Abstract
Laparoscopic and endoscopic cooperative surgery (LECS) is a procedure combining laparoscopic gastric resection with endoscopic submucosal dissection for local resection of gastric tumors with appropriate, minimal surgical resection margins. The LECS concept was initially developed from the classical LECS procedure for gastric submucosal tumor resection. Many researchers reported that classical LECS was a safe and feasible technique for resection of gastric submucosal tumors, regardless of tumor location, including the esophagogastric junction. Recently, LECS was approved for insurance coverage by Japan's National Health Insurance plan and widely applied for gastric submucosal tumor resection. However, the limitations of classical LECS are the risk of abdominal infection, scattering of tumor cells in the abdominal cavity, and tumor cell seeding in the peritoneum. The development of modified LECS procedures, such as inverted‐LECS, non‐exposed endoscopic wall‐inversion surgery, a combination of laparoscopic and endoscopic approaches to neoplasia with a non‐exposure technique, and closed‐LECS, has almost resolved these drawbacks. This has led to a recent increase in the indication of modified LECS to include patients with gastric epithelial neoplasms. The LECS concept is also beginning to be applied to tumor excision in other organs, such as the duodenum, colon and rectum. Further evolution of LECS procedures is expected in the future. Sentinel lymph node mapping could also be combined with LECS, resulting in a portion of early gastric cancers being treated by LECS with sentinel node mapping.
Keywords: gastric cancer, laparoscopic endoscopic cooperative surgery, sentinel node navigation surgery, submucosal tumor
1. INTRODUCTION
Most gastric tumors, such as gastric cancers and submucosal tumors, are recognized intra‐luminally on the mucosal side and these tumors sometimes cannot be recognized from the outside of the stomach. Therefore, it is difficult to determine accurate resection lines for gastric intraluminal tumors using conventional laparoscopic wedge resection from outside the stomach wall. These excessive or inappropriate resection lines may result in the postoperative alteration of the stomach with consequent gastric stasis, or positive resection margins resulting in postoperative local recurrence.1, 2 Therefore, an accurate cutting line and luminal endoscopic observation from the mucosal side is important for the safe local resection of tumors in the stomach.3
Laparoscopic and intraluminal endoscopic rendezvous surgery for gastric wedge resection has been described in several articles to ensure appropriate dissection margins.4, 5 However, in these studies, intra‐luminal endoscopy was only applied to observe the tumor location and not to determine accurate cutting lines.
We developed a laparoscopic endoscopic cooperative surgery (LECS) procedure, combining the technique of endoscopic submucosal dissection (ESD), to determine the exact cutting line, and laparoscopic gastric wall resection, and have used this procedure to resect gastric submucosal tumors (SMTs). After we first reported LECS in 2008,6 many researchers have used this procedure.7, 8, 9, 10, 11, 12 They reported that LECS is safe and feasible for gastric SMTs, and that LECS can be used in most patients, regardless of tumor location.6 Using the LECS procedure, we can preserve the gastric wall, feeder vessels and nerves which preserves gastric motility and improves the patient's postoperative quality of life. Moreover, LECS has been approved for insurance coverage by Japan's National Health Insurance plan since 2014.
The initial indication for LECS was gastric SMTs without ulcerative lesions and the procedure was named “classical LECS”.13 Then the LECS procedure was expanded to include gastric SMTs with ulceration and gastric cancer without the possibility of lymph node metastasis.14 There are some drawbacks with classical LECS—postoperative peritoneal infection from gastric content and the possibility of tumor cells seeding into the peritoneal cavity. To expand the indication of LECS to include gastric epithelial neoplasms, some modified LECS procedures were developed, such as inverted‐LECS,14 nonexposed endoscopic wall‐inversion surgery (NEWS),15, 16, 17, 18, 19 a combination of laparoscopic and endoscopic approaches to neoplasia with a non‐exposure technique (CLEAN‐NET),20 and closed‐LECS,21 and these modified procedures are currently used in patients with gastric epithelial neoplasms.
Today, the LECS concept is also applied to other organs, such as the duodenum,22 colon, and rectum.23 However, the efficacy and safety of these modified LECS procedures in other organs have not yet been fully investigated. In this review, we describe the concept of LECS in the gastrointestinal tract and discuss its future potential.
2. CLASSICAL LAPAROSCOPIC ENDOSCOPIC COOPERATIVE SURGERY FOR GASTRIC SUBMUCOSAL TUMORS
Hiki et al6 first reported the safe excision of gastric SMTs, such as gastrointestinal stromal tumors, with adequate resection margins using the classical LECS procedure.
2.1. Procedure for classical laparoscopic endoscopic cooperative surgery
2.1.1. Setup
The surgeon stands on the patient's right side, with the first assistant on the patient's left side, the laparoscopist between the patient's legs, and the endoscopist positioned near the patient's head (Figure 1A).1, 6 A camera port is inserted into the umbilicus using an open technique. Four additional ports (one 12 mm port and three 5 mm ports) are inserted into the right upper, right lower, left upper and left lower quadrants with a view of the laparoscopic image under a 10 mmHg pneumoperitoneum by CO2 gas.
Figure 1.
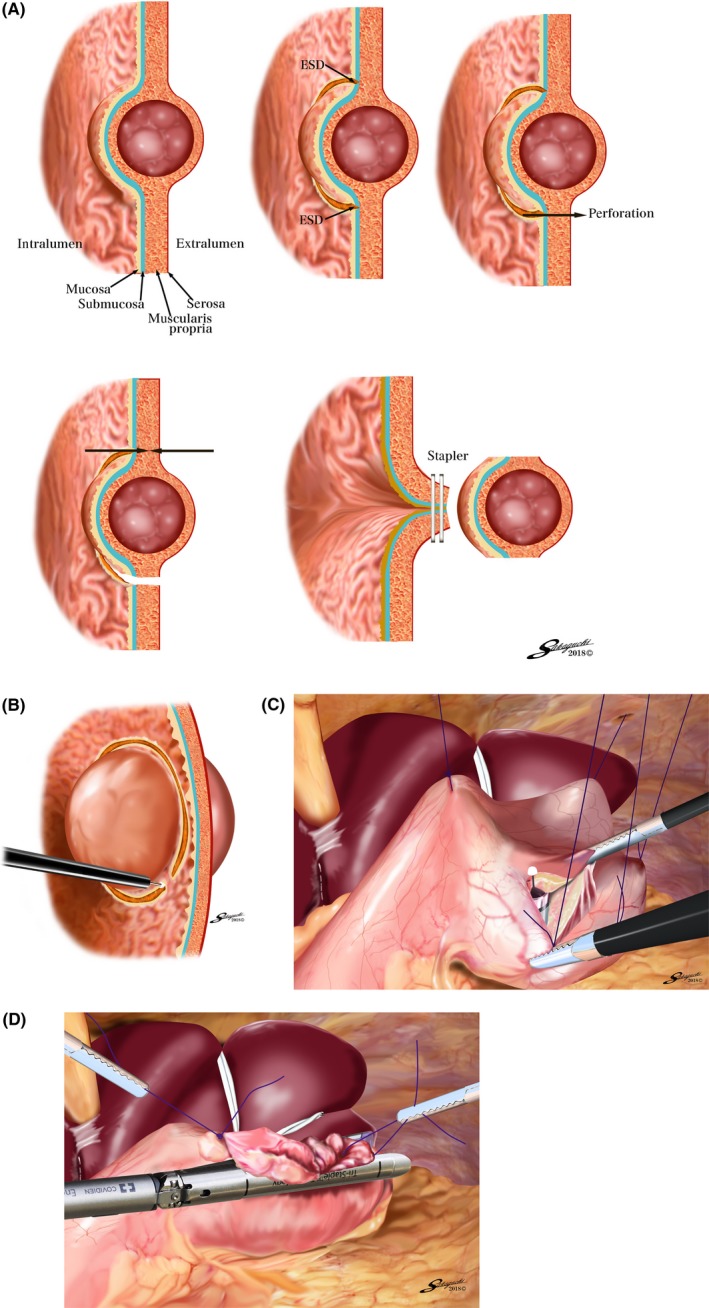
A, Conceptual diagram of the classical laparoscopic and endoscopic cooperative surgery procedure (classical LECS). ESD, endoscopic submucosal dissection. B, Endoscopic submucosal resection around the tumor, using the insulation‐tipped diathermic knife‐2. C, Inverted‐laparoscopic and endoscopic cooperative surgery (inverted‐LECS). The gastric wall is lifted up circumferentially to the dissecting line, like a crown, by several stitches. D, Post‐resection of the tumor with a minimal margin and less stomach deformation
2.1.2. Luminal endoscopic submucosal resection around the tumor
The location of the tumor is confirmed by intraluminal endoscopy and laparoscopic view. The periphery of the tumor is marked on the tumor edge with a 0.5 cm margin using a forced 20‐W coagulation current (ESG‐100; Olympus, Tokyo, Japan) Next, 10% glycerin is injected into the submucosal layer, a small initial incision is made using a standard needle‐knife, and the tip of an insulation‐tipped diathermic knife‐2 (IT‐2; KD‐611L; Olympus) is inserted into the submucosal layer. The marked area is cut circumferentially using the IT‐2 knife (Figure 1B).
2.1.3. Blood vessel preparation in the excision area
Blood vessels around the tumor are prepared using an ultrasonically activated device. The area of blood vessel and nerve manipulation should be minimized to prevent postoperative gastric stasis and ischemia. We have reported a case of suture line leakage due to ischemia caused by excessive vascular preparation.1
2.1.4. Laparoscopic seromuscular incision
Then, a standard needle‐knife from the intraluminal endoscopy is pushed toward the serosa. The tip of the standard needle‐knife is visible on the laparoscopic image beyond the seromuscular layer and is used to perforate the seromuscular layer in the 100‐W Endo‐Cut mode. The tip of the IT‐2 knife or ultrasonically activated device is inserted into the perforated lumen, and the seromuscular dissection is started. The seromuscular layer is dissected along the incision line using either the ultrasonically activated device from the laparoscopic side or the IT‐2 knife from the intraluminal endoscopic side. During dissection, the appropriate dissection line is easily identified because of the endoscopic submucosal resection around the tumor and its confirmation both laparoscopically and endoscopically. The full‐thickness incision is performed endoscopically with laparoscopic assistance as far as possible, and the remaining part of the full‐thickness wall dissection is performed laparoscopically.1
2.1.5. Incision line closure
The edge of the incision line is temporarily closed using hand‐sewn sutures after the tumor has been resected. Then the incision line is closed using a laparoscopic‐stapling device. If the tumor is located in the area close to the esophagogastric junction (EGJ) or pylorus, closure is difficult with a stapler and hand sutures are used to close the incision line. The direction of closure of the stomach incision line is determined by the short axis direction, as much as possible. If the short axis line goes over the lesser curvature, the closure line could be closer to the long axis. The tumor is collected and removed in a bag. An air leak test is performed by endoscopic insufflation, and the absence of the bleeding and stenosis is confirmed using both endoscopy and laparoscopy.
2.2. Superiority and limitation of classical laparoscopic endoscopic cooperative surgery
Many researchers have reported classical LECS as a feasible and safe procedure for gastric SMTs. The benefit of classical LECS is the completeness of the resection with a minimal margin, determined from the mucosal side. In the case of gastric lesions, it is possible to precisely excise the lesion by determining the resection line from the mucosal surface, ensuring pathological complete negative margin resections are obtained by this procedure. Moreover, classical LECS is technically easier than the modified LECS procedures. Thus, classical LECS can be applied to any tumor location including the EGJ. The advantage of LECS can be maximized in patients with gastric SMTs located at the EGJ by avoiding conventional total gastrectomy or proximal gastrectomy. Hoteya et al8 reported the feasibility and safety of classical LECS for gastric SMTs located at the EGJ.
The limitation of classical LECS includes the possibility of tumor and gastric juice contamination into the abdominal cavity because of the opening of the gastric wall during the procedure. Therefore, classical LECS should only be applied to gastric SMTs without a mucosal defect.
3. MODIFIED LAPAROSCOPIC ENDOSCOPIC COOPERATIVE SURGERY PROCEDURES FOR GASTRIC EPITHELIAL NEOPLASMS
3.1. Inverted‐laparoscopic endoscopic cooperative surgery
We have developed a LECS technique, called inverted‐LECS, to avoid the scattering of gastric juice or contact between the tumor and the surrounding tissue.14
After determining the resection line by endoscopic mucosal incision, the gastric wall around the tumor is pulled up circumferentially like a crown by several stitches. Each of the stitches is pulled out of the abdominal cavity using the Endo Close™ site‐closure device (Covidien, Tokyo, Japan) and fixed at skin level by clamping forceps. A full‐thickness incision is carried out endoscopically and laparoscopically (Figure 1C). During the full‐thickness incision, the tumor is inverted to face the intragastric cavity to prevent gastric juice contamination and contact between the tumor and abdominal wall. After the tumor has been resected into the gastric cavity, it is collected via the per‐oral route (Figure 1D).
This technique theoretically minimizes the risk of gastric contents spilling out into the abdominal cavity and prevents the tumor touching any intra‐abdominal tissue. Moreover, inverted‐LECS is less complicated than the other modified LECS procedures. However, this method also requires opening the gastric wall during the procedure. Thus, a slight risk of gastric content contamination cannot be ruled out. Additionally, an essential part of the LECS procedure for epithelial neoplasms is to prevent the seeding of tumor cells into the peritoneal cavity. Therefore, to prevent contact of the tumor with the visceral tissue, the tumor is turned inward toward the intragastric cavity by traction on the stitches on the edge of the resected specimen, and the resection line of the stomach is pulled up like a bowl by several stitches. A previous study has reported no peritoneal dissemination after gastric perforation during ESD for gastric cancer, even after long‐term observation.24 So the inverted‐LECS procedure in the present case would be safe from an oncological point of view. On the other hand, Han et al25 reported that cancer cells were detected by a cytology wash of the inside of the stomach during gastric cancer surgery. Therefore, LECS without opening the stomach wall would be the best procedure for the purpose of preventing cancer cells spreading to the peritoneal cavity.
.
3.2. Nonexposed endoscopic wall‐inversion surgery
Nonexposed endoscopic wall‐inversion surgery, or NEWS, was developed as a novel full‐thickness resection technique without intentional perforation, mainly aimed at early gastric cancer (Figure 2).15, 16, 17, 18, 19
Figure 2.
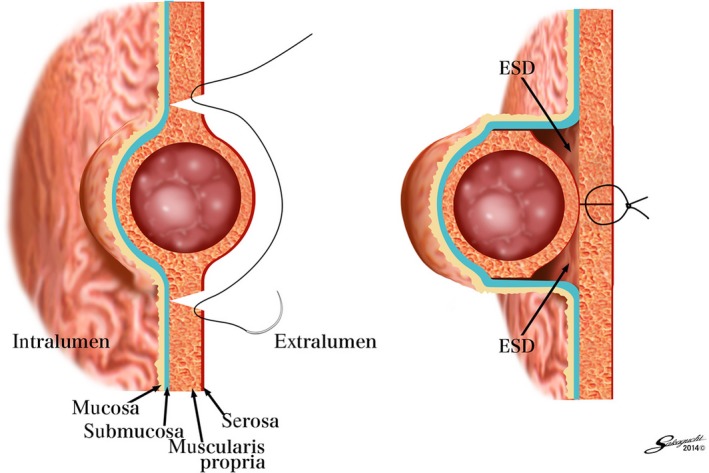
Conceptual diagram of a combination of nonexposed endoscopic wall‐inversion surgery (NEWS)
With this procedure, mucosal markings are first placed around the tumor, followed by serosal markings by laparoscope under endoscopic navigation. Then, sodium hyaluronate solution with indigocarmine dye is injected into the submucosal layer circumferentially by endoscope. A circumferential seromuscular incision is performed laparoscopically around the serosal markings. The seromuscular layers are linearly sutured with the lesion inverted into the inside of the stomach. Before suturing, a laparoscopic surgical sponge is inserted as a spacer between the suture layer and the serosal layer of the inverted lesion. This spacer provides a counter‐traction to the mucosa and protects the suture during the subsequent endoscopic procedure. Finally, the circumferential mucosal and submucosal tissue incisions are made around the inverted lesion by endoscopy. The resected specimen and the spacer are retrieved per‐orally and the mucosal edges are closed with several endoscopic clips.
The superiority of this technique is that both serosal and mucosal layers can be resected precisely under direct visualization by laparoscopy or endoscopy. However, a limitation of NEWS is the tumor size, as NEWS is only indicated for gastric SMTs 30 mm or less in diameter because the resected specimen is retrieved per‐orally. In addition, the operation time of NEWS is known to be long and even proficient NEWS teams sometime need more than 5 hours for the local dissection of a small‐sized tumor.26
3.3. Combination of laparoscopic and endoscopic approaches to neoplasia with non‐exposure technique
Inoue et al20 developed a nonexposed full‐thickness resection after seromuscular incision, preserving the continuity of the mucosa, which works as a barrier; they referred to this as CLEAN‐NET (Figure 3).
Figure 3.
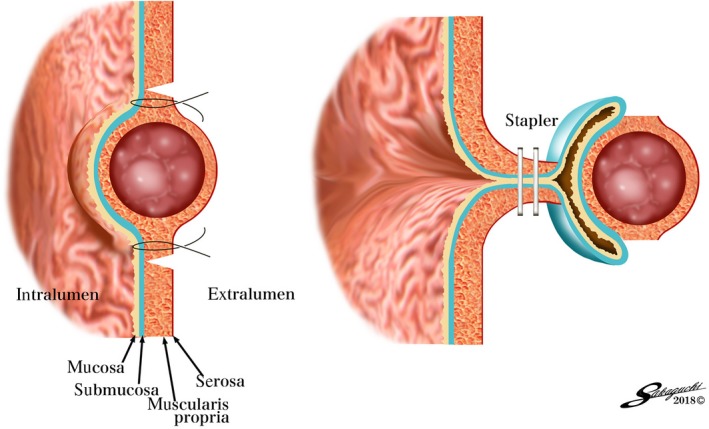
Conceptual diagram of a combination of laparoscopic and endoscopic approaches to neoplasia with a nonexposure technique (CLEAN‐NET)
In this technique, after the endoscopic marking, the mucosal layer is fixed to the seromuscular layer with four full‐layer stay sutures. The seromuscular dissection is performed laparoscopically along the outside of the stay sutures. The full‐layer specimen is lifted by the stay sutures and the mucosa surrounding the full‐layer specimen is also pulled up. The continuity of the mucosal layer prevents the gastric contents from flowing out into the peritoneal cavity. Using a laparoscopic‐stapling device, the full‐layer specimen is dissected with enough surgical margin.
The CLEAN‐NET procedure is unique and one of the attractive non‐exposure techniques for full‐thickness resection of the stomach wall. However, if the tumor is located at the cardia or posterior wall of the upper third of the stomach, CLEAN‐NET might be difficult to apply. Additionally, in this procedure, the incision line is finally determined from the serosal side. Therefore, compared to other modified LECS procedures, the appropriate resection line might be difficult to determine, especially for epithelial neoplasms, such as gastric cancer.
3.4. Closed‐laparoscopic endoscopic cooperative surgery
Nishizaki et al.21 developed a new nonexposed full‐thickness resection named “closed‐LECS”. In this procedure, mucosal markings are first made at the periphery of the tumor, and the marked area is cut circumferentially endoscopically. Then serosal markings are made corresponding to the submucosal dissection line using the guide of the endoscopic light. Next, a spongy spacer is put at the center of the suture line on the serosal surface and the seromuscular sutures are made with inversion of the marked lesion and spacer into the inside of the stomach (Figure 4A). Finally, the circumferential seromuscular dissection is performed endoscopically (Figure 4A). The resected specimen and sponge spacer are retrieved per‐orally.
Figure 4.
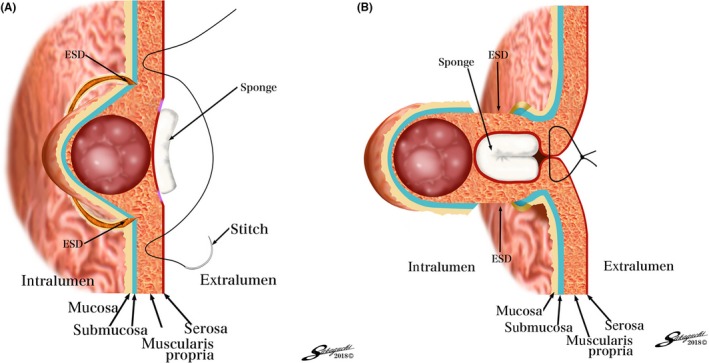
A and B, Conceptual diagrams of closed‐laparoscopic and endoscopic cooperative surgery (closed‐LECS). ESD, endoscopic submucosal dissection
The closed‐LECS procedure is superior due to the appropriate resection line. Because the resection line is determined from the mucosal side by endoscopy, this procedure is similar to classical LECS and inverted‐LECS. One limitation is the tumor size, as closed‐LECS is indicated for gastric SMTs 30 mm or less in diameter because the resected specimen is retrieved per‐orally. However, if the tumor is a gastric cancer, closed‐LECS is a suitable procedure even if the tumor size is more than 30 mm.
4. FUTURE POTENTIAL FOR LAPAROSCOPIC ENDOSCOPIC COOPERATIVE SURGERY PROCEDURES FOR GASTRIC LESIONS
As we have described, LECS has been safely applied to patients with gastric SMTs, while modified LECS procedures (inverted‐LECS, NEWS, CLEAN‐NET and closed‐LECS) were developed mainly for gastric epithelial neoplasms.
Currently, modified LECS procedures can be used in patients with early gastric cancer that would be technically difficult to treat with endoscopic mucosal resection (EMR)/ESD, as long as the tumor is within the indication for EMR/ESD. If EMR/ESD is not indicated for the tumor, then the possibility of lymph node metastasis exists, which necessitates a gastrectomy with lymphadenectomy.
Goto et al16, 27 reported NEWS for early gastric cancer in combination with sentinel node (SN) navigation surgery. The SN is defined as the first lymph node that receives lymphatic drainage from the primary tumor site. Thus, the SN status may predict the pathological status of all regional lymph nodes. If the SNs are confirmed to be negative for cancer metastasis, then unnecessary lymph node dissection may be avoided. SN navigation surgery for gastric cancer has been validated in a prospective multicenter trial.28 In addition, a prospective multicenter trial has commenced to compare personalized gastrectomy with conventional distal/total gastrectomy, based on the intraoperative SN biopsy data (UMIN000014401). When modified LECS procedures and SN navigation surgery are performed together, a minimally invasive surgical technique for adequate radical oncological resection of early gastric cancer could be achieved.
5. LAPAROSCOPIC ENDOSCOPIC COOPERATIVE SURGERY CONCEPT APPLIED TO OTHER ORGANS
5.1. Laparoscopic endoscopic cooperative surgery for duodenal tumors
Endoscopic submucosal dissection for early duodenal cancer is associated with a high risk of perforation during and after surgery, occurring at rates up to 35.7%.29, 30 Furthermore, delayed perforation in the duodenum in particular may cause potentially fatal peritonitis, requiring emergency open surgery to perform adequate peritoneal lavage to counter leakage of bile and pancreatic juices.31
The LECS procedure has been used successfully for duodenal neoplasms (D‐LECS). The indication for D‐LECS is primarily the same as that for ESD in duodenal tumors; however, tumors located near the ampulla of Vater are not indicated. An endoscope is inserted into the duodenum to confirm the precise location of the lesion. Endoscopic submucosal dissection is performed using an insulation‐tipped electrosurgical knife, with the detailed procedure described elsewhere.30 The specimen is retrieved intraluminally and removed, with the endoscope, through the mouth. After ESD is completed, the mucosal defect is reinforced with seromuscular sutures to prevent exposure of the seromuscular layer to duodenal juices, and subsequent digestion of the duodenal wall after surgery. The D‐LECS procedure for extraction of duodenal tumors seems to be feasible and helps to ensure the safety of ESD in the duodenum. Although D‐LECS is a safe and useful technique, it is presently not approved for insurance coverage by Japan's National Health Insurance plan. A further feasibility study is necessary to prove the safety of this procedure.
5.2. Laparoscopic endoscopic cooperative surgery for colorectal tumors
The LECS concept can be also applied to colorectal tumors.23 The appropriate indication of LECS for colorectal tumors is: (a) intra‐mucosal carcinoma (Tis) and adenoma with high‐grade atypia accompanied by a severe degree of fibrosis in the submucosal layer (tumor recurrence after endoscopic or surgical resection); (b) intra‐mucosal carcinoma (Tis) and adenoma with high‐grade atypia involving the appendix or diverticulum; or (c) intraluminal or intramural growth‐type submucosal tumors.
Following confirmation of the tumor location by endoscopy and laparoscopy, the colon wall at this site is exposed. First, a mucosa‐to‐submucosa dissection circumferential to the lesion with an appropriate safety margin is endoscopically performed. Complete full‐thickness dissection and excision are then performed by using ultrasonic‐activating scissors, endoscopy, and laparoscopy cooperatively. The excised lesion is withdrawn intraluminally with endoscopic forceps. The opened colon is then closed with laparoscopic linear staplers.
Compared to combined endoscopic and laparoscopic surgery (CELS)32 another local dissection technique of the colon, LECS for the colon, is a safe and feasible procedure. While CELS has resulted in a residual or local recurrence rate of between 10% (n = 65) and 13% (n = 23),33, 34 LECS for colorectal tumors has shown 0% residual or local recurrence (n = 17).
Currently, LECS for colorectal tumors is not approved for insurance coverage by Japan's National Health Insurance plan. In addition, more studies are required to prove the safety and efficacy of this procedure.
6. CONCLUSION
The LECS concept was initially developed from the classical LECS procedure for gastric SMTs. The limitations of classical LECS, namely the risk of abdominal infection and seeding of tumor cells in the abdominal cavity and peritoneum, have been almost resolved by the modified LECS procedures. However, further advancement of LECS procedures is expected in the future.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Author contributions: (1) conception and design, Naoki Hiki; (2) administrative support, Souya Nunobe; (3) provision of study material or patients, Souya Nunobe and Naoki Hiki; (4) collection and assembly of data, Souya Nunobe and Naoki Hiki; (5) data analysis and interpretation, Souya Nunobe and Naoki Hiki; (6) manuscript writing, Naoki Hiki; and (7) final approval of manuscript, all authors.
Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: Updated indications. Ann Gastroenterol Surg. 2019;3:239–246. 10.1002/ags3.12238
REFERENCES
- 1. Matsuda T, Hiki N, Nunobe S, et al. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video). Gastrointest Endosc. 2016;84:47–52. [DOI] [PubMed] [Google Scholar]
- 2. Hu J, Or BH, Hu K, Wang ML. Comparison of the post‐operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: a multi‐center prospective cohort study. Int J Surg. 2016;33(Pt A):65–71. [DOI] [PubMed] [Google Scholar]
- 3. Eisenberg D, Bell R. Intraoperative endoscopy: a requisite tool for laparoscopic resection of unusual gastrointestinal lesions‐a case series. J Surg Res. 2009;155:318–20. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic‐endoscopic rendezvous resection of gastric tumors. Surg Endosc. 2002;16:1561–5. [DOI] [PubMed] [Google Scholar]
- 5. Ridwelski K, Pross M, Schubert S, et al. Combined endoscopic intragastral resection of a posterior stromal gastric tumor using an original technique. Surg Endosc. 2002;16:537. [DOI] [PubMed] [Google Scholar]
- 6. Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–35. [DOI] [PubMed] [Google Scholar]
- 7. Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327–30. [DOI] [PubMed] [Google Scholar]
- 8. Hoteya S, Haruta S, Shinohara H, et al. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538–44. [DOI] [PubMed] [Google Scholar]
- 9. Obuchi T, Sasaki A, Baba S, Nitta H, Otsuka K, Wakabayashi G. Single‐port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg Today. 2015;45:641–6. [DOI] [PubMed] [Google Scholar]
- 10. Waseda Y, Doyama H, Inaki N, et al. Does laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors preserve residual gastric motility? Results of a retrospective single‐center study. PLoS One. 2014;9:e101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu WQ, Zhuang J, Wang M, et al. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. 2013;14:469–73. [DOI] [PubMed] [Google Scholar]
- 12. Kawahira H, Hayashi H, Natsume T, et al. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology. 2012;59:415–7. [DOI] [PubMed] [Google Scholar]
- 13. Hiki N, Nunobe S, Matsudan T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic and endoscopic cooperative surgery. Dig Endosc. 2015;27:197–204. [DOI] [PubMed] [Google Scholar]
- 14. Nunobe S, Hiki N, Gotoda T, et al. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral‐spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338–42. [DOI] [PubMed] [Google Scholar]
- 15. Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full‐thickness resection: a pilot study of non‐exposed endoscopic wall‐inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183–7. [DOI] [PubMed] [Google Scholar]
- 16. Goto O, Takeuchi H, Kawakubo H, et al. First case of non‐exposed endoscopic wall‐inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434–9. [DOI] [PubMed] [Google Scholar]
- 17. Mitsui T, Goto O, Shimizu N, et al. Novel technique for full‐thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall‐inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217–21. [DOI] [PubMed] [Google Scholar]
- 18. Mitsui T, Niimi K, Yamashita H, et al. Non‐exposed endoscopic wall‐inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goto O, Takeuchi H, Kawakubo H, et al. Feasibility of non‐exposed endoscopic wall‐inversion surgery with sentinel node basin dissection as a new surgical method for early gastric cancer: a porcine survival study. Gastric Cancer. 2015;18:440–5. [DOI] [PubMed] [Google Scholar]
- 20. Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full‐layer resection for gastric cancer with nonexposure technique (CLEAN‐NET). Surg Oncol Clin N Am. 2012;21:129–40. [DOI] [PubMed] [Google Scholar]
- 21. Kikuchi S, Nishizaki M, Kuroda S, et al. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. 2017;20:553–7. [DOI] [PubMed] [Google Scholar]
- 22. Irino T, Nunobe S, Hiki N, et al. Laparoscopic‐endoscopic cooperative surgery for duodenal tumors: a unique procedure that helps ensure the safety of endoscopic submucosal dissection. Endoscopy. 2015;47:349–51. [DOI] [PubMed] [Google Scholar]
- 23. Fukunaga Y, Tamegai Y, Chino A, et al. New technique of en bloc resection of colorectal tumor using laparoscopy and endoscopy cooperatively (laparoscopy and endoscopy cooperative surgery – colorectal). Dis Colon Rectum. 2014;57:267–71. [DOI] [PubMed] [Google Scholar]
- 24. Ikehara H, Gotoda T, Ono H, et al. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94:992–5. [DOI] [PubMed] [Google Scholar]
- 25. Han TS, Kong SH, Lee HJ, et al. Dissemination of free cancer cells from the gastric lumen and from perigastric lymphovascular pedicles during radical gastric cancer surgery. Ann Surg Oncol. 2011;18:2818–25. [DOI] [PubMed] [Google Scholar]
- 26. Mitsui T, Yamashita H, Aikou S, Niimi K, Fujishiro M, Seto Y. Non‐exposed endoscopic wall‐inversion surgery for gastrointestinal stromal tumor. Transl Gastroenterol Hepatol. 2018;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto O, Takeuchi H, Kitagawa Y, Yahagi N. Hybrid surgery for early gastric cancer. Transl Gastroenterol Hepatol. 2016;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–10. [DOI] [PubMed] [Google Scholar]
- 29. Jung JH, Choi KD, Ahn JY, et al. Endoscopic submucosal dissection for sessile, nonampullary duodenal adenomas. Endoscopy. 2013;45:133–5. [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non‐ampullary duodenal tumor. Dig Endosc. 2014;26(Suppl 2):50–6. [DOI] [PubMed] [Google Scholar]
- 31. Inoue T, Uedo N, Yamashina T, et al. Delayed perforation: a hazardous complication of endoscopic resection for non‐ampullary duodenal neoplasm. Dig Endosc. 2014;26:220–7. [DOI] [PubMed] [Google Scholar]
- 32. Garrett KA, Lee SW. Combined endoscopic and laparoscopic surgery. Clin Colon Rectal Surg. 2015;28:14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee SW, Garrett KA, Shin JH, Trencheva K, Sonoda T, Milsom JW. Dynamic article: long‐term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum. 2013;56:869–73. [DOI] [PubMed] [Google Scholar]
- 34. Yan J, Trencheva K, Lee SW, Sonoda T, Shukla P, Milsom JW. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic‐colonoscopic approach. Dis Colon Rectum. 2011;54:753–246. [DOI] [PubMed] [Google Scholar]


