Abstract
Background
Megaesophagus (ME) carries a poor long‐term prognosis in dogs. In people, lower esophageal sphincter (LES) achalasia is a rare cause of ME that may respond to targeted intervention. Dogs with lower esophageal sphincter achalasia‐like syndrome (LES‐AS) have been described recently, warranting investigation of analogous targeted treatment.
Hypothesis/Objectives
Evaluate response of dogs with LES‐AS to LES mechanical dilation and botulinum toxin A (BTA) injections, with or without surgical myotomy and fundoplication. We hypothesized that clinical and videofluoroscopic swallow study (VFSS) features of LES‐AS would improve after treatment targeting functional LES obstruction.
Animals
Fourteen client‐owned dogs with LES‐AS diagnosed by VFSS.
Methods
Retrospective study. Dogs diagnosed with LES‐AS underwent treatment between April 2015 and December 2017. Outcome measures included client perception of clinical severity, body weight (BW), body condition score (BCS), regurgitation frequency, and VFSS parameters (ME, esophageal motility, gastric filling). Dogs with positive responses were considered candidates for LES myotomy with fundoplication.
Results
By a median IQR of 21 (IQR, 14‐25) days after mechanical dilation and BTA, clients reported clinical improvement in 100% of dogs, BW increased 20.4% (IQR, 12.7%‐25%), pre‐ and post‐treatment BCS was 3 (IQR, 3‐4) and 5 (IQR, 4‐5), respectively, and frequency of regurgitation decreased by 80% (IQR, 50%‐85%). Duration of effect was 40 (IQR, 17‐53) days. Despite clinical improvement, ME and abnormal esophageal motility persisted in 14 dogs. Six dogs subsequently underwent myotomy and fundoplication and maintained improvement observed after mechanical dilation and BTA.
Conclusions and Clinical Importance
Dogs with LES‐AS experienced significant, temporary, clinical improvement after mechanical dilation and BTA. Preliminary results suggest myotomy with fundoplication provide lasting clinical benefit despite persistence of ME.
Keywords: balloon dilation, botox, Dor fundoplication, Heller myotomy, megaesophagus, videofluoroscopic swallow study
Abbreviations
- BCS
body condition score
- BTA
botulinum toxin A
- BW
body weight
- HRM
high‐resolution manometry
- IQR
interquartile range
- LES
lower esophageal sphincter
- LES‐AS
LES achalasia‐like syndrome
- ME
megaesophagus
- MU‐VHC
University of Missouri Veterinary Health Center
- SNAP
synaptosomal‐associated protein
- VFSS
videofluoroscopic swallow study
1. INTRODUCTION
Megaesophagus (ME) in dogs is a motility disorder of the esophagus that carries a poor long‐term prognosis with death frequently reported secondary to respiratory complications, malnutrition or both, or euthanasia because of poor quality of life.1, 2, 3 For dogs with idiopathic ME, interventions are limited in part because of a lack of understanding of the underlying disease mechanisms. Recently, with a free‐feeding videofluoroscopic swallow study (VFSS) protocol,4 we identified a subpopulation of dogs with functional obstruction of the lower esophageal sphincter (LES) analogous to LES achalasia in people.5 In people, LES achalasia is diagnosed by high‐resolution manometry (HRM) and is characterized by a failure of the LES to relax in response to pharyngeal swallowing.6 This functional obstruction results in esophageal dilatation, retention of ingesta and oral secretions in the esophagus, loss of esophageal motility, and associated clinical signs of esophageal dysphagia.6, 7, 8 Like achalasia in people, dogs with LES‐AS lacked LES relaxation in response to pharyngeal swallow on VFSS.5 Identifying LES achalasia in dogs is critically important because, in people, it may respond to treatment targeting the LES and esophageal outflow obstruction.6, 9, 10, 11, 12, 13 The analogous functional LES obstruction in dogs, LES achalasia‐like syndrome (LES‐AS), likewise may represent a condition responsive to targeted intervention, although therapeutic studies in this population are critically lacking.
In people, achalasia is subcategorized into a spectrum of different disease phenotypes by HRM. Unfortunately, routine use of HRM in veterinary patients is impractical because of high cost, limited availability, poor patient compliance, and the need for substantial operator expertise.14, 15 These limitations led to VFSS being successfully evaluated as a surrogate for the diagnosis of functional LES obstruction in dogs.5 In people, regardless of clinical phenotype or method of diagnosis, the core treatment objective is to relieve the esophageal obstruction by mechanical disruption of the LES or lowering LES tone. In people, positive clinical responses are seen after mechanical dilation (pneumatic dilation or bougienage), botulinum toxin A (BTA) injections, LES myotomy with fundoplication, or some combination of these.6, 9, 10, 11, 12, 13 Given the positive response to targeted treatment in people with LES achalasia, our objective was to evaluate the response of dogs with LES‐AS to targeted intervention with mechanical dilation and LES BTA injections, with or without surgical myotomy with fundoplication. We hypothesized that clinical and VFSS features of LES‐AS would improve after treatment targeting the LES and esophageal outflow obstruction.
2. MATERIALS AND METHODS
2.1. Case selection and criteria
Dogs presented to the University of Missouri Veterinary Health\Center (MU‐VHC) between April 2015 and December 2017 had medical records retrospectively reviewed. Dogs were included if they had complete medical records, were diagnosed with LES‐AS by free‐feeding VFSS, and underwent targeted treatment by mechanical LES dilation (pneumatic dilation or bougienage), LES BTA injections, with or without LES myotomy with fundoplication. Dogs receiving prokinetics or opioids, those with a secondary form of ME, and those with focal ME or evidence of mechanical obstruction (eg, pseudoachalasia, stricture, hiatal hernia) at the time of diagnosis were excluded. For most dogs, additional testing for relevant endocrinopathies (hypothyroidism, hypoadrenocortisolism) and myasthenia gravis was performed at the discretion of the attending clinician, based on supporting clinical evidence. Dogs meeting inclusion criteria were evaluated for a number of clinical and VFSS outcome variables to determine the response to treatment (Table 1). The VFSS features at the time of diagnosis were used to compare post‐treatment VFSS outcomes for each dog. Dogs with positive response to LES mechanical dilation with BTA injections were considered candidates for and offered the option of surgical intervention (LES myotomy with fundoplication) as a longer term treatment.
Table 1.
Clinical and VFSS outcome parameters for dogs having undergone mechanical dilation and BTA injections for LES‐AS
| Clinical outcome parameters | VFSS outcome parameters |
|---|---|
| Overall client perception of clinical improvement | Presence of ME (yes or no) |
| Body weight | Esophageal motility
|
| Body condition score (9‐point scale) | Volume of gastric filling
|
| Frequency of regurgitation | |
| Duration of clinical improvement | |
| Complications |
Abbreviations: BTA, botulinum toxin A; LES‐AS, lower esophageal sphincter achalasia‐like syndrome; ME, megaesophagus; VFSS, videofluoroscopic swallow study.
2.2. Data extracted from the medical record
Demographic data, clinical features, prior medical management for ME, duration of clinical signs, clinical perception of post‐treatment disease control (positive or negative treatment response), body weight (BW), body condition score (BCS), frequency of regurgitation, and complications after treatment were retrieved from the medical record or from follow‐up calls to clients. Body condition score was evaluated according to the American Animal Hospital Association guidelines. Clients were requested to quantify frequency of regurgitation (episodes per day) before treatment as part of pretreatment evaluation and after treatment as part of follow‐up evaluation. No changes were made to the patient's pretreatment regimen after targeted intervention to avoid confounding treatment effects.
2.3. Videofluoroscopic swallow studies
Videofluoroscopic swallow studies were performed both to confirm diagnosis of LES‐AS and 2‐3 weeks post‐mechanical dilation and BTA injection as previously described.4 Briefly, after being fasted for 12 hours, dogs were placed in 1 of 4 kennels selected according to patient body size. The polycarbonate kennels are designed to accommodate small or toy (≤35 lbs), medium (>35 lbs to ≤65 lbs), large (>65 lbs to ≤85 lbs), and giant breed (≥85 lbs) dogs. These kennels were designed to permit unrestrained free‐feeding behavior, maximize the ease of patient visualization, and perform contrast video fluoroscopy. Dogs were fed 3 standardized food consistencies containing a contrast agent: puree (25% iohexol [350 mg/mL]), liquid (25% iohexol [350 mg/mL]), and kibble (barium 40% wt/vol). Studies were performed at 30 frames per second using a GE Advantx or GE OEC 9900 Elite Mobile C‐Arm system (GE Healthcare, Chicago, Illinois) at the MU‐VHC. The VFSS videos were evaluated by a panel of trained reviewers including 2 board certified internal medicine specialists (M. Grobman, C. Reinero), a PhD and board‐certified speech‐language pathologist specializing in translational deglutology (T. E. Lever), and a senior radiology resident (James Schachtel). The VFSS was considered diagnostic for LES‐AS if a lack of LES relaxation was observed in response to pharyngeal swallowing. The LES was actively challenged (contrast abutting the LES) during active swallowing with the dog in a sitting or standing position or both to mitigate the effect of esophageal weakness on the passage of contrast through the LES. Evaluation also was performed when the dog was not actively swallowing to assess bolus passage secondary to hydrostatic pressure. Some dogs sat during active swallows to add additional challenge to the LES. The VFSS parameters used for pre‐ and post‐treatment comparisons were selected based on studies in humans showing improved gastric filling, improved ME, and improved esophageal motility after treatment for achalasia.9, 16, 17 The VFSS outcome parameters are provided in Table 1.
Megaesophagus: Dogs were assessed pre‐ and post‐ treatment for subjective changes in esophageal diameter.
- Esophageal motility and peristalsis (contraction and propulsion): The esophagus was assessed for the presence or absence of the following clinical features:
- Primary peristalsis, defined as a wave of bolus movement beginning in the proximal esophagus, initiated by a pharyngeal swallow.
- Secondary peristalsis, defined as a wave initiated by esophageal distention, evaluated when the dog was not actively eating or drinking to avoid confounding by concurrent primary peristalsis and clearance initiated by a subsequent food bolus.
- Esophageal contraction, referred to as the inward movement of the dorsal and ventral esophageal walls. Dogs without VFSS evidence of contraction were referred to as “acontractile.”
- Propulsion, referred to as the ability of either primary or secondary peristaltic activity to conduct a food bolus aborally toward the LES.
- Amotile: Referred to dogs without evidence of primary or secondary peristalsis.
- Hypomotile: Referred to dogs with evidence of primary, secondary peristaltic waves, or both that were unable to conduct a food bolus aborally toward the LES.
- Hypermotile: Referred to spastic or excessive esophageal motility.
- Spasticity: Transient segmental decrease in the esophageal diameter, proximal to the LES and resulting in a narrowed contrast column.
- Excessive motility: Robust contraction against a closed LES, during or between pharyngeal swallows.
- Normal motility: Referred to normal primary and secondary peristalsis that transferred swallowed boluses unimpeded to the LES.
- Gastric filling: Passage of ingesta into the stomach in response to pharyngeal swallowing or hydrostatic pressure (sitting or standing). If residual food or contrast remained in the esophagus, dogs were held upright for 5 minutes to increase hydrostatic pressure and facilitate emptying into the stomach.
- The extent of gastric filling was evaluated before and after treatment and graded as small (<25%), medium (25‐75%), or large (>75%).
2.4. Targeted intervention for LES‐AS
All procedures requiring general anesthesia (endoscopy, mechanical disruption of the LES [pneumatic dilation and bougienage], BTA injections of the LES, and LES surgery) were performed for a minimum of 12 hours after VFSS. Anesthetic protocols and monitoring were performed under the direction and supervision of a board‐certified anesthesiologist.
2.5. Endoscopy
Esophagoscopy and abbreviated gastroscopy were performed with a Fujinon EG‐450HR, 10.7 mm gastroscope (Fujifilm, Wayne, New Jersey). Endoscopy was performed before mechanical dilation and BTA injections to evaluate for evidence of an occult mechanical obstruction of the LES. Esophagoscopy included evaluation of the esophageal body for wall defects, mucosal changes, and residual food or fluid. The LES and cardia were assessed for a distal esophageal stricture and to determine the ease of passage of the endoscope through the LES. Strong resistance to passage of the endoscope was considered suspicious for mechanical obstruction of the LES (pseudoachalasia).18 A “J maneuver” was performed to evaluate for pseudoachalasia capable of causing esophageal outflow obstruction. Because a diagnosis of LES‐AS was made based on a failure of LES relaxation in response to pharyngeal swallowing, an open LES observed under anesthesia was not considered to contradict a VFSS diagnosis of LES‐AS nor was it a contraindication to targeted treatment. Fluid and food were suctioned from the esophagus to permit visualization before mechanical LES dilation and BTA injections.
2.6. Mechanical dilation
Mechanical dilation was performed either by pneumatic dilation (CRE Pro Wireguarded Balloon Dilation Catheter; Boston Scientific, Marlborough, Massachusetts) or bougienage. Balloon diameter, ranging from 1 to 3 cm, was subjectively adjusted according to patient size to prevent overdistension and perforation. Under endoscopic guidance, the balloon was passed through the LES19 making sure to span the entire length, inflated, and then held in place for 90 seconds. This process was repeated 2‐3 times. Blanching of the mucosa at the LES was observed through the transparent balloon (Figure 1). The endoscope did not simultaneously span the LES during deployment of the balloon to allow uniform radial force to be applied to the LES. In 1 dog, after the only appropriately sized available balloon was determined to be damaged, rubber bougies (40‐50 French) were inserted sequentially through the LES and each held in place for 90 seconds. This process was repeated twice. Confirmation of bougie placement and mucosal blanching were performed as for pneumatic dilation.
Figure 1.
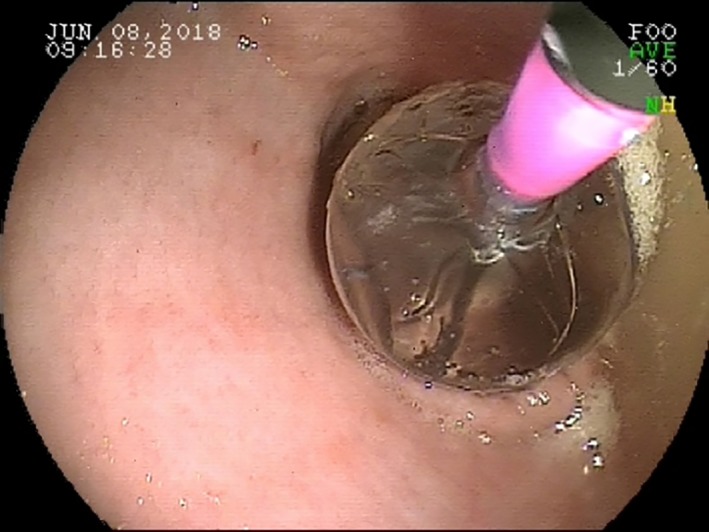
Balloon spanning the lower esophageal sphincter (LES) of a dog diagnosed with LES achalasia‐like syndrome. It is necessary for the balloon to span the entire LES for adequate dilation20
2.7. BTA injection
After mechanical dilation, BTA (Botox [onabotulinumtoxinA]; Allergan, Madison, New Jersey) diluted to 40 U/mL in 0.9% sterile saline was administered using an endoscopic injection needle (Interject Sclerotherapy Needle; Boston Scientific) at 8 sites around the LES (4 U/site) (Figure 2A, B). The first 4 injections (Set 1) were made circumferentially at 90° intervals at the esophagogastric junction. The second 4 injections (Set 2) were made 1 cm distal to Set 1, also circumferentially at 90° intervals. Set 2 was rotated 45° from Set 1, as shown in Figure 2B. A small bleb was visible after each injection with no visually detectable losses.
Figure 2.
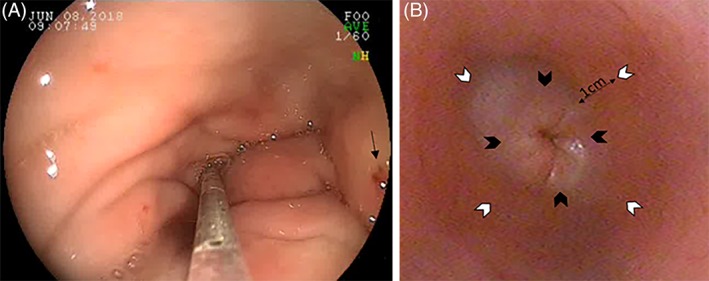
A, Botulinum toxin A (40 U/mL) is injected into 8 sites (4 U/site) circumferentially around the lower esophageal sphincter (LES) using an endoscopic injection needle. A small bleb should be visible after injection (black arrow). B, Four injections (Set 1, black arrowheads) are placed at 90° immediately adjacent to the LES. The remaining 4 sites (Set 2, white arrowheads) should be placed at 90° 1 cm distal to Set 1. Set 2 should be rotated 45° relative to Set 1
2.8. Surgical LES myotomy with fundoplication
Surgical myotomy of the LES (Heller procedure) was performed followed by Dor fundoplication as previously reported in the human surgical literature.21 A standard ventral midline celiotomy was performed, and the LES was isolated from its attachments within the crus of the diaphragm. The definitive location of the LES was determined by intraoperative endoscopy (5 mm × 65 mm Storz Bidirectional Intubation Fiberscope, Tuttlingen, Germany) and marked with monopolar electrosurgery on the serosa of the stomach at the esophagogastric junction. A full‐thickness myotomy of the LES was performed extending 3 cm orad in the esophagus and 3 cm aborad in the stomach. Complete myotomy of the LES was confirmed by retromucosal illumination by intraoperative endoscopy to assess for residual muscle fibers overlying the submucosa. After completion of the myotomy, a Dor fundoplication was performed using polypropylene suture. The right and left crura of the diaphragm were apposed and residual air within the thoracic cavity was removed by suction. Finally, adequate patency of the LES was confirmed by endoscopic visualization before closure. A gastric tube was placed to facilitate feeding as needed during recovery. Postoperative analgesic protocols were carried out at the discretion of the attending clinician. All dogs were treated postoperatively with omeprazole (1 mg/kg PO q12h) for 10‐14 days.
2.9. Statistics
Statistical analysis was performed using SigmaPlot data analysis software (version 14.0). Descriptive statistics were performed where appropriate. Nonparametric analysis was performed because of the small sample size. Wilcoxon Signed Rank tests were performed on pre‐ and post‐treatment variables of BW, BCS, and frequency of regurgitation. Pretreatment data was collected from dogs with LES‐AS at the time of diagnosis. Post‐treatment data was collected at the time of the first evaluation after mechanical dilation and BTA injections. Data are presented as median and interquartile range (IQR). A P value of ≤0.05 was considered significant.
3. RESULTS
3.1. Animals
One‐hundred and thirty VFSS were performed at the MU‐VHC between April 2015 and December 2017 (Figure 3). Nineteen dogs were diagnosed with LES‐AS based on VFSS criteria, and 14 of the 19 dogs met inclusion criteria for the study. Ages ranged from 5 weeks to 12 years with a median (IQR) age of 2.5 years (0.9‐5.8 years). Five dogs were spayed females, 3 were intact females, 2 were castrated males, and 4 were intact males. Breeds represented included mixed breeds (n = 3), Australian Shepherd (n = 2), Chihuahua (n = 1), Golden Retriever (n = 1), Miniature Schnauzer (n = 1), Miniature Dachshund (n = 1), Doberman Pinscher (n = 1), German Shepherd (n = 1), Irish Wolfhound (n = 1), German Shorthair Pointer (n = 1), and English Cocker Spaniel (n = 1).
Figure 3.
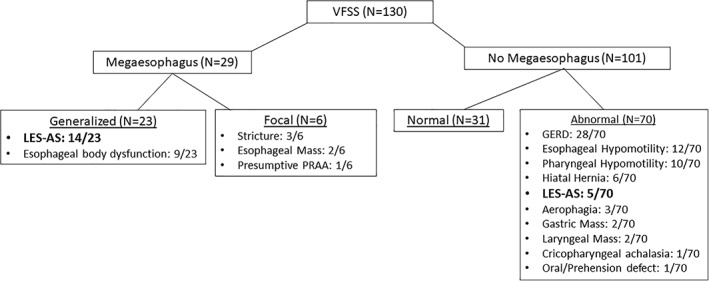
Final videofluoroscopic swallow study (VFSS) diagnosis for all patients evaluated by VFSS at the University of Missouri VHC between April 2015 and December 2017
Presenting complaints included regurgitation (n = 11), regurgitation and cough (n = 2), and cough alone (n = 1). The duration of clinical signs before presentation ranged from 5 weeks to 4 years with a median (IQR) of 8 months (4‐18 months). Twelve of 14 dogs had ≥2 weeks of medical management for regurgitation before presentation including upright feeding and treatment with proton pump inhibitors with or without other gastroprotectants. Thirteen of the 14 dogs had ME at the time of diagnosis for LES‐AS. Hypothyroidism was ruled out in 12 of 14 dogs by measurement of serum total thyroxine (T4) and thyroid stimulating hormone concentrations. Hypoadrenocortisolism was ruled out in 12 of 14 dogs either by baseline serum cortisol concentrations (>2 μg/dL, >55 nmol/L) or ACTH stimulation test. Acetylcholine receptor antibody testing was performed and found to be negative in 12 of 14 dogs. In the remaining dogs, clinicians elected to forgo testing based on lack of supporting clinical signs. A history of aspiration pneumonia was reported in 5 of 14 dogs.
3.2. Endoscopy
Esophagoscopy and abbreviated gastroscopy were performed uneventfully in all 14 dogs. Esophageal diverticula were identified at the thoracic inlet in 2 of 14 dogs corresponding to lesions observed on VFSS. Bone fragments and ingesta were present in the dependent region of the diverticulum in 1 dog. Despite being fasted for ≥12 hours, esophageal fluid was present in all 14 dogs. Roughened texture and esophageal hyperemia were observed in all 14 dogs. According to our inclusion criteria, no evidence of mechanical obstruction was identified in any dog.
3.3. Post‐mechanical dilation and BTA injection (clinical variables)
Dogs were presented for follow‐up with a median (IQR) of 21 days (14‐25 days) after treatment. Total post‐treatment follow‐up was a median (IQR) of 3.5 months (2‐4.8 months). At the time of the first post‐treatment evaluation, 100% of owners described subjective clinical improvement. Body weight was significantly (P < .001) increased after treatment. Median (IQR) pre‐ and post‐treatment BWs (kg) were 7.3 kg (5‐15.8 kg) and 8.1 kg (4.7‐23.25 kg), respectively. Median (IQR) percent increase in BW was 20.4% (12.7%‐25%). No dog lost weight during the evaluation period. Median BCS also was significantly (P < .001) increased after treatment. Median (IQR) pre‐ and post‐treatment BCS (9‐point scale) were 3 (3‐3.5) and 5 (4‐5) respectively. Frequency of regurgitation was significantly (P < .001) decreased after treatment. The median (IQR) decrease in regurgitation as recorded by owners was 80% (50%‐85%). Median (IQR) duration of effect was 40 (17–53) days.
3.4. Post‐mechanical dilation and BTA injection (VFSS parameters)
Pre‐ and post‐treatment VFSS findings are presented in Table 2. After LES mechanical dilation with BTA, all 14 dogs lacked detectable change to esophageal diameter or motility. Gastric filling was markedly improved in 12 of 14 dogs after treatment (Figure 4A, B).
Table 2.
Videofluoroscopic swallow study outcome parameters for dogs with lower esophageal sphincter achalasia‐like syndrome pre‐ and post‐treatment with lower esophageal sphincter mechanical dilation (pneumatic/bougienage) + botulinum toxin A injections
| Pretreatment (out of 14 dogs) | Posttreatment (out of 14 dogs) | |
|---|---|---|
| Presence of ME | 13 | 13 |
| Esophageal motility | Amotile: 7 | Amotile: 7 |
| Hypomotile: 5 | Hypomotile: 5 | |
| Hypermotile: 2 | Hypermotile: 2 | |
| Normal: 0 | Normal: 0 | |
| Volume of gastric filling (sitting or standing) | Small: 10 | Small: 0 |
| Medium: 4 | Medium: 10 | |
| Large: 0 | Large: 4 | |
| Volume of gastric filling (5 min upright) | Small: 5 | Small: 0 |
| Medium: 7 | Medium: 3 | |
| Large: 2 | Large: 11 |
For consistency, dogs were evaluated in the same position (sitting or standing) before and after treatment.
Figure 4.
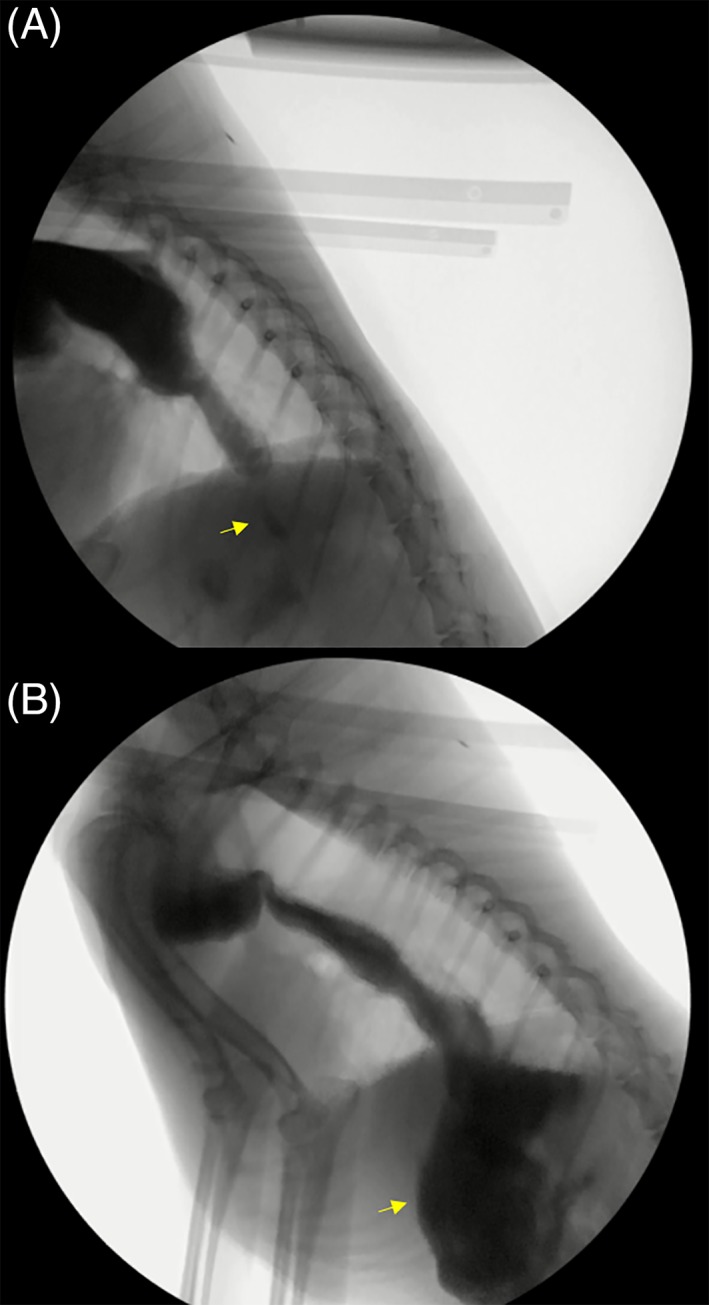
Still lateral image of a 6‐year‐old female spayed mixed breed dog from a videofluoroscopic swallow study showing maximal gastric filling (arrows) in response to hydrostatic pressure before (A) and after (B) treatment with mechanical dilation and botulinum toxin A. Only a small amount of gastric filling was appreciated before intervention; after treatment, there was a large amount of gastric filling observed, indicative of improvement in the functional lower esophageal sphincter obstruction
3.5. Complications after BTA injection with mechanical dilation
Complications after BTA and mechanical dilation were reported for 2 of the 14 dogs. One dog developed post‐treatment aspiration pneumonia. This dog responded well to medical management and recovered uneventfully. No long‐term consequences related to aspiration pneumonia were identified. Improvement in clinical signs and VFSS were observed for this dog after recovery from aspiration pneumonia. In another dog, a gastroduodenal‐esophageal intussusception with a Type IV hiatal hernia was identified 3 weeks after mechanical dilatation with BTA injections (Figure 5A‐D). The dog underwent surgery in which the stomach, spleen, and a portion of the duodenum and pancreas were identified in the distal esophagus. The hernia was surgically corrected during exploratory celiotomy and left‐sided gastropexy. Substantial clinical improvement in clinical signs had been recorded for this dog before it developed complications.
Figure 5.
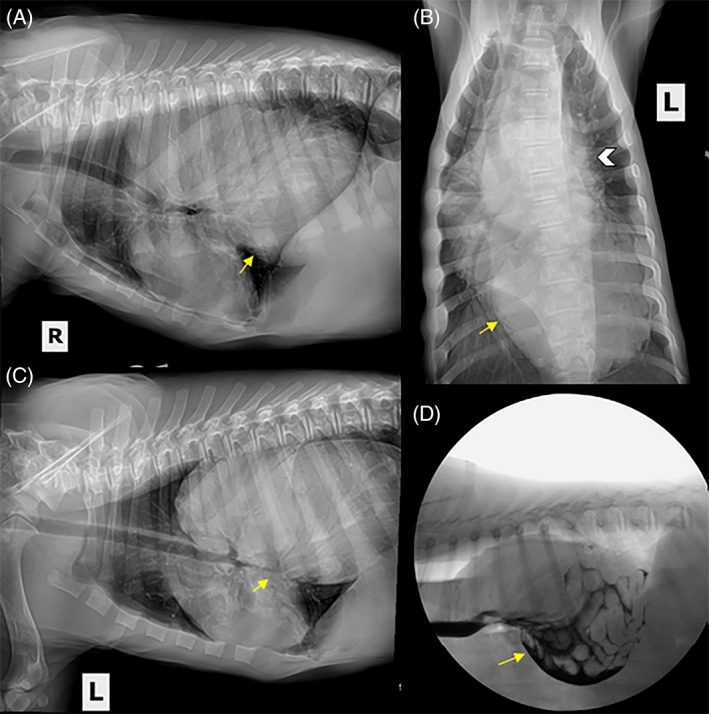
Three view (A: right lateral, B: dorsoventral, C: left lateral) thoracic radiographs of an 8‐week‐old MI Irish Wolfhound presenting for lethargy, regurgitation, and decreased appetite after treatment with mechanical dilation and botulinum toxin A. A large soft tissue opaque structure is present in the distal esophagus (arrows). The cardiac silhouette is obscured by the esophageal contents (white arrowhead, B). D, A still lateral image from a videofluoroscopic swallow study showing a gastroduodenal‐esophageal intussusception with Type IV hiatal hernia. The dog is in sternal recumbency and freely consumed liquid containing 25% iohexol (350/mg iodine/mL). Contrast outlines intestinal loops within the dilated distal esophagus (arrow)
3.6. Heller myotomy and Dor fundoplication
Dogs with documented improvement after mechanical dilation with BTA injections were considered candidates for surgical intervention. Six of 14 dogs underwent surgery (Heller myotomy with Dor fundoplication). The median (IQR) follow‐up for patients undergoing Heller myotomy with Dor fundoplication was 7 months (1‐21 months). In all 6 dogs, postsurgical clinical signs and VFSS features were similar to those at the first evaluation after mechanical dilation and BTA injections (ie, improved over baseline). In addition, 2 dogs had improvement in esophageal diameter and esophageal motility as measured by VFSS >6 months postoperatively, indicative of a delayed positive functional response to surgery. Of the dogs that showed improved motility, 1 dog was considered to have an amotile esophagus and the other a hypomotile esophagus. Evaluation of response with respect to patient age, duration of clinical signs, and LES substage could not be performed because of small sample size.
4. DISCUSSION
Idiopathic ME in dogs is a disorder with high morbidity and mortality that historically has lacked effective targeted treatment. A subgroup of these dogs with LES‐AS however has excellent clinical responses to mechanical dilation and BTA injections, with surgery being a more definitive, long‐term option. To identify which dogs with ME may benefit from these targeted treatments, use of a free‐feeding VFSS protocol is crucial to identify functional LES obstruction.5 In people with LES achalasia, relieving the esophageal‐gastric outflow obstruction significantly improves clinical signs.6, 8, 9, 11, 22 Comparably, significant clinical improvement, based on our previously described outcome variables, was observed in our population of dogs with LES‐AS that underwent targeted intervention using mechanical dilation and BTA injections, although the response was temporary and shorter than is typical in people.19, 21, 23 Surgery provided sustained clinical improvement compared to baseline, despite not resolving the ME and esophageal motility defects detected by VFSS. In all dogs, VFSS showed improvement in gastric filling after surgery. By promoting gastric filling, improvement in the aforementioned outcome variables (BW and BCS as well as decreased regurgitation frequency) would be expected even with continued defects in esophageal motility. Our data suggest that targeted intervention in dogs with LES‐AS may provide substantial clinical benefit in this patient population, providing hope for dogs that are refractory to traditional medical management.1
Megaesophagus is characterized by diffuse dilatation of the esophagus with decreased peristalsis. Unless, and sometimes even if, an underlying cause can be identified, treatment is largely supportive and carries a guard to poor prognosis. Reported median survival times range from 1 to 3 months with an overall fatality rate of 74%.1, 3 Unfortunately, most cases of ME are idiopathic with no clear understanding of an underlying pathologic process.1 In humans, esophageal motility disorders are better classified allowing for identification of patients with conditions that may benefit from targeted intervention.7 Achalasia, a primary esophageal motility disorder in people, results from a selective loss of inhibitory myenteric neurons leading to a failure of the LES to relax in response to pharyngeal swallowing and impaired esophageal peristalsis.24 It represents a rare cause of ME that responds to targeted intervention and is considered distinct from conditions that cause esophageal hypomotility without functional LES obstruction. This condition has been suspected in dogs, with a few case reports over the last 4 decades and most presumptive diagnoses being made without manometry or dynamic imaging studies.22, 25, 26, 27, 28, 29, 30 The lack of recognition of this syndrome in dogs may in part be a consequence of limitations in available diagnostic tests. High‐resolution manometry is considered the gold standard for diagnosis of LES achalasia in people. Unfortunately, this modality is not routinely performed in veterinary medicine because of limited availability, high cost, need for operator expertise, and patient compliance.29, 31 However, before the adoption of the HRM in people, contrast swallow studies were used to diagnose LES achalasia and, although only moderately sensitive, were considered highly specific for this condition.7, 16, 32 Only a few manometric studies in dogs with ME have been performed, and these did not identify LES achalasia to the extent documented in our study.31, 33 The response to treatment in our patient population makes misdiagnosis unlikely, and this discrepancy between our study and previous studies may reflect limitations in available diagnostic tests at the time those studies were performed. Alternatively, it may highlight the point that this condition reflects subpopulations of dogs and not all dogs with ME, making accurate diagnosis crucially important.
Videofluoroscopic swallow studies have long been considered the gold standard for evaluation of dysphagia in veterinary medicine, but because VFSS traditionally have been performed with dogs in lateral recumbency, and often by force‐feeding, the risk of aspiration made such studies relatively contraindicated in dogs with ME, a diagnosis that could be made by routine thoracic radiography. However, thoracic radiography provides essentially no information regarding esophageal motility, which has previously limited our understanding of the pathogenesis of the disease in dogs with ME. Using a protocol that allows dogs to stand and free‐feed, we documented that 61% of dogs with ME that would have been classified as “idiopathic” had underlying LES‐AS. These findings contrast with those of a large study of 216 dysphagic dogs in which VFSS performed with dogs in in lateral recumbency failed to identify any dogs with functional LES obstruction and historical manometric studies that failed to identify functional LES obstruction.33, 34 Furthermore, the use of unrestrained and free‐feeding VFSS protocols decreases the risk of aspiration to no more than would be expected in the dogs at home. To date, no dog at our institution has developed aspiration pneumonia after these unrestrained free‐feeding VFSS, including those with ME. Thus, expanding the population that can be evaluated safely by VFSS has allowed identification and characterization of dogs with LES‐AS, allowing for specific treatment.5
The primary goal in treating LES‐AS is early patient identification and treatment of esophageal‐gastric outflow obstruction. In people, this goal is achieved either by mechanical disruption of the LES by dilation or surgical myotomy or by lowering LES tone. Sildenafil has been used with variable effect in humans to lower LES tone.35 A recent study indicated improved clinical signs in puppies with congenital ME treated with sildenafil (without documentation of functional LES‐obstruction), perhaps supporting the role of increased LES tone in the pathogenesis of ME in dogs.36 In people, treatment is dictated largely by clinical variables, patient risk factors, and LES achalasia subclassification.20, 37 Subclassifications for LES achalasia in people are based on HRM, and treatment responses have been shown to vary based on achalasia subtype.37, 38 Although subtypes have been identified in dogs with LES‐AS based on fluoroscopy,5 numbers of affected animals are insufficient to attempt treatment based on subclassification. Furthermore, because achalasia subtypes in people are established by HRM rather than fluoroscopy, direct comparisons between subtypes in humans and dogs are not possible. As such, a combination of mechanical dilation and BTA injections was selected to maximize the likelihood of a response, and any dog with a positive response then subsequently was offered surgical intervention as a more lasting treatment.
In our study, treatment for LES‐AS resulted in significant clinical and VFSS improvement after treatment targeting LES functional obstruction. Clients perceived clinical improvement in 100% of dogs by 2‐3 weeks after mechanical dilation and BTA injections. This finding is crucial, considering the degree to which perception of quality of life impacts treatment decisions in veterinary patients.39 Although placebo effect may have played some role, this perceived clinical improvement is supported by pre‐ and post‐treatment BW, BCS, frequency of regurgitation, and extent of gastric filling. All the aforementioned metrics were significantly improved from baseline making a substantial placebo effect unlikely. Videofluoroscopic swallow studies documented improvement in passage of food from the esophagus to the stomach in the majority of dogs despite the persistence of ME and abnormal esophageal motility. This finding suggests that clinical improvement is secondary to relieving the functional LES obstruction and supports the role of functional LES obstruction in the pathogenesis of ME in a subpopulation of dogs. The persistence of ME and esophageal dysmotility in these patients may reflect the short time frame between treatment and evaluation. Alternatively, irreversible structural changes may have occurred secondary to chronic dilatation. As such, clients should be informed that complete resolution of ME, esophageal dysmotility, or both may not occur with targeted treatment but this does not diminish the importance of the often dramatic clinical improvement. Two of the 14 dogs did not show improved gastric filling after mechanical dilation and BTA injection despite improvement of other clinical variables. This finding has also been reported in people with achalasia after treatment and in 1 study was predictive of a long‐term treatment failure.40 More study is needed before conclusions regarding this finding can be made in dogs with LES‐AS.
Despite strongly positive responses to mechanical dilation with BTA injections, the relatively short duration of effect precludes their use as definitive (permanent) treatment for LES‐AS. The increased risk of aspiration under general anesthesia makes multiple repeated endoscopic treatments less appealing. As such, mechanical dilation with BTA may be best reserved as a test for definitive surgical intervention, for temporary treatment for patients considered too high risk for myotomy (eg, poor wound healing because of malnutrition), or as a temporary measure before surgery, particularly in young dogs before reaching skeletal maturity.
Although more data are needed, our study suggests that surgical intervention may provide sustained clinical improvement in dogs with LES‐AS and that mechanical dilation and BTA injections may help identify patients that may benefit from surgical intervention. Interestingly, 2 dogs showed delayed (>6 months later) evidence of decreased esophageal diameter and improved esophageal motility after surgical intervention compared to their initial VFSS evaluation. This observation is supported by findings in the human medical literature that suggest that esophageal pathology is secondary to increased LES tone, and treatment may result in a return to peristalsis in some patients without permanent damage.41, 42
The age range of dogs with LES‐AS was wide and inclusive of dogs with both congenital and acquired ME. Surgical intervention cannot be recommended until dogs reach skeletal maturity because gastric motility may be impacted by surgery, and final position of anatomic structures cannot be predicted in a growing animal. For these dogs, repeated treatment with mechanical dilation and BTA merits further evaluation. In people, mechanical dilation of the LES and injections of BTA can be repeated, but submucosal fibrosis may occur with repeated treatment, potentially complicating future surgical intervention.43, 44
Overall treatment complications in people with achalasia are approximately 6.3% with a peri‐procedural mortality of 0.1%. The most commonly reported complications after mechanical dilation and BTA injections in people include chest pain and mild heartburn, managed by antacids.45, 46 Of note, chest pain and heartburn cannot be specifically detected in dogs. More serious complications in people including mediastinitis, allergic reactions to BTA and LES perforation are rare.46 Complications after mechanical dilation with BTA in dogs included aspiration pneumonia and a Type IV hiatal hernia. Review of medical records could not identify a potential cause of the complications in the dog with the hiatal hernia, but concurrent diffuse gastrointestinal dysmotility should be considered.
In people, LES mechanical dilation and BTA injections are performed as independent interventions.13, 18, 38 However, despite combining these 2 procedures in dogs, the duration of effect was considerably shorter than has been reported in people for either procedure alone.10, 13, 19, 47 Dosing of BTA for achalasia is variable in people but ranges between 20 and 100 units.48 A total of 32 U (4 U/site) were selected based on the smaller size of dogs compared with humans and to minimize the risk of complications associated with excessive administration.45 Although doses were uniform for all dogs regardless of size, the study was not powered to detect a dose‐dependent response. Additional studies would be needed to determine if higher doses result in a longer duration of effect.49 Another explanation for the shorter duration of efficacy in dogs may involve anatomic differences between the canine and human esophagus. The entire canine esophagus consists of skeletal muscle compared to humans where the distal two‐thirds of the esophagus comprise smooth muscle. Botulinum toxin A acts by interacting with several proteins including synaptosomal‐associated protein (SNAP) 25 in the nerve terminal to prevent vesicle fusion and inhibiting the release of acetylcholine.50 Differences in regional expression of SNAP‐25 in the esophagus have been reported in other species and could account for differences in treatment response.51 Upregulation of SNAP‐25 mRNA also has been identified in rats after BTA injections into skeletal muscle.52 This finding suggests a possible role for SNAP‐25 in functional muscle recovery and could account for variable responses if species differences are documented. Alternatively, differences in collagen composition, due either to species variation or extent of fibrosis at the time of diagnosis, may impact local diffusion of BTA and subsequent response to treatment.
Anatomic differences also may contribute to a shortened response to mechanical dilation. The purpose of mechanical dilation is to weaken the LES by tearing muscle fibers.18 Differences in collagen vs muscle content may have made the extent of radial pressure applied by pneumatic dilation insufficient to achieve a sustained treatment response. Given the sustained treatment response seen after surgical myotomy, a greater extent of LES disruption may be needed. Mechanical dilation in adults is most commonly performed with balloons ranging from 3 to 4 cm in diameter. Smaller balloons are recommended in children, with balloons >3.5 cm being reserved for children older than 8 years.53 In our population, balloon and bougienage diameter were selected based on patient size and ranged between 1 and 3 cm. Selecting balloon size based on patient size was performed to decrease the risk of LES perforation. Blanching of the mucosa surrounding the LES was observed in all patients, suggesting resistance across the LES in response to balloon dilation and bougienage. Multiple dilation cycles were performed for each dog to maximize disruption of the LES. Multiple dilations with progressively increasing balloon diameter may result in a more sustained response but may be associated with increased risk of LES perforation.
5. CONCLUSIONS
Dogs with LES‐AS experienced marked clinical improvement after targeted intervention with mechanical dilation and BTA injections of the LES. Although the response was temporary, this finding establishes the role of functional LES obstruction in the pathogenesis of ME and esophageal dysphagia in dogs. Preliminary results from dogs with LES‐AS after surgical myotomy suggest that a positive response to mechanical dilation with BTA may identify dogs that could benefit from surgery, and that surgery may provide lasting clinical benefit despite persistence of ME. These interventions allow for often dramatic clinical improvement (improved quality of life, decrease episodes of regurgitation, weight gain, and improved BCS) in a subpopulation of dogs with ME associated with LES‐AS.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 Cranial (Left), Caudal (Right). Lateral projection of a still image from a VFSS. A baseline fluid line (arrow) is visible in the esophagus after a ≥ 12 hour fast prior to administration of oral contrast material. The top lip of the food bowl is marked by brackets. The dashed arrow points to a 1 cm calibration marker worn around the patient's neck.
Grobman ME, Hutcheson KD, Lever TE, Mann FA, Reinero CR. Mechanical dilation, botulinum toxin A injection, and surgical myotomy with fundoplication for treatment of lower esophageal sphincter achalasia‐like syndrome in dogs. J Vet Intern Med. 2019;33:1423–1433. 10.1111/jvim.15476
Funding information Mizzou Advantage Fund; Upright Canine Brigade
REFERENCES
- 1. McBrearty AR, Ramsey IK, Courcier EA, et al. Clinical factors associated with death before discharge and overall survival time in dogs with generalized megaesophagus. J Am Vet Med Assoc. 2011;238:1622‐1628. [DOI] [PubMed] [Google Scholar]
- 2. Mace S, Shelton G, Eddlestone S. Megaesophagus. Compendium. 2012;34:E1‐E1. [PubMed] [Google Scholar]
- 3. Harvey C, O'Brien J, Durie V, Miller DJ, Veenema R. Megaesophagus in the dog: a clinical survey of 79 cases. J Am Vet Med Assoc. 1974;165:443‐446. [PubMed] [Google Scholar]
- 4. Harris R, Grobman M, Allen M, et al. Standardization of a videofluoroscopic swallow study protocol to investigate dysphagia in dogs. J Vet Intern Med. 2017;31:383‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grobman M. Videofluoroscopic swallow study features of obstructive lower esophageal sphincter (LES) disorders in dogs ACVIM Forum 2017.
- 6. Gyawali C. Achalasia: new perspectives on an old disease. Neurogastroenterol Motil. 2016;28:4‐11. [DOI] [PubMed] [Google Scholar]
- 7. Gyawali CP, Bredenoord AJ, Conklin JL, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil. 2013;25:99‐133. [DOI] [PubMed] [Google Scholar]
- 8. Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high‐resolution manometry. Gastroenterology. 2013;145:954‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pohl D, Tutuian R. Achalasia: an overview of diagnosis and treatment. J Gastrointestin Liver Dis. 2007;16:297. [PubMed] [Google Scholar]
- 10. Pasricha PJ, Rai R, Ravich WJ, Hendrix TR, Kalloo AN. Botulinum toxin for achalasia: long‐term outcome and predictors of response. Gastroenterology. 1996;110:1410‐1415. [DOI] [PubMed] [Google Scholar]
- 11. Vaezi MF, Felix VN, Penagini R, et al. Achalasia: from diagnosis to management. Ann N Y Acad Sci. 2016;29:13176. [DOI] [PubMed] [Google Scholar]
- 12. Boeckxstaens G, Zaninotto G. Achalasia and esophago‐gastric junction outflow obstruction: focus on the subtypes. Neurogastroenterol Motil. 2012;24:27‐31. [DOI] [PubMed] [Google Scholar]
- 13. Eckardt V, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut. 2004;53:629‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ullal TV, Kass PH, Conklin JL, Belafsky PC, Marks SL. High‐resolution manometric evaluation of the effects of cisapride on the esophagus during administration of solid and liquid boluses in awake healthy dogs. Am J Vet Res. 2016;77:818‐827. [DOI] [PubMed] [Google Scholar]
- 15. Kempf J, Heinrich H, Reusch CE, Kook PH. Evaluation of esophageal high‐resolution manometry in awake and sedated dogs. Am J Vet Res. 2013;74:895‐900. [DOI] [PubMed] [Google Scholar]
- 16. Neyaz Z, Gupta M, Ghoshal UC. How to perform and interpret timed barium esophagogram. J Neurogastroenterol Motil. 2013;19:251‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huh CW, Youn YH, Chung H, Lee YC, Park H. Functional restoration of the esophagus after peroral endoscopic myotomy for achalasia. PLoS One. 2017;12:e0178414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238‐1249. [DOI] [PubMed] [Google Scholar]
- 19. Dobrucali A, Erzin Y, Tuncer M, Dirican A. Long‐term results of graded pneumatic dilatation under endoscopic guidance in patients with primary esophageal achalasia. World J Gastroenterol. 2004;10:3322‐3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uppal DS, Wang AY. Update on the endoscopic treatments for achalasia. World J Gastroenterol. 2016;22:8670‐8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonavina L, Nosadini A, Bardini R, Baessato M, Peracchia A. Primary treatment of esophageal achalasia: long‐term results of myotomy and Dor fundoplication. Arch Surg. 1992;127:222‐227. [DOI] [PubMed] [Google Scholar]
- 22. Knecht C, Eaddy J. Canine esophageal achalasia corrected by retrograde dilatation—a case report. J Am Vet Med Assoc. 1959;135:554. [PubMed] [Google Scholar]
- 23. Kahrilas PJ, Pandolfino JE. Treatments for achalasia in 2017: how to choose among them. Curr Opin Gastroenterol. 2017;33:270‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furuzawa‐Carballeda J, Torres‐Landa S, Valdovinos M, et al. New insights into the pathophysiology of achalasia and implications for future treatment. World J Gastroenterol. 2016;22:7892‐7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer CS. Achalasia or cardiospasm in great Dane puppies. Vet Med Small Anim Clin. 1968;63:574‐576. [PubMed] [Google Scholar]
- 26. Poppel MH, Lust FJ. Achalasia of the esophagus in a dachshund. Am J Roentgenol Radium Ther Nucl Med. 1962;88:741‐742. [PubMed] [Google Scholar]
- 27. Stack W, Thomson J, Suyama A. Achalasia of the esophagus with megaesophagus in a dog. J Am Vet Med Assoc. 1957;131:225‐226. [PubMed] [Google Scholar]
- 28. Boria PA, Webster CR, Berg J. Esophageal achalasia and secondary megaesophagus in a dog. Can Vet J. 2003;44:232. [PMC free article] [PubMed] [Google Scholar]
- 29. Kempf J, Beckmann K, Kook P. Achalasia‐like disease with esophageal pressurization in a myasthenic dog. J Vet Intern Med. 2014;28:661‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Earlam RJ, Zollman PE, Ellis FH. Congenital oesophageal achalasia in the dog. Thorax. 1967;22:466‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kempf J, Lewis F, Reusch CE, Kook PH. High‐resolution manometric evaluation of the effects of cisapride and metoclopramide hydrochloride administered orally on lower esophageal sphincter pressure in awake dogs. Am J Vet Res. 2014;75:361‐366. [DOI] [PubMed] [Google Scholar]
- 32. El‐Takli I, O'Brien P, Paterson WG. Clinical diagnosis of achalasia: how reliable is the barium x‐ray? Can J Gastroenterol. 2006;20:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diamant N, Szczepanski M, Mui H. Manometric characteristics of idiopathic megaesophagus in the dog: an unsuitable animal model for achalasia in man. Gastroenterology. 1973;65:216‐223. [PubMed] [Google Scholar]
- 34. Pollard RE, Marks SL, Cheney DM, Bonadio CM. Diagnostic outcome of contrast videofluoroscopic swallowing studies in 216 dysphagic dogs. Vet Radiol Ultrasound. 2017;58:373‐380. [DOI] [PubMed] [Google Scholar]
- 35. Bortolotti M, Mari C, Lopilato C, Porrazzo G, Miglioli M. Effects of sildenafil on esophageal motility of patients with idiopathic achalasia. Gastroenterology. 2000;118:253‐257. [DOI] [PubMed] [Google Scholar]
- 36. Quintavalla F, Menozzi A, Pozzoli C, et al. Sildenafil improves clinical signs and radiographic features in dogs with congenital idiopathic megaoesophagus: a randomised controlled trial. Vet Rec. 2017;180:404‐404. [DOI] [PubMed] [Google Scholar]
- 37. Kahrilas PJ, Bredenoord AJ, Fox M, et al. Advances in the management of oesophageal motility disorders in the era of high‐resolution manometry: a focus on achalasia syndromes. Nat Rev Gastroenterol Hepatol. 2018;15:323. [DOI] [PubMed] [Google Scholar]
- 38. Moonen A, Boeckxstaens G. Finding the right treatment for achalasia treatment: risks, efficacy, complications. Curr Treat Option Gastroenterol. 2016;14:420‐428. [DOI] [PubMed] [Google Scholar]
- 39. Yeates J, Main D. Assessment of companion animal quality of life in veterinary practice and research. J Small Anim Pract. 2009;50:274‐281. [DOI] [PubMed] [Google Scholar]
- 40. Vaezi MF, Baker ME, Achkar E, Richter JE. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50:765‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roman S, Kahrilas PJ, Mion F, et al. Partial recovery of peristalsis after myotomy for achalasia: more the rule than the exception. JAMA Surg. 2013;148:157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patti M, Galvani C, Gorodner M, et al. Timing of surgical intervention does not influence return of esophageal peristalsis or outcome for patients with achalasia. Surg Endosc Other Interv Tech. 2005;19:1188‐1192. [DOI] [PubMed] [Google Scholar]
- 43. Horgan S, Hudda K, Eubanks T, McAllister J, Pellegrini CA. Does botulinum toxin injection make esophagomyotomy a more difficult operation? Surg Endosc. 1999;13:576‐579. [DOI] [PubMed] [Google Scholar]
- 44. Patti MG, Feo CV, Arcerito M, et al. Effects of previous treatment on results of laparoscopic Heller myotomy for achalasia. Dig Dis Sci. 1999;44:2270‐2276. [DOI] [PubMed] [Google Scholar]
- 45. Yamaguchi D, Tsuruoka N, Sakata Y, Shimoda R, Fujimoto K, Iwakiri R. Safety and efficacy of botulinum toxin injection therapy for esophageal achalasia in Japan. J Clin Biochem Nutr. 2015;57:239‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eckardt AJ, Eckardt VF. Current clinical approach to achalasia. World J Gastroenterol. 2009;15:3969‐3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Annese V, Bassotti G, Coccia G, et al. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. Gut. 2000;46:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuhn MA, Belafsky PC. Management of cricopharyngeus muscle dysfunction. Otolaryngol Clin North Am. 2013;46:1087‐1099. [DOI] [PubMed] [Google Scholar]
- 49. Kozarek R, Gelfand M, Patterson D, et al. Randomized prospective trial of 50 vs 100 IU BoTox (R) for achalasia—long term follow‐up. Gastroenterology. 1997;112(4):A184‐A184. [Google Scholar]
- 50. Nigam PK, Nigam A. BOTULINUM toxin. Indian J Dermatol. 2010;55:8‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ji J, Lau H, Sheu L, Diamant NE, Gaisano HY. Distinct regional expression of SNARE proteins in the feline oesophagus. Neurogastroenterol Motil. 2002;14:383‐394. [DOI] [PubMed] [Google Scholar]
- 52. Ma J, Shen J, Lee CA, et al. Gene expression of nAChR, SNAP‐25 and GAP‐43 in skeletal muscles following botulinum toxin a injection: a study in rats. J Orthop Res. 2005;23:302‐309. [DOI] [PubMed] [Google Scholar]
- 53. Franklin AL, Petrosyan M, Kane TD. Childhood achalasia: a comprehensive review of disease, diagnosis and therapeutic management. World J Gastrointest Endosc. 2014;6:105‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cranial (Left), Caudal (Right). Lateral projection of a still image from a VFSS. A baseline fluid line (arrow) is visible in the esophagus after a ≥ 12 hour fast prior to administration of oral contrast material. The top lip of the food bowl is marked by brackets. The dashed arrow points to a 1 cm calibration marker worn around the patient's neck.


