10.15585/mmwr.mm6631a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggieri V, Paz MI, Peretti MG, et al. Enterovirus D68 infection in a cluster of children with acute flaccid myelitis, Buenos Aires, Argentina, 2016. Eur J Paediatr Neurol. 2017;21:884–90. doi: 10.1016/j.ejpn.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Cabrerizo M, Garcia-Iniguez JP, Munell F, et al. First cases of severe flaccid paralysis associated with enterovirus D68 infection in Spain, 2015–2016. Pediatr Infect Dis J. 2017;36:1214–16. doi: 10.1097/INF.0000000000001668. [DOI] [PubMed] [Google Scholar]
- 21.Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017;89:129–37. doi: 10.1212/WNL.0000000000004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holm-Hansen CC, Midgley SE, Fischer TK. Global emergence of enterovirus D68: A systematic review. Lancet Infect Dis. 2016;16:e64–75. doi: 10.1016/S1473-3099(15)00543-5. [DOI] [PubMed] [Google Scholar]
- 23.Pavlik D, Huber M, Justice M. A young woman with numbness in her feet. JAAPA. 2016;29:38–40. doi: 10.1097/01.JAA.0000508204.02557.9a. [DOI] [PubMed] [Google Scholar]
- 24.Saltzman EB, Rancy SK, Sneag DB, et al. Nerve transfers for enterovirus D68-associated acute flaccid myelitis: A case series. Pediatr Neurol. 2018;88:25–30. doi: 10.1016/j.pediatrneurol.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Mallet J. [Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Priority for the treatment of the shoulder. Method for the expression of results] Rev Chir Orthop Reparatrice Appar Mot. 1972;58(Suppl. 1):166–68. [in French] [PubMed] [Google Scholar]
- 26.Nath RK, Lyons AB, Paizi M. Successful management of foot drop by nerve transfers to the deep peroneal nerve. J Reconstr Microsurg. 2008;24:419–27. doi: 10.1055/s-0028-1082894. [DOI] [PubMed] [Google Scholar]
- 27.Togashi T, Baba H, Kitazawa M, et al. Surgical treatment of enterovirus D68 brainstem encephalitis-induced dysphagia. Auris Nasus Larynx. 2018;45:1093–97. doi: 10.1016/j.anl.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Nath RK, Kumar N, Somasundaram C. Modified Quad surgery significantly improves the median nerve conduction and functional outcomes in obstetric brachial plexus nerve injury. Ann Surg Innov Res. 2013;7:5. doi: 10.1186/1750-1164-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nath RK, Paizi M. Improvement in abduction of the shoulder after reconstructive soft-tissue procedures in obstetric brachial plexus palsy. J Bone Joint Surg Br. 2007;89:620–26. doi: 10.1302/0301-620X.89B5.18403. [DOI] [PubMed] [Google Scholar]
- 30.Nath RK, Lyons AB, Bietz G. Microneurolysis and decompression of long thoracic nerve injury are effective in reversing scapular winging: Long-term results in 50 cases. BMC Musculoskelet Disord. 2007;8:25. doi: 10.1186/1471-2474-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broekx S, Weyns F. External neurolysis as a treatment for foot drop secondary to weight loss: A retrospective analysis of 200 cases. Acta Neurochir (Wien) 2018;160:1847–56. doi: 10.1007/s00701-018-3614-9. [DOI] [PubMed] [Google Scholar]
- 32.Wilson TJ, Maldonado AA, Amrami KK, et al. The anatomic location and importance of the tibialis posterior fascicular bundle at the sciatic nerve bifurcation: Report of 3 cases. J Neurosurg. 2018 doi: 10.3171/2018.8.JNS181190. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]