Abstract
Synergy is a process in which some substances cooperate to reach a combined effect that is greater than the sum of their separate effects. It can be considered a natural “straight” strategy which has evolved by nature to obtain more efficacy at low cost. In this regard, synergistic effects may be observed in the interaction between herbal products and conventional drugs or biochemical compounds. It is important to identify and exploit these interactions since any improvement brought by such kind of process can be advantageously used to treat human disorders. Even in a complex disease such as cancer, positive synergistic plant–drug interactions should be investigated to achieve the best outcomes, including providing a greater benefit to patients or avoiding adverse side effects. This review analyzes and summarizes the current knowledge on the synergistic effects of plant–drug interactions with a focus on anticancer strategies.
Keywords: synergy, synergistic effects, anticancer, plant-drug interaction, conventional chemotherapeutic agents, plant derivatives
1. Introduction
Multidrug therapy is a helpful strategy focused on the direct blocking or killing of damaging agents (such as cancer cells or pathogens) as well as on the activation of human body defenses or repair mechanisms. It derives from a gradual withdrawal of the previously adopted dogma of mono-drug therapy; for decades, pharmacological research was based on the identification of a single active principle [1]. As regards phytotherapy research, only recently have the contributions of traditional Chinese medicine, Ayurveda and traditional Western phytomedicines begun to be scientifically confirmed and appreciated. Furthermore, during the last 20 years, the world has encountered an increasing rate of the use of conventional medicines combined with complementary and alternative medicine (CAM), which are represented not only by homeopathy, naturopathy, chiropractic, and energy medicine, among others, but also ethnopharmacology and phytotherapy [2]. It is becoming evident that many diseases have a multifaceted etiology, which could be treated more successfully with a drug combination strategy than a single administration. In Western countries, for multifactorial or complex disease treatment (e.g., cancer, hypertension, metabolic and inflammatory diseases, acquired immune deficiency syndrome (AIDS) and infections), an effective multidrug therapy is commonly adopted [3]. In this context, phytotherapy and ethnopharmacology play a key “natural” role, as they find their effectiveness from herbs or plants, which are “secundum naturam”, a complex pool of millions of molecules. Of note, human pharmacotherapy started with the use of plants in ancient times, probably imitating animal self-medication, while first written documents date back to the third millennium with the Sumerian empire [4].
Because of the enormous popularity of CAM (including ethnopharmacology and phytotherapy), it is necessary to focus our attention on the risk–benefit profile of herbal remedies and updated information. Thus, the primary aim of this short review is to provide an overview of plant–drug interactions, with a special update on the synergistic anticancer effects between herbal medicines and conventional chemotherapeutic drugs, relying on recently published works and systematic reviews following the guidelines of Pautasso [5]. The literary search strategy was done interrogating PubMed, Web of Knowledge, Google Scholar and ScienceDirect databases, selecting the following keywords: “herb (s)”, “plant (s)”, “phyto*”, “drug (s)”, “medicine”, “interaction (s)”, “cancer”, “tumour (tumor)”, and “chemotherapy”. Algae derivatives or extracts were intentionally excluded, as their investigation would require a dedicated review.
2. Synergy
Paying attention to the quote from Buckminster Fuller (1968), “Universe is synergetic. Life is synergetic” [6], synergy is broadly defined as the interaction or cooperation of two or more substances, organizations or other agents to produce a combined effect greater than the sum of their separate portions [7]. Synergy comes from the Greek word “synergos”, which means “working together.” More precisely, synergism or synergy according to the McGraw–Hill Concise Dictionary of Modern Medicine is indicated as “the cooperative interaction between two or more components of a system, such that the combined effect is greater than the sum of each part”. In anatomy, it is the combined action of muscle groups which results in a force greater than that which could be generated by the individual muscles, while in pharmacology, synergism is described as an approach to multidrug-resistant bacterial infections or virulent malignancies, among others, in which the use of therapeutic agents may affect different pathways, making the treatment more efficient [8]. Besides this, Mosby’s Dictionary of Complementary and Alternative Medicine defines synergism or pharmacological synergy as the effect of combined components interacting to produce new and different effects than individual components, referring typically to the action of whole plants, as opposed to the active constituents in isolation [9]. As the above statements clearly show, the definition of synergy may overlap to potentiation, a term that means a synergistic action, in which the effect of two drugs administered simultaneously is greater than the sum of the effects of each drug given separately [9]. Nevertheless, a concrete definition derives only from a mathematical approach, demonstrated and proven by pioneering works of Berenbaum, that provided the basis for its use in pharmacology and phytopharmacology [10,11,12]. Of note, the isobole method of Berenbaum is not a unique method that emerged from applied sciences; different methodologies have been developed to understand drug–drug interactions, such as the isobologram method of Loewe [13], the fractional product method of Webb [14] and the combination index method of Chou and Talalay [15].
3. The Isobole Method
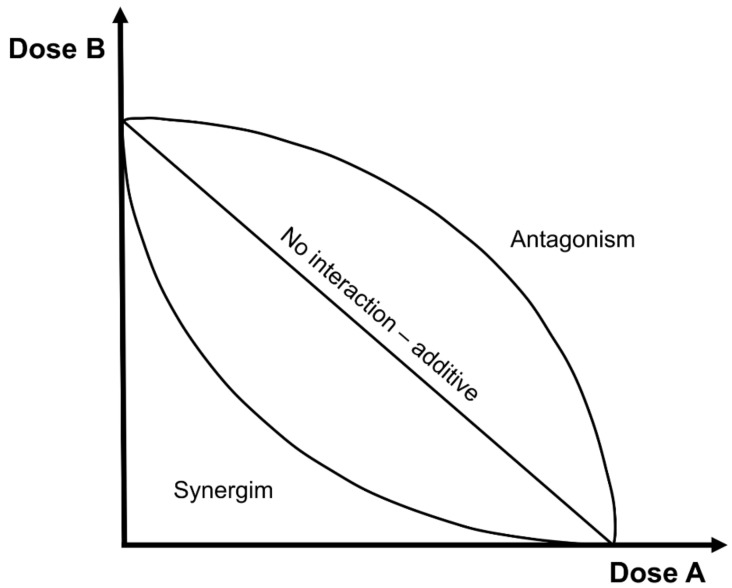
The isobole method is the most convenient and experimentally suitable method for evaluating synergistic effects. This method is represented by a graph in which a curve is constructed by plotting different drug dose combinations; in the x-axis, the dose rates of the first given drug or are is plotted (X-compound dose), while in the y-axis, the dose rates of the second given drug or compound are imposed (Y-compound dose) (Figure 1).
Figure 1.
Representation of the isobole method.
The points derived from the combination of the two dose rates lie on three different isobole curves. Briefly, an isobole is an “iso-effect” curve, in which a combination of compounds is represented on a graph, as follows:
-
(1)
No interaction curve: according to Berenbaum, this is an additive curve (straight line), in which the effects of 2 compounds are the simple sum of the single effects, and they do not interact.
-
(2)
Synergistic curve: this concave-shaped curve represents the real synergism (or potentiation), where the effects of 2 compounds are more than the simple sum of the single effects when the 2 compounds are given together.
-
(3)
Antagonistic curve: this convex shape curve represents the opposite effects of synergism; i.e., the effects of 2 compounds are less than the simple sum of the single effects.
In mathematical terms, three equations may be derived:
| OE (da, db) = OE (da) + OE (db) | (1) |
| OE (da, db) > OE (da) + OE (db) | (2) |
| OE (da, db) < OE (da) + OE (db) | (3) |
where OE means the observed effects, while da and db are the doses of the X-compound and Y-compound, respectively. Note that the isobole method is independent of the mechanism of action of drugs and may apply to most circumstances. Furthermore, it is possible to obtain a synergistic effect at one specific dose combination and antagonistic effects at another, even with the same compounds, and certainly, this fact may complicate the isobole curve with a wave-like or non-homogeneous shape. When a synergistic effect is observed, the number of compounds to use is less than expected, and this turns to dose reduction, a goal that should be pursued when considering the clinical management of human diseases (i.e., the reduction of adverse effects and of costs for expensive drugs).
4. Mechanical Bases of Synergistic Effects
From a scientific point of view, it is not sufficient to know that a synergistic effect exists. Pharmacological and clinical research has led to the conception that at least four mechanisms may be involved.
4.1. Synergistic Multi-Target Effects
A compound, a mixture or a plant extract may act not only, as expected, on a single target, but may impact on different targets; i.e., all functional or structural cell constituents, such as metabolites, receptors, enzymes, ion channels, transporters, nucleic acids, ribosomes, and proteins [16].
4.2. Modulation of Pharmacokinetic or Physicochemical Effects
Synergistic effects may also impact on the physicochemical properties, including solubility, of a compound or mixture, providing an improvement of the so-called bioavailability [17].
4.3. Interference with Resistance Mechanisms
Synergistic effects may be observed on drug-resistant microorganisms (bacteria, fungi) or cancer cells, thanks to the presence of natural derivatives that may antagonize the development of drug resistance (supplied together with antibiotics or cancer drugs) [18].
4.4. Elimination or Neutralization Potential
A compound or a plant extract may have the ability to remove or neutralize the toxic effect of a drug (synthetic or not), even reducing or nullifying its adverse effects and resulting in treatment amelioration.
With no comprehensive claims, the possible mechanisms of synergistic effects are summarized in Table 1. However, it is of note that the most commonly reported mechanism in the literature is synergistic multi-target effects.
Table 1.
Literature examples of the four mechanisms involved in synergistic effects.
| Mechanism | Plants Involved | References |
|---|---|---|
| Synergistic multi-target effects | Herbal pair Chuanxiong rhizome and Paeonia albifora | [19] |
| Ocimum sanctum flavonoid vicenin-2 | [20] | |
| Cannabis extract delta9-trans-tetrahydrocannabinol | [21] | |
| St. John’s wort (Hypericum perforatum) | [22] | |
| Pharmacokinetic or physicochemical effect modulations | Yin-Chen-Hao-Tang (YCHT), a Chinese herbal formula (Herba artemisiae Yinchenhao + fructus gardeniae gasminoidis + radix et rhizoma rhei) | [23] |
| Ammi visnaga aqueous extract | [24] | |
| Hypericum perforatum flavonoids | [17] | |
| Grapefruit juice (Citrus × paradise) | [25] | |
| Panax ginseng | [26] | |
| Interference with resistance mechanisms | Seven commercially available terpenoids | [27] |
| Three commercially available flavonoids (apigenin, quercetin, naringenin) | [28] | |
| Pelargonium graveolens essential oil | [29] | |
| Nine herbal extracts and 23 isoflavonoids | [30] | |
| Elimination or neutralization potential | Ocimum basilicum constituent nevadensin | [31] |
| PHY906, a mixture of Scutellaria baicalensis, Glycyrrhiza uralensis, Paeonia lactiflora, Ziziphus jujube | [32] | |
| Silybum marianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) extracts | [33] |
5. An Introduction to Generic Plant–Drug Interactions
5.1. The Social Problem
Drug interaction is a well-known clinical issue. With the growing number of newly discovered diseases (in the last 50 years), the simultaneous elongation of life expectancy and the detection capacity of new medical technologies has increased the number of prescribed therapies, and this problem should not be undervalued. Besides, if we consider drug interaction as the interaction between pharmacological substances and herbal products or plant extracts (for example, self-prescribed natural products), one could argue that only the tip of the iceberg is emerging, although a huge hidden problem exists. In a larger perspective, it has been estimated that more than 80% of people in Africa and Asia and 60–70% of the American population are using herbal medicines, together with an increasing number in developed countries [2]. Moreover, the amount of related business is increasing day after day. According to the 2007 National Health Interview Survey (NHIS), US adults spent $33.9 billion out of pocket on CAM (in the previous year), and this amount was equivalent to 1.5% of total US health care expenditure and 11.2% of out-of-pocket spending in 2007 [34]. Therefore, the use of plant extracts or natural compounds across the entire world seems to be expected to increase in the next few years and, consequently, the risk of natural products–drug interaction remains challenging.
A critical approach to herbal–drug interactions, adverse effects, and clinical efficacy should be always borne in mind when recommending or taking botanical products, with special attention on serious adverse events [35]. Sometimes, professionals forget that a substance being natural does not mean that it is automatically harmless or innocuous (as many non-professionals believe). Another point to consider is the high variability in plant–drug interactions, due to the distinct methods of preparation or natural product extraction techniques, which are often not standardized and in some countries are not under appropriate regulatory controls.
Marked plant–drug interactions are derived from physiological and pathological individual differences, such as inter-individual variances in drug metabolism, age, gender and the presence of co-morbidities in elderly patients [36]. It is worth mentioning that it is not easy to discern when a patient uses a conventional drug together with a plant-derived compound. Only through a patient interview is some necessary information accessible, as reported by different works [37,38].
5.2. Most Common Examples of Detrimental Interactions
The vast majority of natural products and drugs are administered orally, which is a comfortable and easy way to administer a substance. The efficacy of compounds relies on oral bioavailability, which in turn depends on formulation design, biochemical properties, and gastrointestinal system pathophysiology [39]. In this context, the preclinical model Caco-2 cells have been investigated in plant–drug interactions, considering drug bioavailability [40]. Caco-2 cells can predict the probable gastrointestinal permeability of drugs. Indeed, they express cytochrome P450 enzymes and transporters typical of human small intestine enterocytes. For these reasons, Caco-2 cells represent a good screening tool in plant–drug interactions, where they are becoming more attractive [41]. Furthermore, an interesting attempt has been made regarding their ability to inhibit the membrane solute carrier (SLC) family, that together with ATP-binding cassette (ABC) transporters makes up the plasma membrane transporters [42]. The medicinal plant Salvia miltiorrhiza L., which consists of lithospermic acid (LSA), rosmarinic acid (RMA), salvianolic acid A (SAA), salvianolic acid B (SAB), and tanshinol (TSL), components of the herbal medicine Danshen, has been assessed regarding the function of different organic anion transporters (OATs) that belong to SLC transporters. The authors demonstrated a strong interaction potential for RMA and TSL, where both act on hOAT1 and hOAT3: in addition the authors showed that LSA could act on hOAT3 [43]. Similar results were obtained in a recent work, in which the organic anion transporting polypeptides (OATP) family was investigated (OATP derives from the SLC family and may transport organic anions through the plasma membrane). The researchers found that the flavonoids apigenin, kaempferol, and quercetin may inhibit OATP1A2 and OATP2B1-mediated drug uptake (i.e., atorvastatin and fexofenadine) in HEK293 cells [44], and as flavonoids may reach high concentrations in the gastrointestinal tract, a real plant–drug interaction may exist.
St John’s wort (Hypericum perforatum) is one of the most common natural remedies for depression. It has been demonstrated that St John’s wort is a potent inducer of CYP3A4 and P-glycoprotein (P-gp), influencing the blood concentrations of several drugs, such as amitriptyline, cyclosporine, digoxin, fexofenadine, indinavir, methadone, midazolam, nevirapine, phenprocoumon, simvastatin, and theophylline, whereas it did not alter the pharmacokinetics of carbamazepine, dextromethorphan, mycophenolic acid, and pravastatin. Many other relevant clinical interactions have been observed, such as with warfarin, verapamil, tacrolimus, and so on (for a comprehensive review, see [45]). Also, Ginkgo plant has been intensively studied; several reports showed effects of bleeding after assumption of Gingko biloba extract with simultaneous use of aspirin or warfarin [46]. In another case, Ginkgo biloba negatively influenced the effect of the antiretroviral drug, Efavirenz (EFV), in an HIV-infected male patient, probably interacting with the EFV metabolism enzymes CYP2B6 and CYP3A4 [47]. Grapefruit juice is another botanical extract that may affect the bioavailability of different drugs ingested with it, such as statins, antihypertensives, and antiretrovirals. This juice causes important drug interactions through CYP3A inhibition, to the extent that the Food and Drug Administration (FDA) warned physicians about the potential and demonstrated interaction of drugs with grapefruit [48].
5.3. Most Common Examples of Beneficial Interactions
Not all plant–drug interactions are detrimental. Auspiciously, concrete cases may demonstrate a positive interaction between a plant or herbal product and a conventional drug, which results in a potential increase in drug efficacy or in a possible reduction of adverse effects. For example, the Chinese medicinal plant Tripterygium wilfordii has been shown to have immunosuppressive activity by prolonging heart and kidney allograft survival, exhibiting synergy with cyclosporine in a rat model [49]. Moreover, Acacia confusa bark extract and its active compound gallic acid were used in a rat model of liver injury, and demonstrated hepatoprotective effects due to antioxidant enzyme modulation, lipid peroxidation inhibition and CYP2E1 activation [50]. Positive effects were also observed in rats co-administrated with herbal medicines and metronidazole. The authors used natural antimalarial products isolated from Nauclea lafifolia (Nifadin, Niprisan, and Niprd/92/001/1-1) and found an increase in the serum concentration of metronidazole, thus enhancing the antibiotic potential [51]. In a rat model, garlic (250 mg/kg) combined with captopril demonstrated a synergistic interaction in diminishing blood pressure and inducing angiotensin-converting enzyme (ACE) inhibition [52]. Moreover, a 3-month randomized, double-blind, clinical trial was conducted in beta-thalassemia major patients receiving silymarin, a flavonolignan complex isolated from Silybum marianum, in combination with conventional desferrioxamine therapy [53]. The authors showed beneficial results on thalassemia, especially in the treatment of iron-load effects. Another study investigated the concomitant administration of three first-line antiretroviral drugs (lamivudine, stavudine, and nevirapine) and a plant Andrographis paniculata, used as an immunostimulant. The researchers showed a beneficial anti-anorexia effect, as well as the modulation of hematological and biochemical indices, with erythrocytes and leucocytes increasing without an associated increase in cholesterol and high-density lipoproteins levels [54].
5.4. Controversial and Incomplete Data on Plant–Drug Interactions
Alongside detrimental and beneficial interactions, the vast majority of plant–drug interactions fall into this category, whereas the incomplete data represent the issue we need to face and solve in the near future. Besides this, almost all previously cited works are based on preclinical studies, so that caution should be taken when considering the administration or self-administration of natural products in humans. Consequently, it is crucial to distinguish basic research, with no patient inclusion in which all incomplete data interactions fall, from a clinical trial or meta-analysis, where the results should be regarded as more reliable. In this last case, many examples exist and are frequently associated with detrimental interactions (for a comprehensive review, see [35]). However, controversial results do exist: an example is a ginger, the rhizome of Zingiber officinale, a plant traditionally used in the digestive system for its antispasmodic and anti-nausea properties [55]. It has been shown that ginger may interfere with the anticoagulant warfarin, as well as ginkgo, but a strong demonstration is still lacking, and some works have reported that ginkgo and ginger (at recommended doses) did not affect clotting status [56,57].
These short reports about beneficial, detrimental, controversial and incomplete data regarding plant–drug interactions are not exhaustive, and they are outside of the scope of this review. They are merely included to show the extent to which the picture is still complex. For a more detailed discussion, see the works of Liu, et al. [58], and Izzo [59].
6. Plant–Drug Interaction: Classical Chemotherapeutic Agents and Plant Derivatives
A cancer cell is broadly conceived as a biological entity characterized by continuous uncontrolled growth and abnormal proliferation. This kind of cell may generate a tumor, an irregular mass of tissue, which may be solid or not, that can be differentiated as malignant or benign forms [60]. While the benign form is usually non-progressive and not harmful, malignant entities may grow quickly, invade and metastasize, and potentially result in death. Indeed, cancer is the second-leading cause of death in the USA and developed countries, pushing many researchers and physicians to work hard to fight against cancer [61]. In this context, it is important to use multiple strategies to achieve clinical success, to impact on human survival and to ameliorate life quality. One option is phytomedicine, which can potentially have beneficial effects when properly associated with conventional drugs, both as a preventive approach or targeted-oriented strategy.
6.1. The Synergy between Classical Chemotherapeutic Agents and Plant Derivatives
As mentioned above, the synergistic effects between plant-derived bioactives and conventional chemotherapeutic agents may lie in their resistance mechanisms, namely the ability of natural derivatives to antagonize drug resistance or to enhance drug properties, or possibly in the mitigation of side effects.
Several plant-derived products have been studied in association with conventional drugs to find a synergistic effect, and this review attempts to summarize the progress reported to date. It is of note that 20–30% of frequently used chemotherapeutics derive from plants [62].
We may subdivide literature works into three partially overlapping categories: essential oil derivatives, polyphenol derivatives, and a more general class of biochemical plant derivatives. This review focuses on the synergistic combination of the most often used classical chemotherapeutic agents, such as paclitaxel, docetaxel, irinotecan, topotecan, vinblastine, doxorubicin, 5-fluorouracil, mitomycin C and cisplatin with plant-derived bioactives. Synergistic combination requires the use of two compounds together, i.e., association in the same experiment (mixing) of a chemotherapeutic agent and plant derivatives, and not simply the comparison or the use of substances in parallel.
6.1.1. Essential Oil Derivatives
Essential oils are complex mixtures of molecules that belong to two different biosynthetic families, phenylpropanoids and terpenoids, but only the latter (which is volatile) constitutes the main components of essential oils [63]. Different works have shown the significant anticancer effects of plant products in combination with chemotherapy agents. This review analyzed the most common and purchasable essential oils used worldwide and found in the literature, such as geraniol, β-elemene, eugenol, β-caryophyllene, d-limonene, and thymoquinone.
In 2011, geraniol was reported to be effective in diminishing tumor mass volume (70% compared to control), acting on cell cycle and apoptosis pathways and enhancing docetaxel chemosensitivity. This essential oil was associated with docetaxel or taxotere in a xenograft mouse model of PC-3 prostate cancer cells [64]. The platinum-resistant ovarian cancer cell line A2780/CP70 cells were treated with β-elemene, a sesquiterpene derived from a variety of plants, plus a taxane, and resulted in a striking reduction in cell viability and increased cell apoptosis [65]. Moreover, β-elemene markedly inhibited cell growth and proliferation and increased cisplatin cytotoxicity in human bladder cancer 5637 and T-24 cells. It also enhanced cisplatin sensitivity and amplified cisplatin cytotoxicity in in vitro cell models (such as brain, cervix, breast, colorectal, ovary, and small-cell lung cancer) [65,66]. Eugenol is an essential oil extracted from clove, nutmeg, cinnamon, basil, and bay leaf, while sulforaphane is a compound obtained from cruciferous vegetables, such as broccoli, Brussels sprouts or cabbages. Both were tested together with gemcitabine in HeLa cervical cancer cells and showed no significant cell death increase, indicating that cell cytotoxicity was proportional to gemcitabine alone [67]. Moreover, cisplatin combined with methyl eugenol significantly enhanced the anticancer activity of the drug against HeLa cells, acting on cell apoptosis induction, caspase-3 activity, cell cycle arrest and mitochondrial membrane potential loss [68]. β-caryophyllene, a sesquiterpene present in essential oils of various plants, and paclitaxel were tested against MCF-7 (human breast cells), DLD-1 (human colon cells) and L-929 (mouse fibroblasts) tumor cell lines. β-caryophyllene potentiated the anticancer activity of paclitaxel on those cell lines, probably facilitating the passage of paclitaxel through the plasma membrane [69]. β-caryophyllene oxide was also combined with doxorubicin in 2 ovarian cancer cell lines (sensitive A2780 and partly resistant SKOV3) and 2 lymphoblast cancer cell lines (sensitive CCRF/CEM and completely resistant CEM/ADR) [70]. The authors claimed that doxorubicin acted synergistically with β-caryophyllene oxide in SKOV3 and CCRF/CEM cells, while no effect was appreciable on CEM/ADR resistant cells. Similar to the previous work, β-caryophyllene and β-caryophyllene oxide were evaluated for their potential chemosensitizing properties in Caco-2, CCRF/CEM, and CEM/ADR5000 cancer cell lines in association with doxorubicin [70]. The study reported that both sesquiterpenes interfered with the ABC pump function, enhancing doxorubicin cytotoxicity by increasing its intracellular accumulation and oxidative stress status. Moreover, d-limonene, a cyclic terpene obtained commercially from citrus fruits, was used in human prostate carcinoma DU-145 and normal prostate epithelial PZ-HPV-7 cells in combination with docetaxel. The synergistic effects resulted in higher reactive oxygen species (ROS) generation, glutathione depletion and in caspase activity increase [71]. Thymoquinone, a constituent of the Nigella sativa essential oil, has been studied in its association with doxorubicin on human cells of HL-60 leukemia, 518A2 melanoma, HT-29 colon, KB-V1 cervix, and MCF-7 breast carcinomas. Thymoquinone improved the anti-neoplastic properties of doxorubicin by inhibiting cancer cell growth [72]. Thymoquinone was also combined with paclitaxel to study the genetic networks involved in their actions using pathway-focused real-time protein chain reaction (PCR) panels, which investigated apoptosis, cell proliferation, growth factor activity and cytokine activity [73]. The work proved that in triple-negative breast cancer cells, thymoquinone caused cytotoxicity and apoptosis increase, and wound healing inhibition, besides sensitizing cancer cells to paclitaxel through extrinsic apoptosis, tumor suppressor genes, and p53 signaling. The synergistic effects were demonstrated in mice breast cancer models, as cancer cell growth decreased. Besides, thymoquinone was associated with docetaxel in DU-145 hormone- and drug-refractory prostate cancer cells, inducing significant synergistic cytotoxicity and apoptosis through the PI3K/Akt signaling pathway [74]. Similarly, thymoquinone enhanced cisplatin- and docetaxel-induced cytotoxicity in 2 triple-negative breast cancer cell lines (with mutant p53) [75], again underlining the synergistic effects of thymoquinone. Two independent works of the same group used irinotecan (CPT-11)-resistant LoVo colon cancer cells to study the effects of thymoquinone, and both demonstrated as this phytochemical can modulate JNK (c-Jun N-terminal kinase) and p38 pathways affecting ERK1/2, PI3K or mitochondrial outer membrane permeability and the autophagy process [76,77]. Also, topotecan was used in combination with thymoquinone in acute myelogenous leukemia cells. The combination regimen (which was better with a pre-exposure) reduced cell proliferation with an increase in Bax/Bcl2, p53, and caspase-3 and -9 expression levels [78]. Similarly, in human colorectal cancer cells, thymoquinone synergistically increased topotecan effectiveness with p53- and Bax/Bcl2-independent mechanisms, by decreasing proliferation and lowering cytotoxicity [79].
6.1.2. Polyphenol Derivatives
Polyphenols are natural substances characterized by the presence of multiple phenol structural units with the ability to modulate oxidative stress in cancer cells [80]. A wide variety of studies have investigated the anticancer effects of plant products in combination with conventional chemotherapy drugs. This review focused on the most common polyphenols reported in the literature, such as resveratrol, genistein, curcumin and quercetin.
In cell models of acute myeloid leukemia, resveratrol (a stilbene that is present in grapes, wine, peanuts, soy, and many other products) [81] induced cell growth arrest and apoptotic death in doxorubicin-resistant AML (Acute Myeloid Leukemia) cells and downregulated MRP1 (ABCC1 gene codifying for ABC transporter) expression. Three doxorubicin-resistant AML cell lines (AML-2/DX30, AML-2/DX100, AML-2/DX300) were usedk suggesting that resveratrol could overcome doxorubicin resistance or sensitize doxorubicin-resistant AML cells to anti-leukemic agents [82]. Furthermore, resveratrol inhibited the proliferation of multiple human myeloma cell lines and potentiated the apoptotic effects of bortezomib and thalidomide [83,84].
Genistein (a phytoestrogen occurring in Glycine soja seeds) was tested in numerous works [85]. Its synergistic combination with cisplatin was evaluated with camptothecin in two human cancer cell lines (HeLa and OAW-42) [86,87], with gemcitabine in two murine xenografts of human pancreatic carcinoma cells (COLO 357 and L3.6pl) [88], and with hydroxycamptothecin in murine xenografts of human bladder carcinoma cells (TCC-SUP) [89]. Moreover, the micronutrient mineral selenium (Se) and genistein together with conventional drugs cisplatin and mitomycin C were used in human peripheral lymphocytes used against cytotoxic agent-induced apoptosis, underlining the protective role of Se and genistein in blood cells [90].
Curcumin, derived from Curcuma longa (turmeric) rhizomes, has a long history of use, and its anticancer potential [91,92] was investigated in human (T98G, U87MG, and T67) and rat (C6) glioma cell lines. It was demonstrated that curcumin was able to suppress growth and chemoresistance induced by chemotherapeutic agents (cisplatin, etoposide, camptothecin, and doxorubicin) and radiation [93]. Also, in human ovarian carcinoma cells, curcumin associated with cisplatin or oxaliplatin increased the resistant ovarian cancer cells’ sensitivity to drugs, both in wild-type and in cisplatin-resistant cells, eliciting a reduction in cell cycling and increased apoptosis [94]. Besides this, curcumin inhibited human colon cancer cell line HT-29 growth, when synergically treated with 5-fluorouracil [95]. In MDA-MB-231 cells (highly metastatic breast cancer cells), curcumin associated with ixabepilone, cisplatin, vinorelbine, or everolimus showed cell cycle arrest, a decrease in cell viability and apoptosis induction [96]. Paclitaxel-resistant breast cancer cells and a human breast cancer xenograft model were used to test the synergistic effects of curcumin and paclitaxel. The results indicated that curcumin had a therapeutic action in preventing breast cancer metastasis in a preclinical model by suppressing nuclear factor kappa B (NF-kB) [97].
Quercetin, a flavonol with multiple benefits found in many plants, fruits and vegetables [98], was shown to exhibit synergistic effects with cisplatin in human malignant mesothelioma cell line, with doxorubicin in neuroblastoma and Ewing’s sarcoma cell lines, with temozolomide in human astrocytoma cell line, and with doxorubicin in an established breast cancer in mice [99,100,101,102].
6.2. Biochemical Plant Derivatives
Aside from essential oil and polyphenol derivatives, many other herbal compounds or complex mixtures may exert synergistic anticancer properties associated with conventional chemotherapeutic drugs. For example, l-canavanine, an antimetabolite found in several plants of the Fabaceae family, which is hardly toxic alone, potentiated the cytotoxicity of vinblastine and paclitaxel in 2 cell models (HeLa and hepatocellular carcinoma cells) [103]. Moreover, in an orthotopic pancreatic cancer mouse model with PANC-1 cells, β-carboline (an alkaloid from the plant Rauwolfia vomitoria)-enriched extract in combination with gemcitabine reduced tumor burden and metastatic potential in gemcitabine non-responsive tumor [104]. The same plant, Rauwolfia vomitoria, in combination with carboplatin, was able to increase chemosensitivity in ovarian cancer cells (OVCAR-5, OVCAR-8, SHIN-3) and to inhibit tumor growth in a mouse model with intraperitoneal metastasis and massive ascites formation [105]. Garcinia benzophenones (obtained from Garcinia species) was tested on HT29 colon cancer cells in association with different chemopreventive agents and demonstrated a great ability to block cancer cell growth [106]. Another work showed that red beetroot (Beta vulgaris) extract together with doxorubicin induced synergistic antiproliferative effects against pancreatic (PaCa), breast (MCF-7) and prostate (PC-3) tumor cells [107].
7. Concluding Remarks
Phytomedicine and ethnopharmacology have increased in popularity in the last decades, especially when considering their application in the vast field of human diseases [108,109,110,111,112]. The results of the above-reported literature studies suggest that plant-derived compounds have a high impact as therapeutic agents, both alone or in combination with conventional drugs. Thus, evidence-based phytotherapy should be considered a valid option when treating human disorders, not only for low or mild-grade diseases but also for more complex and problematic diseases, such as cancer. Certainly, the pre-clinical data shown in this review need to be confirmed by robust clinical trials (randomized double-blind), and along this line of scientific research, a good number of clinical trials has been reported. Indeed, bibliographic research using “cancer and herbal” and “cancer and plant” as keywords retrieved 78 and 149 studies on the website of ClinicalTrials.gov (December 2018), even if not strictly related to synergy [113]. This gives us an idea of the undoubtedly increasing interest for CAM and should bring new attention to this field of research, which is sometimes neglected by the main financing channels. Also, it should be kept in mind that the growing use of herbal products, either self-prescribed or integrated with conventional drugs, will eventually contribute to intensifying the incidence of plant–drug interactions. Thus, every health practitioner should be aware of this important issue. However, considering cancer patients, there is the obvious need to invest in more clinical work exploring the administration of herbal remedies, focusing not only on avoiding harmful interactions but also on providing valid support if a scientific synergistic demonstration guarantees effectiveness. Indubitably, an accessible, worldwide, free database of herb–drug interactions is not only desirable but is now becoming a concrete need for clinicians; a need not strictly related to cancer treatment.
Acknowledgments
N. Martins would like to thank the Portuguese Foundation for Science and Technology (FCT–Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020—Programa Operacional Regional do Norte” (NORTE-01-0145-FEDER-000012).
Author Contributions
All authors contributed equally to this work. B.S., N.M. and J.S.-R., critically reviewed the manuscript. All the authors read and approved the final manuscript.
Funding
This work was partially supported by CONICYT PIA/APOYO CCTE AFB170007.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Doos L., Roberts E.O., Corp N., Kadam U.T. Multi-drug therapy in chronic condition multimorbidity: A systematic review. Fam. Pract. 2014;31:654–663. doi: 10.1093/fampra/cmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obodozie-Ofoegbu O. Readings in Advanced Pharmacokinetics—Theory, Methods and Applications. IntechOpen; London, UK: 2012. Pharmacokinetics and Drug Interactions of Herbal Medicines: A Missing Critical Step in the Phytomedicine/Drug Development Process. [Google Scholar]
- 3.Katselou M.G., Matralis A.N., Kourounakis A.P. Multi-target drug design approaches for multifactorial diseases: From neurodegenerative to cardiovascular applications. Curr. Med. Chem. 2014;21:2743–2787. doi: 10.2174/0929867321666140303144625. [DOI] [PubMed] [Google Scholar]
- 4.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pautasso M. Ten simple rules for writing a literature review. PLoS Comput. Biol. 2013;9:e1003149. doi: 10.1371/journal.pcbi.1003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckminster Fuller R. Operating Manual for Spaceship Earth. 1st ed. Lars Muller; Baden, Switzerland: 2008. p. 152. [Google Scholar]
- 7.Tennakoon P.L.K. Master’s Thesis. University of Agriculture Sciences; Dharwad (Institute), Karnataka, India: 2007. Studies on Plant Growth Promoting Rhizomicroorganisms of Tea (Camellia sinensis (L.) kuntze) Plants. [Google Scholar]
- 8.Segen J.C. Concise Dictionary of Modern Medicine. McGraw-Hill; New York, NY, USA: 2005. [Google Scholar]
- 9.Jonas W.B. Mosby’s Dictionary of Complementary & Alternative Medicine. 1st ed. Elsevier Mosby; St. Louis, MO, USA: 2005. [Google Scholar]
- 10.Berenbaum M.C. Synergy, additivism and antagonism in immunosuppression. A critical review. Clin. Exp. Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- 11.Berenbaum M.C. Criteria for analyzing interactions between biologically active agents. Adv. Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 12.Berenbaum M.C. What is synergy? Pharmacol. Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 13.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittel-Forschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 14.Leyden Webb J. Enzyme and Metabolic Inhibitors. Academic Pres; New York, NY, USA: London, UK: 1963. [Google Scholar]
- 15.Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enz. Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 16.Imming P., Sinning C., Meyer A. Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006;5:821–834. doi: 10.1038/nrd2132. [DOI] [PubMed] [Google Scholar]
- 17.Butterweck V., Jurgenliemk G., Nahrstedt A., Winterhoff H. Flavonoids from hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000;66:3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 18.Hemaiswarya S., Kruthiventi A.K., Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–652. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Ye H.Z., Zheng C.S., Xu X.J., Wu M.X., Liu X.X. Potential synergistic and multitarget effect of herbal pair chuanxiong rhizome-paeonia albifora pall on osteoarthritis disease: A computational pharmacology approach. Chin. J. Integr. Med. 2011;17:698–703. doi: 10.1007/s11655-011-0853-5. [DOI] [PubMed] [Google Scholar]
- 20.Nagaprashantha L.D., Vatsyayan R., Singhal J., Fast S., Roby R., Awasthi S., Singhal S.S. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochem. Pharmacol. 2011;82:1100–1109. doi: 10.1016/j.bcp.2011.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson J.D., Whalley B.J., Baker D., Pryce G., Constanti A., Gibbons S., Williamson E.M. Medicinal cannabis: Is Δ9-tetrahydrocannabinol necessary for all its effects? J. Pharm. Pharmacol. 2003;55:1687–1694. doi: 10.1211/0022357022304. [DOI] [PubMed] [Google Scholar]
- 22.Simmen U., Higelin J., Berger-Buter K., Schaffner W., Lundstrom K. Neurochemical studies with St. John’s wort in vitro. Pharmacopsychiatry. 2001;34:S137–S142. doi: 10.1055/s-2001-15475. [DOI] [PubMed] [Google Scholar]
- 23.Zhang A., Sun H., Yuan Y., Sun W., Jiao G., Wang X. An in vivo analysis of the therapeutic and synergistic properties of chinese medicinal formula yin-chen-hao-tang based on its active constituents. Fitoterapia. 2011;82:1160–1168. doi: 10.1016/j.fitote.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Haug K.G., Weber B., Hochhaus G., Butterweck V. Pharmacokinetic evaluation of visnagin and ammi visnaga aqueous extract after oral administration in rats. Planta Med. 2012;78:1831–1836. doi: 10.1055/s-0032-1315393. [DOI] [PubMed] [Google Scholar]
- 25.Banfield C., Gupta S., Marino M., Lim J., Affrime M. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin. Pharmacokinet. 2002;41:311–318. doi: 10.2165/00003088-200241040-00004. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z., Wang J.R., Niu T., Gao S., Yin T., You M., Jiang Z.H., Hu M. Inhibition of p-glycoprotein leads to improved oral bioavailability of compound k, an anticancer metabolite of red ginseng extract produced by gut microflora. Drug Metab. Dispos. Biol. Fate Chem. 2012;40:1538–1544. doi: 10.1124/dmd.111.044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida N., Takada T., Yamamura Y., Adachi I., Suzuki H., Kawakami J. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2- and breast cancer resistance protein-mediated transport. Drug Metab. Dispos. Biol. Fate Chem. 2008;36:1206–1211. doi: 10.1124/dmd.107.019513. [DOI] [PubMed] [Google Scholar]
- 28.Eumkeb G., Chukrathok S. Synergistic activity and mechanism of action of ceftazidime and apigenin combination against ceftazidime-resistant enterobacter cloacae. Phytomedicine. 2013;20:262–269. doi: 10.1016/j.phymed.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Rosato A., Vitali C., de Laurentis N., Armenise D., Antonietta Milillo M. Antibacterial effect of some essential oils administered alone or in combination with norfloxacin. Phytomedicine. 2007;14:727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki H., Satoh H., Hori S., Ohtani H., Sawada Y. Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab. Pharmacokinet. 2010;25:170–179. doi: 10.2133/dmpk.25.170. [DOI] [PubMed] [Google Scholar]
- 31.Alhusainy W., Paini A., Punt A., Louisse J., Spenkelink A., Vervoort J., Delatour T., Scholz G., Schilter B., Adams T., et al. Identification of nevadensin as an important herb-based constituent inhibiting estragole bioactivation and physiology-based biokinetic modeling of its possible in vivo effect. Toxicol. Appl. Pharmacol. 2010;245:179–190. doi: 10.1016/j.taap.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Liu S.H., Cheng Y.C. Old formula, new rx: The journey of phy906 as cancer adjuvant therapy. J. Ethnopharmacol. 2012;140:614–623. doi: 10.1016/j.jep.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 33.Rasool M., Iqbal J., Malik A., Ramzan H.S., Qureshi M.S., Asif M., Qazi M.H., Kamal M.A., Chaudhary A.G., Al-Qahtani M.H., et al. Hepatoprotective effects of Silybum marianum (silymarin) and Glycyrrhiza glabra (glycyrrhizin) in combination: A possible synergy. eCAM. 2014;2014:641597. doi: 10.1155/2014/641597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahin R.L., Barnes P.M., Stussman B.J., Bloom B. Costs of complementary and alternative medicine (cam) and frequency of visits to cam practitioners: United states, 2007. Natl. Health Stat. Rep. 2009;18:1–14. [PubMed] [Google Scholar]
- 35.Izzo A.A., Hoon-Kim S., Radhakrishnan R., Williamson E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016;30:691–700. doi: 10.1002/ptr.5591. [DOI] [PubMed] [Google Scholar]
- 36.Zhou S., Gao Y., Jiang W., Huang M., Xu A., Paxton J.W. Interactions of herbs with cytochrome p450. Drug Metab. Rev. 2003;35:35–98. doi: 10.1081/DMR-120018248. [DOI] [PubMed] [Google Scholar]
- 37.Ramos-Esquivel A., Viquez-Jaikel A., Fernandez C. Potential drug-drug and herb-drug interactions in patients with cancer: A prospective study of medication surveillance. J. Oncol. Pract. 2017;13:e613–e622. doi: 10.1200/JOP.2017.020859. [DOI] [PubMed] [Google Scholar]
- 38.Alsanad S.M., Williamson E.M., Howard R.L. Cancer patients at risk of herb/food supplement-drug interactions: A systematic review. Phytother. Res. 2014;28:1749–1755. doi: 10.1002/ptr.5213. [DOI] [PubMed] [Google Scholar]
- 39.Huang W., Lee S.L., Yu L.X. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11:217–224. doi: 10.1208/s12248-009-9098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung W.T., Subramaniam G., Lee J., Loh H.M., Leung P.H. Assessment of extracts from red yeast rice for herb-drug interaction by in-vitro and in-vivo assays. Sci. Rep. 2012;2:298. doi: 10.1038/srep00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Awortwe C., Fasinu P.S., Rosenkranz B. Application of caco-2 cell line in herb-drug interaction studies: Current approaches and challenges. J. Pharm. Pharma. Sci. 2014;17:1–19. doi: 10.18433/J30K63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hediger M.A., Romero M.F., Peng J.B., Rolfs A., Takanaga H., Bruford E.A. The abcs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsintroduction. Eur. J. Physiol. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 43.Wang L., Sweet D.H. Competitive inhibition of human organic anion transporters 1 (slc22a6), 3 (slc22a8) and 4 (slc22a11) by major components of the medicinal herb Salvia miltiorrhiza (danshen) Drug Metab. Pharmacokinet. 2013;28:220–228. doi: 10.2133/dmpk.DMPK-12-RG-116. [DOI] [PubMed] [Google Scholar]
- 44.Mandery K., Bujok K., Schmidt I., Keiser M., Siegmund W., Balk B., Konig J., Fromm M.F., Glaeser H. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1a2 and 2b1. Biochem. Pharmacol. 2010;80:1746–1753. doi: 10.1016/j.bcp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhou S., Chan E., Pan S.Q., Huang M., Lee E.J. Pharmacokinetic interactions of drugs with st john’s wort. J. Psychopharmacol. 2004;18:262–276. doi: 10.1177/0269881104042632. [DOI] [PubMed] [Google Scholar]
- 46.Diamond B.J., Bailey M.R. Ginkgo biloba: Indications, mechanisms, and safety. Psychiatr. Clin. N. Am. 2013;36:73–83. doi: 10.1016/j.psc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Naccarato M., Yoong D., Gough K. A potential drug-herbal interaction between ginkgo biloba and efavirenz. J. Int. Assoc. Phys. AIDS Care. 2012;11:98–100. doi: 10.1177/1545109711435364. [DOI] [PubMed] [Google Scholar]
- 48.Dolton M.J., Roufogalis B.D., McLachlan A.J. Fruit juices as perpetrators of drug interactions: The role of organic anion-transporting polypeptides. Clin. Pharmacol. Ther. 2012;92:622–630. doi: 10.1038/clpt.2012.159. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Xu R., Jin R., Chen Z., Fidler J.M. Immunosuppressive activity of the chinese medicinal plant tripterygium wilfordii. I. Prolongation of rat cardiac and renal allograft survival by the pg27 extract and immunosuppressive synergy in combination therapy with cyclosporine. Transplantation. 2000;70:447–455. doi: 10.1097/00007890-200008150-00010. [DOI] [PubMed] [Google Scholar]
- 50.Tung Y.T., Wu J.H., Huang C.C., Peng H.C., Chen Y.L., Yang S.C., Chang S.T. Protective effect of acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem. Toxicol. 2009;47:1385–1392. doi: 10.1016/j.fct.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Bakare-Odunola M.T., Mustapha K.B., Garba M., Obodozie O.O., Enemali I.S. The influence of nifadin, niprisan and niprd/92/001/1–1 (AM-1) on the pharmacokinetics of metronidazole in rats. Eur. J. Drug Metab. Pharmacokinet. 2010;35:55–58. doi: 10.1007/s13318-010-0008-7. [DOI] [PubMed] [Google Scholar]
- 52.Asdaq S.M., Inamdar M.N. Potential of garlic and its active constituent, s-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine. 2010;17:1016–1026. doi: 10.1016/j.phymed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Gharagozloo M., Moayedi B., Zakerinia M., Hamidi M., Karimi M., Maracy M., Amirghofran Z. Combined therapy of silymarin and desferrioxamine in patients with beta-thalassemia major: A randomized double-blind clinical trial. Fundam. Clin. Pharmacol. 2009;23:359–365. doi: 10.1111/j.1472-8206.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 54.Obodozie O.O., Adelakun T.A., Tarfa F.D., Tijani A.Y., Busu S.M., Inyang U.S. Evaluation of the effect of co-administration of selected first line antiretroviral agents with an investigational herbal immune booster in healthy rats; Proceedings of the World Congress in Association with the AAPS Annual Meeting and Exposition; New Orleans, LA, USA. 15 November 2010; Abstract #3804. [Google Scholar]
- 55.Sharifi-Rad M., Varoni E.M., Salehi B., Sharifi-Rad J., Matthews K.R., Ayatollahi S.A., Kobarfard F., Ibrahim S.A., Mnayer D., Zakaria Z.A., et al. Plants of the genus zingiber as a source of bioactive phytochemicals: From tradition to pharmacy. Molecules. 2017;22:2145. doi: 10.3390/molecules22122145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang X., Williams K.M., Liauw W.S., Ammit A.J., Roufogalis B.D., Duke C.C., Day R.O., McLachlan A.J. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005;59:425–432. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaes L.P., Chyka P.A. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: Nature of the evidence. Ann. Pharmacother. 2000;34:1478–1482. doi: 10.1345/aph.10031. [DOI] [PubMed] [Google Scholar]
- 58.Liu M.Z., Zhang Y.L., Zeng M.Z., He F.Z., Luo Z.Y., Luo J.Q., Wen J.G., Chen X.P., Zhou H.H., Zhang W. Pharmacogenomics and herb-drug interactions: Merge of future and tradition. ECAM. 2015;2015:321091. doi: 10.1155/2015/321091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izzo A.A. Interactions between herbs and conventional drugs: Overview of the clinical data. Med. Princip. Pract. 2012;21:404–428. doi: 10.1159/000334488. [DOI] [PubMed] [Google Scholar]
- 60.Kufe D.D., Pollock R.E., Weichselbaum R.R., Bast R.C., Gansler T.S., Holland J.F., Frei E. Holland-Frei Cancer Medicine. 6th ed. B.C. Decker Publications; Hamilton, ON, Canada: 2003. [Google Scholar]
- 61.American Cancer Society. [(accessed on 31 December 2018)]; Available online: http://www.cancer.org/research/cancerfactsstatistics/
- 62.Gupta D., Lis C.G., Birdsall T.C., Grutsch J.F. The use of dietary supplements in a community hospital comprehensive cancer center: Implications for conventional cancer care. Support. Care Cancer. 2005;13:912–919. doi: 10.1007/s00520-005-0820-9. [DOI] [PubMed] [Google Scholar]
- 63.Gautam N., Mantha A.K., Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. BioMed. Res. Int. 2014;2014:154106. doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim S.H., Bae H.C., Park E.J., Lee C.R., Kim B.J., Lee S., Park H.H., Kim S.J., So I., Kim T.W., et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem. Biophys. Res. Commun. 2011;407:129–134. doi: 10.1016/j.bbrc.2011.02.124. [DOI] [PubMed] [Google Scholar]
- 65.Zou B., Li Q.Q., Zhao J., Li J.M., Cuff C.F., Reed E. Beta-elemene and taxanes synergistically induce cytotoxicity and inhibit proliferation in ovarian cancer and other tumor cells. Anticancer Res. 2013;33:929–940. [PubMed] [Google Scholar]
- 66.Liu Y., Jiang Z.Y., Zhou Y.L., Qiu H.H., Wang G., Luo Y., Liu J.B., Liu X.W., Bu W.Q., Song J., et al. β-elemene regulates endoplasmic reticulum stress to induce the apoptosis of NSCLC cells through PERK/IRE1α/ATF6 pathway. Biomed. Pharmacother. 2017;93:490–497. doi: 10.1016/j.biopha.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 67.Hussain A., Priyani A., Sadrieh L., Brahmbhatt K., Ahmed M., Sharma C. Concurrent sulforaphane and eugenol induces differential effects on human cervical cancer cells. Integr. Cancer Ther. 2012;11:154–165. doi: 10.1177/1534735411400313. [DOI] [PubMed] [Google Scholar]
- 68.Yi J.L., Shi S., Shen Y.L., Wang L., Chen H.Y., Zhu J., Ding Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against hela cervical cancer cell lines. Int. J. Clin. Exp. Pathol. 2015;8:1116–1127. [PMC free article] [PubMed] [Google Scholar]
- 69.Legault J., Pichette A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007;59:1643–1647. doi: 10.1211/jpp.59.12.0005. [DOI] [PubMed] [Google Scholar]
- 70.Ambroz M., Matouskova P., Skarka A., Zajdlova M., Zakova K., Skalova L. The effects of selected sesquiterpenes from myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules. 2017;22:1021. doi: 10.3390/molecules22061021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabi T., Bishayee A. d-limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Effenberger-Neidnicht K., Schobert R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother. Pharmacol. 2011;67:867–874. doi: 10.1007/s00280-010-1386-x. [DOI] [PubMed] [Google Scholar]
- 73.Sakalar C., Izgi K., Iskender B., Sezen S., Aksu H., Cakir M., Kurt B., Turan A., Canatan H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016;37:4467–4477. doi: 10.1007/s13277-015-4307-0. [DOI] [PubMed] [Google Scholar]
- 74.Dirican A., Atmaca H., Bozkurt E., Erten C., Karaca B., Uslu R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in du-145 human prostate cancer cells by modulating pi3k-akt pathway. Clin. Trans. Oncol. 2015;17:145–151. doi: 10.1007/s12094-014-1206-6. [DOI] [PubMed] [Google Scholar]
- 75.Sutton K.M., Greenshields A.L., Hoskin D.W. Thymoquinone, a bioactive component of black caraway seeds, causes g1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer. 2014;66:408–418. doi: 10.1080/01635581.2013.878739. [DOI] [PubMed] [Google Scholar]
- 76.Chen M.C., Lee N.H., Hsu H.H., Ho T.J., Tu C.C., Hsieh D.J., Lin Y.M., Chen L.M., Kuo W.W., Huang C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015;63:1540–1546. doi: 10.1021/jf5054063. [DOI] [PubMed] [Google Scholar]
- 77.Chen M.C., Lee N.H., Hsu H.H., Ho T.J., Tu C.C., Chen R.J., Lin Y.M., Viswanadha V.P., Kuo W.W., Huang C.Y. Inhibition of nf-kappab and metastasis in irinotecan (CPT-11)-resistant lovo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017;32:669–678. doi: 10.1002/tox.22268. [DOI] [PubMed] [Google Scholar]
- 78.Khalife R., El-Hayek S., Tarras O., Hodroj M.H., Rizk S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin. Lymph. Myel. Leuk. 2014;14:S46–S55. doi: 10.1016/j.clml.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 79.Khalife R., Hodroj M.H., Fakhoury R., Rizk S. Thymoquinone from nigella sativa seeds promotes the antitumor activity of noncytotoxic doses of topotecan in human colorectal cancer cells in vitro. Planta Med. 2016;82:312–321. doi: 10.1055/s-0035-1558289. [DOI] [PubMed] [Google Scholar]
- 80.Mileo A.M., Miccadei S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell. Longev. 2016;2016:6475624. doi: 10.1155/2016/6475624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burns J., Yokota T., Ashihara H., Lean M.E., Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 82.Kweon S.H., Song J.H., Kim T.S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010;395:104–110. doi: 10.1016/j.bbrc.2010.03.147. [DOI] [PubMed] [Google Scholar]
- 83.Bhardwaj A., Sethi G., Vadhan-Raj S., Bueso-Ramos C., Takada Y., Gaur U., Nair A.S., Shishodia S., Aggarwal B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of stat3 and nuclear Factor-κB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 84.Salehi B., Mishra A.P., Nigam M., Sener B., Kilic M., Sharifi-Rad M., Fokou P.V.T., Martins N., Sharifi-Rad J. Resveratrol: A double-edged sword in health benefits. Biomedicines. 2018;6:91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spagnuolo C., Russo G.L., Orhan I.E., Habtemariam S., Daglia M., Sureda A., Nabavi S.F., Devi K.P., Loizzo M.R., Tundis R., et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohammad R.M., Banerjee S., Li Y., Aboukameel A., Kucuk O., Sarkar F.H. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BXPC-3 pancreatic tumor xenografts. Cancer. 2006;106:1260–1268. doi: 10.1002/cncr.21731. [DOI] [PubMed] [Google Scholar]
- 87.Papazisis K.T., Kalemi T.G., Zambouli D., Geromichalos G.D., Lambropoulos A.F., Kotsis A., Boutis L.L., Kortsaris A.H. Synergistic effects of protein tyrosine kinase inhibitor genistein with camptothecins against three cell lines in vitro. Cancer Lett. 2006;233:255–264. doi: 10.1016/j.canlet.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 88.Banerjee S., Zhang Y., Ali S., Bhuiyan M., Wang Z., Chiao P.J., Philip P.A., Abbruzzese J., Sarkar F.H. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Wang H., Zhang W., Shao C., Xu P., Shi C.H., Shi J.G., Li Y.M., Fu Q., Xue W., et al. Genistein sensitizes bladder cancer cells to HCPT treatment in vitro and in vivo via ATM/NF-κB/IKK pathway-induced apoptosis. PLoS ONE. 2013;8:e50175. doi: 10.1371/journal.pone.0050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sonaa E., Usha S., Ja In J. An ex vivo study of selenium, genistein on the morphological and nuclear changes in anticancer drug-induced apoptosis in human peripheral blood lymphocytes. BioFactors. 2013;39:279–293. doi: 10.1002/biof.1069. [DOI] [PubMed] [Google Scholar]
- 91.Chen J., He Z.M., Wang F.L., Zhang Z.S., Liu X.Z., Zhai D.D., Chen W.D. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharmacol. 2016;772:33–42. doi: 10.1016/j.ejphar.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 92.Salehi B., Stojanović-Radić Z., Matejić J., Sharifi-Rad M., Anil Kumar N.V., Martins N., Sharifi-Rad J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019:527–545. doi: 10.1016/j.ejmech.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Dhandapani K.M., Mahesh V.B., Brann D.W. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via ap-1 and nfkappab transcription factors. J. Neurochem. 2007;102:522–538. doi: 10.1111/j.1471-4159.2007.04633.x. [DOI] [PubMed] [Google Scholar]
- 94.Montopoli M., Ragazzi E., Froldi G., Caparrotta L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Prolif. 2009;42:195–206. doi: 10.1111/j.1365-2184.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du B., Jiang L., Xia Q., Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52:23–28. doi: 10.1159/000090238. [DOI] [PubMed] [Google Scholar]
- 96.Ke C.S., Liu H.S., Yen C.H., Huang G.C., Cheng H.C., Huang C.Y., Su C.L. Curcumin-induced aurora-a suppression not only causes mitotic defect and cell cycle arrest but also alters chemosensitivity to anticancer drugs. J. Nutr. Biochem. 2014;25:526–539. doi: 10.1016/j.jnutbio.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Aggarwal B.B., Shishodia S., Takada Y., Banerjee S., Newman R.A., Bueso-Ramos C.E., Price J.E. Curcumin suppresses the paclitaxel-induced nuclear factor-kappab pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin. Cancer Res. 2005;11:7490–7498. doi: 10.1158/1078-0432.CCR-05-1192. [DOI] [PubMed] [Google Scholar]
- 98.D’Andrea G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–271. doi: 10.1016/j.fitote.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Demiroglu-Zergeroglu A., Basara-Cigerim B., Kilic E., Yanikkaya-Demirel G. The investigation of effects of quercetin and its combination with cisplatin on malignant mesothelioma cells in vitro. J. Biomed. Biotechnol. 2010;2010:851589. doi: 10.1155/2010/851589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zanini C., Giribaldi G., Mandili G., Carta F., Crescenzio N., Bisaro B., Doria A., Foglia L., di Montezemolo L.C., Timeus F., et al. Inhibition of heat shock proteins (HSP) expression by quercetin and differential doxorubicin sensitization in neuroblastoma and ewing’s sarcoma cell lines. J. Neurochem. 2007;103:1344–1354. doi: 10.1111/j.1471-4159.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- 101.Jakubowicz-Gil J., Langner E., Wertel I., Piersiak T., Rzeski W. Temozolomide, quercetin and cell death in the moggccm astrocytoma cell line. Chem. Biol. Int. 2010;188:190–203. doi: 10.1016/j.cbi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 102.Du G., Lin H., Yang Y., Zhang S., Wu X., Wang M., Ji L., Lu L., Yu L., Han G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 103.Nurcahyanti A.D., Wink M. Cytotoxic potentiation of vinblastine and paclitaxel by l-canavanine in human cervical cancer and hepatocellular carcinoma cells. Phytomedicine. 2015;22:1232–1237. doi: 10.1016/j.phymed.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Yu J., Chen Q. Antitumor activities of rauwolfia vomitoria extract and potentiation of gemcitabine effects against pancreatic cancer. Integr. Cancer Ther. 2014;13:217–225. doi: 10.1177/1534735414532010. [DOI] [PubMed] [Google Scholar]
- 105.Yu J., Ma Y., Drisko J., Chen Q. Antitumor activities of rauwolfia vomitoria extract and potentiation of carboplatin effects against ovarian cancer. Curr. Ther. Res. Clin. Exp. 2013;75:8–14. doi: 10.1016/j.curtheres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Einbond L.S., Mighty J., Kashiwazaki R., Figueroa M., Jalees F., Acuna U.M., le Gendre O., Foster D.A., Kennelly E.J. Garcinia benzophenones inhibit the growth of human colon cancer cells and synergize with sulindac sulfide and turmeric. Anti Cancer Agents Med. Chem. 2013;13:1540–1550. doi: 10.2174/18715206113139990095. [DOI] [PubMed] [Google Scholar]
- 107.Kapadia G.J., Rao G.S., Ramachandran C., Iida A., Suzuki N., Tokuda H. Synergistic cytotoxicity of red beetroot (Beta vulgaris L.) extract with doxorubicin in human pancreatic, breast and prostate cancer cell lines. J. Complement. Integr. Med. 2013;10:113–122. doi: 10.1515/jcim-2013-0007. [DOI] [PubMed] [Google Scholar]
- 108.Salehi B., Valussi M., Jugran A.K., Martorell M., Ramírez-Alarcón K., Stojanović-Radić Z.Z., Antolak H., Kręgiel D., Mileski K.S., Sharifi-Rad M., et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018;80:104–122. doi: 10.1016/j.tifs.2018.07.030. [DOI] [Google Scholar]
- 109.Sharifi-Rad M., Fokou P.V.T., Sharopov F., Martorell M., Ademiluyi A.O., Rajkovic J., Salehi B., Martins N., Iriti M., Sharifi-Rad J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules. 2018;23:1751. doi: 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mishra A.P., Saklani S., Salehi B., Parcha V., Sharifi-Rad M., Milella L., Iriti M., Sharifi-Rad J., Srivastava M. Satyrium nepalense, a high altitude medicinal orchid of indian himalayan region: Chemical profile and biological activities of tuber extracts. Cell. Mol. Biol. 2018;64:35–43. doi: 10.14715/cmb/2018.64.8.6. [DOI] [PubMed] [Google Scholar]
- 111.Salehi B., Sharopov F., Martorell M., Rajkovic J., Ademiluyi A.O., Sharifi-Rad M., Fokou P.V.T., Martins N., Iriti M., Sharifi-Rad J. Phytochemicals in helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018;19:2361. doi: 10.3390/ijms19082361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharifi-Rad M., Roberts T.H., Matthews K.R., Bezerra C.F., Morais-Braga M.F.B., Coutinho H.D.M., Sharopov F., Salehi B., Yousaf Z., Sharifi-Rad M., et al. Ethnobotany of the genus taraxacum—phytochemicals and antimicrobial activity. Phytother. Res. 2018;32:2131–2145. doi: 10.1002/ptr.6157. [DOI] [PubMed] [Google Scholar]
- 113.U.S. National Institutes of Health. [(accessed on 31 December 2018)]; Available online: https://clinicaltrials.Gov/