The spikelet is the specific unit of the grass inflorescence, and produces a variable number of florets (1–40) depending on whether the species displays determinate or indeterminate growth. Spikelet morphogenesis therefore plays an important role in crop yield; for example in wheat (Triticum aestivum) inflorescences with the indeterminate spikelet, the spikelet meristem is continuously active and produces a plethora of lateral floral meristems, leading to the formation of variable numbers of florets. In inflorescences of rice (Oryza sativa) and maize (Zea mays) with the determinate spikelet, the spikelet meristem produces the fixed numbers of lateral floral meristems, after which its fate is terminated as a terminal floral meristem, resulting in the formation of one or two florets. In rice, MFS1, SNB, EG1/DF1, TOB1 and OsMADS22 regulates the spikelet determinacy or indeterminacy (Lee et al., 2007; Ren et al., 2013). The loss of function of any of these genes induces the production of two complete florets or extra lemmas (secondary florets) within a single spikelet. In this study, we described a new mutant allele of FON4, named fon4‐7.
In the previous studies, the fon4 mutant produced increased inner floral organs and elongated sterile lemmas with the indeterminate identity (Chu et al., 2006), whereas we confirmed that the fon4‐7 mutant showed two florets and induced the reversion of sterile lemmas into lemmas (lateral florets). We discuss two possibilities for the formation of multi‐floret spikelet in rice, which may facilitate the breeding of rice cultivars with increased grain number per rice panicle.
Rice strictly produces a pair of sterile lemmas and one floret per spikelet, each containing one lemma and one palea, two lodicules adjacent to the lemma, six stamens and a pistil (Figure 1A1–A5).
Figure 1.
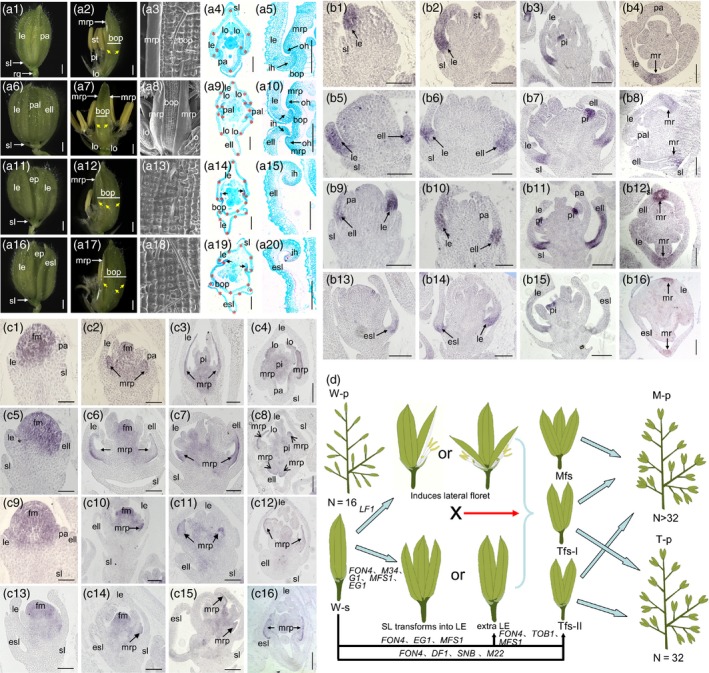
A, phenotypes investigations of spikelets in the wild type and fon4‐7 mutant. A1 and A2, spikelet of the wild type. A3, epidermal surface of palea in the wild type. A4 and A5, histological observations of spikelet in the wild type. A6 and A7, fon4‐7 spikelet with an extra lemma‐like organ, two reduced paleae and four lodicules. A8, epidermal surface of the reduced palea in A7. A9 and A10, histological analysis of an fon4‐7 spikelet with an extra lemma‐like organ, two degenerated paleae and four lodicules. A11 and A12, fon4‐7 spikelet with an extra lemma‐like organ and an enlarged palea. A13, epidermal surface of the extra lemma‐like organ in A12. A14 and A15, histological analysis of an fon4‐7 spikelet with an extra lemma‐like organ and an enlarged palea. A16 and A17, fon4‐7 spikelet with an elongated sterile lemma and an enlarged palea. A18, epidermal surface of the elongated sterile lemma in A17. A19 and A20, histological analysis of an fon4‐7 spikelet with an elongated sterile lemma and an enlarged palea. B, expression of DL gene in the wild type and fon4‐7 mutant. B1‐B4, wild‐type spikelet. B1, Sp4; B2, Sp5‐6; B3, Sp7, B4, Sp8. B5‐B8, fon4‐7 spikelet with an extra lemma‐like organ and two reduced paleae. B5, Sp4; B6, Sp5‐6; B7, Sp7, B8, Sp8. B9‐B12, fon4‐7 spikelet with an extra lemma‐like organ and an enlarged palea. B9, Sp4; B10, Sp5‐6; B11, Sp7, B12, Sp8. B13‐B16, fon4‐7 spikelet with an elongated sterile lemma and an enlarged palea. B13, Sp4; B14, Sp5‐6; B15, Sp7, B16, Sp8. C, expression of OsMADS6 gene in the wild type and fon4‐7 mutant. C1‐C4, wild‐type spikelet. C1, Sp4; C2, Sp5‐6; C3, Sp7, C4, Sp8. C5‐C8, fon4‐7 spikelet with an extra lemma‐like organ and two reduced paleae. C5, Sp4; C6, Sp5‐6; C7, Sp7, C8, Sp8. C9‐C12, fon4‐7 spikelet with an extra lemma‐like organ and an enlarged palea. C9, Sp4; C10, Sp5‐6; C11, Sp7, C12, Sp8. C13‐C16, fon4‐7 spikelet with an elongated sterile lemma and an enlarged palea. C13, Sp4; C14, Sp5‐6; C15, Sp7, C16, Sp8. D, model of hypothesized molecular design breeding and putative function of FON4. In the modle, LF1 induces the lateral florets in the single rice spikelet with normal sterile lemma; FON4,TOB1, M34,SNB, G1,MFS1,EG1/DF1 repress the formation of lemma‐like sterile lemma or extra lemma or two‐floret spikelet in the single rice spikelet with normal sterile lemma or without sterile lemma; M22 induces the two‐floret spikelet in the single rice spikelet with normal sterile lemma. esl, elongated sterile lemma; ell, extra lemma‐like organ; le, lemma; lo, lodicule; st, stamen; pi, pistil; pa, palea; pal, palea‐like organ; rg, rudimentary glume; sl, sterile lemma; ep, enlarged palea; mrp, marginal regions of the palea; bop, body of the palea; oh, outward hook‐like structure; ih, inward hook‐like structure, fm, floral meristem; W‐p, wild‐type panicle; M‐p, panicle with multi‐floret spikelet; T‐p, panicle with two‐floret spikelet; W‐s, wild‐type spikelet; Mfs, multi‐floret spikelet; Tfs‐I, two‐floret spikelet without sterile lemma; Tfs‐II, two‐floret spikelet with sterile lemma; M22, OsMADS22; M34, OsMADS34. Black arrows represent mrp in A14 and A19. Yellow arrows and red stars represent vascular bundles in A. N indicates the floret number in panicle in D. “X” in D indicates the cross in breeding. Bars = 1000 mm in A1, A2, A6, A7, A11, A12, A16 and A17; 100 μm in A3‐A5, A8‐A10, A13‐A15, and A18‐A20; and 50 μm in B and C.
The group‐I fon4‐7 spikelets developed extra lemma‐like organs, and two palea‐like organs (Figure 1A5–A10). Four lodicules were observed in these spikelets, two on either side of each lemma (Figure 1A7–A9). This phenotype indicated that these spikelets formed two florets. The group‐II spikelets produced a secondary floret containing a lemma‐like organ (Figure 1A11–A15), whereas the group‐III spikelets developed a terminal floret with a lemma‐like sterile lemma (lateral florets) (Figure 1A16–A20). Our detailed phenotypic observations revealed that all lemma‐like organs, lemma‐like sterile lemmas and enlarged body of the paleae had similar cell structures and vascular bundle numbers as the wild‐type lemmas (Figure 1A4, A14, A19). OsMADS1, OsMADS14 and OsMADS15, were expressed in the typical lemmas, additional lemma‐like organs, elongated sterile lemmas and palea‐like organs of fon4‐7 mutant. DL expression was also detected in the above organs except the palea‐like organs in the fon4‐7 mutant. OsMADS6 was expressed in the fon4‐7 paleae and palea‐like organs. These changes in expression revealed that the extra lemma‐like organs and the elongated sterile lemmas of the fon4‐7 spikelets had the lemma identity, and that the palea‐like organ was formed of a degraded palea. The increased expressions of OsMADS2 and OsMADS6 in the fon4‐7 mutant may be due to the relative abundance of the corresponding organs of this mutant.
We monitored the development of early spikelets. At spikelet stage 4 (Sp4), some of the fon4‐7 spikelets produced extra lemma‐like primordia and broad or narrow palea primordial. During stages Sp5 and Sp6, the fon4‐7 spikelets formed higher numbers of stamens and maintained the growth of their defective palea. At Sp7 and Sp8, extra lemma‐like, palea‐like organs and elongated sterile lemmas were obviously observed in the fon4‐7 spikelets, and the paleae were broader and had more than one bumped tops. No obvious differences of the sterile lemmas were found between the wild type and fon4‐7 mutant at stages Sp4 to Sp7. At Sp8, the sterile lemmas of the fon4‐7 mutant dramatically differentiated and were comparable in size to the wild‐type lemmas.
We next examined the expression patterns of floral organ identity genes using in situ hybridization. In the wild type, DL expression was detected in the lemma at stages Sp4 to Sp7, and in the midrib of the lemma at Sp8 (Figure 1B1–B4). In the fon4‐7 mutant, DL expression was detected in the normal lemmas, extra lemma‐like organs and elongated sterile lemmas (Figure 1B5–B16). These results confirmed that the mutant spikelets indeed formed an additional lemma or underwent the homologous transformation of a sterile lemma into a lemma. During the Sp4 to Sp7 stages, OsMADS6 expression in the fon4‐7 mutant resembled that of the wild type, with transcription signals observed in the floral meristem, marginal regions of the palea (mrp), lodicule and pistil (Figure 1C1–C3, C5–C7, C9–C11, C11–C15). At Sp8, fon4‐7 expressed this gene in the four mrps of the two palea‐like organs (C4, C8, C12, C16). These findings further indicated that the two palea‐like structures in the single fon4‐7 spikelet were derived from normal paleae. The OsMADS2 signals were detected in the stamens and the lodicules adjacent to the lemma in the wild type during stages Sp4 to Sp8; however, in group‐I fon4‐7 spikelets, the transcripts of this gene were detected in the typical lodicules and extra lodicules at stages Sp4 to Sp7. At Sp8, the OsMADS2 transcription signals were pronounced in the two lodicules on each side of the lemma in the individual fon4‐7 spikelet, confirming that four lodicules were produced in the single fon4‐7 spikelet containing two lemmas. These results supported the conclusion that two independent florets were formed in the single fon4‐7 spikelet.
The FON4 locus was narrowed between the markers M7 and M13. DNA sequencing led to the identification of an amino acid mutation in the fon4‐7 mutant within the Os11g38270. A complementation assay showed that the fon4‐7 phenotypes were completely rescued. The CRISPR/Cas9 knocked out fon4‐8 and fon4‐9 mutants exhibited the phenotypes that resembled the fon4‐7 mutant. These results further supported that the FON4 is the Os11g38270 gene. Further investigations revealed that fon4‐8 and fon4‐9 induced a variable number of multi‐floret spikelet, suggesting that the different variation sites or genetic background in the FON4 gene led to more and less multi‐floret spikelet.
About 41% of fon4‐7 spikelets produced a typical lemma and an extra lemma, two palea‐like organs, increased stamens and pistils, and four lodicules in which each two on either side of each lemma, suggesting that two florets are formed. About 16% of fon4‐7 spikelets bore normal florets with additional lemmas, which implied that these spikelets comprised a terminal floret and a secondary floret containing only the lemma. These findings suggest that the spikelet meristem determinacy was lost in the fon4‐7 mutant. This phenotype of two florets or an extra lemma‐like organ (secondary floret) was also observed previously in the tob1, mfs1, snb and eg1/df1 mutants, as well as the snb+osids1 double mutant and OsMADS22‐overexpressing plants (Lee et al., 2007; Li et al., 2009; Ren et al., 2013, 2018; Sentoku et al., 2005; Tanaka et al., 2012). A recent study revealed that the mutation of LF1 results in the formation of one or two fertile lateral florets (Zhang et al., 2017). These studies suggest that FON4, LF1, TOB1, MFS1, SNB and OsMADS22 regulate the spikelet meristem determinacy, or their mutations or ectopic expressions induce the spikelet meristem indeterminacy. In wheat and oats (Avena sativa), the spikelet meristem exhibits indeterminacy and develops multiple florets, forming more than one seed per spikelet. These findings demonstrate that it is possible to generate two or more florets in each individual rice spikelet, raising the possibility of increasing the number of seeds produced per plant. Furthermore, we revealed that the fon4 mutants produced a variable number of multi‐floret spikelet, indicating that the mutation of different nucleotides in FON4 results in variable effects on its ability to control the spikelet determinacy, thereby producing more and less multi‐floret spikelet.
Previous studies have shown that the mutation of G1, DF1/EG1 or OsMADS34 caused the formation of lemma‐like sterile lemmas, whereas the mutation of LF1 induced the formation of lateral florets without lemmas (Li et al., 2009; Lin et al., 2014; Yoshida et al., 2009; Zhang et al., 2017). These morphological findings strongly supported the three‐floret spikelet hypothesis (Zhang et al., 2017). The loss of FON4, DF1/EG1, TOB1, MFS1 and SNB function causes the development of an extra lemma‐like organ or the formation of two florets within a single spikelet, potentially enabling the development of the residual spikelet meristems or transformation of determinacy into indeterminacy. These findings provide two possibilities for breeding rice cultivars with multi‐floret spikelet. One option is to cross the lf1 mutant with our fon4 mutant or other mutants (m34, g1, mfs1 and eg1) that produce a lemma‐like sterile lemma or an extra lemma‐like organ within a single spikelet, with the aim of eventually generating cultivars with two‐ or three‐floret spikelet (Figure 1D). The other possibility is to identify different gene‐editing target sites in the genes (FON4, DF1/EG1, TOB1, MFS1, SNB and M22) involved in the regulation of spikelet meristem determinacy and indeterminacy (Figure 1D). Multi‐floret spikelet has the potential to increase grain number per panicle, thereby increasing rice yield.
Acknowledgements
This work was supported by the Zhejiang natural science foundation (LY18C130007), the Central Public‐interest Scientific Institution Basal Research Fund of China National Rice Research Institute (2017RG001‐4), the National Natural Science Foundation of China (31521064 and 91735304), the National Science and Technology Major Project (2016ZX08009003‐003‐008) and the National GMO New Variety Breeding Program of PRC (2016ZX08011‐001).
The authors declare that they have no competing interests.
Contributor Information
Deyong Ren, Email: rendeyong616@163.com.
Qian Qian, Email: qianqian188@hotmail.com.
References
- Chu, H.W. , Qian, Q. , Liang, W.Q. , Yin, C.S. , Tan, H.X. , Yao, X. , Yuan, Z. et al (2006) The FLORAL ORGAN NUMBER4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol. 142, 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D.Y. , Lee, J. , Moon, S. , Park, S.Y. and An, G. (2007) The rice heterochronic gene SUPERNUMERARY BRACT regulates the transition from spikelet meristem to floral meristem. Plant J. 49, 64–78. [DOI] [PubMed] [Google Scholar]
- Li, H.G. , Xue, D.W. , Gao, Z.Y. , Yan, M.X. , Xu, W.Y. , Xin, Z. , Huang, D.N. et al (2009) A putative lipase gene EXTRA GLUME1 regulates both empty‐glume fate and spikelet development in rice. Plant J. 57, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X.L. , Wu, F. , Du, X.Q. , Shi, X.W. , Liu, Y. , Liu, S.J. , Hu, Y.X. et al (2014) The pleiotropic SEPALLATA‐like gene OsMADS34 reveals that the ‘empty glumes’ of rice (Oryza sativa) spikelets are in fact rudimentary lemmas. New Phytol. 202, 689–702. [DOI] [PubMed] [Google Scholar]
- Ren, D.Y. , Li, Y.F. , Zhao, F.N. , Sang, X.C. , Shi, J.Q. , Wang, N. , Guo, S. et al (2013) MULTI‐FLORET SPIKELET1, which encodes an AP2/ERF protein, determines spikelet meristem fate and sterile lemma identity in rice. Plant Physiol. 162, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, D.Y. , Yu, H.P. , Rao, Y.C. , Xu, Q.K. , Zhou, T.T. , Hu, J. , Zhang, Y. et al (2018) ‘Two‐floret spikelet’ as a novel resource has the potential to increase rice yield. Plant Biotechnol. J. 16, 351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentoku, N. , Hideki, Kato. , Kitano, H. and Imai, R. (2005) OsMADS22, an STMADS11‐like MADS‐box gene of rice, is expressed in non‐vegetative tissues and its ectopic expression induces spikelet meristem indeterminacy. Mol. Genet. Genomics, 273, 1–9. [DOI] [PubMed] [Google Scholar]
- Tanaka, W. , Toriba, T. , Ohmori, Y. , Yoshida, A. , Kawai, A. , Mayama‐Tsuchida, T. , Ichikawa, H. et al (2012) The YABBY gene TONGARI‐BOUSHI1 is involved in lateral organ development and maintenance ofmeristemorganization in the rice spikelet. Plant Cell, 24, 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, A. , Suzaki, T. , Tanaka, W. and Hirano, H. (2009) The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet. Proc. Natl Acad. Sci. USA, 106, 20103–20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Li, Y.F. , Ma, L. , Sang, X.C. , Ling, Y.H. , Wang, Y.T. , Yu, P. et al (2017) LATERAL FLORET 1 induced the three‐florets spikelet in rice. Proc. Natl Acad. Sci. USA, 114, 9984–9989. [DOI] [PMC free article] [PubMed] [Google Scholar]


