Abstract
Background
Hypertrophic cardiomyopathy (HCM) is the most common cardiac disease in cats. However, most cats are not diagnosed until they develop congestive heart failure, arterial thromboembolism (ATE), or sudden cardiac death. Thus, an affordable screening test for early detection of HCM is desirable.
Hypothesis/Objectives
Evaluation of a sensitive cardiac troponin I (cTnI) assay as a screening test for HCM in cats and determination of a cutoff for its early detection.
Animals
One hundred sixty‐six client‐owned cats (male, n = 97) of various breeds were evaluated and classified using echocardiography as being healthy (n = 87), equivocal (n = 15), or having HCM (mild, n = 16; moderate, n = 10; severe, n = 34) or ATE (n = 4).
Methods
All cats were prospectively evaluated by echocardiography, and serum cTnI concentration was determined using the currently most sensitive assay (Siemens ADVIA Centaur TnI‐Ultra).
Results
The median cTnI concentration was significantly different between study groups (P < .000001). A cutoff of 0.06 ng/mL provided good discrimination between healthy cats and cats with HCM (sensitivity, 91.7%; specificity, 95.4%; area under the curve [AUC], 0.95; 95% confidence interval [CI], 0.899‐0.978). Even for asymptomatic cats with HCM, sensitivity and specificity for a cutoff of >0.06 ng/mL remained high at 87.8% and 95.4%, respectively (AUC, 0.93; 95% CI, 0.864‐0.964).
Conclusions and clinical importance
Cardiac troponin I can be used as a sensitive and specific screening test for the diagnosis of HCM in otherwise healthy cats (cutoff, >0.06 ng/mL). However, echocardiography is needed to confirm the diagnosis.
Keywords: biomarker, cats, cTnI, left ventricular hypertrophy, screening test
Abbreviations
- ATE
arterial thromboembolism
- AUC
area under the curve
- BSA
body surface area
- CHF
congestive heart failure
- cTnI
cardiac troponin I
- HCM
hypertrophic cardiomyopathy
- IQR
interquartile range
- IVSd
interventricular septum in diastole
- LA/Ao
left‐atrial‐to‐aorta ratio
- LV
left ventricle/ventricular
- LVFWd
left ventricular free wall in diastole
- LVIDd
left ventricular internal diameter in diastole
- LVIDs
left ventricular internal diameter in systole
- NT‐proBNP
N‐terminal pro‐B type natriuretic peptide
- ROC
receiver‐operating characteristic
1. INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is the most common cardiac disease in cats.1, 2, 3 It is defined as hypertrophy of the left ventricle4, 5 without identifiable underlying cause, such as systemic hypertension,6 hyperthyroidism,7, 8 or, less commonly, congenital aortic stenosis,9 multicentric lymphoma,10 acromegaly,11 or pseudohypertrophy as a consequence of dehydration.12 Frequently, cats are not diagnosed until they are presented with congestive heart failure (CHF), arterial thromboembolism (ATE), or sudden cardiac death.13, 14 According to a previous study,3 14.7% of 780 apparently healthy cats had echocardiographic evidence of HCM. Furthermore, in another study, 30.5% of cats with preclinical HCM or hypertrophic obstructive cardiomyopathy developed CHF, ATE, or both, and 27.9% died for cardiovascular reasons,15 thus emphasizing the need for a reliable screening test. Currently, echocardiographic examination remains the gold standard for the diagnosis of HCM,16 but it requires specialized equipment and a trained cardiologist. Many owners may be reluctant to take their apparently healthy cat to a specialist for such a costly examination.17 Therefore, an easily available and affordable screening test would be helpful to identify cats that would benefit from more extensive diagnostic evaluation. During the last decade, biomarkers such as N‐terminal pro‐B type natriuretic peptide (NT‐proBNP) and cardiac troponin I (cTnI) have been studied extensively in the assessment of cardiac diseases in cats.17, 18, 19, 20, 21, 22, 23, 24, 25 For NT‐proBNP, a cutoff already has been proposed for differentiating normal cats from cats only mildly affected with HCM.21, 22 Regarding cTnI, it has already been shown that cats with HCM have significantly higher serum cTnI concentrations than do healthy control cats, and that a correlation exists between left ventricular free wall thickness and the serum concentration of cTnI.18, 19, 26 Some investigators state that cTnI only can be used to differentiate cats with moderate‐to‐severe HCM from healthy cats, thus rendering it less useful as a screening test.27 Recently a cutoff of 0.163 ng/mL has been proposed to distinguish healthy cats from cats with HCM without left atrial dilatation.26 However, because of a sensitivity of 62%, its value as a screening test remains unknown.
Our aim was to evaluate the utility of a sensitive cTnI assay as a screening test for cats with HCM and to determine the appropriate cutoff to identify cats that would benefit from a more extensive echocardiographic evaluation. Second, we aimed to identify factors that might influence the cTnI concentration in these cats as well as to detect correlations between cTnI concentration and certain echocardiographic variables.
2. MATERIALS AND METHODS
2.1. Animals
All cats were client‐owned and evaluated prospectively. Informed owner consent was obtained before participation in the study and all examinations followed German guidelines for animal care and use. Cats were excluded if they had a potential reason for having secondary left ventricular hypertrophy (eg, systemic hypertension and hyperthyroidism), other cardiac disease, or kidney disease.
Healthy control cats had to have left ventricular wall thicknesses <5.0 mm and left‐atrial‐to‐aorta ratio (LA/Ao) ≤1.5. Cats with left ventricular wall thicknesses between 5.0 and 6.0 mm or moderate papillary muscle hypertrophy were classified as equivocal.28 Hypertrophic cardiomyopathy was defined as a generalized or focal end‐diastolic left ventricular wall thickness ≥6.0 mm, severe hypertrophy of the papillary muscles, or both.28, 29 Further classification of cats with HCM into mild, moderate, and severe was done as previously reported.21 Briefly, the following criteria were used: mild HCM was defined as end‐diastolic left ventricular free wall (LVFWd) or septal (IVSd) thickness of 6.0 to 6.5 mm or both with a normal LA/Ao of ≤1.5; moderate HCM was defined as LVFWd or IVSd of 6.0 to 6.5 mm or both in conjunction with LA/Ao of 1.5 to 1.8 or LVFWd or IVSd of 6.5 to 7.0 mm or both with normal or increased LA/Ao up to 1.8; severe HCM was defined as LVFWd or IVSd >6.0 mm or both in conjunction with LA/Ao >1.8 or if LVFWd or IVSd thickness or both were >7.0 mm. Cats with ATE were evaluated as a separate group.
2.2. Examination
All cats received a clinical examination, echocardiography with continuous ECG monitoring and diagnostic blood testing. An oscillometric blood pressure measurement (VET HDO Monitor, S + B medVet GmbH Babenhausen, Germany) was only performed on cats with left ventricular or papillary muscle hypertrophy according to current recommendations.30 The average of 5 measurements was taken and considered to be normal if systolic pressure was ≤160 mmHg. Blood testing consisted of serum urea and creatinine concentration in all cats as well as serum thyroxin (T4) concentration in cats with left ventricular or papillary muscle hypertrophy. Cats were excluded if they had a serum T4 concentration >4.5 μg/dL. The remaining serum was frozen and stored at −80°C for a maximum of 20 months for later evaluations of cTnI using the currently most sensitive assay (ADVIA Centaur TnI‐Ultra, Siemens Healthcare Diagnostics Inc., Erlangen, Germany) by IDEXX laboratories (Ludwigsburg, Germany), which has been proven to be applicable in cats.26 All echocardiographic examinations were performed using an ultrasound unit (Vivid 7 Dimension, General Electric Medical System, Waukesha, Wisconsin) equipped with a 7.0 (3.5/6.9) MHz phased‐array transducer by a board‐certified cardiologist or a resident directly supervised by a cardiologist.
During the echocardiographic examinations, the cats were gently restrained without sedation in right and left lateral recumbency. Standard echocardiographic views were obtained.31 Measurements of left ventricular dimensions were acquired from 2‐dimensional right parasternal long or short axis views. The thickest region was used for left ventricular wall measurements using a leading edge‐to‐leading edge method. For all variables, a mean value was calculated from at least 3 measurements and used for further analysis. Left atrial size was assessed by measuring LA/Ao from the right parasternal short axis view using an inner edge‐to‐inner edge method. The papillary muscles were evaluated subjectively.
2.3. Statistical analysis
Statistical analysis was performed using commercial statistical software (SPSS 18, IBM Inc., New York and MedCal 11.5, Medcalc Software, Ostend, Belgium). Statistical significance was defined as P < .05. All data were analyzed descriptively and presented as median with interquartile range (IQR). Mild outliners were defined as 1.5‐3 times the IQR, whereas marked outlines were defined as >3 times the IQR. Nonnormal distribution of all data was confirmed by the Kolmogorov–Smirnov test. Differences in the cTnI concentration among the different study groups (healthy, equivocal, mild, moderate, severe [including a subgroup analysis: compensated, decompensated, compensated under treatment], ATE) were analyzed and compared among groups using a Kruskal–Wallis test. Post hoc analysis was done according to Conover.32 Spearman's rank correlation was used to evaluate the effect of age, sex, body weight, and total body surface area (BSA) on the serum concentration of cTnI in the healthy control group. Correlations among echocardiographic variables such as LVFWd, IVSd, left ventricular internal diameter in diastole (LVIDd) and systole (LVIDs), LA/Ao, and cTnI concentrations also were determined by Spearman's rank correlation. Cutoffs were calculated using receiver‐operating characteristic (ROC) analysis and dot plots. Positive and negative predictive values were calculated for each cutoff. Cats with ATE were excluded from all correlation and ROC analyses because of small sample size and extremely high median cTnI concentration.
3. RESULTS
A total of 166 cats (male, 97; female, 69) of various breeds were included. European Shorthair was the major breed represented (n = 50), followed by Maine Coon (n = 40), British Shorthair (n = 35), and Norwegian Forest cat (n = 25). Furthermore, there were 5 Persian, 3 Siberian Forest Cats, and 8 cats belonging to other breeds. Of the 166 evaluated cats, 87 were classified as being healthy and 60 had HCM of variable disease severity (mild, 16; moderate, 10; severe, 34). Of the remaining 19 cats,15 cats were classified as equivocal and 4 presented with ATE. A significant age difference was found among study groups (P = .001) with healthy cats (median age, 2.75 years; IQR, 1.5‐5.92 years) being significantly younger than cats with mild (median age, 9.92 years; IQR, 1.56‐12.92 years), moderate (median age, 11.46 years; IQR, 3.31‐12.58 years), and severe (median age, 6.38 years; IQR, 4.46‐12.56 years) HCM. Female cats were overrepresented in the healthy control group (60.9%) and ATE group (25%), whereas male cats dominated in all other groups (equivocal, 73.3%; mild HCM, 68.7%; moderate HCM, 80.0%; severe HCM, 94.1%; P = .000001). No difference in weight was identified among groups (P = .057). Additional clinical data are presented in Table 1.
Table 1.
Population characteristics, echocardiographic variables, and cTnI concentration of the 166 cats enrolled in this study
| Healthy | Equivocal | Mild HCM | Moderate HCM | Severe HCM | ATE | P‐value | |
|---|---|---|---|---|---|---|---|
| Total number | 87 | 15 | 16 | 10 | 34 | 4 | |
| Age (years) | 2.75 (1.5–5.92) | 3.33 (1.58‐8.58) | 9.92 (1.56–12.92)a | 11.46 (3.31–12.58)a | 6.38 (4.46–12.56)a | 5.46 (2.15‐6.52) | P = .001b |
| Body weight (kg) | 4.5 (4.00‐5.4) | 6.4 (4.5‐7.5) | 4.67 (3.5‐5.8) | 4.75 (3.95‐5.33) | 5.0 (4.0‐5.75) | 4.25 (3.63‐4.88) | P = .057b |
| Sex male / female |
34/53 | 11/4a | 11/5a | 8/2a | 32/2 | 1/3c | P = .000001b |
| IVSd (mm) | 4.36 (4.12‐4.76) | 5.10 (4.88‐5.42) | 6.07 (6.02‐6.21) | 6.49 (5.38‐6.72) | 6.45 (4.97‐7.5 | 6.44 (5.95‐7.26) | P < .0001d |
| LVIDd (mm) | 15.87 (14.76‐17.33) | 15.9 (14.64‐17.65) | 14.38 (12.74‐15.76) | 13.07 (12.31‐5.6) | 14.69 (11.5‐17.52) | 12.49 (10.24‐14.72) | P < .0001d |
| LVFWd (mm) | 4.39 (4.0‐4.71) | 5.17 (4.9‐5.43) | 5.28 (4.92‐6.1) | 6.29 (5.46‐6.66) | 8.39 (7.22‐8.81) | 7.74 (7.07‐8.43) | P < .0001d |
| LVIDs | 9.53 (8.49‐10.71) | 10.2 (7.35‐11.7) | 7.44 (4.98‐9.01) | 7.27 (5.77‐9.88) | 7.55 (6.18‐9.72) | 8.99 (7.15‐9.12) | P < .0001d |
| LA/Ao | 1.29 (1.19‐1.37) | 1.28 (1.21‐1.34) | 1.3 (1.26‐1.37) | 1.47 (1.38‐1.54) | 1.9 (1.57‐2.28) | 2.59 (1.78‐2.65) | P < .0001d |
| cTnI | 0.013 (0.006‐0.025) | 0.022 (0.013‐0.037)a | 0.1 (0.028‐0.58)a , e | 0.174 (0.121‐0.356)a , e | 0.760 (0.407‐2.53)a , e , f | 6.413 (4.336‐11.266)a , e , f , g | P < .000001b |
Data are expressed as median (IQR).
P < .05, if compared to the healthy control group. Post hoc analysis according to Conover.32
Kruskal–Wallis test followed by a post hoc analysis according to Conover.32
P < .05, if compared to the severe HCM group. Post hoc analysis according to Conover.32
Spearman rank correlation evaluating the effect of echocardiographic parameters on the cTnI concentration excluding ATE.
P < .05, if compared to the equivocal group. Post hoc analysis according to Conover.32
P < .05, if compared to the mild HCM group. Post hoc analysis according to Conover.32
P < .05, if compared to the moderate HCM group. Post hoc analysis according to Conover.32
Abbreviations: ATE, arterial thromboembolism; cTnI, cardiac troponin I; HCM, hypertrophic cardiomyopathy; IVSd, interventricular septum in diastole; LVIDd, left ventricular internal diameter in diastole; LVFWd, left ventricular free wall in diastole; LVIDs, left ventricular internal diameter in systole; LA/Ao, left‐atrial‐to‐aorta ratio.
3.1. cTnI concentration
Twenty‐four cats had a cTnI concentration below the laboratory's detection limit of 0.006 ng/mL. Twenty‐one of these cats belonged to the healthy control group, 1 cat was classified as equivocal and 2 cats had mild HCM. For statistical analysis, a cTnI concentration of 0.006 ng/mL was used for these cats.
The effects of age, sex, body weight, and BSA on the serum concentration of cTnI were evaluated in the group of healthy cats. Body weight (P = .2, r s = −0.14), BSA (P = .24, r s = −0.13), and sex (P = .64, r s = 0.05) did not have an effect on serum cTnl concentration. With increasing age, a slight increase in cTnI concentration was observed, but this difference was not significant (P = .42, r s = 0.09).
The median cTnI concentration was significantly different among patient groups (P < .000001). Healthy cats and equivocal cats had significantly lower median cTnI concentration compared to all other groups. Furthermore, the median cTnI concentration was significantly different between cats with mild HCM compared to those with severe HCM. Cats with ATE had significantly higher median cTnI concentration compared to cats with mild or moderate HCM (Figure 1). The lowest concentrations were found in healthy cats, at 0.013 ng/mL (IQR, 0.006‐0.025 ng/mL), whereas the highest cTnI concentrations were found in cats with ATE with a median cTnI of 6.413 ng/mL (IQR, 4.336‐11.266 ng/mL; Table 1).
Figure 1.
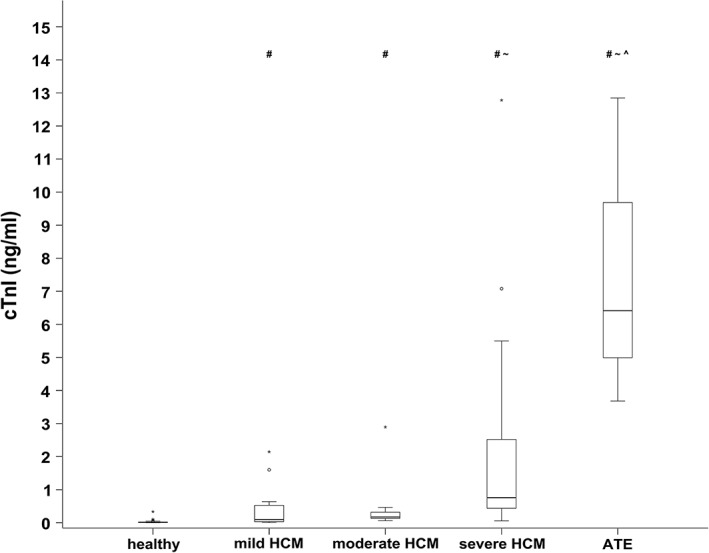
Serum cTnI concentration in healthy cats and cats with HCM of different disease severity as well as with arterial thromboembolism. The middle line of each box stands for the median, whereas the upper line and lower line represent the 75th and 25th percentiles, respectively. Mild outliners (1.5‐3 times the IQR) are marked with a circle (°), whereas marked outlines (>3 times the IQR) are marked with an asterisk (*). The median cTnI concentration was significantly different among most patient groups (P < .000001)
# P < .05, if compared to the healthy control group. Post hoc analysis according to Conover.32
~ P < .05, if compared to the mild HCM group. Post hoc analysis according to Conover.32
^ P < .05, if compared to the moderate HCM group. Post hoc analysis according to Conover.32
cTnI, cardiac troponin I; IQR, interquartile range; HCM, hypertrophic cardiomyopathy; ATE, arterial thromboembolism
To evaluate the effect of decompensation on cTnI concentration, a subgroup analysis was performed in the severe HCM group. Thus, the severe HCM group was divided into compensated (n = 15), decompensated (n = 11), and compensated under treatment (n = 8), and the cTnI concentration compared among these 3 subgroups. The cTnI concentration was significantly lower in the compensated group (median, 0.31 ng/mL; IQR, 0.17‐0.63 ng/mL) than in the decompensated group (median, 4.11 ng/mL; IQR, 2.36‐7.08 ng/mL) and compensated under treatment group (median, 0.64 g/mL; IQR, 0.47‐2.11 ng/mL; P = .00004; Figure 2).
Figure 2.
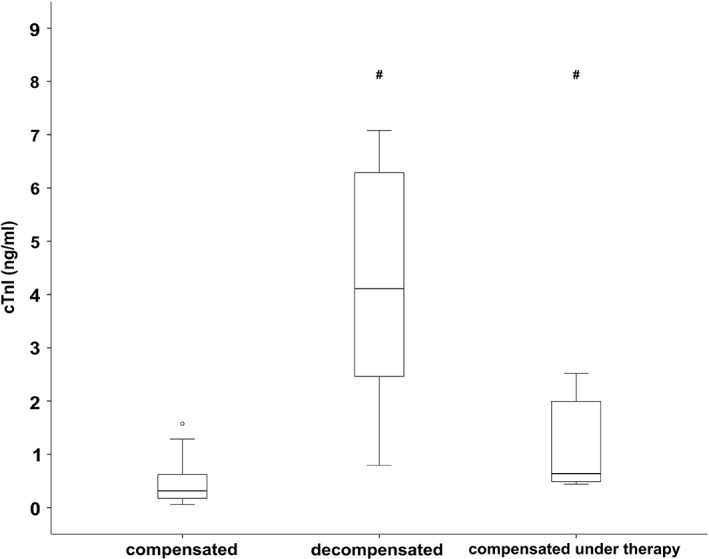
Serum cardiac troponin I concentration of cats with severe HCM that were either compensated (n = 15), acutely decompensated (n = 11), or compensated under treatment (n = 8). The middle line of each box stands for the median, whereas the upper line and lower line represent the 75th and 25th percentiles, respectively. Mild outliners (1.5‐3 times the IQR) are marked with a circle (°), whereas marked outlines (>3 times the IQR) are marked with an asterisk (*). The median cTnI concentration was significantly lower in the compensated group compared to the 2 other groups
# P < .05, if compared to the compensated severe HCM group. Post hoc analysis according to Conover.32
HCM, hypertrophic cardiomyopathy; cTnI, cardiac troponin I
3.2. Correlation of echocardiographic variables and cTnI
A strong and significant correlation was found between cTnI concentration and LVFWd (P < .0001, r s = 0.69). The correlation between cTnI concentration and IVSd and LA/Ao was mild, but significant (P < .0001, r s = 0.43 and 0.52, respectively). A significant negative correlation was found between cTnI concentration and LVIDd (P < .0001, r s = −0.36) and LVIDs (P < .0001, r s = −0.43).
3.3. Cutoffs
Healthy cats and cats with HCM were used for the calculation of a cutoff. Equivocal cats initially were excluded from the analysis. However, a second analysis was performed in which equivocal cats first were regarded as healthy and secondly as having HCM. Cats with ATE were excluded from all ROC analyses. A cutoff of >0.06 ng/mL differentiated healthy cats from cats with HCM with a sensitivity of 91.7% and specificity of 95.4% (AUC, 0.95; 95% CI, 0.899‐0.978; Figure 3) if equivocal cats were excluded from the analysis. By applying this cutoff, 3 cats were classified as false positive and 4 cats as false negative, whereby the false negative cats all had mild HCM only (Figure 4). The optimal cutoff to differentiate healthy cats from cats with asymptomatic HCM also was >0.06 ng/mL (sensitivity, 87.8%; specificity, 95.4%; AUC, 0.93; 95% CI, 0.864‐0.964). Regarding the detection of asymptomatic cats with severe HCM, the cutoff of >0.06 ng/mL had a sensitivity of 100% and specificity of 95.4% (AUC, 0.99; 95% CI, 0.947‐1.0). Additional results are shown in Table 2.
Figure 3.
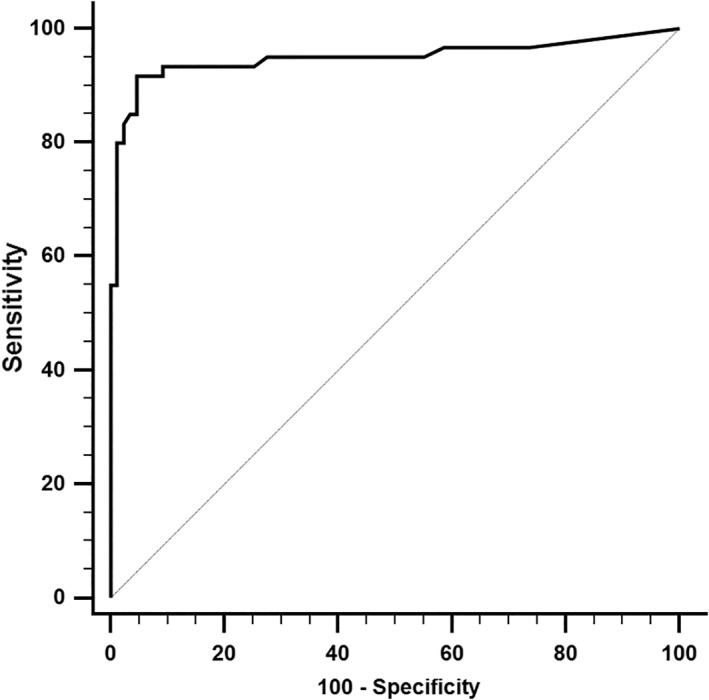
Receiver‐operating characteristic (ROC) curve displaying the sensitivity and specificity of serum cardiac troponin I concentration (cTnI) distinguishing healthy cats from cats with hypertrophic cardiomyopathy of different disease severity. The area under the curve is 0.95
Figure 4.
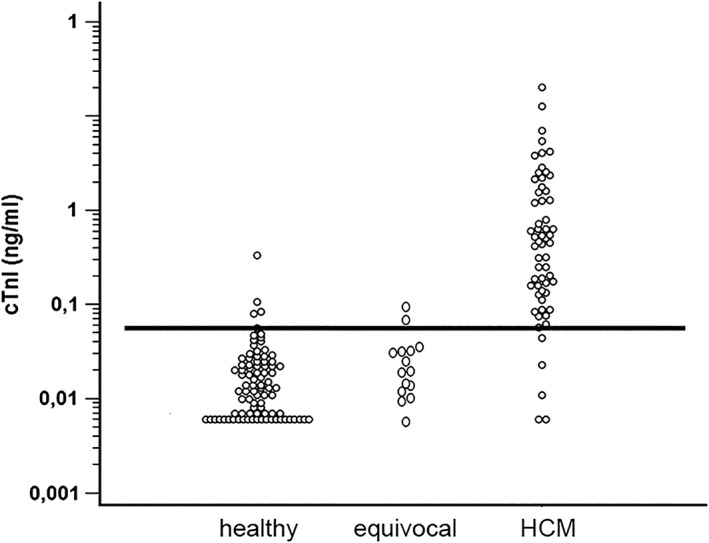
Dot plot with the black line demonstrating the cutoff of 0.06 ng/mL. Dots above the line in the healthy control group demonstrate the false positive, whereas dots below the line in cats with HCM represent false negatives
cTnI, cardiac troponin I; HCM, hypertrophic cardiomyopathy
Table 2.
Results of the receiver‐operating curve analyses to determine cutoffs for cTnI
| Cutoff (ng/ml) | Sensitivity | Specificity | Area under the curve | 95% confidence interval | |
|---|---|---|---|---|---|
| Healthy versus HCM | 0.06 | 91.7% | 95.4% | 0.95 | 0.899‐0.978 |
| Healthy versus asymptomatic HCM | 0.06 | 87.8% | 95.4% | 0.93 | 0.864‐0.964 |
| Healthy versus severe asymptomatic HCM | 0.06 | 100% | 95.4% | 0.99 | 0.947‐1.0 |
| Healthy with equivocal versus HCM | 0.06 | 91.7% | 94.1% | 0.95 | 0.899‐0.975 |
| Healthy versus equivocal with HCM | 0.06 | 76% | 95.4% | 0.9 | 0.838‐0.938 |
Cats with arterial thromboembolism were excluded from all analyses.
Abbreviations: cTnI, cardiac troponin I; HCM, hypertrophic cardiomyopathy.
Assuming a prevalence of 14.7%3 in the general population, the positive and negative predictive values for cTnI to differentiate healthy cats from cats with HCM were 77.4% and 98.5%, respectively, whereas for asymptomatic cats with HCM, the values were 76.7% and 97.8%, respectively.
4. DISCUSSION
To our knowledge, our study is the first to evaluate the usefulness of a sensitive cTnI assay as a screening test for HCM in cats.
A screening test should be an easily applicable procedure that can identify individuals from an apparently healthy population that are likely to have a disease or defect.33 It is recommended that middle aged to older cats receive laboratory assessments as part of their routine health care.34 Therefore, cTnI, which can be analyzed in the blood as a marker of myocardial injury,35 appears to be an ideal measurement. Earlier studies have shown that first‐generation cTnI test results are increased in cats with HCM compared to healthy controls.18, 19, 26, 36 Some concerns have been raised that only cats with moderate‐to‐severe HCM can be identified, thus rendering the assay less useful as a screening test. This statement however was related to first‐generation cTnI tests.27 In our study, we were able to show that median cTnI concentration increased with increasing disease severity and that this difference already was significant between healthy cats and cats with mild HCM. A significant difference also was found between asymptomatic cats with severe HCM and those that were decompensated or compensated under treatment. These findings are similar to those previously reported in which a sensitive cTnI assay also was used.26 Only between cats with moderate HCM compared to cats with mild or severe HCM as well as between cats with severe HCM compared to cats with ATE did this difference not reach significance. This observation might be a consequence of the small sample size of the moderate HCM and ATE groups. In agreement with a previous study,19 the highest cTnI concentrations were found in the ATE group. However, some decompensated cats with severe HCM also had very high cTnI concentrations. Because follow‐up was not part of our study design and no histopathology was done, myocarditis cannot be ruled out completely in these cats.
The determined cutoff of >0.06 ng/mL reliably differentiated between healthy cats and cats with HCM (sensitivity, 91.7%; specificity, 95.4%). This cutoff also showed good discrimination between healthy and asymptomatic cats with HCM (sensitivity, 87.8%; specificity, 95.4%), but it should be kept in mind that the reported sensitivities and specificities only apply for cats in which comorbidities have been excluded. Our determined cutoff is lower than that previously reported.18, 19, 26 Similar to a previous study,26 we used the currently most sensitive cTnI assay with a detection limit of 0.006 ng/mL, whereas the lowest detection limits in other previous studies were 0.03 ng/ml19 and 0.2 ng/ml,18 respectively. Furthermore, a previous study19 used twice the SD above the mean as a cutoff to detect moderate‐to‐severe HCM. Additionally, only 16% of the healthy control cats in that study19 received an echocardiographic examination, whereas the other cats were regarded as healthy based on physical examination, potentially allowing a high number of false negatives. The difference between our cutoff and that determined in a previous study26 might be explained by the different grading system and exclusion criteria used in our study. Because impaired renal function,35, 37 hyperthyroidism,38 and hypertension39 cause increases in cTnI, we excluded such cats from our study. However, the previous study26 included cats with concomitant chronic kidney disease and used higher blood pressure and higher serum T4 concentration as exclusion criteria. Furthermore, in our study cTnI was measured in serum, whereas the previous study26 used heparinized plasma. A recent study performed in dogs showed no statistically significant difference between mean cTnI concentration in serum versus plasma in ethylenediamine tetra‐acetate.40 However, the same might not be true for heparinized plasma.
A significant difference was found between the cTnI concentration of the healthy control group compared to the equivocal group. This might indicate that some of the equivocal cats already had myocardial damage as a consequence of developing HCM. According to the PawPeds screening criteria,27 some of these equivocal cats would have been classified as mild HCM, whereas others still would be regarded as healthy if the criteria of the ACVIM Registry of Cardiac Health were applied.27 Therefore, we excluded the equivocal group from the initial ROC analysis. Because doing so could influence our results, we performed a second analysis in which we regarded the equivocal group as either being healthy or as having HCM. Either way, this did not change the cutoff of >0.06 ng/mL, and both specificity and AUC remained high. Sensitivity remained equally high (91.7%) if the equivocal group was regarded as being healthy, but decreased to 76% if these cats were regarded as having HCM.
N‐terminal pro‐B type natriuretic peptide already has been evaluated in terms of differentiating normal cats from cats only mildly affected with HCM.21, 22 However, the NT‐proBNP assay is almost twice as expensive as the cTnI assay in some countries. Furthermore, NT‐proBNP predominantly is secreted in response to myocardial stretch as well as volume and pressure overload,41, 42 whereas cTnI is a marker of myocardial injury itself35 and thus might be more suitable in cats with HCM. On the other hand, cTnI, like NT‐proBNP,17 can be influenced by various noncardiac diseases that sometimes cause greater increases in the cTnI concentration than do primary cardiac diseases themselves.17, 35 Therefore, further diagnostic evaluation is required if increased biomarker concentrations are identified. It would have been interesting to determine, how both biomarkers would perform against each other, but doing so was not part of our study design.
During our study, no follow‐up was performed to evaluate the role of cTnl as an early marker for cats at risk of later developing HCM. Further research is needed regarding this matter. Assessing the role of cTnl based on different definition criteria for HCM also could be of interest, especially considering the results in the group defined as equivocal in our study.
Conflicting results have been reported regarding the difference in cTnI between asymptomatic cats with severe HCM and those with CHF.18, 19 We found a significant difference between the cTnI concentration of asymptomatic cats and acutely decompensated cats with severe HCM. Similar data are available for humans, where cTnI has been shown to correlate with the severity of CHF and remains a prognostic marker for adverse outcome and longer hospitalization.43, 44, 45, 46, 47, 48, 49, 50 Whereas a previous study19 did not find a significant difference; in our study, a significant difference was found between the cTnI concentration of cats with compensated severe HCM and those with compensated disease under treatment. This finding could implicate ongoing damage of the myocardium with disease progression despite clinical stabilization under treatment and supports the findings of another study,36 in which cTnI concentration at admission was prognostic for survival. In humans with stable chronic heart failure, 1 study51 found that higher baseline cTnI concentration and an increase over a 6‐month period were independent significant prognostic predictors of adverse outcome.
Factors influencing cTnI concentration were evaluated in the healthy control group. Similar to a previous report,26 no significant difference in terms of sex could be found. The observed increase in the proportion of male cats with increased disease severity is in agreement with the current literature.3, 14, 15, 52, 53
In our study, age did not significantly influence cTnl concentration. However, the median age of the healthy control group was only 4 years, possibly underrepresenting older, healthy cats. Dogs and humans also tend to have higher cTnI concentrations with increasing age.54, 55, 56
As described previously, the cTnI concentration correlated strongly with LVFWd18, 19, 26, 36 and mildly with IVSd.26 Additionally, we found a mild positive correlation with LA/Ao as well as a significant negative correlation with LVIDd and LVIDs. These findings partially can be explained by the larger sample size of our study compared to some previous studies.18, 19 Furthermore, a different severity categorization system was used in our study, which included LA/Ao as an independent variable.
Our study had several limitations. First, cTnI concentration was only evaluated once in every cat. Therefore, no individual or circadian changes in cTnI concentration could be taken into account. In healthy dogs, a within‐subject coefficient of variability of 48.1% has been reported.57 Second, because follow‐up echocardiography was not part of the study design, some cats might have had transient myocardial thickening instead of a classical HCM. However, transient myocardial thickening in cats so far has only be reported in conjunction with CHF.58, 59 An influence on our determined cutoff therefore seems unlikely. No histopathology was done to assess the extent of myocardial damage and to rule out myocarditis. Thus, some cats in the control group could have had myocardial changes consistent with HCM that were not yet evident on echocardiography. This would lead to a falsely increased median cTnI concentration, decreasing the specificity of the test. However, echocardiography still represents the current antemortem gold standard for the diagnosis of HCM.27 Lastly, many cats in the healthy control group consisted of animals being evaluated for breeding, which explains the higher proportion of younger female cats in that group. This design feature could have influenced our results, because age might affect cTnI concentration. Furthermore, older cats are more likely to have comorbidities, which potentially could affect cTnI concentration as well. Finally, we did not evaluate the effect of comorbidities on our test results. Various cardiac and noncardiac diseases can influence cTnI concentration.35 However, the presence of unknown comorbidities in an apparently healthy population of cats is a general limitation for the use of biomarkers as a screening test.
Our study showed that a cutoff of >0.06 ng/mL for cTnI can be used as a screening test for HCM in otherwise healthy cats. With a negative predictive value of 97.8% for asymptomatic cats only 2% of affected cats would have been missed. On the other hand, because of the positive predictive value of 76.7% almost 25% of the positive cats in an otherwise healthy screening population would be false positive, even if all other causes for increased cTnI concentration had been excluded. This observation highlights the need for subsequent echocardiography to confirm the diagnosis if increased cTnI concentration is detected in an otherwise healthy cat.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study involved client‐owned animals only with either a spontaneous disease or healthy cats presenting for routine healthcare checks or breeding examination. All examinations followed the German guidelines for animal care and use. Informed owner consent was obtained prior to participating in the study.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Hertzsch S, Roos A, Wess G. Evaluation of a sensitive cardiac troponin I assay as a screening test for the diagnosis of hypertrophic cardiomyopathy in cats. J Vet Intern Med. 2019;33:1242–1250. 10.1111/jvim.15498
REFERENCES
- 1. Ferasin L, Sturgess CP, Cannon MJ, Caney SMA, Gruffydd‐Jones TJ, Wotton PR. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994‐2001). J Feline Med Surg. 2003;5:151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riesen SC, Kovacevic A, Lombard CW, Amberger C. Prevalence of heart disease in symptomatic cats: an overview from 1998 to 2005. Schweizer Archiv Tierheilkunde. 2007;149:65‐71. [DOI] [PubMed] [Google Scholar]
- 3. Payne JR, Brodbelt DC, Luis Fuentes V. Cardiomyopathy prevalence in 780 apparently healthy cats in rehoming centres (the CatScan study). J Veter Cardiol. 2015;17(Suppl 1):S244‐S257. [DOI] [PubMed] [Google Scholar]
- 4. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation Committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807‐1816. [DOI] [PubMed] [Google Scholar]
- 5. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29:270‐276. [DOI] [PubMed] [Google Scholar]
- 6. Nelson L, Reidesel E, Ware WA, Christensen WF. Echocardiographic and radiographic changes associated with systemic hypertension in cats. J Vet Intern Med. 2002;16:418‐425. [DOI] [PubMed] [Google Scholar]
- 7. Bond BR, Fox PR, Peterson ME, Skavaril RV. Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc. 1988;192:1546‐1549. [PubMed] [Google Scholar]
- 8. Liu SK, Peterson ME, Fox PR. Hypertropic cardiomyopathy and hyperthyroidism in the cat. J Am Vet Med Assoc. 1984;185:52‐57. [PubMed] [Google Scholar]
- 9. Stepien RL, Bonagura JD. Aortic stenosis: clinical findings in six cats. J Small Animal Practice. 1991;32:341‐350. [Google Scholar]
- 10. Carter TD, Pariaut R, Snook E, Evans DE. Multicentric lymphoma mimicking decompensated hypertrophic cardiomyopathy in a cat. J Vet Intern Med. 2008;22:1345‐1347. [DOI] [PubMed] [Google Scholar]
- 11. Peterson ME, Taylor RS, Greco DS, et al. Acromegaly in 14 cats. J Vet Intern Med. 1990;4:192‐201. [DOI] [PubMed] [Google Scholar]
- 12. Campbell FE, Kittleson MD. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med. 2007;21:1008‐1015. [DOI] [PubMed] [Google Scholar]
- 13. Rush JE, Freeman LM, Fenollosa NK, Brown DJ. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990‐1999). J Am Vet Med Assoc. 2002;220:202‐207. [DOI] [PubMed] [Google Scholar]
- 14. Payne J, Luis Fuentes V, Boswood A, Connolly D, Koffas H, Brodbelt D. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract. 2010;51:540‐547. [DOI] [PubMed] [Google Scholar]
- 15. Fox PR, Keene BW, Lamb K, et al. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the REVEAL study. J Vet Intern Med. 2018;32:930‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645‐2651. [DOI] [PubMed] [Google Scholar]
- 17. Borgeat K, Connolly DJ, Luis Fuentes V. Cardiac biomarkers in cats. J Vet Cardiol. 2015;17(Suppl 1):S74‐S86. [DOI] [PubMed] [Google Scholar]
- 18. Connolly DJ, Cannata J, Boswood A, Archer J, Groves EA, Neiger R. Cardiac troponin I in cats with hypertrophic cardiomyopathy. J Feline Med Surg. 2003;5:209‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Herndon WE, Kittleson MD, Sanderson K, et al. Cardiac troponin I in feline hypertrophic cardiomyopathy. J Vet Intern Med. 2002;16:558‐564. [DOI] [PubMed] [Google Scholar]
- 20. Hsu A, Kittleson MD, Paling A. Investigation into the use of plasma NT‐proBNP concentration to screen for feline hypertrophic cardiomyopathy. J Vet Cardiol. 2009;11(Suppl 1):S63‐S70. [DOI] [PubMed] [Google Scholar]
- 21. Wess G, Daisenberger P, Mahling M, Hirschberger J, Hartmann K. Utility of measuring plasma N‐terminal pro‐brain natriuretic peptide in detecting hypertrophic cardiomyopathy and differentiating grades of severity in cats. Vet Clin Pathol. 2011;40:237‐244. [DOI] [PubMed] [Google Scholar]
- 22. Fox PR, Rush JE, Reynolds CA, et al. Multicenter evaluation of plasma N‐terminal probrain natriuretic peptide (NT‐pro BNP) as a biochemical screening test for asymptomatic (occult) cardiomyopathy in cats. J Vet Intern Med. 2011;25:1010‐1016. [DOI] [PubMed] [Google Scholar]
- 23. Connolly DJ, Magalhaes RJS, Syme HM, et al. Circulating natriuretic peptides in cats with heart disease. J Vet Intern Med. 2008;22:96‐105. [DOI] [PubMed] [Google Scholar]
- 24. Zimmering TM, Meneses F, Nolte IJ, Simon D. Measurement of N‐terminal proatrial natriuretic peptide in plasma of cats with and without cardiomyopathy. Am J Vet Res. 2009;70:216‐222. [DOI] [PubMed] [Google Scholar]
- 25. Machen MC, Oyama MA, Gordon SG, et al. Multi‐centered investigation of a point‐of‐care NT‐proBNP ELISA assay to detect moderate to severe occult (pre‐clinical) feline heart disease in cats referred for cardiac evaluation. J Vet Cardiol. 2014;16:245‐255. [DOI] [PubMed] [Google Scholar]
- 26. Hori Y, Iguchi M, Heishima Y, et al. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2018;32:922‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Häggström J, Luis Fuentes V, Wess G. Screening for hypertrophic cardiomyopathy in cats. J Vet Cardiol. 2015;17(Suppl 1):S134‐S149. [DOI] [PubMed] [Google Scholar]
- 28. Gundler S, Tidholm A, Häggström J. Prevalence of myocardial hypertrophy in a population of asymptomatic Swedish Maine coon cats. Acta Vet Scand. 2008;50:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in Maine coon cats: an animal model of human disease. Circulation. 1999;99:3172‐3180. [DOI] [PubMed] [Google Scholar]
- 30. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542‐558. [DOI] [PubMed] [Google Scholar]
- 31. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J Vet Intern Med. 1993;7:247‐252. [DOI] [PubMed] [Google Scholar]
- 32. Conover WJ, lman RL. On multiple‐comparisons procedures. Tech. Rep. LA‐7677‐MS, Los Alamos Scientific Laboratory 1979.
- 33. Chronic Illness in the United States . Volume I: prevention of chronic illness. JAMA. 1957;165:1513. [Google Scholar]
- 34. Epstein M, Kuehn NF, Landsberg G, et al. AAHA senior care guidelines for dogs and cats. J Am Anim Hosp Assoc. 2005;41:81‐91. [DOI] [PubMed] [Google Scholar]
- 35. Langhorn R, Willesen JL. Cardiac troponins in dogs and cats. J Vet Intern Med. 2016;30:36‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langhorn R, Tarnow I, Willesen JL, Kjelgaard‐Hansen M, Skovgaard IM, Koch J. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2014;28:1485‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Porciello F, Rishniw M, Herndon WE, Birettoni F, Antognoni MT, Simpson KW. Cardiac troponin I is elevated in dogs and cats with azotaemia renal failure and in dogs with non‐cardiac systemic disease. Aust Vet J. 2008;86:390‐394. [DOI] [PubMed] [Google Scholar]
- 38. Sangster JK, Panciera DL, Abbott JA, Zimmerman KC, Lantis AC. Cardiac biomarkers in hyperthyroid cats. J Vet Intern Med. 2014;28:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bijsmans ES, Jepson RE, Wheeler C, Syme HM, Elliott J. Plasma N‐terminal probrain natriuretic peptide, vascular endothelial growth factor, and cardiac troponin I as novel biomarkers of hypertensive disease and target organ damage in cats. J Vet Intern Med. 2017;31:650‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klüser L, Maier ET, Wess G. Evaluation of a high‐sensitivity cardiac troponin I assay compared to a first‐generation cardiac troponin I assay in Doberman Pinschers with and without dilated cardiomyopathy. J Vet Intern Med. 2018;33(1):54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hasegawa K, Fujiwara H, Doyama K, et al. Ventricular expression of brain natriuretic peptide in hypertrophic cardiomyopathy. Circulation. 1993;88:372‐380. [DOI] [PubMed] [Google Scholar]
- 42. Mueller C, Breidthardt T, Laule‐Kilian K, Christ M, Perruchoud AP. The integration of BNP and NT‐proBNP into clinical medicine. Swiss Med Wkly. 2007;137:4‐12. [DOI] [PubMed] [Google Scholar]
- 43. Missov E, Calzolari C, Pau B. Circulating cardiac troponin I in severe congestive heart failure. Circulation. 1997;96:2953‐2958. [DOI] [PubMed] [Google Scholar]
- 44. Peacock WF, de Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117‐2126. [DOI] [PubMed] [Google Scholar]
- 45. You JJ, Austin PC, Alter DA, Ko DT, Tu JV. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am Heart J. 2007;153:462‐470. [DOI] [PubMed] [Google Scholar]
- 46. La Vecchia L, Mezzena G, Ometto R, et al. Detectable serum troponin I in patients with heart failure of nonmyocardial ischemic origin. Am J Cardiol. 1997;80:88‐90. [PubMed] [Google Scholar]
- 47. Parenti N, Bartolacci S, Carle F, Angelo F. Cardiac troponin I as prognostic marker in heart failure patients discharged from emergency department. Intern Emerg Med. 2008;3:43‐47. [DOI] [PubMed] [Google Scholar]
- 48. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56:1071‐1078. [DOI] [PubMed] [Google Scholar]
- 49. Xue Y, Clopton P, Peacock WF, Maisel AS. Serial changes in high‐sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail. 2011;13:37‐42. [DOI] [PubMed] [Google Scholar]
- 50. Biolo A, Fisch M, Balog J, et al. Episodes of acute heart failure syndrome are associated with increased levels of troponin and extracellular matrix markers. Circ Heart Fail. 2010;3:44‐50. [DOI] [PubMed] [Google Scholar]
- 51. Kawahara C, Tsutamoto T, Sakai H, et al. Prognostic value of serial measurements of highly sensitive cardiac troponin I in stable outpatients with nonischemic chronic heart failure. Am Heart J. 2011;162:639‐645. [DOI] [PubMed] [Google Scholar]
- 52. Granström S, Godiksen MTN, Christiansen M, et al. Prevalence of hypertrophic cardiomyopathy in a cohort of British Shorthair cats in Denmark. J Vet Intern Med. 2011;25:866‐871. [DOI] [PubMed] [Google Scholar]
- 53. Riesen SC, Kovacevic A, Lombard CW, Amberger C. Echocardiographic screening of purebred cats: an overview from 2002 to 2005. Schweizer Archiv Tierheilkunde. 2007;149:73‐76. [DOI] [PubMed] [Google Scholar]
- 54. Wess G, Simak J, Mahling M, Hartmann K. Cardiac troponin I in Doberman pinschers with cardiomyopathy. J Vet Intern Med. 2010;24:843‐849. [DOI] [PubMed] [Google Scholar]
- 55. Clerico A, Fortunato A, Ripoli A, Prontera C, Zucchelli GC, Emdin M. Distribution of plasma cardiac troponin I values in healthy subjects: pathophysiological considerations. Clin Chem Lab Med. 2008;46:804‐808. [DOI] [PubMed] [Google Scholar]
- 56. Oyama MA, Sisson DD. Cardiac troponin‐I concentration in dogs with cardiac disease. J Vet Intern Med. 2004;18:831‐839. [DOI] [PubMed] [Google Scholar]
- 57. Winter RL, Saunders AB, Gordon SG, et al. Biologic variability of cardiac troponin I in healthy dogs and dogs with different stages of myxomatous mitral valve disease using standard and high‐sensitivity immunoassays. Vet Clin Pathol. 2017;46:299‐307. [DOI] [PubMed] [Google Scholar]
- 58. Novo Matos J, Pereira N, Glaus T, et al. Transient myocardial thickening in cats associated with heart failure. J Vet Intern Med. 2018;32:48‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Joseph JL, Oxford EM, Santilli RA. Transient myocardial thickening in a Bartonella henselae‐positive cat. J Vet Cardiol. 2018;20:198‐203. [DOI] [PubMed] [Google Scholar]


