Abstract
The Arabidopsis root is a dynamic system where the interaction between different plant hormones controls root meristem activity and, thus, organ growth. In the root, a characteristic graded distribution of the hormone auxin provides positional information, coordinating the proliferating and differentiating cell status. The hormone cytokinin shapes this gradient by positioning an auxin minimum in the last meristematic cells. This auxin minimum triggers a cell developmental switch necessary to start the differentiation program, thus, regulating the root meristem size. To position the auxin minimum, cytokinin promotes the expression of the IAA-amido synthase group II gene GH3.17, which conjugates auxin with amino acids, in the most external layer of the root, the lateral root cap tissue. Since additional GH3 genes are expressed in the root, we questioned whether cytokinin to position the auxin minimum also operates via different GH3 genes. Here, we show that cytokinin regulates meristem size by activating the expression of GH3.5 and GH3.6 genes, in addition to GH3.17. Thus, cytokinin activity provides a robust control of auxin activity in the entire organ necessary to regulate root growth.
Keywords: GRETCHEN HAGEN 3 (GH3) IAA-amido synthase group II, root apical meristem, auxin, cytokinin, lateral root cap, auxin minimum, auxin conjugation
1. Introduction
Organ growth in plants is supported by the meristems, regions providing a reservoir of undifferentiated cells whose activity depends on the stem cell niche [1]. In the root, the stem cells daughters proliferate establishing the division zone of the meristem and, more distally from the root tip along the longitudinal axis, those cells differentiate generating the differentiation zone [2,3,4,5]. The boundary between proliferating and differentiating cells is called transition zone (TZ). The position of this cell boundary depends on the coordinated activity of the stem cell niche, the division zone, and the differentiation zone. The activities of these zones are controlled by a dynamic equilibrium between cell division and cell differentiation. The regulation of this equilibrium results in a shoot-ward or a root-ward shift of the TZ position along the root longitudinal axis [2,3,4,6]. The position of the TZ depends on the antagonistic interaction between cytokinin and auxin hormones [7,8]. It has been demonstrated that cytokinin controls TZ localization by positioning an auxin minimum specifically in the last meristematic cells of each root tissue [9]. In particular, cytokinin through the primary cytokinin response transcription factor ARABIDOPSIS RESPONSE REGULATOR 1 (ARR1), positively regulates the expression of the Aux/IAA SHORT HYPOCOTYL 2 (SHY2) gene, which in turn negatively controls the polar auxin efflux carriers PIN1, PIN3 and PIN7 genes at the vascular tissue TZ. At the same time, ARR1 positively regulates the expression of the IAA-amino synthase of the GH3 Group II gene family GRETCHEN HAGEN 3.17 (GH3.17) [8,9].
The roots of Arabidopsis thaliana can be represented as a series of concentric cylinders where the vascular bundles lie in the center [1,2,3,4]. In the radial axis of the root, the lateral root cap (LRC) represents the most external tissue that surrounds all tissues of the root meristem [1,2,3,4]. The LRC serves to facilitate root penetration in the soil, it acts as a physical protective barrier of the root meristem, and it plays an important role in meristem maintenance [10,11,12,13,14,15,16,17]. It was previously demonstrated that a molecular mechanism acting specifically in the LRC controls root meristem size and, thus, root growth, by positioning the TZ [17]. In particular, ARR1, besides GH3.17, regulates auxin levels by promoting the transcription of the auxin intracellular transporter PIN-FORMED 5 (PIN5) gene [17]. GH3.17 irreversibly conjugates free auxin with amino acids specifically in the LRC cells, thus, promoting hormone inactivation, whereas PIN5 operates on auxin intracellular homeostasis mediating auxin compartmentalization in the endoplasmic reticulum. As a result, the LRC acts as an auxin sink where the regulation of auxin levels, controlled by the cytokinin activity, influences auxin distribution within the entire meristem regulating root meristem size and, thus, root growth [17].
Due to the importance of the tissue-specific activity of cytokinin in the LRC, we question whether cytokinin controls meristem size from this tissue by acting on additional genes. It has been already reported that the induction of cytokinin activity in the LRC regulates the expression of GH3.5, GH3.6 and GH3.9 genes [17], members of the GH3 Group II gene family [18,19,20].
Here we show that GH3.5 and GH3.6 genes are expressed in the LRC and that their expression is cytokinin-dependent. We also show that those genes, similarly to GH3.17, are involved in meristem size regulation. These findings highlight the pivotal role of cytokinin in localizing a strong auxin inactivation process in the LRC to regulate meristem activity.
2. Results
In order to unveil cytokinin-dependent mechanisms acting in the LRC to control meristem size, we took advantage of the already published microarray data reporting genes differentially regulated in the LRC in response to ARR1 induction. These data, resulting from the transcriptional profiling of LRC cells upon induction of a constitutive active form of ARR1 (ARR1ΔDDK) in the LRC, revealed that genes belonging to “auxin homeostasis regulation” gene ontology category are highly represented [17]. Interestingly, among these genes, GH3.5, GH3.6, and GH3.9 were positively regulated by ARR1 in the LRC [17]. It was demonstrated that GH3.5, GH3.6, and GH3.9 IAA-amido synthases participate in maintaining auxin homeostasis by conjugating amino acids to the hormone [18,19,21,22,23,24] and thereby affect the levels of free auxin molecules that are biologically active and suited for binding to their receptors.
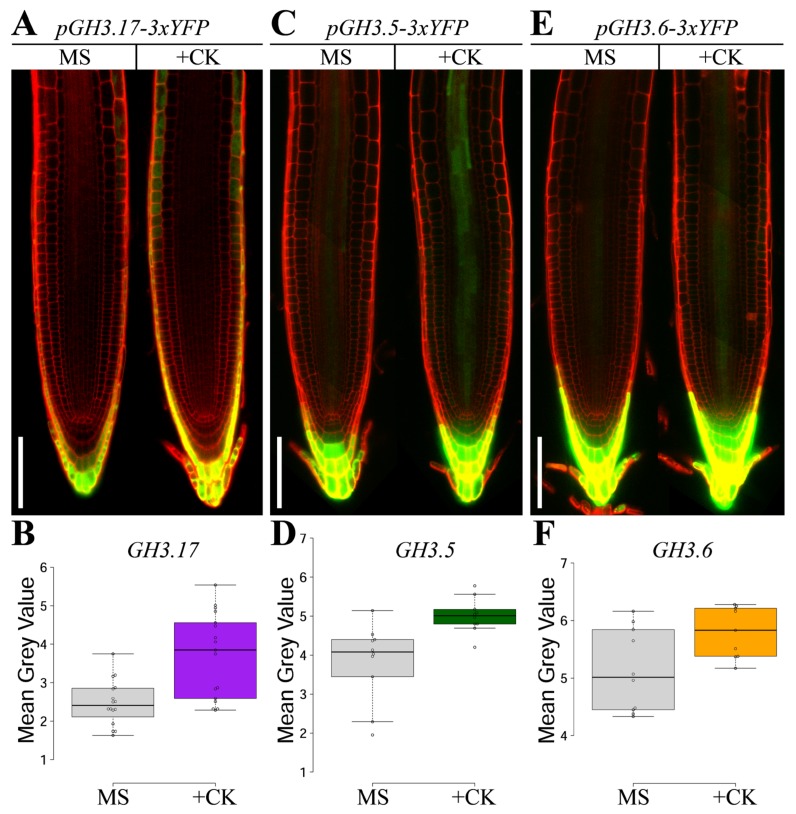
Considering the LRC specific ARR1-dependent positive regulation of GH3.5, GH3.6, and GH3.9, and the LRC specific domain of activity of GH3.17, we thus questioned whether the expression domain of GH3.5, GH3.6, and GH3.9 localizes in the LRC. To this end, we generated GREEN FLUORESCENT PROTEIN (GFP) translational fusions of these three GH3s (pGH3.5::GH3.5-GFP, pGH3.6::GH3.6-GFP, and pGH3.9::GH3.9-GFP lines, respectively). The GFP signal was undetectable for all those lines (data not shown), most likely because of the low expression of those genes in the root as also previously reported [25]. Therefore, we developed transcriptional fluorescent reporters for each of the GH3s using a three-time YELLOW FLUORESCENT PROTEIN (3xYFP) fusion (pGH3.9–3xYFP, pGH3.5–3xYFP, and pGH3.6–3xYFP lines, respectively). Additionally, we also generated a GH3.17 transcriptional fusion line with the same reporter (3xYFP) (pGH3.17–3xYFP line) to verify the overlap of the expression domains of the translational and the transcriptional fusions of GH3.17. The pGH3.17–3xYFP line revealed a localized YFP expression in the more external layer of the LRC and in the differentiated epidermal cells (Figure 1A), resembling that of the pGH3.17:GH3.17-GFP translational fusion [9], and upon cytokinin treatment pGH3.17–3xYFP expression was significantly increased (Figure 1A,B). Although YFP expression was not detectable in pGH3.9–3xYFP line (data not shown), the analysis of pGH3.5–3xYFP and pGH3.6–3xYFP lines revealed, similarly to pGH3.17–3xYFP line, a fluorescent signal in the LRC tissue (Figure 1C,E). Moreover, based on the microarray data [17] and given that cytokinin promotes GH3.17 expression (Figure 1) [9], we verified if the expression of GH3.5 and GH3.6 are responsive to cytokinin analyzing pGH3.5–3xYFP and pGH3.6–3xYFP lines upon cytokinin treatment. The fluorescence signal of pGH3.5–3xYFP and pGH3.6–3xYFP was detected in the youngest cells of the outermost LRC layer, in the columella and in the vascular tissues (Figure 1C,E). After four hours of cytokinin treatment, the fluorescence signal of pGH3.5–3xYFP and pGH3.6–3xYFP lines was significantly increased compared to untreated lines (Figure 1C–F). Furthermore, while GH3.17 expression was localized in the more external tissues of the root (the LRC and the differentiated epidermal cells), GH3.5 and GH3.6 expression was induced also in the vascular tissue (Figure 1C,E). This hinted at the possibility that GH3.5 and GH3.6 are involved in the regulation of auxin levels not only in the LRC, where the regulation of auxin levels affects meristem size but also in the vascular tissue, possibly in coordination with the robust auxin flux active in this tissue.
Figure 1.
Cytokinin induces GH3.5 and GH3.6 expressions. (A,C,E) Confocal images of five days after germination (dag) roots expressing pGH3.17–3xYFP, pGH3.5–3xYFP and pGH3.6–3xYFP constructs untreated (MS) and treated for four hours with 5 μM of cytokinin (+CK) (see Materials and Methods). Scale bar, 100 µm. (B,D,F) Mean Grey Value quantification of pGH3.17–3xYFP, pGH3.5–3xYFP and pGH3.6–3xYFP lines untreated (grey) and treated with cytokinin 5μM for four hours (+CK) (purple-pGH3.17–3xYFP; green-pGH3.5–3xYFP; orange-pGH3.6–3xYFP lines, respectively) at 5 dag where center lines show the medians. Box limits indicate the 25th and 75th percentiles as determined by R software. Whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, data points are plotted as open circles. Statistical significance: (B) Two biological replicates. p-value < 0.005, Student’s t-test, n = 16, 17 sample points, (D) two biological replicates. p-value < 0.05, Student’s t-test, n = 10, 9 sample points, (F) p-value < 0.005, Student’s t-test, n = 10, 10 sample points.
Taken together, these results corroborate the idea that a cytokinin-dependent mechanism regulates the auxin inactivation process by controlling the expression of several members of the GH3 Group II gene family.
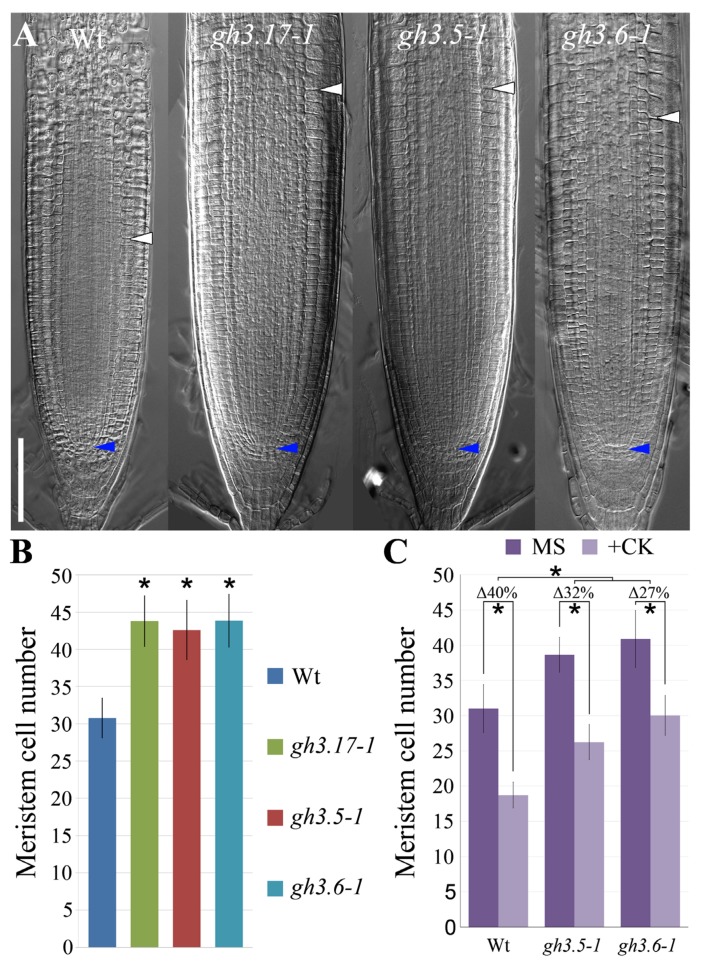
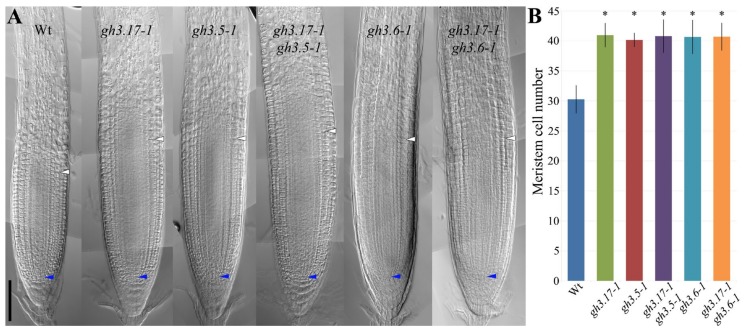
It has been shown that GH3.17 activity in the LRC is necessary and sufficient for the regulation of the meristem size [9,17]. To understand if GH3.5 and GH3.6 are involved in the control of the meristem activity, we analyzed the meristem size of gh3.5–1 and gh3.6–1 loss of function mutants. The meristem size is measured taking into account the number of meristematic cells of the cortex tissue [3]. In a similar way to gh3.17–1 mutant, gh3.5–1 and gh3.6–1 mutants showed increased meristem size when compared to wild type plants (Figure 2A,B). These data indicate that GH3.5 and GH3.6 activities together with GH3.17 are required for meristem size regulation. To unveil if GH3.5 and GH3.6 regulate root meristem size by acting downstream of cytokinin we analyzed the root meristem size of these mutants upon cytokinin treatment. As previously reported, wild type plants treated with cytokinin, show a reduction of the meristem size [7,8], while gh3.17–1 meristem is not affected [9]. Differently from gh3.17–1, gh3.5–1 and gh3.6–1 mutants showed a slight decrease in the number of meristematic cells when comparing the untreated and the cytokinin treated mutant plants (Figure 2C). These data indicate that the root meristem size of those mutants is only partially affected by cytokinin activity, suggesting that cytokinin regulates root meristem size also via GH3.5 and GH3.6. To further investigate the relation between GH3.17, GH3.5, and GH3.6 activities in controlling meristem size, we generated the gh3.17–1;gh3.5–1 and gh3.17–1;gh3.6–1 double mutants. Both the double mutants gh3.17–1;gh3.5–1 and gh3.17–1;gh3.6–1 showed a meristem size similar to that of the parental single mutants gh3.17–1, gh3.5–1 and gh3.6–1 (Figure 3), corroborating the idea that these GH3s act in the same pathway in regulating auxin levels to control meristem size. We, thus, inferred that cytokinin globally promotes the auxin inactivation process triggering GH3.17, GH3.5, and GH3.6 expressions, and as a consequence, determining root meristem size.
Figure 2.
GH3.5 and GH3.6 are involved in the control of root meristem size. (A) Root meristems at 5 dag of Wt, gh3.17–1, gh3.5–1 and gh3.6–1 plants. Blue and white arrowheads indicate the quiescent center (QC) and the cortex transition zone (i.e., meristem size), respectively. Scale bar, 100 µm. (B) Analysis of meristematic cortical cell number of Wt, gh3.17–1, gh3.5–1, and gh3.6–1 plants. Error bars indicate standard deviation (SD). Two biological replicates were performed. Asterisk (*) indicates a significance with a p-value < 0.005, Student’s t-test, n = 18, 15, 16, 17. (C) Analysis of meristematic cortical cell number of Wt, gh3.5–1 and gh3.6–1 untreated (MS) plants and after 22 h of cytokinin treatment (+CK) (see Materials and Methods). “Δ” indicates the relative decrease percentage in the number of meristematic cells of the cortex after cytokinin treatment. Error bars indicate SD. Two biological replicates were performed. * indicates a significance with a p-value < 0.005, Student’s t-test, n(MS) = 14, 14, 18 and n(+CK) = 15, 16, 22.
Figure 3.
GH3.5, GH3.6, and GH3.17 synergistically act in the control of meristem size. (A) Bright field microscopy images of root apical meristems at 5 dag of Wt, gh3.17–1, gh3.5–1, gh3.17–1;gh3.5–1, gh3.6–1 and gh3.17–1;gh3.6–1 plants. Blue and white arrowheads indicate the QC and the cortex transition zone (i.e., meristem size), respectively. Scale bar, 100 µm. (B) Analysis of the number of meristematic cells of the cortex of Wt, gh3.17–1, gh3.5–1, gh3.17–1;gh3.5–1, gh3.6–1 and gh3.17–1;gh3.6–1 plants. Error bars indicate SD. Two biological replicates were performed. * indicates a significance with a p-value < 0.001, Student’s t-test, n = 26, 22, 20, 32, 30, 30.
3. Discussion
In plants, the hormone auxin is distributed as a gradient with morphogenetic properties, similarly to retinoic acid in animals [26,27,28,29]. Indeed, variations in auxin distribution profoundly change cell developmental programs [30]. In the root, an auxin maximum controls stem cell activities [30,31,32] while an auxin minimum establishes the position of the TZ, a cell boundary where stem cell daughters stop to divide and start to differentiate [9]. Indeed, differences in auxin contents between cells of the same tissue are translated into a developmental switch from proliferation to differentiation. The position of the auxin minimum in the root depends on the activity of the GH3.17 enzyme that specifically acts in the LRC tissue [9,17].
Here, we demonstrated that cytokinin supports TZ positioning and, hence, cell differentiation by controlling in the LRC the expression of multiple genes belonging to the GH3 Group II gene family, such as GH3.17, GH3.5, and GH3.6. These GH3s conjugate auxin to different amino acids, thus, adjusting the levels of active auxin within each cell [9,18,19,21,22,23,24].
Cytokinin-dependent control of GH3.17 expression [9] and the simultaneous activation of GH3.5 and GH3.6 gene expression (this work) highlights that auxin inactivation process strongly depends on cytokinin activity in the LRC. Interestingly, it has been already reported that a coordinated GH3.5, GH3.6, and GH3.17 activity is necessary during hypocotyl elongation [33].
Although auxin negatively regulates its own levels by promoting GH3.5 and GH3.6 expression [25,34,35], GH3.17 is not controlled by auxin itself [9]. Thus, GH3.17 cytokinin-dependent control determines a change of auxin levels without suffering from any auxin feedback.
The data collected here show that the specific localized expression of three GH3 Group II genes, regulating auxin inactivation in the LRC tissue, is crucial for meristem activity. Moreover, from these results, the LRC emerges as an important tissue where GH3-dependent auxin conjugation takes place and, hence, the site where the control of auxin levels is finely imposed in the root. Intriguingly, cytokinin-dependent GH3.5 and GH3.6 regulation happens in both LRC and vascular bundle. It will be interesting to know whether GH3.5 and GH3.6 are required in both of those tissues to control root meristem size. Further studies are required to address this crucial point. Nonetheless, the expression domain of the GH3s genes prompts the hypothesis that the control of auxin inactivation has to be confined to specific tissues rather than to the whole root to control root meristem size and, therefore, organ growth.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
The Arabidopsis thaliana ecotypes Columbia-0 (Col-0) was used as a control because the gh3.17–1 [9], gh3.5–1 and gh3.6–1 mutants are in Col-0 background. gh3.5–1 and gh3.6–1 lines were obtained from the NASC collection (SALK_033434C and SALK_082530). Homozygous mutants from the Salk T-DNA were identified by PCR as described (http://signal.salk.edu/tdna primers.html). For growth conditions, Arabidopsis seeds were surface sterilized, and seedlings were grown on one-half strength Murashige and Skoog (MS) medium containing 0.8% agar at 22 °C in long-day conditions (16-h-light/8-h-dark cycle) as previously described [3].
Arabidopsis locus IDs from this article: GH3.17 (AT1G28130), GH3.5 (AT4G27260), GH3.6 (AT5G54510) and GH3.9 (AT2G47750).
4.2. Generation of GH3s Transgenic Plants
Standard molecular biology techniques and the Gateway system (Invitrogen) were used for the cloning procedures. For the pGH3.5::GH3.5-GFP, pGH3.6::GH3.6-GFP, and pGH3.9::GH3.9-GFP transgenic plants, the promoter sequences of GH3.5 (2959 bp), GH3.6 (1993 bp), GH3.9 (2312 bp), and GH3.17 (2128 bp) and genomic sequences of GH3.5 (2189 bp), GH3.6 (2244 bp), and GH3.9 (2668 bp) were amplified from genomic DNA of Arabidopsis thaliana Columbia ecotype using specific primers (pGH3.5 FW 5’-TTTTTCATTGGATGTGAGGAA-3’, pGH3.5 REV 5’-GGTTTAAGAGAAAGAGAGAAGTCTGAG-3’, pGH3.6 FW 5’-AAAACCCATTAACAGCAGACG-3’, pGH3.6 REV 5’-CGTTTAGGTTTTGTGTTTAAAATTC-3’, pGH3.9 FW 5’-TGTCCTTGCAAGTGCAAAAT-3’, pGH3.9 REV 5’-TTCTCAGCTAACCCAAAGAAAG-3’, pGH3.17 FW 5’-GGGCGTTACGTATCAGGAAA-3’, pGH3.17 REV 5’-TGTCTGAAAGCAGACACAAACA-3’, gGH3.5 FW 5’-ATGCCTGAGGCACCAAAGAA-3’, gGH3.5 REV 5’-GTTACTCCCCCACTGTTTGTG-3’, gGH3.6 FW 5’-ATGCCTGAGGCACCAAAG-3’, gGH3.6 REV 5’-GTTACTCCCCCATTGCTTGT-3’, gGH3.9 FW 5’-ATGGATGTAATGAAGCTTGATCA-3’, gGH3.9 REV 5’-TGGAACCCAAGTCGGGTC-3’) and cloned in a pDONOR-P4P1 and pDONOR-221 vectors: pDONOR-P4P1-pGH3.5, pDONOR-P4P1-pGH3.6, pDONOR-P4P1-pGH3.9, and pDONOR-P4P1-pGH3.17, promoter sequences, respectively, pDONOR-221-pGH3.5, pDONOR-221-pGH3.6, and pDONOR-221-pGH3.9, genomic sequences, respectively. The LR reactions were then conducted by using the pDONOR-P4P1-pGH3.5/pGH3.6/pGH3.9, the pDONOR221-gGH3.5/gGH3.6/gGH3.9 and a pDONORP2P3-GFP vectors.
For pGH3.5–3xYFP, pGH3.6–3xYFP, pGH3.9–3xYFP, and pGH3.17–3xYFP transgenic plants, the promoter sequences of GH3.5, GH3.6, GH3.9, and GH3.17 cloned in the pDONOR-P4P1 vectors, as described above, were used. The LR reactions were then conducted by using the pDONOR-P4P1- pGH3.5/pGH3.6/pGH3.9/pGH3.17, a pDONOR221–3xYFP, and pDONORP2P3-NOST2 vectors [36]. The obtained LR products were then sub-cloned in the Gateway pBm43GW destination vector. Plasmids were transformed into Col-0 plants by floral dipping [37]. Each expression domain of the T2 generation of the 3xYFP transcriptional fusion lines was analyzed to verify homogeneous expression. Each transgenic line revealed the same YFP expression pattern.
4.3. Hormonal Treatments
Five days after germination (dag) seedlings were transferred onto solid one-half MS medium containing 0.025% DMSO solvent (mock condition) or onto solid medium containing a final concentration of 5 μM trans-Zeatin (tZ, Duchefa) dissolved in DMSO (0.025% final concentration). A twenty-two-hour hormone treatment was used for meristem size analysis in response to cytokinin and a four-hour hormone treatment was used for GH3s transcriptional reporter lines expression analysis.
4.4. Bright Field and Confocal Microscopy Analysis
Differential interference contrast (DIC) with Nomarski technology microscopy (Zeiss Axio Imager A2 microscope) was used to count meristem cell number with bright field microscopy. Root meristem size of each plant was measured based on the number of cortex cells in a file extending from the quiescent center to the first elongated cortex cell excluded [3]. Plants were mounted in a chloral hydrate solution [3]. Confocal images were obtained using a Zeiss LSM 780 confocal laser scanning microscope. For confocal laser scanning analysis, a propidium iodide 10 μM staining was used. For each experiment, two biological replicates were performed, and the number of samples analyzed were reported in the relative figure legend. Results were comparable in all experiments. The statistical significance was determined by Student’s t-test (http://graphpad.com/quickcalcs/ttest2.cfm), data were reported in the relative figure legend.
4.5. GH3s Reporter Lines Fluorescence Quantification
The fluorescence intensity of pGH3.5–3xYFP, pGH3.6–3xYFP and pGH3.17–3xYFP lines untreated and treated with cytokinin 5 μM for four hours (Figure 1) was quantified as reported in [3]. Mean Grey Value of YFP channel of confocal laser scanning microscope images was measured with the software ImageJ (https://imagej.nih.gov/ij/). Fluorescence signal was measured taking into consideration the same area for untreated and treated lines (length 550 µm × width 187 µm) starting from the tip of the root. Student’s t-test was used to determine the statistical significance (http://graphpad.com/quickcalcs/ttest2.cfm) as reported in the relative figure legend.
4.6. Statistical Analysis Criteria
All the experiments were performed with a number of samples large enough to ensure the statistical significance of the analysis, as reported in corresponding figure legends. Representative sample pictures of the experiments were chosen in all figures.
Author Contributions
Conceptualization, R.D.M; methodology, E.P., S.J.U., E.S., N.S., R.D.I., G.B, and R.D.M.; formal analysis, E.P., S.J.U., E.S., N.S., R.D.I., G.B, and R.D.M.; investigation, E.P., S.J.U., E.S., N.S., R.D.I., G.B, and R.D.M.; data curation, E.P., S.J.U., E.S., N.S., R.D.I., and G.B.; writing – original draft preparation, R.D.M.; writing – review and editing, S.S., R.D.I, and R.D.M.; supervision, R.D.M.; project administration, R.D.M.; funding acquisition, S.S. and R.D.M.
Funding
Postdoc fellowship of the German Academic Exchange Service (DAAD) (to S.J.U.), European Research Council grant 260368 (to S.S.) and MIUR (to S.S. and R.D.M.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Dolan L., Janmaat K., Willemsen V., Linstead P., Poethig S., Roberts K., Scheres B. Cellular organisation of the arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Di Ruocco G., Di Mambro R., Dello Ioio R. Building the differences: A case for the ground tissue patterning in plants. Proc. Biol. Sci. 2018;285 doi: 10.1098/rspb.2018.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Mambro R., Sabatini S. Developmental analysis of arabidopsis root meristem. Methods. Mol. Biol. 2018;1761:33–45. doi: 10.1007/978-1-4939-7747-5_3. [DOI] [PubMed] [Google Scholar]
- 4.Di Mambro R., Sabatini S., Dello Ioio R. Patterning the axes: A lesson from the root. Plants (Basel) 2018;8:8. doi: 10.3390/plants8010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvi E., Di Mambro R., Pacifici E., Dello Ioio R., Costantino P., Moubayidin L., Sabatini S. Scarecrow and shortroot control the auxin/cytokinin balance necessary for embryonic stem cell niche specification. Plant Signal Behav. 2018;13:e1507402. doi: 10.1080/15592324.2018.1507402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacifici E., Di Mambro R., Dello Ioio R., Costantino P., Sabatini S. Acidic cell elongation drives cell differentiation in the arabidopsis root. EMBO J. 2018;37:e99134. doi: 10.15252/embj.201899134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dello Ioio R., Linhares F.S., Scacchi E., Casamitjana-Martinez E., Heidstra R., Costantino P., Sabatini S. Cytokinins determine arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 2007;17:678–682. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Dello Ioio R., Nakamura K., Moubayidin L., Perilli S., Taniguchi M., Morita M.T., Aoyama T., Costantino P., Sabatini S. A genetic framework for the control of cell division and differentiation in the root meristem. Science. 2008;322:1380–1384. doi: 10.1126/science.1164147. [DOI] [PubMed] [Google Scholar]
- 9.Di Mambro R., De Ruvo M., Pacifici E., Salvi E., Sozzani R., Benfey P.N., Busch W., Novak O., Ljung K., Di Paola L., et al. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the arabidopsis root. Proc. Natl. Acad. Sci. USA. 2017;114:E7641–E7649. doi: 10.1073/pnas.1705833114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swarup R., Kramer E.M., Perry P., Knox K., Leyser H.M., Haseloff J., Beemster G.T., Bhalerao R., Bennett M.J. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell. Biol. 2005;7:1057–1065. doi: 10.1038/ncb1316. [DOI] [PubMed] [Google Scholar]
- 11.Bennett T., van den Toorn A., Sanchez-Perez G.F., Campilho A., Willemsen V., Snel B., Scheres B. Sombrero, bearskin1, and bearskin2 regulate root cap maturation in arabidopsis. Plant Cell. 2010;22:640–654. doi: 10.1105/tpc.109.072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xuan W., Audenaert D., Parizot B., Moller B.K., Njo M.F., De Rybel B., De Rop G., Van Isterdael G., Mahonen A.P., Vanneste S., et al. Root cap-derived auxin pre-patterns the longitudinal axis of the arabidopsis root. Curr. Biol. 2015;25:1381–1388. doi: 10.1016/j.cub.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Kanno S., Arrighi J.F., Chiarenza S., Bayle V., Berthome R., Peret B., Javot H., Delannoy E., Marin E., Nakanishi T.M., et al. A novel role for the root cap in phosphate uptake and homeostasis. Elife. 2016;5:e14577. doi: 10.7554/eLife.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xuan W., Band L.R., Kumpf R.P., Van Damme D., Parizot B., De Rop G., Opdenacker D., Moller B.K., Skorzinski N., Njo M.F., et al. Cyclic programmed cell death stimulates hormone signaling and root development in arabidopsis. Science. 2016;351:384–387. doi: 10.1126/science.aad2776. [DOI] [PubMed] [Google Scholar]
- 15.Blancaflor E.B., Fasano J.M., Gilroy S. Laser ablation of root cap cells: Implications for models of graviperception. Adv. Space Res. 1999;24:731–738. doi: 10.1016/S0273-1177(99)00406-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsugeki R., Fedoroff N.V. Genetic ablation of root cap cells in arabidopsis. Proc. Natl. Acad. Sci. USA. 1999;96:12941–12946. doi: 10.1073/pnas.96.22.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Mambro R., Svolacchia N., Dello Ioio R., Pierdonati E., Salvi E., Pedrazzini E., Vitale A., Perilli S., Sozzani R., Benfey P.N., et al. The lateral root cap acts as an auxin sink that controls meristem size. Curr. Biol. 2019;29:1199–1205. doi: 10.1016/j.cub.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa M., Yabe N., Ichikawa T., Yamamoto Y.Y., Yoshizumi T., Hasunuma K., Matsui M. Dfl1, an auxin-responsive gh3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 2001;25:213–221. doi: 10.1046/j.1365-313x.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 19.Staswick P.E., Serban B., Rowe M., Tiryaki I., Maldonado M.T., Maldonado M.C., Suza W. Characterization of an arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen G., Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002;49:373–385. doi: 10.1023/A:1015207114117. [DOI] [PubMed] [Google Scholar]
- 21.Khan S., Stone J.M. Arabidopsis thaliana gh3.9 influences primary root growth. Planta. 2007;226:21–34. doi: 10.1007/s00425-006-0462-2. [DOI] [PubMed] [Google Scholar]
- 22.Westfall C.S., Sherp A.M., Zubieta C., Alvarez S., Schraft E., Marcellin R., Ramirez L., Jez J.M. Arabidopsis thaliana gh3.5 acyl acid amido synthetase mediates metabolic crosstalk in auxin and salicylic acid homeostasis. Proc. Natl. Acad. Sci. USA. 2016;113:13917–13922. doi: 10.1073/pnas.1612635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeClere S., Tellez R., Rampey R.A., Matsuda S.P., Bartel B. Characterization of a family of iaa-amino acid conjugate hydrolases from arabidopsis. J. Biol. Chem. 2002;277:20446–20452. doi: 10.1074/jbc.M111955200. [DOI] [PubMed] [Google Scholar]
- 24.Staswick P.E., Tiryaki I., Rowe M.L. Jasmonate response locus jar1 and several related arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargmann B.O., Vanneste S., Krouk G., Nawy T., Efroni I., Shani E., Choe G., Friml J., Bergmann D.C., Estelle M., et al. A map of cell type-specific auxin responses. Mol. Syst. Biol. 2013;9:688. doi: 10.1038/msb.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimozono S., Iimura T., Kitaguchi T., Higashijima S., Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- 27.Begemann G., Schilling T.F., Rauch G.J., Geisler R., Ingham P.W. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki R., Kuroda K., Ikemoto Y., Ochiai A., Matsumoto A., Kumakiri J., Kitade M., Itakura A., Muter J., Brosens J.J., et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PLoS One. 2017;12:e0173035. doi: 10.1371/journal.pone.0173035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sosnik J., Zheng L., Rackauckas C.V., Digman M., Gratton E., Nie Q., Schilling T.F. Noise modulation in retinoic acid signaling sharpens segmental boundaries of gene expression in the embryonic zebrafish hindbrain. Elife. 2016;5:e14034. doi: 10.7554/eLife.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., Benfey P., Leyser O., Bechtold N., Weisbeek P., et al. An auxin-dependent distal organizer of pattern and polarity in the arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/S0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 31.Grieneisen V.A., Xu J., Maree A.F., Hogeweg P., Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- 32.Petersson S.V., Johansson A.I., Kowalczyk M., Makoveychuk A., Wang J.Y., Moritz T., Grebe M., Benfey P.N., Sandberg G., Ljung K. An auxin gradient and maximum in the arabidopsis root apex shown by high-resolution cell-specific analysis of iaa distribution and synthesis. Plant Cell. 2009;21:1659–1668. doi: 10.1105/tpc.109.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian C.E., Muto H., Higuchi K., Matamura T., Tatematsu K., Koshiba T., Yamamoto K.T. Disruption and overexpression of auxin response factor 8 gene of arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant J. 2004;40:333–343. doi: 10.1111/j.1365-313X.2004.02220.x. [DOI] [PubMed] [Google Scholar]
- 34.Chaiwanon J., Wang Z.Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in arabidopsis roots. Curr. Biol. 2015;25:1031–1042. doi: 10.1016/j.cub.2015.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paponov I.A., Paponov M., Teale W., Menges M., Chakrabortee S., Murray J.A., Palme K. Comprehensive transcriptome analysis of auxin responses in arabidopsis. Mol. Plant. 2008;1:321–337. doi: 10.1093/mp/ssm021. [DOI] [PubMed] [Google Scholar]
- 36.Di Ruocco G., Bertolotti G., Pacifici E., Polverari L., Tsiantis M., Sabatini S., Costantino P., Dello Ioio R. Differential spatial distribution of mir165/6 determines variability in plant root anatomy. Development. 2018;145 doi: 10.1242/dev.153858. [DOI] [PubMed] [Google Scholar]
- 37.Clough S.J., Bent A.F. Floral dip: A simplified method for agrobacterium-mediated transformation of arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]