Abstract
Background
Urine concentration (UC) provides clinically useful information concerning hydration status and renal function of animals.
Objectives
To characterize the clinical performance of urine specific gravity measured by optical refractometry (USG‐R) or Multistix‐SG urine reagent dipstick (USG‐D), urine electrical conductivity using an OAKTON Con 6 conductivity handheld meter (UEC), urine color (UColor) using a custom‐designed 8‐point color chart, and urine creatinine concentration (UCreat) for assessing UC in dairy cattle.
Animals
20 periparturient Holstein‐Friesian cows.
Methods
Urine was obtained by perineal stimulation or urethral catheterization and urine osmolality (UOsm, reference method), USG‐R, USG‐D, UEC, UColor, and UCreat determined. Diagnostic test performance was evaluated using Spearman's rho and logistic regression to determine the area under the receiver operating curve (AUC) and optimal cut point for diagnosing hypohydration (UOsm ≥800 mOsm/kg). P < .05 was considered significant.
Results
The best performing test for diagnosing hypohydration was USG‐R (AUC = 0.90) at an optimal cut point ≥1.030. The second‐best performing test was UEC (AUC = 0.82) at a cut point of ≥23.7 mS/cm, followed by UCreat (AUC = 0.76) at a cut point of ≥95.3 mg/dL, and UColor (AUC = 0.74) at a cut point of ≥4 on an 8‐point scale. Urine specific gravity measured by dipstick performed poorly (AUC = 0.63).
Conclusions and Clinical Importance
USG‐R and UEC provide practical and sufficiently accurate methods for measuring UC in dairy cattle. Urine color had moderate clinical utility as a no‐cost cow‐side method for assessing UC, whereas dipstick refractometry is not recommended for assessing UC.
Keywords: bovine, dehydration, hypohydration, refractometer, urine color chart, urine creatinine, urine dipstick
Abbreviations
- +LR
positive likelihood ratio
- AUC
area under the receiver operating curve
- POC
point‐of‐care
- ROC
receiver operating characteristic
- Se
sensitivity
Sp
specificity
- UC
urine concentration
- UCa
urine total calcium concentration
- UCl
urine chloride concentration
- UColor
urine color
- UCreat
urine creatinine concentration
- UEC
urine electrical conductivity using an OAKTON Con 6 conductivity handheld meter
- Ugluc
urine glucose concentration
- UK
urine concentration of potassium
- UMg
urine magnesium concentration
- UNa
urine concentration of sodium
- UOsm
urine osmolality
- UP
urine inorganic phosphate concentration
- USG‐D
urine specific gravity measured Multistix‐SG urine reagent dipstick
- USG‐R
urine specific gravity measured by optical refractometry
- κ
kappa coefficient.
1. INTRODUCTION
Urine concentration (UC) provides a practical clinical method for assessing hydration status and renal function in adult ruminants.1, 2, 3 The gold standard method for measuring UC is urine osmolality (UOsm),4, 5 and the consensus definition for hypohydration in children and adults, where hypohydration represents a general deficit in body‐water content, is UOsm ≥800 mOsm/kg.6, 7, 8 Measuring UOsm using freezing point depression osmometry is expensive, and a point‐of‐care (POC) device for measuring osmolality is not currently available.9 Urine specific gravity is therefore commonly used as a screening method for evaluating UC because specific gravity can be measured accurately using low‐cost handheld analyzers such as a refractometer.1, 4, 10
Urine specific gravity is a measure of the density of urine relative to the density of distilled water.11, 12 Urine specific gravity measured by refractometry (USG‐R) can falsely suggest a very concentrated urine in the presence of appreciable quantities of large molecules such as glucose, radiocontrast media, or protein, with USG‐R increasing by 0.002 per 10 g/L of glucose and 0.003 per 10 g/L of protein.13 Therefore, urine specific gravity measured by reagent dipstick (USG‐D) was introduced in the early 1980s as an alternative POC method for measuring specific gravity because the dipstick method is minimally affected by the presence of glucose and radiocontrast media.14 However, the dipstick used for measuring specific gravity has been calibrated to human urine, where pH is usually slightly acidic with pH ≈ 6.0.15
Urine electrical conductivity is a nonlinear function of the number of electrically charged particles.16, 17 Urine electrical conductivity (UEC) has been evaluated in several studies as an indirect method for assessing UC in humans.17, 18, 19, 20 However, the clinical performance of UEC does not appear to have been investigated in ruminant urine. Urine color (UColor) provides an attractive no‐cost alternative cow‐side tool for estimating UC.8, 21, 22 Urine color is primarily because of the presence of urochrome pigment, which is a byproduct of hemoglobin breakdown.21 A decrease in the free water component of urine increases the concentration of the urochrome pigment and darkens the urine color.23 To the best of our knowledge, the clinical utility of UColor has not been evaluated for assessing hydration status in ruminants. Finally, the urine creatinine concentration (UCreat) may also provide insight into hydration status because creatinine is formed at a constant rate in mammals from the spontaneous, irreversible, nonenzymatic conversion of creatine in skeletal muscle,24, 25 and is excreted at a constant rate in the urine in dairy cows.26 However, UCreat is dependent on both muscle mass and hydration status,24, 26 and therefore exhibits a large degree of variation between animals.27
Based on the above, we hypothesized that USG‐R, UEC, and UColor provide accurate, practical, and low‐cost methods for assessing UC in dairy cattle. We also hypothesized that UCreat and USG‐D would not provide suitable methods for evaluating UC in dairy cattle because variable muscle mass and urine pH >8.0 are frequently observed in ruminants. The main objective of this study was therefore to evaluate the clinical performance of USG‐R, UEC, UColor, UCreat, and USG‐D for measuring UC in dairy cattle.
2. MATERIALS AND METHODS
All methods were evaluated and approved by the Purdue Animal Care and Use Committee.
2.1. Animals and sampling
Twenty periparturient multiparous Holstein‐Friesian cows were randomly fed 1 of 2 rations of different dietary cation‐anion difference as described elsewhere.28 A total of 390 urine samples were collected by perineal stimulation approximately on days −14, −9, 1, 3, and 5 and by bladder catheterization approximately on days −5, −3, 7, and 14 relative to the expected calving date. Urine samples were collected into 15‐mL polypropylene vials that were completely filled with urine, immediately closed to minimize exposure to air, and placed in a 37 °C water bath for measurement of urine pH. An aliquot of urine was placed in a 2 mL polypropylene vial, stored at −20 °C, thawed at room temperature, and urine specific gravity, electrical conductivity, color, osmolality, and creatinine concentration determined.
2.2. Urine pH
Urine pH was measured within 15 minutes of collection using a glass electrode (M3 internal reference glass pH electrode, Medical Instruments Corp., Solothurn, Switzerland).
2.3. Urine specific gravity
Urine specific gravity was measured in duplicate using an automatic temperature‐calibrated optical refractometer (MASTER‐SUR/Nα, Atago Co Ltd, Bellevue, WA 98005 U.S.A) that had a measurement range of 1.000 to 1.060 in increments of 0.001. For USG‐R, a drop (≥0.3 mL) of urine was placed using a disposable pipette onto a clean dry prism surface at the tip of the refractometer. The cover of the prism was closed and gently pressed to remove any trapped air bubbles and assist in dispersing the urine sample over the prism surface. The specific gravity value was obtained by identifying the line where the blue and light fields met, with the line being visualized by holding the refractometer toward a fluorescent light source. The mean of the 2 readings was used as the measured value.
Urine specific gravity was also measured using Multistix‐10‐SG urine reagent dipsticks (Siemens Medical Solutions Inc.; USG‐D, Malvern, PA 19355, USA) with a measurement range from 1.000 to 1.030 in increments of 0.005. The reagent pad on the test strip was dipped into the urine sample and blotted by touching the edge of the strip to a paper towel to remove excess urine. The test strip was then placed on a flat clean surface with the indicator pad facing up. The color of the test strip was recorded after 45 seconds and compared to the color chart provided by the manufacturer. The measured value for USG‐D was corrected whenever urine pH ≥6.5 by adding 0.005 to the measured value,14, 29, 30 and the corrected value used for statistical analysis.
2.4. Urine electrical conductivity
Urine electrical conductivity was determined using an automatic temperature compensated handheld meter (CON 6, Oakton Instrument, Illinois) that the manufacturer reports is accurate to ±1.0%. Electrical conductivity was measured by immersing the tip of the unit in the urine sample and applying an alternating current between 2 electrodes spaced by a known distance. The change in voltage across the 2 electrodes reflects the resistance of the urine sample, with conductivity representing the reciprocal of resistance. The unit was calibrated each day using 12 and 80 mS/cm standards and the probe rinsed with deionized water and air dried before each measurement to avoid carry‐over effects in measurement. The temperature compensated measured value for conductance (milliSiemens per centimeter, mS/cm) at 25 °C displayed on a screen became stable within a few seconds of placement in the urine sample, and this was the value used for analysis.
2.5. Urine color
The urine sample was placed in a clear, glass 5‐mL tube and urine color was determined under fluorescent lighting by comparing the color of the urine sample placed against a white background next to the 8‐color chart.8 Urine color was determined using a custom‐designed urine color 8‐point chart (hex codes; 1 = #ffffeb, 2 = #fff9be, 3 = #ffeb6b, 4 = #ffd759, 5 = #ffc430, 6 = #ffb907, 7 = #ffaa00, and 8 = #bc7d00). This urine color chart appeared similar to standardized color samples for human urine based on plate/grid numbers of 17/B1, 9/H1, 17/J1, 17/L1, 9/I3, 9/L3, 12/K6, 23/L1, respectively,22 and house paint colors named Pale Sunshine, Cornsilk, Sunbeam, Banana Peel, Snapdragon, Crown Yellow, Yellow Tulip, and Tarnished Brass. Urine colors 1 to 3 were light and 6 to 8 were dark (Figure 1).
Figure 1.
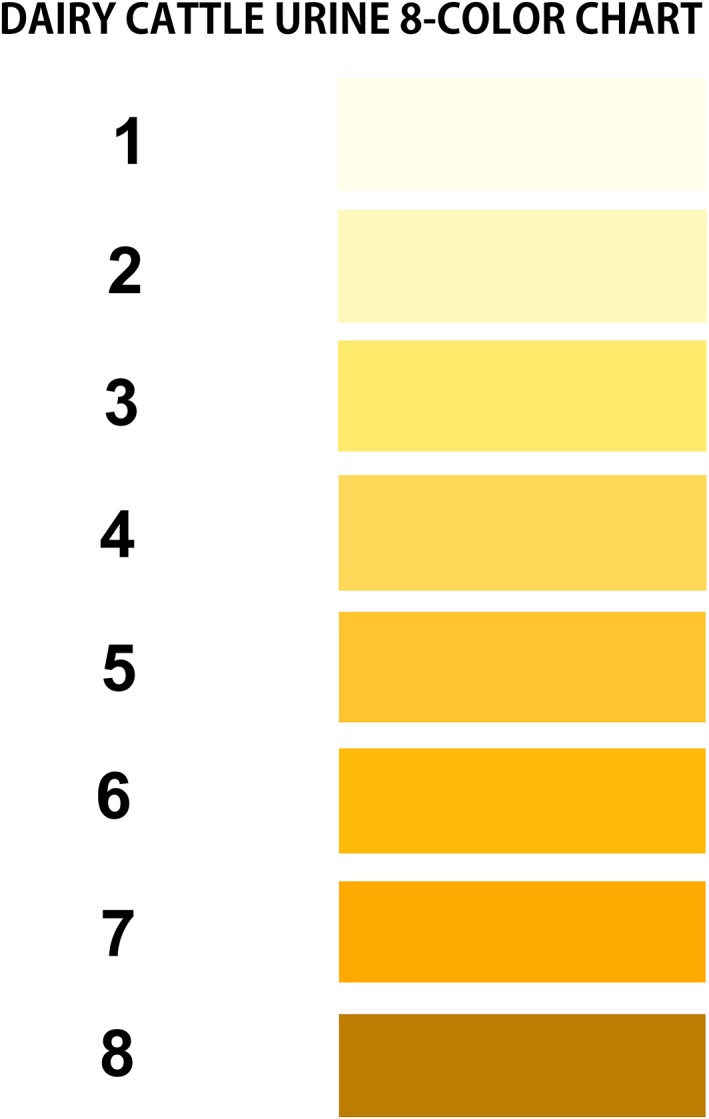
Eight‐color urine chart for assessing urine concentration in periparturient multiparous dairy cows
2.6. Urine osmolality and biochemical analysis
Urine osmolality was measured in triplicate using freezing point depression (Advanced 3MO, Advanced Instruments Inc., Norwood, Massachusetts) on urine samples that had been stored at −20 °C for up to 2 months. The mean value from the 3 measurements was used for statistical analysis.
Urine biochemical analysis was performed within 3 months on urine samples stored at −20 °C. Stored samples were thawed at room temperature and vortexed for 10 seconds immediately before biochemical analysis. Urine concentrations of creatinine (UCreat, picric acid method), total calcium (UCa, cresolphthalein method), magnesium (UMg, Arsenazo dye binding method), inorganic phosphate (UP, ammonium molybdate method), and glucose (Ugluc, hexokinase method) were determined spectrophotometrically (Hitachi 911, Roche Diagnostics, Switzerland). Urine chloride concentration (UCl) was measured using a mercuric nitrate spectrophotometric method. Urine concentration of sodium (UNa), and potassium (UK) were determined using ion‐selective electrodes and appropriate dilutions (Hitachi 911, Roche Diagnostics, Switzerland).
2.7. Statistical analysis
Statistical analyses were performed using MedCalc Statistical Software version 18.6 (MedCalc Software bvba, Ostend, Belgium, 2018) and SAS 9.4 (SAS Inc., Cary, North Carolina). P < .05 was considered significant. Spearman's correlation coefficients were used to evaluate the associations between UOsm, UCreat, USG‐R, USG‐D, UColor, UEC, UpH, UNa, UK, UCa, UMg, UCl, UP, and UGluc. The association of USG‐D with urine pH was explored graphically. Mixed models analysis with an unstructured covariance matrix, random intercept, and cow as the subject was used to characterize the relationship between USG‐R, corrected USG‐D, UEC, and UCreat (dependent variables) and UOsm (predictor variable).
A threshold of UOsm ≥800 mOsm/kg was used in this study to define the presence of hypohydration based on expert opinion and research studies in humans.6, 7, 8 A consensus is currently not available as to whether this cut point is also applicable to adult cattle; however, a recent study identified a mean value for Uosm of 781 mOsm/kg in healthy dairy cattle,3 suggesting that hypohydration in cattle is equal to, or greater than, a Uosm of 800 mOsm/kg. Binary logistic regression was used to characterize the relationship between UOsm (1, ≥800 mOsm/kg; 0, <800 mOsm/kg) and USG‐R, USG‐D, UEC, UColor, and UCreat. The adequacy of the logistic regression model fit was evaluated using plots of deviance influence statistics against the predicted values.31 Receiver‐operating characteristic (ROC) curves were constructed for each logistic regression model and the area under the curve (AUC) calculated as a global index of test performance; AUC values for ROC curves >0.9 typically indicate a highly accurate test, whereas AUC values of 0.7 to 0.9 indicate moderate accuracy, 0.5 to 0.7 low accuracy, and 0.5 represents a chance result.32 Sensitivity (Se) and specificity (Sp) were calculated at the optimal cut point of the ROC determined by the Youden index (the cut point where the following expression has its maximum value: Se + Sp − 1), which equally weights Se and Sp. The positive likelihood ratio (+LR) was calculated as: +LR = Se/(1 − Sp); values >10 indicate that a positive test is good at ruling in a diagnosis such as hypohydration.33 The Kappa coefficient (κ) was calculated at the optimal cut point to characterize the level of agreement between the tests (PROC FREQ). Values for κ <0.2 indicate poor agreement, whereas 0.2 < κ < 0.4 indicates fair agreement, 0.4 < κ < 0.6 indicates moderate agreement, 0.6 < κ < 0.8 reflects good agreement, and κ >0.8 indicates excellent agreement.34 Ninety‐five percent confidence intervals were calculated for ROC, Se, Sp, +LR, and κ as described elsewhere.35
3. RESULTS
Associations between variables of interest are summarized in Table 1. Urine osmolality was most strongly associated with urine specific gravity measured by refractometry, followed by UEC, UK, UCreat, and UColor. Urine osmolality was weakly associated with USG‐D, and was not associated with urine glucose or protein concentration.
Table 1.
Spearman correlation coefficients (with n and P in parenthesis) among variables of interest for 390 urine samples from 20 periparturient multiparous Holstein‐Friesian cows during the transition period
| USG‐R | UEC | UK | UCreat | UColor | UCl | UCa | UMg | USG‐D | UpH | Ugluc | UP | UNa | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UOsm | 0.85 | 0.70 | 0.62 | 0.60 | 0.58 | 0.39 | 0.37 | 0.36 | 0.31 | 0.19 | 0.15 | 0.06 | 0.02 |
| (242, P < .001) | (237, P < .001) | (217, P < .001) | (217, P < .001) | (244, P < .001) | (211, P < .001) | (216, P < .001) | (159, P < .001) | (241, P < .001) | (216, P < .001) | (99, P = .14) | (217, P = .34) | (217, P = .82) | |
| USG‐R | 0.46 | 0.60 | 0.70 | 0.58 | 0.11 | 0.27 | 0.48 | 0.19 | 0.29 | 0.25 | 0.16 | −0.16 | |
| (247, P < .001) | (271, P < .001) | (271, P < .001) | (299, P < .001) | (265, P = .08) | (270, P < .001) | (211, P < .001) | (178, P = .012) | (294, P < .001) | (130, P < .001) | (271, P < .001) | (271, P = .011) | ||
| UEC | 0.57 | 0.28 | 0.39 | 0.56 | 0.16 | 0.26 | 0.22 | 0.14 | −0.18 | −0.06 | 0.16 | ||
| (222, P < .001) | (222, P < .001) | (249, P < .001) | (216, P < .001) | (221, P = .022) | (162, P < .001) | (175, P < .001) | (244, P = .032) | (104, P = .08) | (222, P = .37) | (160, P = .041) | |||
| Uk | 0.67 | 0.41 | 0.24 | 0.04 | 0.51 | 0.17 | 0.40 | 0.23 | 0.15 | −0.15 | |||
| (389, P < .001) | (273, P < .001) | (384, P < .001) | (389, P = .39) | (328, P < .001) | (160, P < .033) | (387, P < .001) | (192, P < .001) | (389, P < .001) | (389, P < .001) | ||||
| UCreat | 0.52 | 0.14 | 0.25 | 0.47 | 0.12 | 0.16 | 0.25 | 0.15 | −0.14 | ||||
| (273, P < .001) | (383, P = .014) | (388, P < .001) | (327, P < .001) | (160, P = .14) | (386, P < .001) | (192, P < .001) | (390, P < .001) | (389, P < .001) | |||||
| UColor | 0.04 | 0.15 | 0.33 | 0.14 | 0.25 | 0.23 | 0.16 | −0.21 | |||||
| (267, P = .47) | (272, P = .024) | (213, P < .001) | (180, P = .06) | (296, P < .001) | (130, P < .001) | (273, P < .001) | (273, P < .001) | ||||||
| UCl | 0.47 | 0.02 | 0.55 | −0.35 | 0.06 | −0.02 | 0.46 | ||||||
| (383, P < .001) | (324, P = .72) | (154, P < .001) | (381, P < .001) | (189, P = .41) | (383, P = .75) | (383, P < .001) | |||||||
| UCa | 0.15 | 0.67 | −0.63 | 0.13 | 0.12 | 0.15 | |||||||
| (328, P < .001) | (160, P < .001) | (386, P < .001) | (224, P = .05) | (388, P < .021) | (389, P < .001) | ||||||||
| UMg | −0.16 | 0.28 | 0.31 | 0.51 | 0.00 | ||||||||
| (116, P = .08) | (328, P < .001) | (192, P < .001) | (328, P < .001) | (328, P = .97) | |||||||||
| USG‐D | −0.53 | 0.09 | 0.23 | 0.17 | |||||||||
| (177, P < .001) | (67, P = .48) | (160, P < .001) | (160, P = .032) | ||||||||||
| UpH | 0.15 | −0.05 | −0.13 | ||||||||||
| (192, P = .043) | (386, P = .34) | (387, P = .011) | |||||||||||
| Ugluc | 0.09 | −0.22 | |||||||||||
| (192, P = .23) | (192, P < .001) | ||||||||||||
| UP | −0.14 | ||||||||||||
| (389, P < .001) |
Abbreviations: UCa, urine total calcium concentration; UCl, Urine chloride concentration; UColor, urine color; UCreat, urine creatinine concentration; UEC, urine electrical conductivity; UK, urine potassium concentration; UMg, urine magnesium concentration; UNa, urine sodium concentration; UOsm, urine osmolality; UP, urine inorganic phosphorus concentration; UpH, urine pH measured by pH meter; UProt, urine protein concentration; UGluc, urine glucose concentration; USG‐D, urine specific gravity measured by Multistix‐SG dipstick; USG‐R, urine specific gravity measured by optical refractometry.
3.1. Urine specific gravity measured by optical refractometry
Mixed models regression between urine USG‐R and UOsm (reference method) for 242 urine samples indicated a linear relationship (Figure 2), such that USG‐R = 0.000030 × UOsm + 1.0056. A linear relationship also existed between UCreat and USG‐R (n = 215; P < .001, data not shown), such that UCreat = 4682 × USG‐R − 4713. Logistic regression analysis indicated that USG‐R accurately identified dairy cows with hypohydration (UOsm ≥800 mOsm/kg), with AUC of 0.90 (95% CI, 0.86‐0.94). The optimal cut point for USG‐R to diagnose hypohydration was ≥1.030, with Se = 0.84 (95% CI, 0.76‐0.90), Sp = 0.79 (95% CI, 0.71‐0.87), and +LR of 4.1 (95% CI, 2.8‐5.9). A good agreement was found between USG‐R and UOsm for detecting hypohydration in dairy cattle (κ = 0.63; 95% CI, 0.54‐0.73).
Figure 2.
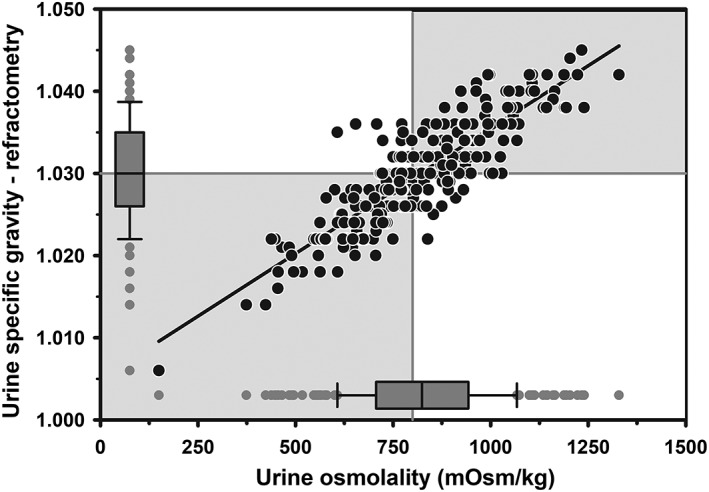
Scatterplot of the linear relationship between urine specific gravity measured by optical refractometry (USG‐R) and urine osmolality (UOsm, reference method) for 242 urine samples obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows from late gestation to early lactation. Some data points are superimposed. The solid black line is the line of regression. The solid gray vertical line indicates the recommended threshold value for diagnosing hypohydration (UOsm ≥800 mOsm/kg), and the solid gray horizontal line indicates the optimal cut point of USG‐R (≥1.030) identified by logistic regression for diagnosing hypohydration. The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10% to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles)
3.2. Urine specific gravity measured by Multistix‐SG dipstick
Mixed models regression between corrected USG‐D and UOsm (reference method) for 241 urine samples indicated a linear relationship (Figure 3), such that USG‐D = 0.0000085 × UOsm + 1.005. Logistic regression analysis indicated that the AUC for corrected USG‐D was 0.63 (95% CI, 0.56‐0.70). The optimal cut point for corrected USG‐D to identify hypohydration was ≥1.015, with Se of 0.51 (95% CI, 0.42‐0.60), Sp of 0.76 (95% CI, 0.67‐0.83), and +LR of 2.1 (95% CI, 1.5‐3.1). A poor agreement was found between corrected USG‐D and UOsm for detecting hypohydration in dairy cattle (κ = 0.26, 95% CI, 0.15‐0.38). The poor agreement was due, at least in part, to the confounding effect of urine pH on the measured value for USG‐D (Figure S1).
Figure 3.
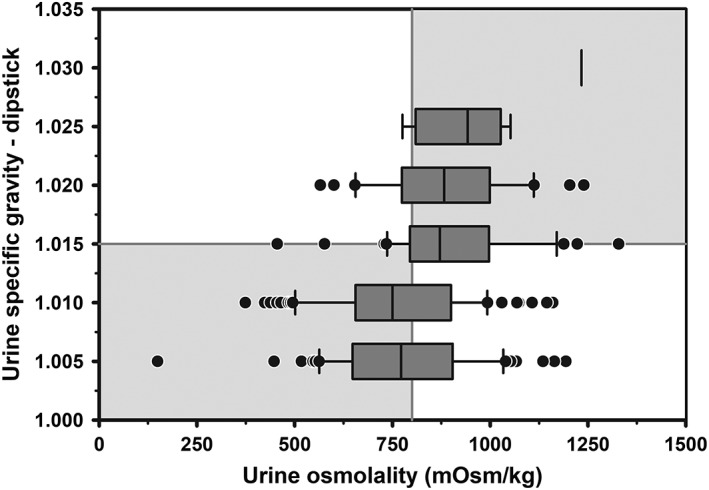
Box and whiskers plot of the association between corrected urine specific gravity measured by urine dipstick (USG‐D) and urine osmolality (UOsm, reference method) for 241 urine samples obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows. The solid gray vertical line indicates the recommended cut point for UOsm (≥800 mOsm/kg) for diagnosing hypohydration, and the solid gray horizontal line indicates the optimal cut point for corrected USG‐D (≥1.015) identified by logistic regression for diagnosing hypohydration. The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10% to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles)
3.3. Urine electrical conductivity
Mixed models regression revealed that UEC was linearly associated with UOsm (n = 237; Figure 4), such that UEC = 0.021 × UOsm + 5.79. Logistic regression analysis indicated that the AUC for UEC was 0.82 (95% CI, 0.77‐0.87). The optimal cut point of UEC for diagnosing hypohydration was ≥23.7 mS/cm, with Se of 0.72 (95% CI, 0.64‐0.80), Sp of 0.73 (95% CI, 0.64‐0.81), and +LR of 2.7 (95% CI, 1.9‐3.7). A moderate agreement was found between UEC and UOsm for detecting hypohydration in dairy cattle (κ = 0.45; 95% CI, 0.34‐0.56).
Figure 4.
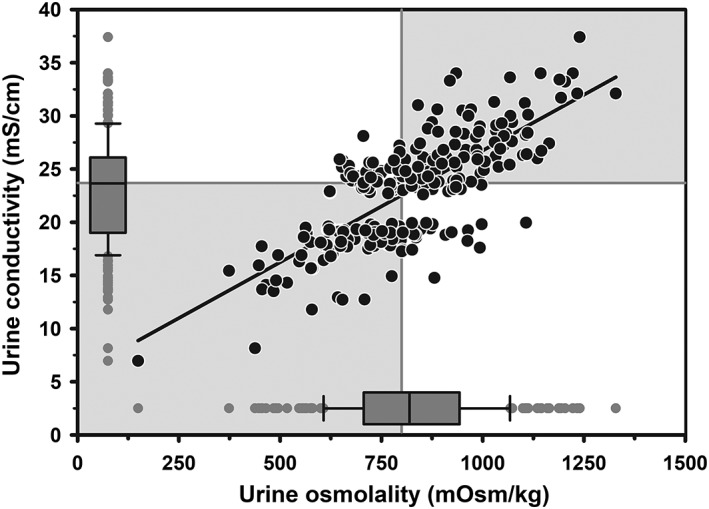
Scatterplot of the linear relationship between urine electrical conductivity (UEC) and osmolality (UOsm) for 237 urine sample obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows. Some data points are superimposed. The solid line is the line of regression. The solid gray vertical line indicates the recommended cut point for UOsm (≥800 mOsm/kg), and the solid gray horizontal line indicates the optimal cut point for UEC (≥23.7 mS/cm) identified by logistic regression for diagnosing hypohydration. The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10% to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles)
3.4. Urine color
The median UOsm was 262, 692, 812, 848, 962, 993, and 1109 mOsm/kg for urine color (UColor) scores of 1 (very light yellow), 2, 3, 4, 5, 6, and 7 (dark yellow), respectively (Figure 5). Logistic regression analysis indicated that the AUC for UColor for diagnosing hypohydration was 0.74 (95% CI, 0.69‐0.80) at an optimal color score ≥4, with Se of 0.53 (95% CI, 0.45‐0.62), Sp of 0.81 (95% CI, 0.73‐0.88), and +LR of 2.9 (95% CI, 1.9‐4.4). Moderate agreement was found between UColor and UOsm for detecting hypohydration in dairy cattle (κ = 0.34; 95% CI, 0.23‐0.45).
Figure 5.
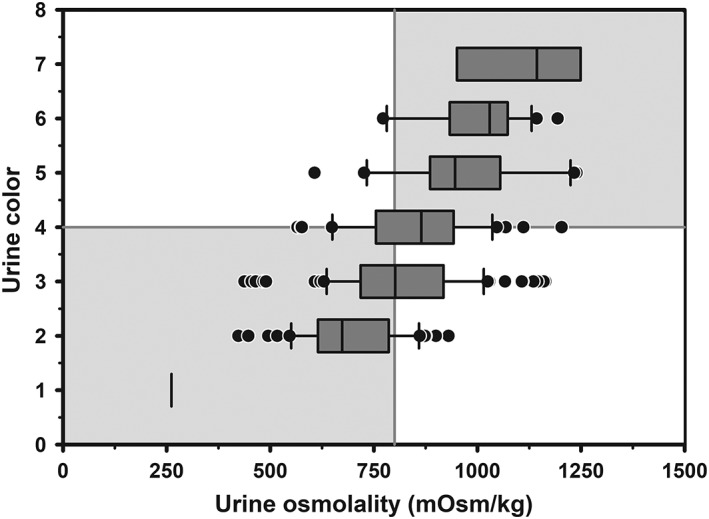
Box and whiskers plot of the association between urine color (8 levels) and urine osmolality (UOsm; reference method) for 237 urine samples obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows. The solid gray vertical line indicates the recommended cut point for UOsm (≥800 mOsm/kg) for diagnosing hypohydration, and the solid gray horizontal line indicates the optimal cut point for color (≥4) identified by logistic regression for diagnosing hypohydration. The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10% to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles)
3.5. Urine creatinine concentration
Mixed models regression of the relationship between UCreat and UOsm (reference method) for 217 urine samples obtained periodically from 20 periparturient multiparous Holstein‐Friesian cows indicated a linear relationship (Figure 6), such that UCreat = 0.152 × UOsm − 13.93. Logistic regression analysis indicated that the AUC for UCreat to identify hypohydration (UOsm ≥800 mOsm/kg) in dairy cattle was 0.76 (95% CI, 0.70‐0.82). The optimal cut point of UCreat for hypohydration was ≥95.3 mg/dL with Se of 0.68 (95% CI, 0.58‐0.76), Sp of 0.78 (95% CI, 0.69‐0.86), and +LR of 3.1 (95% CI, 2.1‐4.5). Moderate agreement was found between UOsm and UCreat for detecting hypohydration in dairy cattle (κ = 0.45; 95% CI, 0.33‐0.57).
Figure 6.
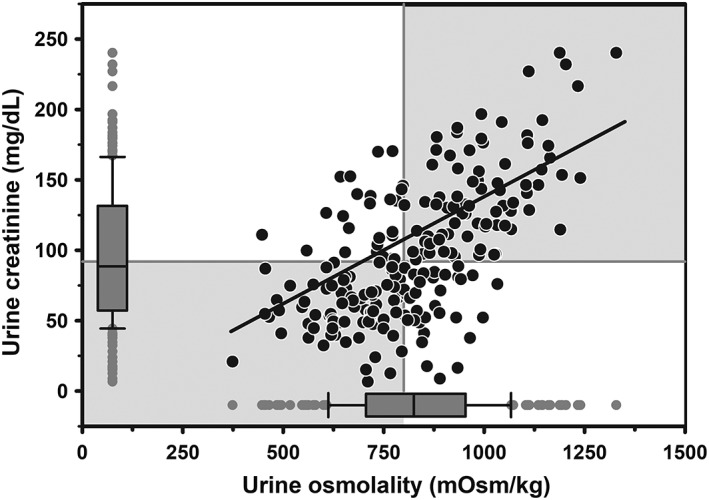
Scatterplot of the linear relationship between urine creatinine concentration (UCreat) and urine osmolality (UOsm; reference method) for 217 urine samples from 20 multiparous periparturient Holstein‐Friesian cows. Some data points are superimposed. The solid line is the line of regression. The solid gray vertical line indicates the recommended threshold value for detecting hypohydration (UOsm, ≥800 mOsm/kg), and the solid gray horizontal line indicates the optimal cut point value for UCreat (≥95.3 mg/dL) identified by logistic regression for diagnosing hypohydration. The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10% to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles)
3.6. Comparison of hypohydration predictors
Urine specific gravity measured by optical refractometry had a greater AUC (0.90) for identifying cows with hypohydration (UOsm ≥800 mOsm/kg) than that for UEC (AUC = 0.82; P = .006), UCreat (AUC = 0.76; P < .001), UColor (AUC = 0.74; P < .001), and USG‐D (AUC = 0.63; P < .001; Figure 7). Urine specific gravity measured by optical refractometry had the highest Se (0.84), +LR (4.1), and κ value (0.65) for identifying cows with hypohydration when compared to all other methods. However, UColor had the highest Sp (0.81) among the evaluated methods.
Figure 7.
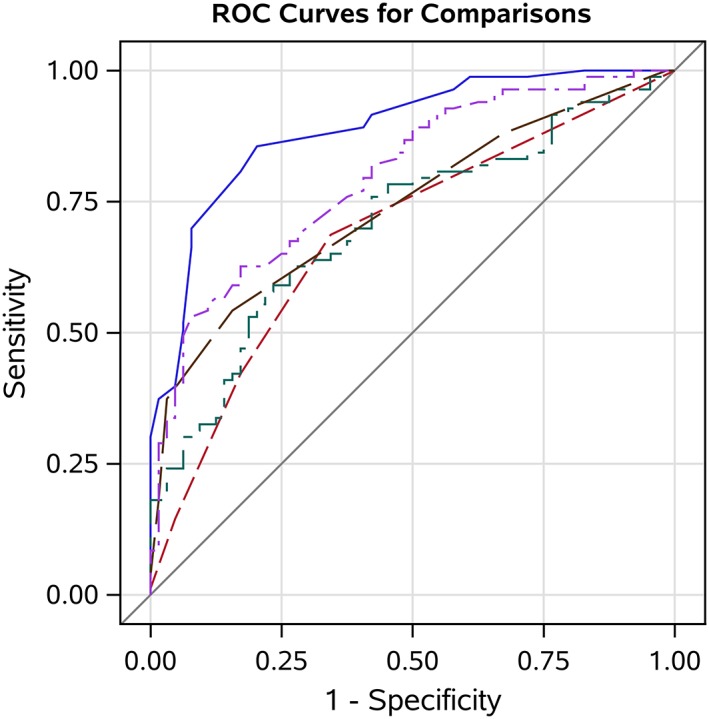
Receiver operating characteristic (ROC) curves and the area under the curve (AUC) demonstrating the diagnostic ability of urine specific gravity measured by optical refractometry (USG‐R, blue solid line; AUC = 0.90), urine electrical conductivity (UEC, purple 2‐dashed line; AUC = 0.82), urine creatinine concentration (UCreat, green dash‐dot line; AUC = 0.76), urine color (UColor, brown long‐dashed line; AUC = 0.74), and urine specific gravity measured by Multistix‐SG urine dipstick (USG‐D, red short‐dashed line; AUC = 0.63) to detect hypohydration (≥800 mOsm/kg) in dairy cattle. The diagonal line represents the ROC curve for a test of no predictive ability. The optimal cut points for detecting hypohydration were ≥1.030, ≥23.7 mS/cm, ≥4, ≥95.3 mg/dL, and ≥1.015, for the USG‐R, UEC, UColor, UCreat, and USG‐D, respectively
4. DISCUSSION
This study evaluated the clinical performance of 4 on‐farm tests for detecting high UC and hypohydration (UOsm ≥800 mOsm/kg) in periparturient dairy cows, including optical refractometry, OAKTON Con 6 conductivity meter, a custom‐designed urine color 8‐point chart, and Multistix‐SG urine dipstick. A laboratory test for detecting high UC, UCreat, was also examined. To the best of our knowledge, this is the first study to characterize the clinical utility of UEC and UColor for predicting UC in dairy cattle. The first major finding of this study was that optical refractometry provided the most accurate method for monitoring UC in dairy cattle. The second major finding was that UEC provided a moderately accurate method for monitoring UC in dairy cows. The third major finding was that an 8‐level urine color scale was predictive of UC in dairy cows; however, the AUC = 0.72 and +LR of 2.9 at the optimal color score cut point ≥4 suggested moderate clinical utility. The fourth major finding was that the urine reagent dipstick performed poorly when estimating UC in dairy cattle.
The strong association between USG‐R and UOsm observed in this study (r s = 0.85) was consistent with the findings of studies involving calf urine (r = 0.72),1 dairy cow urine (r = 0.92),3 human urine (r = 0.83),9 and dog urine (r = 0.93).5 The slope of the USG‐R‐UOsm relationship was 0.000030, which approximated that for calf urine (0.000024).1 The value of urine specific gravity measured by refractometry is dependent on the number, size, and weight of urine solutes, with the most abundant molecules in urine from healthy mammals being urea, electrolytes, and creatinine.36 Two previous studies reported a strong correlation (r = 0.83‐0.88, P < .001) between USG‐R and UCreat,37, 38 that was consistent with the results of this study. Surprisingly, we could not identify any research studies that statistically examined whether USG‐R was correlated with UCreat in cattle. The results of this study suggest that USG‐R can be used as an alternative to UCreat to correct the concentration of urinary metabolites for changes in urine free water.39, 40 However, further studies are required to validate this supposition.
The results of this study indicated that the urine reagent dipstick provided an inaccurate method for evaluating UC (r s = 0.31). To our knowledge, only 1 study had investigated the clinical performance of the urine reagent dipstick for measuring specific gravity in urine from dairy cattle; the results of that study indicated that the urine reagent dipstick measured 0.014 lower than USG‐R.3 Urine specific gravity was poorly correlated (r = 0.36) with UOsm in dog urine (r = 0.39),41, 42 and human urine.13, 43, 44 The dipstick pad has 3 main ingredients: a cation exchanger, pH color indicator (bromothymol), and buffers. Reagent dipstick principle is based on the ionic strength of the urine, whereby urine cations are attracted to negatively charged carboxyl groups of a partially dissociated polymer embedded in the strip pad. Dissociation of carboxyl groups depends on the ionic strength and temperature (which impact the value for the equilibrium constant, K a) of the urine sample as well as the pH; protons are released from the strip pad reagent poly (methyl vinyl ether/maleic anhydride) in response to an increase in ionic strength because of increased UOsm. The protons react with a pH indicator (bromothymol blue) in the strip pad, changing the pad color from deep blue green through different shades of green to yellow green.4, 29, 45 Urine pH therefore impacts the analytical performance of the reagent dipstick, particularly in alkaline urine, by promoting the release of protons from the polymer. Urine pH <6.0 or >8.0 interferes with the analytical accuracy of the reagent dipstick by altering the release of protons because of change in ionic strength.13, 14, 30, 46 Because UpH in dairy cattle fed nonacidogenic diets is alkaline with a pH exceeding 8.0 and can be below 6.0 in cattle fed an acidogenic diet,28 the results of this study indicate that the reagent dipstick method should not be used to measure urine specific gravity in dairy cattle.
Our results indicated that UEC is a clinically useful method for assessing UC in dairy cattle. The median value for UEC in periparturient dairy cattle was 23.7 mS/cm, which was similar to the mean value for urine from adult humans in India (21.6 mS/cm),17 and Turkey (25.5 mS/cm),19 but greater than that reported for children in Turkey (9.1 mS/cm).18 We found a good association (r s = 0.70) between UEC and UOsm that was consistent with studies in human urine, where correlation coefficients ranged from 0.67 to 0.91.18, 19, 20 Interestingly, based on a cut point of UOsm ≥800 mOsm/kg as the optimal threshold for defining hypohydration in humans,6, 7, 8 UEC had the second highest AUC (0.82) for diagnosing hypohydration in the study reported here after USG‐R (0.90). This result suggests that the majority of particles in bovine urine are charged.16
Our finding of the association between UColor and UOsm (r s = 0.58), and between UColor and USG‐R (r s = 0.58) was similar to that observed in humans,47 and consistent with results from other human studies.19, 21, 48 Urine color has been recently evaluated in dog urine as an indicator of UC, and a similar correlation was reported between UColor and USG‐R.49 Urine color is independent of diet because the color is generated by the concentration of urochrome pigment that is a byproduct of hemoglobin breakdown.21 However, UOsm is generated by the concentration of solutes, mainly urea, Na, K, and Cl, and therefore is diet dependent.50 The ROC curve analysis indicated that UColor provided a moderately accurate diagnostic test for assessing UC in dairy cattle. The diagnostic ability of a cut point of UColor ≥4 identified in this study was the same optimal threshold value for defining hypohydration in humans.8 Because urine color can be assessed easily and at no cost, the results of this study suggest that UColor has some clinical value as an alternative cow‐side tool to USG‐R for identifying the individual cow with hypohydration. For screening purposes, combining urine color with other diagnostic tests may improve the clinical utility of urine color to detect cows with hypohydration.
Urine creatinine is routinely measured to evaluate renal function because creatinine is excreted at a reasonably constant rate.51 The linear relationship reported in this study between UOsm and UCreat has been previously reported in human urine.52 The AUC for UCreat identified in this study suggests that UCreat could be of value in assessing UC in dairy cattle. However, UCreat is a nonspecific indicator of hydration status as it depends on several factors, including the muscle mass (which determines the rate of creatine metabolism), renal blood flow, and glomerular filtration rate.24, 27, 51 This is likely to negatively impact its clinical usefulness in evaluating hydration status in lactating dairy cows.
The main limitation of this study was that it was conducted in 20 periparturient dairy cattle in 1 herd fed an acidogenic diet during late gestation. As such, the study was not able to explore the effect of breed, season, and diet on urinary predictors of UC.
5. CONCLUSIONS
We conclude that USG‐R provides an accurate on‐farm method for assessing UC in dairy cattle; however, the urine reagent dipstick test is not a suitable method for evaluating UC. Urine electrical conductivity and UColor have moderate clinical utility as low‐cost (<20 cents/test for UEC) or no‐cost (UColor) cow‐side tests for assessing UC in dairy cattle.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All methods were evaluated and approved by the Purdue Animal Care and Use Committee.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
FIGURE S1 Box and whiskers plot of the association between corrected urine specific gravity measured by urine dipstick reagent (USG‐D) for a range of urine pH values for 241 urine samples obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows. Note that corrected USG‐D is associated with urine pH to a greater extent than urine osmolality (see Figure 3). The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10 to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles).
Megahed AA, Grünberg W, Constable PD. Clinical utility of urine specific gravity, electrical conductivity, and color as on‐farm methods for evaluating urine concentration in dairy cattle. J Vet Intern Med. 2019;33:1530–1539. 10.1111/jvim.15502
REFERENCES
- 1. Thornton JR, English BP. Specific gravity and osmolality as measures of urine concentration in the calf. Aust Vet J. 1976;52:335‐337. [DOI] [PubMed] [Google Scholar]
- 2. Silanikove N. The struggle to maintain hydration and osmoregulation in animals experiencing severe dehydration and rapid rehydration: the story of ruminants. Exp Physiol. 1994;79:281‐300. [DOI] [PubMed] [Google Scholar]
- 3. Alcántara‐Isidro GJ, García‐Rodríguez MB, Diez‐Prietoa IM, et al. Urine concentration in healthy and diseased dairy cows during the first month after calving: comparison of the refractometry and reagent strip methods. J Vet Sci. 2015;1:34‐41. [Google Scholar]
- 4. Chadha V, Garg U, Alon U. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol. 2001;16:374‐382. [DOI] [PubMed] [Google Scholar]
- 5. Ayoub JA, Beaufrere H, Acierno MJ. Association between urine osmolality and specific gravity in dogs and the effect of commonly measured urine solutes on that association. Am J Vet Res. 2013;74:1542‐1545. [DOI] [PubMed] [Google Scholar]
- 6. Manz F, Wentz A. 24‐h hydration status: parameters, epidemiology and recommendations. Eur J Clin Nutr. 2003;57:S10‐S18. [DOI] [PubMed] [Google Scholar]
- 7. Perrier ET, Buendia‐Jimenez I, Vecchio M, et al. Twenty‐four hour urine osmolality as a physiological index of adequate water intake. Dis Markers. 2015;2015:231063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kavouras SA, Johnson EC, Bougatsas D, et al. Validation of a urine color scale for assessment of urine osmolality in healthy children. Eur J Nutr. 2016;55:907‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wyness SP, Hunsaker JJ, Snow T, Genzen JR. Evaluation and analytical validation of a handheld digital refractometry for urine specific gravity measurement. J Appl Lab Med. 2016;5:65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voinescu GC, Shoemaker M, Moore H, et al. The relationship between urine osmolality and specific gravity. Am J Med Sci. 2002;323:39‐42. [DOI] [PubMed] [Google Scholar]
- 11. Stuempfle KJ, Drury DG. Comparison of 3 methods to assess urine specific gravity in collegiate wrestlers. J Athletic Training. 2003;38:315‐319. [PMC free article] [PubMed] [Google Scholar]
- 12. Haynes WM, Lide DR, Bruno TJ. CRC Handbook of Chemistry and Physics. 96th ed. CRC Press, Taylor & Francis Group: Boca Raton, Florida USA; 2015. [Google Scholar]
- 13. Roessingh AB, Drukker A, Guignard JP. Dipstick measurements of urine specific gravity are unreliable. Arch Dis Child. 2001;85:155‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adams LJ. Evaluation of Ames Multistix‐SG for urine specific gravity versus refractometry specific gravity. Am J Clin Pathol. 1983;80:871‐873. [DOI] [PubMed] [Google Scholar]
- 15. Welch AA, Mulligan A, Bingham SA, Khaw KT. Urine pH is an indicator of dietary acid‐base load, fruit and vegetables and meat intakes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)‐Norfolk population study. Br J Nutr. 2008;99:1335‐1343. [DOI] [PubMed] [Google Scholar]
- 16. Polashegg HD. On‐line dialyser clearance using conductivity. Pediatr Nephrol. 1995;9:S9‐S11. [DOI] [PubMed] [Google Scholar]
- 17. Marickar YF. Electrical conductivity and total dissolved solids in urine. Urol Res. 2010;38:233‐235. [DOI] [PubMed] [Google Scholar]
- 18. Kavukcu SM, Turkmen A, Soylu F, Kuralay F. Could conductivity be used as a parameter in urinalysis? J Pak Med Assoc. 1998;48:238‐240. [PubMed] [Google Scholar]
- 19. Kutlu M, Guler G. Assessment of hydration status by urinary analysis of elite junior taekwon‐do athletes in preparing for competition. J Sports Sci. 2006;24:869‐873. [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Wen C, Lin C, et al. Evaluating the performance of urine conductivity as screening for early stage chronic kidney disease. Clin Lab. 2014;60:635‐643. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong LE, Maresh CM, Castellani JW, et al. Urinary indices of hydration status. Int J Sport Nutr. 1994;4:265‐279. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong LE, Soto JA, Hacker FT, et al. Urinary indices during dehydration, exercise, and rehydration. Int J Sport Nutr. 1998;8:345‐355. [DOI] [PubMed] [Google Scholar]
- 23. Ehrig F, Waller S, Misra M, Twardowski ZJ. A case of ‘green urine. Nephrol Dial Transplant. 1999;14:190‐192. [DOI] [PubMed] [Google Scholar]
- 24. Wyss M, Kaddurah‐Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107‐1213. [DOI] [PubMed] [Google Scholar]
- 25. Braun JP, Lefebvre HP, Watson AJ. Creatinine in the dog: a review. Vet Clin Path. 2003;32:162‐179. [DOI] [PubMed] [Google Scholar]
- 26. De Groot T, Aafjes JH. On the constancy of creatinine excretion in the urine of the dairy cow. Br Vet J. 1960;116:409‐418. [Google Scholar]
- 27. Lee C, Morris DL, Dieter PA. Validating and optimizing spot sampling of urine to estimate urine output with creatinine as a marker in dairy cows. J Dairy Sci. 2019; In press;102:236‐245. [DOI] [PubMed] [Google Scholar]
- 28. Grünberg W, Donkin SS, Constable PD. Periparturient effects of feeding a low dietary cation‐anion difference diet on acid‐base, calcium, and phosphorus homeostasis and on intravenous glucose tolerance test in high‐producing dairy cows. J Dairy Sci. 2011;94:727‐745. [DOI] [PubMed] [Google Scholar]
- 29. Burkhardt AE, Johnston KJ, Waszak CE. A reagent strip for measuring the specific gravity of urine. Clin Chem. 1981;28:2068‐2072. [PubMed] [Google Scholar]
- 30. Moon DS, Chung YH, Park YC. Evaluation of reagent strip (Ames N‐Multistix SG) for urine specific gravity. Korean J Clin Pathol. 1984;4:69‐76. [Google Scholar]
- 31. Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989:1‐307. [Google Scholar]
- 32. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285‐1293. [DOI] [PubMed] [Google Scholar]
- 33. Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. The Lancet. 2005;365:1500‐1505. [DOI] [PubMed] [Google Scholar]
- 34. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159‐174. [PubMed] [Google Scholar]
- 35. Kandeel SA, Megahed AA, Ebeid MH, Constable PD. Ability of milk pH to predict subclinical mastitis and intramammary infection in quarters from lactating dairy cattle. J Dairy Sci. 2019;102(2):1417‐1427. [DOI] [PubMed] [Google Scholar]
- 36. Muscat JE, Liu A, Richie JP Jr. A comparison of creatinine vs. specific gravity to correct for urinary dilution of cotinine. Biomarkers. 2011;16:206‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration‐dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63‐67. [DOI] [PubMed] [Google Scholar]
- 38. Parikh CR, Gyamlani GG, Carvounis CP. Screening for microalbuminuria simplified by urine specific gravity. Am J Nephrol. 2002;22:315‐319. [DOI] [PubMed] [Google Scholar]
- 39. Gaines LT, Fent KW, Flack SL. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6‐hexamethylene diamine. J Environ Monit. 2010;12:591‐599. [DOI] [PubMed] [Google Scholar]
- 40. Jacob CC, Dervilly‐Pinel G, Biancotto G, Le Bizec B. Evaluation of specific gravity as normalization strategy for cattle urinary metabolome analysis. Metabolomics. 2014;10:627‐637. [Google Scholar]
- 41. van Vonderen IK, Kooistra HS, de Bruijne JJ. Evaluation of a test strip for the determination of urine specific gravity in the dog. Tijdschr Diergeneeskd. 1995;120:400‐402. [PubMed] [Google Scholar]
- 42. Dossin O, Germain C, Braun JP. Comparison of the techniques of evaluation of urine dilution/concentration in the dog. J Vet Med A. 2003;50:322‐325. [DOI] [PubMed] [Google Scholar]
- 43. McCrossin T, Roy LP. Comparison of hydrometry, refractometry, osmometry and Ames N‐multistix SG in estimation of urinary concentration. Aust Paediatr J. 1985;21:185‐188. [DOI] [PubMed] [Google Scholar]
- 44. Gouyon JB, Houchan N. Assessment of urine specific gravity by reagent strip test in newborn infants. Pediatr Nephrol. 1993;7:77‐78. [DOI] [PubMed] [Google Scholar]
- 45. Hesse A, Wuzel H, Classen A, Vahlensieck W. An evaluation of test sticks used for the measurement of the specific gravity of urine from patients with stone disease. Urol Res. 1985;13:185‐188. [DOI] [PubMed] [Google Scholar]
- 46. Kirschbaum BB. Evaluation of a colorimetric reagent strip assay for urine specific gravity. Am J Clin Pathol. 1983;79:722‐725. [DOI] [PubMed] [Google Scholar]
- 47. Wakefield B, Mentes J, Diggelmann L, Culp K. Monitoring hydration status in elderly veterans. West J Nurs Res. 2002;24:132‐142. [DOI] [PubMed] [Google Scholar]
- 48. Mentes JC, Wakefield B, Culp K. Use of a urine color chart to monitor hydration status in nursing home residents. Biol Res Nurs. 2006;7:197‐203. [DOI] [PubMed] [Google Scholar]
- 49. Cridge H, Wills RW, Lathan P. Correlation between urine color and urine specific gravity in dogs: can urine color be used to identify concentrated urine? Can Vet J. 2018;59:178‐180. [PMC free article] [PubMed] [Google Scholar]
- 50. Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol. 2009;29:178‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environ Health Persp. 2005;113:192‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeh HC, Lin YS, Kuo CC, et al. Urine osmolality in the US population: implications for environmental biomonitoring. Environ Res. 2015;136:482‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Box and whiskers plot of the association between corrected urine specific gravity measured by urine dipstick reagent (USG‐D) for a range of urine pH values for 241 urine samples obtained periodically from 20 multiparous periparturient Holstein‐Friesian cows. Note that corrected USG‐D is associated with urine pH to a greater extent than urine osmolality (see Figure 3). The box and whiskers plot represents the median (middle line), interquartile range (ends of the shaded rectangle), 10 to 90% confidence interval (whiskers), and values outside this confidence interval (small gray circles).


