Abstract
Purpose
To determine when the risk of lymphedema is highest following treatment for breast cancer and which factors influence the time-course of lymphedema development.
Patients and Methods
Between 2005–2017, 2,171 women (with 2,266 at-risk arms) who received surgery for unilateral or bilateral breast cancer at our institution were enrolled. Perometry was used to objectively assess limb volume preoperatively, and lymphedema was defined as ≥10% relative arm volume increase arising >3 months postoperatively. Multivariable regression was used to uncover risk factors associated with lymphedema, the Cox proportional hazards model was used to calculate lymphedema incidence, and the semiannual hazard rate of lymphedema was calculated.
Results
With four years median follow-up, the overall estimated five-year cumulative incidence of lymphedema was 13.7%. Significant factors associated with lymphedema on multivariable analysis were high preoperative body mass index (BMI), axillary lymph node dissection (ALND), and regional lymph node radiation (RLNR). Those receiving ALND with RLNR experienced the highest 5-year rate of lymphedema (31.2%), followed by ALND without RLNR (24.6%), followed by sentinel lymph node biopsy (SLNB) with RLNR (12.2%). Overall, the risk of lymphedema peaked between 12–30 months postoperatively; however, the time-course varied as a function of therapy received. Early-onset lymphedema (<12 months postoperatively) was associated with ALND (HR 4.75, p<0.0001) but not RLNR (HR 1.21, p=0.55). In contrast, late-onset lymphedema (>12 months postoperatively) was associated with RLNR (HR 3.86, p=0.0001), and to a lesser extent, ALND (HR 1.86, p=0.029). The lymphedema risk peaked between 6–12 months in the ALND-without-RLNR group, between 18–24 months in the ALND-with-RLNR group, and between 36–48 months in the SLNB-with-RLNR group.
Conclusion
The time-course for lymphedema development depends on breast cancer treatment received. ALND is associated with early-onset lymphedema, and RLNR is associated with late-onset lymphedema. These results can influence clinical practice to guide lymphedema surveillance strategies and patient education.
Summary
Breast cancer treatment-associated lymphedema is a potentially devastating complication but the time-course for developing lymphedema is unknown. We conducted a prospective study of 2,171 women to determine when patients are at greatest risk for lymphedema in the context of modern surgical and radiation techniques. We observed that the time-course of lymphedema development depends on therapy received: with early-onset lymphedema associated with axillary lymph node dissection and late-onset lymphedema more associated with regional lymph node radiation.
Introduction
Breast cancer treatment-related upper extremity lymphedema is a feared and potentially devastating complication with potential to impact emotional well-being and quality of life.1,2 Estimates of lymphedema incidence in prior studies have ranged widely (e.g., 6%−50% at any time following surgical treatment),3–7 and discrepancies in the reported incidence of lymphedema are likely related to variability in measurement techniques used (e.g., subjective patient self-reported assessments versus objective measurements), whether baseline arm measurements are obtained, and the length of follow-up.3,4,8–10 Previous studies have established that axillary lymph node dissection (ALND), regional lymph node irradiation (RLNR), higher pre-operative BMI, infection, and arm trauma increase the risk of developing lymphedema.3,9,11 However, the time-course for developing lymphedema is not well-established in the context of modern surgical and radiation techniques, and clarifying when patients are at-risk would allow for improved patient education and optimization of lymphedema surveillance strategies.
Therefore, we undertook a prospective study utilizing objective baseline and serial arm volume measurements to establish the time-course, incidence, and risk factors associated with lymphedema in a cohort of patients treated with modern surgical and radiation techniques for breast cancer. The purpose of this study was to assess the impact of both axillary surgery and radiation on the incidence and time-course of lymphedema.
Materials and Methods
Patient population and treatment
2,171 women who received surgery for unilateral or bilateral ductal-carcinoma-in-situ (DCIS) or invasive breast cancer were recruited from our institution between 2005–2017 to participate in the study. All women were recruited at the time of initial pre-surgical consultation in our multidisciplinary breast oncology clinic. All patients received surgery at our institution and had at least three months of postoperative follow-up. Patients with pre-existing lymphedema and prior breast surgery were excluded from the cohort. Treating each breast individually, there were 2,266 cases. This protocol underwent review and was approved by the Partners HealthCare Institutional Review Board (ClinicalTrials.gov Identifier: NCT01521741).12
Surgery
Of the 2,266 breast cases, 1,232 underwent a lumpectomy (54.4%) and 1,034 underwent a mastectomy (45.6%). Regarding axillary lymph node management, 199 (8.8%) patients had no axillary surgery performed, 1520 (67.1%) received a SLNB, and 547 (24.1%) received ALND.
Radiation Treatment
Of the 2,266 breast cases, 1,444 (64%) received radiation. Of the patients who received radiation, 903 (62.5%) received local radiotherapy alone and 541 (37.5%) received local radiotherapy with RLNR. Local radiotherapy encompassed partial breast irradiation (PBI), whole-breast irradiation, or chest wall radiation alone. RLNR included patients who received supraclavicular-only radiation and those who also received axillary radiation through a posterior axillary boost. After 2008, levels I-III of the axilla and the supraclavicular nodes were contoured in the majority of cases in keeping with the Radiation Therapy Oncology Group atlas.13 The treating physician defined the lateral border of the supraclavicular field. Patients receiving whole breast radiation alone generally received 50–50.4 Gy in 25–28 fractions followed by a boost of 10Gy in 5 fractions, or a hypofractionated approach consisting of 42.4 Gy in 16 fractions followed by a boost of 10 Gy in 4 fractions. Patients requiring RLNR received a dose of 50–50.4 Gy in 1.8–2 Gy/fraction to the breast/chest-wall and regional lymph nodes. Until 2009, patients treated with PBI received a total dose of 32, 36, or 40 Gy in 4 Gy/fraction twice daily with three-dimensional conformal mixed photon/electron or photon-alone techniques on a dose-escalation clinical trial ( ClinicalTrials.gov Identifier: NCT00694577).14 Subsequently, PBI patients were treated with 36 Gy in 9 fractions twice daily or 40 Gy once daily for 10 fractions in 2 weeks.
Follow-up and measurement of lymphedema
Upon informed consent, a Perometer was used to measure preoperative/baseline arm volume at the time of initial consultation, and then serially at regular intervals during treatment and in the follow-up setting.15 An optoelectronic Perometer is a validated, reliable instrument for lymphedema measurement and uses infrared light beams to measure limb volume.12,16–19 During treatment, arm volume was assessed after surgery, chemotherapy, and radiation (when given). In the follow-up setting, arm volume was assessed at regular 3 to 8-month intervals for the first 5 years post-operatively, and then annually thereafter (or at any time when a patient experienced new symptoms of concern).15
Lymphedema was defined as a relative increase in arm volume of 10% from baseline occurring greater than three months postoperatively (to avoid mistaking early-onset postoperative swelling for lymphedema).3,12,20 To quantify arm volume change for patients who received unilateral breast surgery, we used the validated relative volume change (RVC) equation which accounts for preoperative asymmetry between arms using the contralateral arm volume as a control as previously described.3,12 For patients who received bilateral breast surgery, and therefore did not have a contralateral control arm, we used the validated weight-adjusted volume change (WAC) method which utilizes changes in patient weight as a control as previously described.3,21
Statistical Methods
Given that prior studies have established the type of axillary surgery and RLNR as important factors for lymphedema development, we focused our analysis to five pre-determined clinically relevant cohorts of interest specified by type of axillary nodal sampling and radiation received. Notably, we chose to group patients who did not receive radiation and patients who received local RT (breast or chest-wall) together because it has been previously shown that local radiation without RLNR does not increase the risk of developing lymphedema.3 The 5 treatment groups comprised patients who received: 1) ALND with RLNR, 2) ALND without RLNR, 3) SLNB with RLNR, 4) SLNB without RLNR, and 5) no axillary surgery. An ANOVA of a mixed effects generalized linear model was used to compare clinical and treatment characteristics across groups.
The cumulative incidence of lymphedema stratified by therapy group was calculated via the Kaplan-Meier method.22 Cumulative incidence estimates of lymphedema were compared using the Cox Proportional Hazards model.23 Semiannual hazard rate of lymphedema was defined as the proportion of patients who developed lymphedema during a 6-month interval.24
Cox proportional hazard models with clusters by patient (to account for within-patient correlation in bilateral cases) were used to determine the risk factors associated with the development of lymphedema in our cohort. We used univariate analysis to identify independent predictors of lymphedema by use of the Cox proportional hazards method.23 Adjusted hazard ratios (AHR) with 95% confidence intervals (95% CI) and p-values were calculated. For multivariable regression, we included all the factors that were shown to be independent predictors of lymphedema (p<0.05) development on univariate analysis, with the exception of the number of lymph nodes removed as this measure was highly correlated with the type of axillary surgery.
We subsequently restricted our analysis to the subset of patients who developed lymphedema to identify risk factors predisposing patients to early-onset versus late-onset lymphedema. We separated the cohort of patients who developed lymphedema into “early-onset” (>3 and ≤12 months postoperatively) and “late-onset” (>12 months) groups and repeated the regression for each subset.
Statistical analyses were conducted using R version 3.3.2 (R Foundation for Statistical Computing, Auckland, New Zealand).
Results
Comparison clinical factors stratified by axillary surgery and radiation treatment groups
Table 1 illustrates the clinical and treatment characteristics of the entire cohort and the number of patients comprising the five treatment groups of interest: 1) ALND with RLNR (n=400, 17.7%), 2) ALND without RLNR (n=147, 6.5%), 3) SLNB with RLNR (n=138, 6.1%), 4) SLNB without RLNR (n=1,382, 60.9%), and 5) no axillary surgery (n=199, 8.8%). Table 2 illustrates the differences in the clinical and treatment characteristics by treatment group. Notably, a greater proportion of patients in the no axillary surgery group had a diagnosis of DCIS compared to the other groups (59% versus 5%, respectively). A greater proportion of patients who received ALND with RLNR received neoadjuvant chemotherapy compared to the other groups (43% versus 8%). A lower proportion of patients in the SLNB without RLNR and no axillary surgery groups received adjuvant chemotherapy compared to the other groups (23% versus 62%). A lower proportion of patients in the no axillary surgery group received hormonal therapy compared to the other groups (53% versus 75%). As expected, a greater number of lymph nodes were removed in the patients who received an ALND as compared to SLNB.
Table 1.
Clinical and treatment characteristics of the study cohort.
| Clinical Factor | No. of patients | % |
| Age at diagnosis, years Median (range) | 55.1 (24.4, 87.9) | |
| BMI at diagnosis Median (range) | 26.5 (16.5, 58.9) | |
| Neoadjuvant chemotherapy | ||
| Yes | 305 | 13.9 |
| No | 1872 | 86.1 |
| Adjuvant chemotherapy | ||
| Yes | 752 | 65.5 |
| No | 1425 | 34.5 |
| Hormonal therapy | ||
| Yes | 1575 | 72.3 |
| No | 602 | 27.7 |
| Clinical Factor | No. of affected sides | % |
| Tumor type | ||
| DCIS | 219 | 9.7 |
| IBC | 2047 | 90.3 |
| Invasive tumor size, cm* Median (range) | 1.2 (0.01, 16) | |
| Breast surgery | ||
| Lumpectomy Mastectomy | 1232 | 54.4 |
| Mastectomy | 1034 | 45.6 |
| Axillary surgery | ||
| None | 199 | 8.8 |
| SLNB | 1520 | 67.1 |
| ALND | 547 | 24.1 |
| Lymph node characteristics median (range) | ||
| No. of LNs removed, SLNB | 2 (1, 50) | |
| No. of LNs removed, ALND | 16 (2, 64) | |
| No. of positive LNs, ALND | 2 (0, 52) | |
| Radiation therapy | ||
| None | 822 | 36.3 |
| Local only (no RLNR) | 903 | 39.8 |
| Local only (no RLNR) | 541 | 28.9 |
| Clinically Relevant Axillary Surgery and Radiation Groups | ||
| ALND with RLNR | 400 | 17.7% |
| ALND without RT | 147 | 6.5% |
| SLNB with RLNR | 138 | 6.1% |
| SLNB without RT | 1382 | 60.9% |
| No axillary surgery | 199 | 8.8% |
Abbreviations: No., number; BMI, body mass index; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; RLNR, regional lymph node radiation; LN, lymph node; RT, radiation therapy; DCIS, ductal carcinoma in situ; IBC, invasive breast cancer
this does not include tumor size for patients who received neoadjuvant chemotherapy
Table 2.
Comparison of the clinical and treatment characteristics across treatment cohorts of interest.
| Axillary Surgery and Radiation Cohorts | F-statistic P-value | |||||
|---|---|---|---|---|---|---|
| No Axillary Surgery | SLNB without RLNR | SLNB with RLNR | ALND without RLNR | ALND with RLNR | ||
| Clinical Factor | No. of patients(%)† | No. of patients(%)† | No. of patients(%)† | No. of patients(%)† | No. of patients(%)† | |
| Age at diagnosis, years | ||||||
| Median (range) | 58.5 (34.1, 87) | 56.8 (27, 87.9) | 51.1 (24.4, 79.5) | 50.4 (28.5, 83.6) | 49.6 (24.8, 83.7) | < 0.001 |
| BMI at diagnosis | ||||||
| Median (range) | 27 (19, 47.2) | 26.3 (17, 58.9) | 25.1 (17.5, 47.5) | 26.2 (17.9, 44.5) | 26.9 (16.5, 58.4) | 0.2592 |
| Tumor type | ||||||
| DCIS | 118 | 97 | 0 | 1 | 3 | < 0.001 |
| IBC | 81 | 1285 | 138 | 146 | 397 | |
| Invasive tumor size, cm* Median (range) | ||||||
| Median (range) | 0.75 (0.05, 7) | 1.1 (0.01, 10.5) | 1.5 (0.15, 13) | 1.5 (0.1, 16) | 2 (0.01, 15) | < 0.001 |
| Lymph node characteristics median (range) | ||||||
| No. LNs removed | n/a | 2 (1, 50) | 2 (1, 18) | 15 (2, 35) | 17 (2, 64) | < 0.001 |
| No. of positive LNs | 0 (0, 3) | 1 (0, 8) | 1 (0, 13) | 3 (0, 52) | ||
| Neoadjuvant chemotherapy | ||||||
| Yes | 28 | 47 | 32 | 37 | 172 | < 0.001 |
| No | 171 | 1335 | 106 | 110 | 228 | |
| Adjuvant chemotherapy | ||||||
| Yes | 14 | 345 | 90 | 88 | 250 | < 0.001 |
| No | 185 | 1037 | 48 | 59 | 150 | |
| Hormonal therapy | ||||||
| Yes | 106 | 1046 | 104 | 105 | 285 | < 0.001 |
| No | 93 | 336 | 34 | 42 | 115 | |
Abbreviations: No., number; BMI, body mass index; SNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; RLNR, regional lymph node radiation; LN, lymph node; RT, radiation therapy; DCIS, ductal carcinoma in situ; IBC, invasive breast cancer
this does not include tumor size for patients who received neoadjuvant chemotherapy
or mean (range) where specified
Lymphedema Incidence and Hazard Rate
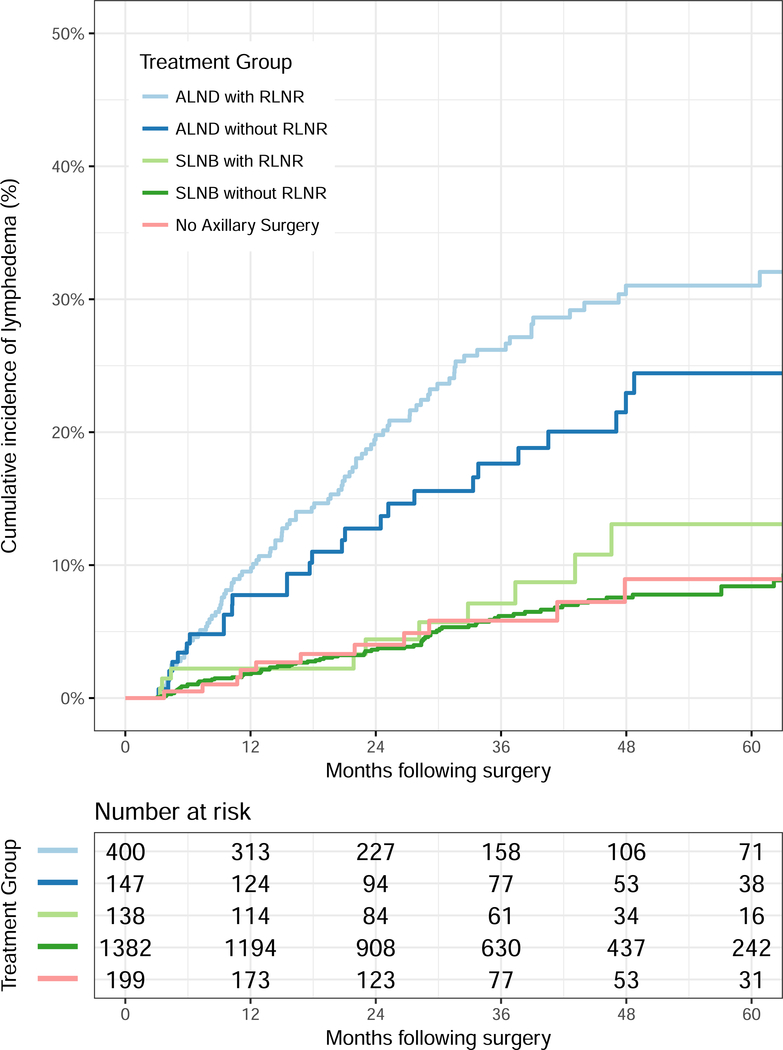
At a median follow-up of four years, the overall estimated two-year cumulative incidence of lymphedema in the cohort was 7.1% and the five-year cumulative incidence estimate was 13.7%. Figure 1 displays the cumulative incidence of lymphedema as a function of the five groups of interest. Specifically, the respective two- and five-year point estimates for lymphedema as shown in Figure 1 were 19.0% (95% CI: 15.3–23.6%) and 31.2% (95% CI: 26.0–37.2%) for patients in the ALND with RLNR group, 12.7% (95% CI: 8.1–19.7%) and 24.6% (95% CI: 17.2–34.5%) for patients in the ALND without RLNR group, 4.3% (95% CI: 1.8–10.0%) and 12.2% (95% CI: 6.5–22.3%) for patients in the SLNB with RLNR group, 3.7% (95% CI: 2.8–4.9%) and 8.3% (95% CI: 6.6–10.5%) for patients in the SLNB without RLNR group, and 4.1% (95% CI: 2.0–8.4%) and 9.4% (95% CI: 4.9–17.7%) for patients in the no axillary surgery group.
Figure 1.
Cumulative incidence of lymphedema by axillary surgery and radiation groups. Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; RLNR, regional lymph node radiation.
Patients in the ALND with RLNR group experienced the highest rates of lymphedema compared to the other four groups, including the next highest group of ALND without RLNR (HR 0.613, 95% CI: 0.403–0.935, p=0.023), followed by those in the SLNB with RLNR group (HR 0.285, 95% CI: 0.149–0.545, p=0.0002).
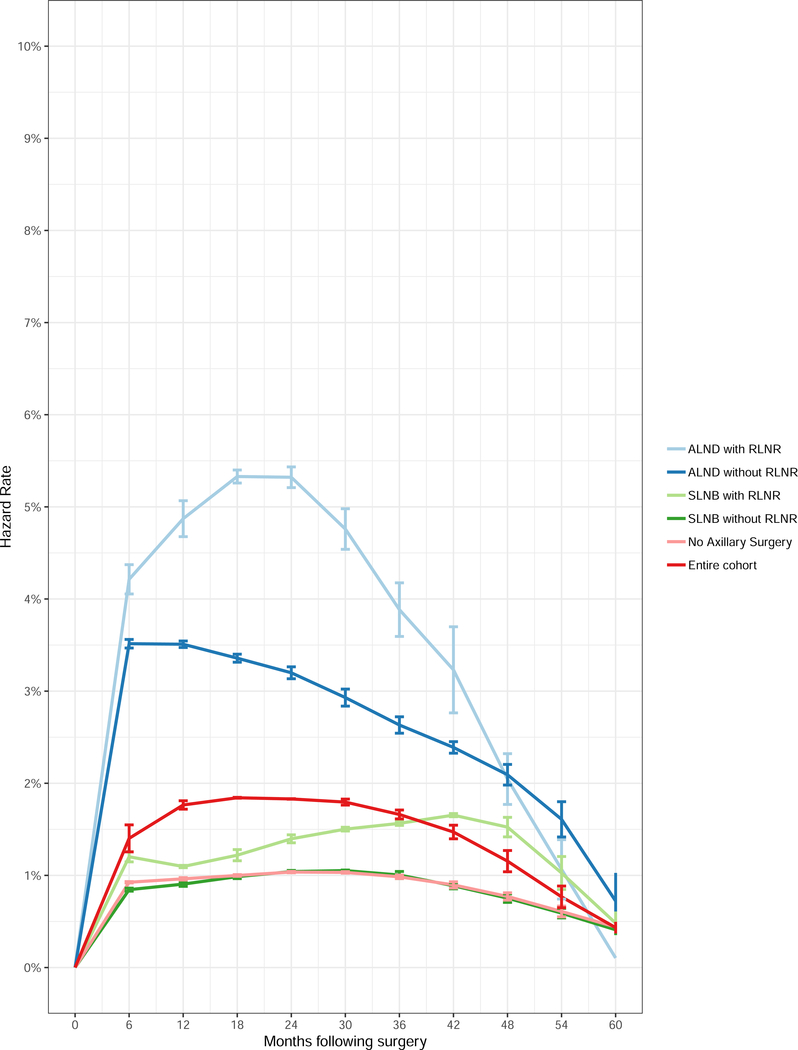
The hazard rate for development of lymphedema over time as a function of treatment group is shown in Figure 2. For the entire cohort, the hazard of lymphedema development is greatest between 12–30 months following surgery but depends on the therapy received. For patients who received ALND without RLNR, the hazard of lymphedema development was greatest in the first 6–12 months. For patients who received ALND with RLNR, the hazard rate peaked between 18–24 months and decreased significantly after 30 months. For patients who received SLNB with RLNR, the hazard rate peaked between 36–48 months and declined significantly thereafter.
Figure 2.
Semiannual hazard rate for development of lymphedema for the entire cohort and by axillary surgery and radiation groups. Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; RLNR, regional lymph node radiation.
Regression Analyses
The results of univariate and multivariate analyses of factors associated with lymphedema development are included in Table 3. On multivariate analysis, the following factors were significantly associated with increased risk of lymphedema: BMI ≥30 compared to BMI <30 (AHR 2.07, p <0.0001), ALND compared to SLNB (AHR 2.71, p<0.0001), and RLNR compared to local RT (AHR 2.1, p=0.0018).
Table 3.
Clinical and Treatment Factor Hazard Ratios for the Risk of Lymphedema
| Clinical Factor | Development of Lymphedema | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| No. of women | No. of LymphED | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Age at diagnosis, years | ||||||
| ≥50 vs | 1477 | 156 | 1.1 (0.83,1.45) | 0.508 | -- | -- |
| <50 | 785 | 77 | ||||
| BMI at diagnosis | ||||||
| ≥30 vs | 532 | 83 | 1.99 (1.49,2.64) | <0.0001 | 2.07 (1.56,2.74) | <0.0001 |
| <30 | 1393 | 120 | ||||
| Tumor type | ||||||
| DCIS vs | 219 | 12 | 0.54(0.29,0.99) | 0.0478 | 0.55 (0.24,1.25) | 0.1512 |
| IBC | 2047 | 221 | ||||
| Breast surgery | ||||||
| Mastectomy vs | 1034 | 89 | 0.55 (0.42,0.72) | <0.0001 | 0.90 (0.53,1.53) | 0.7014 |
| Lumpectomy | 1232 | 144 | ||||
| Axillary surgery | ||||||
| ALND vs | 547 | 130 | 4.09 (3.1, 5.39) | <0.0001 | 2.71 (1.78,4.11) | <0.0001 |
| SLNB | 1520 | 91 | ||||
| Radiation | ||||||
| RLNR vs | 541 | 112 | 3.59 (2.63,4.91) | < 0.0001 | 2.1 (1.32,3.34) | 0.0018 |
| No RLNR | 903 | 61 | ||||
| Lymph nodes removed | ||||||
| ≥3 vs | 980 | 157 | 3.42 (2.4,4.88) | <0.0001 | -- | -- |
| <3 | 1114 | 63 | ||||
| Chemotherapy | ||||||
| Yes vs | 1017 | 145 | 1.98 (1.52,2.58) | <0.0001 | 0.67 (0.45,1.01) | 0.0562 |
| No | 1249 | 88 | ||||
| Hormonal therapy | ||||||
| Yes vs | 1646 | 173 | 1.02 (0.76,1.38) | 0.8922 | -- | -- |
| No | 620 | 60 | ||||
Abbreviations: LymphED, lymphedema; No., number; BMI, body mass index; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; RLNR, regional lymph node radiation; LN, lymph node; RT, radiation therapy; DCIS, ductal carcinoma in situ; IBC, invasive breast cancer
Early-Onset versus Late-Onset Lymphedema Regression Analyses
Table 4 displays the results of multivariable regression restricted to the subsets of patients developing “early-onset” versus “late-onset” lymphedema. As shown in Table 4, on multivariable regression, “early-onset” lymphedema was associated with higher BMI (AHR 2.24, p=0.0001) and ALND compared to SLNB (AHR 4.75, p<0.0001), whereas RLNR was not associated with lymphedema development for the “early-onset” cohort. In contrast, “late-onset” lymphedema was significantly associated with higher BMI (AHR 2.01, p=0.0005), RLNR (AHR 3.86, p=0.0001) and ALND compared with SLNB (AHR 1.86, p = 0.029).
Table 4.
Clinical and Treatment Factor Hazard Ratios for the Risk of Early-Onset (>3 and ≤ 12 months postoperatively) versus Late-Onset (>12 months postoperatively) Lymphedema
| Clinical Factor | Development of Lymphedema | ||||||
|---|---|---|---|---|---|---|---|
| Multivariate (Early) | Multivariate (Late) | ||||||
| No. of women | No. of Early LymphED | No. of Late LymphED | Adjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Age at diagnosis, years | |||||||
| ≥50 vs | 1477 | 66 | 90 | -- | -- | -- | -- |
| <50 | 785 | 45 | 32 | ||||
| BMI at diagnosis | |||||||
| ≥30 vs | 532 | 42 | 41 | 2.24 (1.5,3.36) | 0.0001 | 2.01 (1.35,2.98) | 0.0005 |
| <30 | 1393 | 54 | 66 | ||||
| Tumor type | |||||||
| DCIS vs | 219 | 5 | 7 | 0.52 (0.16,1.71) | 0.2814 | 0.56 (0.18,1.78) | 0.3289 |
| IBC | 2047 | 106 | 115 | ||||
| Breast surgery | |||||||
| Mastectomy vs | 1034 | 36 | 53 | 0.69 (0.43,1.1) | 0.1143 | 0.88 (0.58,1.34) | 0.554 |
| Lumpectomy | 1232 | 75 | 69 | ||||
| Axillary surgery | |||||||
| ALND vs | 547 | 67 | 63 | 4.75 (2.56,8.82) | <0.0001 | 1.86 (1.07,3.24) | 0.0289 |
| SLNB | 1520 | 38 | 53 | ||||
| Radiation | |||||||
| RLNR vs | 541 | 55 | 57 | 1.21 (0.65,2.27) | 0.546 | 3.86 (2.01,7.43) | 0.0001 |
| No RLNR | 903 | 31 | 30 | ||||
| Lymph nodes removed | |||||||
| ≥3 vs | 980 | 80 | 77 | -- | -- | -- | -- |
| <3 | 1114 | 25 | 38 | ||||
| Chemotherapy | |||||||
| Yes | 1017 | 26 | 27 | 0.73 (0.4,1.33) | 0.3061 | 0.7 (0.4,1.22) | 0.2112 |
| No | 1249 | 85 | 95 | ||||
| Hormonal therapy | |||||||
| Yes vs | 1646 | 82 | 91 | -- | -- | -- | -- |
| No | 620 | 29 | 31 | ||||
Abbreviations: LymphED, lymphedema; No., number; BMI, body mass index; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; RLNR, regional lymph node radiation; LN, lymph node; RT, radiation therapy; DCIS, ductal carcinoma in situ; IBC, invasive breast cancer
Discussion
We conducted a large prospective cohort study of 2,171 women (with 2,266 at-risk arms) to investigate the time-course, incidence, and risk factors associated with lymphedema following individualized treatment for breast cancer. Patients in our cohort were treated with modern surgical and radiation techniques and were objectively screened for lymphedema using a Perometer and baseline arm volume measurements. In keeping with prior work, we have shown that ALND, RLNR, and preoperative BMI are associated with increased risk of lymphedema.3,9,25
Our study further describes the time-course for lymphedema development, and the extent to which the timing depends on therapy received. For the entire cohort, the risk of developing lymphedema is greatest within the first 3 years following surgery. Interestingly, we found that ALND, not RLNR, is associated with “early-onset” (≤12 months) lymphedema. In contrast, RLNR emerges as a significant risk factor for lymphedema development only for patients experiencing “late-onset” (>12 months) lymphedema. Although ALND is still a significant predictor for “late-onset” lymphedema, the HR is much smaller for ALND for “late-onset” compared to “early-onset” lymphedema (1.86 versus 4.75, respectively). The risk of lymphedema among patients who receive ALND-alone peaks within 6–12 months postoperatively. In contrast, the lymphedema risk for patients who receive ALND/RLNR is higher by 6 months compared to the ALND-alone group, and peaks between 18–24 months. Finally, the lymphedema risk for patients who receive SLNB/RLNR peaks between 36–48 months and is lower than the other two groups overall.
Several points require further discussion. First, although not the purpose of the study, we may speculate regarding why ALND and RLNR influence lymphedema relatively more at different times. Surgery results in direct trauma to the lymphatic system26 and consequently lymphedema associated with ALND may manifest early. It is important to note that this effect is not driven by patients who experienced early postoperative swelling; lymphedema was defined in this study as occurring greater than three months postoperatively to distinguish those who may experience transient swelling from those who would develop chronic lymphedema. In contrast, trauma to the lymphatics following radiation may be associated with radiation-induced fibrosis.27 This process is expected to be more protracted, with the effects of lymphatic fibrosis taking several years to fully manifest, and thus the effect of RLNR on lymphedema development manifests later.
Second, our findings are consistent with the rates of lymphedema reported in EORTC 10981–22023 AMAROS, a phase 3 trial randomizing patients with a positive SLNB to axillary radiation or completion ALND (combined ALND/RLNR therapy was given to patients with ≥4 positive nodes).28 In this study, lymphedema was assessed both clinically and by tape-measurement (defined by an increase ≥10% compared to baseline of the ipsilateral arm). 5-year clinical lymphedema rates in this study were 23% for patients undergoing ALND versus 11% for SLNB with RLNR (p < 0.0001), and these respective estimates for arm-circumference lymphedema were 13% and 6%.28 While utilization of a baseline measurement and an objective arm volume assessment were performed, measuring arm circumference with a tape-measure may be more prone to operator variability than Perometer measurements. Our findings, in conjunction with those of AMAROS, support choosing RLNR over completion ALND in appropriate patients given the similar oncologic control seen in AMAROS and the lower lymphedema risk for SLNB with RLNR compared to ALND observed in both studies.
Our observed rates are also consistent, although slightly higher, with those reported in NCIC-CTG MA.20 trial which randomized high-risk women following SLNB or ALND to receive whole breast irradiation with or without RLNR (10-year clinical rates of lymphedema of 8.4% versus 4.5%, respectively).29 Differences in lymphedema incidence seen in our study compared to MA.20 may be related to quantitative versus qualitative assessment tools and lack of a baseline arm assessment in MA.20, as prior reports have shown significant rates of misdiagnosis without baseline arm measurements.10 In MA.20, lymphedema was assessed by the clinician as grade 2 (moderate lymphedema requiring compression), or grade 3 (severe lymphedema limiting function), and a baseline arm volume assessment was not performed.
Third, our results can inform clinical practice in many ways. To start, our results can be used to counsel patients regarding their individual risk (with respect to specific surgery and radiation received) and time-course of lymphedema development. Furthermore, understanding the timing of lymphedema onset may be useful for risk stratification and to optimize lymphedema surveillance, especially as resource allocation may preclude intense lymphedema monitoring in all patients and clinical settings. In addition, these data may be used to guide future clinical trial design of interventions designed to prevent or mitigate lymphedema to focus these efforts on when patients are at greatest risk.
Fourth, there is an inconsistency with respect to adjuvant chemotherapy and lymphedema risk with some studies showing an association between adjuvant chemotherapy and increased risk of lymphedema,25,30–33 while others have not.34–37 In our study, chemotherapy use (either neoadjuvant or adjuvant) was associated with increased risk of lymphedema development on univariate analysis, but was not a significant predictor on multivariable analysis.
Our study has several strengths. First, we have investigated lymphedema with an objective arm volume measurement tool, including a baseline arm measurement. Second, our cohort is large, with sufficient follow-up period to accurately elucidate the time-course for development of lymphedema. Finally, we were able to define lymphedema rates for modern clinically relevant cohorts, and a particular strength is that we have a sufficiently large group of women who received RLNR after SLNB.
Our study has potential limitations. First, while prospective, our study was not randomized and there is the possibility that the differences we observed in the timing and incidence of lymphedema are driven by an unaccounted for clinical or pathologic factor. Indeed, patients in the SLNB without RLNR and no axillary surgery groups experienced a 1% semiannual rate of lymphedema for the first 3 years postoperatively, potentially due to breast surgery itself or unaccounted factors in our study (e.g., cellulitis, arm trauma, etc.). Another limitation is that our study does not address the impact of any interventions (e.g., physical therapy, sleeve, etc.) prescribed following lymphedema diagnosis. Additionally, we do not have sufficient follow-up to depict lymphedema beyond 5 years to understand the rates of lymphedema beyond 60 months, although our results indicate that the rate of lymphedema drops below 2% after 5 years for the entire cohort. Finally, all patients in this cohort received surgery at a single high-volume breast cancer institution and it is possible that our findings may not translate to all settings.
Despite these limitations, we have shown that the time-course for breast cancer treatment-related lymphedema is greatest between 12–30 months following therapy, but depends on therapy received. ALND is associated with early-onset lymphedema and RLNR is associated with late-onset lymphedema. These results can be incorporated into clinical practice to guide lymphedema surveillance strategies and patient education. Early identification, continuous monitoring, and intervention for lymphedema should be key components of breast cancer treatment.
Acknowledgement
Presented at the 58th Annual Meeting of the American Society for Radiation Oncology (ASTRO), Boston, MA, September 25–28, 2016. The study was supported by Award Number R01CA139118 (AGT), Award Number P50CA089393 (AGT) from the National Cancer Institute and the Adele McKinnon Research Fund for Breast Cancer-Related Lymphedema.
Footnotes
Conflict of Interest: The authors have no conflict of interests to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vassard D, Olsen MH, Zinckernagel L, Vibe-Petersen J, Dalton SO, Johansen C. Psychological consequences of lymphoedema associated with breast cancer: A prospective cohort study. Eur J Cancer. 2010;46(18):3211–3218. doi: 10.1016/j.ejca.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Pusic AL, Cemal Y, Albornoz C, et al. Quality of life among breast cancer patients with lymphedema: A systematic review of patient-reported outcome instruments and outcomes. J Cancer Surviv. 2013;7(1):83–92. doi: 10.1007/s11764-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren LEG, Miller CL, Horick N, et al. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: A prospective cohort study. Int J Radiat Oncol Biol Phys 2014;88:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27(3):390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83(12 Suppl American):2776–2781. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Petrek JA, Senie RT, Peters M, Peterrosen P. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001;92(6):1368–1377. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J Clin Oncol. 2008;26(21):3536–3542. doi: 10.1200/JCO.2007.14.4899. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mclaughlin SA, Wright MJ, Morris KT, et al. Prevalence of Lymphedema in Women With Breast Cancer 5 Years After Sentinel Lymph Node Biopsy or Axillary Dissection : Objective Measurements. J Clin Oncol. 2008;26(32):5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun F, Skolny MN, Swaroop MN, et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat 2016;157:229–240. [DOI] [PubMed] [Google Scholar]
- 11.Chandra RA, Miller CL, Skolny MN, et al. Radiation therapy risk factors for development of lymphedema in patients treated with regional lymph node irradiation for breast cancer. Int J Radiat Oncol Biol Phys 2015;91:760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancukiewicz M, Russell TA, Otoole J, et al. Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys 2011;79:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XA, Tai A, Arthur DW, et al. Variability of Target and Normal Structure Delineation for Breast Cancer Radiotherapy: An RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys. 2009;73(3):944–951. doi: 10.1016/j.ijrobp.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pashtan IM, Recht A, Ancukiewicz M, et al. External beam acceler-ated partial-breast irradiation using 32 Gy in 8 twice-daily fractions: 5-year results of a prospective study. Int J Radiat Oncol Biol Phys 2012; 84:e271–e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunelle C, Skolny M, Ferguson C, et al. Establishing and sustaining a prospective screening program for breast cancer-related lymphedema at the massachusetts general hospital: Lessons learned. J Pers Med 2015;5:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12(4):412–417. doi: 10.1016/S1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee M-J, Boland RA, Czerniec S, Kilbreath SL. Reliability and Concurrent Validity of the Perometer for Measuring Hand Volume in Women With and Without Lymphedema. Lymphat Res Biol. 2011;9(1):13–18. doi: 10.1089/lrb.2010.0021. [DOI] [PubMed] [Google Scholar]
- 18.Ridner SH, Montgomery LD, Hepworth JT, Stewart BR, Armer JM. Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007;40:35–46. [PubMed] [Google Scholar]
- 19.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer). Lymphology. 1997;30(2):77–97. http://www.ncbi.nlm.nih.gov/pubmed/9215977. [PubMed] [Google Scholar]
- 20.Specht MC, Miller CL, Russell TA, et al. Defining a threshold for intervention in breast cancer-related lymphedema: what level of arm volume increase predicts progression? Breast Cancer Res Treat 2013; 140:485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller CL, Specht MC, Horick N, et al. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilat-eral breast surgery. Lymphology 2013;46:64–74. [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53(282):457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 23.Breslow NE. Analysis of Survival Data under the Proportional Hazards Model. Int Stat Rev / Rev Int Stat. 1975;43(1):45. doi: 10.2307/1402659. [DOI] [Google Scholar]
- 24.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 25.Asdourian MS, Swaroop MN, Sayegh HE, et al. Association between precautionary behaviors and breast cancer-related lymphedema in patients undergoing bilateral surgery. J Clin Oncol 2017;35:3934–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren AG, Brorson H, Borud LJ, Slavin S a. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59(4):464–472. doi: 10.1097/01.sap.0000257149.42922.7e. [DOI] [PubMed] [Google Scholar]
- 27.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141(11):1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981–22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional Nodal Irradiation in Early-Stage Breast Cancer MA 20. N Engl J Med. 2015. doi: 10.1056/NEJMoa1415340. [DOI] [PubMed] [Google Scholar]
- 30.Togawa K, Ma H, Sullivan-Halley J, et al. Risk factors for self-reported arm lymphedema among female breast cancer survivors: a prospective cohort study. Breast Cancer Res. 2014;16(4):414. doi: 10.1186/s13058-014-0414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: Combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86(3):498–503. doi: 10.1016/j.ijrobp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Shah C, Ben Wilkinson J, Baschnagel A, et al. Factors associated with the development of breast cancer-related lymphedema after whole-breast irradiation. Int J Radiat Oncol Biol Phys. 2012;83(4):1095–1100. doi: 10.1016/j.ijrobp.2011.09.058. [DOI] [PubMed] [Google Scholar]
- 33.Shih Y-CT, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–2014. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 34.Swaroop MN, Ferguson CM, Horick NK, et al. Impact of adjuvant taxane-based chemotherapy on development of breast cancer-related lymphedema: results from a large prospective cohort. Breast Cancer Res Treat 2015;151:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monleon S, Murta-Nascimento C, Bascuas I, Macià F, Duarte E, Belmonte R. Lymphedema Predictor Factors after Breast Cancer Surgery: A Survival Analysis. Lymphat Res Biol. 2015;13(4):268–274. doi: 10.1089/lrb.2013.0042. [DOI] [PubMed] [Google Scholar]
- 36.Gartner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment--a nationwide study of prevalence and associated factors. Breast. 2010;19(6):506–515. doi: 10.1016/j.breast.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Meeske KA, Sullivan-Halley J, Smith AW, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat. 2009;113(2):383–391. doi: 10.1007/s10549-008-9940-5. [DOI] [PubMed] [Google Scholar]