Abstract
Background
Gastric wall edema has not been reported as a complication of acute pancreatitis in dogs.
Objective
To describe the ultrasonographic features of gastric wall thickening in dogs with acute pancreatitis.
Animals
Fourteen dogs with ultrasonographic evidence and clinical diagnosis of acute pancreatitis, with ultrasonographic evidence of increased gastric wall thickness (>5 mm).
Methods
A retrospective search in the medical records from 2014 to 2016 was performed to identify dogs that had ultrasonographic evidence of acute pancreatitis, that had increased thickness of the gastric wall and that were diagnosed with acute pancreatitis clinically. The gastric wall changes such as thickness, layering appearance, echogenicity, distribution of lesions, and perigastric changes were recorded. Serial ultrasonographic examination and histopathological findings were recorded if available.
Results
Mean gastric wall thickness was 9.9 ± 4.0 mm (SD). A complete loss of wall layering was observed in 2 dogs. Thickening of the submucosal layer was observed in 12 dogs, and 5 of them had concurrent muscularis layer thickening. The echogenicity of thickened submucosal layer was intermediate hyperechoic. Lacy appearances were present within the thickened submucosal layer in 7 dogs and in the muscularis layer of 1 dog. Thickening was focal in 12 dogs and adjacent to the diseased pancreas. Subsequent resolution of gastric wall thickening was observed in 3 dogs (range 3‐28 days) via follow‐up ultrasound. One dog underwent necropsy, and gastric wall edema was confirmed histopathologically.
Conclusions and Clinical Importance
Findings indicated that gastric wall thickening presumably because of edema could be a complication of acute pancreatitis.
Keywords: canine, gastric wall thickening, submucosal edema, ultrasound
Abbreviations
- CT
computed tomography
- GI
gastrointestinal
1. INTRODUCTION
Ultrasonography is an important diagnostic imaging modality in the evaluation of gastrointestinal (GI) tract in dogs.1, 2 Gastric wall neoplasia,3, 4, 5, 6 gastritis7, 8, 9 (including fungal disease10 and ulceration),11 uremic gastropathy,12 polyps/congenital hypertrophy,13, 14 and edema7 are all possible causes for gastric wall thickening.
In humans, gastric wall thickening is commonly seen in computed tomographic (CT) studies, as ultrasound is not as commonly used to examine the GI tract as it is in dogs and cats. Although the causes of gastric wall thickening in humans are similar to the aforementioned causes in dogs, pancreatitis have also been reported to cause gastric wall thickening as seen on CT studies.15
The ultrasonographic appearance of gastric wall thickening because of edema has been described in humans.16, 17 In the veterinary literature, ultrasonographic appearance of presumed gastric wall edema has only been mentioned in a reference textbook as “thickening of gastric wall with alteration of the wall layering.”7 A more precise characteristic ultrasonographic features of gastric wall edema might help differentiate gastric wall edema from a neoplasia.
Ultrasonography of the canine pancreas is an important noninvasive tool in the diagnosis of pancreatitis as well as evaluation of peripancreatic structures. Ultrasonographic appearance of pancreatitis include enlarged, irregularly marginated, and hypoechoic pancreas with peripancreatic hyperechoic mesenteric fat with or without focal peritoneal effusion.18, 19 However, there is lack of description or observation on gastric wall thickening secondary to pancreatitis in dogs. The authors have occasionally observed moderate to severe gastric wall thickening with loss of wall layering in dogs diagnosed clinically with acute pancreatitis, and this gastric wall thickening resolved concomitantly with the clinical improvement or resolution of pancreatitis. The clinical importance of this is unknown. The authors hypothesized that gastric wall thickening because of edema could be present in dogs with acute pancreatitis and would therefore represent 1 of the complications of acute pancreatitis.
The aim of this retrospective study is to describe the ultrasonographic features of presumed gastric wall edema in dogs with acute pancreatitis.
2. MATERIALS AND METHODS
2.1. Experimental design
This is a retrospective case series study.
2.2. Case selection
The Purdue University Veterinary Teaching Hospital Medical Record database from January 1, 2014, through December 31, 2016, was electronically searched to identify dogs that had both ultrasonographic evidence and clinical diagnosis of acute pancreatitis using keyword of “pancreatitis” or “suspected pancreatitis.” Ultrasonographic still images, real‐time recording videos, or both still images and videos of the gastric wall in the form of digital files were reviewed by the radiology resident (M.M.) using an image viewing workstation (Asteris Keystone viewer, Asteris Inc., Stephentown, New York) to identify cases using the following inclusion criteria: (1) ultrasonographic evidence of acute pancreatitis and (2) increased thickness (>5 mm) of the gastric wall. All ultrasonographic studies were performed by board‐certified veterinary radiologists and radiology residents using the same ultrasound machine (Philips iU22 SonoCT system, Phillips Ultrasound, Bothell, Washington). A linear array (5‐12 MHz) or microconvex array (5‐8 MHz) transducer was used. Ultrasonographic evidence of pancreatitis was characterized as the presence of enlarged, irregularly marginated, hypoechoic pancreas and hyperechoic peripancreatic mesenteric fat, as previously described.18 Patients with nodule or mass lesion detected within gastric wall by ultrasound are excluded from the study. Clinical data from medical records of these dogs were then reviewed by a board‐certified veterinary internist (N.K.P.) to confirm the clinical diagnosis of acute pancreatitis. As there is currently no gold standard diagnostic test for pancreatitis in dogs, the clinical diagnosis of acute pancreatitis was determined using a combination of the following findings: clinical signs, physical examination, and clinicopathological data. Dogs were included if 3 or more criteria consistent with acute pancreatitis were met from more than 1 of the categories provided: clinical signs, physical examination, and clinicopathological data. Clinical findings that could support the diagnosis of pancreatitis included, but were not limited to, vomiting, inappetence, and anorexia. Physical examination findings that could support a diagnosis of pancreatitis included abdominal pain, fever, and dehydration. Clinicopathological data supporting the diagnosis of pancreatitis included inflammatory leukogram, elevated serum amylase and lipase concentration, and serum canine pancreatic lipase immunoreactivity concentration greater than 400 μg/L. Dogs were excluded if the serum albumin was less than 1.7 g/dL. Histopathological findings (when available) were recorded.
2.3. Ultrasonographic findings
Ultrasonographic still images, real‐time recording videos, or both still images and videos of the gastric wall of cases that were diagnosed with clinical pancreatitis were further reviewed to characterized features of the presumed gastric wall edema. This is performed by consensus by a board certified radiologist (H.G.H.) and a radiology resident (M.M.) using the same image viewing workstation. Ultrasonographic features of gastric wall such as thickness, appearance of the wall layering (preserved or loss of the 5 layers appearance, layer of gastric wall involvement), echogenicity, echotexture of the abnormal layer (homogeneity and the presence of lacy appearance), vascularity, and distribution of lesions (general versus focal, locations), and perigastric changes were recorded. Overall gastric wall thickness was measured from the echogenic mucosal surface to the outer hyperechoic serosal layer. The individual layer thickness was evaluated between the interfaces of the 2 adjacent layers. When there was loss of the wall layering, it was further characterized into partial or complete. The echogenicity of the abnormal layers were classified into hypoechoic (relative to normal mucosa), intermediate hyperechoic (echogenicity between the normal mucosa and submucosa), and hyperechoic (isoechoic or hyperechoic to the normal submucosa). The abnormal echotexture were further classified as homogeneous or heterogeneous. Lacy appearance of the abnormal gastric wall could be further classified as hypoechoic lacy (hyperechoic background with hypoechoic striations) and hyperechoic lacy (hypoechoic background with hyperechoic striations). The distribution of the abnormal gastric wall thickening such as generalized or focal was noted. If focal, further description of the distribution of lesions such as ventral, dorsal, greater curvature or lesser curvature of the gastric fundus, gastric body, or gastric pylorus was noted. Circumferential thickening was noted if diffuse circumferential wall thickening is observed in 1 of the still image or real‐time recording video. Circumferential wall thickening was not evaluated if dorsal wall is not able to be evaluated because of gastric contents or gas. Presence or absence of perigastric and peripancreatic changes such as hyperechoic peritoneal fat and peritoneal effusion were recorded. Ultrasonographic still images, real‐time recording videos, or both still images and videos of the gastric wall from serial follow‐up ultrasound(s) were also reviewed using the same methods when available.
3. RESULTS
Gastric wall thickening with ultrasonographic evidence of acute pancreatitis was observed in 18 dogs. Among these 18 dogs, clinical diagnosis of acute pancreatitis at the time of ultrasound was confirmed in 14 cases. Two of the cases were excluded from the study because of the presence of severe hypoalbuminemia, characterized as a serum albumin of less than 1.7 g/dL. Two additional cases did not meet the minimum criteria for acute pancreatitis. One dog was diagnosed with metastatic carcinoma and the other dog was diagnosed with diabetic ketoacidosis with no clinical evidence of pancreatitis.
Ultrasonographic real‐time recording videos were available to review in 8 cases and other 6 cases only had ultrasonographic still images of gastric wall thickening.
The mean gastric wall thickness was 9.9 ± 4.0; 5.9‐17.5 (mean ± SD; range) mm.
Complete loss of wall layering within the thickened gastric wall was seen in 2 cases (Figure 1B). Partial loss of wall layering was present in 9 cases, and preserved wall layering was present in 3 cases. Except cases with complete loss of wall layering, well‐defined preserved mucosal layer was observed in the remaining 12 cases (Figure 1C,D).
Figure 1.
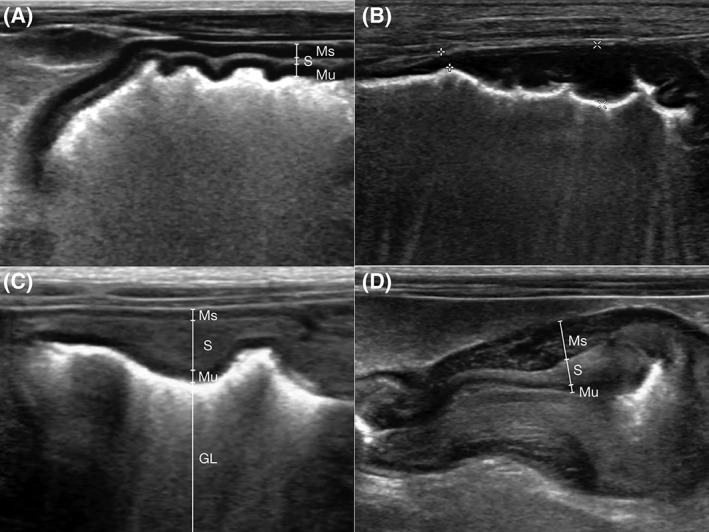
Ultrasonographic appearance of the thickened gastric wall; ventral wall of the gastric body. A, Normal gastric wall without gastric wall thickening. B, Hypoechoic thickening of gastric wall with complete loss of wall layering (between asterics). C, Intermediate hyperechoic thickening of the submucosal layer with preservation of the wall layering. Hyperechoic luminal material is present. D, Nonuniformed thickening of intermediate hyperechoic submucosal layer and hypoechoic muscularis layer. Multiple ill‐defined short linear hyperechoic lacy appearance was present within the thickened muscularis layer. GL, gastric lumen; Ms, muscularis layer; Mu, mucosal layer; S, submucosal layer
For individual gastric layer thickening, submucosal layer is most commonly involved (12/14, Figure 1C,D). In addition to this, 5 dogs had concurrent thickening of muscularis layer (5/14, Figure 1D).
In cases with complete loss of wall layering, the abnormal gastric wall was heterogeneously hypoechoic (Figure 1B). In all cases with thickened submucosal layer, these layers were homogeneously intermediate hyperechoic (Figure 1C,D). The thickened muscularis layer in all 5 cases was homogeneously hypoechoic (Figure 1D).
In the thickened submucosal layer, hyperechoic lacy appearance was present in 4 cases (4/12) and hypoechoic lacy appearance was present in 3 cases (3/12). The remaining 5 cases had no lacy appearance. Hyperechoic lacy appearance was observed in only 1 case in the thickened muscularis layer (1/5, Figure 1D).
Generalized thickening was present in 2 cases (2/14), whereas multifocal thickening was present in 12 cases (12/14). Ventral gastric wall was most commonly involved (13/14), followed by greater curvature of the gastric body to fundus area (12/14) and dorsal gastric wall (11/14). Evaluation of the dorsal wall was not possible in 3 cases because of artifact from the gastric contents (ingesta, gas, or both). Circumferential thickening was present in 6 cases (6/14). The thickness of the gastric wall varies with the thickest wall present adjacent to the abnormal hypoechoic pancreas (9/14), followed by greater curvature of the gastric body (7/14), dorsal gastric wall (5/14), and ventral gastric wall (2/14). Some cases had multifocal areas of gastric wall thickening, and thus the total number of area involved is greater than the number of dogs in this study.
Hyperechoic peritoneal mesentery surrounding the thickened gastric wall was present in all cases (14/14), and peritoneal effusion was present in 13 cases (13/14).
Serial follow‐up ultrasound examinations were performed in 5 cases. Resolution of the gastric wall thickening was seen in 3 cases (3‐28 days after the last ultrasound with gastric wall thickening, Figure 2A,B). Two of these 3 dogs had persistent ultrasonographic features of pancreatitis with resolution of gastric wall thickening (3‐12 days after the last ultrasound with gastric wall thickening). The pancreas and gastric wall were normal in a 28‐days follow‐up ultrasound in the third dog. The other 2 dogs had persistent gastric wall thickening in follow‐up ultrasound (5‐7 days). The owner elected humane euthanasia in 1 dog at the same day the follow‐up ultrasound was performed (Day 5). The other dog had loss to follow‐up 3 weeks after initial ultrasound was performed.
Figure 2.
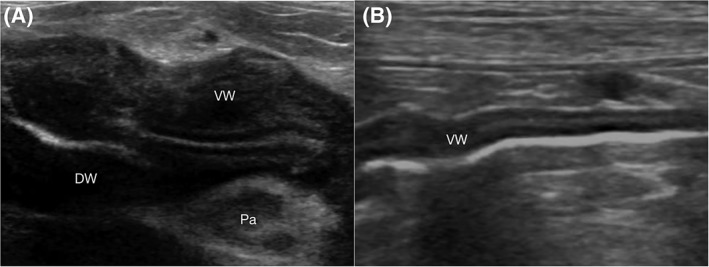
Serial ultrasonographic appearance of the gastric wall in a dog on Day 0 (A) and Day 3 (B) with resolution of gastric wall thickening. A, On Day 0, an intermediate hyperechoic thickening of submucosal layer with partial loss of wall layering between the submucosal and the muscularis layer was present circumferentially. The pancreas (Pa) was diffusely hypoechoic and surrounding fat was hyperechoic. B, Resolution of the gastric wall thickening was observed in Day 3. DW, dorsal wall; VW, ventral wall
Eight of the 14 dogs were alive at the time of discharge from the hospital. Follow‐up phone call survey of those 8 dogs has been performed. One dog was euthanized because of declined health 22 days after discharge, and the remaining 7 dogs recovered from the clinical sign and have lived at least 3 months (3 months, 6 months, 7 months, 19 months, more than 22 months, 24 months, and more than 4 years) after discharge. Two dogs with resolution of the gastric wall thickening by the follow‐up ultrasound have lived more than 22 months after discharge.
The remaining 6 dogs died during hospitalization or the owners elected humane euthanasia. A necropsy was performed on 1 of the 6 dogs, and histopathologic evaluation was performed. The histopathology revealed severe subacute necrotizing pancreatitis and gastric wall edema with mild congestion (Figure 3A,B).
Figure 3.
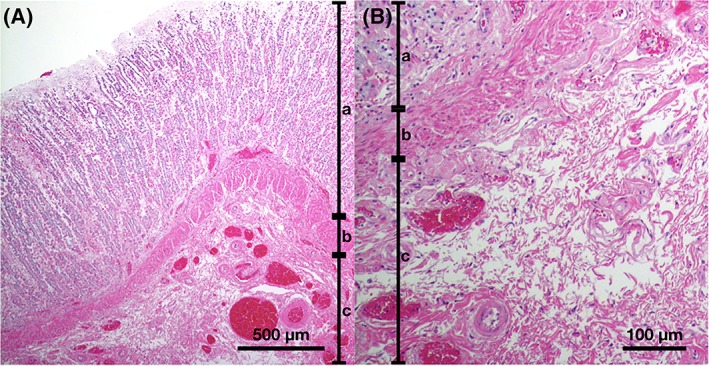
Histopathological appearance of the thickened gastric wall of a dog with low magnification image (A) and close‐up image of submucosal layer (B). There is a diffuse extensive edema in the submucosal layer separating collagen fiber. Hematoxylin and eosin. a, mucosa; b, muscularis mucosa; c, submucosa
4. DISCUSSION
This study describes the ultrasonographic features of presumed gastric wall edema in 14 dogs with acute pancreatitis.
In this present study, all cases with preserved wall layering showed submucosal layer thickening (12/12 cases). Major differential diagnoses for the ultrasonographic submucosal thickening in the GI abnormalities are submucosal edema and hemorrhage.20 Submucosal hemorrhage could take more than 1 week to resolve completely.21 Thus, the rapid resolution and changes of gastric wall thickening, in addition to the histological confirmation in 1 case in our study, support our hypothesis that gastric wall thickening was because of edema. The low number of histological sample is because of relatively good prognosis of the treatment for pancreatitis and owner refused necropsy in the deceased cases. Histopathology would be of great benefit to rule out other causes of gastric wall thickening; however, the rapid changes of gastric wall edema secondary to pancreatitis could be missed because of the lag time between diagnosis and time of death.
Other major differential for gastric wall thickening in dogs includes neoplasia and gastritis. Gastric wall thickening caused by neoplasia has a mean thickness of 16.7 to 43.0 mm.3, 5 In the present study, the thickening of gastric wall was less severe (mean thickness of 9.9 mm; range of 5.9‐17.5 mm). However, there is overlapping of the thickness in these 2 studies, making diagnosis based solely on wall thickness challenging.
Loss or disruption of normal wall layering is 1 of the common ultrasonographic findings in gastric neoplasia, and reflects infiltration and disruption of tissue architecture by the neoplasia.3, 4 In this present study, we found at least partial loss of gastric wall layering with preserved well‐defined mucosal layer in 12 out of 14 cases. The finding of a well‐defined mucosal layer in conjunction with partial disruption of gastric wall layering might differentiate gastric wall edema from the gastric neoplasia though more research needs to occur to determine the validity of this observation.
Complete loss of the wall layering was uncommon in this present study (2/14 cases). This complete loss of wall layering might reflect a more severe state of gastric wall edema.
Lymphedema is the most common cause of submucosal edema of the GI tract caused by obstruction of lymphatic vessels or increased microvascular and lymphatic permeability.22 Increased microvascular and lymphatic permeability has been postulated to be 1 of the causes of pancreatic edema and given the close proximity of the extra‐gastric and pancreatic lymphatic vessels, direct extension of the inflammatory process might result in gastric wall submucosal edema.23, 24 The muscularis layer can become edematous with lacy dissociation of muscularis caused by severe inflammation.25 Concurrent muscularis layer thickening was present in this present study, and could be because of extension of inflammation from the pancreatitis reflecting more severe state of the disease process. As observed in the present study, gastric wall thickening associated with acute pancreatitis has predominantly submucosal or both submucosal and muscularis layer thickening.
Possible differential for gastric wall thickening in the dogs with pancreatitis other than edema include gastritis and hypoalbuminemia. All 14 cases in the present study did not show hypoalbuminemia because cases with hypoalbuminemia had been excluded from the study population.
Gastritis is a common cause of gastric wall edema and can lead to gastric wall thickening with preserved normal wall layering.7, 22 Chronic hypertrophic gastritis and polyps also have mucosal layer thickening although the thickening is predominantly because of mucosal hypertrophy and not edema.8, 9, 13 The normal mucosal layer with abnormal thickness of submucosal layer might help differentiating gastric wall edema because of acute pancreatitis from gastric wall edema because of gastritis. The rapid resolution of gastric wall thickening observed in follow‐up examination could help differentiate gastric wall edema from gastric wall neoplasia and gastritis as observed in this study.
The gastric wall thickening could be generalized, or multifocal. The high percentage of the thickest gastric wall thickening adjacent to the pancreas could be because of close proximity of the abnormal pancreas, thus the severity of inflammation might be marked at this point.
In this present study, acute pancreatitis associated with hazy, hyperechoic peritoneal fat, and peritoneal effusion was present in all cases with gastric wall thickening. Because of the small number of cases that had serial follow‐up ultrasounds performed, correlation between severity of acute pancreatitis and time of resolution of gastric wall thickening could not be established. Further research is needed to study the correlation of the time of resolution of gastric wall thickening with clinical severity of acute pancreatitis. The duration of resolution of the gastric wall thickening because of pancreatitis has not been reported in humans because serial follow‐up CTs are not commonly performed in dogs with pancreatitis.
Six out of 14 dogs had serial ultrasound. One dog only showed gastric wall thickening 3 days after the initial ultrasonographic detection of pancreatitis. Thus, gastric wall thickening could be a common complication of the acute pancreatitis in dogs but was underdiagnosed because of lack of serial ultrasound. The time of occurrence of gastric wall thickening in relation to the time of the clinical presentation of pancreatitis and detection of ultrasonographic evidence of pancreatitis is unknown.
In conclusion, the ultrasonographic gastric wall thickening in dogs with clinical acute pancreatitis is presumably because of gastric wall submucosal edema with or without involvement of the muscularis layer, and with preservation of the mucosal layer. Resolution of gastric wall thickening can be observed with positive response to treatment of acute pancreatitis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
This paper was presented as an oral presentation at the 2017 American College of Veterinary Radiology annual scientific conference in Phoenix, Arizona.
Murakami M, Heng HG, Lim CK, et al. Ultrasonographic features of presumed gastric wall edema in 14 dogs with pancreatitis. J Vet Intern Med. 2019;33:1260–1265. 10.1111/jvim.15507
Present address Nicholas J. Rancilio, Department of Clinical Sciences, College of Veterinary Medicine, Auburn University, Auburn, AL 36849.
REFERENCES
- 1. Penninck DG, Nyland TG, Kerr LY, Fisher PE. Ultrasonographic evaluation of gastrointestinal diseases in small animals. Vet Radiol Ultrasound. 1990;31:134‐141. [Google Scholar]
- 2. Larson MM, Biller DS. Ultrasound of the gastrointestinal tract. Vet Clin North Am Small Anim Pract. 2009;39:747‐759. [DOI] [PubMed] [Google Scholar]
- 3. Kaser‐Hotz B, Hauser B, Arnold P. Ultrasonographic findings in canine gastric neoplasia in 13 patients. Vet Radiol Ultrasound. 1996;37:51‐56. [Google Scholar]
- 4. Rivers B, Walter P, Johnston G, Feeney DA, Hardy RM. Canine gastric neoplasia: utility of ultrasonography in diagnosis. J Am Anim Hosp Assoc. 1997;33:144‐155. [DOI] [PubMed] [Google Scholar]
- 5. Lamb CR, Grierson J. Ultrasonographic appearance of primary gastric neoplasia in 21 dogs. J Small Anim Pract. 1999;40:211‐215. [DOI] [PubMed] [Google Scholar]
- 6. Beck C, O'Neill T, Holloway SA, et al. The use of ultrasound in the investigation of gastric carcinoma in a dog. Aust Vet J. 2001;79:332‐334. [DOI] [PubMed] [Google Scholar]
- 7. Penninck D, d'Anjou M‐A. Atlas of Small Animal Ultrasonography. 2nd ed. Ames, IA: John Wiley & Sons Inc; 2015:xi, 571. [Google Scholar]
- 8. Rallis TS, Patsikas MN, Mylonakis ME, et al. Giant hypertrophic gastritis (Ménétrier's‐like disease) in an Old English Sheepdog. J Am Anim Hosp Assoc. 2007;43:122‐127. [DOI] [PubMed] [Google Scholar]
- 9. Zerbe CA, Boosinger TR, Grabau JH, Pletcher JM, O'Dorisio TM. Pancreatic polypeptide and insulin‐secreting tumor in a dog with duodenal ulcers and hypertrophic gastritis. J Vet Intern Med. 1989;3:178‐182. [DOI] [PubMed] [Google Scholar]
- 10. Graham JP, Newell SM, Roberts GD, Lester NV. Ultrasonographic features of canine gastrointestinal pythiosis. Vet Radiol Ultrasound. 2000;41:273‐277. [DOI] [PubMed] [Google Scholar]
- 11. Pennick D, Matz M, Tidwell A. Ultrasonography of gastric ulceration in the dog. Vet Radiol Ultrasound. 1997;38:308‐312. [DOI] [PubMed] [Google Scholar]
- 12. Grooters AM, Miyabayashi T, Biller DS, Merryman J. Sonographic appearance of uremic gastropathy in four dogs. Vet Radiol Ultrasound. 1994;35:35‐40. [Google Scholar]
- 13. Diana A, Penninck DG, Keating JH. Ultrasonographic appearance of canine gastric polyps. Vet Radiol Ultrasound. 2009;50:201‐204. [DOI] [PubMed] [Google Scholar]
- 14. Kuan S, Hoffmann K, Tisdall P. Ultrasonographic and surgical findings of a gastric hyperplastic polyp resulting in pyloric obstruction in an 11‐week‐old French Bulldog. Aust Vet J. 2009;87:253‐255. [DOI] [PubMed] [Google Scholar]
- 15. Brown BM, Federle MP, Jeffrey RB. Gastric wall thickening and extragastric inflammatory processes: a retrospective CT study. J Comput Assist Tomogr. 1982;6:762‐765. [DOI] [PubMed] [Google Scholar]
- 16. Tomooka Y, Onitsuka H, Goya T, et al. Ultrasonography of benign gastric ulcers. Characteristic features and sequential follow‐ups. J Ultrasound Med. 1989;8:513‐517. [DOI] [PubMed] [Google Scholar]
- 17. Kimmey MB, Martin RW, Haggitt RC, Wang KY, Franklin DW, Silverstein FE. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology. 1989;96:433‐441. [DOI] [PubMed] [Google Scholar]
- 18. Larson MM. Ultrasound imaging of the hepatobiliary system and pancreas. Vet Clin North Am Small Anim Pract. 2016;46:453‐480. v‐vi. [DOI] [PubMed] [Google Scholar]
- 19. Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56:13‐26. [DOI] [PubMed] [Google Scholar]
- 20. Frisoli JK, Desser TS, Jeffrey RB. Thickened submucosal layer: a sonographic sign of acute gastrointestinal abnormality representing submucosal edema or hemorrhage. 2000 ARRS Executive Council Award II. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;175:1595‐1599. [DOI] [PubMed] [Google Scholar]
- 21. Graham DY, Lacey Smith J, Dobbs SM. Gastric adaptation occurs with aspirin administration in man. Dig Dis Sci. 1983;28:1‐6. [DOI] [PubMed] [Google Scholar]
- 22. Granger DN, Barrowman JA. Microcirculation of the alimentary tract. II. Pathophysiology of edema. Gastroenterology. 1983;84:1035‐1049. [PubMed] [Google Scholar]
- 23. Owen DA. Normal histology of the stomach. Am J Surg Pathol. 1986;10:48‐61. [DOI] [PubMed] [Google Scholar]
- 24. Evans HE, Miller ME. Miller's Anatomy of the Dog. 4th ed. St. Louis, MI: Elsevier; 2013:xix, 850. [Google Scholar]
- 25. Jaballah S, Sabri Y, Yacoubi MT, Mustapha K. Phlegmonous gastritis complicated by upper digestive hemorrhage. Dig Dis Sci. 1999;44:2435‐2438. [DOI] [PubMed] [Google Scholar]


