Abstract
Background
One of the remaining questions in treating dogs with internal hydrocephalus is the association between the decrease of ventricular volume and re‐expansion of cerebral parenchyma with clinical improvement.
Hypothesis
A decrease in ventricular volume and re‐expansion of brain tissue occur after ventriculoperitoneal shunting (VPS). Clinical improvement defined by resolution of ≥1 clinical signs is associated with decreased size of cerebral ventricles and that the extent of change in ventricular size is associated with clinical improvement.
Animals
Forty‐five client‐owned dogs with newly diagnosed communicating internal hydrocephalus.
Methods
Ventricular volume, brain volume, and clinical status of dogs that underwent VPS were measured before and 3 months after surgery. Multiple logistic regression analysis was performed to assess the influence of decrease in ventricular size in addition to the covariates “age of the animal” and “duration of clinical signs before surgery” on improvement of clinical signs.
Results
Decreased volume of cerebral ventricles was associated with resolution of ≥1 preoperative clinical sign (P < .003). The covariates “age of the animal” and “duration of clinical signs” were not associated with improvement of clinical signs. The percentage decrease in ventricular size was associated with resolution of ataxia (P = .008) and obtundation (P = .011).
Conclusion and Clinical Importance
The decrease in ventricular volume and increase in brain parenchyma after VPS are associated with improvement in clinical signs.
Keywords: canine, magnetic resonance imaging, ventriculomegaly, volumetry
Abbreviations
- CSF
cerebrospinal fluid
- IVP
intraventricular pressure
- TE
echo time
- TR
repetition time
- FLAIR
fluid attenuated inversion recovery
- MRI
magnetic resonance imaging
- VPS
ventriculoperitoneal shunting
1. INTRODUCTION
Internal hydrocephalus is a common malformation of the central nervous system in dogs1, 2, 3 characterized by accumulation of cerebrospinal fluid (CSF) in the cerebral ventricles and enlargement of the ventricular system at the expense of cerebral parenchyma.4, 5, 6 In rare occasions, CSF accumulates in the subarachnoid space, which is referred to as external hydrocephalus.2 Obstruction within the ventricular system or at the outflow through the lateral apertures of the fourth ventricle prevents communication between the ventricular system and subarachnoid space and is classified as noncommunicating hydrocephalus. Communicating hydrocephalus occurs when impaired circulation, impaired absorption of CSF in the subarachnoid space, or both occurs. Other terms used in the classification of hydrocephalus are hypertensive and normotensive to classify cases of hydrocephalus in which intraventricular pressure (IVP) is increased or normal.2 Hydrocephalus can occur secondary to tumors, brain trauma, hemorrhage, inflammation, or in association with central nervous system malformations (eg, Chiari‐like malformation, Dandy‐Walker malformation) but can also be present without any obvious cause (primary hydrocephalus).2, 3
Neurologic dysfunction in affected dogs can result from stretching of the periventricular white matter, as well as from impaired perfusion by periventricular blood vessels7 and transependymal bulk flow of CSF into the brain tissue leading to interstitial edema.8, 9, 10 Clinical experience with interventional surgery aiming to decrease ventricular dilatation in companion animals is limited. Ventriculoperitoneal shunting (VPS) has the potential to improve clinical signs and long‐term survival.11, 12, 13 However, the efficacy of VPS in reversing the clinical neurological signs is variable.3, 11, 12, 13, 14, 15 Ataxia, obtundation, and circling are somewhat reversible after CSF drainage, whereas impaired vision and seizures can persist.2, 4, 11, 14, 16 It is unclear in this respect, whether absence of clinical improvement is a consequence of insufficient drainage or a result of brain destruction that might persist after a decrease in ventricular volume.17 Although postoperative magnetic resonance imaging (MRI) has been performed in dogs and cats with internal hydrocephalus,12, 13, 14, 18 no study has provided information about postoperative changes in ventricular volume in a large case series.
Ventriculoperitoneal shunting significantly decreases ventricular dilatation and allows for parenchymal reconstitution in hydrocephalic children and adults.18, 19, 20, 21, 22 Postoperatively, unchanged ventricular dimensions usually suggest insufficient CSF drainage with a high probability of postoperative persistence of clinical signs in children with hydrocephalus.22 Preoperative and postoperative MRI and comparison of ventricular size are essential parts of assessment of VPS effectiveness in humans.18, 19, 20, 21, 22 Postoperative MRI to confirm adequate drainage also would be helpful in dogs with hydrocephalus. However, the role of morphological reconstitution of the cerebral parenchyma after VPS in restoring normal brain functions in dogs is unclear.
The purpose of our study therefore was 2‐fold: (1) to prospectively examine whether a decrease in ventricular volume and restoration of cerebral parenchyma occur in dogs undergoing VPS as treatment for internal hydrocephalus during a follow‐up period of 3 months and (2) to assess whether a decrease in volume is associated with clinical improvement.
2. MATERIALS AND METHODS
2.1. Case selection and study criteria
Client‐owned dogs presented between December 2014 and December 2017 to the Clinic for Small Animals of the Department for Veterinary Clinical Sciences, Justus‐Liebig‐University, Giessen, Germany, with newly diagnosed, internal hydrocephalus were prospectively enrolled in the study. A detailed history was obtained for each dog to assess the duration of clinical signs. Clinical evaluation included a standardized neurologic examination performed by a board‐certified neurologist. Age, breed, sex, neuter status, and diagnostic imaging findings of each individual dog, in addition to clinical signs, were recorded. To be included in the study, internal hydrocephalus had to be diagnosed as communicating, based on the absence of visible obstruction to CSF flow. Furthermore, only dogs with primary hydrocephalus without evidence of secondary diseases that cause impairment of CSF flow were included. Dogs with previous medical treatment aimed at decreasing CSF production (eg, omeprazole, acetazolamide, furosemide) or corticosteroids were excluded from the study. Cerebrospinal fluid was analyzed but that data will be presented in a future paper. The CSF analysis did not suggest the presence of an underlying disease process.
Dogs were scheduled for neurologic reexamination and repeat MRI 3 months after VPS implantation as part of the routine treatment plan. Complete physical and neurologic examinations and repeat MRI were recommended to evaluate the status of clinical signs, proper position and patency of the intraventricular catheter, presence of compressing subdural CSF accumulation (ie, CSF hygroma), and for signs of brain inflammation.
2.2. Magnetic resonance imaging
A standardized MRI protocol was used before and 3 months after surgery. Diagnosis of internal hydrocephalus was made from MRI using a 1.0 Tesla scanner (Gyroscan Intera; Phillips, Hamburg, Germany). Sagittal, dorsal, and transverse T2‐weighted (echo time [TE] = 120 milliseconds, repetition time [TR] = 2.900 milliseconds), transverse fluid attenuated inversion recovery sequences (TE = 120 milliseconds, TI = 2400 milliseconds, TR = 7000 milliseconds), and transverse T1‐weighted sequences (TR = 491 milliseconds, TE = 8 milliseconds) before and after contrast were acquired in all animals preoperatively. Slice thickness varied from 2‐3 mm depending on the size of the dog. The field of view measured 180 x 180 mm in small dogs and 210 x 210 mm in large dogs. The matrix was 288 x 288 in small dogs and 384 x 384 in large dogs. The MRI data sets of all dogs were evaluated as DICOM‐formatted images by the use of an image viewer and processing software (EasyImage, easyVET; IFS GmbH, Hannover, Germany).
Dogs with a normal ventricular system have very narrow and slit‐like lateral, third and fourth ventricles. In patients with internal hydrocephalus, the interpreter noted a large proportion of the brain occupied by the ventricles. The closely spaced walls of the temporal horns, third and fourth ventricle, or both, as well as olfactory and fastigial recesses were separated by CSF. In addition, thinning of the periventricular white matter compared to the brains of normal dogs, dorsal deviation of the corpus callosum, compression of the thalamic intermediate mass in the third ventricle, effacement of cerebral sulci, and diminution of the suprasellar cistern indicated an increase in IVP23 (Figure 1). Supracollicular (quadrigeminal) cysts were diagnosed based on the finding of well‐defined space‐occupying lesions with fluid content intensity similar to CSF in a supracollicular location that did not enhance after contrast‐medium administration.24
Figure 1.
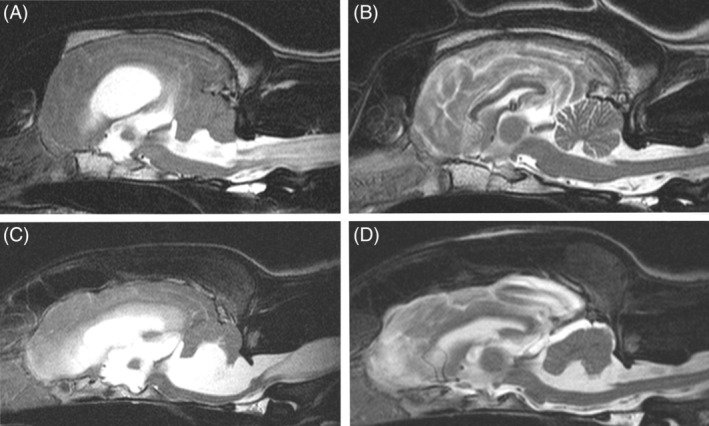
Sagittal T2‐weighted magnetic resonance images of dogs 28 (Boxer) and 14 (German Shepherd dog mix) with tetraventricular hydrocephalus and syringomyelia before (A, C) and 3 months after (B, D) ventriculoperitoneal shunting. Ventricular volume has decreased in all dilated CSF compartments
2.3. Morphometric procedures
Study numbers were assigned to the dogs so that the observer was blind to the preoperative or postoperative condition at the time of volume determination. To assess if a decrease in volume of the ventricular system and increase in volume of the brain parenchyma occurred, and to measure the amount of decreased volume, the CSF inside the ventricles and the brain parenchyma was extracted from the digital images by manually tracking the boundaries using specialized graphical software (AMIRA; Mercury Computer Systems, Berlin, Germany) as described previously.25 This program allows accurate manual image segmentation on a slice‐by‐slice basis. All voxels corresponding to a tissue of interest (eg, ventricles, brain parenchyma) were selected independently and assigned to the same value in the mask. The final mask thus contained information about all of the selected anatomical structures in combination with the original volume data. The total ventricular volume comprised all 4 ventricles, including the choroid plexus. The delineation of both the ventricular system and the brain parenchyma to the spinal cord was set along a vertical line at the obex.25 Polygonal surface reconstruction algorithms allowed determination of the volumes from the 2‐dimensional images. Total ventricular volume was calculated and expressed in relation to the total volume of the brain including cerebrum, cerebellum, and brainstem (ventricle‐brain index, V/B index). All measurements before and after surgery were performed by the same observer (Martin J. Schmidt).
2.4. Shunting procedures
Ventriculoperitoneal shunting was performed using a commercially available shunt system (miniNAV; Miethke GmbH & Co KG, Potsdam, Germany) as described elsewhere.11 The shunt system in all dogs included a ball valve with an opening pressure of 15 cm·H20 (approximately 11 mm Hg).
2.5. Control of intraventricular catheter patency
The miniNAV shunt system includes an SC chamber situated cranially to the valve, from which CSF can be collected transcutaneously without general anesthesia. In the postoperative period, approximately 1 mL CSF was taken from the chamber each day (5‐7 days) to assess patency of the catheter. Aspiration of CSF from the chamber was repeated 3 months after surgery.
2.6. Statistical analysis
Statistical analysis was performed using a commercial statistical software package (BMDP Statistical Software, Inc, Los Angeles, California). The association between the decrease in ventricular size and resolution of ≥1 clinical sign, as well as the influence of the covariates “age of the animal” and “duration of clinical signs” before surgery, in addition to “percentage of ventricular reduction”, were assessed using multiple logistic regression analysis.
In a second step, the same potential associations were evaluated for each specific clinical sign using asymptotic linear regression analysis. For clinical signs that only occurred rarely, the association was tested using exact logistic regression. A significance level of P < .05 was used.
3. RESULTS
3.1. Animals
Forty‐five dogs were included in the study. Age, breed, and sex of each individual dog, as well as clinical signs before and after surgery, are included in the supporting information (see Table S1). The median age of the dogs was 38 weeks (range 8‐288 weeks).
3.2. Clinical signs
Eight dogs (17.7%) had only 1 clinical sign, which was obtundation (7 dogs; 15.6%) or head tilt (1 dog; 2.2%). Ten dogs (22.2%) had a variable combination of 2 clinical signs, and 27 dogs had combinations of 3‐4 clinical signs. The most common clinical sign at the time of presentation was obtundation (36 dogs; 80%). It was often found in combination with ataxia (22 dogs; 48.8%) and central blindness (9 dogs; 20%). Circling was found in 11 dogs (24.4%); aimless barking was found in 11 dogs (17.7%). Head tilt (8 dogs; 17.7%) and nystagmus (7 dogs; 15.5%) also were observed. The clinical signs of each individual dog are summarized in the supporting information (Table S1). The mean duration of clinical signs before surgery was 9 ± 13 weeks.
3.3. Control of intraventricular catheter patency
Cerebrospinal fluid could be aspirated in all dogs in the postoperative period and 3 months after surgery.
3.4. MRI findings before and after surgery
Fifteen dogs (33.3%) had dilatation of the lateral cerebral ventricles (biventricular hydrocephalus), and 16 dogs (35.5%) also had dilatation of the third ventricle (triventricular hydrocephalus). Seven dogs (15.5%) with triventricular hydrocephalus had supracollicular fluid accumulations. Twelve dogs (26.6%) had dilatation of all cerebral ventricles (tetraventricular hydrocephalus) on preoperative MRI. Syringomyelia was found in 10 dogs (22.2%). Periventricular edema in the frontal horn, occipital horn, or both of the ventricular system was seen in 12 dogs (26.6%). In 8 of 11 dogs (72%) that were centrally blind, we observed damage to the caudal part (crus caudale) of the internal capsule in which the optic radiation runs (Figure 2).
Figure 2.
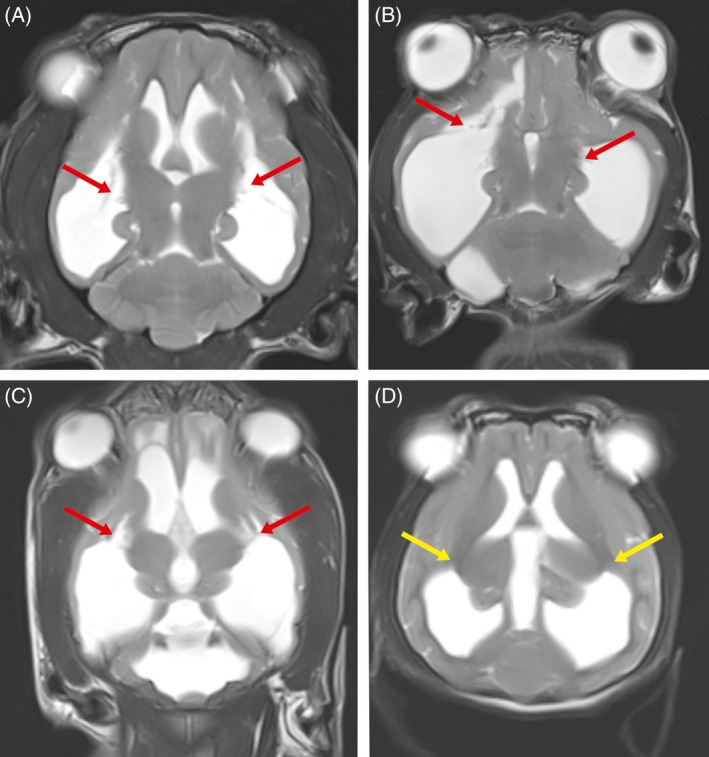
Dorsal T2‐weighted magnetic resonance images through the brain of dogs with internal hydrocephalus (A: dog 4; B dog 13; C dog 40; D: dog 2). In the dogs A‐C in which blindness was diagnosed clinically, a detachment of the white matter containing the optic radiation from the thalamus was found (red arrows). The dog in image D is a dog with internal hydrocephalus but with unimpaired visual function. The yellow arrow highlights the area of the optic radiation in the internal capsule
Comparison of preoperative and postoperative brain and ventricular volumes disclosed decreases in ventricular volume in 38 dogs (84.4%). The volumes of the ventricular system and brain parenchyma as well as percentages of decrease in ventricular volume are presented in supporting information (Table S1). Mean decrease in ventricular volume was 44.16% (±27.3 SD). Supracollicular fluid accumulation was decreased in 2 dogs, and syringomyelia was resolved in all but 3 dogs. Periventricular edema was not seen on postoperative MRI. Seven dogs (15.5%) had unchanged ventricular volumes. Unilateral (6 dogs) or bilateral CSF hygromas (2 dogs) were found in the postoperative images (Figure 3).
Figure 3.
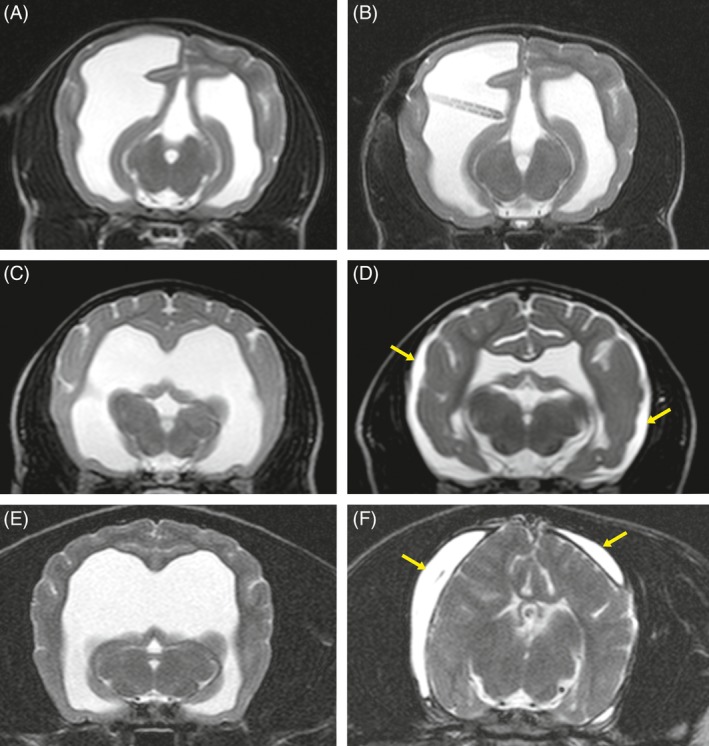
Transverse T2‐weighted magnetic resonance images of dogs 3 (Jack Russell Terrier), 9 (Mini Bull Terrier) and 20 (Cavalier King Charles Spaniel) with biventricular or triventricular hydrocephalus before (A, C, E) and 3 months after (B, D, F) surgery. The images present different variable reduction of the ventricular system from 0% (A, B), over 74.3% (C, D) to 98% (E, F). The yellow arrows mark the cerebrospinal fluid hygroma
3.5. Association between clinical signs and decrease in ventricular size
The majority of the dogs (36) with decreased postoperative ventricular dimensions (38) also showed postoperative improvement of ≥1 clinical sign; 34 dogs showed resolution of all clinical signs. One dog (dog 4) did not improve on any clinical sign, and its ventricular volume decreased to 48.6%. The extent of decrease in ventricular size varied between 18.6% and 100% (Figure 3).
Seven dogs without measurable ventricular diminution also did not show improvement of clinical signs. The presence of decreased cerebral ventricle size was associated with resolution of ≥1 preoperative clinical sign (P < .003). The covariates “age of the animal” and “duration of clinical signs” before surgery were not associated with improvement. On the other hand, the “percentage of ventricular reduction” had a significant effect on improvement (P = .003).
Diminution of the ventricles was not associated with resolution of a specific clinical sign. “Age of the animal” and “duration of clinical signs” also were not associated with resolution of a specific clinical sign. The percentage of decrease in ventricular size again was associated with resolution of obtundation (P = .008) and with resolution of ataxia (P = .011).
4. DISCUSSION
In dogs with internal hydrocephalus, postoperative success after VPS can be relatively simply assessed by means of improvement in neurological signs. However, if postoperative improvement is lacking, it is unclear whether ventricular drainage was insufficient or if brain damage was too severe to allow restoration of normal brain function. We evaluated the occurrence and role of the decrease in ventricular volume and reconstitution of cerebral parenchyma in the improvement of clinical signs in 45 dogs with internal hydrocephalus. The majority of dogs (38) showed visible decreases in ventricular size to a greater or lesser extent (18.6%‐100%), and we demonstrated a significant association between the decrease in ventricular volume and clinical improvement. However, dogs also had a clinical response to shunting despite only little decrease in ventricular volume. The resolution of periventricular white matter edema alone may have caused postoperative clinical improvement without a marked decrease in ventricular volume. However, not all dogs had visible periventricular edema before surgery. Atrophy of brain tissue observed postoperatively did not create any obvious clinical signs in the dogs, but cognitive deficits or subclinical visual impairment might have gone unnoticed clinically. Ideally, inclusion of a control population with blocked shunt systems and absent clinical improvement would have substantiated the documented association, which is a limitation of our study, but all shunt systems were patent 3 months after surgery.
In 7 dogs in our study, brain parenchyma did not re‐expand after shunting and ventricular dimensions remained unchanged. Clinical signs did not resolve in these dogs. A lack of decrease in ventricular volume might be due to inappropriate pressure valves of the shunt system. The persistence of obtundation 3 months after surgery might support this hypothesis, because obtundation resolved very quickly in other dogs of the study. Physiological intracranial (intraventricular) pressure is in the range from 8‐12 mm Hg in dogs,26 whereas pressure in experimental hydrocephalic animals can increase up to 60 mm Hg.27 We decided to use a valve opening pressure of 15 cm H20 (approximately 11 mm Hg), which allows CSF drainage immediately with mildly increased IVP. However, little is known about the actual IVP in hydrocephalic dogs and it is possible to change over time. In the acute stage of development of hydrocephalus, IVP might increase constantly, whereas IVP might return to normal or even decrease below normal after white matter atrophy has occurred in chronically affected animals. One limitation of our study was the missing information about actual IVP, which could be different in each individual dog and might also fall below the opening pressure of the valves. Daily CSF collection from the shunt system is part of our postoperative monitoring procedure to evaluate the patency of the intraventricular catheter. During 5‐7 days of hospitalization, obtundation initially improved in 3 dogs (dogs 13, 19, and 27) but was present again at reexamination 3 months later. Exit of CSF during insertion of the ventricular catheter and manual CSF removal in the early postoperative phase may have allowed IVP to decrease in these dogs despite inappropriate opening pressure of the valve. After surgical replacement of the valve set at 15 cm H2O opening pressure against a low‐pressure valve (10 cm H2O, approximately 7.5 mm Hg), dogs 13 and 19 improved in terms of obtundation but remained blind.
The etiologic factors leading to hydrocephalus differ.8 Although we grouped the dogs in our study together based on the absence of visible obstructions in the ventricular system, it seems unlikely that the underlying pathophysiology of hydrocephalus was the same in every dog. Underlying causes include genetic factors, developmental anomalies (eg, aqueductal stenosis), prenatal or postnatal infection, periventricular hemorrhage, or meningitis.8 It is reasonable to expect that different etiologies affect the speed and severity of ventricular expansion and thereby the grade of functional brain deficits.25 Variable etiologic factors therefore might have an influence on persistent clinical signs in some dogs, which also limit the interpretation of the results of our study. Although unlikely, ventricular dilatation might not be the primary or might only be part of the underlying cause leading to the clinical signs. An undetected secondary brain disease might be a cause for failure to improve despite a decrease in ventricular volume.
The second MRI was performed 3 months after surgery in all dogs, which may not be a long enough time for the hemispheres to recover. In humans, decrease in ventricular volume can be completed soon after surgery in some patients, whereas ventricular volume decreases gradually over months or even years in others.28 It is possible that decrease in ventricular size, parenchymal reconstitution, and clinical improvement might have been present or more pronounced later in the postoperative period. However, shunt failure and other complications occur in the first 3‐5 months after surgery,11, 12 which limited the time for repeat MRI. Volume measurement was based on manual segmentation based on signal intensities, which can be subject to investigator bias. Minor changes of ventricular volume could have been missed in the segmentation process. The validity of our approach would have increased further with multiple examinations, but we did not determine interobserver and intraobserver variability because the manual segmentation process is extremely time‐consuming.25
Studies in humans with hydrocephalus have yielded results comparable to those of our study in dogs. The clinical responses to shunt surgery correlates with a substantial postoperative decrease in ventricular volume.17, 19, 20, 29, 30 However, as in the dogs of our study, there is no strict dependence between decreased ventricular size and improvement in neurological status in some human patients.30, 31, 32, 33, 34, 35, 36 Pediatric patients with reasonably improved clinical status can have persistently enlarged ventricles.28, 29, 30 On the other hand, persistence of neurological symptoms was seen in patients with a complete decrease to physiologically normal volume.28, 29, 30, 36
In dogs, altered vision was a prominent clinical sign and also the sign that most often persisted even after an increase in brain parenchyma. Persistent central blindness probably is a consequence of structural damage to the visual pathways. The optic radiation runs in the white matter that runs next to the caudal horn of the lateral ventricle.37, 38, 39 In dogs with experimentally induced hydrocephalus, the optic radiation was the element of the visual pathway that was mostly damaged by ventricular distension if visual impairment was observed, whereas the visual cortex remained intact.40 In preoperative MRI of 8 dogs (88%) with central visual impairment, we noticed damage of the optic radiation at the level of its emergence from the thalamus (Figure 2). These dogs had persistently impaired vision 3 months after surgery. This finding might be a negative prognostic indicator for postoperative recovery of vision, even if the decrease in ventricular volume is large.
Visual deficits in children can be caused by multiple mechanisms, and the prevalence of visuoperceptual problems in children with internal hydrocephalus can vary considerably (5%‐59%), depending on the study.41, 42, 43, 44 Increased intracranial pressure causes optic nerve traction without causing damage to the visual pathways and visual cortex in hydrocephalic children, probably because children are operated earlier in the course of the disease. Children usually are treated as soon as visual problems occur, whereas the dogs in our study were operated in a chronic stage of the disease (mean, 9 weeks after occurrence of signs). Because of the differences in affected anatomical structures and treatment at a different time point in the disease process, comparison of the postoperative change of this clinical sign in dogs and humans therefore is possible only to a limited extent.
The amount of decrease in ventricular size influenced the resolution of ataxia and obtundation. We consider both to be due to volume decrease of the fourth ventricle and thereby decreased compression of brain stem structures, as well as to resolution of syringomyelia. Many of the dogs with ataxia and obtundation had tetraventricular hydrocephalus that can be associated with a larger amount of CSF in the ventricles that needs to be drained by the shunt system before function of the brain stem and cerebellum can be restored. Other clinical signs were not associated with the percentage of volume decrease.
One dog did not fit into the concept of our hypothesis because its clinical signs did not improve despite the decrease in ventricular volume and increase in brain parenchyma. Pure expansion of neuropil without regeneration of neural elements can explain the absence of clinical improvement. If VPS is performed after axonal destruction, mass increase of brain parenchyma might occur, but this is more likely due to proliferation of astroglial cells45, 46 that may lead to enlargement of the parenchyma but does not reset the cerebral hemisphere to its original state. No assessment of specific gray or white matter reconstitution was done, and thus confirmation of hypotheses relating to glial or axonal components could not be made.
5. CONCLUSION
Decrease in ventricular volume and increase in brain parenchyma after VPS is associated with improvement in the clinical condition in the first 3 months after surgery in dogs with internal hydrocephalus. Blindness, however, can persist even after full reconstitution. The postoperative presence of obtundation can be indicative of insufficient CSF drainage.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was conducted according to the University's institutional guidelines and received ethical approval from the institutional Ethic Commissioners. Re‐evaluation using MRI was part of the standard operating procedure for the treatment of internal hydrocephalus, which is why approval from the Hessen state government (Regierungspräsidium) was not necessary.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Supplemental Table 1 Overview about the epidemiologic data, results of neurologic examination, measurements of brain‐ and ventricular volume as well as ventricular reduction before surgery 3 months after ventriculo‐peritoneal shunting
ACKNOWLEDGMENT
We thank Marion Sparenberg for her help with statistics.
Schmidt MJ, Hartmann A, Farke D, Failling K, Kolecka M. Association between improvement of clinical signs and decrease of ventricular volume after ventriculoperitoneal shunting in dogs with internal hydrocephalus. J Vet Intern Med. 2019;33:1368–1375. 10.1111/jvim.15468
REFERENCES
- 1. Selby LA, Hayes HM, Becker SV. Epizootiologic features of canine hydrocephalus. Am J Vet Res. 1979;40:411‐413. [PubMed] [Google Scholar]
- 2. Thomas WB. Hydrocephalus in dogs and cats. Vet Clin North Am Small Anim Pract. 2010;40:143‐159. [DOI] [PubMed] [Google Scholar]
- 3. Harrington ML, Bagley RS, Moore MP. Hydrocephalus. Vet Clin North Am Small Anim Pract. 1996;26:843‐856. [DOI] [PubMed] [Google Scholar]
- 4. Vite CH. Development disorders Braund's Clinical Neurology in Small Animals: Localization, Diagnosis and Treatment. Ithaca, NY: International Veterinary Information Service; 2006:7‐9. [Google Scholar]
- 5. Deo‐Narine V, Gomez DG, Vullo T, et al. Direct in vivo observation of transventricular absorption in the hydrocephalic dog using magnetic resonance imaging. Invest Radiol. 1994;29:287‐293. [DOI] [PubMed] [Google Scholar]
- 6. Mc Gavin MD, Zachary JF. Pathologic Basis of Veterinary Disease. Vol 805‐908 4th ed. St Louis, Missouri: Mosby Elsevier; 2006. [Google Scholar]
- 7. Schmidt MJ, Kolecka M, Kirberger R, et al. Dynamic susceptibility contrast perfusion magnetic resonance imaging demonstrates reduced periventricular cerebral blood flow in dogs with ventriculomegaly. Front Vet Sci. 2017;22(4):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wünschmann A, Oglesbee M. Periventricular changes associated with spontaneous canine hydrocephalus. Vet Pathol. 2001;38:67‐73. [DOI] [PubMed] [Google Scholar]
- 9. Summers BA, Cummings JF, deLahunta A. Malformations of the central nervous system Veterinary Neuropathology. St. Louis, MO: Mosby; 2000:86‐94. [Google Scholar]
- 10. Rosenberg GA, Saland L, Kyner WT. Pathophysiology of periventricular tissue changes with raised CSF pressure in cats. J Neurosurg. 1983;59:606‐611. [DOI] [PubMed] [Google Scholar]
- 11. Biel M, Kramer M, Forterre F, et al. Outcome of ventriculoperitoneal shunt implantation for treatment of congenital internal hydrocephalus in dogs and cats: 36 cases (2001–2009). J Am Vet Med Assoc. 2013;242:948‐958. [DOI] [PubMed] [Google Scholar]
- 12. Shihab N, Davies E, Kenny PJ, Loderstedt S, Volk HA. Treatment of hydrocephalus with ventriculoperitoneal shunting in twelve dogs. Vet Surg. 2011;40:477‐484. [DOI] [PubMed] [Google Scholar]
- 13. de Stefani A, de Risio L, Platt SR, et al. Surgical technique, postoperative complications and outcome in 14 dogs treated for hydrocephalus by ventriculoperitoneal shunting. Vet Surg. 2011;40:183‐191. [DOI] [PubMed] [Google Scholar]
- 14. Coates JR, Axlund TW, Dewey CW, Smith J. Hydrocephalus in dogs and cats. Comp Cont Educ Pract Vet. 2006;28:136‐146. [Google Scholar]
- 15. Gage ED. Surgical treatment of canine hydrocephalus. J Am Vet Med Assoc. 1970;157:1729‐1740. [PubMed] [Google Scholar]
- 16. Dewey CW. Encephalopathies: disorders of the brain In: Dewey CW, ed. A Practical Guide to Canine and Feline Neurology. Ames, IA: University Press; 2003:99‐178. [Google Scholar]
- 17. Klimo P, Van Poppel M, Thompson CJ, et al. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Pediatric hydrocephalus: systematic literature review and evidence‐based guidelines. Part 10: Change in ventricle size as a measurement of effective treatment of hydrocephalus. J Neurosurg Pediatr. 2014;14(Suppl 1):44‐52. [DOI] [PubMed] [Google Scholar]
- 18. Kolecka M, Ondreka N, Moritz A, Kramer M, Schmidt MJ. Effect of acetazolamide and subsequent ventriculo‐peritoneal shunting on clinical signs and ventricular volumes in dogs with internal hydrocephalus. Acta Vet Scand. 2015;57:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245‐249. [DOI] [PubMed] [Google Scholar]
- 20. McConnell KA, Zou KH, Chabrerie AV, et al. Decreases in ventricular volume correlate with decreases in ventricular pressure in idiopathic normal pressure hydrocephalus patients who experienced clinical improvement after implantation with adjustable valve shunts. Neurosurgery. 2004;55:582‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Imaging correlates of successful endoscopic third ventriculostomy. J Neurosurg. 2000;92:915‐919. [DOI] [PubMed] [Google Scholar]
- 22. Venkataramana NK, Mukundan CR. Evaluation of functional outcomes in congenital hydrocephalus. J Pediatr Neurosci. 2011;6:4‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laubner S, Ondreka N, Failing K, Kramer M, Schmidt MJ. Magnetic resonance imaging signs of high intraventricular pressure‐comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet Res. 2015;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matiasek LA, Platt SR, Shaw S, Dennis R. Clinical and magnetic resonance imaging characteristics of quadrigeminal cysts in dogs. J Vet Intern Med. 2007;21:1021‐1026. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt MJ, Laubner S, Kolecka M, et al. Comparison of the relationship between cerebral white matter and grey matter in normal dogs and dogs with lateral ventricular enlargement. PLoS One. 2015;10:e0124174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bagley RS, Keegan RD, Greene SA, Harrington ML, Moore MP. Pathologic effects in brain after intracranial pressure monitoring in clinically normal dogs, using a fiberoptic monitoring system. Am J Vet Res. 1995;56:1475‐1478. [PubMed] [Google Scholar]
- 27. Strecker EP, Schmidt‐Hieber M, Kauffmann G, et al. Liquordruckschwankungen beim experimentellen kommunizierenden Hydrocephalus. Arch Psychiatry Nervenkr. 1977;233:351‐360. [DOI] [PubMed] [Google Scholar]
- 28. Tuli S, O'Hayon B, Drake J, et al. Change in ventricular size and effect of ventricular catheter placement in pediatric patients with shunted hydrocephalus. Neurosurgery. 1999;45:1329‐1333. [DOI] [PubMed] [Google Scholar]
- 29. Rubin RC, Hochwald GM, Tiell M, Epstein F, Ghatak N, Wisniewski H. Hydrocephalus: III. Reconstitution of the cerebral cortical mantle following ventricular shunting. Surg Neurol. 1976;5:179‐183. [PubMed] [Google Scholar]
- 30. Silva D. Pathophysiology of Hydrocephalus In: Cinalli G, Maixner WJ, Sainte‐Rose C, eds. Pediatric Hydrocephalus. Milano, Italy: Springer; 2004:65‐77. [Google Scholar]
- 31. Takahashi Y. Long‐term outcome and neurologic development after endoscopic third ventriculostomy versus shunting during infancy. Childs Nerv Syst. 2006;22:1591‐1602. [DOI] [PubMed] [Google Scholar]
- 32. Sprung C, Schultz B. Correlation of post‐operative clinical course and ventricular size determined by computed tomography in normal pressure hydrocephalus. Adv Neurosurg. 1982;10:156‐163. [Google Scholar]
- 33. Palm WM, Walchenbach R, Bruinsma B, et al. Intracranial compartment volumes in normal pressure hydrocephalus: volumetric assessment versus outcome. AJNR Am J Neuroradiol. 2006;27:76‐79. [PMC free article] [PubMed] [Google Scholar]
- 34. Meier U, Mutze S. Does the ventricle size change after shunt operation of normal‐pressure hydrocephalus? Acta Neurochir Suppl. 2005;95:257‐259. [DOI] [PubMed] [Google Scholar]
- 35. Meier U, Mutze S. Correlation between decreased ventricular size and positive clinical outcome following shunt placement in patients with normal‐pressure hydrocephalus. J Neurosurg. 2004;100(6):1036‐1040. [DOI] [PubMed] [Google Scholar]
- 36. Kirkpatrick M, Engleman H, Minns RA. Symptoms and signs of progressive hydrocephalus. Arch Dis Childhood. 1989;64:124‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yinon U, Chen M, Milgram A. Hydrocephalus in developing cats: physiological properties of visual cortex cells. Brain Res Bull. 1990;25:651‐663. [DOI] [PubMed] [Google Scholar]
- 38. De Lahunta A. Visual system—special somatic afferent system Veterinary Neuroanatomy and Clinical Neurology. 2nd ed. Philadelphia, PA: WB Saunders Co; 1983:279‐303. [Google Scholar]
- 39. Kurt G, Cemil B, Borcek AO, et al. Infliximab administration reduces neuronal apoptosis on the optic pathways in a rabbit hydrocephalus model: a preliminary report. Br J Neurosurg. 2010;24(3):290‐294. [DOI] [PubMed] [Google Scholar]
- 40. Yamada H, Yokota A, Furuta A. Reconstitution of shunted mantle in experimental hydrocephalus. J Neurosurg. 1992;76:856‐862. [DOI] [PubMed] [Google Scholar]
- 41. Jung JH, Chai YH, Jung S, et al. Visual outcome after endoscopic third ventriculostomy for hydrocephalus. Childs Nerv Syst. 2018;34:247‐255. [DOI] [PubMed] [Google Scholar]
- 42. Andersson S, Persson EK, Aring E, Lindquist B, Dutton GN, Hellström A. Vision in children with hydrocephalus. Dev Med Child Neurol. 2006;48:836‐841. [DOI] [PubMed] [Google Scholar]
- 43. Blohme J, Tornqvist K. Visual impairment in Swedish children. III Diagnoses. Acta Ophthalmol Scand. 1997;75:681‐687. [DOI] [PubMed] [Google Scholar]
- 44. Houliston MJ, Taguri AH, Dutton GN, et al. Evidence of cognitive visual problems in children with hydrocephalus: a structured clinical history‐taking strategy. Dev Med Child Neurol. 1999;41:298‐306. [DOI] [PubMed] [Google Scholar]
- 45. Aoyama Y, Kinoshita Y, Yokota A, Hamada T. Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: a study involving the neurofilament‐immunostaining method. J Neurosurg. 2006. May;104(5 Suppl):332‐339. [DOI] [PubMed] [Google Scholar]
- 46. Kriebel RM, Shah AB, McAllister JP 2nd. The microstructure of cortical neuropil before and after decompression in experimental infantile hydrocephalus. Exp Neurol. 1993;119:89‐98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 Overview about the epidemiologic data, results of neurologic examination, measurements of brain‐ and ventricular volume as well as ventricular reduction before surgery 3 months after ventriculo‐peritoneal shunting


