Abstract
Background
The clinical applicability of sonography and sonoelastography (SOE) in the detection of lymph node malignancy in dogs has not been established.
Objectives
To compare sonographic and sonoelastographic findings between malignant and benign superficial lymph nodes and to evaluate the diagnostic performance of those methods.
Animals
One‐hundred sixteen lymph nodes of 54 dogs.
Methods
A prospective observational study was used to investigate sonographic features and elasticity scores of malignant and benign superficial lymph nodes. Lymph nodes were categorized as malignant or benign according to cytology or histopathology. Quantitative variables were compared using Student's unpaired t test. Prevalence of categorical variables was compared using nonparametric Mann‐Whitney U test. Diagnostic performance was calculated by receiver‐operating characteristic analysis.
Results
Forty‐nine malignant and 67 benign lymph nodes were included. Malignant nodes had larger long axis (LA; P = .0002), short axis (SA; P < .0001) and short‐to‐long axis ratio (P < .0001) in comparison with benign nodes. Malignant nodes had a higher prevalence of mixed vascular distribution on Doppler color flow mapping (P < .005) and on power Doppler (P < .0001) and higher resistivity index (RI; P < .0001), pulsatility index (P < .0001), and elasticity score (P < .0001) in comparison with benign nodes. Short axis, elasticity score, and RI offered the best accuracies, 80.2%, 78.1%, and 77.7% (P < .05), respectively, for malignancy detection.
Conclusions and Clinical Importance
Results support the use of Doppler sonography and SOE as auxiliary methods to brightness mode sonography to detect nodal malignancy.
Keywords: canine, Doppler, elastography, lymphoid tissue, ultrasound
Abbreviations
- ARFI
acoustic radiation force impulse
- AUC
area under the curve
- CI
confidence interval
- DCFM
Doppler color‐flow mapping
- EDV
end‐diastolic velocity
- FNA
fine‐needle aspiration
- LA
long axis
- MV
mean velocity
- NPV
negative predictive value
- PD
power Doppler
- PI
pulsatility index
- PPV
positive predictive value
- PRF
pulse repetition frequency
- PWD
pulsed‐wave Doppler
- RI
resistivity index
- ROC
receiver‐operating characteristic
- SA
short axis
- SE
sensitivity
- SLR
short‐to‐long axis ratio
- SOE
sonoelastography
- SP
specificity
- SPV
systolic peak velocity
1. INTRODUCTION
Accurate identification of malignancy in lymph nodes is essential for therapeutic planning, tumor staging, and prognosis prediction in dogs with neoplasia.1 Fine‐needle aspiration (FNA) cytology is widely used in small animal clinical practice because of its minimal invasiveness, cost‐effectiveness2 and high sensitivity (SE) and specificity (SP) for diagnosis of lymph node lesions.3 However, FNA cytology may be associated with a low percentage of nondiagnostic sampling and may be costly if the assessment of multiple nodes is required.4
Several studies investigated the utility of noninvasive imaging methods, brightness mode (B‐mode), and Doppler sonography, for characterization of lymph node lesions in human patients.5, 6, 7, 8, 9, 10, 11 Sonographic features such as size, shape, margination, echogenicity, texture,5, 8, 9 vascular flow distribution,5, 7, 9, 10, 11 and perfusion indices6, 7 have been used to characterize lesions.
In the veterinary medical literature, although some studies attempted to characterize malignant nodes using ultrasonography, results often have been controversial, particularly with the use of B‐mode sonography.1, 12, 13, 14, 15 Pulsed‐wave Doppler (PWD) sonography has been proposed as a method of differentiating benign from malignant lymphadenopathy in dogs.13, 16 However, the value of the isolated use of this technique remains to be determined, because satisfactory diagnostic accuracy may be dependent on extreme cutoff values.6
Sonoelastography (SOE) was first implemented in human medicine in the early 1990s.17 Among the different modalities available, strain elastography is a real‐time imaging technique that can estimate tissue elasticity according to elasticity stiffness scores18 and is based on the assumption that malignant tissues have stiffer elasticity than do benign tissues.19 Studies performed in human patients suggest that this modality can be used as a complementary tool to ultrasonography with potential to avoid unnecessary biopsies of nodal lesions.20
We are aware of only 1 study in the veterinary medical literature that used strain elastography to try and differentiate malignant from nonmalignant lymph nodes. This study was limited by its small sample size and finding of overlap of the elastographic scores between malignant and nonmalignant lymph nodes.21 The majority (80.4%) of nodes evaluated in that study were intra‐abdominal (deep) nodes.
Although sonography and SOE can provide detailed information regarding dimensions, contours, parenchymal architecture, vascularization, and elasticity of lymph nodes affected by different disease processes, the value of these variables in the detection of malignancy remains unclear in veterinary clinical practice. Our first objective was to compare B‐mode and Doppler sonographic features as well as elasticity stiffness scores between benign and malignant superficial lymph nodes of dogs. We hypothesized that sonographic and elastographic features would be distinct between both categories of nodes. The second objective was to evaluate the diagnostic performance of those methods in the detection of nodal malignancy.
2. MATERIALS AND METHODS
2.1. Animals
This prospective observational study included 116 superficial lymph nodes over a 1‐year period (July 2016 to June 2017). The study population comprised 54 client‐owned dogs. Samples from 53 were submitted to the Pathology Service, Department of Veterinary Clinics, for cytological examination of 114 lymph nodes. Reasons for referral to cytology included investigation of enlarged superficial lymph nodes and regional lymph node staging in patients with local tumors. Immediately after cytology, dogs underwent sonographic and sonoelastographic examination. At this time, cytological specimens were subjected to a rapid on‐site evaluation of sampling quality for later cytological examination and diagnosis. One dog had sonographic evaluation of 2 lymph nodes performed before microscopic evaluation. This dog subsequently was euthanized and the 2 lymph nodes were removed during necropsy, and histopathologic evaluation was performed instead of cytology.
Inclusion criteria consisted of any lymph node of dogs that underwent cytological or histopathological examination and the owners of which allowed sonographic evaluation of the examined node. Exclusion criteria consisted of lymph nodes with nondiagnostic cytology specimens or lymph nodes with diagnosis of a benign lesion in a dog diagnosed with lymphoma by FNA of another node.
The study was performed according to the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and was approved by the Ethics Committee on Animal Use (CEUA) of the School of Veterinary Medicine and Animal Science, Sao Paulo State University (protocol 136/2016). Owners signed consent forms before inclusion of dogs in the study.
2.2. Cytology/Histopathology
Cytological or histopathological examination of the lymph nodes was performed by experienced pathologists. Samples were collected and processed using standard techniques. Cytology slides were air‐dried and subsequently stained with Diff‐Quik (Laborclin, Pinhais, Parana, Brazil). Histologic sections were prepared from formalin‐fixed tissue and stained with hematoxylin‐eosin. Slides were interpreted and, based on the diagnosis, categorized as benign or malignant.
2.3. Ultrasonography
Sonography and SE were performed by a single investigator (A.F. Belotta) who was unaware of the category of each lymph node (benign or malignant), using a MyLab Alpha Unit (Esaote Healthcare, Sao Paulo, SP, Brazil) and multifrequency linear transducer (4‐13 MHz). Images were stored in the equipment and transferred via DICOM for analysis using MyLab Desk3 software (Esaote Healthcare, Sao Paulo, Brazil).
Using B‐mode, maximum short axis (SA) diameter and maximum long axis (LA) diameter were measured, and short‐to‐long axis (SLA) ratio was calculated. Contour regularity (regular or irregular), nodal border definition (well‐defined or ill‐defined), parenchymal uniformity (homogeneous or heterogeneous), nodal hilum definition (present or absent), and perinodal fat echogenicity (isoechoic or hyperechoic) were recorded.
Doppler color‐flow mapping (DCFM) and power Doppler (PD) were used to evaluate vascular supply within the node. Doppler gain was adjusted to the highest without the presence of noise, ensuring high sensitivity for the detection of smaller and low velocity flow vessels. Pulse repetition frequency (PRF) was kept the lowest without aliasing artifact. When detected, vascular flow was analyzed in multiple planes and was subjectively categorized according to intensity (mild, moderate, or intense) and distribution (hilar, peripheral or mixed, hilar and peripheral). When present, anomalous vascular flow was categorized as spotted (scattered vascular spots distributed in a random order within the parenchyma), pericapsular (peripheral vessels, adjacent and outside the lymph node capsule), subcapsular (peripheral vessels, adjacent and inside the lymph node capsule), trans‐capsular (centripetal flow signal, opposite to the hilar flow), exuberant (central or peripheral high flow signals), or deformed (displaced radial vascularity), as previously described in human patients and in dogs.14, 22, 23
Spectral waves of vessels within the nodes were obtained using PWD, and semiquantitative evaluation of nodal perfusion was performed. Frequency varied between 5 and 7.1 MHz, and PRF and Doppler gain were adjusted as reported for DCFM and PWD. Once 3 consecutive and similar arterial peak waveforms within a consistent series were obtained, systolic peak velocity (SPV) and end‐diastolic velocity (EDV) were measured. Resistivity index (RI) and pulsatility index (PI) were calculated according to the following formulas:
where RI is resistivity index; SPV is systolic peak velocity; EDV is end‐diastolic velocity; PI is pulsatility index; and, MV is mean velocity.
For each lymph node, 2 other arterial spectral waves were obtained, and average RI and PI were calculated.
2.4. Sonoelastography
Sonoelastography was performed using the ElaXto sonoelastography function in the same ultrasound unit (MyLabAlpha). Before evaluation of a node, the observer (A.F.B.), who had 5 years of experience with qualitative elastography, evaluated several images to become familiar with the proposed chromatic scale. After acquiring a B‐mode image of the lymph node in the longitudinal plane, a region of interest comprising the lymph node and surrounding tissue was selected. Rhythmic soft movements creating compression and decompression were applied perpendicular to the node. Images were saved when the compression scale became green, indicating that the compressive and decompressive movements performed on the node were uniform and regular.
The elastogram was displayed on the right side of the screen and the B‐mode image on the left side. The degree of deformity of the lymph node was determined according to a qualitative chromatic scale and the estimate of tissue stiffness determined by the same experienced sonographer who subjectively assigned each image of the nodes into 1 of the 4 elasticity stiffness scores previously proposed24:
1: ≥80% of the total area of the node depicted in yellow or red (soft);
2: 50% to 80% of the total area of the node depicted in yellow or red (moderately soft);
3: 50% to 80% of the total area of the node depicted in blue (moderately stiff);
4: >80% of the total area of the node depicted in blue (stiff).
Intranodal anechoic focal areas corresponding to cystic or necrotic changes were not included in the analysis because they were not indicative of the solid component. In those cases, the remaining parenchyma was evaluated. In cases in which acquisition of uniform and regular movements was not feasible, nodes were excluded from the analysis.
Five to 10 elastograms were acquired for each lymph node, and an elasticity stiffness score was assigned for each image. An average score then was calculated for each lymph node. If the resulting average score was a decimal number, it was approximated to the nearest whole number. The mean elasticity stiffness score then was obtained for each group (benign and malignant).
2.5. Statistical analysis
Normal distribution of quantitative variables was determined by the Kolmogorov‐Smirnov test. Quantitative variables including LA, SA, SLA ratio, RI, and PI were compared between neoplastic and nonneoplastic nodes using a Student's unpaired t test (for data with normal distribution). Qualitative variables including contour regularity, nodal border definition, parenchymal uniformity, nodal hilum, perinodal tissue echogenicity, vascular flow intensity and distribution, abnormal vascular flow, and elasticity stiffness score were assessed between the 2 categories of nodes using a nonparametric Mann‐Whitney U test (for data with nonnormal distribution).
To evaluate diagnostic performance of the variables that were significantly different between benign and malignant nodes, optimal cutoff points maximizing SE and SP were determined using receiver‐operating characteristic (ROC) curves. Area under the curve (AUC) was calculated with 95% confidence interval (CI) to quantify the ability of each variable to detect malignancy. In addition, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for each cutoff point. Agreement of malignancy with vascular flow distribution (normal/hilar or abnormal/hilar and peripheral or abnormal/peripheral) on PD was analyzed using Cohen kappa coefficient for each group (benign versus malignant). In addition, a binary logistic regression model was built, using malignancy as the dependent variable. The Nagelkerke pseudo‐R2 value was calculated to assess prediction accuracy, and an ROC curve was built to determine the predictive capacity. Analyses were considered significant when P < .05.
3. RESULTS
Dogs with malignant nodes had a mean age of 9.2 years (median, 10 years; range, 4‐14 years) and mean weight of 22 kg (median, 18.5 kg; range, 5.6‐49 kg), whereas dogs with benign nodes had mean age of 7.6 years (median, 8 years; range, 0.5‐13 years) and mean weight of 20.7 kg (median, 18.5 kg; range, 4.2‐60.5 kg). The malignant nodes group comprised mostly mixed‐breed dogs (10/21, 47.62%), Dachshunds (3/21, 14.29%), and Golden Retrievers (2/21, 9.52%), among others. The benign nodes group comprised mostly mixed‐breed dogs (21/33, 63.64%), Pit Bulls (3/33, 9.09%), and Labradors (2/33, 6.06%), among other breeds.
The evaluated lymph nodes were superficial cervical (n = 40), popliteal (n = 41), mandibular (n = 22), inguinal (n = 12), and axillary (n = 1). According to cytology or histopathology, lymph nodes were categorized into malignant (n = 49) or benign (n = 67) groups. Benign nodes were diagnosed as reactive nodes (42/67, 62.69%), parasitic lymphadenitis (Leishmania spp.; 15/67, 22.39%), eosinophilic lymphadenitis (7/67, 10.45%), neutrophilic lymphadenitis with coccoid bacteria (2/67, 2.98%), and nodal hyperplasia (1/67, 1.49%). Most malignant nodes were diagnosed with lymphoma (39/49, 79.59%) and the remaining nodes with metastasis of local tumors (10/49, 20.41%). With respect to metastatic nodes, most represented metastasis from carcinomas (7/10, 70%), which included metastasis from mammary carcinomas (5/7, 71.43%), sebaceous carcinoma of the prepuce (1/7, 14.28%), and anal glad carcinoma (1/7, 14.28%). The remaining nodes were mast cell tumor (2/10, 20%) and oral melanoma (1/10, 10%) regional lymph node metastases.
Sonographic features of malignant and benign nodes are summarized in Tables 1 and 2 and elasticity scores are summarized in Table 3. Significant differences were seen in LA (P = .0002), SA (P < .0001), and SLA ratio (P < .0001) between both categories. Means for those features were higher in malignant nodes (Table 1). At B‐mode sonography, cutoff SLA ratio was 0.48; ≤0.48 was benign and >0.48 was malignant with 85.7% SE and 67.2% SP (P < .05). No statistical difference was found for contour regularity, nodal border definition, parenchymal uniformity, nodal hilum definition, and perinodal fat echogenicity between benign and malignant nodes (Figure 1). However, an irregular contour and well‐defined nodal border were more frequent in benign nodes, whereas heterogeneous parenchyma, absence of hilum definition, and hyperechoic perinodal fat were more frequent in malignant nodes. Among benign nodes with heterogeneous parenchyma, heterogeneity was a result of hypoechoic areas within the parenchyma (17/33, 51.51%), “target‐like” lesions (8/33, 24.24%), hyperechoic distorted hilum (5/33, 15.15%), and hypoechoic peripheral rim (3/33, 9.09%). Among malignant nodes, heterogeneity was a result of ill‐defined hypoechoic areas within the parenchyma (13/49, 26.53%), focal areas of calcification (11/49, 22.45%), and focal well‐defined nodular areas (3/49, 6.12%).
Table 1.
B‐mode sonographic features of benign and malignant lymph nodes of dogs
| B‐mode sonographic features | Benign | Malignant | P |
|---|---|---|---|
| (N = 67) | (N = 49) | ||
| LA (cm) | 2.61 ± 0.88* | 3.43 ± 1.39* | .0002 |
| SA (cm) | 1.15 ± 0.37** | 2.18 ± 1.12** | <.0001 |
| SLA ratio | 0.46 ± 0.14** | 0.64 ± 0.16** | <.0001 |
| Irregular contour | 40 (59.7%) | 26 (53.06%) | .84 |
| Well‐defined border | 60 (89.55%) | 41 (83.67%) | .79 |
| Heterogeneous parenchyma | 33 (49.25%) | 29 (59.18%) | .36 |
| Nodal hilum definition | 43 (64.18%) | 30 (61.22%) | >.99 |
| Hyperechoic perinodal fat | 19 (28.36%) | 20 (40.82%) | .19 |
Abbreviations: LA, long axis; SA, short axis; SLA ratio, short‐to‐long axis ratio.
P = .0002 between benign and malignant nodes.
P < .0001 between benign and malignant nodes.
Table 2.
Doppler sonographic features of benign and malignant lymph nodes of dogs
| Doppler sonographic features | Benign | Malignant | P |
|---|---|---|---|
| (N = 67) | (N = 49) | ||
| Vascular flow present on DCFM | 61 (91.04%) | 43 (87.75%) | .76 |
| Vascular flow intensity on DCFM | |||
| Mild | 30 (49.18%) | 24 (55.81%) | 0.46 |
| Moderate | 29 (47.54%) | 18 (41.86%) | |
| Intense | 2 (3.28%) | 1 (2.32%) | |
| Vascular flow distribution on DCFM | |||
| Hilar | 47 (77.05%)* | 19 (44.19%)* | .003 |
| Peripheral | 1 (1.64%)* | 4 (9.3%)* | |
| Hilar and peripheral | 13 (21.31%)* | 20 (46.51%)* | |
| Vascular flow present on PD | 65 (97.01%) | 48 (97.96%) | .91 |
| Vascular flow intensity on PD | |||
| Mild | 17 (26.15%) | 16 (33.33%) | .85 |
| Moderate | 42 (64.61%) | 20 (41.67%) | |
| Intense | 6 (9.23%) | 9 (18.75%) | |
| Vascular flow distribution on PD | |||
| Hilar | 45 (69.23%)** | 9 (18.75%)** | <.0001 |
| Peripheral | 0** | 5 (10.42%)** | |
| Hilar and peripheral | 20 (30.77%)** | 32 (66.67%)** | |
| Anomalous vascular flow present | 10 (15.38%)** | 42 (87.5%)** | <.0001 |
| RI | 0.64 ± 0.1** | 0.75 ± 0.1** | <.0001 |
| PI | 1.23 ± 0.42** | 1.84 ± 0.73** | <.0001 |
Abbreviations: DCFM, Doppler color‐flow mapping; PD, Power Doppler; PI, pulsatility index; RI, resistivity index.
P < .05 between benign and malignant nodes.
P < .0001 between benign and malignant nodes.
Table 3.
Distribution of frequencies of elastographic scores of benign and malignant lymph nodes of dogs
| Elastographic score | Benign | Malignant | P |
|---|---|---|---|
| (N = 60) (%) | (N = 36) (%) | ||
| 1 | 24 (40)** | 0** | <.0001 |
| 2 | 21 (35)** | 6 (16.67)** | |
| 3 | 13 (21.67)** | 14 (38.89)** | |
| 4 | 2 (3.33)** | 16 (44.44)** |
1, soft elasticity; 2, soft, intermediate elasticity; 3, stiff, intermediate elasticity; 4, stiff elasticity.
P < .0001 between benign and malignant nodes.
Figure 1.
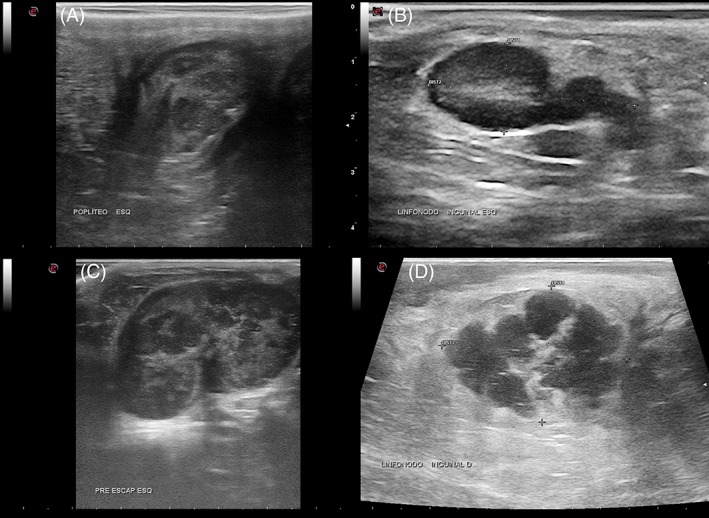
B‐mode sonographic images of reactive (A and B) and neoplastic nodes (C and D) of dogs. Heterogeneous parenchyma is noted in a popliteal reactive node (A) and in a superficial cervical lymphomatous node (C). An increase in echogenicity of peri‐nodal fat is observed around a reactive superficial inguinal node (B) and around a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node
A significant difference was seen in the vascular flow distribution between the groups using DCFM (P < .005) and PD (P < .0001; Figure 2A, B). Hilar flow was the most frequent vascular distribution in the benign nodes category, whereas mixed (hilar and peripheral) flow distribution was most frequent in the malignant nodes (Figure 2C, D). Although vascular flow distribution on PD had low agreement with malignancy (Cohen's kappa = 0.48; 95% CI, 0.29‐0.66), binary logistic regression coefficients were significant (P < .01) and lymph nodes with mixed or peripheral vascular flow distribution on PD had a 9.25 greater risk of malignancy (95% CI, 3.9‐23.8). The presence of abnormal vascular flow within the nodes was significantly different between benign and malignant nodes (P < .0001) and was greater in malignant nodes (42/46, 91.3%). The following types of anomalous flow were identified in malignant nodes: spotted (22/46, 47.83%), pericapsular (14/46, 30.43%), subcapsular (7/46, 15.22%), transcapsular (7/46, 15.22%), exuberant (5/46, 10.87%), and deformed (3/46, 6.52%). In some nodes, >1 type of anomalous flow could be detected (Table 2).
Figure 2.
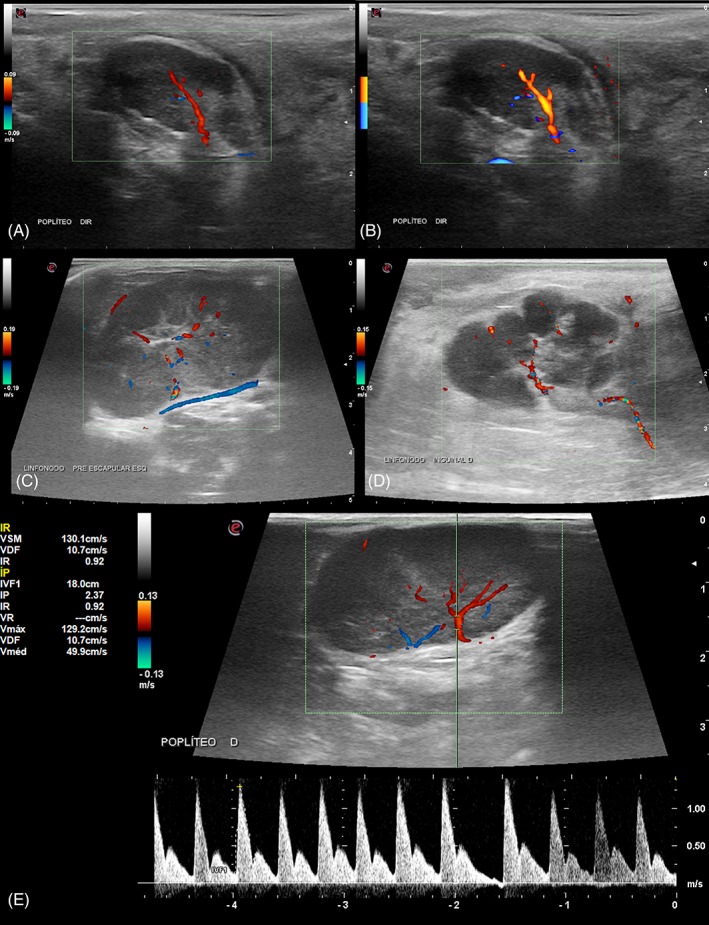
Doppler sonographic images of lymph nodes of dogs. Doppler color flow mapping (A) and Power Doppler (B) images show hilar vascular flow distribution in the same reactive popliteal node, more intense on Power Doppler. Doppler color flow mapping of a superficial cervical lymphomatous node (C) shows hilar and peripheral flow distribution, with an associated abnormal peri‐capsular vessel. Doppler color flow mapping (D) of a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node: abnormal exuberant hilar and peripheral flow. Pulsed wave Doppler image (E) of a popliteal lymph node with high resistivity spectral pattern and perfusion indices (RI: 0.92; and PI: 2.37)
Resistivity and pulsatility indices also differed between the groups (P < .0001), with higher values observed in the malignant nodes in comparison with benign nodes (Figure 2E). When an RI value of 0.69 was taken as the cutoff point, SE and SP of RI were 86.4% and 71.2%, respectively (P < .05). When the PI value of 1.49 was taken as the cutoff point, SE and SP of PI were 70.7% and 78%, respectively (P < .05). No significant differences were found between the groups for other Doppler sonographic features such as the presence of vascular flow and intensity of flow (Table 2).
Elasticity score distribution was significantly different between the groups (P < .0001), with a score of 1, relative to soft elasticity, being most frequent in the benign nodes (Supporting Information Figure S3A, B) and score 4, relative to stiff elasticity, being most frequent in the malignant nodes (Figure 3C, D; Table 3). Satisfactory elastographic images were acquired for 82.8% (96/116) of the nodes. Nearly 89.5% of the benign nodes (60/67) and nearly 73.5% of the malignant nodes (36/49) had satisfactory elastographic images that allowed categorization into 1 of the scores. Reasons for unsatisfactory elastograms included large size of some of the lymphomatous nodes in the malignant group and an anechoic or hypoechoic component corresponding to an extensive portion of the node in both groups.
Figure 3.
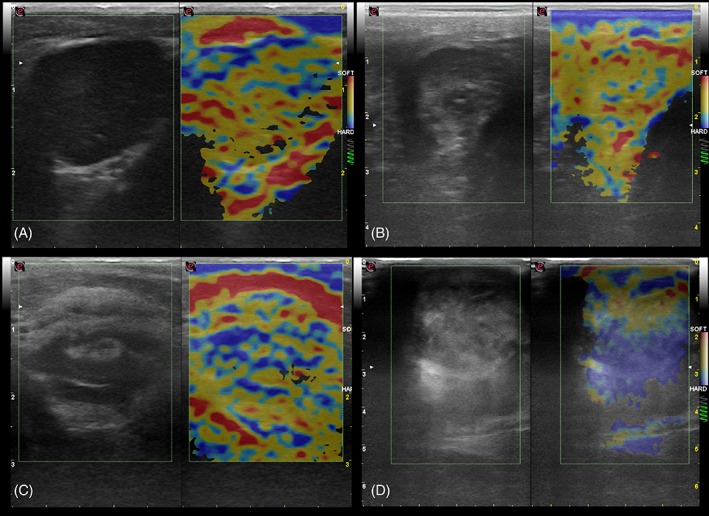
Sonoelastographic images of reactive popliteal nodes (A and B), of a lymphomatous popliteal node (C) and an oral melanoma metastatic mandibular node (D) in dogs. In images (A) and (B), more than 80% of the total area of the node is depicted in red (soft) and yellow (intermediate), with less than 20% of the area depicted in blue (stiff), corresponding to score 1. In (C), 50 to 80% of the total area of the node is depicted in blue and mild areas depicted in red or yellow, corresponding to score 3. In (D), more than 80% of the total are of the node is depicted in blue , correponding to score 4
The diagnostic performances of B‐mode, PD, PWD, and SE variables are summarized in Table 4. Short axis offered the highest diagnostic accuracy, 80.2% (P < .05), for prediction of nodal malignancy, followed by elasticity score, with 78.1% (P < .05), and RI, with 77.7% (P < .05). Long axis offered the highest SP for prediction of nodal malignancy, 85.1% (P < .05). Receiver‐operating characteristic curves for B‐mode, PD, and PWD, relative to SE, are shown in Figure 4. Among the variables, elasticity score showed the highest AUC, 0.87 (95% CI, 0.8‐0.93).
Table 4.
Diagnostic performance of B‐mode, color‐flow mapping Doppler, power Doppler, pulsed‐wave Doppler and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs
| Sonographic features | Cutoff | SE | SP | PPV | NPV | Accuracy | AUC |
|---|---|---|---|---|---|---|---|
| point | (%) | (%) | (%) | (%) | (%) | (95% CI) | |
| SA | 1.73 | 92.5 | 63.3 | 86.1 | 77.5 | 80.2 | 0.83 |
| LA | 3.42 | 51 | 85.1 | 71.4 | 70.4 | 70.7 | 0.68 |
| SLA ratio | 0.48 | 86.7 | 67.2 | 65.6 | 86.5 | 75 | 0.8 |
| Flow distribution PD | … | 80 | 69 | 65 | 83 | 73 | 0.75 |
| RI | 0.69 | 86.4 | 71.2 | 69.1 | 87.5 | 77.7 | 0.82 |
| PI | 1.49 | 70.7 | 78 | 69 | 79.3 | 75 | 0.78 |
| Elasticity score | 2.5 | 83.3 | 75 | 66.7 | 88.2 | 78.1 | 0.87 |
Abbreviations: AUC, area under the curve; LA, long axis; NPV, negative predictive value; PD, Power Doppler; PI, pulsatility index; PPV, positive predictive value; RI, resistivity index; SA, short axis; SE, sensitivity; SLA ratio, short‐to‐long axis ratio; SP, specificity.
Figure 4.
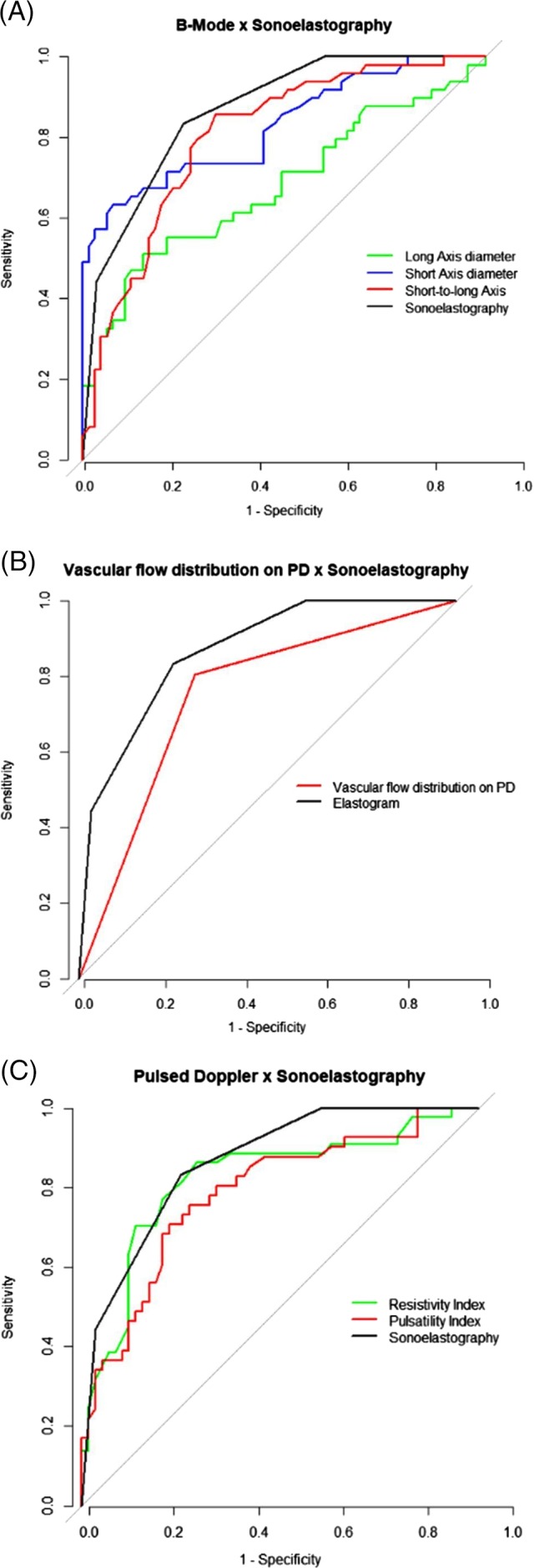
Receiver‐operating characteristic curves assessing the diagnostic efficacy of B‐mode parameters and sonoelastography (A), vascular flow distribution on power Doppler and sonoelastography (B), and pulsed‐wave Doppler parameters and sonoelastography (C), for detection of malignancy in superficial lymph nodes in dogs. Area under the curve for sonoelastography (0.87) is higher than those for short axis (0.83), long axis (0.68), short‐to‐long axis ratio (0.8), vascular flow distribution on power Doppler (0.75), resistivity index (0.82) and pulsatility index (0.78)
4. DISCUSSION
Our hypothesis was that sonographic and elastographic features would be distinct between benign and malignant nodes. Although significant differences were observed in SA, LA, and SLA ratio between both categories, most B‐mode sonographic features including contour regularity, nodal border definition, parenchymal uniformity, nodal hilum definition, and perinodal tissue echogenicity did not differ significantly.
Mean SA and LA for benign and malignant nodes in our study are higher than values reported for presumptively normal superficial cervical lymph nodes in dogs.25 Malignant nodes had significantly higher SA and LA in comparison with benign nodes, as previously reported for deep lymph nodes in dogs.15 To our knowledge, mean values for SA and LA, as well as differences in these values between benign and malignant superficial nodes in dogs, have not been investigated previously. In our study, among all variables, SA offered the best diagnostic accuracy for detection of nodal malignancy. However, the overlap of SA measurements between some benign and malignant nodes and the fact that some metastatic nodes may not increase in size highlight the importance of additional tools such as Doppler modality and SOE in the detection of malignancy. Although LA offered the lowest diagnostic accuracy, it also had the highest SP and thus better ability to avoid unnecessary invasive procedures for the diagnosis of neoplastic nodes.
The best diagnostic accuracy for SLA ratio in our study showed a cutoff point ≤0.48 for reactive and >0.48 for malignant nodes. The higher SLA ratio observed for neoplastic nodes in comparison with benign nodes corroborates previous studies in dogs and human patients,24, 26 which showed that neoplastic nodes tend to have rounded shape. The rounded shape of neoplastic nodes is associated with tumor cell infiltration in part of the node, obliterating lymphatic channels, and leading to focal cortical enlargement.27 However, superficial metastatic nodes in dogs were reported to have a rounded or elongated shape.13 The same was observed in our study, in which half of the metastatic nodes (5/10; 50%) had an SLA ratio <0.5 and, consequently, more elongated shape. The higher proportion of lymphomatous nodes (39/49, 79.59%) included in the malignant group as opposed to metastatic nodes (10/49, 20.41%) may have contributed to a higher mean SLA ratio in this category.
The lack of significant differences in contour regularity, nodal border definition, parenchymal uniformity, and nodal hilum definition corroborates a previous study that compared sonographic features between benign and malignant superficial lymph nodes of dogs.13 With respect to hyperechoic nodal fat, some investigators found that this sonographic variable is more likely associated with lymph nodes with round cell neoplasia, although it also may be a nonspecific finding in lymph nodes with other types of neoplasia or with benign lesions.28 In our study, although hyperechoic perinodal fat was found to be more prevalent in neoplastic nodes, when analyzing only the malignant group, this feature was found to be more prevalent in metastatic nodes (7/10, 70%) than in lymphomatous nodes (13/39, 33.3%), as opposed to the previous study.28
In our study, nodal border was well defined in a high number of lymph nodes in both groups. Well‐defined nodal border, in human patients, was associated with malignancy.29 In another study performed on superficial lymph nodes of dogs, although this feature was highly prevalent in lymphomatous nodes, no statistical difference was observed between malignant and benign nodes, supporting our results.13
Although parenchymal heterogeneity was more frequently observed in malignant nodes because of the presence of focal areas of calcification (11/49, 22.45%), hypoechoic areas (13/49, 26.53%), and focal nodular areas (3/49, 6.12%), no statistical difference was observed between benign and malignant nodes. Some investigators have associated heterogeneity with malignancy in intra‐abdominal lymph nodes of dogs.1, 12 Others reported similar frequencies for parenchymal heterogeneity between benign and malignant lesions in superficial and deep canine lymph nodes.13, 30 Focal areas of decreased echogenicity in benign nodes may be representative of inflammation, whereas in malignant nodes, it may occur as a result of necrosis.30
In our study, nodal hilum was defined in nearly 60% of nodes in both groups and, consequently, no statistical difference was found, corroborating other studies.13, 30 The absence of a nodal hilum definition was described in a high percentage of lymphomatous nodes in dogs.14 It also has been previously associated with malignancy in human patients, because of infiltration of neoplastic tissue.20 However, other researchers showed that nonneoplastic lesions such as inflammatory or infectious lymphoid hyperplasia could lead to formation of new germinal centers within the hilum and, as a result, to its absence.30
Doppler modalities have been shown to be effective in the detection of malignancy in lymph nodes of human and canine patients.11, 13, 15, 31 The same was observed in our study because vascular flow distribution, RI and PI, were significantly distinct between malignant and benign nodes. With regard to vascular distribution, an improvement in statistical significance was observed when PD was used to evaluate vascular flow as opposed to the use of DCFM. This is justified by the higher SE of PD for the detection of weak signals in small vessels that have low‐velocity flow.23 Hilar vascular flow distribution was most frequent in benign lymph nodes, whereas hilar and peripheral were most frequent in malignant lymph nodes, as observed in a previous study.13 In our study, PD did not offer high diagnostic accuracy (73%) in the detection of nodal malignancy. However, nodes with peripheral or mixed vascular distribution were 9.25 times more likely to be malignant (95% CI, 3.9‐23.8, P < .05). Abnormal vascular flow was detected in 87.5% of malignant nodes, including high frequencies of scattered spots of vessel signals, pericapsular (14/46, 30.43%), subcapsular (7/46, 15.22%), and transcapsular (7/46, 15.22%). With the chronicity of neoplastic processes, tumor compression leads to avascular areas within the nodes and capsular vessels are created in order to supply the tumor.23
The high RI and PI observed in the neoplastic nodes may reflect increased intranodal vascular resistance as a result of the compressive effect of the tumor, angioneogenesis, and arteriovenous shunts, which lead to turbulent flow and high perfusion indices,30, 31 corroborating other studies in dogs.13, 16 The ROC analysis showed that cutoff values for RI (0.69) and PI (1.49) to predict nodal malignancy in our study are close to the cutoff values previously reported for RI (0.68) and PI (1.49) to discriminate between normal and metastatic superficial lymph nodes in dogs.13 In addition, RI in our study showed very good diagnostic accuracy in the prediction of malignancy, as opposed to a study performed in human patients that suggested that only extreme cutoff points of perfusion indices are useful in diagnosis and treatment planning.6
With respect to the elastographic examination, the use of a qualitative chromatic scale was effective in the differentiation between superficial benign and malignant nodes. In the benign nodes, a high frequency of score 1 (soft) was found, whereas in malignant nodes a high frequency of score 4 (stiff) was found, similar to results described in a previous study that evaluated a population comprised mostly of deep canine and feline lymph nodes.21 Although variations may occur, particularly in lymphomatous nodes with necrotic areas, malignant nodes of human patients showed >45% of their total area with stiff elasticity because of infiltration by tumor.32, 33 An important proportion of lymph nodes in both groups had overlapping elastographic scores, as previously described,21 limiting the use of SOE individually for the detection of malignancy. However, in human patients, a high diagnostic power was obtained associating the high SP of SOE with the high sensitivity of B‐mode sonography in comparison with the use of each method individually in the detection of malignancy in nodes that had overlapping sonographic scores.32
To our knowledge, ours is the first study to report the diagnostic performance of strain elastography in the detection of nodal malignancy in dogs. Elasticity stiffness score offered the highest AUC value and, consequently, the best diagnostic ability to predict nodal malignancy. This modality also offered very good diagnostic SE (83.3%), and moderate SP (75%) in malignancy detection. These results are supported by those reported in a recent study, in which Acoustic Radiation Force Impulse (ARFI) elastography, a quantitative modality of elastography, had excellent diagnostic accuracy in the detection of axillary and inguinal nodal metastasis in bitches with mammary neoplasms.34 Sonographic and sonoelastographic variables were not accurate enough to replace cytological evaluation of superficial lymph nodes in dogs, which offered SE and SP of 100% and 96%, respectively, in a previous study.3 However, they are useful noninvasive imaging modalities that yield a presumptive diagnosis of malignancy. We believe that combining ≥2 sonographic or sonoelastographic variables would improve the diagnostic accuracy of nodal malignancy detection, and that this association should be investigated.
Variables such as SA, RI, and elasticity stiffness score, which offered the highest diagnostic accuracy in our study, can be used as an aid to determine the need for further and more invasive diagnostic procedures such as FNA or biopsy, avoiding additional costs to clients and unnecessary anesthetic exposure to patients in some cases. Furthermore, sonography and SOE may be used as additional or alternative diagnostic tools when inconclusive or negative cytological results are obtained. In the first case, sonography also can help determine the best area for needle placement, by avoidance of focal anechoic or hypoechoic areas suggestive of necrosis or inflammation and of highly vascularized areas that could negatively influence the adequacy of the aspirates.
A limitation of qualitative real‐time SOE includes the subjectivity associated with the evaluation of elastograms, which may lead to a higher interobserver variability in comparison with other elastographic modalities. This limitation could be avoided by use of a semiquantitative measurement such as strain ratio, which measures the elasticity of the node relative to adjacent tissues and has been shown to improve the diagnostic SE of the elastographic score in humans.18 Although promising results have been shown in individual studies, strain index efficacy still needs further investigation, because cutoff values have been inconsistent across different studies, varying from 0.65 and 2.3 for axillary metastatic nodes22, 35 to 1.5 and 2.39 for cervical24 and superficial36 neoplastic nodes, respectively. In the veterinary medical literature, another quantitative elastographic variable, ARFI shear wave velocity, recently has been proposed to differentiate metastatic from benign nodes in bitches with mammary neoplasms.34 In that study, metastatic nodes had a higher mean value (2.99 m/s) for shear wave velocity in comparison with reactive (2.29 m/s) and normal nodes (1.91 m/s).
Sonographic and elastographic evaluation of the lymph nodes in our study was performed immediately after aspiration cytology for most nodes (114/116, 98.28%). Although the insertion of the needle into the lymph nodes potentially could have some effect on sonographic images, in our experience, the effect is limited to a recognizable needle track on sonographic image in a few lymph nodes. However, a needle track was not observed in any of the nodes. To our knowledge, no available studies have evaluated the effects of FNA on sonographic images of the organ being aspirated. On the other hand, FNA effects on lymph node histology have been investigated previously. It was found that approximately 84% of the nodes aspirated had no evidence of architecture distortion and only 8% of the nodes had some evidence of needle tracks on histology.37 In the same study, of the nodes with a needle track, approximately 43% showed histologic changes such as fibrin and inflammatory cells surrounding the needle track and occupying an area <5% of the section. This finding suggests that fine‐needle cytology would cause minimal effects on qualitative and quantitative sonographic variables.
5. CONCLUSIONS
Sonography and SOE are useful imaging modalities for differentiating between malignant and benign lymph node lesions in dogs, particularly using variables such as SA, RI, and elasticity stiffness score, which offered the highest diagnostic accuracies. Results obtained in our study support the use of both techniques as noninvasive screening diagnostic tools in oncologic patients to obtain relevant additional information that will aid in determining treatment and prognosis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the Ethics Committee on Animal Use (CEUA) of School of Veterinary Medicine and Animal Science, Sao Paulo State University (protocol 136/2016).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 B‐mode sonographic images of reactive (A and B) and neoplastic nodes (C and D) of dogs. Heterogeneous parenchyma is noted in a popliteal reactive node (A) and in a superficial cervical lymphomatous node (C). An increase in echogenicity of perinodal fat is observed around a reactive superficial inguinal node (B) and around a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node
Figure S2 Doppler sonographic images of lymph nodes of dogs. Doppler color flow mapping (A) and Power Doppler (B) images show hilar vascular flow distribution in the same reactive popliteal node, more intense on Power Doppler. Doppler color flow mapping of a superficial cervical lymphomatous node (C) shows hilar and peripheral flow distribution, with an associated abnormal pericapsular vessel. Doppler color flow mapping (D) of a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node: abnormal exuberant hilar and peripheral flow. Pulsed wave Doppler image (E) of a popliteal lymph node with high resistivity spectral pattern and perfusion indices (RI: 0.92; and PI: 2.37.
Figure S3 Sonoelastographic images of reactive popliteal nodes (A and B), of a lymphomatous popliteal node (C) and an oral melanoma metastatic mandibular node (D) in dogs. In images (A) and (B), more than 80% of the total area of the node is depicted in red (soft) and yellow (intermediate), with less than 20% of the area depicted in blue (stiff), corresponding to score 1. In (C), 50 to 80% of the total area of the node is depicted in blue and mild areas depicted in red or yellow, corresponding to score 3. In (D), more than 80% of the total are of the node is depicted in blue, corresponding to score 4
ACKNOWLEDGMENTS
This study was supported by the Coordination for the Improvement of Higher Education Personnel, Brazilian Ministry of Education (CAPES PhD scholarship to Alexandra F. Belotta) and Sao Paulo Research Foundation (FAPESP, process 2012/02484‐2).
Belotta AF, Gomes MC, Rocha NS, et al. Sonography and sonoelastography in the detection of malignancy in superficial lymph nodes of dogs. J Vet Intern Med. 2019;33:1403–1413. 10.1111/jvim.15469
Funding information Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Grant/Award Number: CAPES PhD scholarship to Alexandra F.Belotta; Fundação de Amparo à Pesquisa do Estado de São Paulo, Grant/Award Number: FAPESP, process 2012/02484‐2
REFERENCES
- 1. Kinns J, Mai W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. J Vet Radiol. 2007;48:565‐569. [DOI] [PubMed] [Google Scholar]
- 2. MacNeill AL. Cytology of canine and feline cutaneous and subcutaneous lesions and lymph nodes. Top Companion Anim Med. 2011;26(2):62‐76. [DOI] [PubMed] [Google Scholar]
- 3. Langenbach A, Mac Manus PM, Hendrick MJ, et al. Sensitivity and specificity of methods of assessing the regional lymph nodes for evidence of metastasis in dogs and cats with solid tumors. J Am Vet Med Assoc. 2001;218:1424‐1428. [DOI] [PubMed] [Google Scholar]
- 4. Amores‐Fuster I, Cripps P, Graham P, et al. The diagnostic utility of lymph node cytology samples in dogs and cats. J Small Anim Pract. 2015;56(2):125‐129. [DOI] [PubMed] [Google Scholar]
- 5. Adibelli ZH, Ünal G, Gül E, et al. Differentiation of benign and malignant cervical lymph nodes: value of B‐mode and color Doppler sonography. Eur J Radiol. 1998;28:230‐234. [DOI] [PubMed] [Google Scholar]
- 6. Brnic Z, Hebrang A. Usefulness of Doppler waveform analysis in differential diagnosis of cervical lymphadenopathy. Eur Radiol. 2003;13:175‐180. [DOI] [PubMed] [Google Scholar]
- 7. Ying M, Ahuja A, Brook F. Accuracy of sonographic vascular features in differentiating different causes of cervical lymphadenopathy. Ultrasound Med Biol. 2004;30:441‐447. [DOI] [PubMed] [Google Scholar]
- 8. Rosário PWS, Faria S, Bicalho L, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385‐1389. [DOI] [PubMed] [Google Scholar]
- 9. Leboullex S, Girard E, Rose M, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590‐3594. [DOI] [PubMed] [Google Scholar]
- 10. Dangore‐Khasbage S, Degwekar SS, Bhowate RR, et al. Utility of color Doppler ultrasound in evaluating the status of cervical lymph nodes in oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:255‐263. [DOI] [PubMed] [Google Scholar]
- 11. Chammas MC, Macedo TAA, Lo VW, et al. Predicting malignant neck lymphadenopathy using color duplex sonography based on multivariate analysis. J Clin Ultrasound. 2016;0:1‐8. [DOI] [PubMed] [Google Scholar]
- 12. Llabrés‐Díaz FJ. Ultrasonography of the medial iliac lymph nodes in the dog. Vet Radiol Ultrasound. 2004;45:156‐165. [DOI] [PubMed] [Google Scholar]
- 13. Nyman HT, Kristensen AT, Skovgaard IM, McEvoy FJ. Characterization of normal and abnormal canine superficial lymph nodes using gray‐scale B‐mode, color flow mapping, power, and spectral Doppler ultrasonography: a multivariate study. Vet Radiol Ultrasound. 2005;46:404‐410. [DOI] [PubMed] [Google Scholar]
- 14. Salwei RM, O'Brien RT, Matheson JS. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and power Doppler ultrasound. Vet Radiol Ultrasound. 2005;46:411‐416. [DOI] [PubMed] [Google Scholar]
- 15. Swarte M, Alexander K, Rannou B, et al. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound. 2011;52:451‐456. [DOI] [PubMed] [Google Scholar]
- 16. Prieto S, Gomez‐Ochoa P, Blas I, et al. Pathologic correlation of resistivity and pulsatility indices in canine abdominal lymph nodes. Vet Radiol Ultrasound. 2009;50(5):525‐529. [DOI] [PubMed] [Google Scholar]
- 17. Arda K, Ciledag N, Gumusdag PD. Differential diagnosis of malignant cervical lymph nodes at real‐time ultrasonographic elastography and Doppler ultrasonography. Hungarian Radiol Online. 2010;10:1‐4. [Google Scholar]
- 18. Ying L, Hou Y, Zheng H, et al. Real‐time elastography for the differentiation of benign and malignant superficial lymph nodes: a meta‐analysis. Eur J Radiol. 2012;81:2576‐2584. [DOI] [PubMed] [Google Scholar]
- 19. Bhatia KSS, Cho CCM, Yuen Y, et al. Real‐time qualitative ultrasound elastography of cervical lymph nodes in routine clinical practice: interobserver agreement and correlation with malignancy. Ultrasound Med Biol. 2010;36:1990‐1997. [DOI] [PubMed] [Google Scholar]
- 20. Tan SM, The HS , Mancer JF, Poh WT. Improving B mode ultrasound evaluation of breast lesions with real‐time ultrasound elastography: a clinical approach. Breast. 2008;17:252‐257. [DOI] [PubMed] [Google Scholar]
- 21. Seiler GS, Griffith E. Comparisons between elastographic stiffness scores for benign versus malignant lymph nodes in dogs and cats. Vet Radiol Ultrasound. 2018;59:79‐88. [DOI] [PubMed] [Google Scholar]
- 22. Na DG, Lim HK, Byun HS, et al. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol. 1997;168:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 23. Steinkamp HJ, Wissgott C, Rademaker J, Felix R. Current status of power Doppler and color Doppler sonography in the differential diagnosis of lymph node lesions. Eur Radiol. 2002;12:1785‐1793. [DOI] [PubMed] [Google Scholar]
- 24. Tan R, Xiao Y, He Q. Ultrasound elastography: its potential role in assessment of cervical lymphadenopathy. Acad Radiol. 2010;17(7):849‐855. [DOI] [PubMed] [Google Scholar]
- 25. Silver TI, Lawson JA, Mayer MN. Sonographic charcateristics of presumptively normal main axillary and superficial cervical lymph nodes in dogs. Am J Vet Res. 2012;73:1200‐1206. [DOI] [PubMed] [Google Scholar]
- 26. Nyman HT, Kristensen AT, Flagstad A, McEvoy FJ. A review of the sonographic assessment of tumor metastases in liver and superficial lymph nodes. Vet Radiol Ultrasound. 2004;45:438‐448. [DOI] [PubMed] [Google Scholar]
- 27. Choi MY, Lee JW, Jang KJ. Distinction between benign and malignant causes of cervical, axillary, and inguinal lymphadenopathy: value of Doppler spectral waveform analysis. AJR Am J Roentgenol. 1995;165:981‐984. [DOI] [PubMed] [Google Scholar]
- 28. Davé AC, Zekas LJ, Auld DM. Correlation of cytologic and histopathologic findings with perinodal echogenicity of abdominal lymph nodes in dogs and cats. Vet Radiol Ultrasound. 2017;58(4):463‐470. [DOI] [PubMed] [Google Scholar]
- 29. Esen G. Ultrasound of superficial lymph nodes. Eur J Radiol. 2006;58:345‐359. [DOI] [PubMed] [Google Scholar]
- 30. Vassallo P, Edel G, Roos N, et al. In‐vitro high‐resolution ultrasonography of benign and malignant lymph nodes. A sonographic‐pathologic correlation. Invest Radiol. 1993;28:698‐705. [DOI] [PubMed] [Google Scholar]
- 31. Chang D, Yuan A, Yu C, et al. Differentiation of benign and malignant cervical lymph nodes with color Doppler sonography. AJR Am J Roentgenol. 1994;162:965‐968. [DOI] [PubMed] [Google Scholar]
- 32. Alam F, Naito K, Horiguchi J, et al. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B‐mode sonography. AJR Am J Roentgenol. 2008;191:604‐610. [DOI] [PubMed] [Google Scholar]
- 33. Wojcinski S, Dupont J, Schmidt W, et al. Real‐time ultrasound elastography in 180 axillary lymph nodes: elasticity distribution in healthy lymph nodes and prediction of breast cancer metastases. BMC Med Imaging. 2012;12(35):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silva P, Uscategui RAR, Maronezi MC, et al. Ultrasonography for lymph nodes metastasis identification in bitches with mammary neoplasms. Nature. 2018;8:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor K, O'Keeffe S, Britton PD, et al. Ultrasound elastography as an adjuvant to conventional ultrasound in the preoperative assessment of axillary lymph nodes in suspected breast cancer: a pilot study. Clin Radiol. 2011;66(11):1064‐1071. [DOI] [PubMed] [Google Scholar]
- 36. Zhang YR, Lv Q, Yin YH, et al. The value of ultrasound elastography in the differential diagnosis of superficial lymph nodes. Front Med China. 2009;3(3):368‐374. [Google Scholar]
- 37. Prasad RRA, Narasimhan R, Sankaran V, Veliath AJ. Fine‐needle aspiration cytology in the diagnosis of superficial lymphadenopathy: an analysis of 2418 cases. Diagn Cytopathol. 1996;15(5):382‐386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 B‐mode sonographic images of reactive (A and B) and neoplastic nodes (C and D) of dogs. Heterogeneous parenchyma is noted in a popliteal reactive node (A) and in a superficial cervical lymphomatous node (C). An increase in echogenicity of perinodal fat is observed around a reactive superficial inguinal node (B) and around a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node
Figure S2 Doppler sonographic images of lymph nodes of dogs. Doppler color flow mapping (A) and Power Doppler (B) images show hilar vascular flow distribution in the same reactive popliteal node, more intense on Power Doppler. Doppler color flow mapping of a superficial cervical lymphomatous node (C) shows hilar and peripheral flow distribution, with an associated abnormal pericapsular vessel. Doppler color flow mapping (D) of a malignant mesenchymal tumor metastasis in a superficial inguinal lymph node: abnormal exuberant hilar and peripheral flow. Pulsed wave Doppler image (E) of a popliteal lymph node with high resistivity spectral pattern and perfusion indices (RI: 0.92; and PI: 2.37.
Figure S3 Sonoelastographic images of reactive popliteal nodes (A and B), of a lymphomatous popliteal node (C) and an oral melanoma metastatic mandibular node (D) in dogs. In images (A) and (B), more than 80% of the total area of the node is depicted in red (soft) and yellow (intermediate), with less than 20% of the area depicted in blue (stiff), corresponding to score 1. In (C), 50 to 80% of the total area of the node is depicted in blue and mild areas depicted in red or yellow, corresponding to score 3. In (D), more than 80% of the total are of the node is depicted in blue, corresponding to score 4


