Abstract
Purpose of the Review:
This review summarizes literature pertaining to the dawning field of therapeutic targeting of mitochondria in hypertension, and discusses the potential of these interventions to ameliorate hypertension-induced organ damage.
Recent Findings:
In recent years, mitochondrial dysfunction has been reported as an important contributor to the pathogenesis of hypertension-related renal, cardiac, and vascular disease. This in turn prompted development of novel mitochondria-targeted compounds, some of which have shown promising efficacy in experimental studies and safety in clinical trials. In addition, drugs that do not directly target mitochondria have shown remarkable benefits in preserving these organelles in experimental hypertension.
Summary:
Enhancing mitochondrial health is emerging as a novel feasible approach to treat hypertension. Future perspectives include mechanistic experimental studies to establish a cause-effect relationship between mitochondrial dysfunction and hypertension and further clinical trials to confirm the reno-, cardio-, and vasculo-protective properties of these compounds in hypertension.
Keywords: Blood pressure, Hypertension, Mitochondria, Heart, Kidney, Vasculature
Introduction
Hypertension remains a major a major cardiovascular risk factor that affects approximately one third of adults in the US and 1.2 billion people worldwide [1]. It is associated with significant cardiovascular morbidity and mortality, and a high economic burden, with annual estimated direct and indirect costs in the US around $50 billion and $4 billion, respectively [2]. Elevated blood pressure leads to long-term organ damage, including hypertensive heart disease, nephropathy, vasculopathy, and cerebrovascular disease, thereby increasing risk for cardiovascular events [3]. Therefore, it is critical to develop novel therapeutic interventions to prevent hypertensive end-organ damage and improve cardiovascular disease outcomes.
Mitochondria are double-membrane organelles primarily responsible for producing cellular energy in the form of adenosine triphosphate (ATP). Besides being the power plants of the cell, these organelles modulate important cellular functions, including proliferation, survival, apoptosis, calcium signaling, and redox state [4]. Over the last couple of decades, experimental studies have reported mitochondrial abnormalities and dysfunction in the heart, kidneys, and vasculature exposed to hypertension [5, 6]. For example, renin-induced hypertension in rats is associated with myocardial mitochondrial structural abnormalities and apoptosis [7]. Increased mitochondrial production of reactive oxygen species (ROS) was reported in the renal medulla of mice with salt-induced hypertension [8], whereas impaired mitochondrial bioenergetics and antioxidant activity were observed in glomerular and tubular cells from spontaneously hypertensive rats [9]. In line with this, we have shown that renovascular hypertension induced mitochondrial damage in renal tubular cells and cardiomyocytes in pigs, associated with renal and cardiac dysfunction [10–13]. Similarly, obesity-induced hypertension led to mitochondrial alterations in renal artery and coronary vessels that impaired microvascular endothelial function in pigs [14, 15]. Taken together, these studies underscore the contribution of dysfunctional mitochondria to the pathophysiology of hypertension-induced organ damage.
There are several mechanisms by which hypertension may affect mitochondria, including activation of angiotensin-II receptors expressed in the inner mitochondrial membrane, increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, mechanical stretch, and extracellular matrix turnover [6]. However, despite few reports of mitochondrial DNA mutations in maternally inherited hypertension [16, 17], a cause-effect relationship between mitochondrial injury and hypertension remains to be established. Nevertheless, treatment strategies that preserve mitochondrial structure and function might represent good options to ameliorate organ damage due to hypertension. This includes interventions that target mitochondria (e.g. mitochondria-targeted compounds), as well as therapies that do not directly target the organelle, but exert mitoprotective actions (e.g. antihypertensive drugs). This review summarizes the current status of therapeutic strategies to preserve mitochondrial structure and function in hypertension, and discusses the potential of these interventions to ameliorate hypertension-induced organ damage.
Mechanisms of mitoprotection
Stimulation of mitochondrial energy production
Mitochondria are the primary organelles responsible for energy production, particularly in target organs commonly exposed to the effects of hypertension, such as the kidneys and the heart. Cardiomyocytes and renal tubular cells are highly dependent upon mitochondria to generate ATP. Congruently, they are equipped with the largest number of mitochondria per cell among the body. Mitochondrial respiration encompasses a series of concatenated steps in the inner mitochondrial membrane, including generation of a proton gradient, transportation of electrons through the electron transport chain (ETC), and phosphorylation of adenosine diphosphate (ADP) to form ATP [18].
The integrity of the inner mitochondrial membrane is essential to sustain mitochondrial respiration. The phospholipid cardiolipin is an important constituent of the inner mitochondrial membrane that plays a key role in ETC assembly and function. Its unique conical structure, with 2 phosphate head groups and 4 acyl-side chains, favors formation of cristae membranes in the inner mitochondrial membrane [19]. Furthermore, cardiolipin modulates the structural organization of the respiratory complexes into supercomplexes, allowing optimal oxidative phosphorylation.
Studies have shown that the major cardiac cardiolipin species (tetralinoloyl) decreased significantly in spontaneously hypertensive heart failure rats [20, 21]. In agreement, we have found that both cardiac and renal cardiolipin content is reduced in pigs with renovascular hypertension [11, 12]. Importantly, oxidation and consequent loss of cardiolipin destabilizes the ETC, triggering local generation of ROS and mitochondrial dysfunction [22]. Therefore, treatment strategies oriented to stabilize cardiolipin and protect mitochondria might attenuate cellular damage in hypertension.
Elamipretide (ELAM) is a novel cell-permeable tetrapeptide that concentrates in the inner mitochondrial membrane binding and stabilizing cardiolipin, preventing its oxidation and loss. In addition, this compound binds cytochrome-c, favoring the shuttling of electrons from complex-III to complex-IV [23]. Over the last few of years, the feasibility of ELAM to ameliorate cardiac and renal injury has been evaluated in several preclinical models of hypertension. Chronic treatment with ELAM has been shown to attenuate mitochondrial damage and ROS production in murine models of angiotensin-II-induced cardiac hypertrophy and pressure overload [24, 25]. In line with this, subcutaneous administration of ELAM for 4 weeks attenuated structural and functional changes that obesity-induced hypertension imposed in the renal medullary mitochondria [26] and renal vasculature [15]. Notably, the beneficial effects of ELAM in this model were extended to the heart where it preserved mitochondrial organization [27] and decreased myocardial vascular impairment [14].
ELAM also exerted important cardio- and reno-protective properties in renovascular hypertension. Preservation of mitochondrial cardiolipin attenuated swine stenotic-kidney microvascular loss and injury, and improved renal oxygenation, hemodynamics, and function [12]. ELAM also blunted myocardial hypertrophy, improved left ventricular relaxation, and attenuated myocardial cellular and microvascular remodeling in these pigs [11]. Intravenous administration of ELAM in conjunction with renal revasculatization also improved cardiac and renal outcomes in pigs with renovascular hypertension [10, 13]. What is more, a recent pilot study demonstrated that adjunctive ELAM during renal revascularization attenuated post-procedural hypoxia and improved kidney function [28], supporting a role for cardiolipin targeted interventions to improve outcomes of revascularization for human renovascular hypertension. It is important to note that ELAM had no effect on blood pressure levels, suggesting that it was directly targeting cellular mitochondria.
Besides cardiolipin protective approaches, therapies targeting key enzymes and regulators of fatty acid oxidation may additionally promote mitochondrial ATP production. Oxidation of fatty acids is the primary energy source for high energy-demanding tissues, generating a significant amount of ATP. Long-chain fatty acids are translocated across the plasma membrane and activated to acyl-CoA in the cytosol [29]. The outer mitochondrial membrane protein carnitine palmitoyltransferase (CPT)-1 is the rate-limiting enzyme in fatty acid oxidation, responsible for the conversion of acyl-CoA to acylcarnitine. The latter enters the mitochondrial matrix and is reconverted to acyl-CoA, which then undergoes β-oxidation, generating acetyl-CoA and subsequently nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide-2, serving as electron donors to the ETC.
The peroxisome proliferator-activated receptor (PPAR)-α is a key transcription factor that regulates CPT-1 and is expressed at high levels in the heart and kidneys. Treatment with the PPAR-α agonist fenofibrate improved PPAR-α tissue expression and mitochondrial biogenesis, and attenuated cardiac remodeling and fibrosis in mice with aldosterone-induced left ventricular hypertrophy [30]. Similarly, fenofibrates decreased blood pressure in Goldblatt hypertensive rats [31] and ameliorated oxidative stress, inflammation, and fibrosis in the kidney of spontaneously hypertensive rats [32]. Although fenofibrate may be beneficial in hypertension-mediated organ damage, it has opposite effects on blood pressure in salt-sensitive and salt-resistant hypertension [33] and exacerbates left ventricular dilation and fibrosis in PPAR-α-deficient mice models [34]. Evidently, additional mechanistic studies are needed to explore their mechanisms of action, safety, and efficacy in hypertension.
More recently, the novel indole acetic acid derivative mitochonic acid (MA)-5, which targets the mitochondrial protein mitofilin, has been shown to ameliorate renal tubular and cardiac myocyte damage in murine models of renal disease [35]. Mitofilin forms a core complex in the inner mitochondrial membrane required for the formation of crista junctions. By binding mitofilin, MA-5 promotes oligomerization of the ATP synthase and supercomplex formation, favoring mitochondrial ATP synthesis. Interestingly, this agent modulates mitochondrial bioenergetics independently of oxidative phosphorylation and the ETC. However, whether MA-5 can improve cardiac and renal mitochondrial respiration in hypertension has never been investigated.
Regulation of mitochondrial redox status
Mitochondria are major producers of ROS. There are more than 10 different mitochondrial sites capable of leaking electrons to oxygen to produce ROS. Superoxide is predominantly produced from complex-I and its generation is associated with changes in membrane potential, reduced levels of the antioxidant coenzyme-Q, and high NADH/NAD+ ratio, reflecting impaired ATP production [36]. Importantly, mitochondrial superoxide has the capacity to damage several components of the ETC, impairing ATP synthesis. Furthermore, mitochondrial ROS activates mitochondria-dependent apoptosis and exacerbates cellular oxidative stress, affecting the activity of several cellular functions. Therefore, strategies for targeted delivery of antioxidants to mitochondria may confer protection against overall cellular injury.
Triphenylalkylphosphonium cation (TPP+)-conjugated compounds that concentrate in the mitochondrial matrix have demonstrated promising antioxidants properties in experimental models of hypertension. Administration of the mitochondria-targeted antioxidant MitoQ, which results from the conjugation of TPP+ to the scavenger of free radicals coenzyme-Q, improved endothelial function, and reduced cardiac hypertrophy in spontaneously hypertensive rats [37]. Similarly, MitoQ decreased hydrogen peroxide formation and improved mitochondrial respiration in rats with heart failure induced by pressure overload [38]. Importantly, clinical trials have shown that MitoQ can be safely delivered to patients with liver conditions, including fatty liver (NCT01167088) and hepatitis-C (NCT00433108) with promising efficacy to decrease liver damage. However, MitoQ concentrates in the mitochondrial matrix in a membrane potential-dependent manner, limiting its efficacy in conditions of significant mitochondrial injury, like in severe forms of hypertension.
To counterbalance the constant generation of ROS, mitochondria are provisioned with important antioxidant defense systems. Superoxide dismutase (SOD)-2 is the most effective antioxidant enzyme in mitochondria, and neutralizes superoxide into the relatively more stable and less toxic hydrogen peroxide. Previous studies have shown that administration of an antioxidant mimetic of the SOD-2 (tempol) prevented NADPH oxidase-induced oxidative stress and renal damage in spontaneously hypertensive rats [39]. Similarly, treatment with the mitochondrial targeted antioxidant mitoTEMPO, a piperidine nitroxide conjugated to a TPP+, decreased mitochondrial superoxide production and cellular NADPH oxidase activity, and restored vascular nitric oxide production in angiotensin II-induced and DOCA-salt hypertension models [40]. Collectively, these studies show that antioxidant strategies specifically targeting SOD-2 could have therapeutic benefit in hypertensive patients.
Mitochondria-targeted compounds based on the natural antioxidant vitamins have been synthesized and tested in model of renal disease. For example, conjugated TPP+ with α-tocopherol (MitoE) improved renal mitochondrial respiration and reduced oxidative stress in rats with acute sepsis [41]. However, MitoE remains to be tested in experimental hypertension. Supplementation of vitamin E and C decreased oxidative stress and ameliorated hypertension-induced renal dysfunction in rats [42], but increased oxidative stress in the normal pig heart and kidneys [43, 44]. Clearly, experimental and clinical research is needed to explore the protective properties of mitoE in hypertension.
In addition, mitochondria-targeted antioxidants with iron chelator properties have been tested in experimental models of hypertension. Mitochondria play a key role in maintaining cellular iron homeostasis, representing the primary site for synthesis of heme and iron-sulfur cluster-containing proteins involved in oxidative phosphorylation. However, excess of mitochondrial iron triggers generation of ROS and oxidative damage. The catechol-based 4,5-dihydroxy-1,3-benzenedisulfonic, also known as tiron, is an iron chelator agent with ROS scavenging properties. Studies have shown that co-incubation with tiron decreased hydrogen peroxide-induced contractile responses in mesenteric resistance arteries from spontaneous hypertensive rats [45]. Furthermore, its administration improved baroreflex sensitivity and decreased oxidative stress in hypertensive rats [46], underscoring the potential of mitochondria-targeted iron sequestering compounds in experimental hypertension.
Modulation of mitochondrial homeostasis
Mitochondria are dynamic organelles that constantly adapt their morphology and function in response to cellular, metabolic, and signaling changes. Mitochondrial homeostasis refers to the interplay among pathways that regulate mitochondrial content and metabolism, including biogenesis, dynamics, and mitophagy, crucial for the proper functioning of the cell [47]. Mitochondrial biogenesis is the mechanism by which cells increase their mitochondrial mass and copy number. This complex process includes synthesis, import, and incorporation of proteins encoded in the nucleus into the organelle, lipid synthesis, and assembly of ETC subunits [48]. PPAR-γ coactivator (PGC)-1α is the master regulator of mitochondrial biogenesis. This transcription coactivator induces the expression of downstream transcription factors, regulating the expression of several components of the mitochondrial respiratory complexes. Thus, pharmacological approaches that activate PGC-1α might act as biogenesis activators. β2-adrenergic receptor agonists like formoterol, which induces PGC-1α and consequently mitochondrial biogenesis, increased oxygen consumption rate and mitochondrial-DNA copy number in primary rabbit renal tubular cells and feline cardiomyocytes [49]. Likewise, treatment with PPAR-γ, which also induces PGC-1α, prevented necrosis in the clipped kidneys of 2 kidney 1 clip (2K1C) rats [50], supporting novel therapeutic targets for enhancing mitochondrial biogenesis in hypertension.
Modulation of mitochondrial dynamics has been also proposed as a therapeutic approach to attenuate the deleterious effects that hypertension imposes on several organs. Mitochondria fuse and divide repeatedly to regulate their shape. Fusion of mitochondrial membranes allows complementation of functional components in the organelle, whereas mitochondrial fragmentation promotes oxidative stress and cell death. Therefore, therapies oriented to enhance mitochondrial fusion or prevent fission might be beneficial to attenuate hypertension-induced organ damage. Indeed, hydroxylamine derivatives, which activate optic atrophy-1 and promote the fusion of inner mitochondrial membranes, ameliorated lung damage in a murine model of pulmonary arterial hypertension [51]. Recently, treatment with a drug that prevents activation of the mitochondrial fission marker dynamin related protein (DRP)-1 ameliorated vascular remodeling and inflammation in spontaneously hypertensive rats [52], in accordance with previous observations in cardiomyocytes subjected to renal [53] and cardiac [54] reperfusion injury.
Changes in mitochondrial fusion and fission may also alter mitophagy, the selective degradation of damaged mitochondria. Importantly, defective mitophagy increases production of mitochondrial ROS and apoptosis, amplifying cellular damage. Studies in mice have shown that down-regulation of DRP-1 inhibited mitophagy and mitochondrial dysfunction, thereby promoting cardiac dysfunction [55]. Preservation of the integrity of the inner mitochondrial membrane is also important to sustain mitophagy. We have previously shown in swine renovascular hypertension that restoration of myocardial cardiolipin improved mitophagy [27], in agreement with the previous observation of decreased mitophagy in cardiolipin-deficient cells [56]. Therefore, pharmacological agents that target mitochondrial homeostasis might offer the prospect of decreasing hypertension related end-organ damage. However, the benefits of modulating these pathways in experimental hypertension warrants further investigation.
Attenuation of mitochondrial-mediated apoptosis
Mitochondria are important regulators of apoptosis. Intrinsic apoptosis or mitochondria- dependent apoptosis is triggered by mitochondrial outer membrane permeabilization, which occurs in response to certain intracellular insults. Opening of the mitochondrial permeability transition pore (mPTP) is an early event during intrinsic apoptosis and leads to translocation of pro-apoptotic proteins, cytochrome-c release, and subsequent caspase-9 and −3 activation. Experimental studies have shown that the immunosuppressive drug cyclosporine, which binds to the mPTP constituent cyclophilin-D, prevented mPTP opening and protected the heart and kidneys from ischemia-reperfusion injury [57]. However, cyclosporine therapy did not result in better clinical outcomes following percutaneous coronary intervention [58]. Furthermore, the use of cyclosporine in patients with hypertension is limited by numerous adverse effects, including nephrotoxicity, vasoconstriction, LV hypertrophy, endothelial dysfunction, and hypertension [59, 60].
Previous studies have shown that targeting the glycogen synthase kinase (GSK)-3β confers cytoprotection by phosphorylating cyclophilin-D and increasing the threshold for mPTP opening [61]. GSK-3β-induced mPTP opening has been linked to increased vulnerability to infarction in hypertensive hypertrophied hearts [62], and its inhibition improves post-ischemic recovery in pressure-overload hypertrophied hearts [63]. Similarly, treatment with GSK-3 β inhibitors attenuated mitochondrial permeability and improved antioxidant activity in hearts from salt hypertensive rats [64]. However, additional studies are needed to confirm these findings and validate GSK-3β as a target therapy in hypertension.
Lastly, mitochondrial antioxidants and cardiolipin-protective strategies may also attenuate mitochondrial-mediated apoptosis, as increased production of mitochondrial ROS and cardiolipin oxidation are important triggers for mPTP opening. Treatment with the mitochondria-targeted antioxidant mitoQ exerted important antiapoptotic properties in cardiomyocyte-like cells and isolated hearts [65], and improved mitochondrial dysfunction in heart failure induced by pressure overload [38]. Likewise, cardiolipin protection with ELAM attenuated renal and cardiac apoptosis in experimental renovascular [10–13] and obesity-induced [14, 15] hypertension, supporting the notion that mitochondria-targeted interventions may attenuate hypertension-related apoptosis.
Renin-angiotensin-aldosterone system (RAAS) blockade
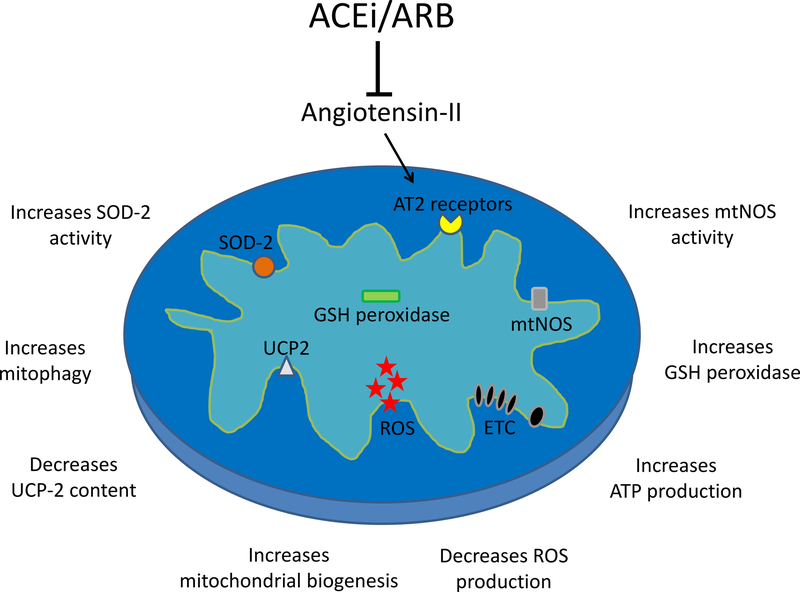
Several RAAS blockers routinely used in clinical practice have shown protective effects towards mitochondrial injury secondary to hypertension. These drugs have greater mitoprotective properties compared to other antihypertensives, and can improve mitochondrial function by multiple mechanisms (Figure 1). Although conventional triple therapy (reserpine + hydralazine + hydrochlorothiazide) and the angiotensin receptor blocker (ARB) valsartan are effective in reducing blood pressure, only the latter improved myocardial mitochondrial biogenesis and mitophagy and attenuated left ventricular remodeling in pigs with renovascular hypertension [66]. Similarly, renal mitochondrial dysfunction in spontaneously hypertensive rats was attenuated by losartan, but not by the calcium channel blocker amlodipine [9].
Figure 1: Mechanisms of mitoprotection by renin angiotensin aldosterone system blockade:
Both angiotensin-I converting enzyme inhibitors (ACEi) and angiotensin-II AT1 receptor blockers (ARB) decrease angiotensin-II levels, thereby preventing its actions through AT2 receptors located in the inner mitochondrial membrane. Experimental studies have shown that treatment with ACEi and ARBs decreases mitochondrial reactive oxygen species (ROS) production and uncoupling protein (UCP)-2 content, and increases biogenesis, nitric oxide synthase (mtNOS), gluthatione (GSH), and superoxide dismutase (SOD) activity, mitophagy, and adenosine triphosphate (ATP) production.
The mitoprotective effects of these drugs remain unclear, but may be linked to the presence of a functional renin-angiotensin system in human mitochondria, characterized by mitochondrial angiotensin-II type-1 (AT2R1) and type-2 (AT2R2) receptors [67]. Activation of AT2R2 increases nitric oxide production and reduces mitochondrial respiration, whereas AT2R1 decreases the number of mitochondria and is associated with aging. Possibly, blocking the AT2R1 on the mitochondria may alleviate hypertensive cellular injury. In addition, angiotensin-I converting enzyme inhibitors (ACEi) and ARBs are known to modulate mitochondrial oxidative stress. In rat cardiomyocytes, enalapril and losartan increased mitochondrial production of NO, regulating mitochondrial respiration and ROS production [68]. Likewise, enalapril enhanced SOD-2 [9] and glutathione-dependent [69] antioxidant defenses, and increased renal expression of the mitochondrial ROS modulator uncoupling protein (UCP)-2, resulting in decreased production of hydrogen peroxide [70]. Therefore, blocking the RAAS confers important mitoprotective benefits beyond their antihypertensive effect, which may help ameliorate hypertension-induced organ damage. However, the mechanisms implicated in mitoprotection secondary to angiotensin-II blockade remain to be elucidated, and whether ACEi and ARBs have direct effects on the mitochondrial membrane or act through cell surface-signaling and intracellular mediators warrants further investigation.
Conclusions
Compelling evidence indicates that mitochondrial dysfunction contributes to hypertension-induced end-organ damage, which inspired development of novel therapeutic approaches that have direct effects on mitochondria. Studies in experimental models of hypertension have shown promising results using mitochondria-targeted therapies, and some of them are currently being tested in clinical trials.
Interestingly, targeting mitochondria with the antioxidants MitoQ and MitoTEMPO protected against the development of hypertension, which was partly attributed to the attenuation of oxidative damage in endothelial cells [37, 40]. Contrarily, treatment with ELAM, which restored cardiolipin and preserved mitochondrial function in renal artery and coronary artery endothelial cells, did not affect blood pressure levels in either obesity-induced [14, 15] or renovascular hypertension [12]. Differences in the effect on blood pressure among different classes of mitochondria-targeted therapies likely reflect distinct mechanisms of actions, but justify further investigation.
Lastly, combining antihypertensives and mitochondria-targeted peptides may provide complementary therapeutic benefit for attenuating the effects of hypertension in the heart. In spontaneously hypertensive rats, a combination of MitoQ and low-dose losartan provided additive therapeutic benefit, attenuating development of hypertension and reducing left ventricular hypertrophy [71]. These observations position mitochondria-targeted compounds as a potential adjuvant therapy to standard antihypertensives in the treatment of end-organ damage associated with hypertension. Nevertheless, further experimental studies are needed to provide important information regarding their safety, efficacy, and mechanisms of action for future clinical trials in patients with hypertension.
Table 1:
Mechanisms of mitoprotection in hypertension
| Mechanisms of mitoprotection | Target |
|---|---|
| Stimulation of mitochondrial energy production | |
|
Cardiolipin |
|
CPT-1, PPAR-α |
|
Mitofillin |
| Regulation of mitochondrial redox status | |
|
Superoxide, Hydrogen peroxide |
|
Superoxide |
|
Iron, Superoxide |
| Modulation of mitochondrial homeostasis | |
|
PGC-1α |
|
OPA-1 |
|
DRP-1 |
| Attenuation of mitochondrial-mediated apoptosis | |
|
Cyclophilin-D, Cardiolipin |
CPT: carnitine palmitoyltransferase, PPAR: peroxisome proliferator-activated receptors, MA: mitochonic acid, TTP: triphenylalkylphosphonium cation, PGC: peroxisome proliferator-activated receptor-gamma coactivator, OPA: optic atrophy, DRP: dynamin related protein, mPTP: mitochondrial permeability transition pore.
Acknowledgements
This study was partly supported by NIH grant numbers DK104273, HL123160, DK100081, DK102325, and DK106427.
Footnotes
Competing interests
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R et al. : Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Merai R, Siegel C, Rakotz M, Basch P, Wright J, Wong B, Dhsc, Thorpe P: CDC Grand Rounds: A Public Health Approach to Detect and Control Hypertension. MMWR Morb Mortal Wkly Rep 2016, 65(45):1261–1264. [DOI] [PubMed] [Google Scholar]
- 3.Schmieder RE: End organ damage in hypertension. Dtsch Arztebl Int 2010, 107(49):866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duchen MR : Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 2004, 25(4):365–451. [DOI] [PubMed] [Google Scholar]
- 5.Eirin A, Lerman A, Lerman LO: Mitochondrial injury and dysfunction in hypertension-induced cardiac damage. Eur Heart J 2014, 35(46):3258–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eirin A, Lerman A, Lerman LO: Mitochondria: a pathogenic paradigm in hypertensive renal disease. Hypertension 2015, 65(2):264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walther T, Tschope C, Sterner-Kock A, Westermann D, Heringer-Walther S, Riad A, Nikolic A, Wang Y, Ebermann L, Siems WE et al. : Accelerated mitochondrial adenosine diphosphate/adenosine triphosphate transport improves hypertension-induced heart disease. Circulation 2007, 115(3):333–344. [DOI] [PubMed] [Google Scholar]
- 8.Jin K, Vaziri ND: Salt-sensitive hypertension in mitochondrial superoxide dismutase deficiency is associated with intra-renal oxidative stress and inflammation. Clin Exp Nephrol 2014, 18(3):445–452. [DOI] [PubMed] [Google Scholar]
- 9.de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F: Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol 2006, 290(6):R1616–1625. [DOI] [PubMed] [Google Scholar]
- 10.Eirin A, Li Z, Zhang X, Krier JD, Woollard JR, Zhu XY, Tang H, Herrmann SM, Lerman A, Textor SC et al. : A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 2012, 60(5):1242–1249. [DOI] [PubMed] [Google Scholar]
- 11.Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS et al. : Restoration of Mitochondrial Cardiolipin Attenuates Cardiac Damage in Swine Renovascular Hypertension. J Am Heart Assoc 2016, 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO: Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 2014, 103(4):461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eirin A, Williams BJ, Ebrahimi B, Zhang X, Crane JA, Lerman A, Textor SC, Lerman LO: Mitochondrial targeted peptides attenuate residual myocardial damage after reversal of experimental renovascular hypertension. J Hypertens 2014, 32(1):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan F, Hedayat AF, Ferguson CM, Lerman A, Lerman LO, Eirin A: Mitoprotection attenuates myocardial vascular impairment in porcine metabolic syndrome. Am J Physiol Heart Circ Physiol 2018, 314(3):H669–H680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eirin A, Hedayat AF, Ferguson CM, Textor SC, Lerman A, Lerman LO: Mitoprotection preserves the renal vasculature in porcine metabolic syndrome. Exp Physiol 2018, 103(7):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Li R, Fettermann A, Li Z, Qian Y, Liu Y, Wang X, Zhou A, Mo JQ, Yang L et al. : Maternally inherited essential hypertension is associated with the novel 4263A>G mutation in the mitochondrial tRNAIle gene in a large Han Chinese family. Circ Res 2011, 108(7):862–870. [DOI] [PubMed] [Google Scholar]
- 17.Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI et al. : A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science 2004, 306(5699):1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osellame LD, Blacker TS, Duchen MR: Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 2012, 26(6):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szeto HH: First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 2014, 171(8):2029–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC: Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res 2005, 46(6):1196–1204. [DOI] [PubMed] [Google Scholar]
- 21.Zachman DK, Chicco AJ, McCune SA, Murphy RC, Moore RL, Sparagna GC: The role of calcium-independent phospholipase A2 in cardiolipin remodeling in the spontaneously hypertensive heart failure rat heart. J Lipid Res 2010, 51(3):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schug ZT, Gottlieb E: Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta 2009, 1788(10):2022–2031. [DOI] [PubMed] [Google Scholar]
- 23.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH: Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 2014, 171(8):2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW 2nd, Kang YJ, Prolla TA et al. : Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 2011, 108(7):837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai DF, Hsieh EJ, Chen T, Menendez LG, Basisty NB, Tsai L, Beyer RP, Crispin DA, Shulman NJ, Szeto HH et al. : Global proteomics and pathway analysis of pressure-overload-induced heart failure and its attenuation by mitochondrial-targeted peptides. Circ Heart Fail 2013, 6(5):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eirin A, Woollard JR, Ferguson CM, Jordan KL, Tang H, Textor SC, Lerman A, Lerman LO: The metabolic syndrome induces early changes in the swine renal medullary mitochondria. Transl Res 2017, 184:45–56 e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan F, Woollard JR, Jordan KL, Lerman A, Lerman LO, Eirin A: Mitochondrial targeted peptides preserve mitochondrial organization and decrease reversible myocardial changes in early swine metabolic syndrome. Cardiovasc Res 2018, 114(3):431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ••.Saad A, Herrmann SMS, Eirin A, Ferguson CM, Glockner JF, Bjarnason H, McKusick MA, Misra S, Lerman LO, Textor SC: Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients With Atherosclerotic Renal Artery Stenosis. Circ Cardiovasc Interv 2017, 10(9).This pilot study showed that adjunctive mitoprotection during renal revascularization attenuates post-procedural hypoxia and improved kidney function in human renovascular hypertension.
- 29.Szeto HH: Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J Am Soc Nephrol 2017, 28(10):2856–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrasseur NK, Duhaney TA, De Silva DS, Cui L, Ip PC, Joseph L, Sam F: Effects of fenofibrate on cardiac remodeling in aldosterone-induced hypertension. Hypertension 2007, 50(3):489–496. [DOI] [PubMed] [Google Scholar]
- 31.Sporkova A, Certikova Chabova V, Dolezelova S, Jichova S, Kopkan L, Vanourkova Z, Kompanowska-Jezierska E, Sadowski J, Maxova H, Cervenka L: Fenofibrate Attenuates Hypertension in Goldblatt Hypertensive Rats: Role of 20-Hydroxyeicosatetraenoic Acid in the Nonclipped Kidney. Am J Med Sci 2017, 353(6):568–579. [DOI] [PubMed] [Google Scholar]
- 32.Hou X, Shen YH, Li C, Wang F, Zhang C, Bu P, Zhang Y: PPARalpha agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochem Biophys Res Commun 2010, 394(3):653–659. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert K, Nian H, Yu C, Luther JM, Brown NJ: Fenofibrate lowers blood pressure in salt-sensitive but not salt-resistant hypertension. J Hypertens 2013, 31(4):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duhaney TA, Cui L, Rude MK, Lebrasseur NK, Ngoy S, De Silva DS, Siwik DA, Liao R, Sam F: Peroxisome proliferator-activated receptor alpha-independent actions of fenofibrate exacerbates left ventricular dilation and fibrosis in chronic pressure overload. Hypertension 2007, 49(5):1084–1094. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Yamaguchi H, Kikusato M, Hashizume O, Nagatoishi S, Matsuo A, Sato T, Kudo T, Matsuhashi T, Murayama K et al. : Mitochonic Acid 5 Binds Mitochondria and Ameliorates Renal Tubular and Cardiac Myocyte Damage. J Am Soc Nephrol 2016, 27(7):1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy MP: How mitochondria produce reactive oxygen species. Biochem J 2009, 417(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ••.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF: Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54(2):322–328.This article reported that treatment with mitochondria-targeted antioxidants protected against the development of hypertension and reduced cardiac hypertrophy in hypertensive rats.
- 38.Ribeiro Junior RF, Dabkowski ER, Shekar KC, KA OC, Hecker PA, Murphy MP: MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic Biol Med 2018, 117:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB: Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol 2009, 29(4):309–318. [DOI] [PubMed] [Google Scholar]
- 40.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI: Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 2010, 107(1):106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowes DA, Webster NR, Murphy MP, Galley HF: Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br J Anaesth 2013, 110(3):472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifi B, Kadkhodaee M, Karimian SM, Zahmatkesh M, Shams S, Bakhshi E: Reduction of kidney damage by supplementation of vitamins C and E in rats with deoxycorticosterone-salt-induced hypertension. Iran J Kidney Dis 2009, 3(4):197–202. [PubMed] [Google Scholar]
- 43.Versari D, Daghini E, Rodriguez-Porcel M, Sattler K, Galili O, Pilarczyk K, Napoli C, Lerman LO, Lerman A: Chronic antioxidant supplementation impairs coronary endothelial function and myocardial perfusion in normal pigs. Hypertension 2006, 47(3):475–481. [DOI] [PubMed] [Google Scholar]
- 44.Daghini E, Zhu XY, Versari D, Bentley MD, Napoli C, Lerman A, Lerman LO: Antioxidant vitamins induce angiogenesis in the normal pig kidney. Am J Physiol Renal Physiol 2007, 293(1):F371–381. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Redondo AB, Briones AM, Beltran AE, Alonso MJ, Simonsen U, Salaices M: Hypertension increases contractile responses to hydrogen peroxide in resistance arteries through increased thromboxane A2, Ca2+, and superoxide anion levels. J Pharmacol Exp Ther 2009, 328(1):19–27. [DOI] [PubMed] [Google Scholar]
- 46.Guimaraes DD, Carvalho CC, Braga VA: Scavenging of NADPH oxidase-derived superoxide anions improves depressed baroreflex sensitivity in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2012, 39(4):373–378. [DOI] [PubMed] [Google Scholar]
- 47.Palikaras K, Tavernarakis N: Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 2014, 56:182–188. [DOI] [PubMed] [Google Scholar]
- 48.Hock MB, Kralli A: Transcriptional control of mitochondrial biogenesis and function. Annu Rev Physiol 2009, 71:177–203. [DOI] [PubMed] [Google Scholar]
- 49.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG: The beta2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 2012, 342(1):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fedorova LV, Sodhi K, Gatto-Weis C, Puri N, Hinds TD Jr., Shapiro JI, Malhotra D: Peroxisome proliferator-activated receptor delta agonist, HPP593, prevents renal necrosis under chronic ischemia. PLoS One 2013, 8(5):e64436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabo A, Sumegi K, Fekete K, Hocsak E, Debreceni B, Setalo G Jr., Kovacs K, Deres L, Kengyel A, Kovacs D et al. : Activation of mitochondrial fusion provides a new treatment for mitochondria-related diseases. Biochem Pharmacol 2018, 150:86–96. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Tan H, Liu X, Wu Q: Correlation between the expression of Drp1 in vascular endothelial cells and inflammatory factors in hypertension rats. Exp Ther Med 2018, 15(4):3892–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumida M, Doi K, Ogasawara E, Yamashita T, Hamasaki Y, Kariya T, Takimoto E, Yahagi N, Nangaku M, Noiri E: Regulation of Mitochondrial Dynamics by Dynamin-Related Protein-1 in Acute Cardiorenal Syndrome. J Am Soc Nephrol 2015, 26(10):2378–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ: Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121(18):2012–2022. [DOI] [PubMed] [Google Scholar]
- 55.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M et al. : Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 2015, 116(2):264–278. [DOI] [PubMed] [Google Scholar]
- 56.Shen Z, Li Y, Gasparski AN, Abeliovich H, Greenberg ML: Cardiolipin Regulates Mitophagy through the Protein Kinase C Pathway. J Biol Chem 2017, 292(7):2916–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh D, Chander V, Chopra K: Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 2005, 207(3):339–347. [DOI] [PubMed] [Google Scholar]
- 58.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N et al. : Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N Engl J Med 2015, 373(11):1021–1031. [DOI] [PubMed] [Google Scholar]
- 59.Bloom IT, Bentley FR, Garrison RN: Acute cyclosporine-induced renal vasoconstriction is mediated by endothelin-1. Surgery 1993, 114(2):480–487; discussion 487–488. [PubMed] [Google Scholar]
- 60.Calo L, Semplicini A, Davis PA, Bonvicini P, Cantaro S, Rigotti P, D’Angelo A, Livi U, Antonello A : Cyclosporin-induced endothelial dysfunction and hypertension: are nitric oxide system abnormality and oxidative stress involved? Transpl Int 2000, 13 Suppl 1:S413–418. [DOI] [PubMed] [Google Scholar]
- 61.Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S, Sollott SJ: Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 2009, 104(11):1240–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yano T, Miki T, Tanno M, Kuno A, Itoh T, Takada A, Sato T, Kouzu H, Shimamoto K, Miura T: Hypertensive hypertrophied myocardium is vulnerable to infarction and refractory to erythropoietin-induced protection. Hypertension 2011, 57(1):110–115. [DOI] [PubMed] [Google Scholar]
- 63.Barillas R, Friehs I, Cao-Danh H, Martinez JF, del Nido PJ: Inhibition of glycogen synthase kinase-3beta improves tolerance to ischemia in hypertrophied hearts. Ann Thorac Surg 2007, 84(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez Arbelaez LF, Perez Nunez IA, Mosca SM: Gsk-3beta inhibitors mimic the cardioprotection mediated by ischemic pre- and postconditioning in hypertensive rats. Biomed Res Int 2013, 2013:317456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuzil J, Widen C, Gellert N, Swettenham E, Zobalova R, Dong LF, Wang XF, Lidebjer C, Dalen H, Headrick JP et al. : Mitochondria transmit apoptosis signalling in cardiomyocyte-like cells and isolated hearts exposed to experimental ischemia-reperfusion injury. Redox Rep 2007, 12(3):148–162. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Li ZL, Crane JA, Jordan KL, Pawar AS, Textor SC, Lerman A, Lerman LO: Valsartan regulates myocardial autophagy and mitochondrial turnover in experimental hypertension. Hypertension 2014, 64(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS et al. : Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 2011, 108(36):14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costa LE, La-Padula P, Lores-Arnaiz S, D’Amico G, Boveris A, Kurnjek ML, Basso N: Long-term angiotensin II inhibition increases mitochondrial nitric oxide synthase and not antioxidant enzyme activities in rat heart. J Hypertens 2002, 20(12):2487–2494. [DOI] [PubMed] [Google Scholar]
- 69.de Cavanagh EM, Inserra F, Ferder L, Fraga CG: Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol 2000, 278(3):R572–577. [DOI] [PubMed] [Google Scholar]
- 70.Piotrkowski B, Fraga CG, de Cavanagh EM: Mitochondrial function and nitric oxide metabolism are modified by enalapril treatment in rat kidney. Am J Physiol Regul Integr Comp Physiol 2007, 292(4):R1494–1501. [DOI] [PubMed] [Google Scholar]
- 71. ••.McLachlan J, Beattie E, Murphy MP, Koh-Tan CH, Olson E, Beattie W, Dominiczak AF, Nicklin SA, Graham D: Combined therapeutic benefit of mitochondria-targeted antioxidant, MitoQ10, and angiotensin receptor blocker, losartan, on cardiovascular function. J Hypertens 2014, 32(3):555–564.This article revealed that combining antihypertensives and mitochondria-targeted peptides has additional therapeutic benefit for attenuating hypertension-induced myocardial damage.