Abstract
Background
Whether underweight calves respond differently to transport stress, enhancing their disease risk, is currently unknown.
Objective
To determine the effects of low body weight and transport stress on immune variables.
Animals
Twenty‐one 2‐ to 4‐week‐old male Holstein calves, housed on a commercial farm.
Methods
Randomized clinical trial. Full factorial design with 4 treatment groups: low body weight (≤46 kg)/no transport (LOWCON); low body weight/transport (LOWTRANS); normal body weight (>46 kg)/no transport (NORMCON), and normal body weight/transport (NORMTRANS). Transport duration was 2 hours.
Results
Transport significantly increased serum cortisol concentration (77.8 μg/mL; 95% confidence interval [CI], 37.8‐131.6; P < .001), interleukin (IL)‐17A (344.9 pg/mL; 95% CI, 32.2‐556.5; P = .04), and tumor necrosis factor‐α (TNF‐α) (218.2 pg/mL; 95% CI, 32.5‐368.3; P = .03) production after lipopolysaccharide (LPS) stimulation. Body weight did not affect any of the studied variables. However, the interaction of transport and body weight was significant. LOWTRANS calves showed increased monocyte count (2.0 × 109/L; 95% CI, 0.6‐4.2; P < .05) and interleukin IL‐17A production (106.0 pg/mL; 95% CI, 4.2‐306.9; P = .03) compared to normal weight calves and increased TNF‐α production (275.6 pg/mL; 95% CI, 2.6‐463.0; P = .02) compared to LOWCON calves in unstimulated peripheral blood mononuclear cells (PBMCs) after transport.
Conclusion and Clinical Importance
These findings contribute to our understanding of increased disease susceptibility of underweight calves when transported. Gamma globulin concentration was identified as important interfering factor in studies on immune variables in neonatal calves.
Keywords: cortisol, modulated leukocyte functions, preweaned calves, respiratory disease, welfare
Abbreviations
- BRD
bovine respiratory disease
- CF
crude fat
- CI
confidence interval
- ConA
Concanavalin A
- CP
crude protein
- CPM
counts per minute
- EDTA
ethylene diamine tetraacetic acid
- fpt
failure of transfer of passive immunity
- GC
glucocorticoids
- GR
glucocorticoid receptor
- IL‐10
interleukin‐10
- IL‐17A
interleukin‐17A
- IL‐1β
interleukin‐1β
- IL‐6
interleukin‐6
- IL‐8
interleukin‐8
- LOWCON
low body weight and no transport
- LOWTRANS
low body weight and transport
- LPS
lipopolysaccharide
- MG
macrogard, a ß‐glucan
- NORMCON
normal body weight and no transport
- NORMTRANS
normal body weight and transport
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate‐buffered saline
- PMA
phorbol myristate acetate
- RLU
relative light units
- ROS
reactive oxygen species
- Th 17
T helper 17
- TNF‐α
tumor necrosis factor‐α
- WBC
white blood cell
1. INTRODUCTION
Neonatal enteritis and pneumonia are the leading causes of morbidity, mortality, and antimicrobial use in calves.1, 2, 3 Especially in settings where neonatal calves (<4 weeks old) are commingled and transported, antimicrobial use is markedly higher than in closed herds.4 Transportation is a well‐known predisposing factor for bovine respiratory disease (BRD) through acquisition of pathogens by commingling of calves from many different farms as well as through activation of the hypothalamic‐pituitary‐adrenal axis resulting in increased plasma cortisol concentrations.5, 6, 7 As a consequence, neutrophil traffic and functions are affected,5, 6 the protein composition of the pulmonary epithelial lining fluid is altered8 and alveolar macrophage count in bronchoalveolar lavage fluid is decreased.9 This suggests a stress‐induced increase in respiratory disease susceptibility. Stress responses in calves during or after transport have extensively been studied in cattle ranging from 3 to 14 months old and in particular for longer transport durations (≥4 hours).5, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Only a few researchers investigated the effects of short transport (from 45 minutes to 4 hours) and only in animals older than 3 months.21, 22, 23, 24 Despite the fact that millions of male and female dairy calves are transported all over the world between their birth and 4 weeks of age, information about the effects of short transport on these animals is lacking.
Next to transport stress, body weight apparently influences disease susceptibility of preweaned calves. Underconditioned or so‐called “underweight” calves are at an increased disease and death risk.25 Veal calves weighing <43 kg at arrival at 2‐4 weeks of age had a higher risk for BRD at the early stage of fattening.26 This association is probably due to a poorer energy or immunological status impairing the calves' ability to cope with their new environment (housing, climate and feed) and multiple stressors (mixing of unfamiliar animals, handling, transport, restraint, and crowding).26 This hypothesis is supported by a few studies, which investigated the association between nutritional plane and immune function in young calves. Preweaned calves fed on a lower nutritional plane displayed increased neutrophil responses.27, 28 Moreover, blood mononuclear leukocytes from calves fed higher planes of nutrition for the first 60 days of life produced lower mitogen‐induced interferon gamma and more inducible nitric oxide on day 60 compared with preweaned calves fed a lower energy and protein density.29
Based on these observations, we hypothesized that underweight calves might show a different innate immune response to short transport than calves with normal body weight. Insights in the effects of short transport and body weight on preweaned calf immunity are useful for the development of preventive programs for the veal and dairy industry, resulting in reduced antimicrobial need/consumption.
Therefore, the objectives of this study were to determine (1) whether a short (2 hours) transport induces a stress response or altered white blood cell (WBC) dynamics and function in 2‐ to 4‐week‐old dairy calves and (2) whether these responses are affected by the body weight of the animals.
2. MATERIALS AND METHODS
2.1. Study design and animals
A randomized controlled field trial was conducted with a 2 × 2 factorial design and a 1 : 1 allocation ratio. Sample size was based on 1 of the primary outcome variables, the neutrophil reactive oxygen species (ROS) production test. To detect a difference of 2400 relative light units (RLU) between 2 test groups, with 95% certainty, 80% power, and based on an SD of 1200 RLU, 5 animals per group were needed.
The study was performed in August 2016 on a commercial dairy farm with 350 dairy cows, located in the province of Antwerp. Female calves born on this farm were raised for future milk production. Male calves were reared in another part of the farm for rosé meat production until 7 months of age. A total of 21 male Holstein Friesian calves were selected based on the first inclusion criterion age: not younger than 2 weeks and not older than 4 weeks. The second criterion was clinically healthy animals (temperature <39.5°C and no clinical abnormalities; special attention was paid to clinical signs such as diarrhea, depression, nasal discharge, eye discharge, coughing, dyspnea, and dropped ears). Both low and normal body weight calves originated from first and second lactation dams. The studied calves were pair‐housed in igloos (1.8 m2 per calf) with straw bedding. All calves received at least 5 L of fresh colostrum within 24 hours after birth. Thereafter, feeding consisted of 2 times daily 4 L (500 g solids) of commercially available milk replacer containing 21% crude protein (CP) and 17.7% crude fat (CF) on a dry matter base in individual drinking buckets. In addition, a texturized calf starter (CP = 16%; CF = 2.8%) and hay was available ad libitum.
On the farm, we awaited a period when a sufficient number of calves was born in a short period of time (to limit the age differences). The available calves were weighed the day before the experiment started. Calves were divided into “normal weight” (>46 kg) and “underweight” calves (≤46 kg) and randomized using Excel rand function (random number allocated to each calf, ranked from small to large and sequentially divided over each group) within their category (normal versus light) to be exposed to a 2‐hour truck transportation. Four test groups were made: (1) underweight/no transport (LOWCON); (2) underweight/transport (LOWTRANS); (3) normal weight/no transport (NORMCON); and (4) normal weight/transport (NORMTRANS). Allocation of animals to the test group was done by the primary investigator. Other investigators sampled blood and clinically evaluated the animals at all time points. The study was double blinded with the caretakers uninformed on group divisions and the statistician blinded from group identification. Calves were transported with a commercial truck, approved for animal transportation, at a stocking density of 0.85 m2 per calf and straw bedding, but no drinking water available. Calves were transported for 2 hours under a variety of road conditions, speed, and traffic, representative of normal traffic in Belgium. At arrival, the transported calves were blood sampled on the truck, unloaded, and returned to their original igloo and pen mates. The person sampling the blood on arrival was not the same person who sampled the blood at later time points. Control animals were blood sampled in front of the igloo. For practical reasons, the trial was split into 3 sessions with 7 calves each. Calves were divided as follows over the 4 treatment groups: LOWCON (n = 5), LOWTRANS (n = 5), NORMCON (n = 5), and NORMTRANS (n = 6).
2.2. Blood collection
All calves were blood sampled at 6 time points: (1) just before transport (−2 hours), (2) immediately on return to the home facility (0 hours), and at (3) 5 hours, (4) 24 hours, (5) 48 hours, and (6) 72 hours after transport.
Blood sampling of the pair‐housed calves occurred in front of the calf igloos at the indicated time points. Per igloo 1 calf of the pair was selected to go on transport, was blood sampled first, and loaded onto the truck. Thereafter, the control calf was blood sampled and left in the igloo. At all time points except for immediately after transport (0 hours), a total of 60 mL of peripheral blood was collected from the jugular vein of all calves into a 60 mL syringe and thereafter aliquoted into 25 mL evacuated tubes coated with anticoagulant lithium‐heparin (Vacutest Kima, Arzergrande, Italy), kept on ice for subsequent isolation of neutrophils, 25 mL anticoagulant lithium‐heparin on environmental temperature to isolate blood mononuclear cells, 5 mL into evacuated tubes coated with K3‐ethylene diamine tetraacetic acid (EDTA) (Vacutest Kima) to determine total and differential leukocyte counts, and 5 mL into evacuated serum tubes (BD Vacutainer, New Jersey) for measuring serum cortisol concentration, total protein, and protein fractions.
At time point 0 hour (=immediately after transport), a total of 10 mL blood from the jugular vein of all calves was collected and divided between a 5 mL serum vacuum tube (for analysis of serum cortisol concentration, total protein, and protein fractions) and a 5 mL K3‐EDTA‐coated tube (for determining cell counts).
2.3. Serum cortisol concentration, cell counts, and electrophoresis
Serum cortisol concentration was determined by a competitive electrochemiluminescence immunoassay and subsequent measurement of chemiluminescence by an automated analyzer (Cobas 6000; Roche Diagnostics, Rotkreuz, Switzerland) at an accredited laboratory (Zoolyx, Aalst, Belgium).
Total WBC count, differential WBC (neutrophil, lymphocyte, monocyte, and eosinophil) count, and platelet numbers were determined using an automatic cell counter (ProCyte analyzer; IDEXX Laboratories, Westbrook, Maine).
The amount of albumin, α1‐, α2‐, β‐ and γ‐globulins in serum samples was determined by solid phase extraction (Capillarys, Sebia, France) at an accredited laboratory (Zoolyx) using optimized settings for bovine samples.30 Additionally, total protein content was measured colorimetrically on an automated biochemistry analyzer (Cobas 6000; Roche Dignostics, Mannheim, Germany). Quality controls previously run were within quality assurance specifications.
2.4. Isolation of peripheral blood mononuclear cells
Heparin‐blood (12.5 mL) was diluted 1 : 1 with PBS, underlayered with 15 mL Lymphoprep (AXIS‐SHIELD PoC AS, Oslo, Norway) and centrifuged at 800g for 30 minutes at 18°C. Peripheral blood mononuclear cells (PBMCs) were aspirated, washed with phosphate‐buffered saline (PBS) + 1 mM EDTA and centrifuged at 300g for 10 minutes at 4°C. After lysis of erythrocytes in ammonium chloride and subsequent centrifugation at 300g for 10 minutes at 4°C, pelleted cells were washed in PBS‐EDTA and resuspended in leukocyte medium RPMI‐1640 supplemented with 10% (vol/vol) fetal bovine serum (Gibco, Merelbeke, Belgium), 100 IU/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL kanamycin, 100 mM nonessential amino acids (Gibco, Merelbeke, Belgium), 1 mM sodium pyruvate (Gibco, Merelbeke, Belgium), 2 mM L‐glutamin (Gibco, Merelbeke, Belgium) at 107 cells/mL.
2.5. Isolation of neutrophils
Neutrophils were isolated as previously described.5 In short, ice‐cold heparin‐blood (25 mL) was centrifuged at 1000g for 20 minutes at 4°C. Plasma, buffy coats, and approximately 2/3 of the red cell pack were aspirated and discarded. The remaining red cell pack was diluted to 25 mL with ice‐cold PBS and underlayered with 12 mL Percoll (1.084 g/mL; GE Healthcare Bio‐Sciences AB, Uppsala, Sweden) and centrifuged at 400g for 40 minutes at 22°C. PBS, mononuclear cells, and Percoll were aspirated and discarded. Erythrocytes in the remaining granulocyte pellet were lysed with ammonium chloride and centrifuged at 800g for 5 minutes at 4°C. The pelleted cells were washed in ice‐cold PBS and resuspended at 106 cells/mL in RPMI‐1640 without phenol red.
2.6. Production of reactive oxygen species by monocytes and neutrophils
Reactive oxygen species production was measured using a chemiluminescence assay as described.31 Neutrophils were seeded in a 96‐well plate (Nunc Technologies, Rochester, New York) at 2 × 105 cells/wells in 200 μL of RPMI, whereas the PBMCs were seeded at a concentration of 2 × 106 cells/well. The plate was incubated at 37°C for 2 hours in a humidified atmosphere with 5% CO2, allowing cells to adhere to the plastic surface of the plate. The supernatant was removed, and 175 μL luminol was added to a final concentration of 0.5 mM. After 5 minutes of background measurement at 37°C, 25 μL of cell stimulants and control agent were added. The following agents were used: Hanks Balanced Salt Solution with Ca2+/Mg2+ (HBSS) as negative control (Gibco Life Technologies Corp, Grand Island), phorbol myristate acetate (PMA; Sigma‐Aldrich, Bornem, Belgium) 25 μg/mL as positive control, and 200 μg/mL Macrogard (MG; Biotech Pharmacon ASA, Norway), a ß‐1,3/1,6‐glucan which is able to stimulate ROS production in these cells.31, 32 Each stimulation was performed in duplicate. Reactive oxygen species production was measured during 120 minutes using a luminometer and was expressed as RLU.
2.7. PBMC proliferation assay
Peripheral blood mononuclear cells from normal and low body weight calves were stimulated with leukocyte medium supplemented with 5 × 10−5 M β‐mercaptoethanol (Sigma‐Aldrich, Overijse, Belgium) as negative control, 10 μg/mL Concanavalin A (ConA, positive control; Sigma‐Aldrich), 0.3 μg/mL ConA, and a combination of 0.3 μg/mL ConA and 50 μg/mL MG. The proliferation assay was performed as described.32 The different agents were added (100 μL/well) to the wells of a 96‐well flat‐bottomed microtiter plate (Nunc Technologies) containing 5 × 105 PBMC per well at a final volume of 200 μL. Each stimulation was performed in duplicate. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. After 72 hours, the cells were pulse labeled with 1 μCi of [3H]‐thymidine (Amersham ICN, Bucks, United Kingdom) per well, and the incubation was continued for 18 hours. Thereafter, cells were harvested onto glass fiber filters (PerkinElmer, Life Science, Brussels, Belgium). The radioactivity incorporated into the DNA was measured using a ß‐scintillation counter (PerkinElmer, Life Science, Brussels, Belgium) and was expressed as counts per minute (CPM).
2.8. Cytokine secretion by PBMCs
To evaluate the effect of transport stress and body weight on cytokine production by PBMCs, cytokine concentration in the supernatant of unstimulated, LPS‐ and BG‐stimulated PBMCs was determined. The concentration of pro‐inflammatory (tumor necrosis factor [TNF]‐α, interleukin [IL]‐1β, IL‐6, IL‐17A) and anti‐inflammatory cytokines (IL‐10) and the chemokine IL‐8 was measured. Peripheral blood mononuclear cells were seeded in a 24‐well plate at a concentration of 5 × 106 cells/well in 1 mL leukocyte medium and incubated with medium (as a negative control), lipopolysaccharide (LPS, Escherichia coli 0111:B4; 10 μg/mL, Sigma‐Aldrich) and a particulate beta‐glucan (40 μg/mL) for 24 hours at 37°C in a humidified atmosphere with 5% CO2. Subsequently, cell‐free supernatant was harvested and stored at −80°C. The concentrations of the following interleukins (IL‐1ß, IL‐8, IL‐6, IL‐10, IL‐17A) and TNF‐α were determined using commercially available ELISA kits: TNF‐α, IL‐6 (R&D Systems Inc, Minneapolis, Minnesota), IL‐10 (Bio‐Rad Laboratories, N.V. Temse, Belgium), IL‐1β, IL‐8 and IL‐17A (Kingfisher Biotech, Inc, St. Paul, Minnesota) following the manufacturer's instructions.
2.9. Statistical analyses
Results are presented as mean ± SD. Data were analyzed with SAS 9.4 (SAS Institute Inc, Cary, North Carolina). The experimental unit was the individual calf. All ROS production and PBMC proliferation tests were performed in duplicate, and the mean value was used in the analysis. For all continuous outcome variables, except the serum protein fractions, a log+1 transformation was needed to obtain a normal distribution. For cortisol, the relative serum cortisol concentration was calculated by the value at a given time point divided by the concentration at time point −2 hours multiplied by 100%. The primary outcome variables were ROS production (in RLU), PBMC proliferation (in CPM), and the different cytokine concentrations (in pg/mL and for IL‐8 only in ng/mL). The secondary outcomes were serum cortisol concentration (in μg/mL), WBC differential counts (in cells × 109/L) and protein fractions (in g/L).
To determine the effects of body weight and transport (0/1) on serum cortisol concentrations, WBC counts (total, neutrophil, monocyte, and lymphocytes), ROS production by neutrophils/monocytes, PBMC proliferation, and the different cytokines produced (TNF‐α, IL‐1ß, IL‐6, IL‐17A, IL‐10, IL‐8) linear mixed models (PROC MIXED) with repeated measurements were used. The study session (1–3) was added as a fixed factor to each model to account for clustering of calves within a session. The γ‐globulin concentration (g/L) of the first sampling point was added to each model as a continuous variable to be evaluated as a potential confounder or interfering variable. To better visualize the relationship between γ‐globulin and the different outcome variables, a binary variable was constructed based on an internationally well excepted cutoff (<10 g/L; ≥10 g/L).33 The models were rerun with the binary variable instead of the continuous variable. A maximum likelihood model with Satterthwaite approximation and a variance components covariance structure was used. Sampling time was added as the repeated within‐subjects effect. Between‐subjects effects were the test groups (LOWCON, LOWTRANS, NORMCON, and NORMTRANS). Post hoc comparisons were done with Bonferroni corrections. Normality of residuals was confirmed by the Shapiro‐Wilk statistic and normal probability plots. Significance was set at P < .05, and .05 < P < .10 was considered a trend.
3. RESULTS
3.1. Animals and observations
Over 3 sessions, a total of 21 calves (10 underweight and 11 normal weight animals) were included, of which 11 were transported. Their mean age was 18 ± 5.5 days and mean body weight 46.3 ± 5.3 kg. All calves were initially healthy, but 6 calves developed diarrhea during the trial. Significantly more underweight calves developed diarrhea (40% [4/10] versus 18% [2/11]; P < .01). No diarrhea cases occurred in the transported group compared to 60% (6/10) in the nontransported group. No significant age effect on the occurrence of diarrhea or body weight could be demonstrated.
3.2. Effects on serum cortisol concentration, WBC counts, and serum protein fractions
Both transported groups showed a significant increase in serum cortisol concentration (77.8 μg/mL; 95% confidence interval [CI], 37.8‐131.6; P < .001) after transport (Figure 1). Body weight did not influence serum cortisol concentrations at any stage of the trial. The peak in serum cortisol concentration in both transported groups was only short‐lived, and serum cortisol concentration was back at basal concentrations 5 hours after transport. There was a positive association between γ‐globulin concentration and serum cortisol concentration (P < .001) before transport. The stress response was accompanied by an increase in total WBC count (Figure 2A). The LOWTRANS group showed a significant rise in monocyte count (2.0 × 109/L; 95% CI, 0.6‐4.2; P < .05) compared to the 3 other groups, and a numerical rise in neutrophil and lymphocyte count was present 5 hours after transport (Figure 2B‐D). Animals with lower γ‐globulin concentrations showed higher lymphocyte counts (P = .01). The LOWCON group showed significant lower albumin concentrations compared to all other groups (P < .001) (Table 1). Also, the session was significantly associated with the γ‐globulin concentration of calves. In session 3, 80% (4/5) of the calves had failure of transfer of passive immunity (γ‐globulin concentration <10 g/L), whereas in sessions 1 and 2, this was 25% (2/8). The session effect was significant for all protein fractions (P < .001). No other significant differences in serum protein fractions among the 4 test groups were detected.
Figure 1.
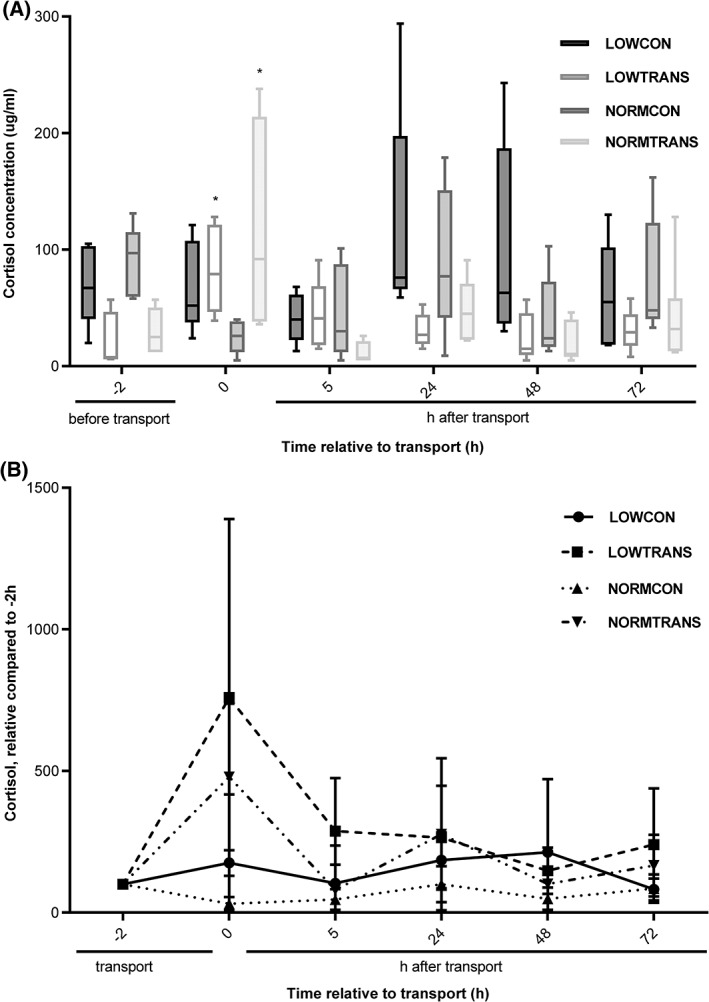
Serum cortisol concentration in underweight and normal weight dairy calves, exposed to short transport or no transport in (A) LOWCON (low body weight, no transport [n = 5]), LOWTRANS (low body weight, transport [n = 5]), NORMCON (normal body weight, no transport [n = 5]), and NORMTRANS (low body weight, transport [n = 6]) calves. Values are measured at time points before and after transport. And (B) the relative serum cortisol concentration changes in the 4 groups of calves related to the initial serum cortisol concentration at time point −2 hours (before transport); data are shown in mean ± SD; * represents a significant (P < .05) effect between groups
Figure 2.
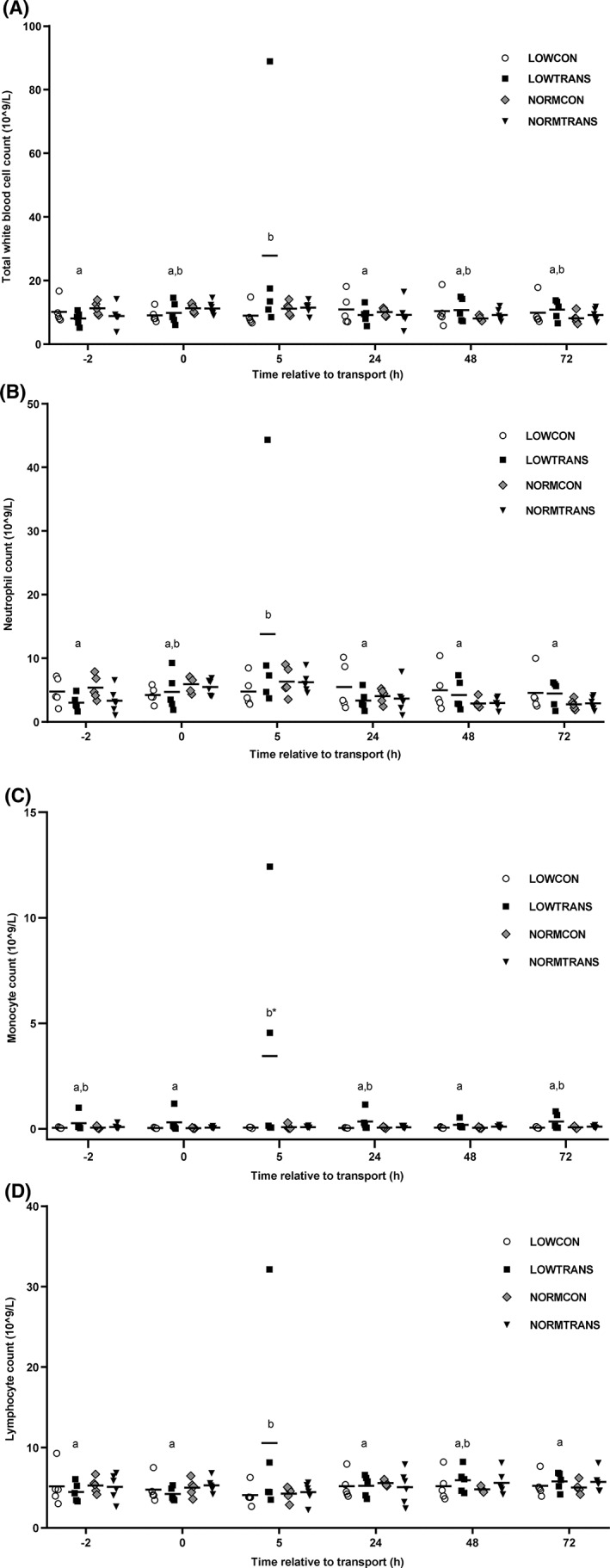
White blood cell counts of underweight and normal weight groups of calves after short transport of 2 hours or no transport. Groups were as followed: LOWCON (low body weight, no transport [n = 5]), LOWTRANS (low body weight, transport [n = 5]), NORMCON (normal body weight, no transport [n = 5]), and NORMTRANS (low body weight, transport [n = 6]). (A) Total white blood cell count, (B) neutrophil count, (C) monocyte count, and (D) lymphocyte count. Results are shown as mean ± SD; * represents a significant (P < .05) effect between groups. Items with different letters are statistically different within a series (P < .05)
Table 1.
Effect of body weight and short transport on serum protein concentrations (mean ± SD) in 2‐ to 4‐week‐old male dairy calves (n = 21)
| Variable | Low body weight | Normal body weight | |||||
|---|---|---|---|---|---|---|---|
| (≤46 kg) | (>46 kg) | P‐value | |||||
| No transport | Transport | No transport | Transport | Time | Test group | Session | |
| Total protein, g/L | 54.3 ± 6.3 | 54.7 ± 2.8 | 59.6 ± 5.8 | 58.0 ± 4.3 | .98 | .36 | <.01 |
| Albumin, g/L | 22.0a ± 1.3 | 24.2b ± 1.0 | 25.1b ± 1.7 | 25.3b ± 2.9 | .42 | <.001 | <.01 |
| α‐globulin, g/L | 15.2 ± 2.6 | 14.2 ± 2.0 | 13.7 ± 4.3 | 14 ± 2.4 | <.001 | .10 | <.001 |
| β‐globulin, g/L | 7.6 ± 2.8 | 7.3 ± 1.7 | 8.5 ± 2.0 | 7.8 ± 1.9 | <.001 | .81 | <.001 |
| γ‐globulin, g/L | 9.5 ± 3.8 | 8.7 ± 2.5 | 11.5 ± 4.5 | 10.9 ± 3.4 | .98 | .36 | <.01 |
Items with different letters are statistically different (P < .05).
3.3. Effects on WBC function
The LOWTRANS animals showed increased ROS production from 5 hours after transport onward (Figure 3A‐C). Significant time and session effects were present for neutrophils in all models (P < .05). No significant effects of body weight or transport on ROS production by monocytes could be detected (data not shown).
Figure 3.
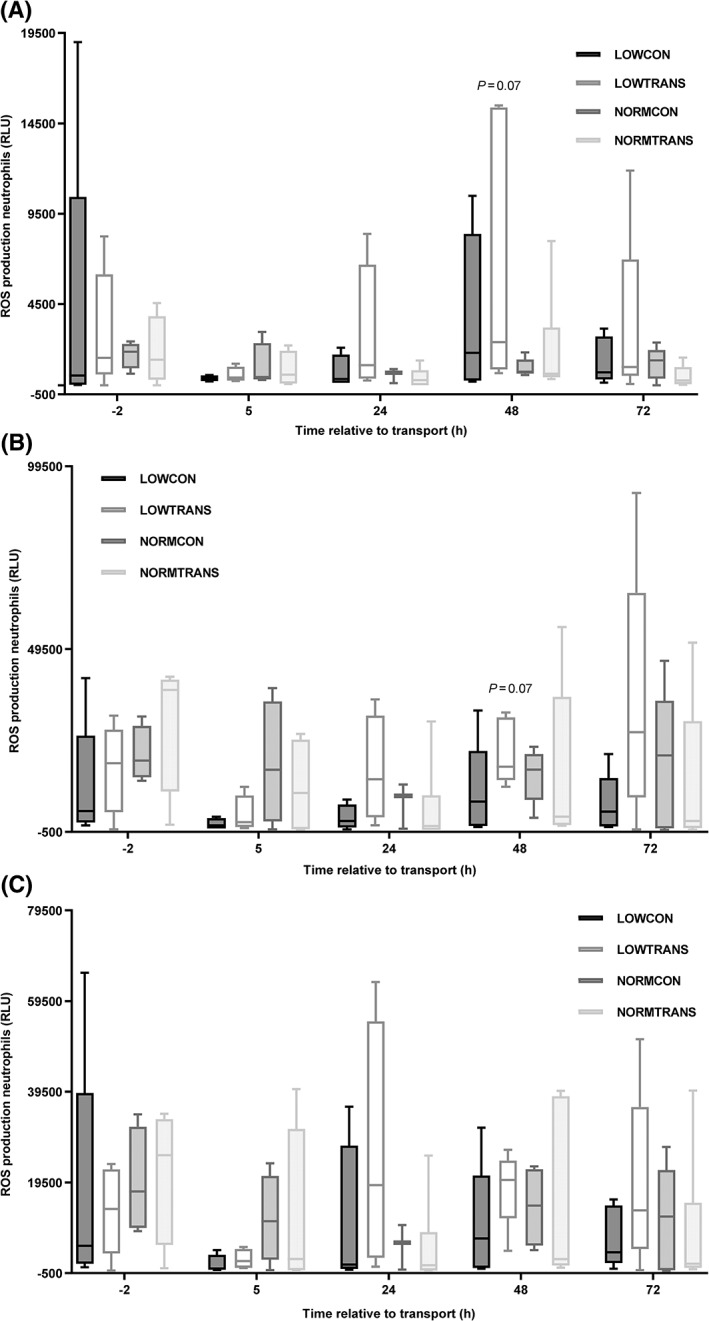
Reactive oxygen species (ROS) production of neutrophils from underweight and normal weight calves subjected to short transport of 2 hours or no transport. Groups were as followed: LOWCON (low body weight, no transport [n = 5]), LOWTRANS (low body weight, transport [n = 5]), NORMCON (normal body weight, no transport [n = 5]), and NORMTRANS (low body weight, transport [n = 6]). A, Reactive oxygen species production of neutrophils without stimulation (negative control; cells suspended in Hanks balanced salt solution (HBSS), (B) after stimulation with 25 μg/mL phorbol myristate acetate (PMA), and (C) after stimulation of the same groups of calves with 200 μg/mL Macrogard. P = .07 represents a trend towards higher ROS production in the LOWTRANS animals compared to the NORMTRANS calves. Results are shown as mean ± SD
To evaluate the proliferation response of lymphocytes, PBMC proliferation was measured in unstimulated cells and after LPS and MG stimulation. Both in unstimulated, LPS, or MG‐stimulated PMBCs, no significant differences among the 4 test groups could be demonstrated. However, the γ‐globulin concentration was negatively associated with PBMC proliferation in unstimulated cells (P = .06), and after stimulation with 0.3 μg/mL ConA (P < .03), 0.3 μg/mL ConA + 50 μg/mL MG (P = .01) and 10 μg/mL ConA (P < .001) as positive control (Figure 4). The time effect was significant (P < .001) for 0.3 μg/mL ConA but not for 0.3 μg/mL ConA + 50 μg/mL BG stimulation. The session effect was significant (P < .001) on any occasion.
Figure 4.
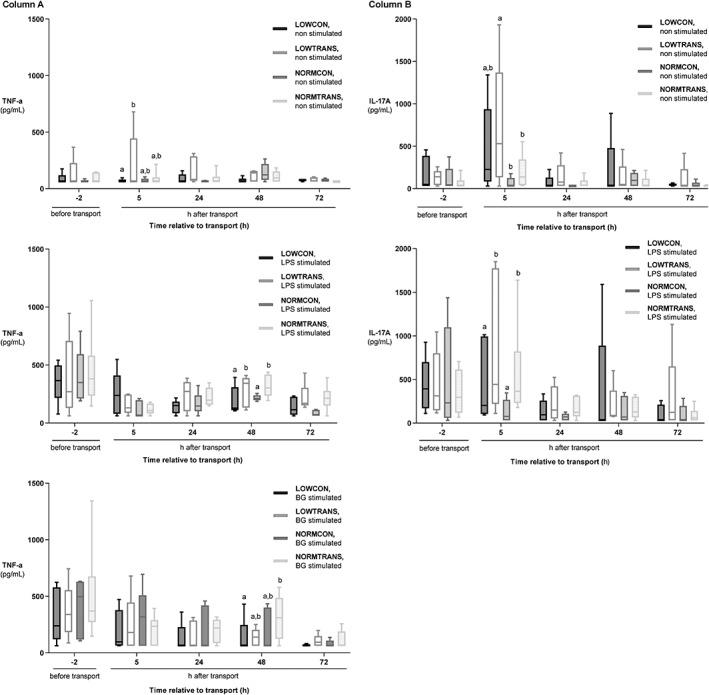
Cytokine production in low body weight and normal body weight dairy calves after short transport of 2 hours or no transport. Groups were as followed: LOWCON (low body weight, no transport [n = 5]), LOWTRANS (low body weight, transport [n = 5]), NORMCON (normal body weight, no transport [n = 5]), and NORMTRANS (low body weight, transport [n = 6]). Column (A) shows the cytokine production of peripheral blood mononuclear cells (PBMC) from the 4 examined groups of calves in unstimulated (blanco), lipopolysaccharide (LPS), and beta‐glucan (BG)–stimulated cells. Column (B) depicts the cytokine production of PBMCs of the same groups of calves in unstimulated and in LPS‐stimulated cells. Results are expressed as mean ± SD. Items with different letters are statistically different (P < .05)
3.4. Effects on cytokine release by PBMCs
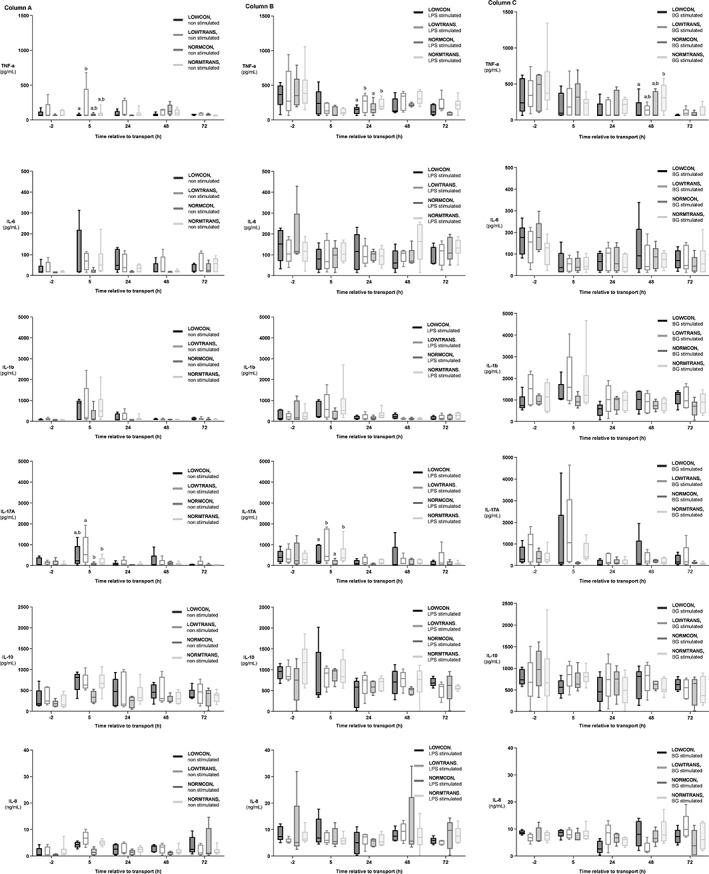
The increases in all tested pro‐ and anti‐inflammatory cytokines and chemokine IL‐8 in unstimulated PBMCs were highest 5 hours after transport and normalized 24 hours after transport (Appendix 1). In unstimulated cells, LOWTRANS animals showed significantly higher concentrations of TNF‐α (275.6 pg/mL; 95% CI, 2.6‐463.0; P = .02) compared to the LOWCON group. Also, IL‐17A was increased compared to the NORMTRANS (106.0 pg/mL; 95% CI, 4.2‐306.9; P = .03) and NORMCON (139.0 pg/mL; 95% CI, 15.2‐395.7; P < .01) calves 5 hours after transportation. No other effects on cytokines were observed in unstimulated PBMCs. After stimulation with LPS, both transported groups exhibited increased TNF‐α (218.2 pg/mL; 95% CI, 32.5‐368.3; P = .03) and IL‐17A (344.9 pg/mL; 95% CI, 32.2‐556.5; P = .04) concentrations 48 hours after transport and 5 hours after transport, respectively, which was independent from body weight. After BG‐stimulation, 48 hours after transport NORMTRANS calves showed significantly higher TNF‐α concentrations compared to LOWCON animals (381.1 pg/mL; 95% CI, 37.2‐632.1; P = .02) (Figure 5). Neither transport nor body weight did affect any of the other studied cytokines. Time effect was significant in all models except for IL‐10 (both LPS and BG), IL‐8 (LPS only), and IL‐6 (LPS only). The session effect was significant in all models except for IL‐17A (unstimulated and LPS), IL‐10 (LPS), IL‐8 (LPS), and TNF‐α (LPS).
Figure 5.
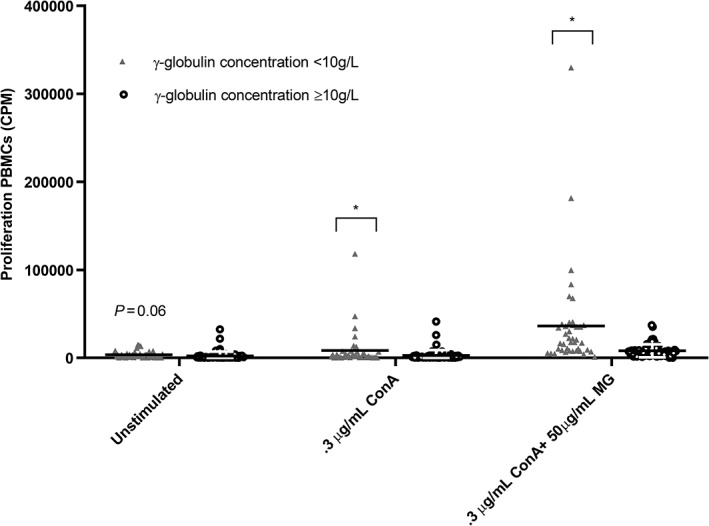
Association of serum γ‐globulin concentration with proliferation ability of peripheral blood mononuclear cells (PBMC) in calves with low γ‐globulin content (<10 g/L) or normal γ‐globulin level (≥10 g/L), after stimulation with leukocyte medium only (used as negative control = unstimulated), 0.3 μg/mL concanavalin A (0.3 ConA), and the combination of 0.3 μg/mL Concanavalin A plus 50 μg/mL Macrogard (0.3 ConA + 50 μg/mL MG). All cells also reacted to the positive control (10 μg/mL ConA) (data not shown). All proliferation ability appeared to be negatively associated with serum γ‐globulin content of the calves. Results are shown as mean ± SD; * represents a significant (P < .05) effect between groups
Gamma globulin concentration was an important confounding or interfering variable in the relationship of body weight/transport and cytokine concentrations. In unstimulated PBMCs, γ‐globulins were positively associated with IL‐17A (P < .001) and negatively with IL‐10 (P = .04). After stimulation with LPS, a negative association of γ‐globulin concentration with TNF‐α (P < .01), IL‐17A (P = .02), and IL‐6 (P < .01) was seen. Also after BG stimulation, an increasing γ‐globulin concentration was associated with a decreasing IL‐10 (P = .04) and IL‐6 (P = .05) concentration. The difference in cytokine concentration between calves with low (<10 g/L) and normal (≥10 g/L) γ‐globulin concentrations is visualized in unstimulated, LPS, and BG‐stimulated PBMCs in Figure 6.
Figure 6.
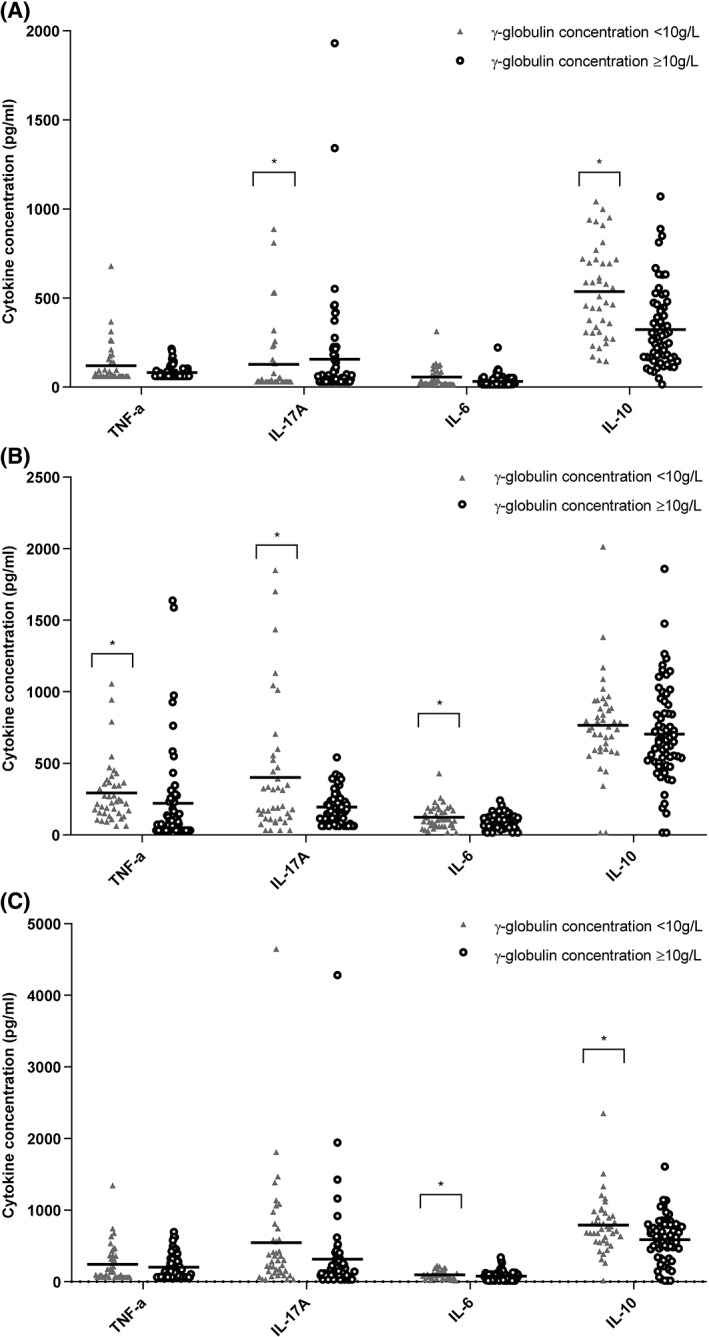
Association of serum γ‐globulin concentration with the cytokine production of peripheral blood mononuclear cells (PBMC) in calves with low γ‐globulin content (<10 g/L) or normal γ‐globulin content (≥10 g/L). (A) In unstimulated cells, (B) in PBMCs stimulated with 10 μg/mL of lipopolysaccharide (LPS), and (C) in PBMCs stimulated with 40 μg/mL beta‐glucan (BG). All cytokine production appeared negatively associated with the γ‐globulin content of calves (lower γ‐globulin meant increased cytokine production), with the exception of interleukin (IL)‐17A in unstimulated cells. Here, higher γ‐globulin content signified higher IL‐17A production. Results are shown as mean ± SD; * represents a significant (P < .05) effect between groups
4. DISCUSSION
In the present study, we demonstrated that young calves showed a classical stress response after short transport, which is comparable to older animals. However, the peak in serum cortisol concentration after 2 hours of transport was not as pronounced as in most previous studies in older animals or on longer transports.5, 6, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 The observed lower stress response in this study might be due to the young age of the animals, breed, or character effects, but most probably due to a relatively mild stressor compared to previous studies. Transport was performed under ideal conditions, with a higher space allowance in the truck than required by EU legislation and possibly more animal friendly handling than under current commercial conditions. Calves were able to lie down, which has a stress reducing effect.13 Previous studies in older animals showed that the rise in serum cortisol concentration during/after transport is associated with a transient increase in total WBC and neutrophil count.5, 12, 15, 16, 17, 18, 19, 20, 23 In contrast, in the present study, only low body weight calves showed this increase 5 hours after transport. The significant rise in circulating monocytes 5 hours after transport in LOWTRANS animals contradicts other studies in older animals, which reported unaltered or decreased monocyte counts.10, 15, 23 Because the rise in circulating WBCs only occurred in the LOWTRANS group 5 hours after transport and untransported underweight animals did not show this leukocytosis, it is plausible to presume a connection between low body weight and a different response to transport stress. It is likely that glucocorticoids (GCs) influence neutrophil production and release from bone marrow in cattle34 or cause a robust leukocyte redistribution by inducing increased expression of surface receptors on stress‐activated monocytes.35, 36 Possibly a combination of both will exist.
Previous studies demonstrated that oxidative burst activity of neutrophils from stressed animals due to transport is lowest at the time neutrophilia peaks,37 followed by enhanced activity (rebound effect) 96 hours after transport.19 In the present study, we observed similar effects: ROS production was lowest in both low body weight groups 5 hours after transport, followed by increased ROS production mainly in LOWTRANS calves until 48 hours after transport in unstimulated (P = .07) and PMA stimulated (P = .07) neutrophils, in MG‐stimulated (P = .09) neutrophils until 24 hours after transport. Owing to a large individual variation in the ROS production, only slight differences between test groups existed. However, the observed activity in neutrophil ROS production is in accordance with previous work in older cattle.12, 19
After ex vivo stimulation with LPS, PBMCs of the transported groups produced more IL‐17A and TNF‐α compared to the control groups. Because TNF‐α is a major pro‐inflammatory cytokine, chiefly released by activated macrophages, it acts on several different signaling pathways to regulate activation of inflammation.38 Upon activation, it can also be produced by other cell types such as T‐lymphocytes, natural killer cells, monocytes, or dendritic cells present in the PBMC fraction. These findings agree with research in older cattle, where TNF‐α concentration was increased 48 hours after transport.19 Also, IL‐17A is considered to be a predominantly pro‐inflammatory cytokine, which is only recently included in cattle research. The increased concentrations in this study might signify that also T helper 17 (Th17) cells are in an activated state because of increased stress‐related serum cortisol concentration. Th17 cells play a vital role in protecting the host from bacterial and fungal infections, particularly at mucosal sites such as lung, gut, and the oral cavity.39, 40, 41 On the other hand, Th17 cells only might not be adequate to explain the very early IL‐17A increase in the PBMC supernatant 5 hours after transport. Possibly, this increase is also due to production of IL‐17A by activated innate IL‐17‐producing cells such as γδT cells, invariant natural killer T cells, natural killer cells, and myeloid cells present in the isolated PBMC, which have crucial roles during stress responses and host defense.42 Early IL‐17A production activates several signaling pathways that in turn lead to the induction of chemokines which recruit monocytes and neutrophils to the site of injury or pathogen invasion, promoting inflammation.43 For this, IL‐17 acts in concert with TNF‐α, IL‐1,40 and IL‐6,42 cytokines which were numerically increased in low body weight groups and the NORMTRANS group after transport in LPS or BG‐stimulated cells or in both stimulations (Appendix 1). Moreover, the function of IL‐17A is not only pro‐inflammatory, because it also protects the epithelium by stimulating the production of antimicrobial and adhesion molecules by epithelial cells lining the mucosa.44
For the second part of the hypothesis, we examined the influence of calves' body weight on transport stress. We expected that the effects of transport stress would be more severe or at least different in underweight calves. Nevertheless, analysis only showed small differences between treatment groups when accounting for γ‐globulin level. The only major difference that remained was that underweight calves apparently react to transport stress with a more severe release of IL‐17A and TNF‐α in unstimulated cells. Because IL‐17A release is one of the earliest events in the inflammatory cascade, this might signify that transport induced a more pro‐inflammatory state in underweight animals. Also, the observation that 5 hours after transport unstimulated as well as LPS‐stimulated cells of underweight calves exhibited comparable concentrations of cytokines when exposed to stress points in this direction (Appendix 1). Whether this pro‐inflammatory state leads to better protection or excessive inflammation in these animals is currently unknown.
To explain the observed differences between transported and control animals and low and normal body weight calves, the most likely mechanism is the effect of serum cortisol concentration. Depending on the concentration, cortisol can have suppressive, stimulatory,45, 46 and also preparatory effects47, 48 on the immune system. Preparatory effects can be observed when a transient (hours) in vivo exposure to stress‐induced GC concentrations is followed by a return to normal, basal concentrations47 and are caused by transcriptional and phenotypic changes in immune effector cells that prime them for enhanced responses to a subsequent immune stimulus.48 These effects typically manifest several hours after transient exposure to stress‐increased GCs and might last for up to a week.49 They appear to enhance an organism's resistance to a subsequent stressful event, for example, injury or infection with pathogens,47 and involve both innate49, 50, 51 and adaptive immune cells..52, 53 In our study, preparatory effects are suspected from 5 hours after transport on, when serum cortisol concentration is back to basal concentrations. With LPS‐ and BG‐stimulation, we mimicked an inflammatory stimulus within this period, resulting in a pro‐inflammatory reaction48 (IL‐17A and TNF‐α). Possibly, low body weight calves respond earlier and more vigorously than normal body weight calves. Transport could here represent a repeated (and intermittent) exposure to increased GC concentrations, as the serum cortisol concentration in calves is also increased during a period of fasting54 or disease. These repeated, transient increases of serum cortisol concentration might also account for a changed concentration, a changed sensitivity of GRs or a combination of both adaptations in target cells.
There are many possible explanations on why animals have a low body weight for their age such as genetics,55 fetal programming effects (eg, heat stress, maternal influence),56 nutritional plane, and disease. Because both underweight and normal weight calves consumed the same amount of feed, nutritional plane is likely not the reason why some calves remained/became thin on this farm. Disease resulting in reduced feed uptake and energy/protein loss as a result of maintaining an acute phase response is a major factor of influence on growth and body weight.57 The significantly lower albumin concentrations in the LOWCON group were most likely caused by not meeting nutritional demands due to loss of appetite, increased protein loss due to enteritis, or a combination of both conditions, because especially this group was affected by diarrhea. The higher albumin concentration in transported animals could be due to transport, although such effect did not occur in the calves with normal body weight.
Gamma globulin concentration was of major influence on several of the outcome variables studied, such as cortisol release into serum, PBMC proliferation and the production of IL‐17A, TNF‐α, IL‐6, and IL‐10. Possibly high IL‐10 concentrations are needed to enable the development of humoral immune responses in neonatal calves.58 At 2‐4 weeks of age, γ‐globulin concentrations predominantly represent maternal antibodies next to endogenous antibodies, produced by the calf itself in response to infections. In our study, animals with low γ‐globulin concentrations likely suffered from failure of transfer of passive immunity. Especially the underweight calves showed lower γ‐globulin concentrations (although not significant in this study), increasing their disease susceptibility and resulting in less efficient growth.59 In this study, calves with lower γ‐globulin concentrations had higher concentrations of the pro‐inflammatory cytokines IL‐17A, TNF‐α, and IL‐6 after LPS stimulation. Most probably, in calves with sufficient maternal antibodies, the calf will be protected by these maternal antibodies resulting in an energy efficient process to control the threat, based on neutralization, opsonization, and phagocytosis. In contrast, in calves with low maternal immunity, activation of the innate immune system is needed, which is more energy/protein consuming at the expense of growth. Alternatively, the observed effects might be due to other colostrum‐associated factors like easily digestible nutrients, hormones, and immunological components, including cytokines and maternal leukocytes.35, 59 Then γ‐globulin concentration would only be a proxy variable. Reber and co‐workers showed that in contrast to calves fed with fresh colostrum from their own dam, cell‐free colostrum induced a monocytosis and increased surface expression of CD11a, CD11c, and CD14.35 These receptors are upregulated upon cellular activation and are likely good indicators of the calves' response to general physiological stress.35 Because in our study all calves received fresh colostrum from their own dam, the observed effects on cytokine production and proliferation activity are most likely due to activation of innate immunity, in the absence of specific immunity (maternal antibodies). Although the present study illustrates the complex interplay among body weight, serum cortisol concentration, and colostrum uptake, it was not designed to unravel the relationship between these 3 variables.
The present study faced a number of limitations. The main limiting factor to increase sample size was the laboratory capacity (8 animals) for leukocyte function tests. To avoid possible time interference, the study period was limited to 3 weeks (=3 sessions). The study was conducted on a single dairy farm to avoid additional variation of feeding, housing, and environment. Immune variables have large variation;60 therefore, some variables were underpowered in the present study. A consequence of opting for a single farm was that less variation in body weight was present. Therefore, an artificial cutoff of 46 kg was used to make the 2 groups. However, a weight of <47 kg was associated with a 2.4 times higher odds to develop hampered respiration.26 An unforeseen limitation was the diarrhea episode, especially in the untransported animals. Finally, another unfortunate event which might account for some bias was that control calves (low and normal body weight) already showed a slightly increased serum cortisol concentration before transport. Calves at this farm were pair‐housed in igloos and when 1 calf from the pairs was assigned to transport, the calf left behind probably experienced stress by “losing” their companion temporarily for transport.
5. CONCLUSIONS
Independent from body weight, short transport causes a stress induced increase in serum cortisol concentration‐ and the release of TNF‐α and IL‐17A after LPS stimulation in calves aged 2‐4 weeks. However, even in unstimulated PBMCs, transported low body weight calves are presumably in a more pro‐inflammatory state given their increased production of IL‐17A and TNF‐α. These findings might contribute to our understanding of the increased disease susceptibility in underweight animals after transport. The γ‐globulin concentration was identified as an important interfering variable of serum cortisol concentration release, cell proliferation, and release of TNF‐α, IL‐17A, IL‐6, and IL‐10 after stimulation. The complex interplay among body weight, colostrum uptake, and serum cortisol concentration needs to be further explored and accounted for in future studies on neonatal calf immunology.
CONFLICTS OF INTEREST DECLARATION
The authors declare that Proviron and Ghent University collaborate within the framework of this project. No professional or personal relationships between individuals belonging to both organizations exist which might influence the results of the present study. Mrs. Masmeijer is specifically employed to perform the research of grant IWT.150705 and has no further activities at Proviron. Bert. Devriendt is supported by a postdoctoral grant from F.W.O‐Vlaanderen.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study protocol was approved by the ethical committee of the Faculty of Veterinary Medicine (Ghent University) under license EC 2016/01.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
This research was supported by a Baekeland grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders, awarded to C. Masmeijer (IWT 150705) with Proviron and Ghent University as research partners. The authors thank Mrs. Griet De Smet (Department of Immunology, Ghent University) for her help with the laboratory assays, Dr. Bonny Van Ranst for the opportunity to perform this field trial on his dairy farm, and Randy Boone (Veterinary Practice Venhei) for kindly providing his scale for calves.
Appendix 1 A.
Cytokine production in low body weight and normal body weight dairy calves after short transport of 2 hours or no transport. Groups were as followed: LOWCON (low body weight, no transport [n = 5]), LOWTRANS (low body weight, transport [n = 5]), NORMCON (normal body weight, no transport [n = 5]), and NORMTRANS (low body weight, transport [n = 6]). Results of unstimulated (blanco) peripheral blood mononuclear cells (PBMC) from the 4 examined groups of calves are shown in column (A). Cytokine production of the same groups after stimulation with lipopolysaccharide (LPS) are shown in column (B) and results of beta‐glucan (BG) stimulation of the 4 groups of calves are shown in column (C). Results are expressed as mean ± SD. Items with different letters are statistically different (P < .05).
Masmeijer C, Devriendt B, Rogge T, et al. Randomized field trial on the effects of body weight and short transport on stress and immune variables in 2‐ to 4‐week‐old dairy calves. J Vet Intern Med. 2019;33:1514–1529. 10.1111/jvim.15482
Funding information Baekeland grant, Grant/Award Number: IWT.150705
REFERENCES
- 1. Pardon B, Catry B, Dewulf J, et al. Prospective study on quantitative and qualitative antimicrobial and anti‐inflammatory drug use in white veal calves. J Antimicrob Chemother. 2012;67:1027‐1038. [DOI] [PubMed] [Google Scholar]
- 2. Windeyer MC, Leslie KE, Godden SM, Hodgins DC, Lissemore KD, LeBlanc SJ. Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age. Prev Vet Med. 2014;113:231‐240. [DOI] [PubMed] [Google Scholar]
- 3. Curtis GC, Argo CMG, Jones D, Grove‐White DH. Impact of feeding and housing systems on disease incidence in dairy calves. Vet Rec. 2016;179:512‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardon B, De Bleecker K, Hostens M, Callens J, Dewulf J, Deprez P. Longitudinal study on morbidity and mortality in white veal calves in Belgium. BMC Vet Res. 2012;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buckham Sporer KR, Burton JL, Earley B, Crowe MA. Transportation stress in young bulls alters expression of neutrophil genes important for the regulation of apoptosis, tissue remodeling, margination, and anti‐bacterial function. Vet Immunol Immunopathol. 2007;118:19‐29. [DOI] [PubMed] [Google Scholar]
- 6. Buckham Sporer KR, Xiao L, Tempelman RJ, Burton JL, Earley B, Crowe MA. Transportation stress alters the circulating steroid environment and neutrophil gene expression in beef bulls. Vet Immunol Immunopathol. 2008;121:300‐320. [DOI] [PubMed] [Google Scholar]
- 7. Cernicchiaro N, White BJ, Renter DG, Babcock AH, Kelly L, Slattery R. Associations between the distance traveled from sale barns to commercial feedlots in the United States and overall performance, risk of respiratory disease, and cumulative mortality in feeder cattle during 1997 to 2009. J Anim Sci. 2012;90:1929‐1939. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell GB, Al‐Haddawi MH, Clark ME, Beveridge JD, Caswell JL. Effect of corticosteroids and neuropeptides on the expression of defensins in bovine tracheal epithelial cells. Infect Immun. 2007;75:1325‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishizaki H, Hanafusa Y, Kariya Y. Influence of truck‐transportation on the function of bronchoalveolar lavage fluid cells in cattle. Vet Immunol Immunopathol. 2005;105:67‐74. [DOI] [PubMed] [Google Scholar]
- 10. Kelley KW, Osborne CA, Evermann JF, Parish SM, Hinrichs DJ. Whole blood leukocyte vs. separated mononuclear cell blastogenesis in calves: time‐dependent changes after shipping. Can J Comp Med. 1981;45:249‐258. [PMC free article] [PubMed] [Google Scholar]
- 11. Kent JE, Ewbank R. The effect of road transportation on the blood constituents and behavior of calves. II. One to three weeks old. Br Vet J. 1986;142:131‐140. [DOI] [PubMed] [Google Scholar]
- 12. Murata H, Takahashi H, Matsumoto H. The effects of road transportation on peripheral blood lymphocyte subpopulations, lymphocyte blastogenesis and neutrophil function in calves. Br Vet J. 1987;143:166‐174. [DOI] [PubMed] [Google Scholar]
- 13. Todd ZE, Mellor DJ, Stafford KJ, Gregory NG, Bruce RA, Ward RN. Effects of food withdrawal and transport on 5‐ to 10‐day‐old calves. Res Vet Sci. 2000;68:125‐134. [DOI] [PubMed] [Google Scholar]
- 14. Odore R, D'Angelo A, Badino P, Bellino C, Pagliasso S. Road transportation affects blood hormone levels and lymphocyte glucocorticoid and β‐adrenergic receptor concentrations in calves. Vet J. 2004;168:297‐303. [DOI] [PubMed] [Google Scholar]
- 15. Gupta S, Earley B, Crowe MA. Effect of 12‐hour road transportation on physiological, immunological and haematological parameters in bulls housed at different space allowences. Vet J. 2007;173:605‐616. [DOI] [PubMed] [Google Scholar]
- 16. Buckham Sporer KR, Weber PSD, Burton JL, Earley B, Crowe MA. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J Anim Sci. 2008;86:1325‐1334. [DOI] [PubMed] [Google Scholar]
- 17. Riondato F, D'Angelo A, Miniscalco B, Bellino C, Guglielmino R. Effects of road transportation on lymphocyte subsets in calves. Vet J. 2008;175:364‐368. [DOI] [PubMed] [Google Scholar]
- 18. Earley B, Murray M, Prendiville DJ. Effect of road transport for up to 24 hours followed by twenty‐four hour recovery on live weight and physiological responses of bulls. BMC Vet Res. 2010;6:38‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hulbert LE, Carroll JA, Burdick NC, Randel RD, Brown MS, Ballou MA. Innate immune responses of temperamental and calm cattle after transportation. Vet Immunol Immunopathol. 2011;143:66‐74. [DOI] [PubMed] [Google Scholar]
- 20. Earley B, Murray M, Prendiville DJ, Pintado B, Borque C, Canali E. The effect of transport by road and sea on physiology, immunity and behavior of beef cattle. Res Vet Sci. 2012;92:531‐541. [DOI] [PubMed] [Google Scholar]
- 21. Murata H, Takahashi H, Matsumoto H. Influence of truck transportation of calves on their cellular immune function. Japan J Vet Sci. 1985;47:823‐827. [DOI] [PubMed] [Google Scholar]
- 22. Arthington JD, Eicher SD, Knukle WE, Martin FG. Effect of transportation and commingling on the acute‐phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci. 2003;81:1120‐1125. [DOI] [PubMed] [Google Scholar]
- 23. Ishizaki H, Kariya Y. Road transportation stress promptly increases bovine peripheral blood absolute NK cell counts and cortisol levels. J Vet Med Sci. 2010;72:747‐753. [DOI] [PubMed] [Google Scholar]
- 24. Odore R, Badino PRG, Barbero R, et al. Effects of housing and short‐term transportation on hormone and lymphocyte receptor concentrations in beef cattle. Res Vet Sci. 2011;90:341‐345. [DOI] [PubMed] [Google Scholar]
- 25. Renaud D, Duffield T, Leblanc S, Haley D, Kelton D. Clinical and metabolic indicators associated with early mortality at a milk‐fed veal facility: a prospective case control study. J Dairy Sci. 2017;101:2669‐2678. [DOI] [PubMed] [Google Scholar]
- 26. Brscic M, Leruste H, Heutinck LF, et al. Prevalence of respiratory disorders in veal calves and potential risk factors. J Dairy Sci. 2012;95:2753‐2764. [DOI] [PubMed] [Google Scholar]
- 27. Obeidat BS, Cobb CJ, Sellers MD, Pepper‐Yowell AR, Earleywine TJ, Ballou MA. Plane of nutrition during preweaning period but not the grower phase influences the neutrophil activity of Holstein calves. J Dairy Sci. 2013;96:7155‐7166. [DOI] [PubMed] [Google Scholar]
- 28. Ballou MA, Hanson DL, Cobb CJ, et al. Plane of nutrition influences the performance, innate leukocyte responses, and resistance to an oral Salmonella enterica serotype typhimurium challenge in Jersey calves. J Anim Sci. 2015;98:1972‐1982. [DOI] [PubMed] [Google Scholar]
- 29. Nonnecke BJ, Foote MR, Smith JM, Pesch BA. Composition and functional capacity of blood mononuclear leukocyte populations from neonatal calves on standard and intensified milk replacer diets. J Dairy Sci. 2003;86:3592‐3604. [DOI] [PubMed] [Google Scholar]
- 30. Pardon B, Alliët J, Boone R, Roelandt S, Valgaeren B, Deprez P. Prediction of respiratory disease and diarrhea in veal calves based on immunoglobulin levels and the serostatus for respiratory pathogens measured at arrival. Prev Vet Med. 2015;120:169‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baert K, Sonck E, Goddeeris BM, Devriendt B, Cox E. Cell type‐specific differences in β‐glucan recognition and signalling in porcine innate immune cells. Dev Comp Immunol. 2015;48:192‐203. [DOI] [PubMed] [Google Scholar]
- 32. Sonck E, Stuyven B, Cox E. The effect of beta‐glucans on porcine leukocytes. Vet Immunol Immunopathol. 2010;15:199‐207. [DOI] [PubMed] [Google Scholar]
- 33. Godden S. Colostrum management for dairy calves. Vet Clin Food Anim. 2008;24:19‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burton JL, Madsen SA, Chang L‐C, et al. Gene expression signatures in neutrophil exposed to glucocorticoids: a new paradigm to help explain “neutrophil dysfunction” in parturient dairy cows. Vet Immunol Immunopathol. 2005;105:197‐219. [DOI] [PubMed] [Google Scholar]
- 35. Reber AJ, Donovan DC, Gabbard J, et al. Transfer of maternal colostral leukocytes promotes development of the neonatal immune system. I Effects on monocyte lineage cells. Vet Immunol Immunopathol. 2008;123:186‐196. [DOI] [PubMed] [Google Scholar]
- 36. Reber AJ, Donovan DC, Gabbard J, et al. Transfer of maternal colostral leukocytes promotes development of the neonatal immune system. Part II Effects on neonatal lymphocytes. Vet Immunol Immunopathol. 2008;123:305‐313. [DOI] [PubMed] [Google Scholar]
- 37. Mehrzad J, Duchateau L, Pyörälä S, Burvenich C. Blood and milk neutrophil chemiluminescence and viability in primiparous and pluriparous dairy cows during late pregnancy, around parturition and early lactation. J Dairy Sci. 2002;85:3268‐3276. [DOI] [PubMed] [Google Scholar]
- 38. Zhang JM, Jianxiong A. Cytokines, inflammation and pain. Int Anesthesiol Clin. 2007;45:27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17‐producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123‐1132. [DOI] [PubMed] [Google Scholar]
- 40. Miossec P, Korn T, Kuchroo VK. Interleukin‐17 and type 17 helper T cells. N Engl J Med. 2009;361:888‐898. [DOI] [PubMed] [Google Scholar]
- 41. Onishi RM, Gaffen SL. Interleukin‐17 and its target genes: mechanisms of interleukin‐17 function in disease. Immunology. 2010;129:311‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cua DJ, Tato CM. Innate IL‐17‐producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479‐489. [DOI] [PubMed] [Google Scholar]
- 43. Gaffen SL. An overview of IL‐17 function and signaling. Cytokine. 2008;43:402‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsai H‐C, Velichko S, Hung L‐Y, Wu R. IL‐17A and Th17 cells in lung inflammation: an update on the role of Th17 cell differentiation and IL‐17R signaling in host defense against infection. Clin Dev Immunol. 2013;13:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Munck A, Náray‐Fejes‐Tóth A. The ups and downs of glucocorticoid physiology permissive and suppressive effects revisited. J Mol Cell Endocrinol. 1992;90:C1‐C4. [DOI] [PubMed] [Google Scholar]
- 46. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55‐89. [DOI] [PubMed] [Google Scholar]
- 47. Yeager MP, Pioli PA, Guyre PM. Cortisol exerts bi‐phasic regulation of inflammation in humans. Dose Response. 2011;9:332‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeager MP, Pioli PA, Collins J, Barr F, Metzler S, Sites D. Glucocorticoids enhance the in vivo response of human monocytes. Brain Behav Immun. 2016;54:86‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang T, Daynes R. Glucocorticoid conditioning of myeloid progenitors enhances TLR4 signaling via negative regulation of the phosphatidylinositol 3‐kinase‐Akt pathway. J Immunol. 2007;178:2517‐2526. [DOI] [PubMed] [Google Scholar]
- 50. Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2009;24:19‐30. [DOI] [PubMed] [Google Scholar]
- 52. Sorrells SF, Sapolsky RM. Glucocorticoids can arm macrophages for innate battle. Brain Behav Immun. 2010;24:17‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frank M, Watkins L, Maier S. Stress‐induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gardy‐Godillot M, Durand D, Bauchart D. Diurnal pattern of plasma cortisol in preruminant calves fasted or fed different milk proteins. J Dairy Sci. 1989;72:1842‐1846. [DOI] [PubMed] [Google Scholar]
- 55. Mujibi FD, Crews DH Jr. Genetic parameters for calving ease, gestation length, and birth weight in Charolais cattle. J Anim Sci. 2009;87:2759‐2766. [DOI] [PubMed] [Google Scholar]
- 56. Van Eetvelde M, Opsomer G. Innovative look at dairy heifer rearing: effect of prenatal and post‐natal environment on later performance. Reprod Domest Anim. 2017;52(Suppl 3):30‐36. [DOI] [PubMed] [Google Scholar]
- 57. Zaias CJ, Altmann NH. Acute phase response in animals: a review. Comp Med. 2009;59:517‐526. [PMC free article] [PubMed] [Google Scholar]
- 58. Chase CCL, Hurley DJ, Reber AJ. Neonatal immune development in the calf and its impact on vaccine response. Vet Clin Food Anim. 2008;24:87‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Langel SN, Wark WA, Garst SN, et al. Effect of feeding whole compared with cell‐free colostrum on calf immune status: the neonatal period. J Dairy Sci. 2015;98:3729‐3740. [DOI] [PubMed] [Google Scholar]
- 60. Kampen AH, Olsen I, Tollersrud T, Storset AK, Lund A. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet Immunol Immunopathol. 2006;113:53‐63. [DOI] [PubMed] [Google Scholar]


